Abstract
Opioid use disorders (OUDs) are a growing problem in the United States. When OUDs co-occur with problematic drinking and posttraumatic stress disorder (PTSD), negative drug-related mental and physical health outcomes may be exacerbated. Thus, it is important to establish whether PTSD treatments with established efficacy for dually diagnosed individuals also demonstrate efficacy in individuals who engage in problematic drinking and concurrent opioid misuse. Adults who met DSM-IV-TR criteria for PTSD and alcohol dependence were recruited from a substance use treatment facility and were randomly assigned to receive either modified prolonged exposure (mPE) therapy for PTSD or a non-trauma-focused comparison treatment. Compared to adults in a non-OUD comparison group (n = 74), adults with OUD (n = 52) were younger, reported more cravings for alcohol, were more likely to use amphetamines and sedatives, were hospitalized more frequently for drug- and alcohol-related problems, and suffered from more severe PTSD symptomatology, depressive symptoms, and anxiety (standardized mean differences = 0.36–1.81). For participants with OUD, mPE was associated with large reductions in PTSD symptomatology, sleep disturbances, and symptoms of anxiety and depression, ds = 1.08–2.56. Moreover, participants with OUD reported decreases in alcohol cravings that were significantly greater than those reported by the non-OUD comparison group, F(1, 71.42) = 6.37, p = .014. Overall, our findings support the efficacy of mPE for PTSD among individuals who engage in problematic drinking and concurrent opioid misuse, despite severe baseline symptoms.
Approximately 46% of individuals with posttraumatic stress disorder (PTSD) meet current diagnostic criteria for a comorbid substance use disorder (SUD; Pietrzak, Goldstein, Southwick, & Grant, 2011). Recently, Blanco and colleagues (2013) reported that individuals with comorbid PTSD and SUD suffered from more severe PTSD symptoms, were more likely to use substances to relieve PTSD symptoms, and experienced higher rates of psychiatric comorbidity relative to individuals with PTSD alone. Individuals with comorbid PTSD and SUD also reported more severe SUD symptoms and lower levels of physical and psychosocial functioning in comparison to individuals with SUD alone (Blanco et al., 2013).
The United States is in the midst of an opioid epidemic that has resulted in an increasing number of drug overdose deaths (Rudd, Aleshire, Zibbell, & Gladden, 2016) and economic costs that exceed $56 billion (USD) annually (Birnbaum et al., 2011). In addition, individuals with opioid use disorders (OUDs) are more likely to suffer from comorbid psychiatric disorders (Conway, Compton, Stinson, & Grant, 2006) and report higher rates of homelessness, criminal justice involvement, polysubstance use, substance use treatment, and healthcare utilization relative to individuals who misuse other substances (e.g., alcohol and cannabis; Lubman et al., 2016).
Individuals who misuse opioids are frequently diagnosed with PTSD and suffer from more severe PTSD symptoms relative to individuals with other drug problems (Meier et al., 2014). Individuals with PTSD may be at risk for opioid misuse, given that stress enhances the rewarding properties of opioids and exacerbates the aversive effects of opioid withdrawal (Logrip, Zorrilla, & Koob, 2012). Although PTSD may increase the positively reinforcing effect of opioids, escalate opioid intake, and increase the odds of relapse, exposure-based psychotherapy has been associated with significant reductions in PTSD symptoms among individuals diagnosed with PTSD who were enrolled in methadone treatment for OUD (Schacht, Brooner, King, Kidorf, & Peirce, 2017). Despite these promising findings, Kedia, Sell, and Relyea (2007) found that many individuals (48%) seeking SUD treatment endorsed polysubstance use. Moreover, Hartzler, Donovan, and Huang (2010) found that the negative psychosocial outcomes associated with OUD were particularly pronounced for the 38% of individuals in their study who received treatment for co-occurring OUD and alcohol use disorder (AUD) compared to individuals who received treatment for OUD alone. Accordingly, research that examines the efficacy of exposure-based therapy for PTSD in representative samples of individuals seeking treatment for polysubstance use in community-based SUD treatment facilities is needed.
Exposure-based psychotherapies, such as prolonged exposure (PE), are highly effective treatments for PTSD (Powers, Halpern, Ferenschak, Gillihan, & Foa, 2010) that use imaginal and real-life exposure to help patients reduce trauma symptoms through confrontation of trauma-related thoughts, situations, and activities that individuals previously avoided but were not inherently harmful (Foa, Hembree, & Rothbaum, 2007). Exposure-based treatments hold promise for the treatment of comorbid PTSD and SUD given that negative reinforcement models of addictive behavior suggest that repeated imaginal and real-life exposure to trauma-specific negative emotional cues in the absence of alcohol and drugs should decrease the magnitude of conditioned emotional and drug-related responses (Stasiewicz & Maisto, 1993). Accordingly, several randomized clinical trials have shown that interventions that combine exposure-based psychotherapy for PTSD with SUD-focused treatment are associated with large reductions in PTSD symptom severity (e.g., Coffey et al., 2016; Foa et al., 2013; Mills et al., 2012). Although the participants in these trials who received exposure-based therapies also reported marked improvements in substance use severity (e.g., percentage of days drinking and substance craving), substance use outcomes did not differ by treatment (Coffey et al., 2016; Foa et al., 2013; Mills et al., 2012). Even with the established efficacy of exposure-based therapies, little is known about the efficacy of exposure-based therapies for PTSD among individuals who use substances other than alcohol.
Coffey and colleagues (2016) examined whether adding an exposure-based treatment for comorbid PTSD to traditional 12-step programming improved mental health outcomes more than a health-focused control treatment matched for therapist time. The exposure-based intervention was associated with significantly greater reductions in trauma and depressive symptoms relative to the control treatment. However, the authors observed no significant between-group differences in terms of substance use outcomes. Although most individuals in the study endorsed polysubstance use, drug type was not examined as a predictor of symptom severity or treatment outcome. Therefore, we conducted the present study to provide a cross-sectional characterization of adults with comorbid AUD, OUD, and PTSD on a wide array of variables and to examine whether opioid use was associated with pre- to posttreatment changes in relevant outcome measures.
