Abstract
The poor correlation of mutational landscapes with phenotypes limits our understanding of pancreatic ductal adenocarcinoma (PDAC) pathogenesis and metastasis. Here we show a critical role of oncogenic dosage-variation in PDAC biology and phenotypic diversification. We found gene-dosage increase of mutant KRASMUT in human PDAC precursors, driving both early tumorigenesis and metastasis, thus rationalizing early PDAC dissemination. To overcome limitations posed to gene-dosage studies by PDAC´s stroma-richness we developed large cell culture resources of metastatic mouse PDAC. Integration of their genomes, transcriptomes and tumor phenotypes with functional studies and human data, revealed additional widespread effects of oncogenic dosage-variation on cell morphology/plasticity, histopathology and clinical outcome, with highest KrasMUT levels underlying aggressive undifferentiated phenotypes. We also identify alternative oncogenic gains (Myc, Yap1 or Nfkb2), which collaborate with heterozygous KrasMUT in driving tumorigenesis, yet with lower metastatic potential. Mechanistically, different oncogenic gains and dosages evolve along distinct evolutionary routes, licensed by defined allelic states and/or combinations of hallmark tumor-suppressor alterations (Cdkn2a, Trp53, Tgfβ-pathway). Thus, evolutionary constraints and contingencies direct oncogenic dosage gain and variation along defined routes to drive early progression and shape downstream PDAC biology. Our study uncovers universal principles in Ras-driven oncogenesis with potential relevance beyond pancreatic cancer.
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death and is expected to become the second within the next decade1. Whilst treatments constantly improved for many other cancer types, 5-year survival rates in PDAC stayed around 5%1. Genome sequencing revealed extensive genetic heterogeneity beyond a few frequently mutated drivers2–8 like KRAS, TP53, CDKN2A or TGFβ-pathway alterations. Disappointingly however, genomic changes could so far not be broadly linked to biologic, morphologic or clinical phenotypes. In addition, the molecular basis of cancer cell dissemination is poorly understood, and genetic comparisons of primary/metastasis pairs could not identify recurrent alterations linked to metastasis3,8. Critical limitations to human PDACs (hPDAC) genomics are (i) the cancer genome complexity, posing challenges to their interpretation, (ii) the high (and variable) stromal content, which confounds particularly gene-dosage analyses and transcriptome interpretation, (iii) the limited availability of human cell culture-based resources to overcome this problem and (iv) the scarcity of paired primary/metastasis tissues, particularly of treatment-naive ones, e.g. for evolutionary studies. Here we characterized large murine PDAC cell line resources and combined the results with cross-species comparisons and functional studies to unravel molecular principles underlying PDAC evolution and phenotypic diversification.
Genetic landscapes of mouse PDAC
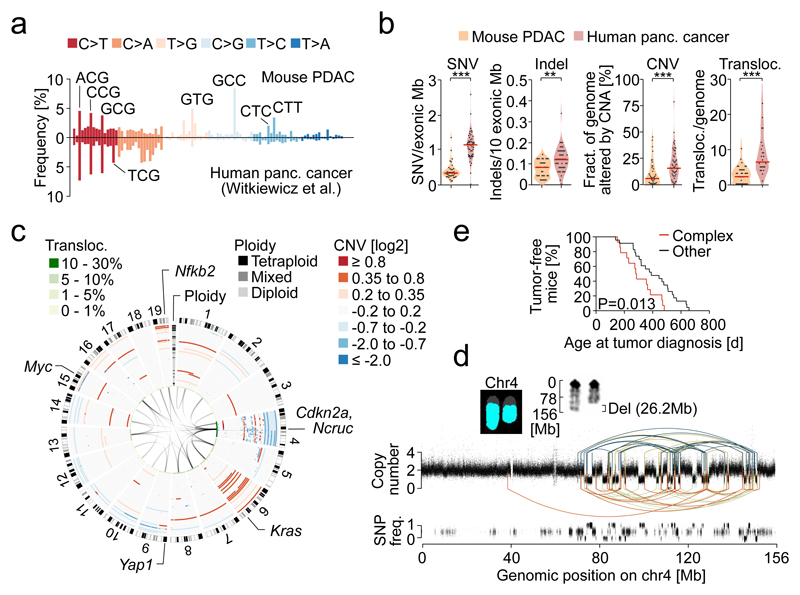
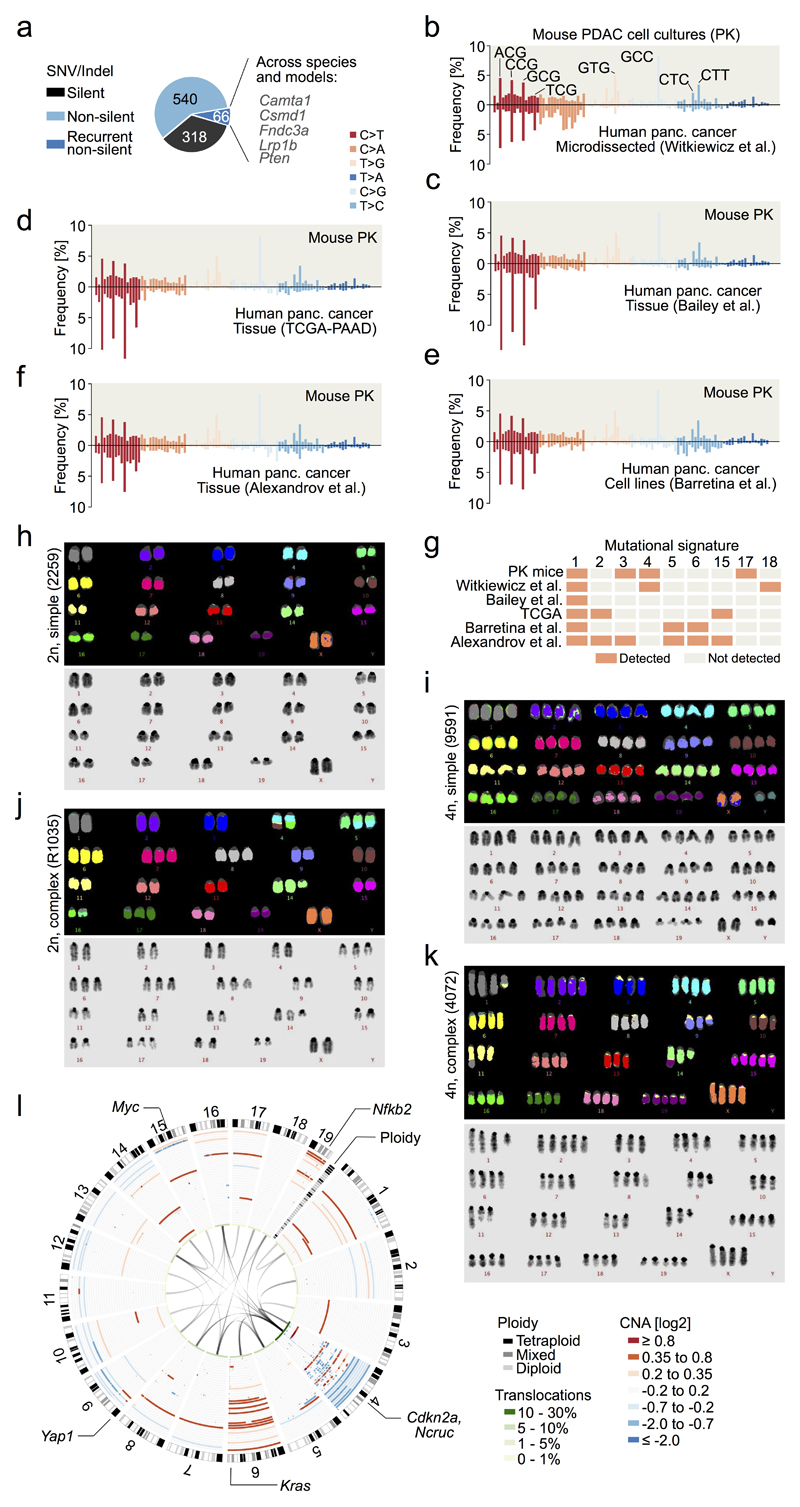
We initially characterized primary PDAC cell cultures from 38 mice expressing KrasG12D conditionally in the pancreas (PK mice)9,10 by multiplex FISH (M-FISH), whole-exome sequencing (WES) and array comparative genomic hybridization (aCGH). We developed a pipeline for WES data analysis allowing mouse/human comparisons using identical parameter settings. A WES study on microdissected human PDAC (reduced stromal “contamination”) served as the reference human data set6. Somatic mutation calling identified 318 synonymous and 606 non-synonymous mutations in 38 mPDACs (Extended Data Fig. 1a and Supplementary Table 1). Mutational patterns were similar, (Fig. 1a; Supplementary Table 2, Extended Data Fig. 1b-g) but mutational burdens were significantly higher in hPDAC than mPDAC, with 3.3 and 1.5 fold increased median SNV and indel numbers, respectively (Fig. 1b and Supplementary Tables 1, 3-5). Recurrently altered genes were infrequent in mice. A subset overlapped with recurrently mutated genes in human cancers and/or common insertion sites in pancreas-specific transposon screens11–13 (Extended Data Fig. 1a).
Figure 1. Genetic landscape of mouse PDAC and comparison to the human disease.
a, Trinucleotide context-dependent SNV frequencies in mouse (n=38 PK mice) and human PDAC (n=51 patients from6) derived from WES. b, SNV, indel, CNA and translocation burdens by WES, aCGH and M-FISH in PK mice (n=38) and human PDAC (n=51 patients for SNV, indel, CNA [data from6] and n=24 cell lines for translocations). **P=0.002, ***P≤0.001, two-sided Mann-Whitney test; bars, median. c, CNAs, ploidy and translocations in PK mice (n=38), detected by aCGH and M-FISH. Mixed ploidy, n≥3 diploid/tetraploid cells in 10 karyotypes. d, Rearrangement graph showing chr4 chromothripsis in mPDAC S821, based on WGS. Haplotype-specific chromosome content loss confirmed by M-FISH (n=10/10 karyotypes). e, Age at tumor diagnosis of mice having cancers with (n=14) or without (n=23) complex/clustered chromosomal rearrangements (n≥10 CNAs/chromosome). Two-sided log-rank test.
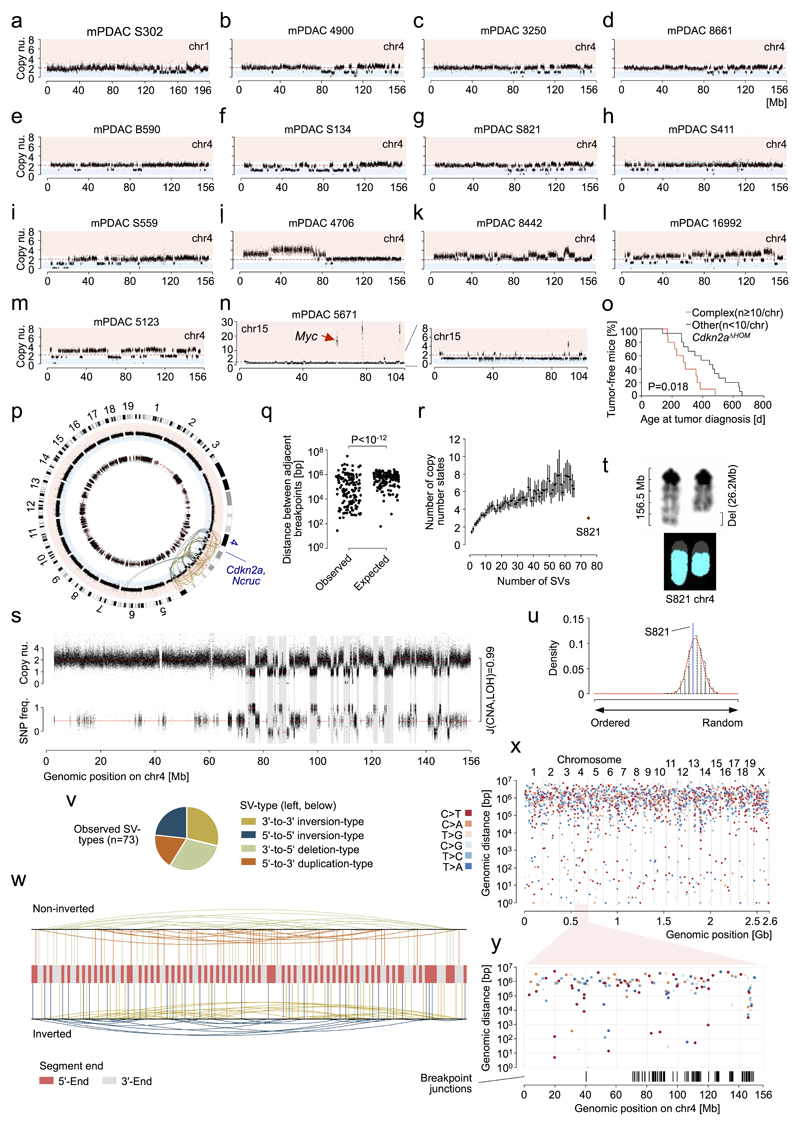
Structural changes were also less common in mPDAC (Fig. 1b, Supplementary Tables 6-8). There was however substantial variation between cancers, with some mPDACs having only few focal alterations, but others showing extensive changes, including clustered intra-chromosomal alterations, aneuploidy and inter-chromosomal translocations (Fig. 1c and Extended Data Fig. 1h-l). Notably, 34% (14/38) tumors had complex rearrangements, with ten or more alterations per affected chromosome. The majority (12/14) of such events affect chr4, invariably involving Cdkn2a. One cancer showed massively rearranged chr15 with high-level Myc amplification and another tumor had clustered chr1 rearrangements (Extended Data Fig. 2a-n). These findings reflect selection of complex rearrangements that affect cancer drivers.
The regularity of oscillating copy number states in most cancers suggested chromothripsis as the predominant process underlying these complex alterations. Whole-genome sequencing, followed by rearrangement analysis and computational simulations confirmed all hallmarks defining the one-off nature of chromothripsis14, including clustering of breakpoints, regularity of oscillating copy number states, identical CNA and LOH patterns, randomness of DNA segment order/joints and alternating head-tail sequences (Fig. 1d and detailed analyses in Extended Data Fig. 2p-y. In addition, M-FISH confirmed chr4 content loss affecting only one haplotype (Fig. 1d).
Complex rearrangements were proposed to trigger accelerated evolution of human PDAC15. The mouse model allows experimental interrogation of this hypothesis due to the “synchronized” nature of tumor initiation (KrasG12D-mutation). We found that time-to-tumor development was indeed shorter in animals with Cdkn2a loss through catastrophic events (Fig. 1e and Extended Data Fig. 2o). A subset (16%) of complex rearrangements in hPDAC disrupts multiple known tumor suppressors through translocations15. Chromothripsis-associated chr4 translocations were also frequent in mice (Fig. 1c), although no recurrent translocation partners were found.
