Abstract
Glioblastoma is a highly malignant disease in critical need of expanded treatment options. The AURKA inhibitor alisertib exhibits antiproliferative activity against glioblastoma in vitro and in vivo. Unlike current clinically used taxane drugs, the novel taxane TPI 287 penetrates the CNS. We tested for interactions between three selective AURKA inhibitors and TPI 287 against standard U87 and U1242 cells and primary glioblastoma neurospheres using colony formation assays. Bliss and Chou-Talalay analyses were utilized to statistically test for synergism. Morphological analysis, flow cytometry and annexin V binding were employed to examine cell cycle and apoptotic effects of these drug combinations. TPI 287 not only potentiated the cytotoxicity of the AURKA inhibitors alisertib, MLN8054 and TC-A2317, but was often potently synergistic. Morphologic and biochemical analysis of the combined effects of alisertib and TPI 287 consistently revealed synergistic induction of apoptosis. While each agent alone induces a mitotic block, slippage occurs allowing some tumor cells to avoid apoptosis. Combination treatment greatly attenuated mitotic slippage, committing the majority of cells to apoptosis. Alisertib and TPI 287 demonstrate significant synergism against glioblastoma cells largely attributable to a synergistic effect in inducing apoptosis. These results provide compelling rationale for clinical testing of alisertib and/or other AURKA inhibitors for potential combination use with TPI 287 against glioblastoma and other CNS neoplasms.
Keywords: TPI 287, alisertib, apoptosis, synergy, glioblastoma
Introduction
Glioblastoma is the most common malignant primary brain tumor of adults [1]. Due to its pervasive and highly invasive growth characteristics it is not amenable to surgical resection. Standard treatment includes surgical debulking followed by radiation and alkylating agent chemotherapy. Although newer glioblastoma treatments have shown promise, average overall survival remains only 15 months [2]. Development of new treatments is therefore critical.
Aurora-A kinase (AURKA) is a serine-threonine kinase that plays multiple key roles in the cell, including maintenance of stemness and regulating mitotic spindle formation [3, 4]. AURKA inhibitors may cause senescence, differentiation and/or apoptosis of tumor cells [5–7]. Treatment of glioblastoma cells, including tumor stem cells, with AURKA inhibitors inhibits their growth in vitro and in vivo [7–9]. AURKA inhibitors also potentiate the effects of ionizing radiation and the standard alkylating agent temozolomide [8, 10].
Taxanes stabilize polymerized microtubules, and like AURKA inhibitors, interfere with formation of normal mitotic spindles and mitotic progression to anaphase [11–13], which may lead to mitotic catastrophe, or abnormal mitotic exit, and apoptosis [14]. Because microtubule dynamics are important in all phases of the cell cycle, taxanes may also adversely affect interphase cells [15]. Although widely used against cancers, the taxanes paclitaxel and docetaxel have certain limitations [16], including acquired drug resistance via P-glycoprotein and mutations in tubulin’s taxane-binding sites [17, 18]. Also, for agents to be effective against brain tumors they must cross the blood-brain barrier. Despite high lipophilicity, conventional taxanes only poorly enter the central nervous system (CNS) [19]. TPI 287 is a novel taxane that crosses the blood-brain barrier [20]. Structural modifications confer higher lipophilicity and the abilities to avoid P-glycoprotein and bind mutant tubulin [21]. TPI 287 could thus be useful for treating CNS neoplasms.
The AURKA inhibitor alisertib is in clinical trials for a variety of neoplasms [22, 23], including atypical teratoid rhabdoid tumor [24] and recurrent glioblastoma [25]. TPI 287 was evaluated in phase I in combination with temozolomide for neuroblastoma and medulloblastoma and was well tolerated [21]. It is now in trials with and without bevacizumab for recurrent glioblastoma [26–28]. Due to the therapeutic challenges of glioblastoma, effective treatments may require a combination agent approach. Here we show that the highly selective AURKA inhibitors alisertib (MLN8237) [29], MLN8054 [5], and TC-A2317 [30] act synergistically with TPI 287 to inhibit glioblastoma cell proliferation. We further demonstrate that the mechanism of this interaction is synergistic induction of apoptosis.
Materials and Methods
Cell Culture
U87 and U1242 cells were grown in DMEM/10% FBS (GE Healthcare, Chicago, IL) with 1% penicillin/streptomycin (Thermo Fisher). GB30 neurosphere cells <20 passages were cultured in DMEM/F12 (Corning) with 1% N2 supplement, 1% penicillin/streptomycin, and 20 ng/mL bFGF and EGF (R&D Systems). All cells were incubated in 5% CO2/air at 37°C. Patient-derived GB30 cells were obtained as previously described [7]. U87 cells were purchased from the American Type Culture Collection. STR profiling of GB30 and U1242 cells was performed at the University of Arizona Genetics Core for authentication.
Chemicals
TPI 287 was provided by Cortice Biosciences and dissolved in ethanol. MLN8054 and alisertib (MLN8237) were purchased from Selleckchem and diluted in DMSO or sterile water, respectively. TC-A2317 was purchased from Tocris Bioscience and dissolved in DMSO.
Colony Formation Assay (CFA)
U87 or U1242 cells were seeded at 600 cells/60 mm dish (Corning) and treated the following day with TPI 287, the AURKA inhibitors alisertib, MLN8054 or TC-A2317, or both TPI 287 and an AURKA inhibitor. Drug doses were chosen as multiples of the approximate IC50s from dose range finding CFA experiments performed for each single drug and cell line combination. A volume of drug equivalent to 0.1% of media volume was added to dishes. Equal volumes of ethanol, DMSO, or sterile water were added to controls. Dishes were incubated for 3 d, the media aspirated, dishes rinsed with DPBS, and fresh media added. Three to 4 d later, dishes were rinsed with DPBS, methanol fixed and Giemsa stained. Colonies containing >20 cells were counted using a dissecting microscope. Percent survival was calculated as the mean number of colonies in 3 replicate treatment dishes divided by the mean of 3 untreated control dishes.
For GB30 CFAs, cells were seeded at 3×103 cells/well in 0.4% low melting point agarose (Thermo Fisher) into 6-well plates (Corning) as described [8]. Neurosphere media (3 ml/well) was added and triplicate wells treated with TPI 287, AURKA inhibitors, or both. Media and drugs were changed every 3 d over 10 d. Plates were fixed with methanol and stained with 0.0125% crystal violet. Colonies >20 cells were counted.
