Abstract
Background
Functional neuroimaging techniques can provide a unique window into the neural basis of language recovery after a stroke. The functional neuroimaging literature on post-stroke language recovery is complex; multiple factors such as the time post-stroke, degree of initial impairment, nature of the task, and lesion location and size, influence recovery patterns. Some of these factors may not be applicable across different stroke participants, and therefore, influence recovery trajectories in vastly different manners across patients.
Aims
The aim of this paper is to examine longitudinal changes in brain activation patterns of reading and naming recovery in participants with posterior cerebral artery (PCA) strokes with varying degrees of initial language impairment.
Methods & Procedures
Five participants with PCA strokes and 5 healthy controls underwent language testing and functional MRI with a covert reading task and an overt picture-naming task. Stroke participants underwent language testing and scanning at the three time points: 2–5 weeks (T1, subacute phase), 4–7 months (T2, chronic phase), and 11–13 months (T3, chronic phase). Healthy controls underwent language testing and fMRI once.
Outcomes & Results
Language testing indicated that there were varying degrees of reading and naming recovery or decline from the subacute to the chronic phase. With regard to task-based fMRI, we found that for most participants, naming consistently activated a diffuse bilateral network of frontal, temporal, parietal, and occipital regions across the three time points. In contrast, for the reading task, functional activation across the three time points was more left lateralized with a right to left shift in peak activation from the subacute to the chronic phase.
Conclusions
These results indicate that the patterns of activation during language processing is highly dependent on the task and phase of recovery, and these results may have implications for neurally targeted non-invasive brain stimulation techniques.
Keywords: Stroke recovery, reading, naming, fMRI
Introduction
Stroke leaves hundreds of thousands of survivors with lasting disabilities and deficits across an array of cognitive and motor functions (Hillis & Tippett, 2014; Langhorne, Coupar, & Pollock, 2009). Aphasia is among the most well-documented and commonly encountered cognitive impairments after a stroke. Major scientific efforts continue to be made to understand the mechanisms of language recovery in the days and months following a stroke. A quick survey of the extant literature reveals several potential recovery processes that operate at distinct time periods with a striking degree of individual heterogeneity (for a detailed review see Anglade, Thiel, & Ansaldo, 2014). This complexity of recovery, along with considerable individual variability, often makes it difficult to obtain fully generalizable theories of language recovery that can be used to develop effective treatments to facilitate recovery. The principal recovery mechanisms at play in language recovery are reperfusion of affected areas, recovery from diaschisis (dysfunction in a brain region due to loss of long-range inputs from lesioned areas), and neuroplastic functional reorganization (Jarso et al., 2013; Thiel & Zumbansen, 2016). Reperfusion is generally responsible for early reversal of language deficits, whereas neuroplastic reorganization of language functions is thought to govern later, more gradual recovery (e.g., Anglade, Thiel, & Ansaldo, 2014; Marsh & Hillis, 2006).
Functional neuroimaging work suggests that neuroplastic reorganization will occur by either (1) recruiting the cortex adjacent to the lesion in the left hemisphere, or (2) engaging the intact right hemisphere homologues of the affected left hemisphere regions (for reviews see Gainotti, 2015; Saur & Hartwigsen, 2012). The exact role of right hemisphere in stroke recovery is still an active area of discussion, though it is clear that right hemisphere activation should not be characterized as necessarily detrimental to recovery nor necessarily beneficial. Moreover, we have previously reported data showing that functional connectivity between right and left hemispheres, rather than observed activity in the right hemisphere, correlated with language recovery (Sebastian et al, 2016). There is no consensus on a single pathway that can explain the recovery trajectories for a majority of stroke patients, and operative mechanisms of neuroplastic reorganization have not been fully clarified.
Another potential factor that may further complicate studying language recovery after stroke centers on the issue of the type of language task (Sebastian & Kiran, 2011). The type of task used to assess language processing during neuroimaging is likely to have a major impact on brain activation patterns. We intuitively would not expect language tasks across different domains or difficulty levels to activate the same brain areas to the same extent. In general, we assume that a stroke participant or a healthy control would not activate the same brain regions in the same way for two different language functions. Even within a single language domain, we would anticipate divergent activation patterns if two tasks demand dissimilar levels of cognitive investment. For example, asking someone to read the word dog should not be functionally identical to asking someone to read and understand an archaic sentence of old English.
It is already well documented that reading and naming have some shared underlying cognitive architecture but do not use all of the same brain areas (Price, 2012). Reading words activates the left angular gyrus, supramarginal gyrus, left fusiform gyrus, left inferior prefrontal gyrus, and occipital cortex (e.g., Joubert et al., 2004), whereas naming pictures activates a more bilateral network including frontal, temporal, parietal, and occipital regions (e.g., Price et al., 2005). Functional imaging experiments by Price and colleagues have suggested that activation is higher during picture naming than reading in healthy participants (Price & Devlin, 2003).
These points about language tasks are relevant to studying language recovery after stroke, because different language tasks might show dramatically different trajectories of recovery over time. Despite the intuitive need to investigate differences between language tasks, very few of the handful of longitudinal neuroimaging studies of language recovery have fully investigated this issue. Some studies make claims about language reorganization in general, despite only studying a single task. Without exploring differences in language recovery trajectories for separate language tasks, it is difficult to arrive at a critically needed complete conception of language recovery that can be used to inform the development and application of new aphasia treatments.