Method
Participants and Procedure
As part of a previously published trial (Coffey et al., 2016), adults (N = 126) who met Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM-IV-TR; American Psychiatric Association [APA], 2000) criteria for alcohol dependence and PTSD were recruited from a community-based residential substance use treatment facility. Individuals with an acute psychotic disorder, those in the midst of a manic episode, and those determined to be at imminent risk for suicide were excluded from the study. Additional exclusion criteria included: medical conditions that could limit cooperation or compromise the integrity of the data, including dementia and head injury; and illiteracy in English. Although a current prescription for methadone, buprenorphine, naltrexone, or disulfiram was not a formal exclusion criterion, the treatment facility from which participants were recruited did not allow current use of opioid replacement therapy or medications that reduce craving or alcohol use. As such, none of the participants in the present study were prescribed the aforementioned medications.
Potential participants were approached within their first week of treatment to determine whether they were interested in the trial. Interested participants were then briefly screened for DSM-IV-defined alcohol dependence and PTSD. The Alcohol Use Disorder Identification Test (AUDIT; Saunders, Aasland, Babor, de la Fuente, & Grant, 1993) was administered to screen potential participants for problematic alcohol use. The AUDIT has strong psychometric properties (Saunders et al., 1993) and consists of 10 items rated on scale of 0 to 4. Interested individuals also completed the DSM-IV version of the PTSD Checklist (PCL; Weathers, Litz, Herman, Huska, & Keane, 1993). The PCL assesses the degree to which PTSD symptoms associated with the participant’s most distressing traumatic event have been present during the past month and consists of 17 items rated on a scale of 1 to 5; it has demonstrated excellent psychometric properties (Weathers et al., 1993). Individuals who reported the experience of an “emotional, physical, or sexual trauma at some point in their lives,” scored 8 or higher on the AUDIT (Saunders et al., 1993), or scored 44 or higher on the PCL (Blanchard, Jones-Alexander, Buckley, & Forneris, 1996) were invited to complete a more comprehensive assessment to fully assess inclusion and exclusion criteria.
Participants who passed the brief screening were scheduled for an appointment, during which they provided informed consent and completed a more comprehensive assessment to determine study eligibility. Using urn randomization (Wei, 1978), eligible participants were then assigned to receive one of two treatment conditions: modified prolonged exposure (mPE; n = 85) or a healthy lifestyles (HLS) control condition (n = 41).
The mPE treatment protocol utilized in the present study was similar to PE and included psychoeducation, breathing retraining, and imaginal and real-life exposure exercises to reduce symptoms of PTSD (Foa et al., 2007). In accordance with Foa et al. (2005), participants were offered nine to 12 sessions. Nine sessions were initially offered and, if PTSD symptomatology as measured by the Impact of Event Scale–Revised (IES-R) did not decrease by at least 70%, three additional mPE sessions were offered. Although the intervention was largely based on the work of Foa and colleagues (2007), we made the following modifications to increase the likelihood of adoption of exposure-based therapy in substance use treatment facilities (see Coffey et al., 2016): (a) because outcomes of 60-min PE sessions do not differ from traditional 90-mine PE sessions (Nacasch et al., 2015), sessions were 60 min in length; (b) participants received psychoeducation about the association between PTSD and SUD symptoms; and (c) participants completed diaphragmatic breathing at the end of each imaginal exposure exercise session in order to reduce distress to baseline levels.
The HLS control condition is an active health information–based, nontrauma intervention similar to the control condition used by Stasiewicz and colleagues (2013). Healthy lifestyles provided education about a variety of health-related topics including sleep, progressive muscle relaxation, exercise, personal role identification, nutrition, diabetes, goals and values, cancer, and HIV. Sessions were 60 min in length and designed to provide a similar amount of therapist contact as mPE. Participants who received the HLS intervention were yoked with participants in the mPE group in order to equalize treatment dose between treatment conditions. Accordingly, HLS participants were assigned to complete nine or 12 sessions depending on the number of sessions completed by the mPE participant with whom they were yoked.
All participants received treatment as usual (TAU) for SUD within the context of a 6-week community residential treatment facility. Substance use TAU was mandatory and consisted of the programming offered by the treatment facility at which recruitment occurred. Treatment components included daily group therapy for approximately three hours each day, daily recreation therapy, Alcoholics Anonymous and Narcotics Anonymous meetings, individual drug counseling sessions, and completion of drug counseling homework. Substance use TAU was largely based on the 12-step model; however, session content also emphasized teaching participants how to improve relationships, secure gainful employment, and enhance their level of spirituality. Counselors who provided TAU were state-certified drug and alcohol counselors unaffiliated with the study.
All participants completed self-report measures at pre- and posttreatment to assess for the effects of treatment. Participants received $60 USD for completing the pretreatment assessment and $90 USD for completing the posttreatment assessment in person, or $35 USD for completing the posttreatment assessment by phone. At the assessment session, participants completed a University of Mississippi Medical Center Institutional Review Board-approved informed consent procedure.
Measures
Trauma exposure.
At baseline, trauma exposure was assessed using the National Women’s Study (NWS) PTSD Module (Resnick, 1996). The NWS PTSD module is a commonly used structured interview that assesses participants’ trauma history and establishes DSM-IV PTSD Criterion A (APA, 2000). The NWS PTSD module has been used to identify PTSD Criterion A events in numerous studies involving both men and women (Coffey, Stasiewicz, Hughes, & Brimo, 2006).
PTSD.
As noted in the Participants and Procedures section, potential participants completed the DSM-IV version of the PCL (Weather et al., 1993) as part of the brief screening prior to study entry. At baseline, PTSD symptoms were assessed using the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995). The CAPS (Blake et al., 1995) was used to assess the 17 symptoms of PTSD according to DSM-IV criteria. The CAPS is a well-established and psychometrically sound structured clinical interview that is considered to be the “gold standard” for PTSD assessment (Weathers, Cane, & Davidson, 2001). Symptom frequency and severity are rated from 0 to 4 and a total severity score is calculated by summing the frequency and severity ratings of all 17 items (Weathers et al., 2001). The Impact of Event Scale–Revised (IES-R) was administered at both pre- and posttreatment in the present study and is a widely used self-report measure that assesses the PTSD symptomatology participants have experienced during the past week (Weiss & Marmar, 1997). The IES-R consists of 22 items and has demonstrated acceptable internal consistency and convergent validity among substance-dependent individuals both with and without PTSD (Rash, Coffey, Baschnagel, Drobes, & Saldin, 2008). In the present study, the IES-R had a Cronbach’s alpha value of .90.
Alcohol use.
