Abstract
Polycystic ovary syndrome (PCOS), the most common endocrinopathy in women of reproductive age, is characterized by hyperandrogenism, anovulation, and polycystic ovaries. Although its etiology is unknown, excess androgens are thought to be a critical factor driving the pathology of PCOS. We previously demonstrated that continuous exposure to the aromatase inhibitor letrozole (LET) in mice produces many hallmarks of PCOS, including elevated testosterone (T) and luteinizing hormone, anovulation, and obesity. In the current study, we sought to determine whether androgen receptor (AR) actions are responsible for any of the phenotypes observed in LET mice. C57BL/6 female mice were subcutaneously implanted with LET or placebo control and subsequently treated with the nonsteroidal AR antagonist flutamide or vehicle control. Flutamide treatment in LET females reversed elevated T levels and restored ovarian expression of Cyp17a1 (critical for androgen synthesis) to normal levels. Pituitary expression of Lhb was decreased in LET females that received flutamide treatment, with no changes in expression of Fshb or Gnrhr. Flutamide treatment also restored estrous cycling and reduced the number of ovarian cyst-like follicles in LET females. Furthermore, body weight and adipocyte size were decreased in flutamide-treated LET females. Altogether, our findings provide strong evidence that AR signaling is responsible for many key reproductive and metabolic PCOS phenotypes and further establish the LET mouse model as an important tool for the study of androgen excess.
Female mice were treated with letrozole or placebo and then exposed to 2.5 weeks of flutamide or control. We found that flutamide treatment reversed many PCOS phenotypes induced by letrozole.
Polycystic ovary syndrome (PCOS) is the most common reproductive disorder in women of reproductive age, affecting 5% to 10% of the population (1–4). PCOS is a complex and heterogeneous disorder characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovaries and frequently presents with metabolic comorbidities, including obesity and insulin resistance (5). In women, elevated androgens are associated with obesity (6), which can exacerbate the pathology of PCOS (5). In addition to hyperandrogenism, PCOS is characterized by a distinct neuroendocrine phenotype involving persistent, rapid gonadotropin-releasing hormone (GnRH) pulsatility, which favors synthesis and secretion of luteinizing hormone (LH) over follicle-stimulating hormone (FSH) and leads to an increased ratio of LH to FSH in women with PCOS (4, 7, 8). Elevated LH stimulates ovarian theca cells to produce excess androgens, whereas low FSH contributes to impaired folliculogenesis and anovulation (4, 9). These changes in GnRH and gonadotropin release are likely due to alterations in sex steroid feedback caused by abnormal steroidogenesis of the PCOS ovary. Indeed, women with PCOS display increased activity of 17α-hydroxylase (encoded by Cyp17a1), the rate-limiting enzyme in androgen biosynthesis (10, 11), and also exhibit decreased sensitivity to progesterone (P4) negative feedback (12, 13). Although the etiology of PCOS is unknown, excess androgens are thought to be a key factor driving the pathology of this disorder, evidenced by data showing that loss of androgen receptor (AR) signaling improves or prevents PCOS phenotypes in animal models (14–16), and CAG repeat polymorphisms in the AR gene may contribute to hyperandrogenism in women with PCOS (17–20). Furthermore, treatment with AR antagonists improves many symptoms in women with PCOS, including responsiveness to P4 (7, 21). Thus, one possibility is that excess androgens may be acting through AR, which is expressed throughout the hypothalamic-pituitary-gonadal axis, to decrease sensitivity to P4 negative feedback. Impaired negative feedback would in turn promote neuroendocrine dysfunction and contribute to the ovarian features of PCOS. In addition to improving neuroendocrine feedback responses, antiandrogen treatment has been shown to restore ovulatory cycles, improve hirsutism scores, decrease androgen levels, and improve lipid profiles in women with PCOS (22, 23), further implicating androgen actions through AR as important contributors to PCOS pathophysiology.
Animal models have been a valuable resource in elucidating possible mechanisms underlying the pathology of PCOS in women. Several strategies have been used to develop animal models of PCOS, including exposure to androgens, estrogens, progesterone receptor antagonists, and genetic manipulations (24). Mouse models of PCOS have been of particular interest and are advantageous due to the ease with which mice can be genetically manipulated. Dihydrotestosterone (DHT), a potent and nonaromatizable androgen, has been shown to induce many symptoms of PCOS when administered to mice. Prenatal DHT treatment [prenatal androgenization (PNA)] results in several endocrine phenotypes associated with PCOS, including irregular estrus cycles and resistance to P4 (25–29), although it fails to faithfully reproduce many of the metabolic features of PCOS, including obesity (28, 30), and thus may best represent lean women with PCOS (31). Conversely, chronically administered DHT from puberty to adulthood primarily recapitulates the metabolic phenotypes associated with PCOS, including obesity, increased adiposity, enlarged adipocytes, and insulin resistance (32), but only produces a subset of the many reproductive endocrine phenotypes observed in women with PCOS. Altogether, these models demonstrate that exogenous androgen administration at different developmental stages in mice is sufficient to produce some but not all aspects of human PCOS.
We previously developed a new mouse model of PCOS using the nonsteroidal aromatase inhibitor letrozole (LET) (33), following a similar model in rats (34, 35). Aromatase is encoded by Cyp19a1 and is the cytochrome p450 enzyme necessary for conversion of androgens to estrogens. LET models of PCOS are based on the findings that genetic variants of Cyp19a1 are associated with PCOS in women (36, 37), and follicular fluid from the ovaries of women with PCOS exhibits an increased ratio of androgens to estrogens due to increased activity of 17α-hydroxylase (10, 11). We demonstrated that female mice continuously treated with LET from 4 weeks of age are hyperandrogenic and display many PCOS phenotypes, including increased testosterone (T) and LH, polycystic ovaries, and acyclicity after 5 weeks of treatment (33). LET females also exhibit increased body weight and adiposity. Interestingly, estrogen was not depleted in LET females after 5 weeks, likely due to their ∼10-fold upregulation of Cyp19a1. Altogether, LET successfully recapitulated many reproductive and metabolic features of human PCOS.
Although the LET mouse model exhibits hyperandrogenemia, it remains to be determined which, if any, aspects of the PCOS phenotype in LET mice can be attributed to androgen actions. Understanding the specific contributions of androgens to LET PCOS phenotypes will allow insight into the mechanisms underlying reproductive and metabolic dysfunction in this model and lay the groundwork for combining transgenic mouse models with LET treatment to study the pathogenesis of PCOS. In the current study, we hypothesized that several PCOS phenotypes in the LET mouse model would be reversed with antiandrogen treatment if AR signaling is a primary factor causing the phenotypes in this model. To approach this question, we treated LET mice with the nonsteroidal AR antagonist flutamide and found that many PCOS phenotypes were ameliorated with flutamide treatment, further establishing the role of AR and the importance of the LET mouse as a model of androgen excess.
Methods
Animals and treatment
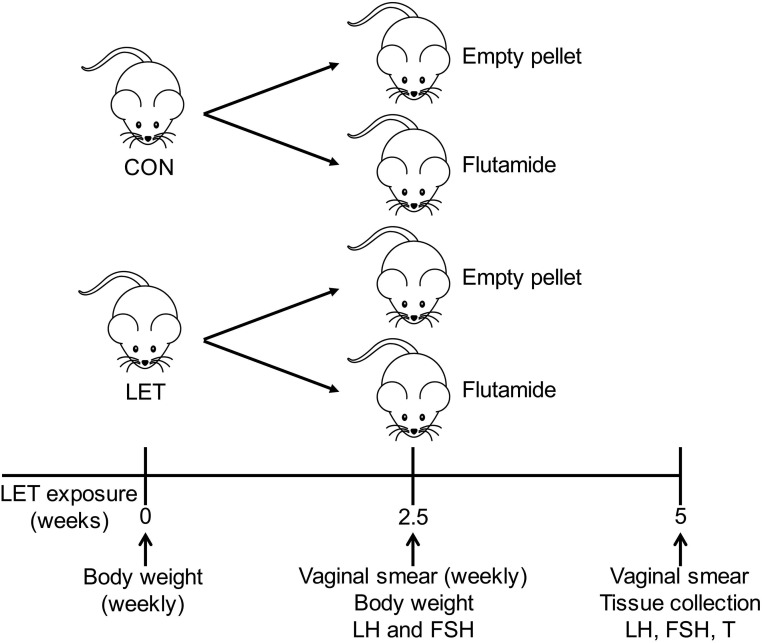
Female C57BL/6 mice (Harlan Laboratories) were maintained on a 12:12 light/dark cycle with ad libitum standard chow diet and water. All experimental procedures were approved by the University of California, San Diego Institutional Animal Care and Use Committee. Mice were housed two females per cage according to treatment. Based on our previous study (33), we subcutaneously implanted 4-week-old females with custom-made continuous-release pellets (Innovative Research of America) containing 50 μg/d LET (Fitzgerald) or placebo control (CON) (n = 20 per treatment). Mice were weighed weekly from the time of LET or CON pellet implantation, and 2.5 weeks after mice received a LET or CON pellet, they were subcutaneously implanted with a second pellet containing 20 mg flutamide (38) or an empty pellet (n = 10 per group) (Fig. 1). The 20-mg flutamide pellets were made in-house by packing 1.5 cm flutamide (Sigma; catalog no. F9397) into 2-cm Silastic tubing (1.47 mm inner diameter, 1.96 mm outer diameter) and sealing with silicone sealant. This dose of flutamide was validated by implantation in intact male mice for 3 weeks, after which we observed a significant increase in both serum LH and pituitary expression of Lhb, indicating that 20 mg flutamide was sufficient to block the negative feedback effects of endogenous androgens (Supplemental Fig. 1).