In this paper, we begin to address this knowledge gap by conducting longitudinal task-based fMRI of two language tasks in individual stroke patients across a recovery period. The aims of this study are to: 1) examine the neural basis of recovery of reading and naming in participants with left posterior cerebral artery (PCA) strokes; and 2) compare neural activation patterns during reading and naming in participants with language impairment to determine potential differences in recovery across tasks. We hypothesize that overt picture naming will involve a distributed bilateral network including frontal, temporal, parietal, and occipital regions at all time points, consistent with our previous findings (Sebastian et al., 2016). In contrast, we hypothesize that reading will show more lateralization and more restricted activation to occipitotemporal and frontal areas, based on previous imaging studies.
Methods
Participants
Five participants (1 female) with ischemic left hemisphere stroke participated in this study. All patients were right-handed prior to the stroke. Participants were in the subacute phase post stroke (mean days post stroke = 23 days, SD = 3.9). These participants are part of an ongoing longitudinal study, the Stroke Outcome and REcovery (SCORE) project. Participants were between 46 and 64 years in age (mean age = 55.6 years, SD = 6.8). All stroke participants had completed at least 12th grade education (mean education = 13.2 years, SD = 1.7). Exclusion criteria for stroke participants included: known uncorrected visual or hearing loss, primary hemorrhage on imaging, reduced level of consciousness or sedation, contraindication for MRI, and previous neurological disorders affecting the brain or psychiatric disorders (other than depression).
Five (1 female) healthy controls were also recruited for this study. Controls were all right handed and were between 52 and 62 years in age (mean age = 56.6, SD = 3.9). All controls had completed at least 12th grade education (mean education = 14.4 years, SD = 2.6). Exclusion criteria included known uncorrected visual or hearing loss, neurological disorders such as stroke, Parkinson’s disease, Alzheimer’s disease, psychological illness, learning disability, and attention deficit disorders. There was no significance difference in age (U = 11, p = 0.83) and education (U = 11, p = 0.83) between the stroke participants and controls. All participants provided written informed consent under the Human Subjects Protocol for the Johns Hopkins University School of Medicine.
All stroke participants had lesions involving the left PCA territory (see Figure 1 for lesion information). P1, P4, and P5 had lesions involving the left thalamus; P2’s lesion was in the left occipital cortex, and P4’s lesion included the occipital cortex, fusiform, lingual gyrus, and splenium. P2 also had lesions in the right occipital, parietal and cingulate regions. However, the participant did not have evidence of hemispatial neglect, left visual field cut, left motor or sensory deficits, or left sided visual, motor, or sensory extinction to double simultaneous stimulation on detailed neurological exam. Stroke participants underwent language testing at three time points: 2–5 weeks (T1), 4–7 months (T2), and 11–13 months (T3) post stroke. Controls underwent language testing once. Language tests included the Boston Naming Test-short (BNT, Kaplan, Goodglass, & Weintraub, 2001; Mack, Freed, Williams, & Henderson, 1992), Hopkins Action Naming Test (HANA, Breining et al., 2015), and Johns Hopkins University (JHU) Dyslexia Battery: Regularity subtest (Beeson & Hillis, 2001; Goodman & Caramazza, 1986). The HANA consists of 30 black and white pictures of actions, with names matched in surface frequency and length to the object names in the Boston Naming Test. The Regularity subtest of the JHU Dyslexia Battery consists of 90 words and 50 nonwords matched in length to the words. The 90 words include: 30 regular consistent words (15 high frequency and 15 low frequency), 30 regular inconsistent words (15 high frequency and 15 low frequency), and 30 exception words (15 high frequency and 15 low frequency). Regular, irregular, and exception words are matched in length and frequency. The nonwords consisted of 25 nonhomophones and 25 pseudohomophones. Nonwords were created by changing one letter of a real English word. Responses to nonwords are scored as correct if the reader produces any pronunciation that is plausible based on orthography-phonology conversion. For example, jear might be correctly read as /ʤɝ/ rhyming with bear or jeer. Participants underwent additional assessments with standardized and unstandardized language tests. Given the focus of this study is on reading and naming recovery, we are only reporting results for the following language tests: JHU Dyslexia Battery (word and non word), BNT, and HANA. All stroke participants, with the exception of P5, had varying levels of naming and reading deficits at the subacute phase. P5 only had mild word retrieval deficits at the conversational level and his action naming accuracy was lower than his subsequent action naming. There is some overlap in the naming data between the present study and a previous study from our group (Sebastian et al., 2016).
Figure 1.

Lesion maps for the stroke participants based on the Diffusion Weighted Imaging (DWI) scans.
Imaging
All stroke participants underwent imaging at the three time points: 2–5 weeks (T1), 4–7 months (T2), and 11–13 months (T3) post stroke. Imaging and language testing was done on the same day. Controls were scanned once. Scans were acquired on a 3-T Philips Achieva MRI scanner, equipped with a 32-channel head coil. The data that are relevant to this work include the following: magnetization prepared rapid gradient echo (MPRAGE) high-resolution anatomical scan and blood-oxygenation-level dependent (BOLD) functional scan. MPRAGE scans were acquired as 170 sagittal slices, using a multishot, turbo field echo pulse sequence with an in-plane resolution of TR = 6800 ms, TE = 3.1 ms, inversion time (TI) = 850 ms, slice thickness = 1 mm. Functional MRI scans were acquired using EPI sequences in the axial plane (TR = 2000 ms, TE = 30 ms, flip-angle = 90°, FOV = 240×240mm, 35 axial slices, 3×3 mm in plane resolution, 3 mm slice thickness).