Cytology
GB30 cells were seeded into 6-well plates and treated with TPI 287, alisertib, or both. After 3, 5, and 7 d, 3×105 cells were deposited onto glass slides using a Shandon Cytospin 4 centrifuge (Thermo Fisher) for 3 min at 800 rpm, ethanol fixed and H&E stained for light microscopy.
Annexin V binding
GB30 cells were seeded at 2×105 cells/well into 6-well plates and treated with TPI 287, alisertib, or both. After 3, 5, and 7 d, cells were stained with Alexa Fluor 594 annexin V conjugate (Thermo Fisher) and analyzed with a Countess II FL cell counter (Thermo Fisher) per the manufacturer’s instructions.
Flow cytometry
GB30 cells were seeded at 2×105 cells/well into 6-well plates and treated with TPI 287, alisertib, or both for 3, 5, and 7 d and stained with DRAQ5 and CellEvent Caspase-3/7 Green Detection Reagent (Thermo Fisher). An LSR II Flow Cytometer (BD) and FlowJo software were used for analysis of DNA content and caspase 3/7 activity.
Statistical analysis
For CFAs, the effect (E) of a drug or combination at a given dose was the mean of 3 replicates divided by the mean of the untreated replicates. For each concentration, two models were considered to identify potential synergy: an effect based model (Bliss) and a dose-effect based model (Loewe). The Bliss independence model combination index [31–33] was calculated as the expected combination effect (assuming the drugs act independently) divided by the observed combination effect ((Ea + Eb − Ea*Eb)/Eab). The Loewe independence model [34] was assessed using the Chou Talalay combination index [33, 35] (a/A+b/B; where a and b are the doses in combination for a given effect, and A and B are the single doses required for this effect). A combination index >1 indicates antagonism, and an index <1 indicates synergy.
To measure apoptosis via an interaction between two drugs, logit transformed proportion apoptotic data from cytology assays were modeled with a linear model with day effect, main effects for each drug and an interaction effect for the two drugs. Annexin V-positive data were modeled with a linear model with main effects for each drug and an interaction effect for the two drugs. To test the assumption of normality, model residuals were tested with the Shapiro-Wilkes test.
Results
TPI 287 and AURKA inhibitors synergistically inhibit the growth of traditional and glioblastoma stem cell lines
TPI 287 and alisertib
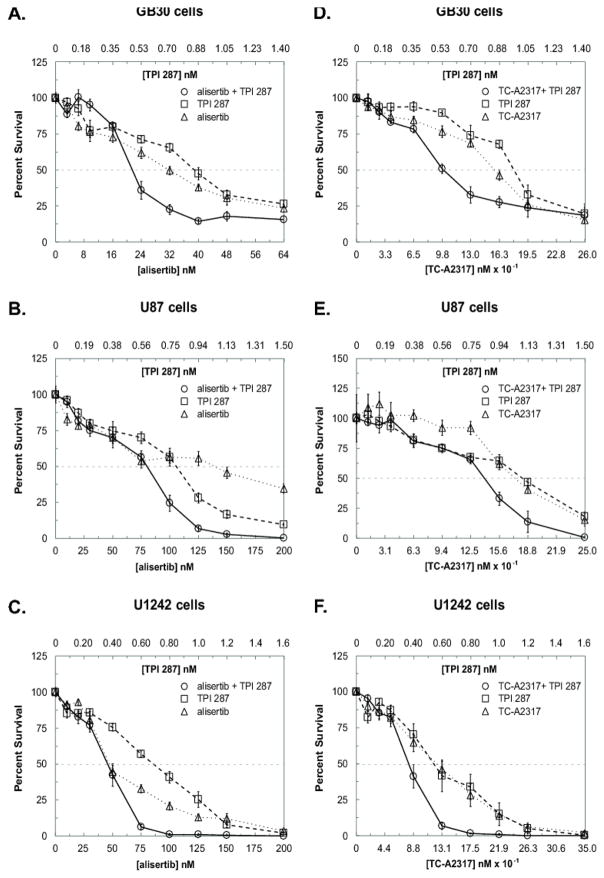
Treatment of GB30 cells in soft agar with TPI 287 and alisertib resulted in synergistic inhibition of colony formation at concentrations ranging from 0.75 to 1.5x the approximate IC50s of these agents alone, or between 0.53–1.23 nM TPI 287 and 24–56 nM alisertib with respect to the average of repeated experiments (Fig. 1A), and was most convincing from 1x to 1.25x the approximate IC50s, or 0.70–0.88 nM TPI 287 and 32–40 nM alisertib as determined by both Chou-Talalay and Bliss analyses (Table 1).
Fig. 1.
TPI 287 and AURKA inhibitors demonstrate cytotoxic synergy in glioblastoma cells. GB30 cells were seeded in soft agar and exposed to drugs for 10 days. U87 and U1242 cells were seeded, treated the following day for 72 h, and cultured an additional 3–4 d. Drug concentrations were chosen as multiples of approximate IC50s for colony formation. For both assays, colonies of ≥20 cells were counted. A–C. CFAs of alisertib and TPI 287 in GB30, U87, and U1242 cells. D–F. CFAs of TC-A2317 and TPI 287 in GB30, U87, and U1242 cells. All experiments were performed twice. The average of both experiments is shown
Table 1.
Synergy analysis of TPI 287 and AURKA inhibitors alisertib and TC-A2317.