As noted previously, the AUDIT (Saunders et al., 1993) was administered to potential participants to screen for problematic alcohol use prior to the start of the study. At both pre- and posttreatment, The Alcohol Craving Questionnaire–Now (ACQ-Now; Singleton, Tiffany, & Henningfield, 2003) was administered to participants. The ACQ is a 47-item questionnaire used to assess craving for alcohol. Total scores are the sum of all 47 items and range from 47 to 329. The ACQ has been shown to be valid, reliable, and able to predict both craving and alcohol dependence (Connolly, Coffey, Baschnagel, Drobes, & Saladin, 2009). In the present study, Cronbach’s alpha was .87.
Psychiatric disorders.
The Computerized Diagnostic-Interview Schedule (C-DIS-IV; Robins et al., 2000) and the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998) were the diagnostic measures administered at baseline to assess psychiatric disorders.
The C-DIS-IV (Robins et al., 2000) is a computerized version of the Diagnostic Interview Schedule, a fully structured and psychometrically sound diagnostic interview for Axis I disorders as defined by the DSM-IV. The C-DIS was used to establish SUD diagnostic status within the past 12 months. The MINI was used to establish current DSM-IV diagnostic status for mood disorders and all anxiety disorders (other than PTSD). The MINI is widely used and has demonstrated reliability and validity for the assessment of DSM-IV Axis I psychiatric disorders (Sheehan et al., 1998).
Anxiety and depression.
Symptoms of anxiety and depression were assessed at baseline and again after treatment had ended. The Beck Anxiety Inventory (BAI; Beck & Steer, 1993) is a commonly used and psychometrically sound 21-item measure that was used to assess the presence and severity of anxiety symptoms at both time points. In the present study, Cronbach’s alpha was .93. The Beck Depression Inventory–II (BDI-II; Beck, Steer, & Brown, 1996) is a commonly used and psychometrically sound 21-item measure that was used to assess the presence and severity of depression symptoms both pre- and posttreatment. In the present study, Cronbach’s alpha was .91.
Insomnia.
Finally, because individuals with PTSD commonly report sleep disturbances, the seven-item Insomnia Severity Index (ISI; Morin, Belleville, Belanger, & Ivers, 2011) was administered both pre- and posttreatment to measure the severity of insomnia during the past 2 weeks. The ISI is a reliable and valid instrument used to detect cases of insomnia and is sensitive to treatment response (Morin et al., 2011). In the present study, Cronbach’s alpha was .85.
Data Analysis
We conducted analyses using SPSS (Version 22.0; IBM, Inc., Chicago, IL, USA). Means, standard deviations (SDs), frequencies, and percentages were used to describe the demographic and baseline characteristics of the sample by presence/absence of OUD. Odds ratios (ORs) with 95% confidence intervals (CIs) also are also presented. To avoid inflated Type I error rates often associated with analyses involving large numbers of predictors, we calculated standardized mean differences (SMDs) to quantify the size of observed between-group differences at baseline. Accordingly, SMD functions as a measure of effect between groups that was calculated in relation to the two groups’ pooled variability (e.g., SD). Similar to Cohen’s d (Cohen, 1992), SMDs of 0.2 to 0.49 are interpreted as small effects, 0.5 to 0.79 as medium effects, and 0.8 or greater as large effects.
Given that the treatment gains associated with mPE were demonstrated to be greater than those of the health information-based intervention (Coffey et al., 2016), a series of mixed-model 2 × 2 repeated-measures analyses of variance (ANOVAs) were run using the linear mixed-effects models procedure in SPSS to examine the combined influence of opioid use and time on relevant clinical outcome measures for individuals who received mPE. Instead of utilizing listwise deletion, the mixed-model approach uses all available data; thus, mixed-model analyses produce unbiased estimates of model parameters when data are missing at random. Because mixed-model approaches assume that data are missing at random, we conducted ANOVAs examining differences on continuous measures and chi-square tests examining differences on dichotomous measures to determine whether participants who were missing end-of-treatment data differed from those who completed the end-of-treatment assessment in terms of demographics or outcome measures at baseline. Dependent variables in mixed models included alcohol craving, PTSD symptoms, sleep, anxiety, and depression. Opioid use (OUD vs. non-OUD) was included as a fixed effect, the time at which outcome measures were obtained (pre- or posttreatment) was included as the repeated-measures factor, and participant identification number was included as a random effect in the model. Finally, an unstructured covariance matrix was specified for the repeated measurements, and model parameters were estimated using restricted maximum likelihood. Results of the model are expressed as means with 95% CIs, F values, and p values. Cohen’s d (Cohen, 1992) was used to express effect-sizes for each of the outcome variables.
Results
Many participants lived in rural counties located near the treatment facility, and a majority were self-referred for treatment. Table 1 shows that the OUD and non-OUD groups were similar on most demographic variables. However, the OUD group was younger on average relative to the non-OUD group.
Table 1.
Participant Demographic Characteristics at Baseline
| OUD (n = 52) |
Non-OUD (n = 74) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | M | SD | % | M | SD | % | ORa | 95% CI | SMD |
| Gender | |||||||||
| Male | 50.0 | 56.8 | 1.31 | [0.64, 2.68] | 0.13 | ||||
| Female | 50.0 | 43.2 | |||||||
| Race/Ethnicity | |||||||||
| White | 78.8 | 79.7 | 0.95 | [0.40, 2.27] | 0.08 | ||||
| Non-White | 21.2 | 20.3 | |||||||
| Education | |||||||||
| ≤ High school | 42.3 | 35.1 | 0.74 | [0.36, 1.53] | 0.15 | ||||
| > High school | 57.7 | 64.9 | |||||||
| Employment status | |||||||||
| Employed | 27.0 | 37.8 | 0.64 | [0.30, 1.34] | 0.21 | ||||
| Unemployed | 73.0 | 62.2 | |||||||
| Age, years | 31.58 | 10.12 | 35.69 | 10.48 | 0.96* | [0.93, 0.99] | 0.40 | ||
Note. OUD = opioid use disorder; OR = odds ratio; SMD = standardized mean difference.
Versus non-OUD group.
p < .05.
Per inclusion criteria, 100% of the sample met DSM-IV criteria for alcohol dependence. Further, all participants in the OUD group met DSM-IV criteria for current opioid abuse (n = 3) or dependence (n = 49). In the OUD group, 51 participants (98.1%) reported primary use of prescription opioids and one (2.0%) OUD group member reported heroin as his or her drug of choice; however, 17 (32.7%) participants in the OUD group reported heroin use during their lifetime and 9 (17.3%) participants in the OUD group reported heroin use within the past 3 months. Table 2 shows that compared to the non-OUD group, individuals in the OUD group were more likely to meet criteria for amphetamine and sedative use disorders. After controlling for the use of alcohol and opioids, the OUD group met criteria for a greater number of SUD diagnoses relative to the non-OUD group. The OUD group also reported higher ACQ scores and a significantly greater number of hospitalizations for alcohol and drug use in comparison to the non-OUD group.
