Abstract
Nuclear spin hyperpolarization overcomes the sensitivity limitations of traditional NMR and MRI, but the most general method demonstrated to date (dynamic nuclear polarization) has significant limitations in scalability, cost, and complex apparatus design. As an alternative, signal amplification by reversible exchange (SABRE) of parahydrogen on transition metal catalysts can hyperpolarize a variety of substrates, but to date this scheme has required transfer of the sample to low magnetic field or very strong rf irradiation. Here we demonstrate “Low-Irradiation Generation of High Tesla-SABRE” (LIGHT-SABRE) which works with simple pulse sequences and low power deposition; it should be usable at any magnetic field and for hyperpolarization of many different nuclei. This approach could drastically reduce the cost and complexity of producing hyperpolarized molecules.
Keywords: NMR and MRI, parahydrogen, hyperpolarization, NMR spectroscopy, magnetic properties
“Hyperpolarization” methods developed over the last few decades give samples much larger nuclear spin polarization than would be obtained by simply putting the samples into a large magnetic field. This enables MRI of biomolecules at low concentrations and NMR studies of complex systems with concentrations in the nanomolar regime. However, the most general molecular hyperpolarization methods (dissolution dynamic nuclear polarization (d-DNP)[1] and a variant of para-hydrogen induced polarization (PHIP)[2, 3] called signal amplification by reversible exchange (SABRE)[4, 5]) generally require ex situ hyperpolarization followed by transfer of the hyperpolarized material into the magnet for detection. Here we demonstrate a method we call Low Intensity Generation of High-Tesla SABRE (LIGHT-SABRE), which should enable SABRE hyperpolarization at any magnetic field used in NMR or MRI, requires low rf power, and drastically simplifies apparatus design.
The original approach to hyperpolarization with parahydrogen (PHIP) involves catalytic addition to a substrate.[2, 3] This approach is limited in several ways: it requires unsaturated precursors, both catalyst and substrate need to be specifically optimized, and the hyperpolarization is inherently induced in protons, which have a relatively short relaxation time T1 (although the polarization can transferred to 13C sites [6–9] to facilitate in vivo imaging).[10–12] SABRE, a more flexible variant of PHIP, works by reversibly binding both para-hydrogen and the to-be-polarized substrate to a metal complex[4, 5] and has been used to polarize a variety of molecules[13–17]. Typically the complex is kept at low magnetic fields (~5 mT) to create strong coupling conditions, where continuous exchange of substrate and para-hydrogen creates large polarization on the free substrate within seconds. However, NMR or MRI then requires fast field-cycling between the low field and high field of the MR magnet, unless detection is performed directly at low field [18–22]; in addition, the specific low field needed may vary with every molecule and catalyst. At the high fields of superconducting magnets only modest enhancements have been observed without irradiation.[23] Several groups have shown that strong irradiation can transfer para-hydrogen order to other hydrogen atoms in symmetric spin systems at high magnetic fields [24–27], but this requires rf locking fields with strengths comparable to or larger than the resonance frequency difference between para-hydrogen and the other target atoms (on the order of ω1=γB1≈104–105 rad/s for ring protons, and essentially impossible for other nuclei).
The key insight in this paper is that low-power CW pulses (less than .01% of the power in reference[24]) can cause large coherent polarization transfer from para-hydrogen to metal-bound ligands at any magnetic field, as long as the para-hydrogen atoms are chemically equivalent but not magnetically equivalent (a very common case). Here we demonstrate a particularly simple example, using 15N labeled pyridine (Figure 1). All that is required is that the CW-power match the sum (or difference) of hydride-hydride and 15N-15N J-coupling (γB1=2π(JHH±JNN)). In this application, LIGHT-SABRE directly creates 15N polarization from the para-hydrogen order. But the technique is very general, as no resonance frequency matching or strong coupling between the hydride and other atoms is required; any nucleus would be usable, and this method could also generate hyperpolarized ortho-hydrogen. Furthermore, in contrast to all previous SABRE work, the coherent dynamics are easy to understand and thus straightforward to optimize.
Figure 1.