| alisertib + TPI 287 | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| IC50x | alisertib (nM) | TPI 287 (nM) | Bliss | Chou-Ta | Lower CI | Upper CI |
| GB30 Colony Formation Assay (1) | ||||||
|
| ||||||
| 0.2 | 6.4 | 0.14 | NA | NA | NA | NA |
| 0.3 | 9.6 | 0.21 | NA | NA | NA | NA |
| 0.5 | 16 | 0.35 | 2.74 | 3.42 | 1.99 | 5.87 |
| 0.75 | 24 | 0.53 | 1.19 | 1.67 | 0.98 | 2.84 |
| 1 | 32 | 0.70 | 0.94 | 0.97 | 0.56 | 1.70 |
| 1.25 | 40 | 0.88 | 0.97 | 0.76 | 0.43 | 1.36 |
| 1.5 | 48 | 1.05 | 1.22 | 1.55 | 0.89 | 2.70 |
| 2 | 64 | 1.40 | 1.18 | 1.76 | 1.00 | 3.08 |
|
| ||||||
| GB30 Colony Formation Assay (2) | ||||||
|
| ||||||
| 0.2 | 6.4 | 0.14 | 3.85 | 4.05 | 1.98 | 8.30 |
| 0.3 | 9.6 | 0.21 | 3.00 | 2.86 | 1.52 | 5.39 |
| 0.5 | 16 | 0.35 | 1.63 | 2.71 | 1.50 | 4.91 |
| 0.75 | 24 | 0.53 | 0.65 | 0.48 | 0.26 | 0.89 |
| 1 | 32 | 0.70 | 0.79 | 0.62 | 0.34 | 1.15 |
| 1.25 | 40 | 0.88 | 0.94 | 0.53 | 0.28 | 0.99 |
| 1.5 | 48 | 1.05 | 0.97 | 0.50 | 0.26 | 0.96 |
| 2 | 64 | 1.40 | 1.04 | 0.63 | 0.33 | 1.21 |
|
| ||||||
| U87 Colony Formation Assay (1) | ||||||
|
| ||||||
| 0.2 | 20 | 0.15 | 1.97 | 2.63 | 1.38 | 5.04 |
| 0.3 | 30 | 0.23 | 2.53 | 3.74 | 1.96 | 7.15 |
| 0.5 | 50 | 0.38 | 2.78 | 4.55 | 2.43 | 8.53 |
| 0.75 | 75 | 0.56 | 1.83 | 3.13 | 1.74 | 5.64 |
| 1 | 100 | 0.75 | 1.21 | 1.50 | 0.86 | 2.62 |
| 1.25 | 125 | 0.94 | 0.94 | 0.44 | 0.24 | 0.80 |
| 1.5 | 150 | 1.13 | 0.96 | 0.25 | 0.13 | 0.50 |
| 2 | 200 | 1.50 | 0.97 | 0.08 | 0.03 | 0.21 |
|
| ||||||
| U87 Colony Formation Assay (2) | ||||||
|
| ||||||
| 0.2 | 20 | 0.15 | 1.49 | 1.30 | 0.32 | 5.24 |
| 0.3 | 30 | 0.23 | 0.86 | 0.99 | 0.31 | 3.17 |
| 0.5 | 50 | 0.38 | 0.78 | 1.34 | 0.44 | 4.06 |
| 0.75 | 75 | 0.56 | 1.10 | 1.34 | 0.50 | 3.60 |
| 1 | 100 | 0.75 | 0.67 | 0.51 | 0.22 | 1.15 |
| 1.25 | 125 | 0.94 | 0.88 | 0.31 | 0.12 | 0.79 |
| 1.5 | 150 | 1.13 | 0.95 | 0.24 | 0.08 | 0.68 |
| 2 | 200 | 1.50 | 0.97 | 0.00 | NA | NA |
|
| ||||||
| U1242 Colony Formation Assay (1) | ||||||
|
| ||||||
| 0.2 | 20 | 0.16 | 2.95 | 2.44 | 0.98 | 6.06 |
| 0.3 | 30 | 0.24 | 1.55 | 2.37 | 0.98 | 5.73 |
| 0.5 | 50 | 0.40 | 0.81 | 1.10 | 0.46 | 2.59 |
| 0.75 | 75 | 0.60 | 0.79 | 0.48 | 0.19 | 1.23 |
| 1 | 100 | 0.80 | 0.89 | 0.27 | 0.09 | 0.76 |
| 1.25 | 125 | 1.00 | 0.98 | 0.29 | 0.10 | 0.85 |
| 1.5 | 150 | 1.20 | 0.99 | 0.29 | 0.09 | 0.87 |
| 2 | 200 | 1.60 | 1.00 | 0.00 | NA | NA |
|
| ||||||
| U1242 Colony Formation Assay (2) | ||||||
|
| ||||||
| 0.2 | 20 | 0.16 | 0.77 | 1.43 | 0.59 | 3.45 |
| 0.3 | 30 | 0.24 | 1.26 | 1.98 | 0.83 | 4.74 |
| 0.5 | 50 | 0.40 | 1.74 | 2.55 | 1.08 | 6.02 |
| 0.75 | 75 | 0.60 | 0.95 | 1.05 | 0.45 | 2.44 |
| 1 | 100 | 0.80 | 0.96 | 0.55 | 0.23 | 1.33 |
| 1.25 | 125 | 1.00 | 0.99 | 0.69 | 0.29 | 1.66 |
| 1.5 | 150 | 1.20 | 1.01 | 0.64 | 0.26 | 1.57 |
| 2 | 200 | 1.60 | 1.00 | 0.51 | 0.20 | 1.29 |
| TC-A2317 + TPI 287 | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| IC50x | TC-A2317 (nM) | TPI 287 (nM) | Bliss | Chou-T | Lower CI | Upper CI |
| GB30 Colony Formation Assay (1) | ||||||
|
| ||||||
| 0.2 | 26.0 | 0.14 | 1.41 | 1.32 | 0.36 | 4.87 |
| 0.3 | 39.0 | 0.21 | 1.14 | 1.31 | 0.37 | 4.66 |
| 0.5 | 65.0 | 0.35 | 0.77 | 1.63 | 0.47 | 5.69 |
| 0.75 | 97.5 | 0.53 | 0.54 | 0.95 | 0.28 | 3.30 |
| 1 | 130 | 0.70 | 0.78 | 1.07 | 0.31 | 3.71 |
| 1.25 | 163 | 0.88 | 1.01 | 1.00 | 0.28 | 3.51 |
| 1.5 | 195 | 1.05 | 1.44 | 1.27 | 0.36 | 4.47 |
| 2 | 260 | 1.40 | 1.38 | 1.31 | 0.37 | 4.71 |
|
| ||||||
| GB30 Colony Formation Assay (2) | ||||||
|
| ||||||
| 0.2 | 26.0 | 0.14 | 1.53 | 1.49 | 0.55 | 4.05 |
| 0.3 | 39.0 | 0.21 | 1.07 | 1.40 | 0.54 | 3.64 |
| 0.5 | 65.0 | 0.35 | 1.17 | 2.00 | 0.78 | 5.16 |
| 0.75 | 97.5 | 0.53 | 0.73 | 1.39 | 0.56 | 3.43 |
| 1 | 130 | 0.70 | 0.72 | 0.88 | 0.36 | 2.18 |
| 1.25 | 163 | 0.88 | 0.89 | 1.06 | 0.43 | 2.62 |
| 1.5 | 195 | 1.05 | 1.04 | 0.85 | 0.33 | 2.14 |
| 2 | 260 | 1.40 | 1.06 | 0.92 | 0.36 | 2.38 |
|
| ||||||
| U87 Colony Formation Assay (1) | ||||||
|
| ||||||
| 0.2 | 25.0 | 0.15 | 10.23 | 7.27 | 2.83 | 18.65 |
