Abstract
In humans, chronic psychological stress is associated with increased intestinal paracellular permeability and visceral hyperalgesia, which is recapitulated in the chronic intermittent water avoidance stress (WAS) rat model. However, it is unknown whether enhanced visceral pain and permeability are intrinsically linked and correlate. Treatment of rats with lubiprostone during WAS significantly reduced WAS-induced changes in intestinal epithelial paracellular permeability and visceral hyperalgesia in a subpopulation of rats. Lubiprostone also prevented WAS-induced decrease in the epithelial tight junction protein Occludin (Ocln). To address the question of whether the magnitude of visceral pain correlates with the extent of altered intestinal permeability, we measured both endpoints in the same animal because of well described individual differences in pain response. Our studies demonstrate that visceral pain and increased colon permeability positively correlate (0.6008, p = 0.0084). Finally, exposure of the distal colon in control animals to Ocln siRNA in vivo, revealed that knockdown of Ocln protein inversely correlated with increased paracellular permeability and enhanced visceral pain similar to the levels observed in WAS-responsive rats. These data support that Ocln plays a potentially significant role in the development of stress-induced increased colon permeability. We believe this is the first demonstration that the level of chronic stress-associated visceral hyperalgesia directly correlates with the magnitude of altered colon epithelial paracellular permeability.
Keywords: water avoidance stress, visceral hyperalgesia, paracellular permeability, colon permeability, siRNA, lubiprostone
1. Introduction
In humans, chronic psychological stress is associated with increased intestinal permeability and visceral hyperalgesia [30] which are also observed in the chronic intermittent water avoidance stress (WAS) rat model [58]. WAS has been show to enhance visceral motor response (VMR) to colorectal distention (CRD), which is indicative of visceral hyperalgesia, in a number or rat strains and in mice [1,7–10,25,31,32,39,40,50,53,56,58]. WAS has also been demonstrated to induce increased intestinal permeability in both in vivo and in vitro assays [6,11,19,42,43,46,49,51,55,57,58]. Specifically, WAS induces increased permeability to macromolecules in the colon [11,42,46,57,58], and reduces epithelial tight junction protein expression in the colon epithelium [58]. Increased paracellular permeability in the colon has been linked to visceral hypersensitivity to colonic distension in an acute stress model [2]. However, it is unknown whether pain and permeability are intrinsically linked and correlate in a validated model of chronic stress.
WAS-associated symptoms have been shown to be driven by activation of the hypothalamus-pituitary-adrenal (HPA) axis. WAS-induced increases in pellet output, VMR to CRD, and paracellular permeability are mediated by elevated corticosterone levels acting on the glucocorticoid receptor [26,27,58]; whereas, CRF has been shown to play a significant role in motility and visceral hyperalgesia [36]. Treatment with an oral antibiotic blocks WAS-induced visceral pain [55], implicating the gut microbiome as a contributing factor to stress-induced visceral hyperalgesia.
Treatment with lubiprostone, which expedites restoration in intestinal barrier function in an ischemic porcine model [37], reduced WAS-induced visceral hyperalgesia in a subpopulation of rats and prevented WAS-induced increases in intestinal permeability to large molecules. We observed that concurrent treatment with lubiprostone during WAS prevented the stress-induced decrease of the tight junction protein Occludin (Ocln) that correlated with increased intestinal epithelial paracellular permeability. While these population studies revealed that blocking WAS-induced increase in intestinal permeability had a significant effect to prevent stress-induced increased in VMR to CRD, it did not causally link the two outcomes. To directly link WAS-induce intestinal permeability and visceral hyperalgesia, we measured both outcomes in the same animal and observed a positive correlation between increased colon paracellular permeability and enhanced VMR to CRD (0.6008, p = 0.0084). To provide additional evidence that increased intestinal epithelial paracellular permeability and visceral pain are causally linked, we exposed the distal colon in healthy control rats to siRNA to knockdown Ocln protein levels and observed increased epithelial paracellular permeability and enhanced VMR to CRD similar to the responses noted in WAS-responsive animals. We believe that this is the first report that the level of visceral hyperalgesia and increased colon permeability positively correlate in a validated rodent model of chronic, intermittent stress. In addition, we provide evidence that a potentially important component in the cellular pathway underlying chronic stress-associated visceral hyperalgesia is decreased expression in the epithelial tight junction protein occludin in the colon.
2. Material and Methods
2.1 Animals
Male Sprague Dawley rats (180 – 200 gm) were obtained from Charles River Breeding Labs (Wilmington, MA). All experiments were in compliance with the National Institute of Health Guide for Care and Use of Laboratory Animals. All experiments were approved by the University of Michigan Committee on Use and Care of Animals (animal protocol number 0005713). Animals were housed in facilities maintained at 22°C with a 12 hour light/dark cycle. Animals had water and PicoLab Laboratory Rodent Diet (5L0D, LabDiet, St. Louis, MO) ad libitum and were group housed unless indicated.
2.2 Water Avoidance Stress (WAS) Protocol
The water avoidance stress protocol is a well-established chronic intermittent stress model that is thought to represent moderate psychological stress associated with elevation in ACTH and corticosterone levels [9,25,27,58]. The stress tank consisted of a plastic container (30 cm wide × 37.5 cm long × 26.5 cm high) with a pedestal 6 cm in diameter and 9.5 cm high attached to the center of the bottom of the tank. Daily, the tank was filled with fresh water (22–24 °C) to approximately 1 cm from the top of the pedestal. Animals were placed on the pedestal for 1 hour/day for 10 consecutive days. Sham WAS animals were placed in the same tanks without water and would curl up in a corner of the tank and sleep. Animals undergoing WAS were individually housed with no enrichment; social housing and enrichment have been shown to influence/ameliorate the effects of WAS [22,32,33].
2.3 Lubiprostone Treatment
Oral administration of drugs to laboratory rodents is usually achieved by gavage; however, when this method is employed twice daily it can cause esophageal irritation, and sometimes injury, as well as lead to restraint-associated distress [3]. Therefore, we used a method that allowed the rats to drink the dose based on the method developed by Atacha, et al. [3]. Briefly, rats were lightly restrained in a restraint bag. The ball portion of a gavage rod, attached to 1 mL syringe filled with 500 μL of medium chain triglycerides (MCT) ± lubiprostone, was placed just inside the rat’s mouth and the rat then began to drink as the liquid was slowly dispensed. Rats did not exhibit signs of distress and after a couple of days were fully cooperative. A 500 μL volume of MCT, with or without a 10 μg/Kg dose of Lubiprostone, was administered twice daily for all experiments.
2.4 Pain Assessment: Visceral Motor Response to Colorectal Distention
i) Surgical Implanted Electrode Method
Electromyography (EMG) response (EMR) to colorectal distension (CRD) was measured to determine visceral pain. Assessment of EMR to CRD was based on previously described experiments [9,25,27,58]. On day 6 of 10-day WAS or sham stress, electrodes were implanted in the external oblique pelvic muscle to measure EMG to CRD. Briefly, rats were deeply anesthetized with a sub-cutaneous injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). Silver wires (0.01 in/30 gauge) coated with PFA/Teflon (0.013 in/28 gauge) (A-M Systems, Sequim, WA) were inserted into the external oblique pelvic muscles superior to the inguinal ligament. Wires were tunneled subcutaneously and externalized at the nape of the neck where they were secured. Wounds were monitored daily for tenderness and infection to ensure complete recovery from surgery prior to testing.
EMG response to CRD was measured 24 hrs after the last WAS or sham stress session. Rats were habituated to the Plexiglas cylinders used for partial restraint during testing. A custom-made distention devise, consisting of a flexible latex balloon (4–5 cm), was inserted intra-anally with its end 1 cm proximal to the anus for 20 min before CRD was initiated. A series of CRDs was conducted at the constant pressures of 0, 20, 40, 60 mmHg. The EMG response for each CRD was recorded using SPIKE2/CED 1401 data acquisition interface (Cambridge Electronic Design Ltd, Cambridge, England). Each distention consisted of three segments: a 20 second pre-distention baseline period, a 20 second distention period, and a 20 second period post-CRD. EMG responses were measured at intervals ≥ 4 minute between CRDs and considered stable if there was ≤ 20% variability between 2 consecutive trials at each CRD pressure.
EMG signals higher than 0.2 mV were regarded as significant and used to estimate the pain response. The EMG activity was rectified, area under the curve of EMG amplitudes determined, and the signal during the CRD baseline period subtracted. The resulting values were then normalized as a percentage of the average of the control values at 60 mmHg. This approach to normalization has been validated in a similar model to account for individual variations [7]. Experiments comparing EMG to CRD and permeability (see below) were performed on separate groups of rats, 24hrs after completion of the WAS protocol.
ii) Non-Invasive Manometric Method
Visceral motor response (VMR) to CRD was also assessed using a non-invasive manometric method validated by Larauche, et al. [34]. This relatively new method is gaining acceptance because it eliminates the potentially confounding effects of surgery and implanted electrodes on baseline stress levels, and provides a more reliable method to perform serial measurements over time. In addition, the EMG method is subject to electrode failure, inflammation and local infection at the site of the electrode placement. A balloon pressure-sensor was made by building a distention balloon around a miniaturized pressure transducer catheter (SPR-524 Mikro-Tip catheter; Millar Instruments, Houston, TX). PE50 catheter tubing was taped 3.5 cm below the pressure sensor to use for inflating and deflating the balloon. Then a balloon, made from latex glove (brand), 2 cm wide × 5 cm long was tied over the catheter tubing at 1 cm and 6 cm below the pressure sensor with silk suture thread. The thread was then covered with parafilm (brand) to protect the rat and prevent air leakage. All balloons were tested to ensure 60 mmHg pressure displaced 5.0 ± 0.1 mL of water.