KrasMUT-iGD links early progression & metastasis
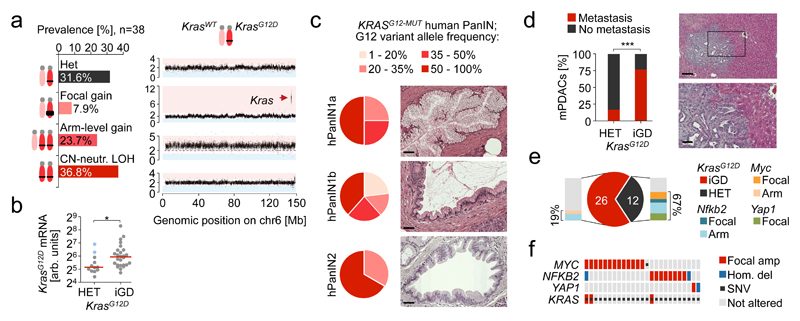
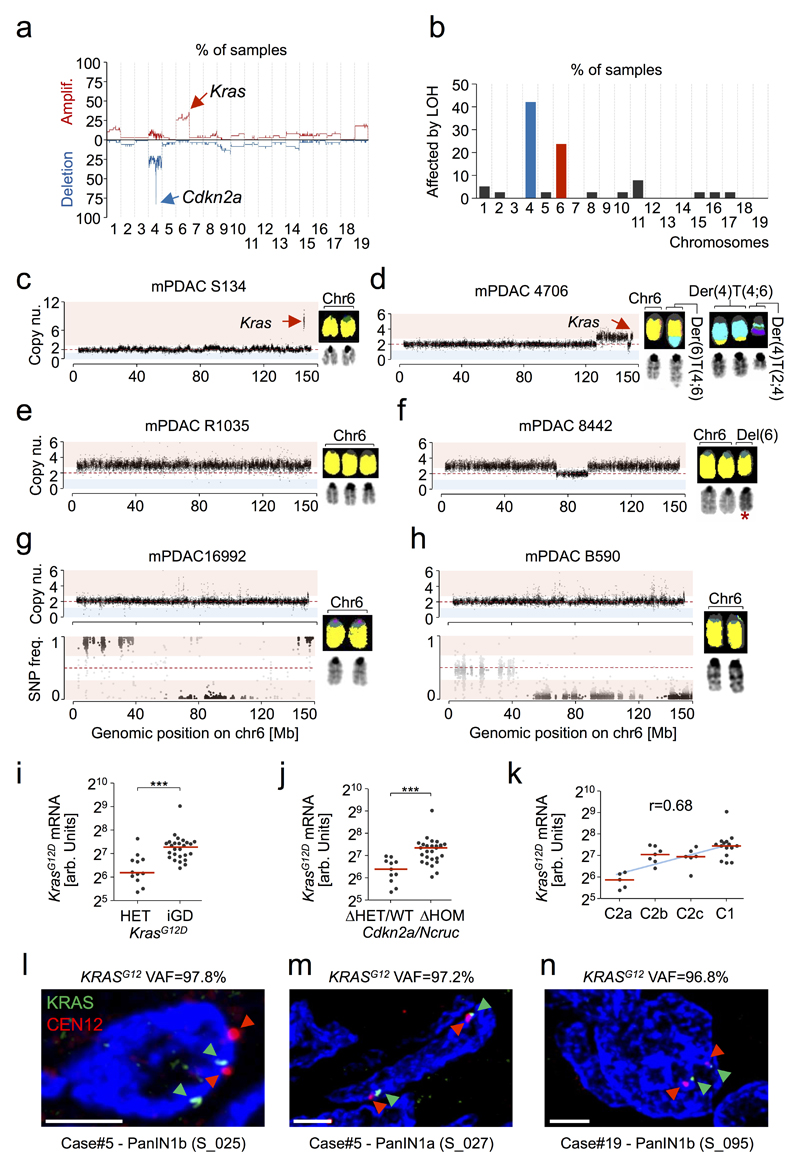
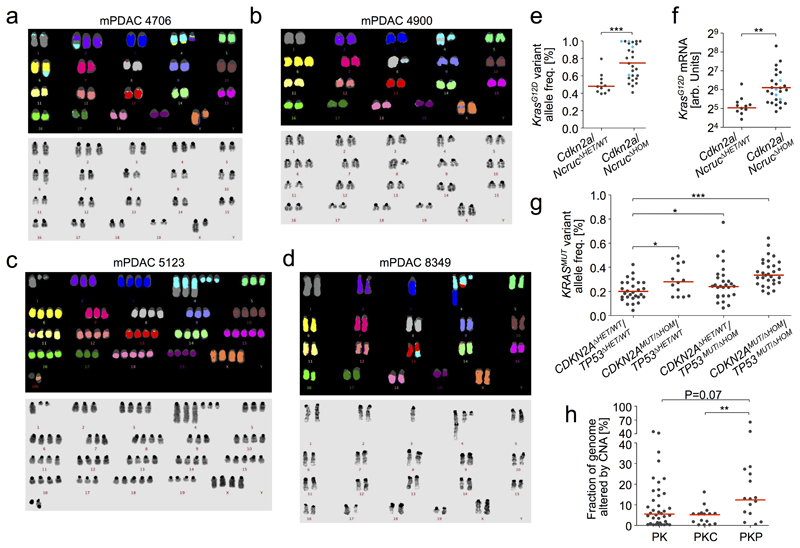
The most common amplification affected the Kras locus (Extended Data Fig. 3a,b), which is also frequent in hPDAC16,17. Combined analyses of M-FISH, aCGH and Kras mutant/wild-type (wt) allele frequencies revealed four different KrasG12D gene dosage “states” (Fig. 2a, Extended Data Fig. 3c-h and Supplementary Table 9): focal gain (KrasG12D-FG, 7.9%), arm-level gain (KrasG12D-AG, 23.7%), copy number neutral loss of wt Kras (KrasG12D-LOH, 36.8%) or no change (KrasG12D-HET, 31.6%). Thus, two thirds of cancers had allelic imbalances causing increased KrasG12D gene dosage (hereafter designated KrasG12D-iGD), suggesting strong selective pressure for its acquisition. In addition, two KrasG12D-HET tumors displayed loss of KrasWT-mRNA, but high KrasG12D expression (blue dots in Fig. 2b), suggesting additional non-genetic mechanisms. Of note, we observed similar KRASG12D-iGD rates/types in human PDAC cell lines (Supplementary Table 10). Gene dosage increase affects transcriptional output, as KrasG12D-iGD mPDAC had higher KrasG12D mRNA expression than KrasG12D-HET cancers (Fig. 2b and Extended Data Fig. 3i).
Figure 2. Mutant KRAS gene dosage increase occurs early in PDAC evolution and drives metastasis.
a, KrasG12D gene dosage “states” defined by aCGH, WES and M-FISH (n=38 PK mice). Exemplary CNV-plot for each “state” on the right, y-axis, copy number b, Allele-specific KrasG12D mRNA expression in KrasG12D-iGD (n=26 mice) and KrasG12D-HET mPDACs (n=12 mice) by combined amplicon-based RNA-Seq and qRT-PCR. *P=0.02, two-sided Mann-Whitney test; bars, median. c, Codon-12 variant allele frequency of microdissected KRASG12 mutant hPanIN (n=20) by amplicon-based deep sequencing. H&E stains show histopathologic stages of microdissected hPanINs. Scale bars, 50 µm. d, Macro-/micro-metastasis prevalence in KrasG12D-HET (n=12) vs. KrasG12D-iGD (n=26) mPDACs. (***P=0.001, two-sided Fisher’s exact test). Liver metastasis, H&E. Scale bars, 150 µm (top) and 50 µm (bottom); square, zoom-in area. e, KrasG12D-HET mPDAC amplify alternative oncogenes (Myc, Nfkb2 or Yap1) to intensify partial aspects of Ras downstream signaling. Focal, focal amplification; Arm, arm-level amplification. f, Amplification of MYC, NFKB2 or YAP1 in KRASMUT human PDAC. Note, these amplified genes can not only collaborate with KRASMUT-Het but also with KRASMUT-iGD. Data from6.
Ras/Raf signaling amplification was observed at different stages of mammary, intestinal or lung tumorigenesis18–21. To identify the stage of KRASMUT-iGD-acquisition in PDAC, we microdissected low-grade human pancreatic intraepithelial neoplasias (hPanIN) from 19 patients and performed amplicon-based deep sequencing of KRAS exon-2 (Fig. 2c and Supplementary Table 11/12). hPanIN with exon-2-mutated KRAS (20/40 hPanINs featured KRASG12 mutations) displayed frequent KRASMUT-iGD: KRASMUT allele frequencies >50% occurred in 50%, 38% and 67% of KRAS exon-2-mutated hPanIN1a, hPanIN1b and hPanIN2, respectively. Given that healthy tissue “contamination” rates in microdissected PanINs ranged between 10-60%, KRASMUT-iGD is likely to be even more frequent. In cases with close to 100% mutant read frequency, KRAS interphase FISH excluded false-positive KRASG12D-iGD arising through chr12 monosomy (Extended Data Fig. 3l-n). Moreover, false-positive KRASMUT-iGD through cross-“contaminating” hPDAC is excluded due to either (i) the large distance of selected hPanINs to associated cancers, (ii) distinct KRAS mutations in hPanINs and associated cancers, or (iii) KRASMUT-iGD in IPMN-related hPanINs without invasive hPDAC. Altogether, these data suggest a critical role of KRASMUT-iGD in early PDAC progression.
Looking at organ dissemination, we found a dramatically increased metastatic potential of KrasG12D-iGD cancers (OR 16.7; 95% CI 2.8-98.0; Fig. 2d): primary mPDACs with KrasG12D-iGD were mostly metastasized (20/26), whereas KrasG12D-HET mPDACs were predominantly non-metastatic (2/12). Thus, KrasG12D-iGD drives both early progression and metastasis. This dual role explains (i) early PDAC dissemination in humans and mice22 and (ii) the high incidence of human PDAC metastasis at diagnosis23. We also mined published data8,24 and invariably found KRASMUT-iGD in human PDAC metastases. However, because KRASMUT-iGD is present in the primary (early acquisition), its contribution to metastasis could not be recognized by primary/metastasis comparisons8.
Alternative oncogenic gains in KrasMUT-HET tumors
Among the 12 cancers without KrasG12D-dosage gain, two cases had Myc amplifications and two had Yap1 gains (Fig. 2e and Extended Data Fig. 4a-d). MYC and YAP1 are known human oncogenes, amplified in 12% (13/109) and 1% (1/109) of hPDAC, respectively (Fig. 2f). In addition, chr19 gain occurred more frequently in KrasG12D-HET (3/12) than KrasG12D-iGD tumors (4/26), although this was not significant. A focal amplification on chr19 contained 20 genes (Extended Data Fig. 4e). Cross-species analyses revealed frequent gains of the syntenic region in hPDAC, with two genes in the minimal peak region: NFKB2 and PSD, both amplified in 7% (8/109) of hPDAC (Extended Data Fig. 4f). NFKB2 (but not PSD) is expressed in human pancreas and hPDAC (Extended Data Fig. 4g,h), suggesting Nfkb2 to be the target proto-oncogene on murine chr19. NFKB2 mediates non-canonical Nfkb signaling. It has not yet been associated with hPDAC, but promotes cell cycle progression in vitro25, and knockout of its interaction partner RelB impairs PanIN formation in PK mice26. Thus, upon Kras mutation, further amplification of partial aspects of Kras downstream signaling (Myc, Yap1 or Nfkb2) seem sufficient to drive early PDAC progression, whereas strong metastatic potential is linked to amplification of the full KrasG12D signaling program.
Evolutionary licensing of oncogenic dosages
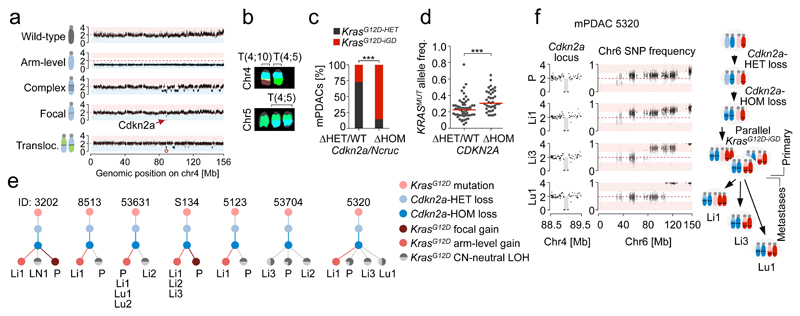
The most frequent deletion in mPDAC affected Cdkn2a and/or the adjacent non-coding Cdkn2a-regulatory region Ncruc: 23 Cdkn2aΔHOM, 4 NcrucΔHOM, 10 Cdkn2aΔHET, 1 Cdkn2aWT (chr4 alteration types shown in Fig. 3a,b, Extended Data Fig. 5a-d and Supplementary Table 9). Notably, the majority of Cdkn2a/NcrucΔHOM cancers were KrasG12D-iGD (23/27) and had high KrasG12D expression. In contrast, Cdkn2aΔHET or Cdkn2aWT cancers were predominantly KrasG12D-HET (8/11) with low KrasG12D expression (Fig. 3c, Extended Data Fig. 3j and 5e,f). Accordingly, in microdissected human PDAC datasets6, KRASMUT variant allele frequencies were higher in CDKN2AΔHOM than in CDKN2AΔHET/WT tumors (Fig. 3d and Extended Data Fig. 5g). Thus, CDKN2AΔHOM-deletion and KRASMUT-iGD are linked, with two possible scenarios: (i) KRASMUT-iGD occurs first, but induces senescence that prevents progression until CDKN2A is lost (as proposed in breast tumorigenesis18), or (ii) KRASMUT-iGD is only tolerated if preceded by CDKN2A deletion.
Figure 3. Cdkn2a alteration “states” dictate distinct evolutionary PDAC trajectories.
a, Chr4 alteration types involving Cdkn2a by aCGH/M-FISH (n=38 PK mice). Complex rearrangements, n≥10 CNAs/chromosome. Examplary CNV plots on the right; y-axis, copy number. b, Translocations affecting chr4/Cdkn2a in mPDAC-R1035 by M-FISH (10/10 karyotypes). c, Prevalence of KrasG12D-iGD in mPDAC with homozygously (ΔHOM, n=27) vs. wild-type/heterozygously (ΔHET/WT, n=11) deleted Cdkn2a/Ncruc. ***P=0.001, two-sided Fisher’s exact test, OR 15.3, 95% CI 2.8-83.9. d, KRAS variant allele frequencies in human PDAC with wild-type/heterozygously (n=56) vs. homozygously deleted (n=38) CDKN2A. Data from6. ***P≤0.001, two-sided Mann-Whitney test; bars, median. e, Sequential order of Cdkn2a and KrasG12D alterations. Chr4 and chr6 CNA/LOH patterns (based on aCGH,WES) of primary mPDACs (n=13 PK mice) and associated metastases (n=25). For seven mPDACs and 16 associated metastases the order of genetic events (dots) could be reconstructed. Bifurcations, divergent evolution of clones; lines, lengths do not represent evolutionary distances; P, primary tumor; Li/Lu/LN, liver/lung/lymph node metastasis. f, Detailed chr4/chr6 CNV/LOH profiles for mPDAC5320 primary/metastases. Cdkn2a deletions are identical in all lesions (y-axis, copy number). SNP frequency analysis by WES shows distinct chr6 SNP patterns in metastases and a composite picture in the primary, showing convergent evolution of different KrasG12D-iGD-gains upon Cdkn2aΔHOM. Scheme, combined interpretation of WES/aCGH data.
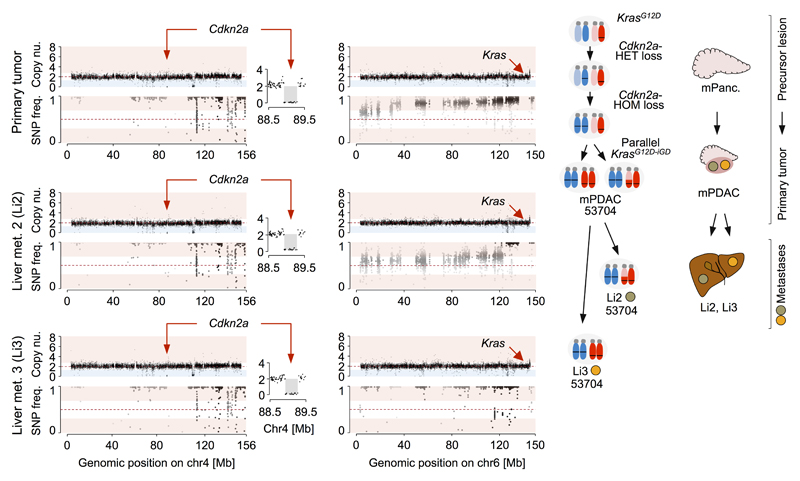
To resolve the sequence, we determined copy-number changes and copy-number neutral allelic imbalance at Cdkn2a and Kras in Cdkn2aΔHOM;KrasG12D-iGD mPDACs (n=13) and associated metastases (n=25). We found identical Cdkn2a deletions in all 13 primary/metastasis pairs, but discordant chr6 CNA/LOH phenotypes in 7/13 pairs (Fig. 3e). In 6/13 pairs the sequence of Cdkn2a-loss/KrasG12D-iGD could not be reconstructed, either because the SNP density was too low (4 cases) or because chr6 profiles in primary/metastasis pairs were identical (2 cases). Thus, in all cases with reconstructable sequence, Cdkn2a deletion preceded KrasG12D-iGD acquisition. For example, mPDAC-53704 (Extended Data Fig. 6) had two liver metastases with identical Cdkn2a deletions, but distinct chr6 SNP patterns: one with KrasG12D-LOH at distal chr6 (through mitotic recombination) and another affecting the whole chromosome (likely through missegregation). This confirms clonal chr6 diversification and convergent evolution following Cdkn2a loss, and explains the primary´s gradual chr6 SNP pattern (Extended Data Fig. 6). Fig. 3f shows another example: mPDAC 5320 and its three metastases had identical Cdkn2a deletions, but distinct chr6 patterns: while liver metastasis-1 had KrasG12D-AG (combined interpretation of aCGH and SNP data), liver metastasis-3 and the lung-metastasis had distinct KrasG12D-LOH events, again showing convergent evolution of Kras-allelic imbalance and explaining the primary´s composite SNP pattern (Fig. 3f).