| 0.3 | 37.5 | 0.23 | NA | NA | NA | NA |
| 0.5 | 62.5 | 0.38 | 1.62 | 2.27 | 1.08 | 4.77 |
| 0.75 | 93.8 | 0.56 | 1.90 | 2.42 | 1.15 | 5.06 |
| 1 | 125 | 0.75 | 1.99 | 2.67 | 1.28 | 5.59 |
| 1.25 | 156 | 0.94 | 1.05 | 1.29 | 0.60 | 2.78 |
| 1.5 | 188 | 1.13 | 0.90 | 0.30 | 0.13 | 0.71 |
| 2 | 250 | 1.50 | 0.97 | 0.12 | 0.04 | 0.31 |
|
| ||||||
| U87 Colony Formation Assay (2) | ||||||
|
| ||||||
| 0.2 | 25.0 | 0.15 | 1.05 | 1.37 | 0.29 | 6.40 |
| 0.3 | 37.5 | 0.23 | 1.89 | 2.32 | 0.48 | 11.29 |
| 0.5 | 62.5 | 0.38 | 1.50 | 2.07 | 0.50 | 8.48 |
| 0.75 | 93.8 | 0.56 | 1.83 | 2.80 | 0.69 | 11.26 |
| 1 | 125 | 0.75 | 1.19 | 2.45 | 0.64 | 9.40 |
| 1.25 | 156 | 0.94 | 0.90 | 1.39 | 0.37 | 5.25 |
| 1.5 | 188 | 1.13 | 1.14 | 1.41 | 0.37 | 5.36 |
| 2 | 250 | 1.50 | 1.01 | 0.23 | 0.06 | 0.90 |
|
| ||||||
| U1242 Colony Formation Assay (1) | ||||||
|
| ||||||
| 0.2 | 35.0 | 0.16 | 0.81 | 1.77 | 0.72 | 4.38 |
| 0.3 | 52.5 | 0.24 | 1.10 | 2.26 | 0.92 | 5.53 |
| 0.5 | 87.5 | 0.40 | 0.92 | 1.75 | 0.73 | 4.17 |
| 0.75 | 131 | 0.60 | 0.71 | 0.95 | 0.39 | 2.33 |
| 1 | 175 | 0.80 | 0.84 | 0.49 | 0.18 | 1.30 |
| 1.25 | 219 | 1.00 | 0.97 | 0.33 | 0.12 | 0.94 |
| 1.5 | 263 | 1.20 | 0.99 | 0.00 | NA | NA |
| 2 | 350 | 1.60 | 1.00 | 0.39 | 0.13 | 1.17 |
|
| ||||||
| U1242 Colony Formation Assay (2) | ||||||
|
| ||||||
| 0.2 | 35.0 | 0.16 | 1.47 | 2.01 | 0.72 | 5.60 |
| 0.3 | 52.5 | 0.24 | 1.75 | 2.80 | 1.01 | 7.73 |
| 0.5 | 87.5 | 0.40 | 0.90 | 1.51 | 0.56 | 4.05 |
| 0.75 | 131 | 0.60 | 0.96 | 0.65 | 0.22 | 1.95 |
| 1 | 175 | 0.80 | 0.98 | 0.77 | 0.25 | 2.34 |
| 1.25 | 219 | 1.00 | 1.01 | 0.87 | 0.28 | 2.68 |
| 1.5 | 263 | 1.20 | NA | NA | NA | NA |
| 2 | 350 | 1.60 | NA | NA | NA | NA |
Bliss and aChou-Talalay values < 1.0 in bold indicate synergy. 95% confidence intervals (CI) are indicated for Chou-Talalay analyses. CI < but not containing 1.0 are of higher statistical significance. When observed percent inhibition was negative or when both obs erved and expected percent inhibition were 1.0, Bliss and Chou-Talalay indices were not valid and are indicated as “NA”.
Synergy was observed in U87 cell CFAs at 1 to 1.5x the approximate IC50s, or between 0.75–1.13 nM TPI 287 and 100–175 nM alisertib with respect to the average of repeated experiments (Fig. 1B). This synergy was most consistent from 1.25 to 1.5x the approximate IC50s, or 0.94–1.13 nM TPI 287 and 125–150 nM alisertib by Chou-Talalay and Bliss analyses, although Chou-Talalay indicated stronger synergism (Table 1). The average of repeated U1242 cell experiments showed synergism over a range of 0.75 to 1.25x the approximate IC50s, or between 0.60–1.00 nM TPI 287 and 75–125 nM alisertib (Fig. 1C), which was corroborated by both analyses, but most convincingly by Chou-Talalay (Table 1). Synergy was also seen between TPI 287 and the alisertib predecessor compound MLN8054 (Fig. S1, Table S1).
TPI 287 and TC-A2317
In the average of repeated GB30 CFAs, synergy was observed between 0.75 and 1.25x the approximate IC50s, or between 0.53–0.88 nM TPI 287 and 97.5–163 nM TC-A2317 (Fig 1D). This synergy was consistently observed between 0.75 and 1x the approximate IC50s, or between 0.53–0.70 nM TPI 287 and 97.5–130 nM TC-A2317 in both models but most notably with the Bliss model (Table 1).
In U87 CFAs, synergy was observed from 1.25 to 1.5x the approximate IC50s, or between 0.94–1.13 nM TPI 287 and 156–188 nM TC-A2317 when repeated experiments are considered together between both the Bliss and Chou-Talalay models, although this interaction was not as strong as that seen with alisertib in this cell line (Fig. 1E, Table 1). CFAs with U1242 cells indicated synergism over a range of 0.5 to 1.25x the approximate IC50s, or between 0.40–1.00 nM TPI 287 and 87.5–219 nM TC-A2317 with respect to the average of repeated experiments (Fig. 1F), and was most convincing across both models between 0.75 and 1x the approximate IC50s, or between 0.60–0.80 nM TPI 287 and 131–175 nM TC-A2317 (Table 1).