VMR to CRD was measured 24 hrs before the start of WAS or sham stress and 24 hrs after the last WAS/sham stress session. Measurements are expressed as the percentage of the pre-stress VMR to the 60 mmHg CRD for each rat. Rats were habituated to the Plexiglass restraint cylinders by placement of the cylinders in their cage for at least one hour the day before measurements, but not restrained in the tube. It was determined in preliminary experiments that if rats were placed in the tube for 1 hour the day before VMR to CRD measurements, pre-stress responses were higher than those after stress. We believe that this is a response to restraint stress.
To measure VMR to CRD, rats were briefly anesthetized with isoflurane (4% in air initial, 1.5% in air maintenance) and the lubricated balloon pressure sensor was inserted intra-anally with its end 1 cm proximal to the anus. The balloon pressure sensor was secured to the tail with tape and in a Plexiglas cylinders used for partial restraint during testing with a light cotton blanket covering. The rat was allowed to fully recover, 15–20 minutes. Pre-distention at 60 mmHg was performed to unfurl the balloon followed by a series of CRDs conducted at constant pressures of 60, 40, and 20 mmHg twice with at least 4 min inter-stimulus interval. Each distention consisted of three segments: a 20 second pre-distention baseline period, a 20 second distention period, and a 20 second period post-CRD. The VMR electrical signal (mV) for each CRD was recorded using SPIKE2/CED 1401 data acquisition interface (Cambridge Electronic Design Ltd, Cambridge, England). VMRs were considered stable if there was ≤ 20% variability between 2 consecutive trials at each CRD pressure. Signals higher than the backroad for each pressure-sensor probe (0.2–0.6 mV) were regarded as significant and used to estimate the pain response. The resulting values were then normalized as a percentage of 60 mmHg readings taken pre-stress/sham stress. This approach to normalization has been validated in a similar model to account for individual variations [32,34]. In summary, in experiments using the non-surgical VMR to CRD method, each rat served as their own control.
2.5 In Vivo Intestinal Permeability Assay
On the day after completion of 10 days of WAS or sham stress, rats were fasted for four hours, gavaged with 400 mg/Kg of 4 KDa FITC-Dextran (Sigma), and after 4 hours blood was collect by heart puncture. Blood was transferred to EDTA coated tubes and samples were centrifuged at 2,000 × g for 15 minutes at 4°C. The plasma fraction of the blood was collected and stored at −80°C until analysis. Plasma FITC-dextran levels were determined using fluorescent spectrometry. To determine FITC-dextran levels, 25 μL of plasma was diluted in 175 μL of PBS in a 96-well black wall microplate in triplicate. FITC-dextran standards in PBS, along with 25 μL of pooled rat serum, were also included. The standard curve was then used to determine the ng/mL of FITC-dextran. Higher FITC-dextran levels indicated increased intestinal permeability. The in vivo intestinal permeability data for each rat was correlated with that animal’s VMR to CRD.
2.6 In vitro Permeability Assay/Ussing Chamber
The Ussing chamber experiments were carried out according to guidelines from Clarke, 2009 [12]. Following euthanasia, the distal colon (5–6 cm just proximal to the anus) was dissected out and immersed in ice cold oxygenated Krebs solution (115 mM NaCl, 25 mM NaHCO3, 2.4 mM K2HPO4, 0.4 mM KH2PO4, 1.2 mM MgCl2, 1.2 mM CaCl2, and 10 mM glucose). The colon was opened longitudinally along the mesenteric attachment remnant, and the serosal musculature stripped away leaving only the submucosal and mucosal (epithelium) layers. Two pieces of adjacent tissue from each colon were mounted in an EasyMount Ussing chamber apparatus with the P2405 slider (Physiologic Instruments, SanDiego, CA). The 0.4 cm2 of tissue was exposed to 4 ml of circulating oxygenated Krebs buffer at 37°C. Inserted into the chambers were standard agar-salt bridges (3.5% agar in 3 M KCl) to monitor the potential difference across the tissue and to inject the required short circuit current (Isc) from a Model EC-825 Epithelial Voltage Clamp (Warner Instruments, Inc., Hamden, CT). Baseline values for spontaneous potential, Isc, and conductance were recorded after equilibrium, 20 minutes after mounting, and then at 25, 35, 60, and 90 minutes after mounting. Resistance was determined by sending pulses of −5, 5 and 10 mV through the tissue, recording the change in Isc, and then calculating resistance by Ohm’s law. Colon tissue segments with signs of poor viability, which included spontaneous potential < 1 mV, unstable Isc or unstable resistance, were excluded from the study.
Mucosal to serosal transport of macromolecules was assessed by measuring the flux of 4 KDa FITC-dextran (Sigma, St. Louis, MO). Briefly, 4 KDa FITC-dextran dissolved in Kreb’s buffer was added to a final concentration of 4 mg/mL into the mucosal side of the Ussing chamber, and equal volume of Kreb’s buffer added to the serosal side of the chamber. Aliquots of 450 μL were taken from each chamber at 30 and 60 minutes post addition of FITC-dextran. FITC-dextran levels were determined using fluorescent spectrometry in a 96-well black wall microplate in duplicate. A FITC-dextran standard curve diluted in Kreb’s buffer was included and was used to determine the ng/mL of FITC-dextran. The apparent permeability coefficient was then calculated using the following formula: Papp = (ΔC/Δt)V/CoA, where ΔC is the change is concentration of 4 KDa FITC-dextran on the serosal side, Δt the time interval (30 minutes), V the volume of the chamber 4 mL, Co the initial concentration of 4 KDa FITC-dextran, and A the area of the exposed tissue (0.4 cm2). The in vitro permeability assay and Ussing chamber data from each animal were correlated with that animal’s VMR to CRD.
2.7 Western Blot
Distal colon sections were taken from rats 24 hrs after WAS or sham stress, snap frozen, and stored at −80°C. Tissue was homogenized in ice-cold RIPA buffer ( Pierce cat# 89900, ThermoFisher Scientific) supplemented with Complete Mini protease inhibitor cocktail (Roche Diagnostics, Indianapolis, Indiana, USA). In siRNA experiments, colon epithelium was collected by mechanical scrapping, spun down in DPBS with calcium and magnesium, the supernatant removed, and the cell pellet lysed with an equal volume of RIPA buffer supplemented with protease inhibitor cocktail. After lysis, samples were centrifuged at 14,000 × g for 10 minutes at 4°C. The protein concentration in the supernatants were determined with Bio-Rad Protein Assay Dye Reagent (cat# 500-0006, Bio-Rad, Hercules, CA) using a standard curve made with BSA protein.
Proteins were resolved with SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Amersham Biosciences, Piscataway, New Jersey, USA) using the Mini Protean 3 apparatus (Bio-Rad, Hercules, CA). PVDF membranes were blocked with 5% non-fat powdered milk in Tris buffer saline with 0.1% Tween (TBST) and probed with occludin specific antibody (mouse anti-occludin 33-1500, Invitrogen; rabbit anti-occludin ABT146, EMD Millipore), mouse anti-β-actin (SC-47778, Santa Cruz Biotechnology), or mouse anti-GAPDH (SC-20356, Santa Cruz Biotechnology) antibodies. Membranes were washed with Tris buffered saline with 0.1% Tween-20. Immunoblots were developed using West Dura Supersignal chemiluminescence kit (Pierce Thermo Fisher) and visualized using X-ray film. Immunoblot films were scanned at 1200 dots per inch using the film (transparency) function of the scanner. Scans were semi-quantified with Image J software (National Institutes of Health) according to the gel analysis method outlined in the ImageJ documentation.
2.8 Immunostaining
Distal colon tissue from a different group of rats were removed 24 hrs after completion of WAS or sham stress and VMR to CRD measurements, fixed in 4% formalin, quenched with 30% sucrose, and embedded in OCT (ThermoFisher Scientific, Waltham, MA). Tissue sections were cut at the thickness of 10–12 μm and immunostained. For immunostaining, sections were blocked with 10% normal goat serum in PBS with 0.1% Triton X-100 over night at 4°C. Sections were then incubated with the polyclonal anti-occludin antibody (ABT146, Millipore) and the goat anti-rabbit Alexa 594-conjugated secondary antibody (Molecular Probes, Carlsbad) for two hours each at room temperature with washing with PBS with 0.1% Triton X-100 after each incubation. Images were visualized using a Zeiss microscope and images processed using Zeiss Black software.