These results reveal several evolutionary principles in PDAC. First, KrasG12D-iGD is contingent on Cdkn2aΔHOM inactivation. Second, Myc, Yap1 or Nfkb2 amplifications can occur on a Cdkn2aΔHET context, suggesting context-dependent Cdkn2a haploinsufficiency. Of note, only one cancer was Cdkn2aWT. Third, evolution of multiple independent KrasG12D-gains in Cdkn2aΔHOM cancers demonstrates functional convergence towards KrasG12D-iGD acquisition upon homozygous Cdkn2a loss.
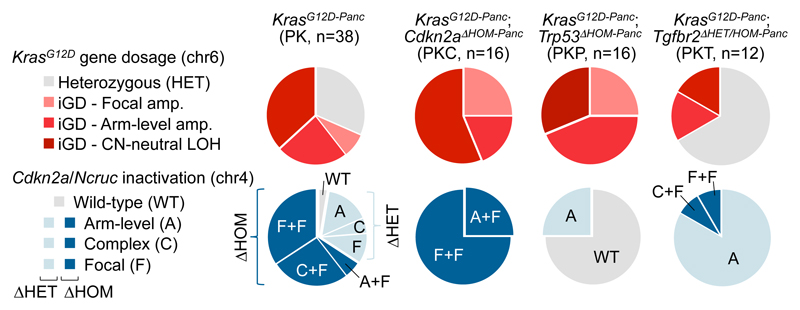
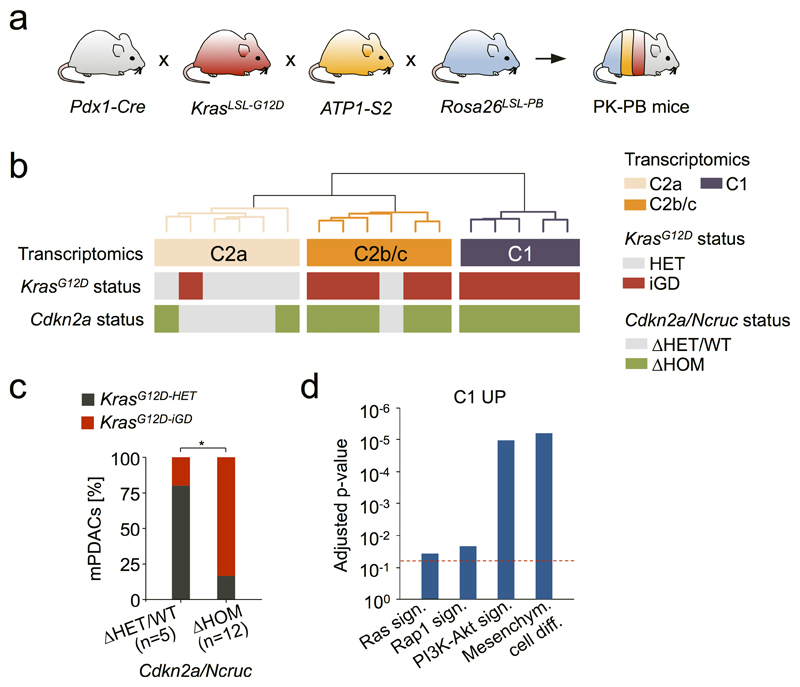
To provide in vivo evidence for functional convergence in Cdkn2aΔHOM contexts, we generated mice with pancreas-specific KrasG12D-expression and Cdkn2a-deletion (PKC). We found KrasG12D-iGD in 100% (16/16) of PKC tumors (Fig. 4 and Supplementary Table 9), confirming that KrasG12D-iGD acquisition is the preferred evolutionary route upon homozygous Cdkn2a loss.
Figure 4. Defined allelic states and/or combinations of hallmark PDAC tumor-suppressor alterations license oncogenic dosage variation.
Types and frequencies of KrasG12D gene dosage gains and Cdkn2a inactivations, defined by aCGH and amplicon-based KrasG12D sequencing in PDAC mouse models expressing pancreas-specific KrasG12D alone (PK) or in combination with engineered Cdkn2aΔHOM (PKC), Trp53ΔHOM (PKP) or Tgfbr2ΔHET/HOM (PKT) inactivation.
Another hPDAC hallmark is TP53 inactivation27. The analysis of cancers from KrasG12D-Panc;Trp53ΔPanc (PKP) mice revealed ubiquitous KrasG12D-iGD (16/16) (Fig. 4 and Supplementary Table 9). Thus, Trp53-loss (like Cdkn2aΔHOM alteration) predisposes to KrasG12D-iGD acquisition (also reflected in hPDAC; Extended Data Fig. 5g). PK/PKC/PKP cross-comparisons revealed higher CNA numbers and a tendency to amplify KrasG12D through arm-level gain (trisomy) in PKP, whereas copy-number neutral LOH predominates in PKC (Fig. 4 and Extended Data Fig. 5h). Of note, PKP or PKC tumors did not have complex chr4 rearrangements, confirming that chromothripsis in PK cancers results from natural selection for Cdkn2a inactivation (Fig. 4).
To address the role of the TGFβ-pathway, we characterized KrasG12D-Panc;Tgfbr2ΔPanc (PKT) mice (Fig. 4 and Supplementary Table 9). Strikingly, all PKT tumors (n=12) had Cdkn2a alterations: two cancers were Cdkn2aΔHOM/KrasG12D-iGD, ten were Cdkn2aΔHET and predominantly KrasG12D-HET (8/10). Overall, the prevalence of KrasG12D-iGD is significantly lower in PKT (4/12) than PK mice (26/38) (P=0.04, Fisher’s exact test, OR 0.23, 95% CI 0.06-0.92). KrasG12D-HET cancers had frequent alternative oncogenic gains (Nfkb2/chr19 trisomy in 4/8 PKT mice), similarly to KrasG12D-HET cancers in the PK cohort. Thus, contrary to Trp53ΔHOM or Cdkn2aΔHOM alterations, which license KrasG12D-iGD-acquisition, Tgfbr2 alterations facilitate the alternative route with Cdkn2a haploinsufficiency.
Altogether these data show that evolutionary contingencies and convergence shape early tumorigenesis: different tumor suppressor genes/pathways (Cdkn2a, Trp53, Tgfβ), their alteration types (ΔHOM/ΔHET) or their combinations (e.g. Cdkn2aΔHET/Tgfbr2ΔHET) direct evolution into different trajectories by licensing distinct types and extents of oncogenic dosage gains.
Integrating genomes, transcriptomes, phenotypes
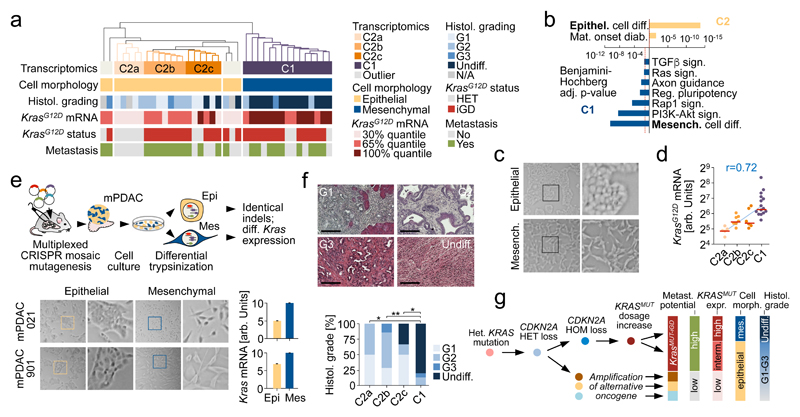
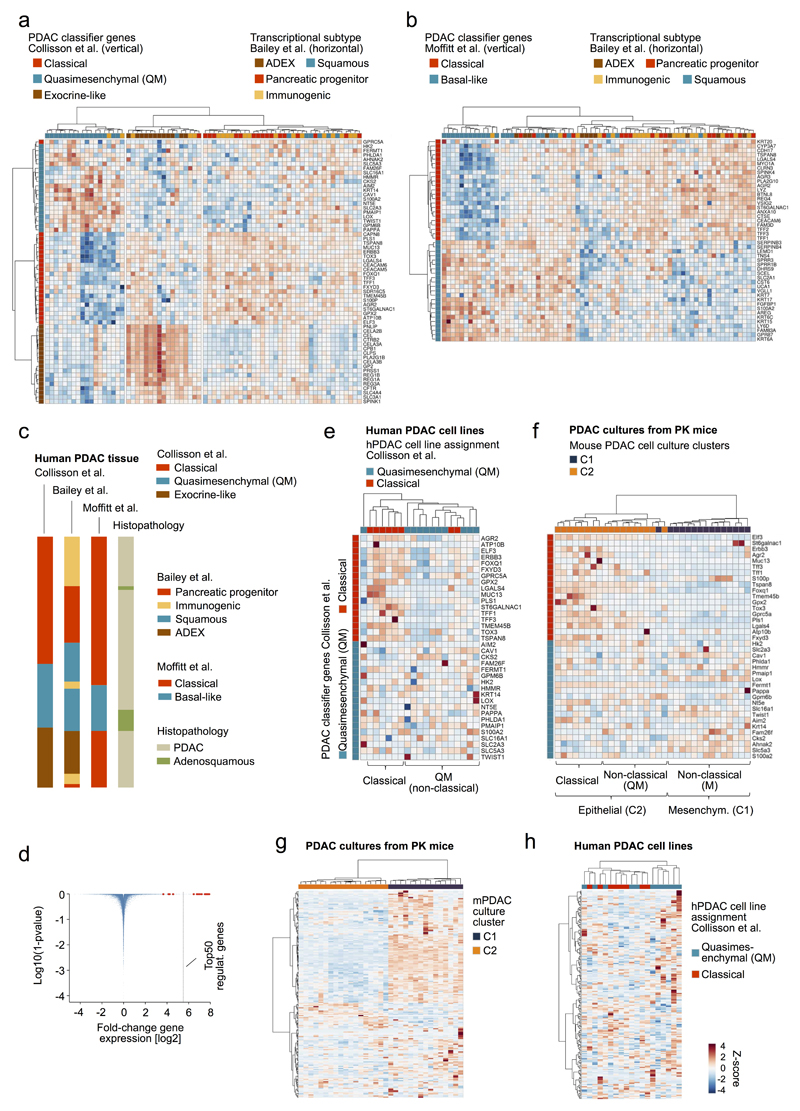
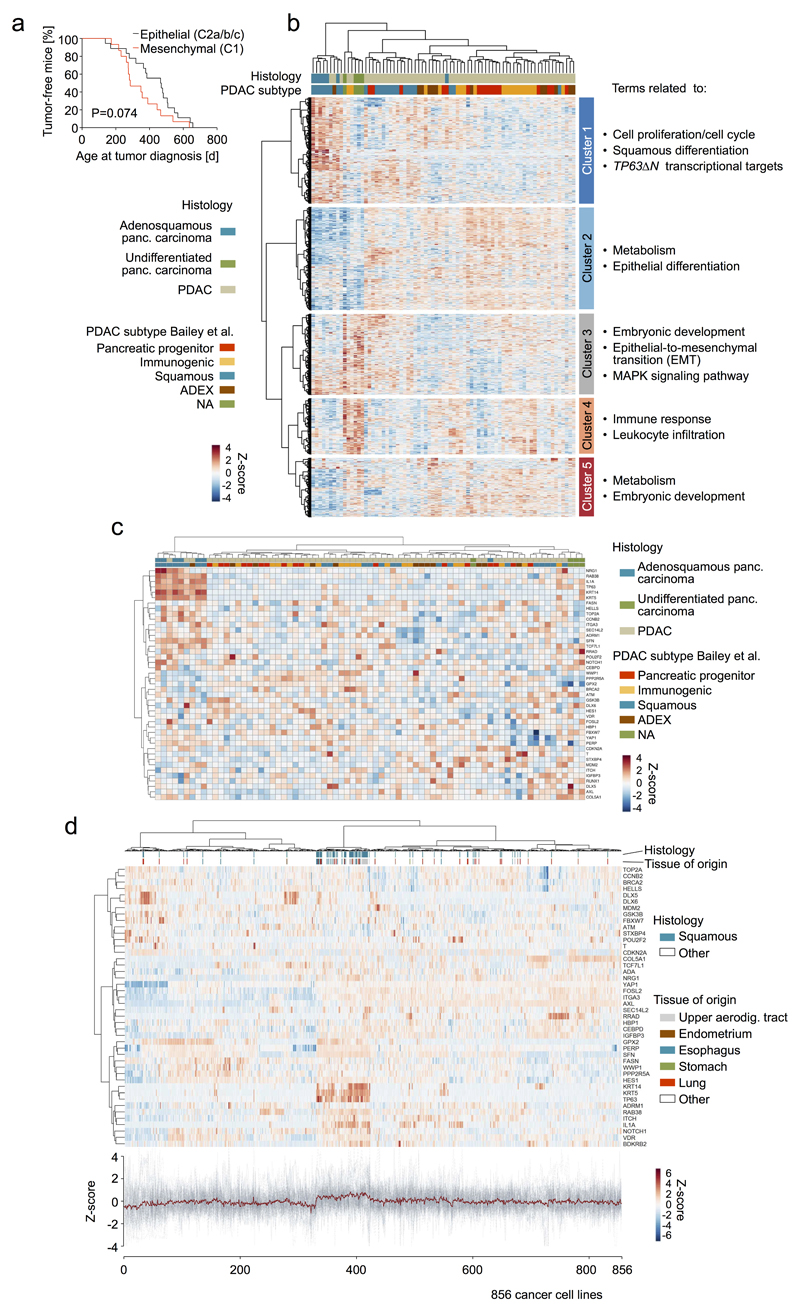
Unbiased hierarchical clustering of RNA-Seq data from mPDAC cell cultures (PK cohort) revealed two clusters C1/C2, with 3 sub-clusters within C2 (Fig. 5a). Pathway analyses identified “epithelial cell differentiation” as the top C2 GO-term, whereas “mesenchymal cell differentiation” was defining C1 (Fig. 5a,b and Supplementary Table 13,14). Notably, all C1 cell lines show mesenchymal cell morphology, while C2 lines are invariably epithelial (Fig. 5a,c).
Figure 5. Integrative analyses of PDAC genomics, transcriptomics, cellular phenotypes and histopathologies link molecular, morphologic and clinical disease characteristics.
a, Unbiased hierarchical clustering of primary mPDAC culture transcriptomes (PK mice). Cell morphology, histopathological grading, KrasG12D mRNA expression, genetic KrasG12D status and presence/absence of metastasis integrated below. b, Selected gene sets from gene-set enrichment analysis of clusters C2 vs. C1. (full list in Supplementary Table 13,14). c, mPDAC cultures with mesenchymal/epithelial morphology from clusters C1/C2, respectively. 100x magnification; squares, zoom-in area. d, KrasG12D-allele-specific mRNA levels in mPDAC transcriptional clusters, combined amplicon-based RNA-Seq and qRT-PCR (C2a/b/c/C1, n=5/7/6/15 mice). P=1.9*10-6, two-sided Pearson correlation; bars, median. e, CRISPR/Cas9-mediated multiplexed somatic inactivation of PDAC-relevant tumor suppressors by electroporation-based transfection to achieve low-frequency mosaicism and clonal tumor outgrowth. Differential trypsinization separates epithelial/mesenchymal cells in mPDACs with mixed morphologies (100x magnification; squares, zoom-in area). CRISPR/Cas9-induced indel signatures are identical in epithelial/mesenchymal pairs (Extended data Fig. 8), indicating common cell of origin. Total Kras mRNA levels in epithelial/mesenchymal pairs (qRT-PCR, normalized to Gapdh, n=2 technical replicates). Bars, mean; error bars, SEM. f, mPDAC histophathological grading in transcriptional clusters (C2a/b/c/C1, n=4/7/6/15, single section per mPDAC). Representative sections (H&E) shown. *Benjamini-Hochberg-adj. P≤0.05, **P=0.005; two-sided Fisher’s exact test; scale bars, 150µm. g, Simplified model of PDAC evolution reconciling molecular, morphologic and clinical disease characteristics. KRASG12D-iGD gain or alternative oncogenic amplifications (Myc/Yap1/Nfkb2) are critical for early disease progression. Different oncogenic gains and dosages evolve along distinct evolutionary routes, licensed by defined allelic states (heterozygous/homozygous) and/or combinations of hallmark tumor-suppressor alterations. For simplicity, only the prototype tumor suppressor gene CDKN2A is shown. Not visualized: TP53ΔHOM loss, also promoting KRASMUT-iGD, or TGFBR2ΔHET/HOM inactivation, supporting evolution through CDKN2AHET/KRASMUT-HET trajectories. Depicted alternative trajectories are typical, but not completely exclusive, e.g. MYC or NFKB2 amplifications, which drive KRASMUT-HET cancers, can also cooperate with KRASMUT-iGD. Major aspects of a cancer´s biology/phenotype are linked to differential evolution.