Combined treatment of glioblastoma tumor stem cells with TPI 287 and alisertib results in markedly increased apoptosis
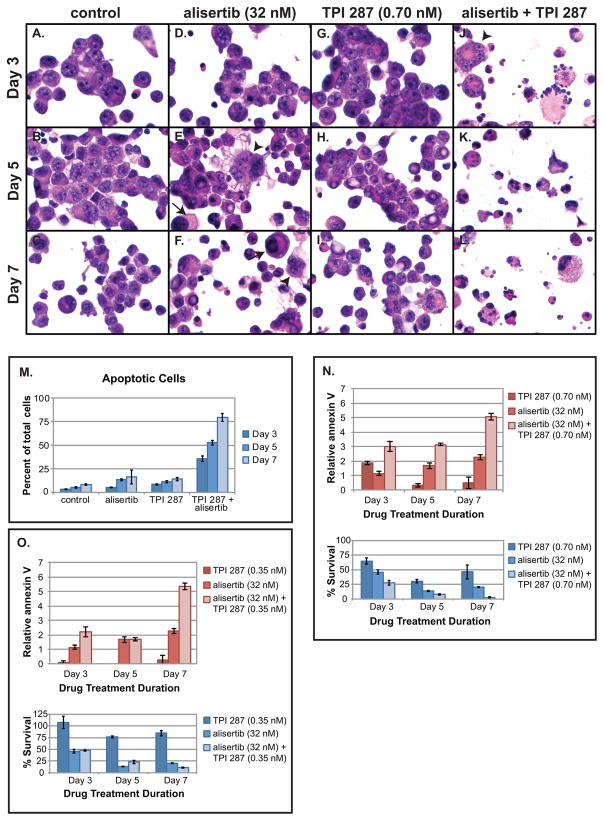
Morphological analysis of H&E-stained GB30 neurosphere cells treated with alisertib (32 nM), TPI 287 (0.70 nM) or both showed an increase in giant mononucleated and multinucleated cells compared to controls, especially after 5 days of treatment (Fig. 2A–2I, Fig. S2A & B), and was more pronounced when drugs were used in combination (Fig. 2J–2L, Fig. S2A & B). The number of mitotic cells was fairly consistent across treatment groups, but increased slightly after 5 days of alisertib alone and after treatment with both drugs (Fig. S2C). This is likely attributable to mitotic arrest caused by AURKA inhibition [5, 36].
Fig. 2.
TPI 287 and alisertib synergistically induce apoptosis in glioblastoma neurosphere cells. GB30 cells were stained with H&E (A-C) after treatment with alisertib (32 nM, approximate IC50) (D-F), TPI 287 (0.70 nM, approximate IC50) (G-I), or both drugs (J-L) for a period of 3, 5, and 7 days. Arrows indicate large mononucleated cells; arrowheads indicate multinucleated cells. Magnification (600x) is identical in all panels. Apoptotic cells were counted, and average values from 2 experiments are shown (M). Similarly treated neurospheres were dissociated with accutase and counted and stained with an Alexafluor 594 annexin V conjugate and analyzed using a Countess II FL equipped with a Texas Red fluorescent light cube. N. Combined effects of alisertib (32 nM) and TPI 287 (0.70 nM) on apoptosis as measured by fold increase in annexin V binding. O. Combined effects of alisertib (32 nM) and TPI 287 (0.35 nM, 0.5x approximate IC50) on apoptosis. GB30 neurosphere cells showed greatly increased annexin V labeling when treated with TPI 287 and alisertib. This experiment was performed twice and showed similar results. A representative example is shown
A dramatic increase in cells exhibiting fragmented and condensed chromatin and multilobulated morphology, characteristic of apoptosis, was seen when cells were treated with TPI 287 and alisertib together (Fig. 2J-2L). Cotreated cells showed a 9.6-fold, 9.0-fold, and 8.7-fold increase in the proportion of apoptotic cells on days 3, 5, and 7, respectively, compared to 0.5-fold to 1.5-fold increases observed with either drug alone. By day 7, apoptotic cells represented nearly 80% of observed cells in the combined treatment group (Fig. 2M). When the effect of treatment time is factored out, this interaction was statistically significant (p = 0.018, Fig. 2a–l), but the substantial increase in apoptosis over time suggests that treatment duration may be an important factor. This, with the increase in mitotic figures seen after 5 days of treatment with both drugs in combination and the notable disappearance of mitotic figures among them after 7 days (Fig. S2C), may suggest that a synergistic induction of apoptosis is occurring at mitosis and/or that the increase in apoptosis occurs after one or more rounds of abnormal mitoses.
To further quantify the increase in apoptosis seen when alisertib and TPI 287 are used in combination, annexin V binding assays were performed with GB30 glioblastoma tumor stem cells. Cultures cotreated with TPI 287 and alisertib showed a marked increase in annexin V positive cells compared to controls and cultures treated with either drug alone (Fig. 2N & 2O). The combined pro-apoptotic effects of these drugs were greater than the sum of their individual effects, and in many cases were consistent with statistically significant synergism. The latter applies to the interactions between alisertib (32 nM) and TPI 287 (0.35 nM) at 3 days (p=0.023) and 7 days (p=0.023) post-treatment (Fig. 2O), and between alisertib (32 nM) and TPI 287 (0.70 nM) on days 5 (p=0.046) and 7 (p=0.0079) (Fig. 2N). Even when TPI 287 alone did not cause a significant increase in apoptosis with respect to controls (i.e., at 0.35 nM), it still potentiated the apoptotic effect of alisertib (Fig. 2O). This provides further evidence that induction of apoptosis is responsible for the synergistic growth inhibition seen with these drugs in combination.