2.9 siRNA Treatment
For rats treated with siRNA, VMR to CRD was performed using the non-invasive manometric method described previously before treatment and 48 hours after treatment with the siRNA reagent. Values are expressed as the percent of the VMR at 60 mmHg distention from the pre-treatment CRD for each rat. The researcher performing the VMR to CRD was blinded to which treatment group was being handled.
To prepare the siRNA, either Silencer Select Pre-designed Ocln-specific siRNA (Ambion by life technologies, Catalog# 4390816) or Silencer Select Negative Control siRNA (Ambion by life technologies, Catalog# 4390844) was prepared by dissolving the 40 nmol of siRNA in 600 μl of deionized water and separated into 100 μl aliquots. A siRNA aliquoted was prepared for each rat immediately before treatment by adding 100 μl of lipofectamine 2000 (ThermoFisher), mixing the tube, and incubating the mixture for 20 minutes at room temperature. After 20 minute incubation, 800 μl of water was added and the sample mixed by inverting the tube.
For siRNA treatment of the rats, the rats were anesthetized with isoflurane and manually stimulated to remove fecal pellets within 8 cm distance of the anal opening. A lubricated 1 mL syringe was then used to flush out fecal matter. Next, the 1 mL of siRNA solution was delivered through the anus using a lubricated 1 mL syringe and the anus sealed using a plastic plug to prevent the liquid from leaking out. After 2 hrs, the plastic plug was removed and the rat removed from anesthetic. Preliminary studies indicated that the 1 ml delivery of the siRNA was retained in the distal colon (5–7 cm proximal to the anus). Two days after treatment, VMR to CRD was determined, the rat sacrificed, and the colon epithelium was mechanically collected from the distal colon (5–7 cm proximal of the anus) and the proximal colon (10–17 cm proximal of the anus). Western blot analysis was performed as described above to assess knockdown of occludin protein.
3. Results
3.1 Lubiprostone prevents WAS-mediated increase in gut permeability and decreases visceral hyperalgesia
To determine whether preventing gut permeability blocks WAS-induced visceral hyperalgesia, we utilized lubiprostone treatment. Lubiprostone is a bicyclic fatty acid derived from prostaglandin E1 that is used to treat chronic constipation in suitable patients by activating the CIC-2 chloride channel on the apical aspect of gastrointestinal epithelial cells, producing a chloride-rich fluid secretion [4,13,17]. A subset of patients taking lubiprostone reported significant improvement in abdominal pain [18]. In animal models, lubiprostone has been shown to restore barrier function and epithelial tight junction protein expression after barrier disruption, and protect the intestine epithelium from damage when administered before insult [15,16,24,38].
In preliminary experiments, the effect of lubiprostone on WAS-induced increase of visceral motor response (VMR) to colorectal distention (CRD) was assessed in rats receiving four different lubiprostone dosing schemes. The recommended therapeutic dose range of lubiprostone for the rat was 10 –100 μg/Kg/day, with 10 μg/Kg/day the suggested starting dose (personal correspondence with Takeda/Sucampo representatives). Dosing schemes included 1 or 10 μg/Kg twice daily, or 2 or 20 μg/Kg once daily. Rats given 1 or 2 μg/Kg lubiprostone twice daily while undergoing WAS demonstrated no significant changes in VMR to CRD compared to untreated WAS rats (data not shown). Rats that received lubiprostone 10 μg/Kg twice daily or 20 μg/Kg once daily during WAS had a significant decrease in VMR to CRD compared to untreated and vehicle treated WAS rats. No differences were observed in untreated and vehicle treated rats. The 10 μg/Kg twice daily dose induced a slightly greater and more consistent decrease in VMR response, therefore, all further experiments were carried out with a lubiprostone dose of 10 μg/Kg twice daily during the ten days of WAS or sham stress.
To test if lubiprostone treatment would prevent WAS-induced visceral hyperalgesia, rats were subjected to sham stress (Ctrl) or WAS while they received vehicle (medium chain triglycerides, MCT) or lubiprostone (Ctrl+L and WAS+L) treatments twice daily. VMR to CRD was determined using the surgically implanted electrodes 24 hrs after completion of WAS/sham stress. In Ctrl rats (n=11), lubiprostone and MCT vehicle did not significantly influence CRD to VMR at any CRD pressure measured (Figure 1A). WAS-treated rats (n=11) showed an increase in VMR to CRD at 40 mmHg (p ≤ 0.02) and 60 mmHg (p ≤ 0.0001) pressure compared to Ctrl (1.9 and 2.13 fold, respectively) and Ctrl+L (2.82 and 1.85 fold, respectively) (Figure 1A). Lubiprostone treatment (n=15) significantly decrease EMG response to CRD in WAS animals at 60 mmHg pressure (WAS 210% versus WAS+L 160%; p ≤ 0.0001) (Figure 1B). In summary, we normalized the data to the average value (using raw data) of the 60 mmHg pressure in control rats, and expressed all the other values in the same group and other groups as a function of this value, e.g. designated 100%. This methodology has been employed in numerous publications. Every data point in Figure 1B represents one individual measurement, which shows the actual variation of the data at 40 and 60 mmHg pressure between the 4 different groups. As shown in the Figure 1, the variation of pain response to colorectal distention at a particular distention pressure could be easily identified in the same group of animals. We also analyzed the difference between these 4 groups using the raw data and determined that there was no statistically significant difference between groups, other than the actual values indicated in Y-axis of Figure 1B. In summary, the trend of changes (mean±SEM) at different distention pressure between the 4 groups were the same using the raw data vs. normalized data.
Fig. 1. WAS-induced visceral hypersensitivity is decreased with lubiprostone treatment.
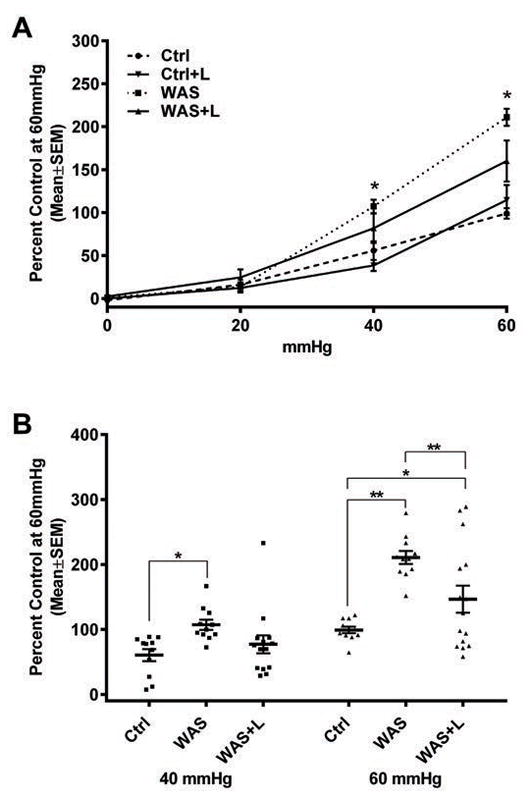
Rats were subjected to sham stress (controls, Ctrl, n=11) or WAS (n=11) for 1 hr/day for 10 consecutive days. WAS rats received MCT vehicle (WAS) or lubiprostone (WAS+L, n=15) treatment twice daily. 24 hrs after WAS/sham stress, electromyographic response (EMR) to colorectal distention at 20, 40, and 60 mmHg of pressure was tested using surgically implanted electrodes in the abdominal wall muscle. Data were analyzed with two-way ANOVA, * p ≤ 0.02, ** p ≤ 0.0001. (A) Differences in EMR were observed between Ctrl/Ctrl+L and WAS at 40 and 60 mmHg of distention. (B) Lubiprostone treatment significantly decreased VMR only at 60 mmHg distention.
Interestingly, eight of the 15 lubiprostone treated WAS rats had EMG responses to CRD that were within two standard deviations from the average value of control animals at both 40 (WAS+L = 50% ± 6.9%; Ctrl = 60.5% ± 9.32%) and 60 mmHg (WAS+L = 84% ± 7.3%; Ctrl = 99% ± 4.9 %) pressures, whereas the rest of the lubiprostone treated rats had significantly higher VMR to CRD levels compared to control rats. Therefore, lubiprostone prevented WAS-induced visceral hyperalgesia in 53% of rats. We next examined whether this outcome was due to lubiprostone only partially blocking gut permeability in a subpopulation of rats.
To assess the effect of lubiprostone on gut permeability, an in vivo gut permeability test was performed. On the day after completion of WAS (n=14) or sham stress controls (n=7), rats were gavaged with 4 KDa FITC-dextran and its presence in blood plasma was assayed 4 hours later (Figure 2). Since 4 KDa FITC-dextran is 14 Å in size, this reagent is used to detect impairment in the leak paracellular pathway [14,45]. No significant differences between treatment-groups were observed when a one-way ANOVA was performed (Figure 2A). However, a subpopulation of rats exhibited intestinal permeability that was greater than two standard deviations from the average permeability observed in control rats. Specifically, WAS-Responsive rats were > 499 ng/mL and the remaining were non-responsive to WAS, e.g. WAS-NR ≤ 2 SDs from the average Ctrl value. When WAS rats were separated into subgroups (WAS-R (n=7) and WAS-NR (n=7)), significant differences were observed between control and WAS-R rats (321 ± 34 versus 872 ± 208 ng/mL, respectively, p = 0.0041), WAS-NR and WAS-R rats (257 ± 26.2 versus 872 ± 208 ng/mL, p = 0.0007), and WAS-R and WAS+L rats (n= 8) (872 ± 208 versus 372 ± 40.5, p = 0.0075) (Figure 2B). These data indicate that lubiprostone treatment prevented WAS-induced intestinal permeability increases in a subpopulation of rats that respond to WAS with significant barrier dysfunction.