Table 2.
Participant Substance Use at Baseline
| OUD (n = 52) |
Non-OUD (n = 74) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | M | SD | % | M | SD | % | ORa | 95% CI | SMD |
| Cannabis diagnosis | 48.1 | 32.4 | 1.93 | [0.93, 4.00] | 0.32 | ||||
| Amphetamine diagnosis | 34.6 | 13.5 | 3.39** | [1.41, 8.15] | 0.52 | ||||
| Sedative diagnosis | 75.0 | 9.5 | 28.71*** | [10.56, 78.06] | 1.81 | ||||
| Cocaine diagnosis | 55.8 | 45.9 | 1.48 | [0.73, 3.03] | 0.19 | ||||
| Hallucinogen diagnosis | 3.8 | 1.4 | 2.92 | [0.26, 33.08] | 0.16 | ||||
| Number of SUD diagnosesb | 2.31 | 1.21 | 1.18 | 1.04 | 2.36*** | [1.64, 3.40] | 1.02 | ||
| ACQ | 164.33 | 58.91 | 142.85 | 61.43 | 1.01* | [1.00, 1.01] | 0.36 | ||
| Outpatient treatment episodesc | 0.90 | 1.13 | 1.34 | 1.73 | 0.81 | [0.61, 1.06] | 0.29 | ||
| Hospitalizationsc | 4.23 | 6.24 | 2.08 | 4.13 | 1.10* | [1.00, 1.20] | 0.42 | ||
Note. OUD = opioid use disorder; OR = odds ratio; SMD = standardized mean difference; SUD = substance use disorder; ACQ = Alcohol Craving Questionnaire.
Versus non-OUD group.
Other than alcohol and opioid use disorders.
For alcohol- or drug-related problems.
p < .05.
p < .01.
p < .001.
As seen in Table 3, participants in the OUD group reported more mean lifetime Criterion A events in comparison to individuals in the non-OUD group (10.27 vs. 8.85; SMD = 0.29); however, the difference was not statistically significant and both groups reported similar levels of distress related to these events. The OUD group also reported more severe PTSD symptoms as measured by the CAPS total score (84.37 vs. 74.57; SMD = 0.57) and Avoidance subscale (36.56 vs. 30.74; SMD = 0.72) relative to the non-OUD group. Additionally, the OUD group reported higher mean scores on the BAI (30.31 vs. 24.45; SMD = 0.44) and BDI (33.75 vs. 28.32; SMD = 0.51) than the non-OUD group.
Table 3.
Posttraumatic Stress Disorder (PTSD) and Other Psychiatric Symptoms at Baseline
| OUD (n = 52) |
Non-OUD (n = 74) |
||||||
|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | ORa | 95% CI | SMD |
| Criterion A events | 10.27 | 4.73 | 8.85 | 5.04 | 1.06 | [0.97, 1.14] | 0.29 |
| IES-R | 52.06 | 14.85 | 50.95 | 14.85 | 1.01 | [0.98, 1.03] | 0.07 |
| CAPS total | 84.37 | 16.38 | 74.57 | 17.80 | 1.03** | [1.01, 1.06] | 0.57 |
| CAPS Reexperiencing |
22.65 | 6.17 | 20.20 | 7.73 | 1.05 | [0.99, 1.11] | 0.34 |
| CAPS Avoidance | 36.56 | 8.16 | 30.74 | 7.95 | 1.10*** | [1.04, 1.15] | 0.72 |
| CAPS Hyperarousal | 25.15 | 6.92 | 23.62 | 6.53 | 1.04 | [0.98, 1.09] | 0.23 |
| ISI | 17.29 | 6.04 | 15.69 | 5.80 | 1.05 | [0.99, 1.12] | 0.27 |
| BAI | 30.31 | 12.66 | 24.45 | 13.96 | 1.03* | [1.01, 1.06] | 0.44 |
| BDI | 33.75 | 10.17 | 28.32 | 10.82 | 1.05** | [1.01, 1.09] | 0.51 |
| Number of anxiety diagnosesb | 1.62 | 1.33 | 1.24 | 1.12 | 1.29 | [0.96, 1.73] | 0.31 |
| Outpatient treatment episodesc | 3.00 | 13.90 | 1.69 | 6.32 | 1.01 | [0.98, 1.05] | 0.13 |
| Hospitalizationsc | 1.15 | 2.07 | 0.70 | 1.82 | 1.13 | [0.93, 1.37] | 0.23 |
Note. OUD = opioid use disorder; OR = odds ratio; SMD = Standardized mean difference; IES-R = Impact of Event Scale–Revised; CAPS = Clinician Administered PTSD Scale; ISI = Insomnia Severity Index; BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory.
Versus non-OUD group.
Other than PTSD.
For a psychiatric problem.
p < .05.
p < .01.
p < .001.
Of the individuals who were assigned to the mPE condition, 34 (40.0%) met DSM-IV criteria for current opioid abuse or dependence and 51 (60.0%) met criteria for non-opioid SUD. At baseline, the two diagnostic groups did not differ significantly on any demographic characteristics. Furthermore, the two groups were statistically equivalent in terms of pretreatment PTSD symptom severity (as measured by the IES-R), p = .612, insomnia severity (as measured by the ISI), p = .098, and anxiety (as measured by the BAI), p = .080. However, participants who met criteria for OUD reported higher pretreatment levels of alcohol craving (as measured by the ACQ), p = .033, PTSD symptomatology (as measured by the CAPS), p = .001, and depressive symptoms (as measured by the BDI-II), p = .038, relative to individuals with nonopioid SUD. Of the 85 participants who received mPE, participants in the OUD group completed a mean of 7.85 (SD = 3.14) therapy sessions and participants in the non-OUD group completed a mean of 8.06 (SD = 3.06) therapy sessions, F(1, 84) = 0.84, p = .772.
Study treatment completion was defined as completion of eight or more therapy sessions (Foa et al., 2005), and all participants were eligible to complete end-of-treatment assessments regardless of whether they completed study treatment. A similar proportion of participants in the OUD (58.8%) and non-OUD (62.7%) groups completed treatment, χ2(1, N = 85) = 0.13, p = .716. Participants who did and did not complete treatment did not differ in terms of age, gender, race, lifetime number of PTSD Criterion A events, or baseline PTSD symptom severity, ps = .341 to .984. Furthermore, participants who did and did not complete the end-of-treatment assessment did not differ significantly in terms of demographics or outcome measures at baseline, ps = .063–.898.