Figure 1.
Experimental design. C57BL/6 female mice were implanted with pellets containing LET or CON at 4 weeks of age. Halfway through the 5-week treatment paradigm, mice received a second pellet containing 20 mg flutamide or an empty pellet, creating four groups. Body weights were measured weekly throughout the study. Vaginal smears were taken at the time of flutamide or empty pellet implantation (and weekly thereafter) to assess estrous cycle stage, and tail blood was taken to measure serum LH and FSH. Mice were euthanized at the end of the 5-week study and tissues and serum collected for analysis.
Estrous cycle assessment
Vaginal smears were taken at the time of flutamide or empty pellet implantation (2.5 weeks of exposure to LET) and weekly thereafter at 3, 4, and 5 weeks of exposure to LET (Fig. 1). Mice were not cycled daily to reduce stress caused by handling. Estrous cycle stage was evaluated for these time points by light microscopic analysis of the predominant cell type in vaginal smear samples. Diestrus was characterized by the presence of predominantly leukocytes, proestrus by the presence of mostly nucleated cells, estrus by the presence of cornified epithelial cells, and metestrus by the presence of leukocytes with some cornified epithelial cells (39).
Tissue collection and histology
After 5 weeks of LET or CON treatment (2.5 weeks of flutamide or empty pellet treatment), mice were weighed and a vaginal smear collected before euthanasia by CO2 inhalation. Blood was collected from the abdominal aorta. Pituitaries and one ovary per animal were collected, rapidly frozen on dry ice, and stored at −80°C. The parametrial fat pad and one ovary from each animal were fixed in 10% acetic acid, 30% formaldehyde, and 60% ethanol.
For histological analysis, fixed ovaries were paraffin embedded and then serially sectioned at 10 µm and stained with hematoxylin and eosin (Sigma-Aldrich). Corpora lutea, atretic cyst-like follicles (large, fluid-filled cysts exhibiting granulosa cell degeneration) (14), and hemorrhagic cysts were counted from every third slide per ovary (seven sections total per ovary). Ovarian features were identified and counted by an investigator blind to treatment group. Fixed parametrial adipose tissue was embedded in paraffin and then serially sectioned at 5 µm before staining with hematoxylin and eosin. Adipocyte number and area were quantified from two sections per sample using ImageJ Fiji software with the Adiposoft plugin (40).
Hormone assays
Tail blood was taken for analysis of serum LH and FSH at 2.5 weeks (time of flutamide or empty pellet implantation), and abdominal aorta blood was taken at the end of the 5-week study for analysis of LH, FSH, T, estradiol (E2), P4, dehydroepiandrosterone (DHEA), and androstenedione (A4). For both time points, serum was collected and stored at −20°C. LH and FSH were measured in-house on a MILIPLEX (MPTMAG-49K; Millipore) from 5 µL serum per sample. LH: lower detection limit: 5.7 pg/mL, intra–assay coefficient of variation (ACOV) 16.8%, and inter-ACOV 7.05%. FSH: lower detection limit: 24.5 pg/mL, intra-ACOV 13.8%, and inter-ACOV 5.84%. Serum T, E2, and P4 were measured by the University of Virginia Ligand Core Facility using mouse enzyme-linked immunosorbent assays (range, 10 to 1600 ng/dL, 3 to 300 pg/mL, and 0.15 to 40 ng/μL, respectively). DHEA and A4 were measured by the University of Virginia Ligand Core Facility using human enzyme-linked immunosorbent assays (range, 0.37 to 30 ng/mL and 0.1 to 10 ng/mL, respectively). For each hormone assay, all data shown are above the level of detection of the assay.
Quantitative real-time polymerase chain reaction of ovary and pituitary genes
Total RNA from pituitary and ovary was isolated using an RNeasy Mini Plus kit, which also removes genomic DNA (Qiagen). Complementary DNA (cDNA) was made by reverse transcription of total RNA using an iScript cDNA synthesis kit (Bio-Rad Laboratories). cDNA products were detected using SYBR Green Supermix (Bio-Rad Laboratories) on a Bio-Rad CFX Connect quantitative real-time polymerase chain reaction system (Bio-Rad Laboratories). Data were analyzed by the 2−ΔΔCt method (41) by normalizing the gene of interest to Gapdh (pituitary) or to Ppia (ovary). Data are represented as mean fold change compared with CON (empty pellet) ± standard error of the mean. Primer sequences are listed in Table 1.
Table 1.
Primers
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Lhb | CTGTCAACGCAACTCTGG | ACAGGAGGCAAAGCAGC |
| Fshb | GCCGTTTCTGCATAAGC | CAATCTTACGGTCTCGTATACC |
| Gnrhr | GCCCCTTGCTGTACAAAGC | CCGTCTGCTAGGTAGATCATCC |
| Fshr | CTGGAGCAGGCAGAAAGCAG | CAGTTCAATGGCGTTCCG |
| Cyp17a1 | CTCCAGCCTGACAGACATTC | CTGAGAACACACTTGGGTCC |
| Cyp19a1 | GAGTCTGGATCAGTGGAGAG | CACGCTTGCTGCCGAATC |
| Ppia | AAGTTCCAAAGACAGCAGAAAAC | CTCAAATTTCTCTCCGTAGATGG |
| Gapdh | TGCACCACCAACTGCTTAG | GGATGCAGGGATGATGTTC |
Statistical analysis
All data are shown as mean ± standard error of the mean unless otherwise stated. Statistical analyses were performed using an unpaired two-tailed Student t test or two-way analysis of variance (ANOVA) (to assess the main effects of LET, flutamide, and LET × flutamide interaction) as indicated in the figure legends. Data collected before flutamide or empty pellet implantation were analyzed by two-way ANOVA to ensure that there were no preexisting differences between animals that would go on to receive flutamide or empty pellet treatment within the LET or CON group. Because no preexisting differences within LET or CON were detected, only the main effects of LET are reported prior to flutamide or empty pellet treatment. When significant LET × flutamide interactions were detected, two-way ANOVA was followed by Tukey post hoc analysis. For all analyses, P < 0.05 was considered statistically significant. One animal was found to be a statistical outlier with circulating T 4.3 standard deviations (SDs) above the mean (Fig. 2A, mean = 49.2 ng/dL and SD = 59.63) and 2.63 to 3.72 times higher than animals with similar LH concentrations. This animal was excluded from all subsequent analysis.
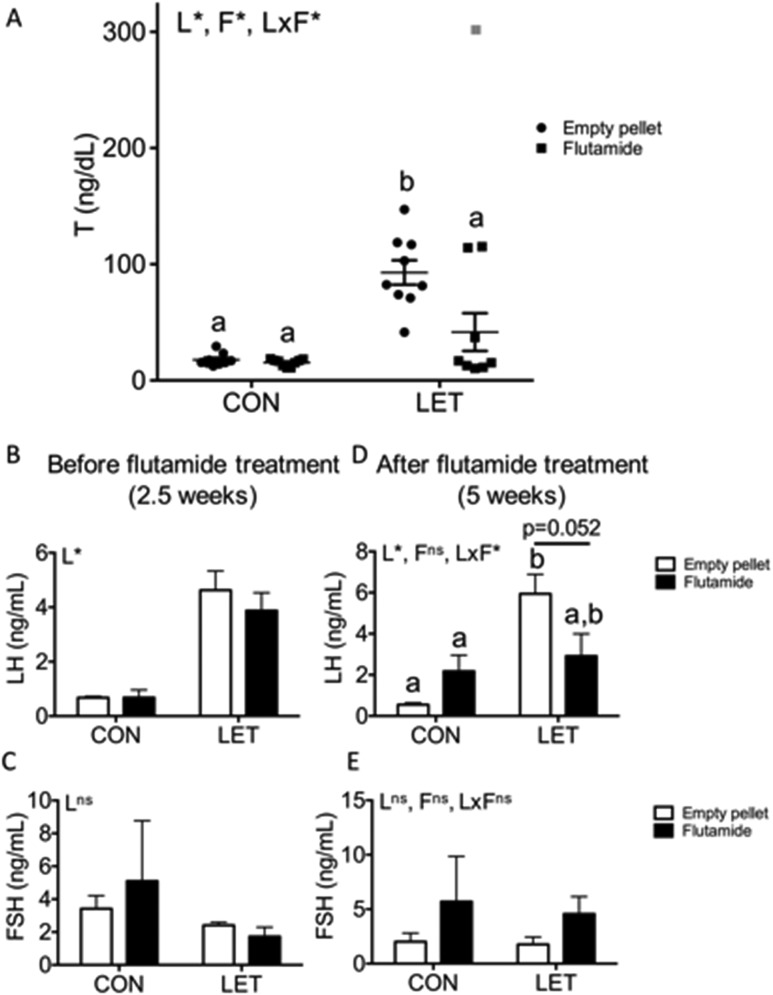
Figure 2.