Fig. 2. WAS-induced gut permeability is prevented with lubiprostone treatment.
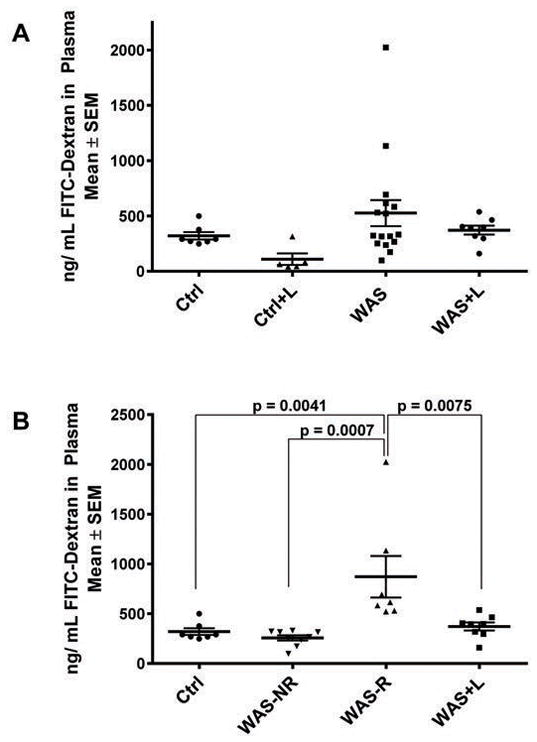
Rats were subjected to sham stress (Ctrl, n=7)) or WAS (n=14) for 1 hr/day for 10 consecutive days while receiving MCT vehicle or lubiprostone (Ctrl+L, n=5) and WAS+L, n=8)) treatment twice daily. On day 11, rats were treated with 4 KDa FITC-Dextran by gavage and after 4 hours blood was collected by heart puncture. Samples were processed, plasma fluorescence levels determined, and the ng/mL calculated using a standard curve. Data was analyzed by one-way ANOVA. A) No significant differences were observed until a sub-group analysis was performed (B). A subset of rats responded to WAS with increased gut permeability (WAS-R, n=7, > 2 SDs from the average Ctrl value, > 499 ng/mL) and the rest were non-responsive to WAS (WAS-NR, n=7, ≤ 2SDs from the average Ctrl value).
3.2 The protein levels and localization of the epithelial tight junction protein occludin inversely correlates with increased gut permeability
Previously, we found that WAS induces increased permeability to PEG-400 (4.7–7.6Å) in the colon, but not the small intestine [58]. In the current study a subpopulation of rats demonstrated WAS-induced increased permeability to 4 KDa FITC-dextran (14Å). Due to the size of this molecular species, these data indicate that the paracellular leak pathway is involved in WAS-induced increased colon permeability. The leak pathway is formed by gaps in the tight junctions between cells, allowing passage of molecules. The level of expression of the intestinal epithelial tight junction protein occludin (Ocln) has been shown to stabilize tight junctions, reducing the number and size of the gaps between cells [14,45]. In addition, cortisol in the human and corticosterone in the rodent, key players in the HPA axis, are known to regulate Ocln gene expression via a glucocorticoid receptor binding site upstream of the Ocln gene [21,23]. Therefore, we analyzed changes in Ocln protein levels in distal colon tissue extracts.
Western blot analysis of Ocln protein levels revealed no significant difference between control rats and WAS rat groups due to the large variation of Ocln levels between individual rats (Figure 3A). However, when WAS protein samples were grouped based on whether the rat of origin responded to WAS with increased permeability, WAS-R, or were non-responsive to stress, WAS-NR (Figure 3B) significant changes were observed. Ocln protein levels were significantly different between WAS-R and ctrl rats (63.7 ± 30.3% versus 123 ± 50.3%, p = 0.0064). Also, colon tissue from WAS-R showed a significant decrease in Ocln levels over colon tissue from WAS+L rats (63.7 ± 30.3 versus 275 ± 175%, p = 0.0098), demonstrating that lubiprostone treatment prevented stress-induced decreases in Ocln protein expression. Although there was no significant difference between WAS-NR and the Ctrl group, 5 of 7 rats in the WAS-NR group exhibited a decreased in Ocln protein from the average Ocln protein level of the Ctrl group (88–117% versus 123%, respectively), with 4 of the 7 WAS-NR rats exhibiting a > 10% decrease from the average ctrl. In addition, despite lack of significance, the average WAS+L group Ocln level (275%) was over 2 fold greater than the average WAS-NR group Ocln level (112%) (Figure 3B).
Fig. 3. Levels of the tight junction protein Occludin inversely correlated with gut permeability.

Western blot analysis of distal colon tissue extracts from control (Ctrl), WAS, and lubiprostone treated WAS (WAS+L) and control (Ctrl+L) rats were performed. A subset of rats responded to WAS with increased gut permeability (WAS-R, > 2 SDs from the average Ctrl value, > 499 ng/mL) and the remaining were non-responsive to WAS (WAS-NR, ≤ 2SDs from the average Ctrl value). A) Representative Western blots of Occludin (Ocln) and β-actin. B) Blots were quantitated, using β-actin as a loading control, with ImageJ. Protein levels are expressed as a percentage of a control sample loaded on all blots (average ± SEM). P-values were calculated using unpaired t-tests, not assuming equal SD due to large, variation between treatment groups, with an adjustment for multiple observations, 2% FDR. C) Occludin (Ocln) protein levels (% control) versus gut permeability to 4 KDa FITC-dextran (ng/mL) was graphed for control (filled circle), control + lubiprostone (open circle), WAS-NR (triangle), WAS-R (square), and WAS+L (diamond) rat groups. The Pearson and Spearman’s rank-order correlations were determined; the Spearman’s correlation was more significant, indicating a non-linear relationship, and is shown.
Since protein levels are not an indication of localization, we next analyzed the localization of Ocln protein in colon tissue using fluorescent immunostaining. Immunostaining of colon tissue from control rats revealed Ocln localization in the expected pattern for its role in stabilization of the tight junction. In control rat colon, Ocln localized mainly at the apical (luminal) side of the epithelium layer and extended between cells decreasing toward the basal side of the epithelium (Figure 4, A–C, yellow arrows). WAS rats that did not exhibit an increase in intestinal permeability, WAS-NR, demonstrated an Ocln localization indistinguishable from controls (Figure 4, A–C compared to G–I). On the other hand, colon tissue from WAS-R rats demonstrated overall less Ocln immunostaining, with varying amounts of apical localization in the sagittal sections (Figure 4J, small white arrows). Immunostaining of WAS-R transverse sections revealed Ocln apical localization varies along the axis of the crypt, with less apical staining toward the crypt base compared to the Ocln localization in control rat tissue (Figure 4, K and L, small white arrows).
Fig. 4. WAS rats with increased intestinal permeability, demonstrated a decrease in Occludin protein levels in colon epithelial cells, which was prevented with lubiprostone treatment.

Immunostaining for Ocln protein is shown for sham stress rats (Ctrl), sham stress rats treated with lubiprostone (Ctrl+L), and WAS rats with and without lubiprostone treatment (WAS+L). Tissue from rats that demonstrated increased gut permeability due to WAS, WAS-R, or did not demonstrate increased gut permeability (WAS-NR), are shown separately. Both sagittal (first column) and transverse (2nd and 3rd column) views are shown. Yellow arrows indicate examples of the normal localization of Ocln protein, small white arrows indicate examples of abnormal localization or decreased stained areas, and large white arrows indicate examples of increased Ocln staining or localization at the basal side of the colon.
Lubiprostone treated control rats demonstrated a similar Ocln localization pattern to that of untreated controls with localization on the apical side and between cells, although slightly lighter staining than control tissue (Figure 4D–F, examples yellow arrows) compared to Figure 4, A–C. In addition, Ocln localization in Ctrl+L rat tissue was increased at the basal side of the epithelium (Figure 4D–F, large white arrows examples). Lubiprostone treatment prevented the decrease in Ocln immunostaining seen in WAS-R rats (Figure 4, J–L compared to Figure 4, M–O), and Ocln immunostaining appears qualitatively greater than observed in control rat colon tissue (Figure 4, A–C). Also, WAS+L colon tissue (Figure 4, M–O) shows qualitatively increased basal Ocln localization, which was also observed in Ctrl+L colon tissue (Figure 4, D–F), large white arrows). Overall, the staining levels observed agreed with the levels determined by Western blot analysis.