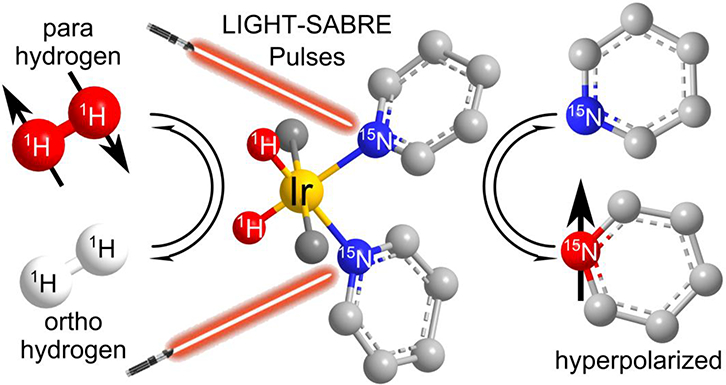
The LIGHT-SABRE (Low Irradiation Generation of High Tesla Signal Amplification by Reversible Exchange) process enables hyperpolarization at any magnetic field. Parahydrogen and 15N-pyridine dissolved in the solvent are in constant chemical exchange with their respective bound forms on the Iridium complex, which can be viewed as an AA’XX’ spin system (with A=1H and X=15N). Singlet hyperpolarization is converted to magnetization on the bound 15N-pyridine by the LIGHT-SABRE pulses and finally accumulated on the free pyridine through the exchange processes. The catalytic system used was [IrCl(COD)(IMes)] (IMes = 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene; COD = cyclooctadiene) as precursor; upon hydrogenation COD and Cl− are replaced by the hydrogen and pyridine to form the catalytically active form [IrH2Py3(IMes)]+.[4, 5]
We start by noting that the SABRE complex binds para-hydrogen and pyridine (and often other targets) without inducing a chemical shift difference between the para-hydrogen atoms, or between the bound pyridine molecules. However, each para-hydrogen atom is coupled differently to the atoms of the two pyridine rings: one 1H-Ir-15N bond angle is ~90° (JNH), the other 1H-Ir-15N bond angle is ~180° (JNH’). In NMR parlance this means the hydrogens (and nitrogens) are chemically equivalent, but not magnetically equivalent. An AA’XX’ spin system is formed (see Fig.1) where ΔJNH = (JNH-JNH’)≠0 breaks the magnetic equivalence and allows for coherent conversion of the singlet order into hyperpolarization. We estimate ΔJNH ~1 Hz and (JHH+JNN) ~12Hz (see supplement), dominated by JHH with a small contribution from JNN.
In the limit that (JHH+JNN)>>ΔJNH (achieved here and likely for many other targets), previous work has shown that AA’XX’ spin systems (and more generally systems of the form AA’XnXn’) often support very long lived nuclear spin states which can be accessed by simple pulse sequences.[28, 29] The Hamiltonian of the spin system is
| (1) |
and for our purposes, the 16 basis states are conveniently expressed as follows. We designate the two hydride atoms by eigenstates of the z-component of the angular momentum:
| (2) |
The first state is the “singlet state”, the remaining three are the “triplet states”. The two nitrogens also have four possible states, but for those we will express the triplet states as eigenstates of the x-component of the angular momentum:
| (3) |
Previous work has shown that continuous irradiation at a specific low power or shaped pulses can transfer population in and out of long-lived spin states (such as the “15N-singlet – 1H singlet” state SNSH) [28], in an extension of an approach referred to as Spin Lock Induced Crossing (SLIC) [30]. Here the problem is different but closely related: we look to transfer the hydride singlet spin order into bulk magnetization on the rings. Specifically, LIGHT-SABRE starts with bound para-hydrogen and unpolarized targets, so there is a large excess of population in the state SH, and essentially equal population in each of the four possible bound pyridine N-states:
| (4) |
In this basis set the 16×16 Hamiltonian of the AA’XX’ system, in the presence of irradiation with amplitude ω1 = γB1 of phase x applied at the nitrogen frequency, only has important dynamics in two 4×4 submatrices.[28]
| (5) |
| (6) |
These matrices would be diagonal, with no interesting dynamics, except for ΔJNH=JNH-JNH’ which breaks the magnetic equivalence.