Previous studies classified human pancreatic cancer based on transcriptional profiles7,28,29. Unbiased hierarchical clustering with published classifiers shows large overlaps of subtypes proposed by Bailey7 and Moffitt29 to the initially proposed three Collisson28 subtypes: classical, exocrine-like, quasimesenchymal (QM). One exception is the lacking exocrine-like signature in the Moffitt classification, which was proposed to be an artefact of acinar cell “contamination” (details in Extended Data Fig. 7a-d). The Collisson classifier28 separates human PDAC cell lines into two subtypes (classical and QM; Extended Data Fig. 7e) and mouse PDAC cell lines into 3 subtypes: classical-equivalent, QM-equivalent (both in epithelial C2) and the mesenchymal M subtype (C1) (Extended Data Fig. 7f). The equivalent of the mouse M subtype with the strong EMT signature has not been described in human cell lines so far, reflecting underrepresentation of mesenchymal phenotypes in human cell line collections (see also Extended Data Fig. 7g,h). As described below, however, mesenchymal mPDACs in C1 represent undifferentiated pancreatic carcinomas with a pronounced EMT signature and human equivalent.
C1 shows strong gene set enrichment for Ras downstream signaling pathways (Fig. 5b and Supplementary Tables 13,14). This cannot be explained by the genetic Kras status alone: only C2a is KrasG12D-HET, whereas C2b, C2c and C1 are mostly KrasG12D-iGD. However, integration of KrasG12D expression revealed its gradual increase from C2a to C2b/c and further substantial elevation in C1 (Fig. 5d and Extended Data Fig. 3k). Thus, the mesenchymal phenotype is associated with KrasG12D expression above a certain threshold.
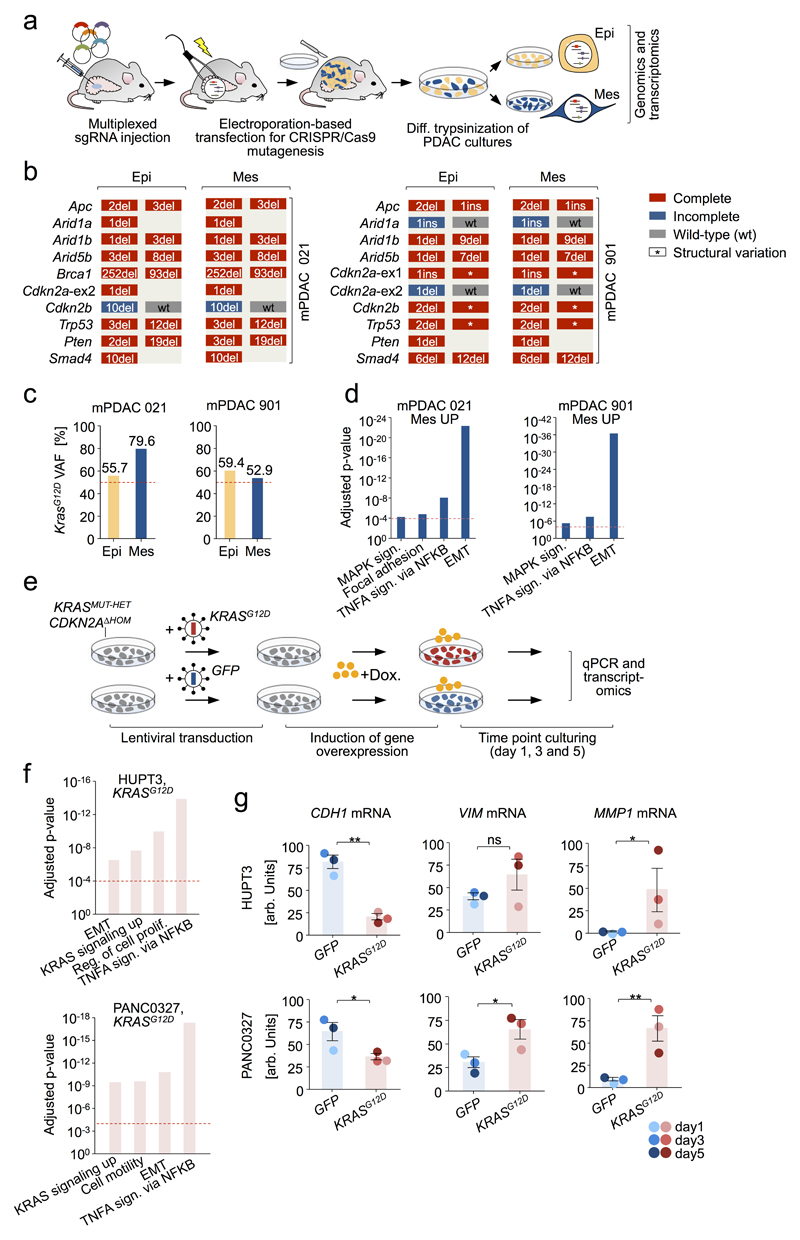
To study this association further, we induced clonal PDACs by CRISPR/Cas9 somatic mutagenesis30 in PK mice (Fig. 5e), screened for simultaneous presence of epithelial and mesenchymal cells, and separated/enriched either phenotype by differential trypsinization. Two such cancers were identified. In each case, indel patterns of epithelial/mesenchymal pairs were identical (Extended Data Fig. 8a,b), showing (i) common clonal origin of epithelial/mesenchymal cells and (ii) independence of epithelial/mesenchymal phenotypes form CRISPR/Cas9-induced TSG alterations. Notably however, mPDAC 021 had KrasG12D-iGD, elevated Kras expression and downstream pathway activation in mesenchymal, but not epithelial cells. In mPDAC 901 both clones were KrasG12D-HET, but mesenchymal cells had increased Kras expression, supporting a role of KrasG12D dosage-variation in shaping cellular phenotypes (Fig. 5e, Extended Data Fig. 8c,d and Supplementary Table 15). Moreover, KRASG12D overexpression in hPDAC cell lines induced an EMT signature, with Vimentin upregulation and E-cadherin repression (Extended Data Fig. 8e-g and Supplementary Table 16).
PDAC histology revealed a striking association with transcriptome clusters (Fig. 5a,f). Histopathologic grade scores increased from C2a to C2b/c and C1, with C2a being well- or moderately differentiated (G1, G2) and C1 being almost exclusively undifferentiated. Undifferentiated cancers are typically advanced and therefore underrepresented (1-3%) in human surgical series or cell line collections, but autopsy series reported up to 16% hPDACs with at least focal undifferentiated components31,32. Dedifferentiation can occur during disease progression or is triggered by treatment. It is associated with poor prognosis32,33, which is also reflected in mice (Extended Data Fig. 9a). Our results link this aggressive PDAC subtype with highest KrasG12D expression levels and Ras-related transcriptional programs (Fig. 5b,d and Supplementary Table 13). We also screened human transcriptome data (ICGC PACA-AU cohort) for undifferentiated pancreatic carcinomas and performed unbiased hierarchical clustering of differentially regulated genes in undifferentiated cancers (Extended Data Fig. 9b). Of note, undifferentiated human pancreatic carcinomas are characterized by reduced expression of genes involved in “epithelial” (cluster-2) or “squamous differentiation” (cluster-1), and a strong upregulation of genes in cluster-3, containing gene sets enriched for EMT and Ras downstream signaling (Extended Data Fig. 9b-d and Supplementary Table 17 and 18).
We exploited the mouse to address complex questions, including cell based resources (overcoming human PDAC´s stroma richness), primary/metastasis resources (phylogenetic tracking, evolution) and in vivo modelling (proof-of-concept functional studies). In addition, discoveries were facilitated by the relatively low complexity of mouse PDAC genomes (easier interpretability). Notably, a transposon-induced PDAC model13 showed that our findings are equally valid in contexts of excessive mutational loads (Extended Data Fig. 10 and Supplementary Table 19).
Conclusions
Our study proposes a novel comprehensive conceptual framework for molecular PDAC evolution and phenotypic diversification. It describes evolutionary trajectories, identifies their genetic hallmarks and shows how oncogenic dosage-variation is differentially licensed along individual routes by the three major PDAC tumor suppressive pathways to control critical disease characteristics, including early progression, histopathology, metastasis, cellular plasticity and clinical behavior (Fig. 5g). RAS gene mutations affect more than 30% of human cancers, often involving their allelic imbalance. We therefore presume that the principles identified here are significant far beyond PDAC.
Methods
Primary mPDAC cultures preparation
For 2D primary cell culturing, primary tumor or metastasis were cut into small pieces and digested 1-2h in 200Units/mL collagenase II (Worthington) in DMEM medium (Thermo Fisher Scientific Inc.) containing 10% fetal calf serum (FCS, Merck) and 1x Penicillin/Streptomycin (P/S, Thermo Fisher Scientific Inc.). After short term expansion, primary cells were frozen in 10% dimethyl sulfoxide (Roth) and 50% FCS. For all primary culture experiments, culturing medium (DMEM supplemented with 10% FCS and 1x P/S) and cultures with less than 10 passages were used. Primary cultures were routinely tested for mycoplasma contamination by PCR and authenticated by re-genotyping of cell cultures and corresponding mice.
gDNA and RNA isolation
gDNA from murine primary cell culture pellets was isolated using the DNeasy Blood & Tissue Kit (Qiagen) according to manufacturer’s instructions. Total RNA was isolated with the RNeasy kit (Qiagen) from 60-80% confluent primary cell lines cultured in a 10cm dish in culturing medium without P/S and immediately transferred into RLT buffer (Qiagen) containing ß-mercaptoethanol.
Histology and micro-metastases screening
For histologic characterization of mPDACs, 2µm thick specimens from formalin-fixed paraffin embedded (FFPE) material were routinely H&E stained and submitted to two veterinary pathologists experienced in comparative pancreatic cancer pathology. Histopathologic grading was performed with respect to the most recent consensus report of genetically engineered mouse models34. For histopathologic examination of micro-metastases, three H&E-stained liver sections (separated by 200µm) were screened for metastatic lesions by a veterinary pathologist.
Animal experiments
Mice were maintained on C57Bl/6;129S6/SvEv mixed background and housed under specific-pathogen-free conditions. Female and male mice were randomly submitted to respective tumor cohorts. For the generation of double- or triple-mutants, pancreas-specific Cre lines10,35,36 were intercrossed with KrasG12D-Panc (PK mice)9,10 only, or in addition with Cdkn2aΔHOM-Panc (PKC mice)37, Trp53ΔHOM-Panc (PKP mice)38,39 or Tgfbr2ΔHET-Panc and Tgfbr2ΔHOM-Panc (PKT mice)40 mice. Kaplan-Meier survival curves were generated using Prism (GraphPad Software v5.01). In case the animal presented a palpable abdominal mass above 1.5cm, ascites, signs of sickness or a weight loss of more than 15% of the body weight, mice were euthanized in compliance with the European guidelines for the care and use of laboratory animals. For necropsy of tumor-bearing mice, the abdominal cavity was macroscopically checked for pancreatic cancer and for metastases at the main metastatic routes (liver, lung, lymph nodes). Animal studies were approved by the Institutional Animal Care and Use Committees (IACUC) of Technische Universität München (Regierung von Oberbayern, Munich, Germany).
Amplicon-based deep sequencing at the Kras locus or of Kras mRNA
Fifty ng of high-quality genomic DNA or reversely transcribed mRNA (cDNA) were subjected to amplicon-based deep sequencing. Briefly, the KrasG12D-mutated locus was amplified using Q5® High-Fidelity DNA Polymerase (New England Biolabs, 40 cycles) and primers with Nextera adapter overhangs (Supplementary Table 20). In a second Q5® PCR step (15 cycles), Nextera index primers (Illumina) were added. After each PCR step, solid phase reversible immobilization cleanup (0.8x) was performed using the Agencourt® AMPure® XP kit (Beckman Coulter GmbH). The pooled library was quantified by SYBR® Green qPCR (Thermo Fisher Scientific Inc.) and the Kapa Biosystems library quantification kit. In total, 8pM of denatured library (20% spiked PhiX DNA) was sequenced in 300bp paired end mode using a MiSeq system (Illumina). Raw reads were mapped to Kras reference sequence (Ensemble release GRCm38p4, Genome Reference Consortium). Variant allele frequencies on chr6 at position 145246771 were calculated.
Microdissection of hPanIN and KRASG12 status analysis
Nineteen patients (Supplementary Table 11) with or without a history of pancreatic cancer were included into hPanIN lesion analysis, according to approval by the Ethics Committee of the Faculty of Medicine of the Technische Universität München. Patients were classified using WHO recommendations and the TNM staging system. Serially cut 10µm thick specimens from FFPE material were air-dried overnight. Paraffin was removed through short incubation with xylene. Specimens were briefly stained with hematoxylin and kept wet for the micro-dissection procedure. Individually diagnosed samples were microdissected under the Axio Imager microscope (Zeiss) using 20 gauche cannula. Pre- and post-sampling microscopic pictures were taken to (i) document dissection performance and (ii) re-identify each specimen on the corresponding H&E-stained slide. gDNA was extracted as described above using MinElute spin columns (Qiagen) for higher sample concentration. Five µl of eluted hPanIN gDNA were submitted to amplicon-based deep sequencing of KRAS exon-2 for detection of KRASG12 hotspot mutations. Briefly, 2 pairs of custom KRAS primers (Supplementary Table 20) were used for nested PCR amplification of the corresponding KRAS region. Illumina Nextera primer pairs were used to add sequencing adapters and indices. PCR steps, library quantification and sequencing were performed as described above. Raw reads were mapped to KRAS reference sequence (GRCh38.p10). Variant allele frequencies were calculated for KRASG12 hotspot mutations (positions 25398284 and 25398285 on chr12).
Whole genome sequencing (WGS)
One µg of high-quality gDNA extracted from primary tumor cell line and corresponding tail were sheared on a Covaris M220 focused ultrasonicator (Covaris, Inc.) to an approximate fragment size of 500bp. Library was prepared from 500ng of fragmented gDNA using the NEBNext® Ultra™ II DNA Library Prep Kit (New England Biolabs, Inc.) in combination with the adapter/primer sequences and PCR conditions published before41. The final library was quantified by qPCR using the Kapa Biosystems library quantification kit. Equimolar amounts of indexed libraries were denatured and diluted to a final concentration of 1.8pM and sequenced in 300bp paired end mode on a NextSeq 500 system (Illumina) to ~20x coverage. Sequencing raw data were converted to fastq format using the bcl2fastq software v2.18.0.12 (Illumina). Raw reads were trimmed with Trimmomatic v0.36 to preserve an average base Phred quality of 25, mapped using the BWA-MEM algorithm v0.7.12 with alternative contig handling and mapped to GRCm38.p5 reference genome.