3.3 Gut permeability inversely correlated with Occludin protein levels
Ocln stabilizes tight junctions, preventing gaps between epithelial cells and decreases permeability via the paracellular leak pathway. We observed large variability for both permeability and Ocln protein levels in the distal colon. Therefore, we analyzed the relationship between permeability and Ocln levels. Ocln protein levels (% control) versus gut permeability to 4 KDa FITC-dextran (ng/mL) were graphed and correlations calculated. The Pearson correlation coefficient was −0.3906 (p = 0.0246) and the Spearman’s rank-order correlation was −0.5859 (p = 0.0026) (Figure 3C). The Spearman’s correlation was both significant and negative, indicating a non-linear, inverse relationship between gut permeability and Ocln protein levels. This correlation supports the conclusion that Ocln plays a significant role in WAS-induced permeability.
3.4 Colon epithelial permeability directly correlates with visceral hyperalgesia
Measuring electromyography response to CRD with surgically implanted electrodes has been shown to be stressful to the animals [32,34] and is technically challenging when multiple measurement (i.e. on the day before and after stress/sham stress) are desired to clarify differences due to variability. Therefore, to determine if there is a direct correlation between barrier function and visceral pain, we utilized a new non-surgical method to measure VMR to CRD that uses a balloon pressure-sensor made by building a distention balloon around a miniaturized pressure transducer catheter (see material and methods) that allows VMR to CRD to be measured before and after stress/sham stress, as well as in vivo colon permeability in the same rat, longitudinally.
On day the day prior to the start of WAS or sham stress, VMR to CRD was measured for all rats. Rats were subjected to sham stress (Ctrl) or WAS for 1 hr/day for 10 consecutive day, and VMR to CRD was measured again 24 hours after the last stress session. Values were expressed as a percentage of the signal measured at 60 mmHg pre-stress (Figure 5C). WAS-treated rats demonstrated an increase in VMR to CRD at 60 mmHg over controls (WAS 135% ± 60% compared to Ctrl 107% ± 9%), but was not significant with a student t-test analysis. This was due to the variable response to WAS with measurements ranging from 61% to 243%.
Fig. 5. Level of visceral pain correlates with the magnitude of change in colon permeability.
Rats were subjected to sham stress (Ctrl, n=8) or WAS (n=10) for 1 hr/day for 10 consecutive days. Visceral motor response (VMR) was measured using the non-invasive manometric method on the day before stress/sham stress and 24 hrs after the last WAS/sham stress session. Each rat served as its own control, with data expressed as a percentage of the average 60 mmHg pre-stress measurement (C and D). 24 hrs post WAS/sham stress, the distal colon was removed, the serosal musculature stripped away, and the tissue put into an Ussing Chamber to determine the mucosal to serosal transport of 4 KDa FITC-dextran, apparent permeability (Papp, A and B), and transepithelial resistance (TER, E and F) for each rat. A subgroup analysis based on permeability was performed to separate rats into WAS-responders (WAS-R, n=3), those with Papp values greater than > 2 SDs from the average Ctrl value, and WAS non-responsive (WAS-NR, n=7), those with Papp values ≤ 2 SDs from the average Ctrl (B,D, and F). All data is expressed as mean ± SD. A student t-test (2 groups) or one-way ANOVA analysis (3 groups) was performed to determine significance. The Pearson and Spearman’s rank-order correlations were determined for Papp vs. VMR (G) and Papp vs. TER (H); the Pearson correlation was more significant, indicating a linear relationship, and is shown (n=17).
The day after the completion of WAS or sham stress, Ussing chamber experiments were performed to determine mucosal to serosal transport of 4 KDa FITC-dextran for colon epithelium and expressed as apparent permeability (Papp). Again, no significant difference were observed between Ctrl and WAS rat groups (2.89 ± 0.74×10−6 cm/s versus 4.1 ± 2.7×10−6 cm/s, respectively) due to variability in the WAS group (Papp values ranged 2.1 to 9.7×10−6 cm/s) (Figure 5A). Transepithelial resistance (TER) was also measured, which is a well-accepted indicator of epithelial barrier integrity [12]. When comparing TER measurements, again, no difference was observed between Ctrl (104.4 ± 13.6 Ω/cm2) and WAS (98.6 ± 14.7 Ω/cm2) rat groups (Figure 5E).
As observed in the in vivo intestinal permeability assay, a subpopulation of rats exhibited intestinal permeability that was greater than two standard deviations from the average Papp value of control rats. When WAS rats were separated into groups that were WAS-responders with increased permeability to 4 KDa FITC-dextran (WAS-R > 2 SDs; n=3 from the average Ctrl value), and those that were non-responsive to stress (WAS-NR, n=7), significant differences were observed using a one-way ANOVA between the WAS-R group and Ctrl and WAS-NR groups not only for permeability (Figure 5B), but also VMR to CRD (Figure 5D) and TER (Figure 5F). Specifically, when WAS rats were placed into subgroups based on the permeability data, colon epithelial permeability for WAS-R rats showed a significant increase over Ctrl rats (7.8 ± 1.8×10−6 cm/s versus 2.1 ± 0.7×10−6 cm/s, p = 0.0064) and the WAS-NR rat group (7.8 ± 1.8×10−6 cm/s versus 2.5 ± 0.4×10−6 cm/s, p= 0.0064). Also, TER was decreased in the WAS-R group compared to Ctrl (77.8 ± 9 versus 104.4 ± 13.6 Ω/cm2, p=0.027) and WAS-NR (77.8 ± 9 versus 103.4 ± 10.8 Ω/cm2, p = 0.0385) groups.
When VMR to CRD data were reanalyzed based on the permeability data, WAS-R rats demonstrated a significant increase in VMR to CRD at 60 mmHg distention over both Ctrl (184% ± 55% versus 107% ± 9%, p<0.0001) and WAS-NR (184% ± 55% versus 104% ± 40%, p<0.0001) rat groups in a one-way ANOVA analysis. Most significantly, when colon permeability (Papp) was plotted as a function of VMR to CRD (Figure 5G), a significant positive correlation of 0.6008 (p = 0.0084) was observed. As expected, TER and permeability revealed a linear negative correlation with a Pearson coefficient of −0.6045 (p = 0.0102) (Figure 5H). Since permeability was determined in an Ussing chamber using tissue obtained from the colon, this shows that the increased permeability involves changes in the colon.
3.5 Knockdown of the epithelial cell tight junction protein Ocln in the colon in control rats leads to Visceral Hyperalgesia
In order to test the hypothesis that increased colon permeability leads to visceral hyperalgesia, we treated control rats with Ocln-specific siRNA. We chose to target Ocln because we demonstrated that the Ocln protein level correlates colon barrier dysfunction (Figure 3), and Ocln has been shown to be a key player in maintaining tight junction stability and barrier function in knockout mice [14], without being lethal. Ocln siRNA treated rats (n=5) showed a significant increase (p < 0.01) in VMR to CRD over rats treated with Control (scrambled) siRNA (n=5) at all distention pressures tested (Figure 6A) (20 mmHg: 155 ± 32% vs. 58 ± 26%, p<0.0001; 40mmHg: 146 ± 20% vs. 106 ± 11%, p = 0.0056; 60 mmHg: 145 ± 35% vs. 96 ± 22%, p = 0.0007; mean ± SD, 1% FDR). The VMR to CRD levels of Ocln siRNA treated rats had significantly higher VMR to CRD levels at 20 and 40 mmHg distention pressures (p <0.0001, 1% FDR) when compared to all WAS rats (compare Figure 5C and 6A: 20 mmHg: 155 ± 32% vs. 41 ± 51%; 40mmHg: 146 ± 20% vs. 76 ± 49%; 60 mmHg: 145 ± 35% vs. 96 ± 22%). However, no significant differences were observed between Ocln-siRNA treated and WAS-R rat groups VMR to CRD data (compare Figure 5C and 6A: 20 mmHg: 155 ± 32% vs. 107 ± 9%; 40mmHg: 146 ± 20% vs. 104 ± 41%; 60 mmHg: 145 ± 35% vs. 184 ± 55%). Overall, Ocln siRNA treatment increased pain in healthy control rats to a level similar to that observed in WAS-R rats.
Fig. 6. Knockdown of Ocln protein with si-RNA in the colon of control rats leads to increased visceral pain.

Control rats were treated intrarectally with Ocln-specific siRNA (Ocln siRNA, n=3) or non-specific, scrambled siRNA (Ctrl siRNA, n=3). A) VMR to CRD was measured one day before treatment and 48 hrs after treatment. Each rat served as its own control, with VMR expressed as the percent of the pre-treatment 60 mmHg distention value. After VMR to CRD measurements, colon epithelium was collected from the distal (D) and proximal (P) colon for each rat. Epithelial extracts were analyzed in duplicate by Western blot at two different exposures within the linear range. C and D) Western blots of Occludin (Ocln) and β-actin are shown. Blots were quantitated, using β-actin as a loading control, with ImageJ. Protein levels are expressed as a percentage of proximal colon epithelial levels. B) In a second group of rats, 48 hrs after Ocln siRNA (n=5) or control siRNA (n=5) treatment, the distal colon was removed, the serosal musculature stripped away, and the tissue put into an Ussing Chamber to determine the mucosal to serosal transport of 4 KDa FITC-dextran, apparent permeability (Papp). Some bars are too small to see. * p < 0.001, t-test with a 1% FDR.