Now, for Eq. (5), continuous irradiation with amplitude ω1 = −2π(JHH + JNN) equalizes the diagonal elements between the first and the second state. The effect of the off-diagonal element, , is to transfer population between SNSH and This two-level problem is mathematically identical to the normal spin two-level system: irradiation for a time will be a “π pulse” which transfers population between the two states, converting 25% of the hydrogen singlet into x-magnetization on 15N. The other two states are not populated in the limit (JHH+JNN) >> ΔJNH.
In Eq. (6) an irradiation with amplitude ω1 = −2π(JHH − JNN) transfers the 25% population in to which does not have any net magnetization. However, now the large nitrogen magnetization from the 25% population in is not cancelled out by the population. Therefore, Eq. 5 and Eq. 6 each convert 25% singlet-polarization into detectable x-magnetization. For the present catalytic system JNN is expected to be small, and then the resonance conditions for matrices 4 and 5 are identical, which leads us to conclude that 50% of the initial singlet polarization could in principle be converted into detectable hyperpolarization.
In practice, the transfer is limited by the exchange rate of both the hydrogen and the free pyridine, both reported to be on the order of ~10/s corresponding to ~0.1s residence times on the catalyst.[14] Under this assumption, during one cycle of exchange only a fraction of the hyperpolarization can be transferred into 15N x-magnetization, but because the exchange keeps refreshing the singlet reservoir the process keeps pumping x-magnetization. However, as soon as the pyridine dissociates the x-magnetization would dephase immediately, no longer being locked by the cw SLIC irradiation. To avoid this problem we simply interleave 90° pulses applied selectively on the bound 15N-pyridine, creating z-magnetization which then accumulates on the free pyridine upon dissociation, as displayed in Fig.2 (the free pyridine in solution sees no effect of the pulse sequence, as that nitrogen’s resonance frequency for the weak pulses to have any impact). To maximize the polarization, the pulse sequence in Figure 2 is repeated n times, at a repetition rate comparable to the exchange rate, and for a time comparable to the solution T1. Upon optimization (see supplement) we found n=15 and τcw = 0.5 s to work well, giving a total LIGHT-SABRE pulse length of 7.5 s. Note that this time plus the time for acquisition (0.8 s) is the total experimental time.
Figure 2.
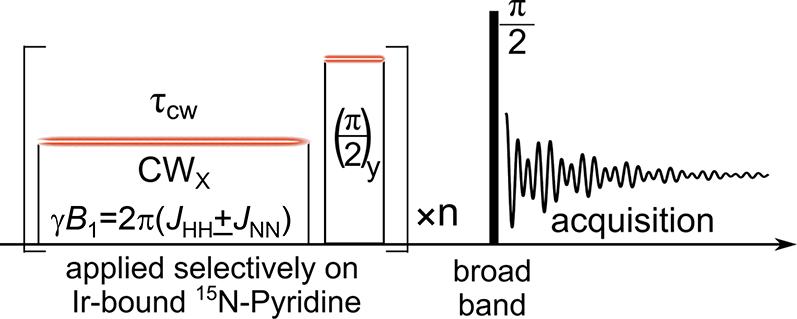
LIGHT-SABRE pulse sequence. A long weak pulse of amplitude ω1 = 2π(JHH ± JNN) is applied, optimally for a time . This pulse converts singlet hydride polarization into x-magnetization on the catalyst bound substrate (15N-pyridine). Subsequently, a 90° pulse selective to the bound substrate converts x-magnetization to z-magnetization, which is retained on the substrate upon dissociation. Repetition of this process n times builds up hyperpolarization on the free substrate. A broad-band 90° pulse and acquisition concludes the experiment. If JNN is negligible, as in the current case, magnetization buildup is twice as fast. Irradiation instead at the Ir-bound p-H2 frequency would create hyperpolarized ortho-H2.
In Fig. 3 we show the experimental results measured for two samples at 2.5 mM 15N-pyridine and 63 mM 15N-pyridine at 9.4 T; catalyst concentration was one tenth of the respective 15N-pyridine concentration. All measurements were carried out under constant bubbling of parahydrogen at 5.2 bar, including during acquisition. This makes this method highly reproducible eliminating all experimental uncertainties resulting from sample transfer and discontinued parahydrogen supply typically experienced in other SABRE experiments. (For full experimental details see the supplementary information.)