Cotreatment with TPI 287 and alisertib interferes with mitotic slippage in GB30 neurosphere cells
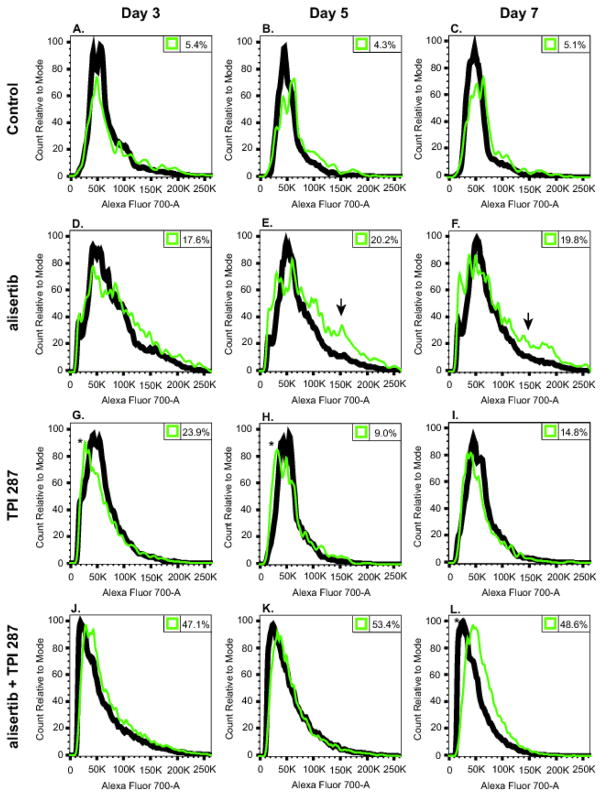
Flow cytometry analysis demonstrates untreated GB30 cells undergoing normal cell cycle progression characterized by a large G0/G1 DNA content peak, a smaller G2/M peak to the right, and low levels of background caspase 3/7 activity (Fig. 3A–C). Treatment with alisertib (32 nM) resulted in a much greater proportion of cells with increased DNA content (Fig. 3D–F), indicating that more cells were in the G2 or M phases of the cell cycle, and/or that drug treatment had resulted in increased aneuploidy or polyploidy (both consistent with the observed morphological changes present in the H&E stained slides). In fact, a small population of cells with DNA content consistent with alisertib-induced G2/M tetraploidy [7] is observable in Fig. 3D–F (arrows).
Fig. 3.
Cell cycle progression of GB30 cells treated with alisertib, TPI 287 alone and in combination. GB30 tumor stem cells were treated with alisertib (32 nM), TPI 287 (0.70 nM) or both for 3, 5, and 7 days, stained with DRAQ5 and CellEvent Caspase-3/7 Green Detection Reagent and analyzed by flow cytometry. Black lines represent DNA content of all single cells analyzed, while green lines represent DNA content of cells positive for caspase 3/7 activity. Counts were normalized to the mode. Numbers in the top right corners of histograms indicate the percentage of cells showing active caspase 3/7 activity. Arrows indicate G2/M tetraploid populations, and asterisks mark sub G1/G0 populations. A replicate experiment yielded similar results. A representative example is shown
As expected, alisertib treatment led to an uptick in caspase 3/7 activity (Fig. 3D–F). Cells treated with TPI 287 (0.70 nM) also showed increased caspase 3/7 activity, but unlike cells treated with alisertib, did not show markedly increased DNA content (Fig. 3G–I). This could be because alisertib-treated cells may be more likely to undergo mitotic slippage, resulting in increased ploidy [37]. TPI 287-treated cells exhibiting caspase 3/7 activity were more likely to have G1/S or even sub G1/G0 DNA content (indicated with an asterisk in Fig. 3G–H) than their alisertib-treated counterparts. Possible explanations for this include the interphase activity of taxanes as well as their tendency to cause the formation of micronuclei with fragmented DNA [15].
Combined treatment resulted in vastly increased levels of caspase 3/7 activity (Fig. 3J–3L), which by day 7 resulted in a large sub G1/G0 peak (asterisk) and nearly half of the cells exhibiting signs of apoptosis (Fig. 3L). The histogram of cotreated cells on day 7 (Fig. 3L) shares features with those of cells treated with individual drugs—namely, the sub-G0/G1 population seen with TPI 287, and an increase in the proportion of cells with G2/M or greater DNA content seen with alisertib alone. An increase in caspase 3/7 activity at G2/M at day 7 provides further evidence that this drug combination is preventing many cells from slipping out of mitosis and instead causing them to undergo apoptosis.
Discussion
Because TPI 287 can penetrate the blood brain barrier and avoid resistance mechanisms, it is an appealing candidate for treating CNS neoplasms. It also has potential advantages for use in combination with AURKA inhibitors. Although both drug classes are antimitotic, they work by different mechanisms. AURKA inhibitors additionally offer inhibitory effects on pro-proliferative cell signaling, while taxanes also interfere with earlier stages of the cell cycle [7, 10, 15, 38].
We found potent synergistic inhibition of glioblastoma colony formation when TPI 287 was used in combination with each of three selective AURKA inhibitors against both traditional and primary glioblastoma neurosphere cell lines. When interpreting Bliss and Chou combination indices for these assays, it is important to consider the middle range of concentrations, most likely to be applicable in vivo, as the most important in the determination of synergy, as opposed to the lowest concentrations that caused little to no inhibition, or the highest concentrations in which nearly all growth was sometimes inhibited. Importantly, data among repeated CFAs was consistent in this intermediate range. Sometimes combination indices indicating antagonism were observed with lower drug concentrations, but this is likely background variability in the data associated with ineffective drug concentrations. Still, the possibility of antagonism at sub-therapeutic concentrations should be considered in future, primarily in vivo studies.
CFAs measure of the ability of a drug to prevent cell division without respect to how division has been impeded. Decreased colony formation can result from multiple cell fates. We have previously shown that the anti-glioma effects of alisertib are attributable to a combination of the induction of apoptosis, differentiation and/or senescence [7]. Although it is widely held that senescent cells cannot regain the ability to proliferate, their accumulation may lead to a pro-tumorigenic microenvironment via the senescence-associated secretory phenotype (SASP) [39]. Furthermore senescence and apoptosis are mutually exclusive fates, and some pro-senescence signaling can actively suppress apoptosis [40]. This provides an opportunity for tumor cells to exploit these conflicting signals and escape death. In fact, alisertib or TPI 287 doses that were approximately equivalent to the IC50s in CFAs did not cause large increases in apoptosis on their own (Fig. 2 and 3). However, when they were used in combination the number of apoptotic cells increased dramatically. Notably, a synergistic potentiation of apoptosis was observed even when alisertib was used in conjunction with a concentration of TPI 287 that caused virtually no apoptosis on its own (Fig. 2O). TPI 287 co-treatment should thus abrogate this theoretic limitation of alisertib and other AURKA inhibitors, while also leading to a significant decrease in proliferation-capable tumor cells due to markedly increased outright tumor cell killing.
Factors interfering with normal chromosome segregation such as antimitotic drugs can lead to mitotic blockade. Some neoplastic cells can escape this blockade through mitotic slippage, leading to the formation of multinucleated and aneuploid cells potentially capable of further cell division [37]. This is a proposed mechanism for the generation of genomic instability and tumor progression. Alisertib treatment was shown to greatly increase ploidy (Fig. 3D–F). However, cells treated with both alisertib and TPI 287 did not exhibit high levels of polyploidy before committing to apoptosis, and when apoptosis was initiated, many cells were in mitosis (Fig. 3L). Thus, this combined treatment abrogated slippage from the mitotic block created by either agent alone. These findings suggest important ramifications for glioblastoma treatment. First, since the vast majority of tumor cells are committed to apoptosis by combined treatment, effective tumor cell burden reduction should be achievable. Second, because mitotic slippage associated with either agent alone is prevented, possible emergence of nascent aneuploid clones from mitotic slippage is also prevented. Thirdly, because this drug combination tilts the scale in favor of apoptosis and inhibits other potential cell fates caused by either drug alone, the potential for continued tumor cell survival and possible senescence-associate protumoral effects is greatly reduced. Thus combination therapy should prevent tumor progression possibly resultant from genomic instability generated by single agent antimitotic therapy.