After VMR to CRD measurements, rats were sacrificed, colon tissue harvested, and Ocln protein levels were analyzed. Since Ocln levels can vary up to 30% between individuals (Figure 3B) and the siRNA intervention was designed to localize the siRNA intervention to the distal 5 cm of the colon, we compared Ocln protein levels from the proximal and distal portion of the colon (Figure 6C and D) in a subpopulation of rats, control (scrambled) siRNA (n=3) and Ocln siRNA (n=3). Control rats demonstrated distal colon epithelial Ocln levels that were 122 ± 32% of the proximal colon epithelium levels (data not shown). This subpopulation of rats underwent VMR to CRD and permeability ± control vs Ocln siRNA using tissue harvested from the proximal and distal colon to confirm that the effect of rectal installation of the siRNA intervention was confined to the distal colon. In this group the Ocln siRNA treatment markedly decreased Ocln protein expression in the rectum in two rats and had no effect in one rat. Specifically, Western blot analysis showed that two of three rats treated with Ocln siRNA had undetectable Ocln levels in the distal colon compared to the proximal (Figure 6C). One Ocln siRNA treated rat showed no Ocln knockdown and this rat demonstrated CRD to VMR at 60 mmHg that was 106 ± 8% of pre-treatment values, e.g. no significant change in VMR to CRD compared to control. We do not know why the Ocln siRNA treatment demonstrated no effect in this rat but speculate that the presence of fecal pellets in the distal colon of this rat interfered with the availability of the Ocln-siRNA reagent to interact with the epithelium.
The control siRNA treated rats Ocln levels (Figure 6C) were decreased in two rats (76% and 68% of control level); however, these levels were within the 33% variance observed in non-treated controls, indicating this was variation in the Ocln protein levels and not actually knock down. No significant knock-down of Ocln protein levels in control siRNA treated rats is supported by VMR to CRD data, e.g. when VMR to CRD data from control sham stressed rats and control siRNA treated rats were compared, no significant differences were observed for all distention pressures (20 mmHg: 33 ± 12% vs. 58 ± 26%; 40mmHg: 79 ± 33% vs. 106 ± 11%; 60 mmHg: 107 ± 9% vs. 96 ± 22%).
Ussing chamber experiments were performed to confirm that siRNA knockdown of Ocln protein in the distal colon leads to increased permeability. Mucosal to serosal transport of 4 KDa FITC-dextran (Papp) and TER were measured in tissue obtained from the distal colon of rats treated with Ocln-specific and control (scrambled) siRNA. Interestingly, no significant differences in TER measurements were observed (data not shown, refer to Discussion section for comments). Permeability was significantly increased (p = 0.0106) in rats treated with Ocln siRNA (6.88 ± 0.71) compared to Ctrl siRNA (3.18 ± 0.35) treated rats (Figure 6B). The permeability (Papp) of distal colon epithelium from WAS-R rats and Ocln siRNA treated control rats were similar (7.82 ± 1.77 vs. 6.88 ± 0.71), as was that of Ctrl (sham stress) and Control siRNA (2.88 ± 0.74 vs. 3.18 ± 0.35), with no significant differences.
4. Discussion
The existence of an association between chronic psychological stress, increased intestinal permeability and visceral hyperalgesia is an active area of investigation and increasingly accepted based on animal and human studies. However, few studies have attempted to causally link chronic stress, impaired permeability and enhanced abdominal pain. In this report we provide compelling data supporting the interpretation that chronic stress-induced visceral hyperalgesia is causally linked to increased intestinal epithelial paracellular permeability. We believe that this is the first study to directly assess both pain and permeability in the same animal, which is necessary to correlate the two endpoints in a rigorous manner because of well-known individual differences in pain and permeability measurements in population studies.
Our results reveal a significant degree of individual variability in the pain response, which correlates with intestinal permeability (Figure 3C). Using measurements in the same animal, our data demonstrate a positive correlation between the level of visceral pain and the magnitude of paracellular permeability (0.6008, p = 0.0084) in a validated model of chronic, intermittent stress. Our data support the interpretation that prevention of WAS-induced increase in intestinal permeability with lubiprostone significantly decreases visceral hyperalgesia (Figure 1 and 2). However, measurements of permeability and visceral pain in the same animal ± lubiprostone will be required to confirm that preventing gut permeability changes with lubiprostone decreases visceral hyperalgesia. Conversely, we also show that increasing colon paracellular permeability in control rats by treating the distal colon with Ocln siRNA in vivo, leads to increased visceral pain similar to levels observed in WAS-R rats (Figure 6). It is potentially noteworthy that the effect of Ocln siRNA treatment in control rats on permeability and VMR to CRD may be more potent than WAS at comparable endpoints. For example, the increase in VMR to CRD at 20 mmHg is significantly higher in the Ocln siRNA treated control rats compared to the WAS rats (Figures 1 and 6). Overall, these findings support that increased colon permeability plays a potentially pivotal role in the development of visceral hyperalgesia.
In addition, our findings demonstrated that the tight junction protein Ocln plays a significant role in the development stress-induced colon permeability. Specifically, reduction in Ocln protein levels in the colon epithelial correlates with increased epithelial permeability (Figure 3C). In addition, when WAS-induced reduction in Ocln levels are prevented with lubiprostone, increased colon epithelial paracellular permeability is not observed (Figure 2).
4.1 Intestinal Permeability
Available data supports involvement of two pathways in intestinal epithelial paracellular permeability, a high capacity pore pathway and a low capacity leak pathway, both of which are passive (not requiring energy) [45,54]. The leak pathway is formed by gaps in the tight junctions allowing passage of molecules between cells, and is charge-independent and to some extent molecular size-independent, with a maximum radius of 17 – 20 Å for intact gut epithelium. The pore pathway is size restrictive (≤ 4.5 Å), due to the pore being created by the epithelial tight junction proteins forming a channel, and charge selective, which is determined by the pore forming claudin proteins expressed [45,54]. Since we examine permeability using 4 KDa FITC-dextran, a large molecule of 14 Å, our data suggest that the leak pathway plays a key role in WAS-induce colon permeability.
We observed significant variability in colon epithelial paracellular permeability to 4 KDa FITC-dextran (14 Å) in response to WAS. Previously we observed that all WAS rats demonstrated an increase in colon permeability to PEG-400 (4.7–7.6 Å) [58]. The observed differences are most likely related to the size differences between the molecules, with the magnitude of flux of different sized molecules determined by the gap size between tight junctions, and the severity of paracellular permeability disruption. Specifically, all WAS rats exhibited an increase in paracellular permeability that allowed molecules between 4.7–7.6 Å to move across the colon epithelium barrier. However, only a subpopulation of WAS rats demonstrated enhanced permeability that allowed 14 Å molecules to move across the colon epithelial barrier. Collectively, these studies suggest that all WAS rats have increased paracellular permeability involving the leak pathway, with a subpopulation of WAS rats demonstrating a more severe colon epithelial barrier dysfunction. The cellular pathways and molecular mechanisms that underlie the individual variability in epithelial barrier dysfunction are unknown but we speculate that differential expression of epigenetic regulatory pathways is a significant contributing factor.
The leak pathway has been shown to be dependent on two epithelial tight junction proteins Occludin and ZO-1 [45,54]. Previously, we have shown that both ZO-1 and Ocln decrease with WAS [58]. In this study, we focused on Ocln since it is associated with tight junction stability rather than formation. We found that the level of Ocln protein in the colon epithelial cells correlated with the level of paracellular permeability. Immunostaining revealed that Ocln was localized to the surface membranes (tight junctions) in untreated and lubiprostone-treated WAS rats, supporting no alteration in intracellular trafficking of the protein. Furthermore, we observed variability in the levels of Ocln protein in the control rats. Our evidence supports that there is a natural variability in the colonic paracellular permeability in rats, which leads to differences in WAS-induced barrier dysfunction. Our data suggests that a potentially key mechanism in WAS-induced permeability is regulation of the Ocln protein levels.
While our data supports the hypothesis that paracellular permeability involving large macromolecules utilizing the leak pathway is a significant contributing factor to the development of stress-induced visceral hyperalgesia, the pore pathway likely plays a role as well. A number of studies have shown that WAS increases the pore pathway paracellular permeability for ionic molecules in the colon [11,42,43,46,51,57]. In addition, lubiprostone has been shown to ameliorate pore pathway paracellular permeability dysfunction in the ischemia-injured porcine ilium mucosa model [38]. Therefore, future studies will need to specifically address the contribution of changes in pore paracellular permeability in stress-induced visceral hyperalgesia.
4.2 Linking Increased Colon epithelial Paracellular Permeability to Enhanced Visceral Pain
In most model systems it is unknown whether changes in intestinal barrier function is a prerequisite for the development of visceral hyperalgesia. Indeed, a review of the literature revealed that, except in the case of acute stress [2], a direct link between the magnitude of visceral hyperalgesia and magnitude of increased intestinal permeability has never been clearly established. Our data indicates that increases in paracellular permeability to larger molecules (14 Å) correlates with the magnitude of visceral pain, and that inducing increased permeability leads to visceral hyperalgesia.