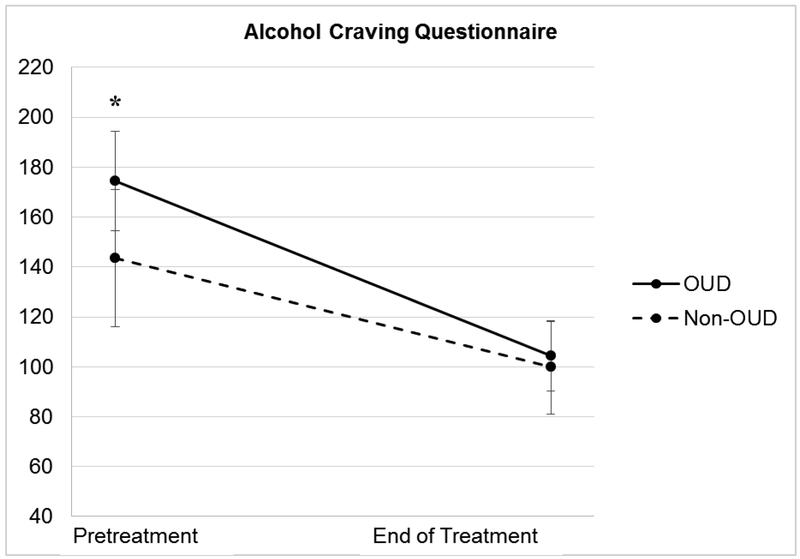
In the first mixed-models analysis, time was a significant predictor of ACQ scores, F(1, 71.42) = 118.88, p <.001, with ACQ scores declining significantly for both groups (see Table 4). The effects indicated that mPE would produce, on average, a 70.11 point decrease, t(70.10) = 8.75, p < .001, d = 1.32, for individuals with OUD. Following treatment, individuals in the nonopioid SUD group reported an average decrease of 43.76 points on the ACQ, t(73.29) = 6.54, p < .001, d = 0.81. When we examined the effect of diagnosis over time, the OUD group reported reductions on the ACQ that were 26.35 points greater on average relative to individuals who met criteria for nonopioid SUD (see Figure 1); thus, the interaction was statistically significant F(1,71.42) = 6.37, p = .014. Although participants with OUD reported pretreatment ACQ scores that were significantly higher than those reported by participants with nonopioid SUD, ACQ scores did not differ significantly between groups following treatment.
Table 4.
Primary Outcomes by Substance Use Disorder Diagnosis, at Baseline and End of Treatment
| Baseline |
Posttreatment |
||||||
|---|---|---|---|---|---|---|---|
| Variable | M | 95% CI | M | 95% CI | F | df | p |
| ACQ | |||||||
| OUD | 174.35 | [152.47, 196.23] | 104.24 | [90.35, 118.13] | |||
| Non-OUD | 143.57 | [125.70, 161.43] | 99.81 | [88.14, 111.48] | 6.37* | 1,71.42 | .014 |
| CAPS | |||||||
| OUD | 87.18 | [81.62, 92.74] | 41.40 | [33.34, 49.46] | |||
| Non-OUD | 75.08 | [70.54, 79.62] | 29.84 | [23.11, 36.58] | 0.11 | 1,65.15 | .919 |
| IES-R | |||||||
| OUD | 52.65 | [47.19-58.11] | 18.09 | [11.78, 24.41] | |||
| Non-OUD | 50.84 | [46.39, 55.30] | 18.00 | [12.73, 23.27] | 0.15 | 1,73.55 | .703 |
| ISI | |||||||
| OUD | 17.91 | [15.90, 19.92] | 10.65 | [7.78, 13.52] | |||
| Non-OUD | 15.73 | [14.08, 17.37] | 9.57 | [7.18, 11.96] | 0.40 | 1,67.35 | .531 |
| BAI | |||||||
| OUD | 30.29 | [25.52, 35.07] | 12.64 | [8.34, 16.94] | |||
| Non-OUD | 24.80 | [20.90, 28.71] | 8.63 | [5.00, 12.26] | 0.22 | 1,72.90 | .644 |
| BDI | |||||||
| OUD | 33.97 | [30.29, 37.65] | 9.23 | [6.12, 12.35] | |||
| Non-OUD | 28.94 | [25.94, 31.94] | 9.08 | [6.45, 11.72] | 3.40 | 1,72.85 | .069 |
Note. OUD = opioid use disorder; ACQ = Alcohol Craving Questionnaire; CAPS = Clinician-Administered PTSD Scale; IES-R = The Impact of Events Scale–Revised; ISI = Insomnia Severity Index; BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory.
p < .05.
Figure. 1.
Alcohol Craving Questionnaire mean scores across the two time points for both groups, with 95% confidence interval bars.
Note. OUD = opioid use disorder. *p < .05 for difference between the two groups (independent samples t test).
In the second mixed model analysis, CAPS scores decreased significantly for individuals in both diagnostic groups over time F(1, 65.15) = 296.89, p < .001 (see Table 4). On average, individuals in the OUD group reported a score reduction of 45.78 points on the CAPS, t(65) = 11.29, p <.001, d = 2.51. Individuals who met criteria for nonopioid SUD reported, on average, a 45.24 point reduction on the CAPS, t(65.36) = 13.36, p <.001, d = 2.49. Although both groups reported significant decreases in PTSD symptomatology, between-group differences observed at baseline were maintained, such that individuals in the non-OUD group reported CAPS scores that were, on average, 11.56 points lower at posttreatment relative to individuals in the OUD group, t(64.90) = 2.20, p = .032, d = 0.56. Thus, the Diagnosis x Time interaction was nonsignificant, F(1, 65.15) = 0.11, p = .919, indicating that groups did not differ significantly in their treatment response.
The effects on IES-R scores supported the finding that PTSD symptoms decreased over time regardless of diagnosis, F(1, 73.55) = 226.45, p < .001 (see Table 4). On average, mPE was associated with an average decrease of 34.56 points for individuals who met criteria for OUD, t(73.20) = 10.04, p < .001, d = 2.12. Similarly, individuals with nonopioid SUD who received mPE endorsed an average decrease of 32.84 points on the IES-R, t(74.04) = 11.47, p < .001, d = 2.01. Despite these large reductions, the interaction effect of Diagnosis x Time was nonsignificant, F(1,73.55) = 0.15, p = .703, indicating that the groups did not differ significantly in their treatment response.