Tasks
Participants underwent two fMRI experiments: reading and naming. Participants were trained outside the MRI scanner using a short form of the in scanner task on a laptop. During training, each participant was asked to overtly perform the tasks (reading and naming) in order to determine performance accuracy. However, during the actual fMRI scans, participants were asked to silently perform the reading task. Participants who showed less than 60% accuracy on a specific task during training were excluded from data acquisition using that task.
For the reading and naming experiments, all of the stimuli were frequent and highly imageable nouns. The stimuli for reading and naming were equated for number of syllables, frequency of occurrence (Kučera & Francis, 1983), length, and imageability (Gilhooly & Logie, 1980). See Supplementary material S1 for details.
The reading experiment involved three conditions: typeface words, checkerboards, and a fixation cross. This paradigm was adapted from the work of Cohen et al. (2002). Each condition was presented in blocks of 20 seconds. For the word and checkerboard conditions, 10 visual stimuli were presented for 500 milliseconds (ms) each, with a 1500 ms fixation interval between items. For the word condition, participants were asked to read each word covertly. For the checkerboard and fixation conditions, participants were instructed to passively attend to all stimuli. Data were acquired across two runs; each run was 4 min and 46 seconds. A covert reading paradigm was chosen to avoid lengthening the inter-trial-interval to accommodate verbal response durations. This allowed us to maintain a block design and also be in line with the original experimental design (Cohen et al., 2002) from which ours was adapted.
The naming experiment involved a cued picture-naming task. This paradigm was adapted from the work of Holland et al. (2011). In the cued picture-naming task, each picture was concurrently presented with an auditory cue in the form of the whole word, the initial phoneme, or auditory noise. The naming experiment involved four conditions: phonemic cued picture naming, whole word cued picture naming, noise cued picture naming, and scrambled picture viewing. A total of 60 pictures were presented and each picture was preceded by a fixation cross for 500 ms and was then displayed for 3500 ms. Participants were asked to overtly name the picture. For the control condition, participants were asked to passively view the scrambled pictures. Trials were presented in blocks of six pictures, separated by the control condition, which was displayed for 7 seconds. There were 10 blocks of picture naming and 10 blocks of scrambled picture viewing in each run. Data were acquired across two runs; each run was 5 minutes and 24 seconds. For a detailed description of the naming task, see Sebastian et al. (2016).
Imaging Analyses
fMRI data were analyzed using FMRIB’s software library (FSL) version 6.0 (Smith et al., 2004; Woolrich et al., 2009). Pre-statistical processing included: non-brain removal (BET); motion correction (MCFLIRT); spatial smoothing (FWHM= 5 mm); and high-pass temporal filtering. Each participant’s fMRI data was registered to their high-resolution MPRAGE using Boundary-Based Registration (BBR) implemented in FSL (Greve & Fischl, 2009) and the high resolution MPRAGE was registered to the MNI standard space using FMRIB’s nonlinear image registration tool (FNIRT; Andersson, Jenkinson, & Smith, 2010; Jenkinson et al., 2012). Registration quality was checked by visual inspection.
General linear model implemented in FSL was used to analyze the BOLD images. The three conditions for the reading task were: words, checkerboards, fixation and the four conditions for the naming task were: phonemic cued naming, word cued naming, noise cued naming, and scrambled picture viewing. Each condition was modeled separately. For the naming task, only correct trials where included in the analysis. Incorrect trials were assigned to a separate regressor of no-interest. For the reading task, the main contrast of interest was passive reading vs. passive checkerboards viewing. For the naming task, the main contrast of interest was phonemic+word cued naming vs. scrambled picture viewing. In all analyses the contrasts between conditions were estimated on the first level for each run, and then the two runs for each participant were combined using a fixed effects model in order to produce an average activation map for each individual. Higher level group analysis for stroke participants and controls were carried out using FMRIB’s Local Analysis of Mixed Effects (Beckmann, Jenkinson, & Smith, 2003; Woolrich, Behrens, Beckmann, Jenkinson, & Smith, 2004). We used cluster level thresholding as implemented in FSL FEAT, with an initial Z > 2.3 (corrected) and p = 0.05 in all analyses.
Results
Behavioral
All healthy controls scored above 90% accuracy on the JHU dyslexia battery, BNT, and HANA. At the subacute phase, participants with stroke had varying levels of reading and naming deficits and showed varying degrees of change over time (reported in Table 1). To summarize:
Table 1.
Percent accuracy on naming and reading tests for the stroke participants.
| Participant | Time point | JHU dyslexia battery | Naming | ||
|---|---|---|---|---|---|
| Word | Nonword | BNT | HANA | ||
| P1 | T1 | 97 | 80 | 87 | 82 |
| T2 | 97 | 88 | 80 | 77 | |
| T3 | 86* | 74 | 86 | 77 | |
| P2 | T1 | 86 | 66 | 73 | 66 |
| T2 | 83 | 72 | 77 | 74 | |
| T3 | 78 | 48* | 60 | 63 | |
| P3 | T1 | 26 | 13 | 47 | 71 |
| T2 | 94 | 94 | 100 | 94 | |
| T3 | 98** | 96** | 97** | 97** | |
| P4 | T1 | 84 | 50 | 57 | 57 |
| T2 | 87 | 60 | 63 | 43 | |
| T3 | 81 | 30* | 70 | 77** | |
| P5 | T1 | 100 | 98 | 96.6 | 94.2 |
| T2 | 100 | 100 | 93.3 | 100 | |
| T3 | 100 | 100 | 100 | 100 | |
Significant decline by chi squared tests (see text)
Significant improvement by chi squared tests (see text)
T1: 2–5 weeks; T2: 4–7 months; T3: 11–13 months
P1 had mild reading (nonword) and naming deficits at T1. P1 continued to have mild reading and naming deficits at T2 and T3, with a decline in reading both words and nonwords at T3. Only his decline in word reading was a significant change (X2 =7.8; p = 0.005).