Our results support the interpretation that a potentially key link between increased permeability and pain is the tight junction protein Ocln that influences paracellular permeability. However, decreased Ocln protein levels are unlikely to be the only factor that leads directly to pain because no differences were observed in TER when Ocln was knocked-down, whereas differences in TER were observed between Ctrl and WAS rat colon tissue. WAS-induced colon paracellular permeability dysfunction can lead to inflammation that triggers visceral pain by sensitizing enteric neurons and dorsal root ganglia upstream [20,35,55]. However, it remains unresolved whether : i) stress leads directly to colon paracellular permeability dysfunction via glucocorticoid release as a result of stimulation of the hypothalamus-pituitary-adrenal axis; ii) increased permeability is secondary to stressed-induced inflammation, or iii) stress-induced increases in permeability and visceral hyperalgesia requires both the effects of glucocorticoid and inflammation.
There is evidence of both inflammation and glucocorticoids being the driving forces in the development of intestinal permeability and visceral pain. Supporting glucocorticoids being the driving force, it has been found that dexamethasone, a powerful glucocorticoid receptor activator, increases gut permeability in chickens [52] and rats [47], and glucocorticoids have been shown to regulate Ocln gene expression via the glucocorticoid receptor binding site upstream of the Ocln gene in human and rodent [21,23]. It should be noted, dexamethasone is a long acting potent pharmacologic agent used in the clinic, which is much different than the effect of cortisol levels in chronic, intermittent stress. On the other hand, inflammation has been reported to be a driving force in increased paracellular permeability in a number of studies looking at antibiotic, pro-biotic, and inflammatory factors [5,28,29,41,44,46,48,51,55]. Longitudinal studies examining the time course of WAS-induced colon paracellular permeability dysfunction and activation of pro-inflammatory mediators will help to resolve this important issue.
4.3 Conclusion
Conditions like irritable bowel syndrome and chronic, intermittent abdominal pain are common and are often aggravated by stress. We believe that the present study provides the first direct evidence that the magnitude of chronic stress-induced visceral hyperalgesia directly correlates with the level of increased colon epithelial paracellular permeability. This observation provides a rationale for the development of therapeutic interventions that specifically target the maintenance or restoration of intestinal epithelial tight junction proteins in the management of chronic stress-induced visceral hyperalgesia.
Acknowledgments
This research was supported by NIH grants: R01DK098205 and R21AT009253 (JWW) and a research grant from Takeda Pharmaceuticals (JWW).
References
- 1.Aguilera M, Vergara P, Martinez V. Stress and antibiotics alter luminal and wall-adhered microbiota and enhance the local expression of visceral sensory-related systems in mice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(8):e515–529. doi: 10.1111/nmo.12154. [DOI] [PubMed] [Google Scholar]
- 2.Ait-Belgnaoui A, Bradesi S, Fioramonti J, Theodorou V, Bueno L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain. 2005;113(1–2):141–147. doi: 10.1016/j.pain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Atcha Z, Rourke C, Neo AH, Goh CW, Lim JS, Aw CC, Browne ER, Pemberton DJ. Alternative method of oral dosing for rats. Journal of the American Association for Laboratory Animal Science : JAALAS. 2010;49(3):335–343. [PMC free article] [PubMed] [Google Scholar]
- 4.Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. American journal of physiology Gastrointestinal and liver physiology. 2008;295(2):G234–251. doi: 10.1152/ajpgi.00366.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharucha AE, Linden DR. Linaclotide - a secretagogue and antihyperalgesic agent - what next? Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22(3):227–231. doi: 10.1111/j.1365-2982.2009.01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boudry G, Cheeseman CI, Perdue MH. Psychological stress impairs Na+-dependent glucose absorption and increases GLUT2 expression in the rat jejunal brush-border membrane. American journal of physiology Regulatory, integrative and comparative physiology. 2007;292(2):R862–867. doi: 10.1152/ajpregu.00655.2006. [DOI] [PubMed] [Google Scholar]
- 7.Bradesi S, Kokkotou E, Simeonidis S, Patierno S, Ennes HS, Mittal Y, McRoberts JA, Ohning G, McLean P, Marvizon JC, Sternini C, Pothoulakis C, Mayer EA. The role of neurokinin 1 receptors in the maintenance of visceral hyperalgesia induced by repeated stress in rats. Gastroenterology. 2006;130(6):1729–1742. doi: 10.1053/j.gastro.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 8.Bradesi S, Martinez V, Lao L, Larsson H, Mayer EA. Involvement of vasopressin 3 receptors in chronic psychological stress-induced visceral hyperalgesia in rats. American journal of physiology Gastrointestinal and liver physiology. 2009;296(2):G302–309. doi: 10.1152/ajpgi.90557.2008. [DOI] [PubMed] [Google Scholar]
- 9.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. American journal of physiology Gastrointestinal and liver physiology. 2005;289(1):G42–53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 10.Bradesi S, Svensson CI, Steinauer J, Pothoulakis C, Yaksh TL, Mayer EA. Role of spinal microglia in visceral hyperalgesia and NK1R up-regulation in a rat model of chronic stress. Gastroenterology. 2009;136(4):1339–1348. e1331–1332. doi: 10.1053/j.gastro.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron HL, Perdue MH. Stress impairs murine intestinal barrier function: improvement by glucagon-like peptide-2. The Journal of pharmacology and experimental therapeutics. 2005;314(1):214–220. doi: 10.1124/jpet.105.085373. [DOI] [PubMed] [Google Scholar]
- 12.Clarke LL. A guide to Ussing chamber studies of mouse intestine. American journal of physiology Gastrointestinal and liver physiology. 2009;296(6):G1151–1166. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cryer B, Katz S, Vallejo R, Popescu A, Ueno R. A randomized study of lubiprostone for opioid-induced constipation in patients with chronic noncancer pain. Pain medicine. 2014;15(11):1825–1834. doi: 10.1111/pme.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummins PM. Occludin: one protein, many forms. Molecular and cellular biology. 2012;32(2):242–250. doi: 10.1128/MCB.06029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuppoletti J, Blikslager AT, Chakrabarti J, Nighot PK, Malinowska DH. Contrasting effects of linaclotide and lubiprostone on restitution of epithelial cell barrier properties and cellular homeostasis after exposure to cell stressors. BMC pharmacology. 2012;12:3. doi: 10.1186/1471-2210-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuppoletti J, Chakrabarti J, Tewari K, Malinowska DH. Methadone but not morphine inhibits lubiprostone-stimulated Cl- currents in T84 intestinal cells and recombinant human ClC-2, but not CFTR Cl- currents. Cell biochemistry and biophysics. 2013;66(1):53–63. doi: 10.1007/s12013-012-9406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. American journal of physiology Cell physiology. 2004;287(5):C1173–1183. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 18.Drossman DA, Morris CB, Schneck S, Hu YJ, Norton NJ, Norton WF, Weinland SR, Dalton C, Leserman J, Bangdiwala SI. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. Journal of clinical gastroenterology. 2009;43(6):541–550. doi: 10.1097/MCG.0b013e318189a7f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ersoy Y, Cikler E, Cetinel S, Sener G, Ercan F. Leukotriene D4 receptor antagonist montelukast alleviates water avoidance stress-induced degeneration of the gastrointestinal mucosa. Prostaglandins, leukotrienes, and essential fatty acids. 2008;78(3):189–197. doi: 10.1016/j.plefa.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Eutamene H, Bradesi S, Larauche M, Theodorou V, Beaufrand C, Ohning G, Fioramonti J, Cohen M, Bryant AP, Kurtz C, Currie MG, Mayer EA, Bueno L. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22(3):312–e384. doi: 10.1111/j.1365-2982.2009.01385.x. [DOI] [PubMed] [Google Scholar]
- 21.Forster C, Silwedel C, Golenhofen N, Burek M, Kietz S, Mankertz J, Drenckhahn D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. The Journal of physiology. 2005;565(Pt 2):475–486. doi: 10.1113/jphysiol.2005.084038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrido P, De Blas M, Ronzoni G, Cordero I, Anton M, Gine E, Santos A, Del Arco A, Segovia G, Mora F. Differential effects of environmental enrichment and isolation housing on the hormonal and neurochemical responses to stress in the prefrontal cortex of the adult rat: relationship to working and emotional memories. Journal of neural transmission. 2013;120(5):829–843. doi: 10.1007/s00702-012-0935-3. [DOI] [PubMed] [Google Scholar]
- 23.Harke N, Leers J, Kietz S, Drenckhahn D, Forster C. Glucocorticoids regulate the human occludin gene through a single imperfect palindromic glucocorticoid response element. Molecular and cellular endocrinology. 2008;295(1–2):39–47. doi: 10.1016/j.mce.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi S, Kurata N, Yamaguchi A, Amagase K, Takeuchi K. Lubiprostone prevents nonsteroidal anti-inflammatory drug-induced small intestinal damage by suppressing the expression of inflammatory mediators via EP4 receptors. The Journal of pharmacology and experimental therapeutics. 2014;349(3):470–479. doi: 10.1124/jpet.114.213991. [DOI] [PubMed] [Google Scholar]