Fig. 3.
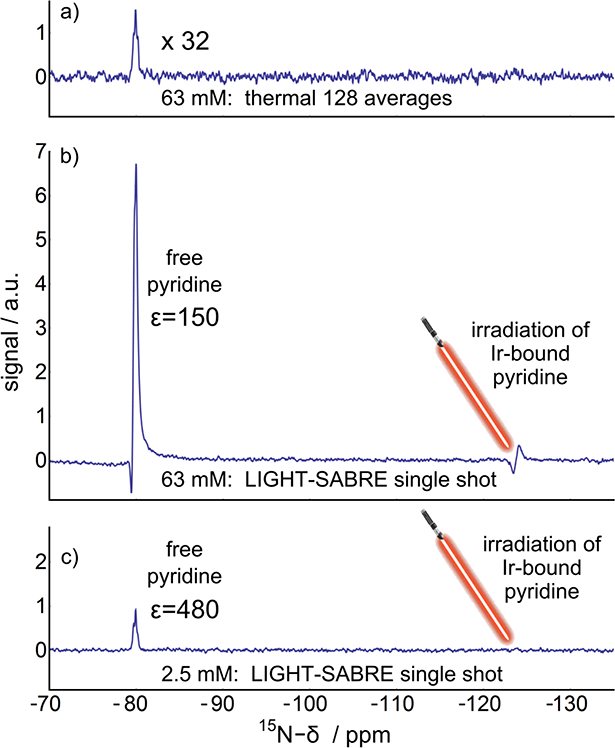
Experimental demonstrations of the LIGHT-SABRE approach at two concentrations. a) Thermal spectrum obtained after 128 averages of 90-acquire on 63mM 15N-pyridine in Methanol at 9.4T b) The LIGHT-SABRE experiment conducted at that same 63 mM concentration as depicted in part (a), yielding a 150-fold enhancement. c) The LIGHT-SABRE experiment conducted at 2.5 mM concentration yielding a 480-fold enhancement. The 15N-pyridine to catalyst concentration was 10:1. The LIGHT-SABRE pulse sequence including acquisition was applied under constant bubbling of parahydrogen making the experiments highly reproducible.
This experiment can be readily improved: for example, the RF-coil did not irradiate the entire sample, which is especially problematic given that the bubbles move the sample in and out of the sensitive region of the coil. Still, with this arrangement, for the 2.5 mM solution an enhancement of 480 is observed over the 9.4T thermal signal. For the higher concentration of 63 mM a larger absolute signal is observed but the enhancement over thermal is only 150-fold, partially reflecting the shorter T1 time of the free pyridine at this catalyst concentration. Notice the partial antiphase character of the signal in Fig 3.b), which results from hyperpolarized terms involving the ortho-protons of the free pyridine, indicating that proton polarization is established as well (currently not included in the presented model).
Note that nothing in the theoretical treatment uses the magnetic field strength or the resonance frequency difference between the hydride and the targeted atoms (except that in using the truncated form of the scalar coupling, we assume it is much larger than 10 Hz). References [28] [31, 32] and [33] present the mathematical treatments of SLIC with AA’XnXn’ and AA’QQ’ spin systems respectively (where Q is a quadrupolar nucleus), and by inspection exactly the same LIGHT-SABRE sequence will work in those cases. Thus, for example, in the case of unlabelled pyridine, polarization could be transferred using either the couplings from the hydride to the first ring protons, or the couplings from the hydride to the 14N nuclei. The most important practical limitation is the T1 time of the target nucleus, which depends on the molecule, concentration and field strength, and limits the total accumulated polarization. For this reason, more typical clinical imaging fields (ca. 1T) will generally give larger enhancements, as T1 increases with lower field for virtually every molecule except water. Also note that, while in this case we irradiated at the bound nitrogen frequency, the matricies in equations [5] and [6] can be trivially rewritten to describe spin locking at the bound para-hydrogen frequency; in that case, the state SHSN is converted to X1HT0N and dissociation then produces hyperpolarized ortho-hydrogen gas.