Inference of chromothripsis
For estimation of copy number states, the Bioconductor HMMcopy package 1.16.0 was used followed by segmentation with the Bioconductor DNAcopy package 1.48.0. For LOH analysis variant positions in control and tumor were computed with samtools mpileup v1.3.1. Only positions in regions with mapping quality of 60 and an average phredscore of 20 were considered for further analysis. Furthermore, positions harboring strand bias and variant allele frequencies less than 20% and above 85% in the control were excluded as they are likely homozygous in the germline. The minimal cutoff coverage for a given polymorphic position in the control was set to eight reads. Segmental duplications (UCSC Genome Browser) and regions with mouse line specific variation (Mouse Genomes Project, REL-1505) were excluded. For this set of somatic nucleotide polymorphisms (SNPs) the difference of frequencies between tumor and control samples were calculated. DELLY v0.7.6 was used for calling structural variations (SVs). SV-classes were defined according to DELLY callings: Deletion-type (3to5), duplication-type (5to3) and inversion-type (5to5 and 3to3). The predicted rearrangements were merged and filtered based on variant frequency, mapping quality and the distance between two connected breakpoints. The existence of chromothripsis was tested by applying the six hallmark criteria proposed by Korbel et al.14. Clustering of SV breakpoints was tested using a χ2-goodness-of-fit test. Regularity of oscillating copy number states in the chromothriptic model was compared to a virtual chromosome generated by a Monte Carlo simulation, as described in42. For each distinct number of breakpoints, 100 simulation runs were completed and mean values as well as 95% CI were calculated. Interspersed loss and retention of heterozygosity was analyzed by calculating the Jaccard index between heterozygously deleted segments and regions comprising LOH and SNP information. Randomness of observed DNA segment order was tested using a Monte Carlo simulation as described in14. The uniform distribution of SV-types was tested using a χ2-goodness-of-fit test. The Wald-Wolfowitz runs test as implemented in R package randtests 1.0 was performed for testing right-sided against the null hypothesis of randomly distributed 5’-to-3’ breakpoint joints sequence.
FISH analyses
For the analysis of copy number status or large structural alterations of human and murine primary cell lines, multicolor fluorescence in situ hybridization (M-FISH) was performed as described before43. For KRAS gene detection in hPanIN specimens, the ZytoLight® SPEC KRAS/CEN12 Dual Color Probe kit was used according to manufacturer’s instructions (ZytoVision GmbH). In brief, 2µm FFPE specimens were deparaffinized, pre-incubated in CC 2 buffer (at 95°C for 24min), treated with pepsin solution (at 37°C for 8min) and denatured by a heat treatment step at 80°C for 8min on an automated Discovery XT system (Ventana Medical Systems, Inc.). KRAS/CEN12 dual color probe hybridization was performed by co-denaturing at 75°C for 10min and by incubating at 37°C overnight in a ThermoBrite system (Abbott Laboratories). Slides were washed, nuclei stained with 4',6-diamidino-2-phenylindole (DAPI), covered in an antifade mounting medium and by a coverslip and stored for confocal laser scanning microscopy (LSM) analyses at 4°C in the dark. ZytoLight® SPEC KRAS/CEN12 Dual Color Probes and DAPI nuclear stain (excitation/emission: DAPI 405nm/415-490nm; ZyGreen, 503nm/510-540nm; ZyOrange, 547nm/560-650nm) were detected by confocal LSM using a Leica TCS SP8; DMi8 CS microscope equipped with a 63x/1.4 oil immersion objective (Leica). Images (z-stacks, covering the whole nucleus) with a magnification factor of 3 and a frame size of 2048 x 2048 pixels were collected. Generated images were processed using the Huygens Essential software (Scientific Volume Imaging) for deconvolution, than merged and maximum projections were converted with Leica LAS X software.
aCGH analysis
Agilent oligonucleotide aCGH (SurePrint G3 Mouse CGH 240K or custom 60K microarray) was performed according to manufactures instructions. Agilent Genomic Workbench software v7.0.4.0 was used for aCGH data preprocessing. Legacy centralization option was used for re-centralization of raw log ratios to the most common ploidy state. ADM-2 algorithm was applied for aberration calling. Segments coordinates were reported for GRCm37 reference genome. Aberrations on chromosome 6 between positions 148719747 and 149503634 were excluded in further downstream analysis as this region resulted likely from an artefact. Normalized and curated data was imported into R.
Whole-exome sequencing (WES) analysis
Coding exons were enriched via whole-exome pull down using Agilent SureSelect Mouse Exon Kit according to manufacturer's instructions and sequenced on the Illumina HiSeq2000 system. Prior to mapping, raw sequencing reads were trimmed using Trimmomatic v0.33. Leading and trailing bases with phred scores below 25 and reads shorter 50 nucleotides were removed. In addition the average base quality within a sliding window of 10 nucleotides should be above 25 to keep the read for further downstream analysis. Reads were aligned to the GRCm38.p3 reference genome using BWA-MEM 0.7.12 with default settings. PCR duplicates were marked with Picard tools v1.130 and realignment around indels was performed with GATK toolkit v 3.4.46. Mutect v 1.1.7 was used for calling somatic mutations with default settings. Potential somatic events were filtered for SNPs by excluding SNVs which were listed in in release 1505 of the Mouse Genome Project SNP database44. Somatic point mutations were included in the final list, if the read coverage for each position was ≥10 in both control and tumor, variant frequency was ≥10% and read count supporting the variant nucleotide is ≥3 in the tumor sample and =0 in the control. Further, SNVs marked as strand or PCR bias artifacts by “DKFZBiasFilter” (https://github.com/eilslabs/DKFZBiasFilter, using default settings) or with a FOXOG-Score of 1 were excluded. Annotation of somatic events was conducted with SNPeff v4.1. SNVs causing variation in splice sites or upstream/downstream of genes were excluded from further analysis. Indels were detected with Pindel45. For each potential indel the read coverage was re-calculated using bedtools v2.17.0. Criteria for further downstream processing were: Variant frequency ≥10% in tumor and =0% in control; and total coverage at the altered position in both control and tumor ≥20. LOH analysis was conducted as described in chapter Inference of Chromothripsis.
WES data analysis from hPDAC
Mapped BAM files from Witkiewicz et al.6 were downloaded from the Sequence Read Archive (accession number PRJNA278883) approved by the Ethics Committee of the Faculty of Medicine of the Technische Universität München. Further downstream analysis was performed as described above. SNPs were filtered by excluding variants with an alternate allele frequency ≥1% in the 1000 Genomes Project, as listed in dbSNP build 146. All available VCF files from the TCGA-PAAD cohort generated by Mutect2 were downloaded from the NIH Genomic Data Commons data. Downstream processing was performed as described above (PCR and strand bias marking by DKFZBiasFilter was not possible using VCF files). SNPs were filtered by excluding variants with an alternate allele frequency ≥1% in the 1000 Genomes Project, as listed in dbSNP build 142. MAF files from other human pancreatic cancer cohorts were downloaded and included in our analysis: All samples, for which whole-exome sequencing data was available, as provided by Bailey et al.7; pancreatic cancer cell lines from the Cancer Cell Line Encyclopedia46 and SNV data from pancreatic cancers as analyzed by Alexandrov et al.47. In these cohorts, SNPs were filtered by excluding variants with an alternate allele frequency ≥1% in the 1000 Genomes Project, as listed in dbSNP Build 146. Remaining SNVs were annotated and filtered with SNPeff as described above.
Analysis of mutational signatures
Mutation spectra for each cohort were compared to a list of 21 signatures previously described by Alexandrov et al.47; Signature 1B was excluded from further analyses because of presumed biological similarity to Signature 1A. The contribution of each individual signature to the mutation spectrum of each cohort was analyzed by using “deconstructSigs” v1.8.0.
qRT-PCR analysis
Reverse transcription was performed with random hexamers using 1µg of total RNA following instructions of the SuperScript II protocol (Thermo Fisher Scientific Inc.). Real-time qPCR was performed either with the TaqMan qPCR chemistry (Thermo Fisher Scientific Inc.) for mouse using Kras-specific primers and probes or with the SYBR® Green master mix (Thermo Fisher Scientific Inc.) using primers for human target genes VIM, CDH1 and MMP1 (Supplementary Table 20). Gapdh or GAPDH in combination with PPIA were used as housekeeping genes for normalization (Supplementary Table 20). qPCR was conducted on a StepOnePlus system (Applied Biosystems). For analyses of mutant KrasG12D mRNA levels in mPDACs, first total (wild-type plus mutant) Kras mRNA levels were determined using qRT-PCR. Second, the identical cDNA was used for amplicon-based deep sequencing to detect the proportion of mutant to wild-type Kras mRNA. Third, the mutant to wild-type Kras mRNA ratio was multiplied with the total Kras mRNA level to calculate the mutant KrasG12D-specific mRNA level.
RNA-Seq analysis
Bulk 3’-prime transcript end RNA-Seq (SCRB-Seq) libraries were prepared as described previously48. Briefly, RNA was reversely transcribed using oligo-dT primers decorated with sample barcodes, unique molecular identifiers (UMIs) and adapters (Integrated DNA Technologies). cDNA from all samples was pooled and un-incorporated primers digested using ExonucleaseI (New England Biolabs). Next, the cDNA pool was amplified with KAPA HiFi ReadyMix (KAPA Biosystems). To obtain sequencing libraries, 0.8ng of cDNA was tagmented and 3’ ends amplified with the Nextera XT Kit (Illumina) using a specific primer for the adapter on the 3’-end. The library was paired-end sequenced on a HiSeq1500 with 16 cycles for read 1 to decode sample barcodes and UMIs and 51 cycles on read 2 into the cDNA fragment. For the preparation of the human pancreatic cancer cell line samples, the flow cell binding sites P5 and P7 were exchanged to allow sequencing of the cDNA in read1 and barcodes and UMIs in read2. Data was processed using the published Drop-seq pipeline (v1.0)49 to generate sample- and gene-wise UMI tables. Reference genome (GRCm38) was used for alignment. Transcript and gene definitions were used according to the ENSEMBL annotation release 75. Further analyses were performed with R version 3.2.2. Initial hierachical clustering (method: Complete linkage, distance measure: Euclidian) of samples was performed for the top 10% variable genes. Bootstrapping was performed to access cluster stability with the pvclust package v2.0. The 4 most prominent clusters were selected and differential expression between these clusters was calculated with DEseq250. A gene was considered to be differentially regulated if the absolute log2-foldchange was above 0.8 and the adjusted P-value was ≤0.05. Gene set enrichment testing was performed with DAVID 6.851 or the hypergeomtric test as implemented on the “Molecular Signature Database” (MSigDB) v6.0 homepage (http://software.broadinstitute.org/gsea/msigdb/annotate). For all MSigDB analyses, top100 enriched terms with a false discovery rate (FDR) of P≤10-4 were included. Published PDAC classifier genes28 and the hallmark EMT gene set (downloaded from MSigDB v5.252) were used for sample clusterings (method: Ward, distance measure: Euclidian).
hPDAC subtyping
Normalized RNAseq data was derived from Bailey et al.7. Samples that were histologically classified as “PDA-Adenosquamous carcinoma” and “Pancreatic Ductal Adenocarcinoma” were used for hierarchical clustering (method: Ward, distance measure: Euclidian) with classifier gene lists published elsewhere28,29.
Microarray data analysis
Affymetrix-based CCLE raw data set was downloaded from (Broad-Novartis Cancer Cell Line Encyclopedia, Version 2.17). Hematopoietic or lymphoid neoplasms were excluded since (1) the primary interest of our study were solid tumors and (2) the overall gene expression signature of these samples was shown to be very distinct from all other samples in the study46. Normalization of the data was performed with RMA. In general, if genes were represented by two or more probe sets, the probe set with the highest mean expression was used for all further microarray data analyses. Mapping between probeset and genes were conducted with the appropriate Bioconductor packages. Target genes for the TP63ΔN network were downloaded from the “Pathway Interaction Database” (PID)53 and hierarchically clustered (method: Ward, distance measure: Euclidian). Gene set enrichment analysis was conducted with DAVID or MSigDB v6.0. All following microarray data sets are Illumina-based and were VST-transformed followed by quantile normalization as implemented in lumi54. Microarray data set of hPDAC cell lines (accession number GSE17891) was downloaded from Gene Omnibus Expression database. PDAC classifier genes and EMT hallmark gene set were used as described above. For the comparison of human wild-type pancreatic tissue and hPDAC cell lines, limma55 was used for detection of differential expression between groups. Differentially expressed genes were determined with an alpha level threshold of 5%. The PACA-AU ICGC data set was downloaded from the repository https://dcc.icgc.org/repositories. Samples that met the following criteria were selected for further analyses: (i) Bailey HistoSubtypes “PDA-Adenosquamous carcinoma” or “Pancreatic Ductal Adenocarcinoma” with available subtype information from Bailey et al.7 and (ii) ICGC WHO Grading “Undifferentiated carcinoma”. Only representative samples, as judged by cluster analysis, from this group were selected for downstream analysis. ANOVA was performed across six defined subgroups of pancreatic cancer: (i) undifferentiated pancreatic carcinoma, (ii) adenosquamous pancreatic carcinoma and (iii-vi) PDAC sub-stratified in pancreatic progenitor, immunogenic, squamous and ADEX subtypes. Genes with an adjusted P-value ≤0.05 were hierarchically clustered (method: Ward, distance measure: Manhattan) and the resulting cluster tree was computationally stratified into five sub-clusters. Genes within subclusters were used for gene enrichment analysis as described above. Seventeen PK-PB primary cultures established elsewhere13 were submitted to RNA extraction and subsequent gene expression profiling analysis on a MouseWG-6 v2.0 Expression BeadChip (Illumina). The 5% of genes with the highest variability across all samples were used for hierarchical clustering using the ward method for aggregation of samples. Limma was used as described above. A gene was called differentially expressed if the adjusted P-value was ≤0.05 and the log2-fold was at least 0.8.
Quantitative transposon insertion site sequencing (QiSeq)
Aforementioned gDNA samples of the PK-PB pancreatic cancer cell cultures13 were sequenced for transposon integration sites and bioinformatics analyses were performed as described elsewhere56. Transposon integration sites that are supported by at least 20 reads and reside in intragenic regions were counted for the computation of the mutational burden. For the assessment of the Cdkn2a/NcrucΔHOM status caused by transposon insertional mutagenesis, only the top hit of each tumor was considered.
KrasG12D induction after lentiviral transduction of hPDAC cell lines
The pINDUCER2057 vector system comprising a puromycin resistance gene was used for doxycycline-inducible KRASG12D overexpression. Briefly, cDNA of oncogenic KRASG12D (CCDS 8702.1, 35G>A) and GFP were cloned into the pINDUCER20 lentiviral vector. Stbl3™ (Thermo Fisher Scientific Inc.) were chemically transformed and pDNA sequence was verified. For lentivirus production, HEK293FT cells were transfected using TransIT®-LT1 (Mirus Bio LLC) with standard virus packaging plasmids and respective pINDUCER20 vectors by following manufacturer’s recommendations. Virus-containing supernatant was pooled 48h and 72h post transfection, concentrated by polyethylene glycol 6000 precipitation58 and stored at -80°C after shock-freezing. 1x105 HUPT3 (COSMIC ID: COSS907285) and PANC0327 (COSMIC ID: COSS925346) hPDAC cells were transduced in presence of 1µg/mL polybrene and selected with puromycin antibiotic. Target gene expression was induced for stated time points by the addition of 100ng/µL doxycycline into P/S-free culturing medium. RNA isolation, qRT-PCR and SCRB-Seq were performed as described above. For differential gene expression analysis, raw sequencing data were mapped to the human reference genome (GRCh 38p10). Transcript and gene definitions were used according to the ENSEMBL annotation release 87. Group comparisons (KRASG12D vs GFP) were conducted with DESeq2.
Somatic CRISPR/Cas9 gene-editing for tumor clone tracking in mice
Multiplexed gene editing of tumor suppressor genes using CRISPR/Cas9 in the pancreas of PK mice was performed as described elsewhere30. Primary cultures of induced mPDACs were isolated as described above and monitored for the simultaneous presence of epithelial and mesenchymal phenotypes. Enrichment of epithelial and mesenchymal cell morphologies was achieved by differential exposition times to trypsin (Thermo Fisher Scientific Inc.). Short-term incubation (2-3 min) at room temperature induced detachment of mesenchymal cells, while epithelial colonies remained adherent. Both cell fractions were subsequently grown to 80% confluency in new flasks. This process was repeated for 3-6 times until homogenous epithelial and mesenchymal cell fractions were enriched. Clonal origin of both phenotypes was confirmed by targeted amplicon-based next generation sequencing of CRISPR/Cas9-edited loci as described earlier30,59. Analyses of the Kras allelic status and mRNA expression were carried out as described above.
Statistics and reproducibility
For each experiment, all statistics were performed as indicated in respective Figure legends and Extended Data Figure legends. Statistical testing across all classes was performed to account for multiple testing. Continuous variables were tested for normal distribution. Non-parametric tests were used for non-normally distributed data. Complex statistical techniques are explained in detail in the Methods section. No animals were excluded from any of the cohorts. The veterinarian pathologists were blinded during histological grading of primary tumors and metastasis screening. The study was of explorative nature. Due to this study design prior knowledge of the expected effect-size was not available and no power calculations were conducted.
Code availability
Source code is available from the authors upon reasonable request.