Serum T and LH were decreased with flutamide treatment in LET females, with no change in FSH. (A) Circulating T levels plotted individually with bars indicating mean and standard error of the mean for each group; n = 8 to 10 per group. One LET animal that received flutamide treatment (shown in gray) was found to be a statistical outlier with an SD 4.3 times above the mean and was excluded from analysis. Circulating (B) LH and (C) FSH levels at 2.5 weeks of LET or CON treatment; n = 8 to 10 per group. Circulating (D) LH and (E) FSH levels at the end of the 5-week study; n = 8 to 10 per group. Data were analyzed by two-way ANOVA with results indicated as follows: L, LET; F, flutamide; LxF, LET × flutamide interaction; *, significant effect; ns, no significant effect. Different letters indicate statistically significant differences between groups by Tukey post hoc analysis.
Results
Circulating hormone levels are improved with flutamide treatment in LET females
To investigate whether flutamide treatment affects hyperandrogenism in LET females, we measured circulating T. One animal was found to be a statistical outlier with T 4.3 SDs outside the mean and was excluded from subsequent analysis (Fig. 2A, outlier shown in gray). We observed that LET [F(1, 31) = 30.22, P < 0.0001], flutamide [F(1, 31) = 8.457, P = 0.0067], and the interaction between these two factors [F(1, 31) = 6.999, P = 0.0127] had significant overall effects on circulating T. As we have previously reported (33), T was significantly increased in LET females compared with both CON groups (Fig. 2A, P < 0.0001). However, with flutamide treatment in LET females, T was significantly decreased compared with LET females that received empty pellets (Fig. 2A, P = 0.0028). There were no differences in T levels between either CON group compared with each other or when compared with LET females that received flutamide treatment. Circulating E2, P4, DHEA, and A4 levels were also measured, but most samples fell below the level of detection of these assays, regardless of treatment group. Thus, there was not sufficient power to detect differences in serum E2, P4, DHEA, or A4 levels among groups, if any existed (data not shown).
Circulating LH and FSH levels were measured from serum taken after 2.5 weeks of LET or CON treatment (on the day of flutamide or empty pellet implantation) and at the end of the 5-week study. At 2.5 weeks, LH was significantly elevated in animals that received LET compared with CON [Fig. 2B; F(1, 30) = 43.53, P < 0.0001], whereas there were no significant effects of LET on FSH [Fig. 2C; F(1, 31) = 2.554, P = 0.1202]. At the end of the study, the overall effect of LET [F(1, 32) = 13.88, P = 0.0008], but not flutamide [F(1, 32) = 0.7196, P = 0.4026], on LH was significant, and the interaction between these two factors was significant [F(1, 32) = 7.967, P = 0.0081]. LH levels remained significantly increased in LET females that received empty pellets compared with CON females that received empty pellet or flutamide treatment (Fig. 2D, P = 0.0001 and P = 0.019, respectively). The mean LH level of LET females that received flutamide treatment was not statistically different from any other group, although there was a nearly significant trend toward decreased serum LH in flutamide-treated LET females compared with LET females that received an empty pellet (Fig. 2D, P = 0.052). Circulating FSH levels remained unaffected by LET [F(1, 32) = 0.1361, P = 0.7146] or flutamide treatment [F(1, 32) = 2.973, P = 0.0943] at the end of the study (Fig. 2E).
Expression of pituitary messenger RNAs is altered with flutamide treatment in LET females
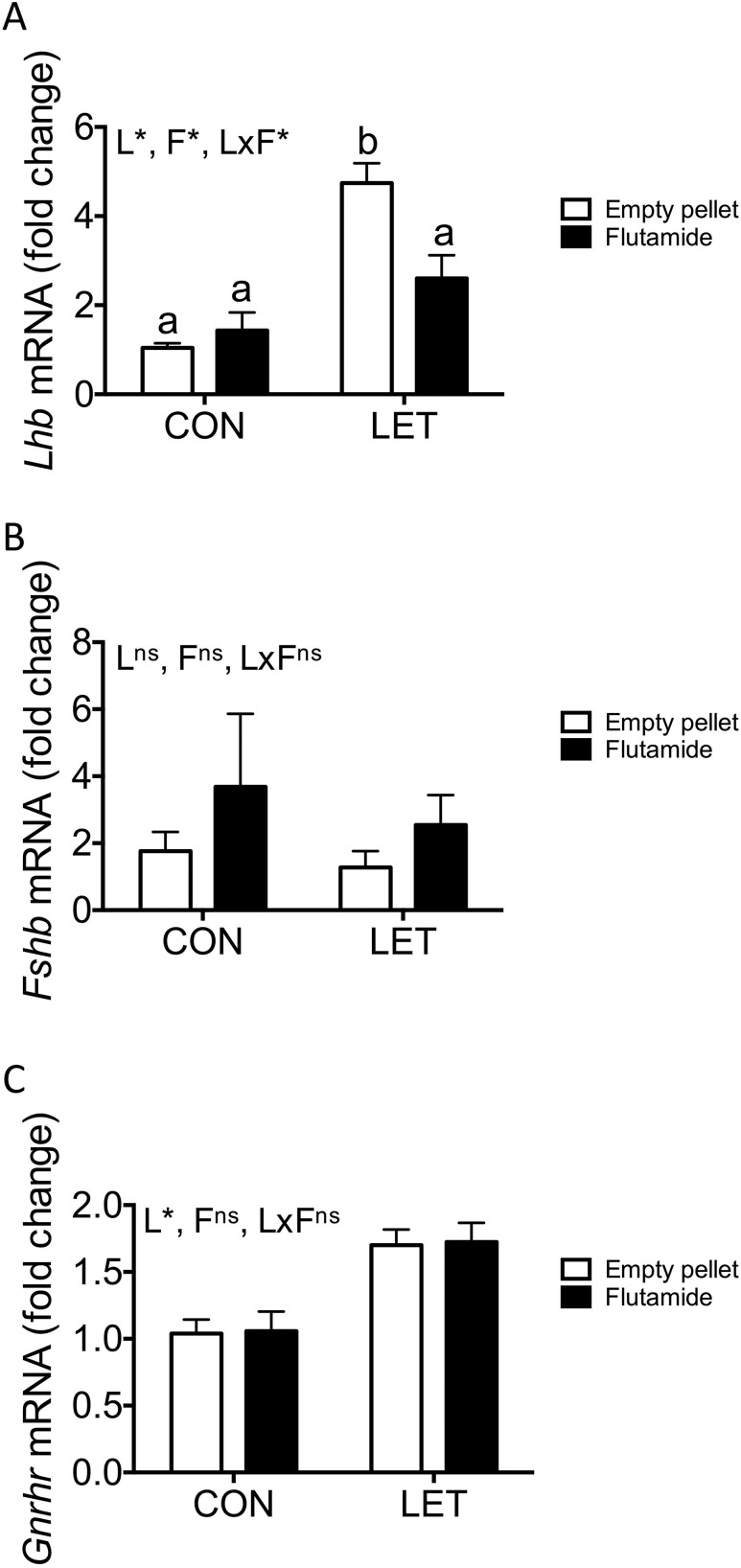
Both LET [F(1, 28) = 33.16, P < 0.0001] and flutamide treatment [F(1, 28) = 4.261, P = 0.0484], as well as the interaction between these two factors [F(1, 28) = 8.960, P = 0.0057], had significant overall effects on pituitary expression of Lhb. Consistent with the observed elevation in serum LH levels, LET females exhibited significantly increased expression of Lhb in the pituitary compared with either CON group (Fig. 3A, P < 0.0001), and this effect was reversed with flutamide treatment (Fig. 3A, P = 0.005). There was a trend toward increased Lhb in flutamide-treated LET mice compared with CON mice that received empty pellets, although this did not reach statistical significance (Fig. 3A, P = 0.0660). There was no effect of flutamide treatment on Lhb expression in CON females. We did not observe any effects of LET [F(1, 28) = 0.4188, P = 0.5228] or flutamide [F(1, 28) = 1.601, P = 0.2162] on expression of Fshb (Fig. 3B). Pituitary expression of Gnrhr was significantly affected by LET [Fig. 3C; F(1, 28) = 22.63, P < 0.0001], as we have previously reported, but not by flutamide treatment [F(1, 28) = 0.0025, P = 0.9602].
Figure 3.
Pituitary expression of key reproductive genes with flutamide treatment in LET females. Mean expression levels of (A) Lhb, (B) Fshb, and (C) Gnrhr pituitary messenger RNAs (mRNAs) from CON and LET females that received flutamide or empty pellet treatment; n = 7 to 10 per group. Data are represented as mean fold change compared with CON (empty pellet) ± standard error of the mean. Data were analyzed by two-way ANOVA with results indicated as follows: L, LET; F, flutamide; LxF, LET × flutamide interaction; *, significant effect; ns, no significant effect. Different letters indicate statistically significant differences between groups by Tukey post hoc analysis.