P2 had mild reading (word and nonword) and naming deficits at T1. He read words significantly better than nonwords (X2 =11.0; p= 0.0009). P2 continued to have mild deficits at T2, followed by a decline in reading and naming at T3. Only his decline in nonword reading was a significant change (X2 =6.6; p = 0.01).
P3 had moderate to severe reading and naming deficits at T1. P3 recovered from much of her reading and naming deficits by T2 and this recovery persisted to T3. Improvements were significant for word reading (X2 =110; p < 0.00001), nonword reading (X2 =139; p<0.00001), object naming (X2 =62; p<0.00001), and action naming (X2 =25; p< 0.00001).
P4 had moderate naming deficits and mild to moderate reading deficits at T1. Reading performance was markedly better for words compared to nonwords (X2 =26; p<0.00001). P4 showed significant improvement in naming actions (from 57% to 77% correct; X2 =9.1; p = 0.003) and nonsignificant improvement in naming objects (from 57% to 70%) by T3. In reading nonwords, he showed a slight improvement at T2, followed by a marked decline in accurate responses at T3 (X2 =8.3; p= 0.004). However, at T3, 50% of his responses at T3 were “don’t know” responses, indicating he just made less of an effort to read nonwords at T3.
P5 had normal word reading and confrontational naming scores; however, he had mild word retrieval deficits during conversation and slightly lower scores on action naming at T1, compared to T2 and T3. Follow up assessments at T2 and T3 revealed normal language skills, even in conversation.
Imaging
Healthy controls
The in scanner naming accuracy for the controls was 100%. For the reading task, group level whole brain analysis for the contrast ‘word reading versus checkerboards viewing’ revealed activation in the left lateral occipital cortex and fusiform gyrus. For the cued picture-naming task, whole brain analysis for the contrast ‘phonemic+word cued naming compared to scrambled picture viewing’ revealed robust bilateral activation. Compared to the reading task, there was a marked increased in activation bilaterally (Figure 2). The two largest clusters were identified bilaterally in the frontal cortex including the inferior frontal gyrus and precentral gyrus. Peak activation for the frontal cluster was located in the precentral gyrus bilaterally. This was followed by clusters in the bilateral temporal cortex. Activated regions included the superior and middle temporal gyrus, Heschl’s gyrus, and planum temporale, with peak activation located in the left superior temporal gyrus. Other regions involved during the cued picture-naming task included the left supramarginal gyrus, bilateral cerebellar and occipital cortex. All images are thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of P = 0.05. See supplementary material S4 for activation coordinates.
Figure 2.
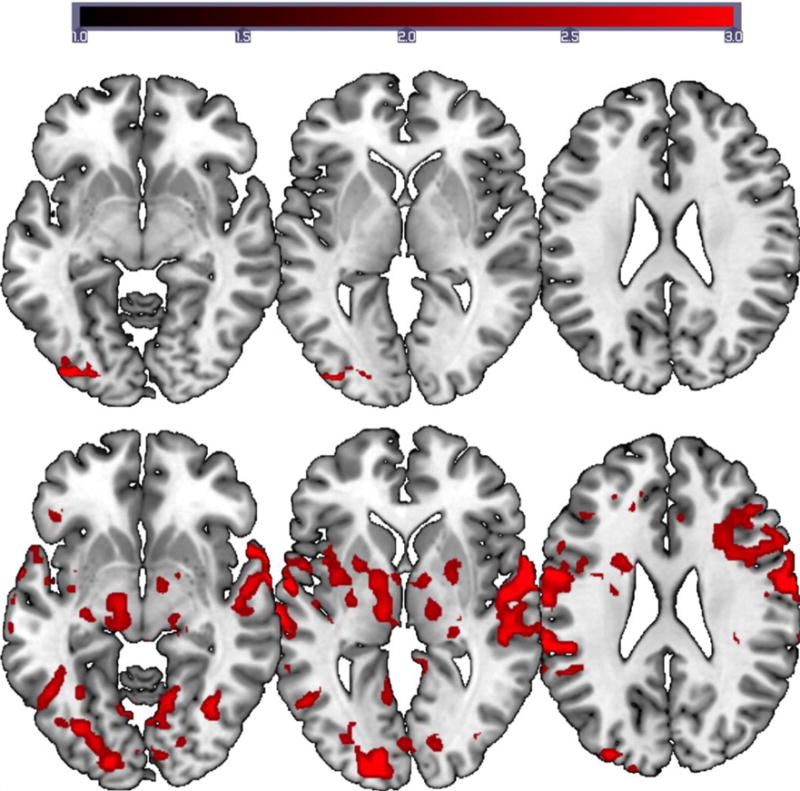
Group activation maps for the reading (top) and naming tasks (bottom) for the normal controls. FMRI data registered in MNI space shows areas of activation associated with word reading compared to viewing checkerboards and correct picture naming (phonemic+word cued naming) compared to viewing scrambled pictures. Images are displayed on MNI brain using MRIcroGL. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of p = 0.05. All images are shown in neurological convention (left in image is left in the brain). The scale on the intensity bar represents the Z scores.