Data Availability
Sequence data have been deposited at EBI European Nucleotide Archive (ENA; https://www.ebi.ac.uk/ena) under accession number PRJEB23787. Microarray data have been deposited in the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE107458. All data are available from the corresponding author upon reasonable request.
Extended Data
Extended Data Figure 1. Mutational patterns, karyotype complexity and structural alterations in primary PDAC.
a, Single nucleotide variants (SNVs) and indels in primary PDAC cultures derived from 38 KrasG12D (PK) mice, as detected by whole-exome sequencing. Recurrently mutated genes that are frequently altered in human cancers and/or genomewide pancreas-specific transposon screens are indicated. b, Frequency of somatic base substitutions based on trinucleotide context in mouse (n=38 PK mice) and human PDAC (n=51 patients, data used for analysis from 6). b-f, Mutation spectra defined by trinucleotide contexts around base substitutions as detected by whole-exome sequencing show similar patterns in PK mice (n=38) and in relevant human pancreatic cancer cohorts. Base substitutions were extracted from BAM, VCF or MAF files from: b, Witkiewicz et al.6, c, Bailey et al.7, d, TCGA-PAAD, e, Barretina et al.46 and f, Alexandrov et al.47. Additional information regarding the analysis of each cohort is provided in Supplementary Table 2. g, Mutational signatures in mouse and human pancreatic cancer cohorts. Information on mutational signatures was used from Alexandrov et al.47, who identified 21 mutational signatures operative in human cancer. The „deconstructSigs“ tool was used to determine the composition of the given set of 21 mutational signatures in each pancreatic cancer cohort. Extraction of mutational signatures strongly depends on SNV load per tumor. Due to the low mutational burden of mPDACs from PK mice (median of 18 SNVs per tumor as detected by WES), the analyses of mutational signatures could not be performed at the level of individual tumors. We have therefore investigated the contribution of each of the 21 mutational signatures to the SNV spectrum at the cohort-level (see Methods). Signature 1, reflecting age-associated C>T transversions at NCG trinucleotides, was the only signature consistently identifiable in all cohorts of human and mouse pancreatic cancer. In comparison to human cohorts, PK mice show C>G substitutions at GCC trinucleotides that cannot be attributed to one of 21 mutational signatures. Note that mutations at the GCC motif are not a general phenomenon of PDAC from PK mice, since only 4 samples are predominantly contributing to this peak. h-i, Representative M-FISH karyotypes with no or few karyotypic changes are shown for a diploid (40 chromosomes) and tetraploid mouse PDAC (81 chromosomes). Tumor 9591 shows gain of chr14. j, Representative karyotype of a complex diploid mPDAC genome with aneuploidy and translocations (46 chromosomes). Both copies of chr4 are involved in translocations: der(4)t(4;10) and der(4)t(4;16); likely affecting Cdkn2a. Further structural alterations and copy number changes are: +5, der(5)t(4;5)*2, +6, +7, +8, del(9), +14, del(14), der(16)t(5;16), +17. k, Representative example of a complex tetraploid mPDAC karyotype (77 chromosomes). Structural alterations are: der(1)t(1;11), dic(9;9), der(11)t(1;11), and der(14)t(14;19). Single chromosomal copy number changes are: +2, -3, -9, -10, -11, -13, -14, +15 and +19. Del, deletion; der, derivative chromosome; dic, dicentric chromosome; t, translocation; „-“, chromosome loss; „+“, chromosome gain. l, (Extension to Fig. 1c.) Circos plot shows CNAs assessed by aCGH as well as translocations and ploidy states detected by M-FISH in 38 primary PDACs derived from PK mice (n=38). CNAs for each mPDAC are displayed as log2 difference from tail control. Frequencies of translocations per chromosome are indicated in green in the inner circle of the graph. Connecting lines indicate individual translocations and involved chromosomes. On chr4, genomic alterations frequently involve Cdkn2a or Ncruc, a Non-coding regulatory region upstream of Cdkn2a (27/38 cancers with homozygous and 10/38 with heterozygous inactivation of Cdkn2a and/or Ncruc). Only one cancer remained Cdkn2aWT. The target of copy number changes on chr6 is KrasG12D, either through arm level gain or focal amplification. In addition, primary mPDAC of PK mice exhibited recurrent genetic amplifications affecting other known oncogenes, such as Myc or Yap1, or Nfkb2, a novel oncogenic PDAC driver identified in this study (see also Fig. 2e,f and Extended Data Figure 4).
Extended Data Figure 2. Characterization of complex rearrangements in PDAC from PK mice and statistical inference of chromothripsis based on whole genome sequencing (WGS).
a-n, Copy number profiles of chromosomes with complex rearrangements (defined as n≥10 CNAs per chromosome) from primary mPDAC cell cultures as detected by aCGH. A total of 14 mPDACs had chromosomes with complex rearrangements. a-i, Nine primary mPDACs show copy number patterns characterized by heterozygous deletions and oscillation of copy number around few states, indicating chromothripsis as the underlying mechanism. g, mPDAC-S821 was subjected to whole genome sequencing for the inference of chromothripsis using previously established criteria14 (see Fig. 1d and Extended Data Figure 2p-w). j-m, Four primary mPDACs showed complex rearrangements with multiple copy number states on chr4, likely acquired through progressive/sequential rearrangement cycles. n, Cancer 5671 carries a complex rearrangement on chr15 characterized by oscillating copy number states and 3 prominent focal amplifications, of which one contained the Myc oncogene. Myc amplification is most likely the result of double minute chromosome formation during chromothriptic rearrangement of chr15. o, Comparison of age at tumor diagnosis in Cdkn2aΔHOM-deleted cancers with (n=10) or without (n=15) complex clustered chromosomal rearrangements (n≥10 CNAs/chromosome). Complex clustered rearrangements are associated with significantly shortened time to tumor diagnosis, indicating accelerated tumor evolution through genetic crisis. Two-sided log-rank test. p, Criteria proposed by Korbel et al.14 were tested for the inference of chromothripsis. Circos plot displays SNP ratio (inner circle, red dashed line indicating heterozygosity), CNV (outer circle, blue area indicating deletion, red amplification) and structural variations (SVs, colors as in v) as detected by WGS. Chr4 shows a complex deletion pattern and massive rearrangements associated with loss of one copy of Cdkn2a. The second copy of Cdkn2a is focally deleted. In addition, a balanced translocation of a ~200Kb segment from trisomic chr6 to chr4 and a far smaller segment of chr4 into chr6 was detected. The Kras locus is not directly affected by this inter-chromosomal translocation. LOH, CNAs and rearrangements are not detected on other chromosomes. q, In a chromothriptic model, DNA breakpoints tend to cluster on a chromosome. Testing against an exponential distribution (parameter λ derived from mean of observed distance between adjacent breakpoints), revealed significantly shorter distances than expected in a progressive model (n=146 breakpoints). P<10-12; χ_-goodness-of-fit test. r, In a progressive model of acquisition of massive rearrangements, copy number states tend to be more complex than in the chromothriptic. Monte Carlo simulations were used to generate a progressive evolution model with sequential accumulation of observed rearrangements (n=100 simulations per number of SVs). mPDAC S821 showed fewer copy number states on chr4 than expected in the progressive model. Mean is indicated as a black point and lines represent the 95% CI. s, Chromothriptic tumors typically feature interspersed loss and retention of heterozygosity. Accordingly, there was a high overlap between deleted regions and LOH segments on chr4 (Jaccard index (J) = 0.99). t, In a chromothriptic model, DNA shattering typically occurs on a single haplotype. M-FISH showed that significant loss of chromosomal content occurred on only one copy of chr4. u, To show random chromothriptic DNA shattering and re-joining, observed segments (n=73) were re-ordered by running Monte Carlo simulations (n=103) that generate a background probability distribution. S821 segment order lies within the chromothriptic null model. Two-sided P=0.78. v, All 4 SV-types are uniformly distributed in a chromothriptic tumor model. P=0.43; χ_-goodness-of-fit test. w, In a chromothriptic model, paired end connection types (as given by the SV-type) induce an alternating sequence of DNA segment ends when ordered according to the genomic position on the original chromosome. Tendency towards this alternating 3’-to-5’ pattern of rearranged DNA segment ends (n=146) was tested by using right-sided Wald-Wolfowitz runs test. P<10-12. x, Mutation clusters in relation to breakpoint junctions involved in chromothripsis are shown as rainfall plot for primary PDAC from PK mouse S821. Each dot represents a single somatic nucleotide variation (SNV) and is ordered on the x-axis according to its position in the mouse genome. The distance of each SNV to the previous SNV in the genome is shown on the y-axis. The coloring of individual SNV dots indicates the type of nucleotide substitution. y, Chr4 “zoom-in” from (x). Breakpoint junctions are shown according to their genomic position on chr4. No mutation clusters - neither in absence nor in combination with breakpoint junctions - were detected, consistent with chromothripsis involving end joining DNA repair mechanisms. This is in contrast to other complex rearrangement types, such as chromoanasynthesis, which arise through replication-based mechanisms with breakpoint-associated high mutation rates (e.g. kataegis).
Extended Data Figure 3. Specificity, timing, mechanisms and impact of KrasG12D gene dosage alterations on gene expression in pancreatic tumorigenesis.
a, Overlay of copy number profiles of primary mPDAC cell cultures from PK mice (n=38) as determined by aCGH. Y-axis shows frequency of a genomic region to be amplified (up) or deleted (down) in the cohort, with Cdkn2a and Kras loci being most frequently affected by CNAs. b, Prevalence of LOH in primary mPDAC cell cultures from PK mice (n=38) based on whole exome sequencing (WES) data. A chromosome was considered to be affected by LOH if the SNP frequency was shifted to ≤0.1 or ≥0.9 in a segment with a size ≥200kb. LOH on chr4 is frequently the consequence of heterozygous deletions involving the Cdkn2a locus. By contrast, LOH on chr6 is predominantly copy number neutral and linked to increase in KrasG12D gene dosage. Chr4 (home of Cdkn2a) and chr6 (home of Kras) show markedly increased rates of LOH as compared to all other chromosomes reflecting their functional importance during tumorigenesis. c-h, Genetic mechanisms of KrasG12D gene dosage alterations as identified by aCGH, M-FISH and whole exome sequencing (WES) in pancreatic cancers from PK mice. The observed types of increased KrasG12D gene dosage acquisition were: (i) focal gain (affecting ≤50% of the chromosome length), arising either through replication-based mechanisms (2 cases, one with high-level KrasG12D amplification [shown in c] and one with low level amplification) or translocation and subsequent amplification of the translocated chromosome (one case [shown in d]), (ii) arm-level gain (affecting ≥50% of the chromosome length) arising through mitotic errors (7 cases of whole-chromosome gain [example shown in e], occasionally [2 cases] with concomitant intra-chromosomal deletions or translocations not affecting Kras [example shown in f]) and (iii) copy-number neutral LOH (CN-LOH, KrasG12D homozygosity, acquired uniparental disomy), arising either through mitotic recombination (affecting parts of chr6 [shown in h]) or chromosomal missegregation (duplication of KrasG12D-mutant chr6 and loss of wild-type chr6 [shown in g]). c, mPDAC S134 shows a high-order focal amplification of KrasG12D. Sharp borders, small size of the amplification (600kb) and strong increase in copy number (4x) indicate that KrasG12D was amplified through multiple cycles of repeated template-switching by a replication-based DNA repair mechanism. KrasG12D mutant allele frequency is 89.1%. d, Tumor 4706 carries a focal amplification of KrasG12D. M-FISH analysis revealed that the mutant KrasG12D allele (chr6) was likely first affected by a reciprocal translocation of chr4 and chr6, resulting in two rearranged chromosomes: Der(4)T(4;6) and Der(6)T(4;6). Subsequently, Der(4)T(4;6) was missegregated through mitotic error resulting in focal gain of the KrasG12D locus. KrasG12D mutant allele frequency is 72.2%. e, mPDAC R1035 shows ‘classical’ whole chromosome gain (trisomy) of chr6, which was likely generated through mitotic error/missegregation. The KrasG12D mutant allele frequency is 69.8%. f, In tumor 8442 arm-level gain of KrasG12D was likely generated through mitotic missegregation of chr6. Intra-chromosomal deletion on one of three chromosomes (19.6Mb) does not affect Kras. KrasG12D mutant allele frequency is 66.4%. Asterisk, chr6 with reduced length resulting from intra-chromosomal deletion. g-h, mPDAC 16992 and B590 display copy-number neutral LOH (CN-LOH) leading to increased KrasG12D gene dosage. KrasG12D mutant allele frequency is 99.2% and 96.3%, respectively. The SNP pattern of chr6 in mPDAC 16992 reveals that the whole chromosome is affected by CN-LOH indicating chromosome missegregation (duplication of the KrasG12D-mutant chr6 and loss of wild-type chr6) as the underlying mechanism. By contrast, in mPDAC B590 only a partial region of chr6 is affected by CN-LOH, therefore probably resulting from mitotic recombination. i, Allele-specific KrasG12D mRNA expression in KrasG12D-HET (n=12) vs. KrasG12D-iGD (n=26) primary PDAC cell cultures from PK mice as detected by combined analysis of amplicon-based RNA-Seq (proportion of mutant/wild-type Kras mRNA) and 3’-prime pA RNA-Seq (amount of total Kras mRNA, but not the proportion of mutant/wild-type Kras mRNA due to sequencing of 3’-prime transcript ends; see Methods section). This figure is related to Fig. 2b. ***P≤0.001, two-tailed Mann-Whitney test; bars, median. j, Mutant KrasG12D mRNA levels in Cdkn2a/NcrucΔHET/WT (n=11) vs. Ckdn2a/NcrucΔHOM (n=27) primary PDAC cell cultures from PK mice as detected by combined amplicon-based RNA-Seq and 3’-prime pA RNA-Seq. This figure is related to Extended Data Figure 5f. ***P≤0.001, two-tailed Mann-Whitney test; bars, median. k, Mutant KrasG12D mRNA levels in transcriptional clusters of mPDAC from PK mice (C2a/b/c/C1, n=5/7/6/15) as detected by combined amplicon-based RNA-Seq and 3’-prime pA RNA-Seq. This figure is related to Fig. 5d. P=1.6*10-5, two-sided Pearson correlation; bars, median. l-n, Interphase fluorescence in situ hybridization (FISH) for the analysis of copy-number and ploidy states at the KRAS locus on chr12 in human pancreatic intra-epithelial neoplasia (PanIN) with KRASG12 variant allele frequencies (VAFs) of ~100%. KRASG12 VAFs are indicated above each FISH profile as detected by amplicon-based deep sequencing. A VAF of ~100% can be caused either by loss of the wild-type KRAS-locus (hemizygosity of KRASG12-MUT: one KRASG12-MUT allele per cell) or by CN-LOH (acquired uniparental disomy; homozygosity of KRASG12-MUT: two KRASG12-MUT alleles per cell). All samples show a diploid genome as suggested by CEN12 (two red signals per nucleus). Neither loss of one KRAS allele nor monosomy of chr12 was observed providing evidence for CN-LOH and increased KRASG12-MUT gene dosage in hPanIN. Scale bars, 2.5µm; CEN12, centromere probe chr12.
Extended Data Figure 4. Enrichment for amplification of alternative oncogenic drivers in mPDACs of PK mice with KrasG12D-HET status.