Flutamide treatment restores estrous cycling and improves ovarian morphology in LET females
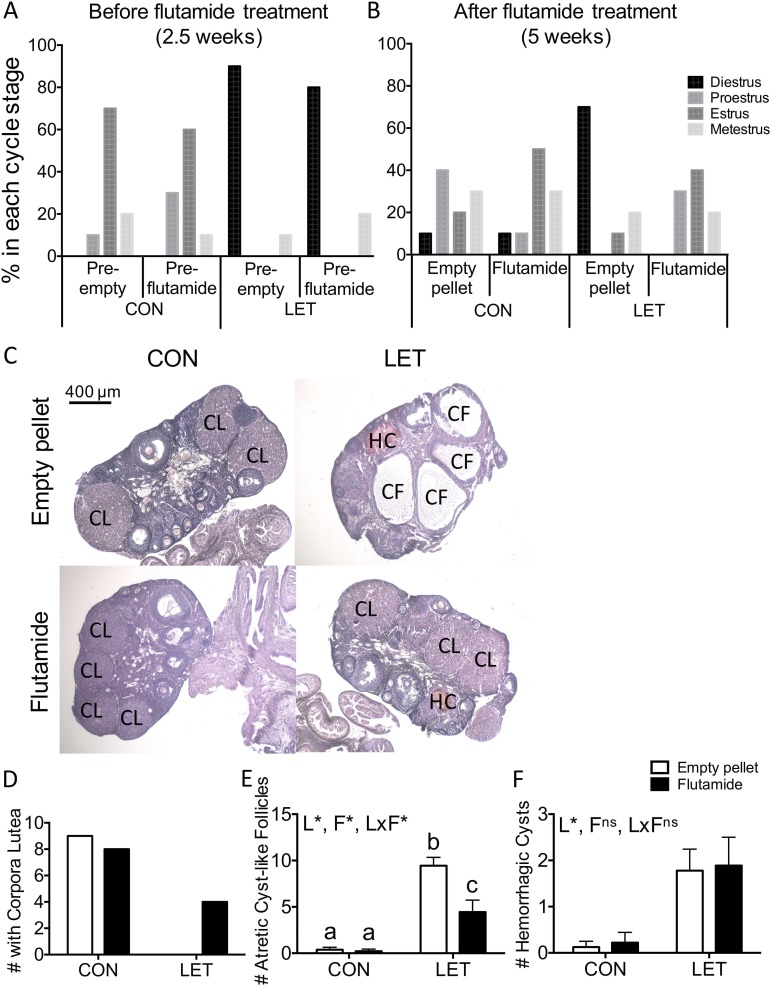
We previously observed that LET treatment disrupts estrous cyclicity in female mice and results in constant diestrus. To determine whether flutamide treatment had any effect on estrous cycling in CON or LET females, cycle stage was evaluated at the time of flutamide or empty pellet implantation and weekly thereafter. At 2.5 weeks of CON or LET treatment, evaluation of vaginal cytology revealed that CON females were in various cycle stages (estrus, metestrus, or proestrus), whereas most LET females were in diestrus, with a few exhibiting vaginal cytology characteristic of metestrus (Fig. 4A). At the end of the study (5 weeks), after 2.5 weeks of flutamide or empty pellet treatment, CON females were in various cycle stages (estrus, metestrus, proestrus, or diestrus), regardless of flutamide treatment (Fig. 4B). However, although LET females that received empty pellets continued to primarily exhibit diestrus, flutamide-treated LET females displayed various different cycle stages similar to CON animals (Fig. 4B), suggesting that flutamide treatment may have restored estrous cycling in LET females. There were no differences in ovary weights among groups (data not shown).
Figure 4.
Flutamide treatment restores estrous cyclicity and improves ovarian morphology in LET females. (A) Estrous cycle stages determined by vaginal cytology 2.5 weeks into the study, before empty pellet or flutamide treatment; n = 9 to 10 per group. CON females exhibited various cycle stages, whereas LET females primarily exhibited diestrus. (B) Estrous cycle stages determined by vaginal cytology after 2.5 weeks of empty pellet or flutamide treatment; n = 9 to 10 per group. LET females that received empty pellets primarily exhibited diestrus, whereas all other groups exhibited various cycle stages. (C) Representative images of hematoxylin and eosin–stained ovaries from CON and LET female mice that received empty pellet or flutamide treatment. n = 9 per group. (D) Number of ovaries that exhibited corpora lutea, an indicator of ovulation. (E) Number of atretic cyst–like follicles and (F) hemorrhagic cysts counted in ovaries by an investigator blind to treatment group. Data were analyzed by two-way ANOVA with results indicated as follows: L, LET; F, flutamide; LxF, LET × flutamide interaction; *, significant effect; ns, no significant effect. Different letters indicate statistically significant differences between groups by Tukey post hoc analysis. CF, atretic cyst–like follicle; CL, corpus luteum; HC, hemorrhagic cyst.
To confirm whether ovulation was restored with flutamide treatment in LET females, we examined ovaries for the presence of corpora lutea. CON ovaries displayed several corpora lutea, with no effect of flutamide treatment in CON animals (Fig. 4C and 4D). No corpora lutea were observed in any ovaries from LET animals that received empty pellet treatment. However, several (4/9) LET females that received flutamide treatment ovulated, evidenced by the presence of corpora lutea in their ovaries (Fig. 4C and 4D). We also examined ovaries for the presence of atretic cyst–like follicles and found significant effects of LET [F(1, 32) = 68.82, P < 0.0001], flutamide [F(1, 32) = 10.11, P = 0.0033], and an interaction between LET and flutamide [F(1, 32) = 9.252, P = 0.0047]. LET ovaries had a significantly increased number of atretic cyst–like follicles compared with either CON group (Fig. 4C and 4E; P < 0.0001). Although flutamide-treated LET mice had more atretic cyst–like follicles compared with both CON mice that received empty pellets (P = 0.0053) and CON mice that received flutamide treatment (P = 0.0041), atretic cyst–like follicles were significantly reduced compared with LET females that received empty pellets (Fig. 4C and 4E; P = 0.0006). LET [F(1, 32) = 17.06, P = 0.0002] but not flutamide [F(1, 32) = 0.0758, P = 0.7848] significantly affected the number of hemorrhagic cysts observed (Fig. 4C and 4F).
Expression of key ovarian messenger RNAs is restored with flutamide treatment in LET females
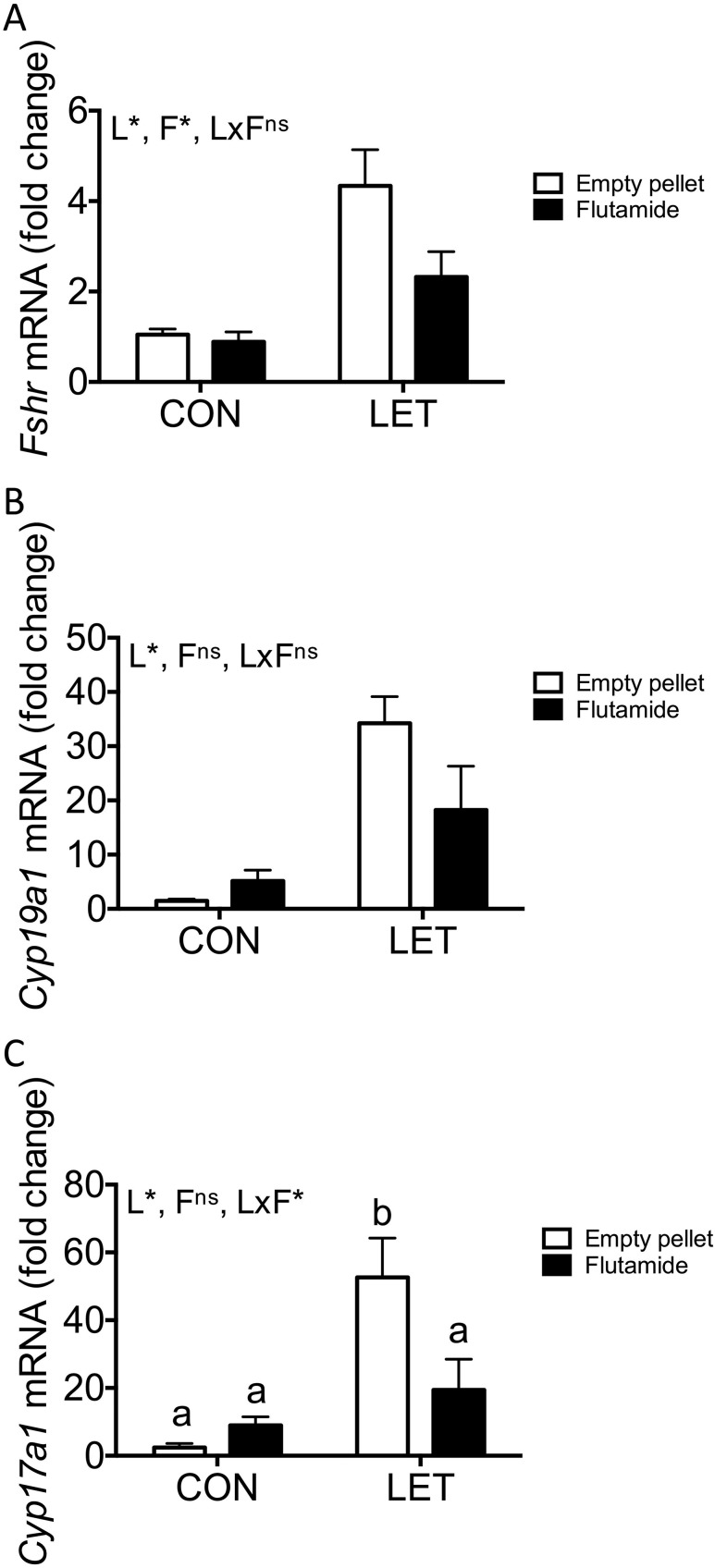
There was a significant overall effect of both LET [F(1, 29) = 21.08, P < 0.0001] and flutamide [F(1, 29) = 4.473, P = 0.0432] on ovarian expression of Fshr, although the interaction between these two factors was not significant [F(1, 29) = 3.264, P = 0.0812]. LET also had a significant effect on expression of Cyp19a1, which encodes aromatase, the enzyme inhibited by letrozole [F(1, 29) = 19.47, P = 0.0001]. However, Cyp19a1 was unaffected by flutamide [F(1, 29) = 1.404, P = 0.2456], and the interaction between LET and flutamide did not reach statistical significance [F(1, 29) = 3.589, P = 0.0682]. We also measured Cyp17a1 messenger RNA (mRNA), which is essential for androgen synthesis, and found that LET [F(1, 29) = 15.61, P = 0.0005] had significant overall effects on its expression, with an interaction between LET and flutamide [F(1, 29) = 6.709, P = 0.0149]. However, there was no significant effect of flutamide on Cyp17a1 [F(1, 29) = 3.003, P = 0.0937]. Although expression of Cyp17a1 was increased in LET females compared with either CON group (Fig. 5C, P < 0.001), flutamide treatment restored normal Cyp17a1 in LET females (P < 0.0203 compared with LET, empty pellet and P = 0.4275 compared with CON, empty pellet).