- 25.Hong S, Fan J, Kemmerer ES, Evans S, Li Y, Wiley JW. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut. 2009;58(2):202–210. doi: 10.1136/gut.2008.157594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong S, Zheng G, Wiley JW. Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system. Gastroenterology. 2015;148(1):148–157. e147. doi: 10.1053/j.gastro.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong S, Zheng G, Wu X, Snider NT, Owyang C, Wiley JW. Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat. Gastroenterology. 2011;140(2):627–637. e624. doi: 10.1053/j.gastro.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorge E, Fernandez JA, Torres R, Vergara P, Martin MT. Functional changes induced by psychological stress are not enough to cause intestinal inflammation in Sprague-Dawley rats. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22(8):e241–250. doi: 10.1111/j.1365-2982.2010.01507.x. [DOI] [PubMed] [Google Scholar]
- 29.Keita AV, Carlsson AH, Cigehn M, Ericson AC, McKay DM, Soderholm JD. Vasoactive intestinal polypeptide regulates barrier function via mast cells in human intestinal follicle-associated epithelium and during stress in rats. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(6):e406–417. doi: 10.1111/nmo.12127. [DOI] [PubMed] [Google Scholar]
- 30.Zhou QQ, Zhang B, Verne GN. Intestinal Membrane Permeability and Hypersensitivity In the Irritable Bowel Syndrome. Pain. 2009;146(1–2):41–46. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larauche M, Bradesi S, Million M, McLean P, Tache Y, Mayer EA, McRoberts JA. Corticotropin-releasing factor type 1 receptors mediate the visceral hyperalgesia induced by repeated psychological stress in rats. American journal of physiology Gastrointestinal and liver physiology. 2008;294(4):G1033–1040. doi: 10.1152/ajpgi.00507.2007. [DOI] [PubMed] [Google Scholar]
- 32.Larauche M, Gourcerol G, Million M, Adelson DW, Tache Y. Repeated psychological stress-induced alterations of visceral sensitivity and colonic motor functions in mice: influence of surgery and postoperative single housing on visceromotor responses. Stress. 2010;13(4):343–354. doi: 10.3109/10253891003664166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larauche M, Mulak A, Tache Y. Stress-related alterations of visceral sensation: animal models for irritable bowel syndrome study. Journal of neurogastroenterology and motility. 2011;17(3):213–234. doi: 10.5056/jnm.2011.17.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larauche M, Mulak A, Yuan PQ, Kanauchi O, Tache Y. Stress-induced visceral analgesia assessed non-invasively in rats is enhanced by prebiotic diet. World journal of gastroenterology WJG. 2012;18(3):225–236. doi: 10.3748/wjg.v18.i3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liebregts T, Adam B, Bertel A, Lackner C, Neumann J, Talley NJ, Gerken G, Holtmann G. Psychological stress and the severity of post-inflammatory visceral hyperalgesia. European journal of pain. 2007;11(2):216–222. doi: 10.1016/j.ejpain.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Million M, Zhao JF, Luckey A, Czimmer J, Maynard GD, Kehne J, Hoffman DC, Tache Y. The newly developed CRF1-receptor antagonists, NGD 98-2 and NGD 9002, suppress acute stress-induced stimulation of colonic motor function and visceral hypersensitivity in rats. PloS one. 2013;8(9):e73749. doi: 10.1371/journal.pone.0073749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moeser AJ, Haskell MM, Shifflett DE, Little D, Schultz BD, Blikslager AT. ClC-2 chloride secretion mediates prostaglandin-induced recovery of barrier function in ischemia-injured porcine ileum. Gastroenterology. 2004;127(3):802–815. doi: 10.1053/j.gastro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Moeser AJ, Nighot PK, Engelke KJ, Ueno R, Blikslager AT. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel agonist of the ClC-2 chloride channel, lubiprostone. American journal of physiology Gastrointestinal and liver physiology. 2007;292(2):G647–656. doi: 10.1152/ajpgi.00183.2006. [DOI] [PubMed] [Google Scholar]
- 39.Myers B, Greenwood-Van Meerveld B. Differential involvement of amygdala corticosteroid receptors in visceral hyperalgesia following acute or repeated stress. American journal of physiology Gastrointestinal and liver physiology. 2012;302(2):G260–266. doi: 10.1152/ajpgi.00353.2011. [DOI] [PubMed] [Google Scholar]
- 40.Nebot-Vivinus M, Harkat C, Bzioueche H, Cartier C, Plichon-Dainese R, Moussa L, Eutamene H, Pishvaie D, Holowacz S, Seyrig C, Piche T, Theodorou V. Multispecies probiotic protects gut barrier function in experimental models. World journal of gastroenterology : WJG. 2014;20(22):6832–6843. doi: 10.3748/wjg.v20.i22.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T. Repeated water avoidance stress induces visceral hypersensitivity; role of IL-1, IL-6 and peripheral corticotropin-releasing factor. Journal of gastroenterology and hepatology. 2017 doi: 10.1111/jgh.13787. [DOI] [PubMed] [Google Scholar]
- 42.Santos J, Benjamin M, Yang PC, Prior T, Perdue MH. Chronic stress impairs rat growth and jejunal epithelial barrier function: role of mast cells. American journal of physiology Gastrointestinal and liver physiology. 2000;278(6):G847–854. doi: 10.1152/ajpgi.2000.278.6.G847. [DOI] [PubMed] [Google Scholar]
- 43.Santos J, Yang PC, Soderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48(5):630–636. doi: 10.1136/gut.48.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saunders PR, Santos J, Hanssen NP, Yates D, Groot JA, Perdue MH. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Digestive diseases and sciences. 2002;47(1):208–215. doi: 10.1023/a:1013204612762. [DOI] [PubMed] [Google Scholar]
- 45.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annual review of physiology. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soderholm JD, Yang PC, Ceponis P, Vohra A, Riddell R, Sherman PM, Perdue MH. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123(4):1099–1108. doi: 10.1053/gast.2002.36019. [DOI] [PubMed] [Google Scholar]
- 47.Spitz J, Hecht G, Taveras M, Aoys E, Alverdy J. The effect of dexamethasone administration on rat intestinal permeability: the role of bacterial adherence. Gastroenterology. 1994;106(1):35–41. doi: 10.1016/s0016-5085(94)94155-6. [DOI] [PubMed] [Google Scholar]
- 48.Tache Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 49.Takadanohara H, Catanzaro R, Chui de H, He F, Yadav H, Ganguli A, Sakata Y, Solimene U, Minelli E, Kobayashi R, Nagamachi Y, Marotta F. Beneficial effect of a symbiotic preparation with S. boulardii lysate in mild stress-induced gut hyper-permeability. Acta bio-medica : Atenei Parmensis. 2012;83(3):208–216. [PubMed] [Google Scholar]
- 50.Tran L, Chaloner A, Sawalha AH, Greenwood Van-Meerveld B. Importance of epigenetic mechanisms in visceral pain induced by chronic water avoidance stress. Psychoneuroendocrinology. 2013;38(6):898–906. doi: 10.1016/j.psyneuen.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 51.Velin AK, Ericson AC, Braaf Y, Wallon C, Soderholm JD. Increased antigen and bacterial uptake in follicle associated epithelium induced by chronic psychological stress in rats. Gut. 2004;53(4):494–500. doi: 10.1136/gut.2003.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vicuna EA, Kuttappan VA, Galarza-Seeber R, Latorre JD, Faulkner OB, Hargis BM, Tellez G, Bielke LR. Effect of dexamethasone in feed on intestinal permeability, differential white blood cell counts, and immune organs in broiler chicks. Poultry science. 2015;94(9):2075–2080. doi: 10.3382/ps/pev211. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z, Ocampo MA, Pang RD, Bota M, Bradesi S, Mayer EA, Holschneider DP. Alterations in prefrontal-limbic functional activation and connectivity in chronic stress-induced visceral hyperalgesia. PloS one. 2013;8(3):e59138. doi: 10.1371/journal.pone.0059138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watson CJ, Rowland M, Warhurst G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. American journal of physiology Cell physiology. 2001;281(2):C388–397. doi: 10.1152/ajpcell.2001.281.2.C388. [DOI] [PubMed] [Google Scholar]
- 55.Xu D, Gao J, Gillilland M, 3rd, Wu X, Song I, Kao JY, Owyang C. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology. 2014;146(2):484–496. e484. doi: 10.1053/j.gastro.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu YB, Yang J, Zuo XL, Gao LJ, Wang P, Li YQ. Transient receptor potential vanilloid-1 (TRPV1) and ankyrin-1 (TRPA1) participate in visceral hyperalgesia in chronic water avoidance stress rat model. Neurochemical research. 2010;35(5):797–803. doi: 10.1007/s11064-010-0137-z. [DOI] [PubMed] [Google Scholar]
- 57.Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55(11):1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng G, Wu SP, Hu Y, Smith DE, Wiley JW, Hong S. Corticosterone mediates stress-related increased intestinal permeability in a region-specific manner. Neurogastroenterology and motility. 2013;25(2):e127–139. doi: 10.1111/nmo.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]