Insomnia severity index scores decreased for both groups following mPE, F(1, 67.35) = 58.59, p < .001 (see Table 4). On average, mPE was associated with a decrease of 7.26 points for participants who met criteria for OUD, t(67.21) = 5.40, p < .001, d = 1.08. Further, mPE was associated with an average decrease of 6.16 points for participants with nonopioid SUD, t(67.56) = 5.48, p < .001, d = 0.92. The interaction effect of Diagnosis x Time was nonsignificant, indicating that groups did not differ significantly in their treatment response.
Anxiety symptoms on the BAI decreased over time for both groups, F(1, 72.90) = 112.07, p < .001 (see Table 4). On average, individuals in the OUD group reported a decrease of 17.66 points on the BAI, t(72.20) = 7.21, p < .001, d = 1.38, and participants in the nonopioid SUD group reported an average decrease of 16.17 points, t(73.85) = 7.88, p < .001, d = 1.26. Using a cutoff score of 10 (the lowest BAI category), 46.2% of participants with OUD and 77.8% of participants with nonopioid SUD who received mPE were no longer in the clinical range on the BAI following treatment. The interaction effect of Diagnosis x Time was nonsignificant, F(1, 72.90) = 0.22, p = .644, indicating that the groups did not differ significantly in their treatment response.
Depressive symptoms on the BDI-II decreased over time for both groups, F(1, 72.85) = 284.22, p < .001 (see Table 4). On average, mPE was associated with a 24.74 point reduction on the BDI-II, t(71.96) = 12.18, p < .001, d = 2.56, for individuals with OUD and a 9.86 point reduction in depressive symptoms for participants with nonopioid SUD diagnoses, t(74.10) = 11.72, p < .001, d = 2.05. Using a cutoff score of 14 (the lowest BDI-II category), 80.8% of participants in the OUD group and 75.0% of participants in the nonopioid SUD group scored below the clinical range at posttreatment. In contrast, only 2.9% of the participants in the OUD group and 5.9% of participants in the nonopioid SUD group fell below this threshold at pretreatment. The nonsignificant Diagnosis x Time interaction effect, F(1, 72.85) = 3.40, p = .069, indicated that mPE was associated with similar reductions in BDI-II scores for both groups.
Discussion
The present study examined adults with DSM-IV-defined substance use dependence who were enrolled in a residential SUD treatment program. We compared individuals diagnosed with both OUD and other SUDs with individuals diagnosed with SUDs other than OUD in order to determine how opioid use affected PTSD symptom severity. Overall, participants with OUD were distinguished by being younger, reporting more cravings for alcohol, being hospitalized more frequently for drug- and alcohol-related problems, and suffering from more severe PTSD symptomatology, depressive symptoms, and anxiety. Similar to the findings of Lubman and colleagues (2016), individuals in the OUD group reported greater SUD-related impairment and a greater total number of SUD diagnoses (2.31 vs. 1.18) relative to the non-OUD comparison group after controlling for alcohol and opioid diagnoses. In terms of specific substances, a greater proportion of the OUD group met diagnostic criteria for methamphetamine and sedative use disorders relative to the non-OUD comparison group. The rate of opioid misuse concurrent with illicit substances, such as methamphetamine and sedatives, is concerning, given that polysubstance use is often associated with serious health risks including overdose (Calcaterra, Glanz, & Binswanger, 2013). With the introduction of abuse deterrent formulations and the establishment of prescription drug monitoring programs, fewer prescription opioids are available for diversion and misuse. In response, many individuals have turned to heroin as a cheaper and more readily available option (Cicero, Ellis, & Surratt, 2014). Accordingly, the prevalence of polysubstance use in the OUD group may reflect that individuals who engage in problematic opioid use may turn to other substances when they lack regular access their drug of choice. However, additional research examining contextual variables, including drug availability and cost, is necessary before meaningful conclusions can be drawn.
In addition to high rates of SUD-related consequences and the concurrent abuse of multiple substances, individuals with OUD reported significantly higher scores on the CAPS, BAI, and BDI relative to participants in the non-OUD comparison group. Thus, it is important to understand the association between trauma-related psychiatric distress and substance use. Some opioid misuse may be interpreted as an attempt to self-medicate psychiatric distress (Khantzian, 1985); however, pain may interact with psychiatric distress in the initiation and maintenance of opioid misuse among individuals with PTSD. Indeed, Sullivan, Edlund, Zhang, Unutzer, and Wells (2006) proposed that some individuals diagnosed with psychiatric disorders use opioids to self-medicate a poorly differentiated state of physical pain and psychiatric distress. This proposal is particularly relevant given that individuals with PTSD are more likely to use opioids for pain compared to individuals without a diagnosis of PTSD (Phifer et al., 2011). Because indices of coping, motivation, and pain were not included in the present study, we were unable to draw many conclusions regarding the function of problematic opioid use. However, future longitudinal research should examine the transition from medical to nonmedical use of prescription opioids among individuals with PTSD.
The present study was among the first to examine exposure-based therapy specifically among participants with comorbid PTSD, alcohol dependence, and OUD. Although the OUD group initially presented with higher levels of alcohol craving, PTSD symptomatology, and depression, mPE produced large reductions in PTSD symptomatology, sleep disturbances, and symptoms of anxiety and depression for individuals with OUD, with effect sizes (Cohen’s d) ranging from 1.08 to 2.56, such that the two groups were nearly identical in symptom severity after completion of treatment. In terms of between-group differences, individuals with OUD reported significantly greater reductions in alcohol craving relative to participants in the non-OUD comparison group. This finding is consistent with the findings of Kaczkurkin, Asnaani, Alpert, and Foa (2016), who demonstrated that individuals with more severe PTSD symptomatology showed greater improvements in alcohol craving over time. Together, these findings suggest that individuals with more severe PTSD and SUD symptomatology have more room for improvement over time. Further, the effectiveness of exposure-based therapies is not limited to individuals diagnosed with one SUD of mild-to-moderate severity. Rather, exposure-based interventions hold great potential for improving mental health and substance craving among individuals who present with severe psychiatric symptoms and drug-related health consequences, such as those observed among individuals who concurrently engage in the use of alcohol and opioids.