Stroke participants (Group level
All stroke participants’ accuracy was greater than 75% for the naming task. Group level activation for the reading task at T1 showed bilateral occipital, cerebellar, and inferior temporal/fusiform gyrus activation. The peak activation was located in the right occipital cortex and fusiform gyrus (homologous to the peak for healthy controls). At T2, the activation pattern was bilateral; however, there was an increase in left hemisphere activation especially in the occipital/parietal cortex. Activation was also observed in the left precentral gyrus. There was a shift in peak activation from the right hemisphere to the left hemisphere at T2, with peak activation located in the left occipital pole. At T3, activation pattern was left lateralized with peaks in the occipital cortex and fusiform region. The group level reading activation data at T3 was very similar to the normal controls’ group data. At T3, the peak activation for the stroke participants and the normal controls was noted in the left lateral occipital cortex/fusiform gyrus. See Figure 3 for activation maps and supplementary material S4 for activation coordinates.
Figure 3.
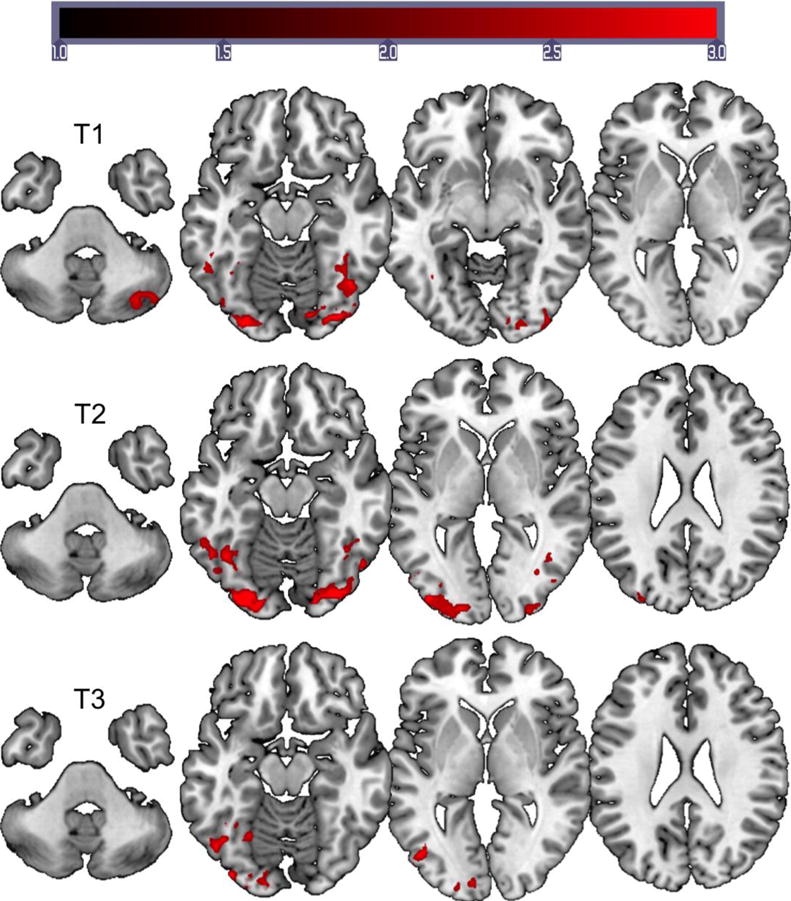
Group activation maps for the reading task for the stroke participants. FMRI data registered in MNI space shows areas of activation associated with passive word reading compared to viewing checkerboards. Top images show activation data for T1 (2–5 weeks), middle images show activation data for T2 (4–7 months), and bottom images show activation data for T3 (11–13 months). Images are displayed on MNI brain using MRIcroGL. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of p = 0.05. All images are shown in neurological convention (left in image is left in the brain). The scale on the intensity bar represents the Z scores.
Group level activation for the naming task at the three time points revealed bilateral activation involving the left frontal, temporal, parietal, and occipital regions (Figure 4). Cerebellar activation was noted at T1 and T2. Activation patterns were very similar for T1and T2; however, there was an overall reduction in activation bilaterally at T3. The peak activation for naming across the three time points was in the left superior temporal gyrus. A marked increase in overall activation was noted for the naming task compared to the reading task.
Figure 4.