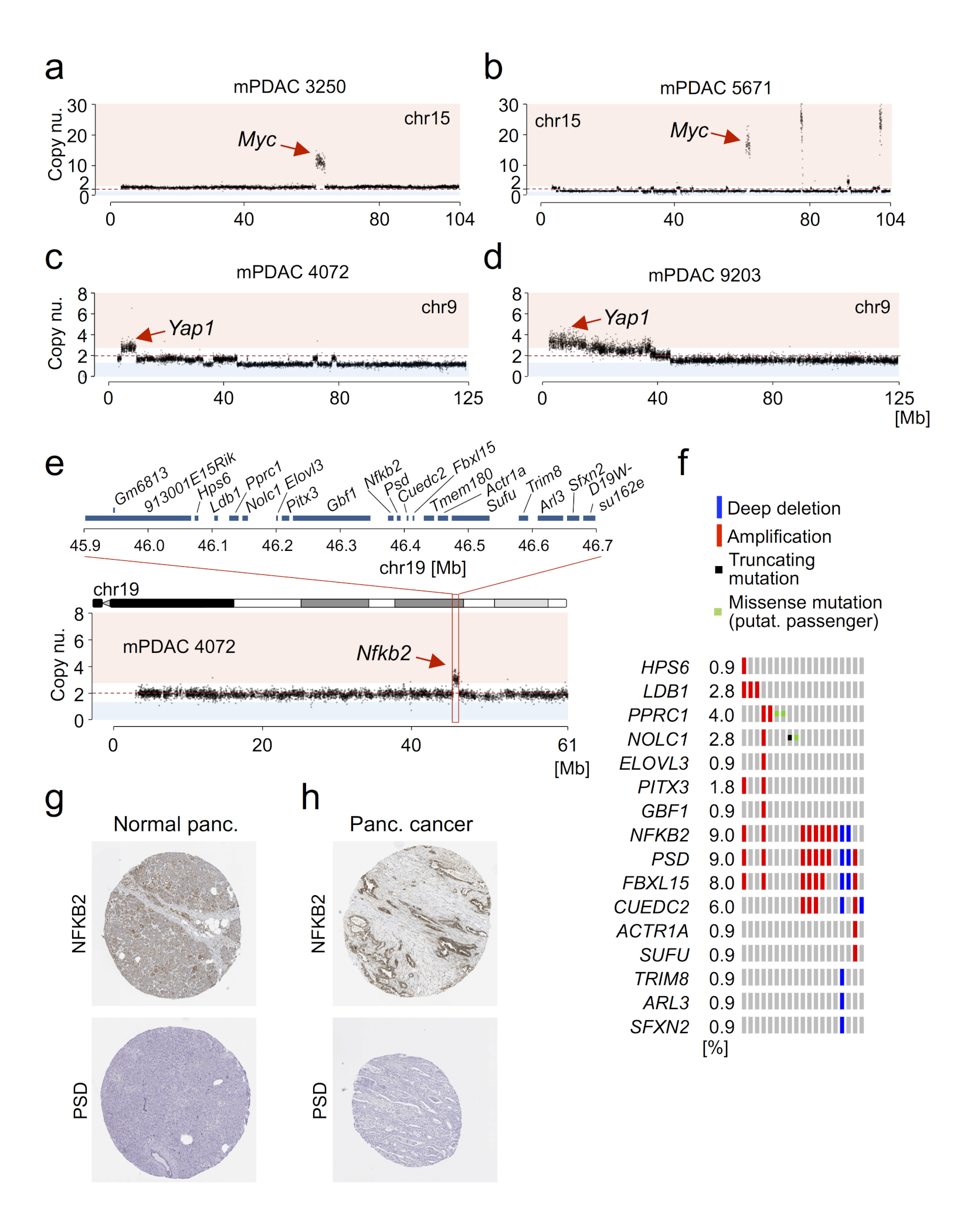
a-b, Two primary mPDACs with strong focal Myc amplification on chr15 are shown, as detected by aCGH. Red dashed line indicates no copy number change. c-d, Focal copy number gains targeting the Yap1 locus on chr9 in primary mPDACs 4072 and 9203 as revealed by aCGH. e, Chr19 was also frequently subject to arm-level gain (see Fig. 1c and Extended Data Figure 1l). Primary mPDAC of PK mouse 4072 harbors a focal gain on chr19 containing 20 genes: 9130011E15Rik, Gm6813, Hps6, Ldb1, Pprc1, Nolc1, Elovl3, Pitx3, Gbf1, Nfkb2, Psd, Fbxl15, Cuedc2, Tmem180, Actr1a, Sufu, Trim8, Arl3, Sfxn2, D19Wsu162e. f, Cross-species analyses revealed that the orthologous region on human chr10 is also subject to recurrent amplifications in human PDAC (8 out of 109 hPDACs have focal amplifications; data from Witkiewicz et al.6). Of the 20 mouse genes, sixteen could be assigned to orthologues in humans. Further analyses revealed that only two genes, NFKB2 and PSD, are within the minimal overlapping region of recurrent amplification (data from6 and oncoplot from cBioPortal60,61). g, NFKB2, but not PSD, shows medium protein expression in exocrine glandular cells of normal pancreatic tissue, as detected by immunohistochemistry (IHC, data from TheHumanProteinAtlas62). h, NFKB2 is highly expressed in 17% (2/12) of stained hPDAC biopsies as shown by IHC. In contrast, there was no PSD expression in any of the analyzed pancreatic cancers (0/12). Protein expression data was used from TheHumanProteinAtlas62.
Extended Data Figure 5. Characterization of Cdkn2a (chr4) alterations and correlation with KrasMUT gene dosage variation and mRNA expression in mouse and human PDAC.
a-d, Cdkn2a alteration on mouse chr4 can occur through arm-level, complex or focal loss as well as uniparental disomy (see Figure 3). In addition, chr4 is frequently involved in inter-chromosomal translocations. Examples of representative karyotypes of primary pancreatic cancer cultures derived from PK mice with translocations involving chr4, likely affecting the Cdkn2a locus. In all 4 cases, chr4 translocations were found in all 10 metaphase spreads of each cancer, indicating their early acquisition during tumor evolution. a, mPDAC 4706 with diploid karyotype: 42, XX, del(X), +2, der(2)t(2;4)is(2;4), der(4)t(4;6)*2, +der(4)t(2;4), der(6)t(4;6). b, mPDAC 4900 also features a diploid karyotype: 41, XX, der(X)is(X;4), der(4)is(4;8), del(4), +6, der(8)t(4;8). c, mPDAC 5123 underwent polyploidization, after translocation of chr4 with chr1 and an deletion on the other copy: 78, XXXX, -1, del(1)*2, -2, +4*2, der(4)t(1;4)*3, del(4)*3, -5, -7, -9, +15, -17, +18 d, mPDAC 8349 shows a diploid karyotype: 40,XX, der(4)t(3;4), der(4)t(4;13), +del(4), der(13)t(4;13). e, KrasG12D variant allele frequencies detected by amplicon-based deep sequencing of the Kras locus are higher in Cdkn2a/NcrucΔHOM mPDAC (n=27) as compared to Cdkn2a/NcrucΔHET/WT (n=11) pancreatic cancers. All cancers are from PK mice. Blue dots indicate tumors with complete Ncruc deletion. ***P≤0.001, two-tailed Mann-Whitney test; bars, median. f, Allele-specific expression of mutant KrasG12D mRNA is increased in primary tumors from PK mice with Cdkn2a/NcrucΔHOM (n=27) background in comparison to Cdkn2a/NcrucΔHET/WT (n=11) cancers. Primary mPDACs with homozygous loss of Ncruc are highlighted in blue. KrasG12D expression was analyzed by combining amplicon-based RNA-Seq and qRT-PCR (as described in the Methods section). **P=0.003, two-tailed Mann-Whitney test; bars, median. g, KRASMUT variant allele frequencies based on WES in a published dataset of microdissected human PDAC (Witkiewicz et al., reduced stromal content) was analyzed with respect to CDKN2A and TP53 status. KRASMUT allele frequency was higher in mutated/homozygous deleted CDKN2A and/or TP53 (CDKN2AMUT/ΔHOM/TP53MUT/ΔHOM; hPDACs as compared to cancers with CDKN2AΔHET/WT/TP53ΔHET/WT status (from left: n=28, n=14, n=28, n=30). Two-sided rank-based ANOVA (P=5.8*10-6); post hoc testing with two-sided Tukey honest significant difference test, *adj. P≤0.05, ***adj. P≤0.001; bars, median. h, Fraction of the genome altered by copy number changes detected by aCGH in primary mPDACs of PK (n=38), PKC (n=16) and PKP (n=16) mice. PKP mice show a significantly increased CNA load as compared to PKC mice. Two-sided rank-based ANOVA (P=0.01); post hoc testing with two-sided Tukey honest significant difference test, **adj. P=0.009, adj. P-values for group wise comparisons are shown; bars, median. Del, deletion; der, derivative chromosome; is, insertion; t, translocation; „-“, chromosome loss; „+“, chromosome gain.
Extended Data Figure 6. Complete Cdkn2a barrier loss precedes KrasG12D-iGD in primary mPDAC of PK mouse 53704.
Copy number alterations at chr4 (Cdkn2a) and chr6 (Kras) in mPDAC 53704 and corresponding metastases, as detected by aCGH (top) and whole-exome sequencing based SNP pattern analysis (bottom). The primary cancer and both liver metastases display identical focal deletions of Cdkn2a and similar SNP patterns on chr4 revealing that all lesions share the same ancestor cell with complete Cdkn2a loss. By contrast, SNP analysis on chr6 revealed discordant patterns in the primary mPDAC and both metastases. Li2 shows partial LOH of a distal region on chr6 involving the Kras locus, while LOH in Li3 involves the whole chr6. This explains the step-wise LOH pattern observed on chr6 in the primary mPDAC. The graphic on the right shows the combined interpretation of CNV/LOH profiles, which suggests the following sequence of genetic events during tumor evolution: The initial KrasG12D mutation was followed by focal deletion of one copy of Cdkn2a. In a subsequent genetic event, the second copy of Cdkn2a was lost by chr4 missegregation and copy-number neutral LOH. Complete barrier loss allowed for convergent evolution of increased KrasG12D gene dosage through copy-number neutral LOH and gave rise to independent metastases in the liver. Note: A major obstacle for equivalent human studies is the limited availability of human matched primary/metastases samples, particularly of treatment naive ones. We performed cross-species analyses using data from a recent study, which analyzed human treatment-naive metastatic PDACs by whole-genome sequencing8 and provided CDKN2A and KRAS copy number data for matched primaries/metastases from 3 patients. In one patient the sequential order of CDKN2A deletion and KRAS amplification could be reconstructed: homozygous CDKN2A deletions were identical in all primaries and metastases, whereas there were 5 different KRAS gains in the 6 metastases. This suggests convergent evolution of mutant KRAS gene dosage gain upon homozygous CDKN2A loss in this patient, in line with similar data in large series of mouse cancers and their metastases (see Figure 3e).
Extended Data Figure 7. Transcriptome-based subtyping of human primary pancreatic cancer and classification of human PDAC cell lines and primary PDAC cell cultures from PK mice.
a-c, Independent cross-comparison of transcriptional classification systems from Collisson et al.28, Moffitt et al.29 and Bailey et al.7. Collisson et al. performed PDAC microdissection and defined 3 transcriptional subtypes: classical, quasimesenchymal (QM) and exocrine-like. Moffitt et al. defined 2 subtypes (classical, basal-like) using (i) virtual separation of tumor and non-tumor gene expression patterns, (ii) transplantation studies and (iii) human PDAC cell lines; and proposed that the exocrine-like signature stems from exocrine pancreatic cells, rather than from the cancer cells. Bailey et al. used bulk tumors and defined 4 subtypes (pancreatic progenitor, immunogenic, squamous, aberrantly differentiated endocrine exocrine [ADEX]). RNA-Seq data from PDAC and adenosquamous pancreatic carcinoma from Bailey et al. was used for cross-comparison of classification systems. Other histological subentities of pancreatic cancer were excluded (e.g. IPMN, MCN, acinar cell carcinoma). The Bailey subtyping for this dataset was available. a, Unbiased hierarchical clustering of primary pancreatic cancer samples (n=71) from Bailey et al. using Collisson classifier genes. b, Subtyping of primary pancreatic cancer samples (n=71) from Bailey et al. using classifier genes defined by Moffitt et al. c, Consensus clustering based on analyses performed in a/b. There is considerable overlap between at least two subtypes, which are in large parts captured by the initially proposed Collisson classical and quasimesenchymal (QM) signatures (which are also detected in mouse and human PDAC cell lines; see Extended Data Figure 7e-h). The Bailey classification (based on bulk tissue analyses) suggests that Collisson classical cancers (microdissected cancer tissue) can be further sub-stratified in some with and some without a strong immune cell infiltration. The Moffitt classification suggests that the Collisson exocrine-like signature (Bailey ADEX subtype) stems from “contaminating” healthy exocrine pancreatic cells, based on the evidence described above. Given that the Collisson exocrine-like signature was derived from microdissected PDAC, such “contamination” is only conceivable, if exocrine-like signature genes were dramatically higher expressed in pancreatic acinar cells as compared to PDAC cells. d, Volcano plot showing strongly upregulated expression of exocrine-like genes in human wild-type pancreas (13 to 241 fold; median: 183-fold upregulation). Note that 15 out of 19 exocrine-like signature genes (red dots) are among the top50 genes upregulated in human wild-type pancreas (n=3) as compared to hPDAC cell lines (n=30) (y axis is calculated on Benjamini-Hochberg adj. P-values derived from R package limma [see Methods section]). Although these data do not exclude the existence of exocrine-like PDACs, they support the possibility that “contamination” with few acinar cells can impose an exocrine-like signature on a cancer. This might explain why human or mouse PDAC cell lines don´t cluster into the exocrine-like subtype (see also Extended Data Figure 7e-f below). e, Hierarchical clustering of microarray-based expression profiles using Collisson identifier genes28 on human PDAC cell lines (n=19, GEO series GSE17891). As also described earlier by Collisson et al., only two subtypes can be detected in human cell line collections: classical and quasimesenchymal (QM). Of note, the most prominent change in the QM cell lines is downregulation (extinction) of the classical assigner genes, whereas expression of QM classifier genes is quite variable. We therefore also use here the terms classical and non-classical. f, Projection of the Collisson classifiers on mouse PDAC cell culture transcriptomes (n=33) also identified classical and non-classical subtypes. The non-classical subtype contained a subset of mPDAC cell cultures from cluster C2a/b/c (epithelial morphology; equivalent of human QM) and all cluster C1 mPDACs (mesenchymal morphology; “M” cluster). g, Application of a human EMT hallmark gene set52 for hierarchical clustering of expression profiles from primary PDAC cultures (PK mice; n=33) resulted in a separation of C1 (mesenchymal) and C2a/b/c (epithelial) cell lines. h, Projection of the EMT hallmark gene set on human PDAC cell line transcriptomes (n=19, GEO series GSE17891) did not result in a clear separation of samples, indicating underrepresentation of the mesenchymal M subtype (equivalent to murine C1/“M”) in available human cell line collections. As shown in Extended Data Figure 9b, however, the EMT signature is detectable in undifferentiated human pancreatic carcinoma, which is the human equivalent of the mesenchymal mouse PDACs in C1.
Extended Data Figure 8. Functional analyses to study the role of KrasG12D gene dosage increase in EMT. a-d, Multiplexed somatic CRISPR/Cas9 mutagenesis for phylogenetic tracking of epithelial/mesenchymal mPDAC clones in vivo.
a, Graphic demonstrates major steps of multiplexed gene editing by pooled delivery of CRISPR/Cas9 vectors, each targeting a different tumor suppressor gene in the pancreas of PK mice. Electroporation-based transfection enables low-frequency mosaic vector delivery (average of 120 cells per pancreas are transfected) to induce clonal tumors. Primary tumor cell cultures were screened for the simultaneous presence of epithelial and mesenchymal cells. Two such cancers were identified (mPDACs from mouse 021 and mouse 901) and subjected to differential trypsinization in order to enrich for each morphology. b, Amplicon-based deep sequencing of all sgRNA-targeted loci revealed identical indel patterns in both epithelial/mesenchymal culture pairs. This shows (i) that epithelial and mesenchymal cells originate from the same clone and (ii) that the CRISPR-induced mutations are not contributing to the differential phenotype. c, KrasG12D variant allele frequencies in epithelial and mesenchymal cell cultures from mPDAC 021 and mPDAC 901, as detected by amplicon-based deep sequencing. Both cancers had increased KrasG12D expression in mesenchymal cells (see Fig. 5e). In mPDAC 021 this is due to selective amplification of the KrasG12D allele in mesenchymal cells. In mPDAC 901 genetic KrasG12D amplification was not observed, suggesting induction of increased Kras expression in mesenchymal cells by other mechanisms. d, Gene set enrichment analysis using “Molecular Signatures Database” (MSigDB) of differentially regulated genes in mesenchymal versus epithelial mPDACs based on RNA-Seq. Mesenchymal clones of mPDAC 021 and mPDAC 901 show an upregulation of genes involved in “MAPK signaling pathway” and “EMT” as compared to the corresponding epithelial clones, in line with increased KrasG12D gene dosage (a full list of enriched gene sets is provided for comparisons in Supplementary Table 15). FDR-adjusted P-values are shown on y axis. Representative data from one experiment are shown. e-g, induction of EMT-like transcriptional programs by KRASG12D overexpression in human PDAC cell lines. e, Graphic of experimental workflow. Two human PDAC cell lines (HUPT3 and PANC0327) with homozygous CKDN2A loss (CDKN2AΔHOM) and heterozygous KRASMUT (KRASMUT-HET) status were transduced with lentivirus carrying doxycycline-inducible KRASG12D or GFP-control expression constructs. KRASG12D or GFP expression was induced by adding doxycycline for 1, 3 and 5 days. f, Gene set enrichment analysis using “Molecular Signatures Database” (MSigDB) of differentially regulated genes in KRASG12D- versus GFP-induced hPDAC cell lines HUPT3 and PANC0327 based on RNA-Seq. Upon doxycycline treatment, both hPDAC cell lines showed a consistent upregulation of genes involved in “KRAS signaling up” and “EMT” (a full list of enriched gene sets is provided for both cell lines in Supplementary Table 16). FDR-adjusted P-values are shown on y axis. g, Expression of marker genes for epithelial (CDH1) or mesenchymal (VIM) cell differentiation and invasion/matrix disassembly (MMP1) was validated by qPCR (normalized to GAPDH and PPIA). In line with RNA-Seq data KRASG12D-induced cells show an increased expression of the mesenchymal marker gene VIM, increased expression of MMP1 and reduced levels of epithelial marker gene CDH1. *P≤0.05, **P≤0.005, ns=not significant, two-tailed t-test; bars=mean; error bars=SEM.