Participants in the OUD group also reported a 47.5% reduction in symptoms of PTSD as measured by the CAPS. Although the two groups did not differ significantly in their reported reductions in PTSD symptomatology from pre- to posttreatment, participants in the OUD group reported significantly higher average posttreatment CAPS scores relative to participants in the non-OUD comparison group (41.40 vs. 29.84). This finding was unexpected and suggests that co-occurring AUD and OUD may in fact result in a modestly less favorable PTSD treatment response. Notably, the two study groups were not equal on all outcome measures at baseline; therefore, a variety of factors may be associated with the observed changes that occurred over the course of treatment. Although it is possible that individuals diagnosed with both AUD and OUD benefit less from exposure-based therapies, the two groups reported nearly identical reductions in PTSD symptomatology and reported similar posttreatment levels of PTSD symptomatology as measured by the IES-R. Therefore, more research that examines the substance-specific effects of exposure-based treatment for PTSD is needed. Another limitation of this study is that substance use TAU is often associated with improvements in both PTSD and SUD outcomes (Torchalla, Nosen, Rostam, & Allen, 2012). Accordingly, treatment gains may have been at least partially attributable to the substance use treatment provided by the residential treatment facility. Future research may include an assessment-only condition to better evaluate the potential benefit associated with TAU. Additionally, the number of participants who met criteria for OUD was relatively small. As a result, the present sample size left some room for chance findings and type II error (i.e., false negative). Another limitation is that we did not assess the number of participants who were court-mandated to attend SUD treatment. Accordingly, it is unclear whether participants who were court-mandated to attend treatment differed from participants who attended treatment voluntarily. Finally, measures of substance use and opioid craving were not included as part of the posttreatment assessment. All participants met the DSM-IV diagnostic criteria for alcohol dependence, and because participants completed the end-of-study assessment while at a residential treatment facility, measures of substance use were expected to be near zero. Therefore, self-reported alcohol craving was determined to be most relevant, as craving was expected to vary over the course of treatment. Although mPE was associated with significant reductions in alcohol craving, future research examining individuals with co-occurring PTSD and OUD should assess opioid craving.
Given the benefits presented in this and previous studies (e.g., Coffey et al., 2016), it may be prudent for community-based SUD treatment programs to integrate exposure-based therapies for PTSD with SUD treatment. Despite the enduring concern that exposure-based treatments will exacerbate existing psychiatric symptoms and prompt relapse or treatment dropout, individuals with OUD who received mPE reported significant reductions in psychiatric symptoms. Adults with OUD also experienced greater reductions in alcohol craving than participants in the non-OUD comparison group. In terms of dropout, participants attended eight treatment sessions on average, and a majority of the participants (58.8%) assigned to receive mPE completed treatment. Despite these positive results, support should be provided for individuals who may experience increased urges to use drugs or alcohol, particularly at the start of treatment. Although individuals who engage in problematic drinking and concurrent opioid use may initially present with high levels of distress, the current study provided preliminary evidence that mPE may be beneficial for this population.
Acknowledgments
This research was supported in part by the National Institute on Alcohol Abuse and Alcoholism (R01AA016816; PI: Coffey). We thank M. Trost Friedler, Jackie Lampley, and the staff and clients of Harbor House Recovery Center for their cooperation on this study.
References
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: Author. [Google Scholar]
- Beck AT & Steer RA (1993). Manual for the Beck Anxiety Inventory. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, & Roland CL (2011). Societal costs of prescription opioid abuse, dependence and misuse in the United States. Pain Medicine, 12, 657–667. https://doi.org/10.1111/j.1526–4637.2011.01075.x [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8, 75–90. 10.1002/jts.2490080106 [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, & Forneris CA (1996). Psychometric properties of the PTSD Checklist (PCL). Behavior Research and Therapy, 34, 669–673. 10.1016/0005-7967(96)00033–2 [DOI] [PubMed] [Google Scholar]
- Blanco C, Xu Y, Brady K, Perez-Fuentes G, Okuda M, & Wang S (2013). Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: Results from National Epidemiological Survey on Alcohol Related Conditions. Drug and Alcohol Dependence, 132, 630–638. 10.1016/j.drugalcdep.2013.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcaterra S, Glanz J, & Binswanger IA (2013). National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999–2009. Drug and Alcohol Dependence, 131, 263–270. 10.1016/j.drugalcdep.2012.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, & Surratt HL (2014). The changing face of heroin use in the United States: A retrospective analysis of the past 50 years. JAMA Psychiatry, 71, 821–826. 10.1001/jamapsychiatry.2014.366 [DOI] [PubMed] [Google Scholar]
- Coffey SF, Schumacher JA, Nosen E, Littlefield AK, Henslee AM, Lappen A & Stasiewicz PR (2016). Trauma-focused exposure therapy for chronic posttraumatic stress disorder in alcohol and drug dependent patients: A randomized controlled trial. Psychology of Addictive Behaviors, 30, 778–790. 10.1037/adb0000201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Stasiewicz PR, Hughes PM, & Brimo ML (2006). Trauma-focused imaginal exposure for individuals with comorbid posttraumatic stress disorder and alcohol dependence: Revealing mechanisms of alcohol craving in a cue reactivity paradigm. Psychology of Addictive Behaviors, 20, 425–435. 10.1037/0893-164X.20.4.425 [DOI] [PubMed] [Google Scholar]
- Cohen J (1992). A power primer. Psychological Bulletin, 112, 155–159. 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Connolly KM, Coffey SF, Bashnagel JS, Drobes DJ, & Saladin ME (2009). Evaluation of the alcohol craving questionnaire-now factor structures: Application of a cue reactivity paradigm. Drug and Alcohol Dependence, 103, 84–91. 10.1016/j.drugalcdep.2009.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, & Grant BF (2006). Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of Clinical Psychiatry, 67, 247–257. 10.4088/JCP.v67n0211 [DOI] [PubMed] [Google Scholar]
- Foa EB, Hembree EA, Cahill SP, Rauch SA, Riggs DS, Feeny NC, & Yadin E (2005). Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: Outcome at academic and community clinics. Journal of Consulting and Clinical Psychology, 73, 953–964. 10.1037/0022-006X.73.5.953 [DOI] [PubMed] [Google Scholar]