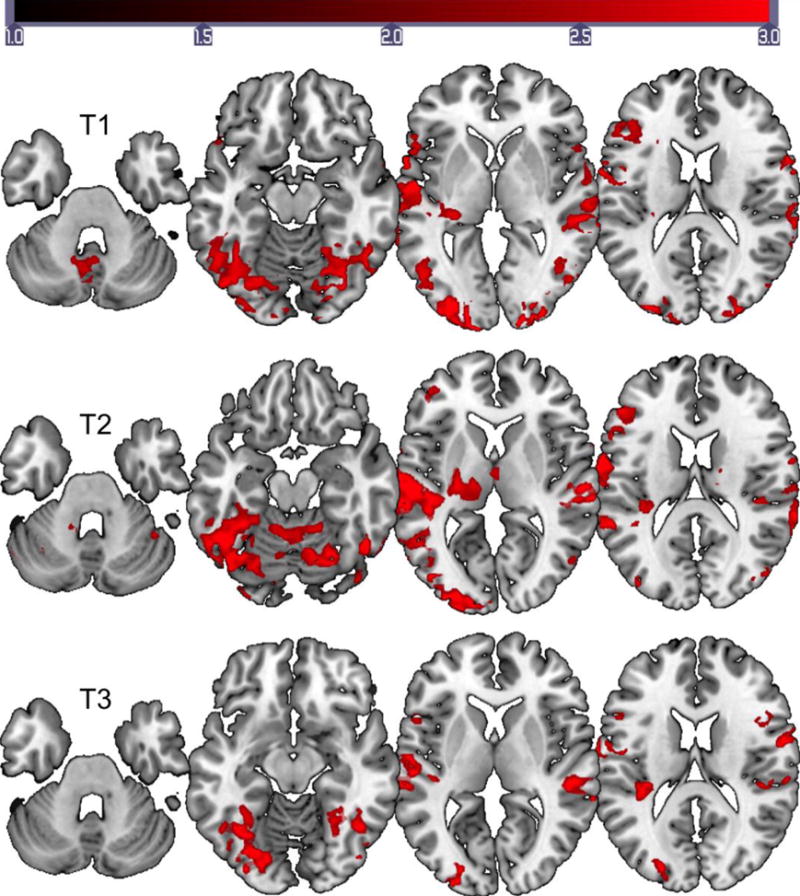
Group activation maps for the naming task for the stroke participants. FMRI data registered in MNI space shows areas of activation associated with correct picture naming (phonemic+word cued naming) compared to viewing scrambled pictures. Top images show activation data for T1 (2–5 weeks), middle images show activation data for T2 (4–7 months), and bottom images show activation data for T3 (11–13 months). Images are displayed on MNI brain using MRIcroGL. All images are shown in neurological convention (left in image is left in the brain). The scale on the intensity bar represents the Z scores.
Stroke participants (Individual activation patterns)
Individual activation patterns were very similar to the group level; however, differences were observed based on the severity of impairment, especially for the reading task. See supplementary materials S2 and S3 for individual activation patterns. Increased right hemisphere activation was noted for participants with more pronounced reading deficits at T1 (e.g., P3). P1, who had a mild reading deficit at T1, showed a predominantly left lateralized activation for the reading task including the left inferior frontal gyrus, bilateral lateral occipital cortex/fusiform gyrus, left inferior temporal gyrus, left occipital pole, and bilateral cerebellum. Peak activation was noted in the left occipital/fusiform gyrus. At T3, P1 showed a slight decline in reading skills. But P1 continued to show a left lateralized activation including the left inferior/middle frontal gyrus, precentral gyrus, lateral occipital cortex/fusiform gyrus, and inferior temporal gyrus. There was a shift in peak activation from the left occipital/fusiform gyrus (T1) to left middle frontal gyrus (T3).
P3, who had a moderate reading deficit at T1, showed activation in the left inferior frontal gyrus, left superior, middle, and inferior temporal gyrus, left lateral occipital cortex, and right superior and middle temporal gyrus. Peak activation was located in the left middle frontal gyrus. P3 showed a complete recovery of her reading impairment at T3. Activation was noted in the left occipital/fusiform cortex (perilesional region for P3), left inferior temporal gyrus, and right lateral occipital cortex. There was a shift in peak activation from the left middle frontal gyrus (T1) to the left occipital/fusiform cortex (T3).
Activation data for the naming task was bilateral and there was no notable difference in activation patterns based on the level of impairment, with the exception of P4. P4 also showed bilateral activation at all three time points; however, greater right hemisphere activation was noted at T1, followed by an increase in left hemisphere activation at follow up (T2 and T3).
Discussion
Our main aim was to compare the task-based activation patterns for reading and naming in PCA stroke participants to assess potential similarities and differences in recovery from the subacute (T1) to chronic time points (T2 and T3). Behavioral assessments showed varying levels of reading and naming impairments at the subacute time point, with different rates of recovery/decline across patients and across time periods. Even two individuals (e.g., P1 and P5) with very similar left thalamic ischemic strokes show very different outcomes one year later (T3).
Imaging analyses in the controls showed that the neural activation patterns associated with passive reading were unsurprisingly distinct from activation patterns associated with overt naming. The reading task recruited the left lateral occipital/fusiform gyrus, whereas the overt naming task recruited a bilateral network of regions in frontal, temporal, occipital, and parietal cortex. This bilateral activation likely reflects the nature of the task, which includes visual recognition, hearing one’s own response, and motor speech, in addition to semantic processing and word-retrieval.
For the stroke participants, there was a shift in peak activation for the reading task from the right lateral occipital and fusiform cortex at the 2–5 weeks time point (T1) to the left hemisphere lateral occipital cortex and fusiform gyrus at the 11–13 months time point (T3), whether or not they improved in reading. The left fusiform gyrus has been the focus of numerous neuroimaging studies that have reported consistent activation selective to reading (e.g., Dehaene, Le Clec, Poline, Le Bihan, & Cohen, 2002; Dehaene & Cohen, 2011; Glezer, Jiang, & Riesenhuber, 2009; Martin, Schurz, Kronbichler, & Richlan, 2015). Naming, on the other hand, showed greater activation levels and consistently recruited a bilateral network of regions in frontal, temporal, occipital, parietal, and cerebellar areas with peak activations in left superior temporal gyrus, the left lateral occipital cortex, and left precentral gyrus (similar to healthy controls). At the group level, there was an overall decrease in intensity in activation across the three time points. However, there was no discernible shift in peak activation from the right hemisphere to the left hemisphere, even for the individual who showed marked improvement in naming (P3). However, in all cases, when participants showed improvement, their activation pattern was closer to the pattern observed in the controls. For example, P3 showed a shift in peak activation from the left middle temporal to left occipital and fusiform cortex during reading, associated with the recovery of reading.