Figure 5.
Ovarian expression of key reproductive genes with flutamide treatment in LET females. Mean mRNA expression levels of (A) Fshr, (B) Cyp17a1, and (C) Cyp19a1 from ovaries of CON and LET females that received flutamide or empty pellet treatment; n = 7 to 10 per group. Data are represented as mean fold change compared with CON (empty pellet) ± standard error of the mean. Data were analyzed by two-way ANOVA with results indicated as follows: L, LET; F, flutamide; LxF, LET × flutamide interaction; *, significant effect; ns, no significant effect. Different letters indicate statistically significant differences between groups by Tukey post hoc analysis.
Flutamide treatment reverses the weight gain phenotype in LET females and restores normal adipocyte size
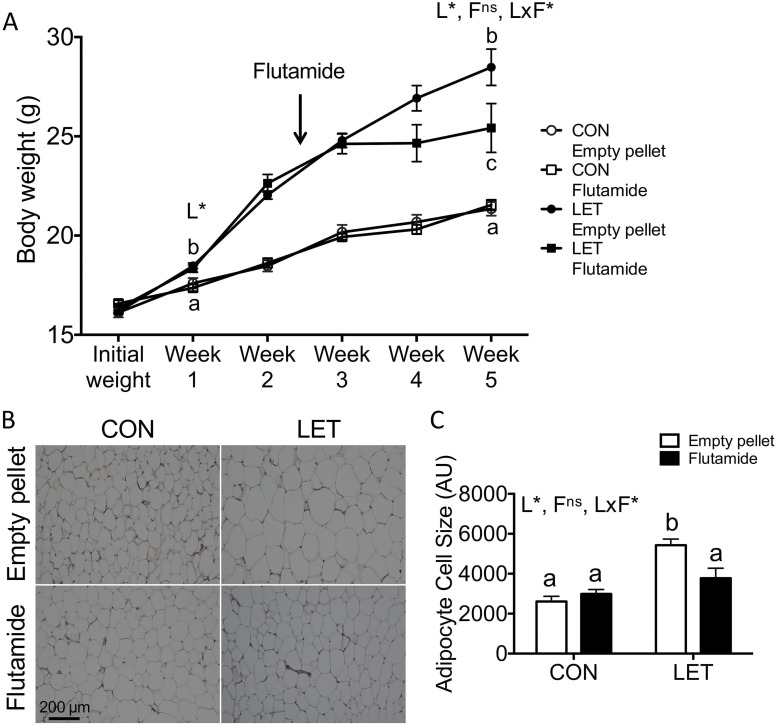
Body weight was measured weekly throughout the study and at the time of flutamide or empty pellet implantation (2.5 weeks). By 1 week of LET treatment, LET females weighed significantly more than CON mice [Fig. 6A; F(1, 36) = 21.79, P < 0.0001]. Change in body weight during the first 1.5 weeks of flutamide or empty pellet treatment (Fig. 6A, difference in weight between weeks 2.5 and 4) was significantly affected by flutamide [F(1, 36) = 15.80, P = 0.0003] but not LET [F(1, 36) = 3.448, P = 0.0715], and there was a significant interaction between LET and flutamide [F(1, 36) = 12.50, P = 0.0011]. After 1.5 weeks of exposure to flutamide, weight gain in LET females was comparable to both CON groups (P ≥ 0.4493), whereas LET mice that received empty pellets continued gaining excess weight (P < 0.01 compared with all other groups). At the end of the 5-week study, LET [F(1, 35) = 51.22, P < 0.0001] but not flutamide [F(1, 35) = 3.474, P = 0.0708] had significant effects on final body weight, and a significant interaction between LET and flutamide was detected [F(1, 35) = 4.455, P = 0.0420]. Final body weight in flutamide-treated LET females remained elevated compared with either CON group (P < 0.01) but was significantly decreased compared with LET females that received empty pellets (P = 0.0419).
Figure 6.
Flutamide treatment reduced body weight and adipocyte cell size in LET females. (A) Body weights were measured weekly throughout the study; n = 9 to 10 per group. LET females weighed significantly more than CON after 1 week of LET, and flutamide-treated LET females weighed significantly less compared with LET mice that received empty pellets after 2.5 weeks of flutamide treatment. (B) Representative images of hematoxylin and eosin (H&E)–stained parametrial adipose tissue from CON and LET female mice that received empty pellet or flutamide treatment; n = 5 to 7 per group. (C) Quantification of mean adipocyte cell size from H&E-stained sections of parametrial adipose tissue; n = 5 to 7 per group. Images were quantified using ImageJ Fiji software with the Adiposoft plugin. Data were analyzed by two-way ANOVA with results indicated as follows: L, LET; F, flutamide; LxF, LET × flutamide interaction; *, significant effect; ns, no significant effect. Different letters indicate statistically significant differences between groups by Tukey post hoc analysis.
We also evaluated parametrial adipose tissue to determine whether adipocyte size was affected by flutamide treatment in LET and CON mice. LET [F(1, 18) = 20.30, P = 0.0003] but not flutamide [F(1, 18) = 2.536, P = 0.1287] had significant overall effects on adipocyte size, and there was a significant interaction between LET and flutamide [F(1, 18) = 6.472, P = 0.0204]. As previously reported, LET females exhibited significantly increased mean adipocyte area compared with either CON group (Fig. 6B and 6C, P < 0.01). In CONs, flutamide treatment had no effect on adipocyte size (Fig. 6B and 6C). However, flutamide-treated LET mice exhibited significantly reduced mean adipocyte area compared with LET mice that received empty pellets (Fig. 6B and 6C, P = 0.0215).
Discussion
PCOS is a complex and heterogeneous reproductive endocrine disorder in women. Although its etiology is unknown, excess androgens are believed to be a key factor driving the pathophysiology of this disorder. Murine models in which exogenous androgens are given to induce features of PCOS have been a valuable research tool to study possible mechanisms underlying this disorder but do not fully recapitulate the spectrum of phenotypes seen in women with PCOS, which typically includes both reproductive and metabolic dysfunction. We previously demonstrated that LET treatment from 4 weeks of age produces many reproductive and metabolic phenotypes similar to human PCOS, including elevated T, increased ratio of LH to FSH, anovulation, obesity, and enlarged adipocytes. Because the LET model relies on increasing endogenous androgens by inhibition of aromatase rather than treating animals with exogenous androgens, it remained to be determined whether any LET phenotypes can be attributed to excess androgen signaling. Here we report that many features of the LET-induced mouse model of PCOS are ameliorated by treatment with the AR antagonist flutamide and thus can be attributed to actions of AR, further establishing the LET mouse as a useful tool in the study of androgen excess.
In women with PCOS, elevated T is typically driven by increased LH pulse frequency (4, 7, 21), which itself is likely a downstream consequence of dysregulated steroidogenesis and sex steroid feedback (12, 13). We found that treatment with flutamide significantly decreased serum T levels in LET mice, indicating that 2.5 weeks of continuous antiandrogen treatment can reverse hyperandrogenemia induced by LET. Similar effects of antiandrogens have been observed in women with PCOS, with chronic flutamide treatment decreasing both T and LH levels throughout the menstrual cycle (22, 42, 43) and improving symptoms of hyperandrogenism, including hirsutism (22, 43). Importantly, decreased secretion of sex hormone–binding globulin (SHBG) in women with PCOS increases T bioavailability (44, 45), making free vs total T a valuable clinical measure (1). Although antiandrogen treatment has been shown to increase serum SHBG in women with PCOS (22, 46), we were unable to evaluate this PCOS phenotype in the LET model because mice do not secrete SHBG postnatally (47). In addition to decreased T, we also observed decreased expression of Lhb mRNA in LET mice that received flutamide treatment and a nearly significant decrease in circulating LH (discussed further below). One mechanism by which LH promotes ovarian androgen production is positive regulation of Cyp17a1 (48, 49), the cytochrome p450 enzyme critical for androgen synthesis (10, 11). Cyp17a1 is upregulated in both women with PCOS (11, 50–52) and the LET model of PCOS. Reductions in circulating T with flutamide treatment in the LET mouse are likely due in part to decreased ovarian expression of Cyp17a1. In addition, there is some evidence that in humans, flutamide itself may weakly inhibit activity of 17α-hydroxylase, the gene product of Cyp17a1 (53, 54). Although flutamide decreases T in women with PCOS, it has not been determined whether this can be directly attributed to decreased Cyp17a1 expression or activity, although flutamide has been shown to decrease Cyp17a1 mRNA in cultured porcine ovarian follicles (55). Although androgens and AR have known roles in the positive regulation of Cyp19a1 (56, 57) and Fshr (58–60), ovarian expression of these genes was not significantly decreased following flutamide treatment in LET females. We previously found that ovarian expression of Lhcgr (encoding the LH/choriogonadotropin receptor) was unaffected by LET treatment in female mice (33). Recently, genetic variants of both FSHR and LHCGR (61–64) have been reported as PCOS risk loci in genome-wide association studies, highlighting critical roles of gonadotropin signaling in PCOS pathophysiology.