We have demonstrated that TPI 287 acts synergistically with AURKA inhibitors to prevent glioblastoma cell growth by synergistic induction of tumor cell apoptosis, and that this synergism abrogates pharmacodynamic limitations of each agent alone. This data provides a strong rational for clinical trials of the combined use of TPI 287 and alisertib or other AURKA inhibitors in glioblastoma patients.
Supplementary Material
GB30 cells were seeded in soft agar and exposed to MLN8054 for 10 days. Colonies of ≥20 cells were counted. Experiments were performed at least twice. A representative example is shown.
GB30 cells treated with TPI 287, alisertib, or both drugs for 3, 5, and 7 d were deposited on slides, H&E stained, and examined for morphological changes. A. Percent of large mononucleated cells following treatment with alisertib and/or TPI 287. B. Percent of multinucleated cells following treatment with alisertib and/or TPI 287. C. Percent of cells undergoing mitosis following treatment with alisertib and/or TPI 287.
Table S1. Synergy analysis of TPI 287 and MLN8054.
Acknowledgments
Funding for this work was supported by NIH Grant RO1 NS081125 (NLL).
Footnotes
Conflicts of Interest: None
References
- 1.Bleeker FE, Molenaar RJ, Leenstra S. Recent advances in the molecular understanding of glioblastoma. J Neurooncol. 2012;108:11–27. doi: 10.1007/s11060-011-0793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau D, Magill ST, Aghi MK. Molecularly targeted therapies for recurrent glioblastoma: current and future targets. Neurosurg Focus. 2014;37:E15. doi: 10.3171/2014.9.FOCUS14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asteriti IA, Giubettini M, Lavia P, Guarguaglini G. Aurora-A inactivation causes mitotic spindle pole fragmentation by unbalancing microtubule-generated forces. Mol Cancer. 2011;10:131. doi: 10.1186/1476-4598-10-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr AR, Gergely F. Aurora-A: the maker and breaker of spindle poles. J Cell Sci. 2007;120:2987–2996. doi: 10.1242/jcs.013136. [DOI] [PubMed] [Google Scholar]
- 5.Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W, Galvin KM, Hoar KM, Huck JJ, LeRoy PJ, Ray ET, Sells TB, Stringer B, Stroud SG, Vos TJ, Weatherhead GS, Wysong DR, Zhang M, Bolen JB, Claiborne CF. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci U S A. 2007;104:4106–4111. doi: 10.1073/pnas.0608798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huck JJ, Zhang M, McDonald A, Bowman D, Hoar KM, Stringer B, Ecsedy J, Manfredi MG, Hyer ML. MLN8054, an inhibitor of Aurora A kinase, induces senescence in human tumor cells both in vitro and in vivo. Mol Cancer Res. 2010;8:373–384. doi: 10.1158/1541-7786.MCR-09-0300. [DOI] [PubMed] [Google Scholar]
- 7.Van Brocklyn JR, Wojton J, Meisen WH, Kellough DA, Ecsedy JA, Kaur B, Lehman NL. Aurora-A inhibition offers a novel therapy effective against intracranial glioblastoma. Cancer Res. 2014;74:5364–5370. doi: 10.1158/0008-5472.CAN-14-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong X, O’Donnell JP, Salazar CR, Van Brocklyn JR, Barnett KD, Pearl DK, deCarvalho AC, Ecsedy JA, Brown SL, Mikkelsen T, Lehman NL. The selective Aurora-A kinase inhibitor MLN8237 (alisertib) potently inhibits proliferation of glioblastoma neurosphere tumor stem-like cells and potentiates the effects of temozolomide and ionizing radiation. Cancer Chemother Pharmacol. 2014;73:983–990. doi: 10.1007/s00280-014-2430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannino M, Gomez-Roman N, Hochegger H, Chalmers AJ. Differential sensitivity of Glioma stem cells to Aurora kinase A inhibitors: implications for stem cell mitosis and centrosome dynamics. Stem Cell Res. 2014;13:135–143. doi: 10.1016/j.scr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehman NL, O’Donnell JP, Whiteley LJ, Stapp RT, Lehman TD, Roszka KM, Schultz LR, Williams CJ, Mikkelsen T, Brown SL, Ecsedy JA, Poisson LM. Aurora A is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle. 2012;11:489–502. doi: 10.4161/cc.11.3.18996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 12.Manfredi JJ, Horwitz SB. Taxol: an antimitotic agent with a new mechanism of action. Pharmacol Ther. 1984;25:83–125. doi: 10.1016/0163-7258(84)90025-1. [DOI] [PubMed] [Google Scholar]
- 13.Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci U S A. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56:816–825. [PubMed] [Google Scholar]
- 15.Shi J, Mitchison TJ. Cell death response to anti-mitotic drug treatment in cell culture, mouse tumor model and the clinic. Endocr Relat Cancer. 2017;24:T83–T96. doi: 10.1530/ERC-17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagiwara H, Sunada Y. Mechanism of taxane neurotoxicity. Breast Cancer. 2004;11:82–85. doi: 10.1007/BF02968008. [DOI] [PubMed] [Google Scholar]
- 17.Brodie SA, Li G, Harvey D, Khuri FR, Vertino PM, Brandes JC. Small molecule inhibition of the CHFR-PARP1 interaction as novel approach to overcome intrinsic taxane resistance in cancer. Oncotarget. 2015;6:30773–30786. doi: 10.18632/oncotarget.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matesanz R, Trigili C, Rodriguez-Salarichs J, Zanardi I, Pera B, Nogales A, Fang WS, Jimenez-Barbero J, Canales A, Barasoain I, Ojima I, Diaz JF. Taxanes with high potency inducing tubulin assembly overcome tumoural cell resistances. Bioorg Med Chem. 2014;22:5078–5090. doi: 10.1016/j.bmc.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 19.Heimans JJ, Vermorken JB, Wolbers JG, Eeltink CM, Meijer OW, Taphoorn MJ, Beijnen JH. Paclitaxel (Taxol) concentrations in brain tumor tissue. Ann Oncol. 1994;5:951–953. doi: 10.1093/oxfordjournals.annonc.a058736. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald DP, Emerson DL, Qian Y, Anwar T, Liewehr DJ, Steinberg SM, Silberman S, Palmieri D, Steeg PS. TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells. Mol Cancer Ther. 2012;11:1959–1967. doi: 10.1158/1535-7163.MCT-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell D, Bergendahl G, Ferguson W, Roberts W, Higgins T, Ashikaga T, DeSarno M, Kaplan J, Kraveka J, Eslin D, Werff AV, Hanna GK, Sholler GL. A Phase 1 Trial of TPI 287 as a Single Agent and in Combination With Temozolomide in Patients with Refractory or Recurrent Neuroblastoma or Medulloblastoma. Pediatr Blood Cancer. 2016;63:39–46. doi: 10.1002/pbc.25687. [DOI] [PubMed] [Google Scholar]
- 22.Graff JN, Higano CS, Hahn NM, Taylor MH, Zhang B, Zhou X, Venkatakrishnan K, Leonard EJ, Sarantopoulos J. Open-label, multicenter, phase 1 study of alisertib (MLN8237), an aurora A kinase inhibitor, with docetaxel in patients with solid tumors. Cancer. 2016;122:2524–2533. doi: 10.1002/cncr.30073. [DOI] [PubMed] [Google Scholar]
- 23.Millennium Pharmaceuticals, Inc. [Accessed May 3, 2016];Phase 2 study of alisertib (MLN 8237 in combination with paclitaxel versus placebo in combination with paclitaxel as second line therapy for small cell lung cancer (SCLC) https://clinicaltrials.gov/ct2/show/NCT02038647 NLM Identifier: NCT02038647.