Extended Data Figure 9. Transcriptional profiles of human undifferentiated pancreatic carcinomas are enriched for signatures of oncogenic signaling intensification and EMT but not for activation of TP63ΔN transcriptional network.
a, Primary pancreatic tumors from PK mice with a mesenchymal phenotype (C1 cluster, n=15) are almost exclusively classified as undifferentiated/sarcomatoid by histopathological evaluation and tend to have a reduced age at diagnosis when compared to epithelial (C2a/b/c cluster, n=18) tumors (histopathological grade 1 to 3 [G1-G3]). This aggressive behavior of undifferentiated pancreatic carcinoma is also observed in human patients and is associated with worse clinical outcome33. P-value calculated by two-sided log-rank test. b, Comparison of publically available expression profiles of human undifferentiated pancreatic carcinoma (n=4), PDAC (WHO grade 1 to 3 [G1-G3], n=64) and adenosquamous pancreatic carcinoma (n=7). Human samples with the above histopathological characteristics for which expression-based subtype information from Bailey et al.7 was available were used and complemented with available undifferentiated pancreatic carcinomas from the ICGC PACA-AU cohort (Supplementary Table 18). Other histological subentities of pancreatic cancer were excluded (e.g. IPMN, MCN, acinar cell carcinoma). ANOVA was performed to select genes which are differentially expressed in at least one of the six defined subgroups of pancreatic cancer: (i) undifferentiated, (ii) adenosquamous pancreatic carcinoma and (iii-vi) PDAC (G1-G3) sub-stratified in pancreatic progenitor, immunogenic, squamous and aberrantly differentiated endocrine exocrine (ADEX) Bailey subtypes. Differentially regulated genes were used for unbiased hierarchical clustering of these pancreatic cancer transcriptional profiles. Five sub-clusters of co-regulated gene expression could be identified according to the cluster tree on the y-axis (separated by white horizontal bars in the heatmap). Gene set enrichment analysis using “Molecular Signatures Database” (MSigDB) was performed for individual sub-clusters and terms related to predominating gene sets/pathways are annotated for each cluster on the right (full list provided in Supplementary Table 17). Undifferentiated pancreatic carcinomas cluster together and are associated with (i) upregulation of genes in cluster 3 (containing MAPK signaling pathway and gene sets relevant during embryonic development or EMT) and (ii) downregulation of genes in clusters 2 and 5, which contain gene sets related to epithelial cell differentiation, embryonic development or metabolic signatures. This reflects the pathway enrichment signature in the equivalent undifferentiated (mesenchymal) mouse PDACs (cluster C1/"M" in PK mice; see Extended Data Figure 7g) and provides further support for the link between KRAS signaling intensification, EMT and the undifferentiated tumor phenotype. The immunogenic PDAC subtype showed high expression of cluster 4 genes, which was also strong (even elevated) in undifferentiated pancreatic carcinomas, suggesting an increased immune cell infiltration in undifferentiated carcinomas. Cluster 1 contained gene sets related to cell proliferation/cell cycle, squamous differentiation and TP63ΔN transcriptional targets, which were most highly overexpressed in pancreatic carcinomas with adenosquamous histology. Undifferentiated pancreatic carcinomas did not show activation of the TP63ΔN transcriptional targets. This suggests that activation of TP63ΔN transcriptional targets is not causally linked to KRAS signaling intensification and EMT (see also Extended Data Figure 9c-d, showing a lack of association of undifferentiated carcinomas withTP63ΔN transcriptional network activation). c, Unbiased hierarchical clustering of human pancreatic carcinomas with adenosquamous histology (n=7) as well as PDACs (WHO grade 1 to 3 [G1-G3], n=64) and undifferentiated pancreatic carcinomas (n=4) (sample set as in Extended Data Figure 9b) using a list of validated TP63ΔN transcriptional targets53. Pancreatic cancers with adenosquamous differentiation were significantly enriched in a cluster showing increased TP63ΔN transcriptional network activity (P≤0.001, two-sided Fisher’s exact test, OR 130, 95% CI 11.6-1452). Undifferentiated pancreatic carcinomas did not contribute to this cluster. In line, pancreatic cancers from PK mice did not show differential regulation of the TP63ΔN network, reflecting the lack of adenosquamous tumors in this cohort (not shown). d, Unbiased hierarchical clustering across solid cancers (Cancer Cell Line Encyclopedia, n=856) using the same gene list showed a strong enrichment of tumors with squamous differentiation in the sub-cluster with highest TP63ΔN transcriptional network expression (P≤0.001, two-sided Fisher’s exact test, OR 28.1, 95% CI 16.4-48.1), in line with the observation of Hoadley et al.63 that TP63ΔN is a signature for squamous differentiation across cancers.
Extended Data Figure 10. KrasG12D-gene dosage is a critical determinant of PDAC biology in a mouse model with high mutational load.
The mutational burden in primary PDAC cultures of PK mice was significantly lower as compared to human PDAC studies (see Fig. 1b). To account for this potential confounding factor and to test if our discoveries in PK mice also apply in a setting of high mutational burden, we used a mouse model combining KrasG12D mutation and PiggyBac transposon-based insertional mutagenesis (PK-PB mice13). PK-PB mice show accelerated tumorigenesis as compared to PK mice. PK-PB derived tumors had an extensive mutational burden (median of 494 transposon insertions per tumor). Primary cultures of PDAC from PK-PB mice (n=17) were subjected to comprehensive genetic characterization using aCGH, microarray-based gene expression profiling, quantitative transposon insertion site sequencing (QiSeq) and amplicon-based deep sequencing of the Kras locus. a, Transcriptome profiles of primary PDAC cultures from PK-PB mice (n=17) were used for unbiased hierarchical clustering that resulted in 2 major clusters (C1 and C2), like in PK mice. KrasG12D gene dosage status (as determined by aCGH and amplicon-based deep sequencing of the Kras locus) and Cdkn2a status (as determined by aCGH and quantitative transposon insertion site sequencing [QiSeq]) are indicated below the cluster tree for each individual tumor. Similarly to PK mice, cluster C2a was characterized by KrasG12D-HET and Cdkn2a/NcrucΔHET/WT status, whereas mPDACs in clusters C2b/c and C1 had increased KrasG12D gene dosage (KrasG12D-iGD) and were Cdkn2a/NcrucΔHOM. The genetic KrasG12D-status was significantly associated with expression clusters (P=0.01, two-sided Fisher’s exact test) providing further evidence that expression clusters are associated with KrasG12D gene dosage. b, Prevalence of KrasG12D-iGD in cultures of primary mPDAC (from PK-PB mice) with homozygous (n=12) or heterozygous/wild-type (n=5) Cdkn2a/Ncruc status. *P=0.03, two-sided Fisher’s exact test, OR 20.0, 95% CI 1.4-287.8. c, Gene set enrichment analysis using DAVID of upregulated genes in cluster C1 (n=5) as compared to cluster C2 (n=12) of primary mPDAC cultures from PK-PB mice. As in PK mice, PK-PB tumors in C1 are characterized by upregulation of genes enriched in gene sets describing mesenchymal cell differentiation and revealed a strong enrichment for Ras downstream signaling pathways (full list in Supplementary Table 19). FDR-adjusted P-values are shown on y axis. Overall, these analyses show that the biological principles discovered in the PK model also apply to pancreatic cancers from PK-PB mice with high mutational load.
Supplementary Material
Acknowledgements
We thank the comparative experimental pathology team for valuable discussions and Aaron Selmeier, Laura Dajka, Olga Seelbach, Petra Meyer, Tatjana Schmidt, Julia Eichinger and Teresa Stauber for excellent technical assistance as well as Maximilian Reichert for vector constructs. The work was supported by the German Cancer Consortium Joint Funding Program, the Helmholtz Gemeinschaft (PCCC Consortium), the German Research Foundation (SFB1243; A13/A14) and the European Research Council (ERC CoG No. 648521). The authors declare no competing financial interests.
Footnotes
Author Contributions
S.M., D.S., R.R. designed the study; S.M., T.E., R.M., D.S., R.R. interpreted and visualized data; T.E., S.La., M.Zw., M.B. conducted bioinformatic analyses. S.M., T.E., R.M., S.La., M.Zw., I.V. developed bioinformatic analyses strategies; S.M., R.M., M.Zu., T.K., A.S., B.S., J.G., K.Sc., Z.H., A.A., N.S., C.V. L.R. isolated mPDAC cell cultures; S.M., R.M., J.H., K.U. performed genomics with help from R.Ö.; R.Ö., C.Z. conducted RNA-Seq; R.B., S.Lo., B.F., S.K., K. St., F.Y. performed cytogenetics; B.K. performed microdissection; B.K., H-Y.Y., G.K., W.W., K.St. carried out pathological assessment; C.Z., S.P., W.E., K.U., I.V. contributed analytic tools; M.Fri., O.M.D., S.E., F.C-C., J.R., M.I.S., M.Fra., J.M., G.K., R.M.S., J.C., P.L., G.V., W.W., K.St., W.E., G.S., A.B., D.S., R.R. provided resources and critical input; D.S., R.R. supervised the study; S.M., R.R. wrote the manuscript; T.E., R.M. and D.S. edited the manuscript.
Author Information
Reprints and permissions information is available at www.nature.com/reprints. Readers are welcome to comment on the online version of the paper.
The authors declare no competing financial interests.
References
- 1.Rahib L, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Jones S, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell PJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biankin AV, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waddell N, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkiewicz AK, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey P, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 8.Makohon-Moore AP, et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat Genet. 2017;49:358–366. doi: 10.1038/ng.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson EL, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schonhuber N, et al. A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nat Med. 2014;20:1340–1347. doi: 10.1038/nm.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Mancera PA, et al. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature. 2012;486:266–270. doi: 10.1038/nature11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann KM, et al. Sleeping Beauty mutagenesis reveals cooperating mutations and pathways in pancreatic adenocarcinoma. Proc Natl Acad Sci U S A. 2012;109:5934–5941. doi: 10.1073/pnas.1202490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rad R, et al. A conditional piggyBac transposition system for genetic screening in mice identifies oncogenic networks in pancreatic cancer. Nat Genet. 2015;47:47–56. doi: 10.1038/ng.3164. [DOI] [PubMed] [Google Scholar]
- 14.Korbel JO, Campbell PJ. Criteria for inference of chromothripsis in cancer genomes. Cell. 2013;152:1226–1236. doi: 10.1016/j.cell.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Notta F, et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature. 2016;538:378–382. doi: 10.1038/nature19823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada H, et al. Amplifications of both c-Ki-ras with a point mutation and c-myc in a primary pancreatic cancer and its metastatic tumors in lymph nodes. Jpn J Cancer Res. 1986;77:370–375. [PubMed] [Google Scholar]
- 17.Heidenblad M, et al. Detailed genomic mapping and expression analyses of 12p amplifications in pancreatic carcinomas reveal a 3.5-Mb target region for amplification. Genes Chromosomes Cancer. 2002;34:211–223. doi: 10.1002/gcc.10063. [DOI] [PubMed] [Google Scholar]
- 18.Sarkisian CJ, et al. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- 19.Junttila MR, et al. Selective activation of p53-mediated tumour suppression in high-grade tumours. Nature. 2010;468:567–571. doi: 10.1038/nature09526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldser DM, et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature. 2010;468:572–575. doi: 10.1038/nature09535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rad R, et al. A genetic progression model of Braf(V600E)-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer Cell. 2013;24:15–29. doi: 10.1016/j.ccr.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhim AD, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 24.Yachida S, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider G, et al. IKKalpha controls p52/RelB at the skp2 gene promoter to regulate G1- to S-phase progression. EMBO J. 2006;25:3801–3812. doi: 10.1038/sj.emboj.7601259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamidi T, et al. Nuclear protein 1 promotes pancreatic cancer development and protects cells from stress by inhibiting apoptosis. J Clin Invest. 2012;122:2092–2103. doi: 10.1172/JCI60144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redston MS, et al. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res. 1994;54:3025–3033. [PubMed] [Google Scholar]
- 28.Collisson EA, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moffitt RA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47:1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maresch R, et al. Multiplexed pancreatic genome engineering and cancer induction by transfection-based CRISPR/Cas9 delivery in mice. Nat Commun. 2016;7:10770. doi: 10.1038/ncomms10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morohoshi T, Held G, Kloppel G. Exocrine pancreatic tumours and their histological classification. A study based on 167 autopsy and 97 surgical cases. Histopathology. 1983;7:645–661. doi: 10.1111/j.1365-2559.1983.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 32.Iacobuzio-Donahue CA, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winter JM, et al. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res. 2008;14:412–418. doi: 10.1158/1078-0432.CCR-07-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hruban RH, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 35.Hingorani SR, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 36.Nakhai H, et al. Ptf1a is essential for the differentiation of GABAergic and glycinergic amacrine cells and horizontal cells in the mouse retina. Development. 2007;134:1151–1160. doi: 10.1242/dev.02781. [DOI] [PubMed] [Google Scholar]
- 37.Aguirre AJ, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonkers J, et al. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 39.Lee CL, et al. Generation of primary tumors with Flp recombinase in FRT-flanked p53 mice. Dis Model Mech. 2012;5:397–402. doi: 10.1242/dmm.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32:73–75. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- 41.Bronner IF, Quail MA, Turner DJ, Swerdlow H. Improved Protocols for Illumina Sequencing. Curr Protoc Hum Genet. 2014;80 doi: 10.1002/0471142905.hg1802s80. 18 12 11-42. [DOI] [PubMed] [Google Scholar]
- 42.Stephens PJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jentsch I, Adler ID, Carter NP, Speicher MR. Karyotyping mouse chromosomes by multiplex-FISH (M-FISH) Chromosome Res. 2001;9:211–214. doi: 10.1023/a:1016696303479. [DOI] [PubMed] [Google Scholar]
- 44.Keane TM, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–294. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25:2865–2871. doi: 10.1093/bioinformatics/btp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barretina J, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parekh S, Ziegenhain C, Vieth B, Enard W, Hellmann I. The impact of amplification on differential expression analyses by RNA-seq. Sci Rep. 2016;6:25533. doi: 10.1038/srep25533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macosko EZ, et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 52.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaefer CF, et al. PID: the Pathway Interaction Database. Nucleic Acids Res. 2009;37:D674–679. doi: 10.1093/nar/gkn653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 55.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friedrich MJ, et al. Genome-wide transposon screening and quantitative insertion site sequencing for cancer gene discovery in mice. Nat Protoc. 2017;12:289–309. doi: 10.1038/nprot.2016.164. [DOI] [PubMed] [Google Scholar]
- 57.Meerbrey KL, et al. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc Natl Acad Sci U S A. 2011;108:3665–3670. doi: 10.1073/pnas.1019736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- 59.Weber J, et al. CRISPR/Cas9 somatic multiplex-mutagenesis for high-throughput functional cancer genomics in mice. Proc Natl Acad Sci U S A. 2015;112:13982–13987. doi: 10.1073/pnas.1512392112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uhlen M, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 63.Hoadley KA, et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158:929–944. doi: 10.1016/j.cell.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data have been deposited at EBI European Nucleotide Archive (ENA; https://www.ebi.ac.uk/ena) under accession number PRJEB23787. Microarray data have been deposited in the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE107458. All data are available from the corresponding author upon reasonable request.