In addition to improved serum T levels, we observed changes in circulating gonadotropins and expression of key reproductive genes in the pituitary in LET females, some of which were ameliorated with flutamide treatment. Serum LH levels were significantly increased in LET females compared with CON at 2.5 weeks of treatment, indicating that this PCOS phenotype was established in LET mice prior to flutamide treatment. Indeed, elevated LH has been observed as early as 1 week after beginning LET treatment (65). After 2.5 weeks of flutamide treatment, LET females exhibited a nearly significant decrease in mean circulating LH levels compared with LET females that received empty pellets. Similar to our result, 3 weeks of flutamide treatment decreased LH in female aromatase knockout mice, although not to the level of wild-type CONs (66). Chronic flutamide administration also decreases circulating LH in women with PCOS and in hyperandrogenic women without PCOS (42). We also observed a significant decrease in expression of Lhb mRNA with flutamide treatment in LET mice compared with LET females that received empty pellets. As we previously reported, Gnrhr mRNA was significantly increased in LET females compared with CON, and this phenotype was not changed with flutamide treatment in LET mice, suggesting that increased Gnrhr is not an AR-dependent phenotype in this model. Although androgens have been implicated in the positive regulation of Gnrhr in mice (67, 68), GnRH is known to have potent stimulatory effects on Gnrhr expression (69–71). Thus, we speculate that rapid frequency GnRH secretion in LET females is sufficient to maintain high Gnrhr regardless of AR antagonism by flutamide. It has not been reported whether LET females exhibit increased GnRH pulsatility similar to women with PCOS (7, 8), although increased LH in LET mice is likely reflective of upstream changes in GnRH secretion patterns. At present, it appears unlikely that Lhb expression is decreased in flutamide-treated LET animals as a result of decreased GnRH receptor levels, but it will be important for future studies to investigate specific contributions of brain vs pituitary AR to PCOS phenotypes in the LET model. Although we previously reported a significant decrease in both circulating FSH and pituitary expression of Fshb in LET mice compared with CON, we did not see differences in levels of circulating FSH or Fshb mRNA between any treatment groups in the current study. This is likely reflective of the variability of FSH and Fshb within treatment groups, as mice were euthanized after 5 weeks of LET or CON treatment regardless of cycle stage. Genetic variants of FSHB have recently been associated with PCOS risk in women by genome-wide association studies (62, 63, 72). Although many reproductive parameters are improved with flutamide treatment in women with PCOS, circulating FSH levels have been reported to either remain unchanged (22, 42) or modestly decrease (21). Likewise, flutamide treatment has no effect on serum FSH levels in female aromatase knockout mice (66).
In women with PCOS who have oligo- or anovulation, flutamide treatment restores menstrual cyclicity and ovulation (22, 42). Acyclicity in the PNA mouse model of PCOS can be reversed with flutamide treatment (27) or by haploinsufficiency or complete loss of genomic AR signaling (15). Similar to PCOS in women, LET mice exhibit acyclicity characterized by persistent diestrus and fail to ovulate (33, 73). In the current study, we found that several but not all LET females that received flutamide treatment ovulated, evidenced by the presence of corpora lutea in their ovaries. Furthermore, although most LET females that received empty pellet treatment were in diestrus at the end of the study, flutamide-treated LET mice displayed various estrous cycle stages similar to CONs. Estrous cycling may have normalized ovarian steroidogenesis in these animals, which may have contributed to decreased T. Altogether, this suggests that blockade of AR can restore estrous cyclicity and ovulation in the LET-induced mouse model of PCOS. At present, it is unclear whether this is due to antagonism of AR signaling in the ovary or neuroendocrine tissues or both, although this will be an important area of future investigation. It has recently been demonstrated that loss of AR signaling in neurons improves follicle health and ovulatory function in a postnatal DHT mouse model of PCOS (14). Another recent study showed that theca cell–specific AR knockout improved estrous cycling, although not to the level of CONs, and restored ovulation in adult mice treated with DHT (16). Together, these studies indicate possible roles of both neuroendocrine and ovarian AR in disrupted cyclicity in the LET model and in human PCOS. Interestingly, although tissue-specific or global loss of AR results in subfertility in female mice (74–78), 2.5 weeks of AR antagonism by flutamide in CON females did not appear to have any effects on estrus cyclicity or ovulation. Although we also observed fewer atretic cyst–like follicles in flutamide-treated LET females compared with LET mice that received empty pellets, flutamide had no effect on the number of hemorrhagic cysts in these mice, indicating that the latter is not an AR-dependent phenotype. Indeed, flutamide treatment similarly fails to improve ovarian hemorrhagic cysts in aromatase knockout females (66).
In addition to improved reproductive endocrine phenotypes, we observed that LET females stopped gaining excess weight after receiving flutamide treatment, indicating that obesity in these mice is androgen dependent. Moreover, decreased body weight in flutamide-treated LET females may also have contributed to decreased T levels in these animals, as obesity promotes increased androgen secretion both in women and in rodents on a high-fat diet (6, 79). Together with previous data demonstrating that E2 levels and many estrogen-sensitive parameters are normal in LET female mice (likely due to upregulation of Cyp19a1) (33, 73), increased body weight in the LET model can most likely be attributed to excess androgens rather than inhibition of estradiol synthesis. Indeed, chronic DHT exposure in intact female mice results in increased body weight, decreased energy expenditure, and alterations in the central melanocortin system, with no change in food intake (80). Although the mechanisms through which androgens may act to affect female body weight have not been elucidated, neuroendocrine AR appears to be involved (14). Food intake and total energy expenditure are unchanged in LET females compared with CON, although LET mice do exhibit decreased activity during the dark cycle (65). Metabolic dysfunction in PCOS is associated with hyperandrogenism regardless of body mass index (81, 82), and flutamide significantly decreases visceral adiposity in obese women with PCOS on calorie-restricted diets (83), further highlighting the importance of androgens in the metabolic phenotypes associated with PCOS. Metabolic features of PCOS include increased adipocyte size (84, 85), which is also exhibited by LET mice. Although the effect of flutamide on adipocyte area in women with PCOS has not been determined, we observed a significant decrease in mean adipocyte size with flutamide treatment in LET females, indicating that this is an androgen-dependent phenotype in the LET model. In line with our finding, global AR knockout prevented increased adipocyte area in the PNA mouse model of PCOS (15) and in a postnatal DHT model (14).
In the current study, we have demonstrated that blockade of AR by the pure AR antagonist flutamide ameliorates or reverses many PCOS phenotypes in the LET mouse after only 2.5 weeks. To our knowledge, there are no known interactions between flutamide and letrozole, although we cannot rule out the possibility that differences in T between treatment groups may have affected metabolism of one or both of these drugs (86). PCOS phenotypes similar to those that we found to be reversed with flutamide treatment in LET females have been improved with antiandrogen treatment in both humans (22, 42, 43, 83) and animal models (27) or prevented with loss of AR signaling in animals (14–16), strongly supporting our findings that androgen actions are responsible for many key features of PCOS. Although AR actions are involved in the maintenance of LET phenotypes, it remains unclear whether AR is necessary for establishment of these PCOS phenotypes and the specific tissues in which androgens may be acting in the LET mouse, both of which will be important areas of future study. Altogether, we have shown that many reproductive and metabolic aspects of the LET PCOS phenotype can be attributed to AR signaling, establishing the relevance and importance of this model for the study of mechanisms underlying PCOS.
Supplementary Material
Acknowledgments
We thank Varykina Thackray and Alexander Kauffman for helpful comments on the manuscript and Jason D. Meadows, Mary Jean Sunshine, Erica L. Schoeller, and Karen J. Tonsfeldt for technical support.