Two patients (P2 and P4) showed a significant decline in nonword reading skills at T3. Decline was also noted for word reading; however, it was less pronounced than nonword reading. P1 also showed a significant decline in word reading. This marked decline in nonword reading could be a potential marker for the onset of decline in other cognitive functions (Hillis, Benzing, Epstein, & Lyketsos, 1995). In our study, P4’s family members reported decline in cognitive functions about six months after the completion of data collection. The patient was diagnosed with probable vascular dementia by a neurologist at that time. Another potential reason for this decline could be due to the lack of speech and language therapy. The three patients (P1, P2, and P4) did not continue with speech and language therapy after discharge from acute care, where as P3 and P5 continued with speech and language therapy on a regular basis even after discharge from acute care.
The results of the imaging data emphasize the considerable differences in activation patterns for the reading and naming task. The diffuse activation network for the overt picture naming task is consistent with the cognitive complexity of this foundational language function. Naming requires several intact cognitive processes such as recognition of the visual stimulus, access to the meaning of the visual stimulus, access to the phonological word form, and motor programming and planning of articulation. Functional neuroimaging studies show widespread activation of left perisylvian cortex, including posterior inferior frontal gyrus, posterior temporal fusiform gyrus, mid and posterior superior and middle temporal gyri, right cerebellum, and thalamus during naming (for reviews see Indefrey and Levelt, 2004; Price, 2012; Race & Hillis, 2015). Naming is one of the most commonly impaired language functions after a left hemisphere stroke and damage to any of the regions involved could result in a naming deficit, albeit at different steps of the process. The broad activation network we see in the group level analysis is consistent with, and is reflective of, the cognitive complexity of picture naming. Furthermore, the group-level activation was largely stable, recruiting similar regions for each of the three time points without a left-right shift as was seen for reading.
The covert reading task, which did not require spoken word production, activated a more restricted network of mostly occipital/fusiform area and did display a much clearer right-left shift across the three time points. The “left to right shift” in reading, which may actually reflect reactivation of the left over time, was not clearly associated with improvement in reading. The participants variously improved, declined, or remained fairly stable in reading performance. Reading changed mostly for pseudowords. However, in all individuals who read words relatively well, activation associated with reading was more lateralized than naming, and involved left or right occipital temporal regions (but not necessarily both homologous regions). It may be the case that either left or right occipitotemporal cortex can support silent reading, while both hemispheres are necessarily engaged in spoken naming. The activation patterns, with respect to the involvement of each hemisphere for the reading task, are very similar to a previous longitudinal fMRI study of language recovery in stoke patients using an auditory comprehension task (Saur et al., 2006). It should be noted that the Saur et al study focused on recovery from the acute to the chronic phase, where as our study focused on recovery from the subacute to the chronic phase.
In summary, the important result to stress is that the two tasks elicited divergent changes in activation patterns from the subacute to the chronic phase post stroke, whether individuals improved or declined in the tasks. These results highlight the fact that there may not be a single unitary recovery path for all language functions after stroke. Task-based fMRI activation will differ based on the type of task used, the time after stroke at which the scans are taken, and the brain regions damaged by the stroke. These factors all complicate the question of how to assess stroke recovery based on MR neuroimaging, and current MR-based models of language recovery may only describe the recovery dynamics of restricted segments of the stroke population for a particular type of task at a specific time interval during recovery.
Limitations
There are several limitations to this study. First, we investigated a relatively small sample size to study longitudinal recovery of reading and naming in patients with relatively similar lesions (involving the left PCA territory). Even greater differences across individuals would likely be observed in participants with damage to one or more of the perisylvian regions critical for naming. Second, because none of our participants has a lesion in the MCA territory, it is unclear if patients with left MCA stroke (which is more common than PCA stroke) would show such marked differences in activation patterns over time across tasks of reading and naming, as we observed in PCA stroke. Third, there were inherent differences in the language tasks utilized. Reading required a covert response, whereas naming required an overt response. We did not require a verbal response from the participants for the reading task so as to avoid lengthening the inter-trial-interval to accommodate verbal response durations thus maintaining a block design; this is in keeping with the original experimental design from which ours was adapted (Cohen et al., 2002). Overt tasks such as the naming task employed in this study are often effortful, particularly for patients with naming deficits. Brain activation measured using complex, effortful tasks could, at least in part, reflect error monitoring or cognitive strategies rather than language processing per se. Effort related activation might be reduced in this study because we used a cued naming task.
Implications
Results of this study support the proposal that neuroimaging of language recovery in the future should be approached with a diversity of language tasks in order to track the different aspects of language that recover. Different conclusions about changes in lateralization of language might be drawn from neuroimaging studies of different tasks. The variations in brain activation patterns across tasks and time points post stroke may have implications for neurally targeted interventions, such as transcranial direct current stimulation and transcranial magnetic stimulation. Several ongoing studies are investigating whether or not stimulating (or inhibiting) right or left hemisphere ensures a positive response to treatment or improves outcome. Our results indicate that the optimum hemisphere to target might vary for reading versus naming, or for subacute versus chronic stroke. Stimulation of perilesional areas or stimulation or inhibition of right hemisphere regions may all facilitate language recovery, depending on the task that is targeted in speech and language therapy and the time post-stroke.