- Foa E, Hembree E, & Rothbaum BO (2007). Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences therapist guide. New York, NY: Oxford University Press. [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA Jr., Oslin D, … Volpicelli J (2013). Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: A randomized clinical trial. JAMA, 310, 488–495. 10.1001/jama.2013.8268 [DOI] [PubMed] [Google Scholar]
- Hartzler B, Donovan DM, & Huang Z (2010). Comparison of opiate-primary treatment-seekers with and without alcohol use disorder. Journal of Substance Abuse Treatment, 39, 114–123. 10.1016/j.jsat.2010.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Asnaani A, Alpert E, & Foa EB (2016). The impact of treatment condition and the lagged effects of PTSD symptom severity and alcohol use on changes in alcohol craving. Behavior Research and Therapy, 79, 7–14. 10.1016/j.brat.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedia S, Sell MA, & Relyea G (2007). Mono- versus polydrug abuse patterns among publicly funded clients. Substance Abuse Treatment, Prevention, and Policy, 2, 33 10.1186/1747-597X-2-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ (1985). The self-medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence. American Journal of Psychiatry, 142, 1259–1264. 10.1176/ajp.142.11.1259 [DOI] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP, & Koob GF (2012). Stress modulation of drug self-administration: Implications for addiction comorbidity with post-traumatic stress disorder. Neuropharmacology, 62, 552–564. 10.1016/j.neuropharm.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Garfield JBB, Manning V, Berends L, Best D, Mugavin JM, … Allsop S (2016). Characteristics of individuals presenting to treatment for primary alcohol problems versus other drug problems in the Australian patient pathways study. BMC Psychiatry, 16 10.1186/s12888-016-0956-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier A, Lambert-Harris C, McGovern MP, Xie H, An M, & McLeman B (2014). Co-occurring prescription opioid use problems and posttraumatic stress disorder symptoms severity. American Journal of Drug and Alcohol Abuse, 40, 304–311. 10.3109/00952990.2014.910519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Teesson M, Back SE, Brady KT, Baker AL, Hopwood S, … Ewer PL (2012). Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: A randomized controlled trial. JAMA, 308, 690–699. 10.1001/jama.2012.9071 [DOI] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Belanger L, & Ivers H (2011). The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34, 601–608. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacasch N, Huppert JD, Su Y-J, Kivity Y, Dinshtein Y, Yeh R, & Foa EB (2015). Are 60-minute prolonged exposure sessions with 20-minute imaginal exposure to traumatic memories sufficient to successfully treat PTSD? A randomized noninferiority clinical trial. Behavior Therapy, 46, 328–341. 10.1016/j.beth.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Phifer J, Skelton K, Weiss T, Schwartz AC, Wingo A, Gillespie CF, … Ressler KJ (2011). Pain symptomatology and pain medication use in civilian PTSD. Pain, 152, 2233–2240. 10.1016/j.pain.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, & Grant BF (2011). Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: Results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Anxiety Disorders, 25, 456–465. 10.1016/j.janxdis.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, & Foa EB (2010). A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clinical Psychology Review, 30, 635–641. 10.1016/j.cpr.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Rash CJ, Coffey SF, Baschnagel JS, Drobes DJ, & Saladin ME (2008). Psychometric properties of the IES-R in a sample of traumatized substance users. Addictive Disorders, 33, 1039–1047. 10.1016/j.addbeh.2008.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick H (1996). Psychometric review of National Women’s Study (NWS) Event History-PTSD Module In Samm BH (Ed.), Measurement of stress trauma, and adaptation (pp. 214–217). Lutherville, MD: Sidrian Press. [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, & Rourke KM (2000). Diagnostic Interview Schedule for the DSM-IV (DIS-IV). St. Louis, MO: Washington University. [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, & Gladden RM (2016). Increases in drug and opioid overdose deaths – United States, 2003–2012. MMWR. Morbidity and Mortality Weekly Report, 64, 50–51. 10.15585/mmwr.mm6450a3 [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, & Grant JR (1993). Development of the Alcohol Use Disorders Screening Test (AUDIT). WHO collaborative project on early detection of persons with harmful alcohol consumption. II. Addiction, 88, 791–04. 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- Schacht RL, Brooner RK, King VL, Kidorf MS, & Peirce JM (2017). Incentivizing attendance to prolonged exposure for PTSD with opioid use disorder patients: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 85, 689–701. 10.1037/ccp0000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar, G. C. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59, 22–23. 10.1016/S0924-9338(97)83297-X [DOI] [PubMed] [Google Scholar]
- Singleton EG, Tiffany ST, & Henningfield JE (2003). The Alcohol Craving Questionnaire (ACQ-Now). In Allen JP & Wilson VB (Eds.), Assessing alcohol problems: A guide for clinicians and researchers (2nd ed.; pp. 271–281). Baltimore, MD: National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Stasiewicz PR, Bradizza CM, Schlauch RC, Coffey SF, Gulliver SB, Gudleski GD, & Bole CW (2013). Affect regulation training (ART) for alcohol use disorders: Development of a novel intervention for negative affect drinkers. Journal of Substance Abuse Treatment, 45, 433–443. 10.1016/j.jsat.2013.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiewicz PR, & Maisto SA (1993). Two-factor avoidance theory: The role of negative affect in the maintenance of substance use and substance use disorder. Behavior Therapy, 24, 337–356. 10.1016/S0005-7894(05)80210-2 [DOI] [Google Scholar]
- Sullivan MD, Edlund MJ, Zhang L, Unutzer J, & Wells KB (2006). Association between mental health disorders, problem drug use, and regular prescription opioid use. Archives of Internal Medicine, 166, 2087–2093. 10.1001/archinte.166.19.2087 [DOI] [PubMed] [Google Scholar]
- Torchalla I, Nosen L, Rostam H, & Allen P (2012). Integrated treatment programs for individuals with concurrent substance use disorders and trauma experiences: A systematic review and meta-analysis. Journal of Substance Abuse Treatment, 42, 65–77. 10.1016/j.jsat.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Weathers F, Keane TM, & Davidson JR (2001). Clinician-Administered PTSD Scale: A review of the first ten years of research. Depression and Anxiety, 13, 132–156. 10.1002/da.1029 [DOI] [PubMed] [Google Scholar]
- Weathers F, Litz B, Herman D, Huska J, & Keane T (1993). The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at the Annual Convention of the International Society for Traumatic Stress Studies, San Antonio, TX. [Google Scholar]
- Wei LJ (1978). An application of an urn model to the design of sequential controlled trials. Journal of the American Statistical Association, 73, 559–563. 10.1080/01621459.1978.10480054 [DOI] [Google Scholar]
- Weiss DS, & Marmar CR (1997). The impact of event scale-revised In Wilson JP & Keane TM (Eds.), Assessing psychological trauma and PTSD (pp. 399–411). New York, NY: Guilford Press. [Google Scholar]