Financial Support: This work was supported by National Institutes of Health (NIH) Grants R01 HD072754 and R01 HD082567 (to P.L.M.). It was also supported by National Institute of Child Health and Human Development (NICHD)/NIH P50 HD012303 as part of the National Centers for Translational Research in Reproduction and Infertility (P.L.M.). P.L.M. was also partially supported by Grants P30 DK063491, P30 CA023100, and P42 ES101337. G.E.R. was partially supported by Grants P42 ES101337 and T32 GM008666. The University of Virginia, Center for Research in Reproduction, Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH Grant P50 HD28934.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- A4
androstenedione
- ACOV
assay coefficient of variation
- ANOVA
analysis of variance
- AR
androgen receptor
- cDNA
complementary DNA
- CON
control
- DHEA
dehydroepiandrosterone
- DHT
dihydrotestosterone
- E2
estradiol
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- LET
letrozole
- LH
luteinizing hormone
- mRNA
messenger RNA
- P4
progesterone
- PNA
prenatal androgenization
- PCOS
polycystic ovary syndrome
- SD
standard deviation
- SHBG
sex hormone–binding globulin
- T
testosterone
References
- 1. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society . The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488. [DOI] [PubMed] [Google Scholar]
- 3.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25. [DOI] [PubMed] [Google Scholar]
- 4. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–231. [DOI] [PubMed] [Google Scholar]
- 5. Chang RJ. The reproductive phenotype in polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3(10):688–695. [DOI] [PubMed] [Google Scholar]
- 6. Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319–1340. [DOI] [PubMed] [Google Scholar]
- 7. Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12(4):351–361. [DOI] [PubMed] [Google Scholar]
- 8. Blank SK, McCartney CR, Helm KD, Marshall JC. Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin Reprod Med. 2007;25(5):352–359. [DOI] [PubMed] [Google Scholar]
- 9. Witchel SF, Tena-Sempere M. The Kiss1 system and polycystic ovary syndrome: lessons from physiology and putative pathophysiologic implications. Fertil Steril. 2013;100(1):12–22. [DOI] [PubMed] [Google Scholar]
- 10. Barontini M, García-Rudaz MC, Veldhuis JD. Mechanisms of hypothalamic-pituitary-gonadal disruption in polycystic ovarian syndrome. Arch Med Res. 2001;32(6):544–552. [DOI] [PubMed] [Google Scholar]
- 11. Nelson VL, Legro RS, Strauss JF III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13(6):946–957. [DOI] [PubMed] [Google Scholar]
- 12. Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. J Clin Endocrinol Metab. 2005;90(5):2810–2815. [DOI] [PubMed] [Google Scholar]
- 13. Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83(2):582–590. [DOI] [PubMed] [Google Scholar]
- 14. Caldwell ASL, Edwards MC, Desai R, Jimenez M, Gilchrist RB, Handelsman DJ, Walters KA. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci USA. 2017;114(16):E3334–E3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caldwell AS, Eid S, Kay CR, Jimenez M, McMahon AC, Desai R, Allan CM, Smith JT, Handelsman DJ, Walters KA. Haplosufficient genomic androgen receptor signaling is adequate to protect female mice from induction of polycystic ovary syndrome features by prenatal hyperandrogenization. Endocrinology. 2015;156(4):1441–1452. [DOI] [PubMed] [Google Scholar]
- 16. Ma Y, Andrisse S, Chen Y, Childress S, Xue P, Wang Z, Jones D, Ko C, Divall S, Wu S. Androgen receptor in the ovary theca cells plays a critical role in androgen-induced reproductive dysfunction. Endocrinology. 2017;158(1):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim JJ, Choung SH, Choi YM, Yoon SH, Kim SH, Moon SY. Androgen receptor gene CAG repeat polymorphism in women with polycystic ovary syndrome. Fertil Steril. 2008;90(6):2318–2323. [DOI] [PubMed] [Google Scholar]
- 18. Schüring AN, Welp A, Gromoll J, Zitzmann M, Sonntag B, Nieschlag E, Greb RR, Kiesel L. Role of the CAG repeat polymorphism of the androgen receptor gene in polycystic ovary syndrome (PCOS). Exp Clin Endocrinol Diabetes. 2011;120(2):73–79. [DOI] [PubMed] [Google Scholar]
- 19. Shah NA, Antoine HJ, Pall M, Taylor KD, Azziz R, Goodarzi MO. Association of androgen receptor CAG repeat polymorphism and polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(5):1939–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skrgatic L, Baldani DP, Cerne JZ, Ferk P, Gersak K. CAG repeat polymorphism in androgen receptor gene is not directly associated with polycystic ovary syndrome but influences serum testosterone levels. J Steroid Biochem Mol Biol. 2012;128(3-5):107–112. [DOI] [PubMed] [Google Scholar]
- 21. Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85(11):4047–4052. [DOI] [PubMed] [Google Scholar]
- 22. De Leo V, Lanzetta D, D’Antona D, la Marca A, Morgante G. Hormonal effects of flutamide in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83(1):99–102. [DOI] [PubMed] [Google Scholar]
- 23. Diamanti-Kandarakis E, Mitrakou A, Raptis S, Tolis G, Duleba AJ. The effect of a pure antiandrogen receptor blocker, flutamide, on the lipid profile in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83(8):2699–2705. [DOI] [PubMed] [Google Scholar]
- 24. Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod. 2012;86(5):149, 1–12. [DOI] [PubMed] [Google Scholar]
- 25. Moore AM, Prescott M, Campbell RE. Estradiol negative and positive feedback in a prenatal androgen-induced mouse model of polycystic ovarian syndrome. Endocrinology. 2013;154(2):796–806. [DOI] [PubMed] [Google Scholar]
- 26. Roland AV, Moenter SM. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology. 2011;152(2):618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA. 2004;101(18):7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roland AV, Nunemaker CS, Keller SR, Moenter SM. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol. 2010;207(2):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci USA. 2014;112(2):596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Witham EA, Meadows JD, Shojaei S, Kauffman AS, Mellon PL. Prenatal exposure to low levels of androgen accelerates female puberty onset and reproductive senescence in mice. Endocrinology. 2012;153(9):4522–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Homa LD, Burger LL, Cuttitta AJ, Michele DE, Moenter SM. Voluntary exercise improves estrous cyclicity in prenatally androgenized female mice despite programming decreased voluntary exercise: implications for polycystic ovary syndrome (PCOS). Endocrinology. 2015;156(12):4618–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Houten EL, Kramer P, McLuskey A, Karels B, Themmen AP, Visser JA. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology. 2012;153(6):2861–2869. [DOI] [PubMed] [Google Scholar]
- 33. Kauffman AS, Thackray VG, Ryan GE, Tolson KP, Glidewell-Kenney CA, Semaan SJ, Poling MC, Iwata N, Breen KM, Duleba AJ, Stener-Victorin E, Shimasaki S, Webster NJ, Mellon PL. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol Reprod. 2015;93(3):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maliqueo M, Sun M, Johansson J, Benrick A, Labrie F, Svensson H, Lönn M, Duleba AJ, Stener-Victorin E. Continuous administration of a P450 aromatase inhibitor induces polycystic ovary syndrome with a metabolic and endocrine phenotype in female rats at adult age. Endocrinology. 2013;154(1):434–445. [DOI] [PubMed] [Google Scholar]
- 35. Mannerås L, Cajander S, Holmäng A, Seleskovic Z, Lystig T, Lönn M, Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148(8):3781–3791. [DOI] [PubMed] [Google Scholar]
- 36. Xita N, Lazaros L, Georgiou I, Tsatsoulis A. CYP19 gene: a genetic modifier of polycystic ovary syndrome phenotype. Fertil Steril. 2010;94(1):250–254. [DOI] [PubMed] [Google Scholar]
- 37. Wang H, Li Q, Wang T, Yang G, Wang Y, Zhang X, Sang Q, Wang H, Zhao X, Xing Q, Shi J, He L, Wang L. A common polymorphism in the human aromatase gene alters the risk for polycystic ovary syndrome and modifies aromatase activity in vitro. Mol Hum Reprod. 2011;17(6):386–391. [DOI] [PubMed] [Google Scholar]
- 38. Gonzalez B, Ratner LD, Di Giorgio NP, Poutanen M, Huhtaniemi IT, Calandra RS, Lux-Lantos VA, Rulli SB. Endogenously elevated androgens alter the developmental programming of the hypothalamic-pituitary axis in male mice. Mol Cell Endocrinol. 2011;332(1-2):78–87. [DOI] [PubMed] [Google Scholar]
- 39. Becker KL. Principles and Practice of Endocrinology and Metabolism. 2nd ed.Philadelphia, PA: Lippincott; 1995. [Google Scholar]
- 40. Galarraga M, Campión J, Muñoz-Barrutia A, Boqué N, Moreno H, Martínez JA, Milagro F, Ortiz-de-Solórzano C. Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J Lipid Res. 2012;53(12):2791–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 42. Paradisi R, Fabbri R, Battaglia C, Venturoli S. Ovulatory effects of flutamide in the polycystic ovary syndrome. Gynecol Endocrinol. 2013;29(4):391–395. [DOI] [PubMed] [Google Scholar]
- 43. Ibáñez L, Potau N, Marcos MV, de Zegher F. Treatment of hirsutism, hyperandrogenism, oligomenorrhea, dyslipidemia, and hyperinsulinism in nonobese, adolescent girls: effect of flutamide. J Clin Endocrinol Metab. 2000;85(9):3251–3255. [DOI] [PubMed] [Google Scholar]
- 44. Pugeat M, Cousin P, Baret C, Lejeune H, Forest MG. Sex hormone-binding globulin during puberty in normal and hyperandrogenic girls. J Pediatr Endocrinol Metab. 2000;13(Suppl 5):1277–1279. [PubMed] [Google Scholar]