Supplementary Material
Acknowledgments
We would like to thank Amy Wright, Cameron Davis, Jeremy Purcell, Samson Jarso, and Joseph Posner for their assistance with data collection. The research reported in this paper was supported by the National Institutes of Health (National Institute on Deafness and Other Communication Disorders) through award R01 DC05375, P50 DC014664, and K99 DC015554. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
References
- Andersson JL, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation. Oxford, UK: FMRIB Analysis Group of the University of Oxford; 2010. (FMRIB technical report TR07JA2). [Google Scholar]
- Anglade C, Thiel A, Ansaldo AI. The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: a critical review of literature. Brain Injury. 2014;28(2):138–145. doi: 10.3109/02699052.2013.859734. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Beeson PM, Hillis AE. Comprehension and production of written words. In: Chapey R, editor. Comprehension and Production of Written Words. 4th. Baltimore, MD: Williams and Wilkin; 2001. [Google Scholar]
- Breining BL, Tippett DC, Davis C, Posner J, Sebastian R, Oishie K, Hillis AE. Assessing dissociations of object and action naming in acute stroke; Paper presented at the Clinical Aphasiology Conference; Monterey, CA. 2015. [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125(5):1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends in cognitive sciences. 2011;15(6):254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec’H G, Poline JB, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 2002;13(3):321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- Gainotti G. Contrasting opinions on the role of the right hemisphere in the recovery of language. A critical survey. Aphasiology. 2015;29(9):1020–1037. doi: 10.1080/02687038.2015.1027170. [DOI] [Google Scholar]
- Gilhooly KJ, Logie RH. Age-of-acquisition, imagery, concreteness, familiarity, and ambiguity measures for 1,944 words. Behavior Research Methods & Instrumentation. 1980;12(4):395–427. doi: 10.3758/BF03201693. [DOI] [Google Scholar]
- Glezer LS, Jiang X, Riesenhuber M. Evidence for highly selective neuronal tuning to whole words in the “visual word form area”. Neuron. 2009;62(2):199–204. doi: 10.1016/j.neuron.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Caramazza A. The Johns Hopkins University Dyslexia Battery. Baltimore, MD: Johns Hopkins University; 1986. Unpublished. [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Benzing L, Epstein C, Lyketsos C. Cognitive gains and losses of patients with Alzheimer's Disease during frequent practice. American Journal of Speech-Language Pathology. 1995;4(4):152–158. doi: 10.1044/1058-0360.0404.152. [DOI] [Google Scholar]
- Hillis AE, Tippett DC. Stroke recovery: Surprising influences and residual consequences. Advances in Medicine. 2014;2014:1–10. doi: 10.1155/2014/378263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland R, Leff AP, Josephs O, Galea JM, Desikan M, Price CJ, Crinion JT. Speech Facilitation by Left Inferior Frontal Cortex Stimulation. Current Biology. 2011;21(16):1403–1407. doi: 10.1016/j.cub.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jarso S, Li M, Faria A, Davis C, Leigh R, Sebastian R, Hillis AE. Distinct mechanisms and timing of language recovery after stroke. Cognitive Neuropsychology. 2013;30(7–8):454–475. doi: 10.1080/02643294.2013.875467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Joubert S, Beauregard M, Walter N, Bourgouin P, Beaudoin G, Leroux JM, Lecours AR. Neural correlates of lexical and sublexical processes in reading. Brain and Language. 2004;89(1):9–20. doi: 10.1016/S0093-934X(03)00403-6. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Austin, TX: Pro-ed; 2001. [Google Scholar]
- Kučera H, Francis WN. Computational analysis of present-day American English. Providence, RI: Brown University Press; 1983. [Google Scholar]
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. The Lancet Neurology. 2009;8(8):741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, Henderson VW. Boston naming test: Shortened versions for use in Alzheimer’s disease. Journal of Gerontology. 1992;47:154–158. doi: 10.1093/geronj/47.3.P154. [DOI] [PubMed] [Google Scholar]
- Marsh EB, Hillis AE. Recovery from aphasia following brain injury: The role of reorganization. Progress in Brain Research. 2006;157:143–156. doi: 10.1016/S0079-6123(06)57009-8. [DOI] [PubMed] [Google Scholar]
- Martin A, Schurz M, Kronbichler M, Richlan F. Reading in the brain of children and adults: A meta-analysis of 40 functional magnetic resonance imaging studies. Human Brain Mapping. 2015;36(5):1963–1981. doi: 10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19(3):473–481. doi: 10.1016/S1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, Moore CJ, Morton C, Laird AR. Meta‐analyses of object naming: Effect of baseline. Human Brain Mapping. 2005;25(1):70–82. doi: 10.1002/(ISSN)1097-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race D, Hillis AE. The Neural Mechanisms Underlying Naming. In: Hillise AE, editor. Handbook of Adult Language Disorders. 2nd. New York, NY: Psychology Press; 2015. [Google Scholar]
- Saur D, Hartwigsen G. Neurobiology of language recovery after stroke: lessons from neuroimaging studies. Archives of Physical Medicine and Rehabilitation. 2012;93(1):S15–S25. doi: 10.1016/j.apmr.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129(6):1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Sebastian R, Kiran S. Task-modulated neural activation patterns in chronic stroke patients with aphasia. Aphasiology. 2011;25(8):927–951. doi: 10.1080/02687038.2011.557436. [DOI] [Google Scholar]
- Sebastian R, Long C, Purcell JJ, Faria AV, Lindquist M, Jarso S, Hillis AE. Imaging network level language recovery after left PCA stroke. Restorative Neurology and Neuroscience. 2016;34(4):473–489. doi: 10.3233/RNN-150621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Thiel A, Zumbansen A. The pathophysiology of post-stroke aphasia: A network approach. Restorative Neurology and Neuroscience. 2016;34(4):507–518. doi: 10.3233/RNN-150632. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modeling for fMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