- 24.Wetmore C, Boyett J, Li S, Lin T, Bendel A, Gajjar A, Orr BA. Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro Oncol. 2015;17:882–888. doi: 10.1093/neuonc/nov017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi W. Alisertib and fractionated stereotactic radiosurgery in treating patients with recurrent high grade gliomas. National Cancer Institute; [Accessed May 2, 2016]. https://clinicaltrials.gov/show/NCT02186509 NLM Identifier: NCT02186509. [Google Scholar]
- 26.Cortice Biosciences, Inc. [Accessed May 3, 2016];Phase 1/2 dose-escalation study of TPI 287 in combination with bevacizumab followed by randomized study of the maximum tolerated dose of TPI 287 in combination with bevacizumab versus bevacizumab alone in adults with recurrent glioblastoma. https://clinicaltrials.gov/ct2/show/NCT01933815 NLM Identifier: NCT01933815.
- 27.M.D. Anderson Cancer Center. [Accessed May 3, 2016];Phase I/II bevacizumab versus bevacizumab plus TPI 287 for recurrent glioblastoma. https://clinicaltrials.gov/ct2/show/NCT01582152 NLM Identifier: NCT01582152.
- 28.ASCO Meeting Library. [Accessed May 25, 2017];Final results from the dose-escalation stage of a phase 1/2 trial of TPI 287, a brain penetrable microtubule inhibitor, plus bevacizumab in patients with recurrent glioblastoma. 2017 Available at http://meetinglibrary.asco.org/record/146708/abstract.
- 29.Manfredi MG, Ecsedy JA, Chakravarty A, Silverman L, Zhang M, Hoar KM, Stroud SG, Chen W, Shinde V, Huck JJ, Wysong DR, Janowick DA, Hyer ML, Leroy PJ, Gershman RE, Silva MD, Germanos MS, Bolen JB, Claiborne CF, Sells TB. Characterization of Alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin Cancer Res. 2011;17:7614–7624. doi: 10.1158/1078-0432.CCR-11-1536. [DOI] [PubMed] [Google Scholar]
- 30.Ando R, Ikegami H, Sakiyama M, Ooike S, Hayashi M, Fujino Y, Abe D, Nakamura H, Mishina T, Kato H, Iwase Y, Tomozane H, Morioka M. 3-Cyano-6-(5-methyl-3-pyrazoloamino)pyridines: selective Aurora A kinase inhibitors. Bioorg Med Chem Lett. 2010;20:4709–4711. doi: 10.1016/j.bmcl.2010.04.119. [DOI] [PubMed] [Google Scholar]
- 31.Bliss CI. The toxicity of poisons applied jointly. Annals of Applied Biology. 1939;26:585–615. doi: 10.1111/j.1744-7348.1939.tb06990.x. [DOI] [Google Scholar]
- 32.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 33.Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 2015;3:e00149. doi: 10.1002/prp2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3:285–290. [PubMed] [Google Scholar]
- 35.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 36.Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, Saya H. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003;278:51786–51795. doi: 10.1074/jbc.M306275200. [DOI] [PubMed] [Google Scholar]
- 37.Wysong DR, Chakravarty A, Hoar K, Ecsedy JA. The inhibition of Aurora A abrogates the mitotic delay induced by microtubule perturbing agents. Cell Cycle. 2009;8:876–888. doi: 10.4161/cc.8.6.7897. [DOI] [PubMed] [Google Scholar]
- 38.Ding YH, Zhou ZW, Ha CF, Zhang XY, Pan ST, He ZX, Edelman JL, Wang D, Yang YX, Zhang X, Duan W, Yang T, Qiu JX, Zhou SF. Alisertib, an Aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer cells. Drug Des Devel Ther. 2015;9:425–464. doi: 10.2147/DDDT.S74062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lujambio A. To clear, or not to clear (senescent cells)? That is the question. Bioessays 38 Suppl. 2016;1:S56–64. doi: 10.1002/bies.201670910. [DOI] [PubMed] [Google Scholar]
- 40.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GB30 cells were seeded in soft agar and exposed to MLN8054 for 10 days. Colonies of ≥20 cells were counted. Experiments were performed at least twice. A representative example is shown.
GB30 cells treated with TPI 287, alisertib, or both drugs for 3, 5, and 7 d were deposited on slides, H&E stained, and examined for morphological changes. A. Percent of large mononucleated cells following treatment with alisertib and/or TPI 287. B. Percent of multinucleated cells following treatment with alisertib and/or TPI 287. C. Percent of cells undergoing mitosis following treatment with alisertib and/or TPI 287.
Table S1. Synergy analysis of TPI 287 and MLN8054.