- 45. Deswal R, Yadav A, Dang AS. Sex hormone binding globulin—an important biomarker for predicting PCOS risk: a systematic review and meta-analysis. Syst Biol Reprod Med. 2017;64(1):12–24. [DOI] [PubMed] [Google Scholar]
- 46. Marugo M, Bernasconi D, Meozzi M, Del Monte P, Zino V, Primarolo P, Badaracco B. The use of flutamide in the management of hirsutism. J Endocrinol Invest. 1994;17(3):195–199. [DOI] [PubMed] [Google Scholar]
- 47. Laurent MR, Hammond GL, Blokland M, Jardi F, Antonio L, Dubois V, Khalil R, Sterk SS, Gielen E, Decallonne B, Carmeliet G, Kaufman JM, Fiers T, Huhtaniemi IT, Vanderschueren D, Claessens F. Sex hormone-binding globulin regulation of androgen bioactivity in vivo: validation of the free hormone hypothesis. Sci Rep. 2016;6:35539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murayama C, Miyazaki H, Miyamoto A, Shimizu T. Luteinizing hormone (LH) regulates production of androstenedione and progesterone via control of histone acetylation of StAR and CYP17 promoters in ovarian theca cells. Mol Cell Endocrinol. 2012;350(1):1–9. [DOI] [PubMed] [Google Scholar]
- 49. Palaniappan M, Menon KM. Luteinizing hormone/human chorionic gonadotropin-mediated activation of mTORC1 signaling is required for androgen synthesis by theca-interstitial cells. Mol Endocrinol. 2012;26(10):1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nelson VL, Qin KN, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JF III, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86(12):5925–5933. [DOI] [PubMed] [Google Scholar]
- 51. Wickenheisser JK, Nelson-Degrave VL, McAllister JM. Dysregulation of cytochrome P450 17alpha-hydroxylase messenger ribonucleic acid stability in theca cells isolated from women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(3):1720–1727. [DOI] [PubMed] [Google Scholar]
- 52. Wickenheisser JK, Quinn PG, Nelson VL, Legro RS, Strauss JF III, McAllister JM. Differential activity of the cytochrome P450 17alpha-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J Clin Endocrinol Metab. 2000;85(6):2304–2311. [DOI] [PubMed] [Google Scholar]
- 53. Ayub M, Levell MJ. Inhibition of rat testicular 17 alpha-hydroxylase and 17,20-lyase activities by anti-androgens (flutamide, hydroxyflutamide, RU23908, cyproterone acetate) in vitro. J Steroid Biochem. 1987;28(1):43–47. [DOI] [PubMed] [Google Scholar]
- 54. Raynaud JP. Antiandrogens in combination with LH-RH agonists in prostate cancer. Am J Clin Oncol. 1988;11(Suppl 2):S132–S147. [DOI] [PubMed] [Google Scholar]
- 55. Duda M, Grzesiak M, Knet M, Knapczyk-Stwora K, Tabarowski Z, Michna A, Slomczynska M. The impact of antiandrogen 2-hydroxyflutamide on the expression of steroidogenic enzymes in cultured porcine ovarian follicles. Mol Biol Rep. 2014;41(7):4213–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84(8):2951–2956. [DOI] [PubMed] [Google Scholar]
- 57. Wu YG, Bennett J, Talla D, Stocco C. Testosterone, not 5α-dihydrotestosterone, stimulates LRH-1 leading to FSH-independent expression of Cyp19 and P450scc in granulosa cells. Mol Endocrinol. 2011;25(4):656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101(12):2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, Bondy CA. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83(7):2479–2485. [DOI] [PubMed] [Google Scholar]
- 60. Walters KA, Allan CM, Handelsman DJ. Androgen actions and the ovary. Biol Reprod. 2008;78(3):380–389. [DOI] [PubMed] [Google Scholar]
- 61. Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, Zhang B, Liang X, Li T, Chen J, Shen J, Zhao J, You L, Gao X, Zhu D, Zhao X, Yan Y, Qin Y, Li W, Yan J, Wang Q, Zhao J, Geng L, Ma J, Zhao Y, He G, Zhang A, Zou S, Yang A, Liu J, Li W, Li B, Wan C, Qin Y, Shi J, Yang J, Jiang H, Xu JE, Qi X, Sun Y, Zhang Y, Hao C, Ju X, Zhao D, Ren CE, Li X, Zhang W, Zhang Y, Zhang J, Wu D, Zhang C, He L, Chen ZJ. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44(9):1020–1025. [DOI] [PubMed] [Google Scholar]
- 62. Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, Bjonnes A, Broer L, Dunger DB, Halldorsson BV, Lawlor DA, Laval G, Mathieson I, McCardle WL, Louwers Y, Meun C, Ring S, Scott RA, Sulem P, Uitterlinden AG, Wareham NJ, Thorsteinsdottir U, Welt C, Stefansson K, Laven JS, Ong KK, Perry JR. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015;6:8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S, Lindgren CM, Welt CK, Diamanti-Kandarakis E, Panidis D, Goodarzi MO, Azziz R, Zhang Y, James RG, Olivier M, Kissebah AH, Stener-Victorin E, Legro RS, Dunaif A. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun. 2015;6:7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Almawi WY, Hubail B, Arekat DZ, Al-Farsi SM, Al-Kindi SK, Arekat MR, Mahmood N, Madan S. Leutinizing hormone/choriogonadotropin receptor and follicle stimulating hormone receptor gene variants in polycystic ovary syndrome. J Assist Reprod Genet. 2015;32(4):607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Skarra DV, Hernández-Carretero A, Rivera AJ, Anvar AR, Thackray VG. Hyperandrogenemia induced by letrozole treatment of pubertal female mice results in hyperinsulinemia prior to weight gain and insulin resistance. Endocrinology. 2017;158(9):2988–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liew SH, Drummond AE, Jones ME, Findlay JK. The lack of estrogen and excess luteinizing hormone are responsible for the female ArKO mouse phenotype. Mol Cell Endocrinol. 2010;327(1–2):56–64. [DOI] [PubMed] [Google Scholar]
- 67. Naik SI, Young LS, Charlton HM, Clayton RN. Pituitary gonadotropin-releasing hormone receptor regulation in mice. I: Males. Endocrinology. 1984;115(1):106–113. [DOI] [PubMed] [Google Scholar]
- 68. Spady TJ, Shayya R, Thackray VG, Ehrensberger L, Bailey JS, Mellon PL. Androgen regulates follicle-stimulating hormone β gene expression in an activin-dependent manner in immortalized gonadotropes. Mol Endocrinol. 2004;18(4):925–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stamatiades GA, Kaiser UB. Gonadotropin regulation by pulsatile GnRH: signaling and gene expression. Mol Cell Endocrinol. 2018;463:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Norwitz ER, Cardona GR, Jeong KH, Chin WW. Identification and characterization of the gonadotropin-releasing hormone response elements in the mouse gonadotropin-releasing hormone receptor gene. J Biol Chem. 1999;274:867–880. [DOI] [PubMed] [Google Scholar]
- 71. Norwitz ER, Jeong KH, Chin WW. Molecular mechanisms of gonadotropin-releasing hormone receptor gene regulation. J Soc Gynecol Investig. 1999;6(4):169–178. [DOI] [PubMed] [Google Scholar]
- 72. Tian Y, Zhao H, Chen H, Peng Y, Cui L, Du Y, Wang Z, Xu J, Chen ZJ. Variants in FSHB are associated with polycystic ovary syndrome and luteinizing hormone level in Han Chinese women. J Clin Endocrinol Metab. 2016;101(5):2178–2184. [DOI] [PubMed] [Google Scholar]
- 73. Kelley ST, Skarra DV, Rivera AJ, Thackray VG. The gut microbiome is altered in a letrozole-induced mouse model of polycystic ovary syndrome. PLoS One. 2016;11(1):e0146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci USA. 2002;99(21):13498–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Walters KA, Middleton LJ, Joseph SR, Hazra R, Jimenez M, Simanainen U, Allan CM, Handelsman DJ. Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility. Biol Reprod. 2012;87(6):151. [DOI] [PubMed] [Google Scholar]
- 76. Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci USA. 2004;101(31):11209–11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wu S, Chen Y, Fajobi T, DiVall SA, Chang C, Yeh S, Wolfe A. Conditional knockout of the androgen receptor in gonadotropes reveals crucial roles for androgen in gonadotropin synthesis and surge in female mice. Mol Endocrinol. 2014;28(10):1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci USA. 2005;103(1):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Delemarre-van de Waal HA, van Coeverden SC, Engelbregt MT. Factors affecting onset of puberty. Horm Res. 2004;57(Suppl 2):15–18. [DOI] [PubMed] [Google Scholar]
- 80. Nohara K, Laque A, Allard C, Münzberg H, Mauvais-Jarvis F. Central mechanisms of adiposity in adult female mice with androgen excess. Obesity (Silver Spring). 2014;22(6):1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Barber TM, Wass JA, McCarthy MI, Franks S. Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: implications for the management of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2007;66(4):513–517. [DOI] [PubMed] [Google Scholar]
- 82. Moghetti P, Tosi F, Bonin C, Di Sarra D, Fiers T, Kaufman J-M, Giagulli VA, Signori C, Zambotti F, Dall’Alda M, Spiazzi G, Zanolin ME, Bonora E. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98(4):E628–E637. [DOI] [PubMed] [Google Scholar]
- 83. Gambineri A, Patton L, Vaccina A, Cacciari M, Morselli-Labate AM, Cavazza C, Pagotto U, Pasquali R. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab. 2006;91(10):3970–3980. [DOI] [PubMed] [Google Scholar]
- 84. Mannerås-Holm L, Leonhardt H, Kullberg J, Jennische E, Odén A, Holm G, Hellström M, Lönn L, Olivecrona G, Stener-Victorin E, Lönn M. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96(2):E304–E311. [DOI] [PubMed] [Google Scholar]
- 85. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–363. [DOI] [PubMed] [Google Scholar]
- 86. Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76(2):215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.