There are many straightforward modifications which would likely be useful; for example, irradiating the entire sample would greatly increase the signal. Also, the weak CW pulse at the bound pyridine frequency can be combined with a stronger locking field at the free pyridine frequency, such that the induced x-magnetization does not dephase upon dissociation. This experiment would then no longer require the selective 90 for storage of the magnetization.
Most importantly, the in situ hyperpolarization we have demonstrated could also be done directly in a clinical MRI magnet-without the need for cryogenic cooling, microwave or high rf irradiation, and without recycling times between experiments. The less-homogeneous magnetic field would not be an issue for 15N (a 1 ppm linewidth at 1 Tesla is about 4 Hz), and could easily be compensated for other nuclei using the “adiabatic SLIC” demonstrated in reference [28], or with an echo pulse train.
In summary, we have demonstrated a strategy to create hyperpolarization (LIGHT-SABRE) at any magnetic field, without field shuttling or cryogenic cooling, and with very low power deposition. The only requirement on the apparatus is the ability to give a simple pulse sequence, which can be conducted on any modern high-resolution NMR spectrometer. We thus believe that LIGHT-SABRE will be a practical solution for production of large quantities of hyperpolarized reagents for in situ and ex situ use, without the requirement for highly specialized, expensive and high-maintenance instrumentation.
Supplementary Material
Acknowledgements
We thank Prof. Boyd M. Goodson and Fan Shi for providing the SABRE catalyst, [IrCl(COD)(IMes)], for this work. We also thank Prof. Kevin W Waddell for access to his parahydrogen generator. This work was supported by NSF under grants CHE-1058727 and CHE-1363008, and by the DOD CDMRP breast cancer award W81XWH-12-1-0159/BC112431.
References
- [1].Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K, Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR, Proceedings of the National Academy of Sciences of the United States of America, 100 (2003) 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bowers CR, Weitekamp DP,Para-Hydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment, J Am Chem Soc, 109 (1987) 5541–5542. [Google Scholar]
- [3].Pravica MG, Weitekamp DP, Net Nmr Alignment by Adiabatic Transport of Para-Hydrogen Addition-Products to High Magnetic-Field, Chem Phys Lett, 145 (1988) 255–258. [Google Scholar]
- [4].Adams RW, Aguilar JA, Atkinson KD, Cowley MJ, Elliott PI, Duckett SB, Green GG, Khazal IG, Lopez-Serrano J, Williamson DC, Reversible interactions with para-hydrogen enhance NMR sensitivity by polarization transfer, Science, 323 (2009) 1708–1711. [DOI] [PubMed] [Google Scholar]
- [5].Cowley MJ, Adams RW, Atkinson KD, Cockett MCR, Duckett SB, Green GGR, Lohman JAB, Kerssebaum R, Kilgour D, Mewis RE, Iridium N-Heterocyclic Carbene Complexes as Efficient Catalysts for Magnetization Transfer from para-Hydrogen, J Am Chem Soc, 133 (2011) 6134–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chekmenev EY, Hovener J, Norton VA, Harris K, Batchelder LS, Bhattacharya P, Ross BD, Weitekamp DP, PASADENA hyperpolarization of succinic acid for MRI and NMR spectroscopy, J Am Chem Soc, 130 (2008) 4212–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shchepin RV, Coffey AM, Waddell KW, Chekmenev EY, Parahydrogen Induced Polarization of 1-(13)C-Phospholactate-d2 for Biomedical Imaging with >30,000,000-fold NMR Signal Enhancement in Water, Analytical chemistry, 86 (2014) 5601–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Haake M, Natterer J, Bargon J, Efficient NMR pulse sequences to transfer the parahydrogen-induced polarization to hetero nuclei, J Am Chem Soc, 118 (1996) 8688–8691. [Google Scholar]
- [9].Goldman M, Jóhannesson H, Conversion of a proton pair para order into 13C polarization by rf irradiation, for use in MRI, Comptes Rendus Physique, 6 (2005) 575–581. [Google Scholar]
- [10].Bhattacharya P, Chekmenev EY, Perman WH, Harris KC, Lin AP, Norton VA, Tan CT, Ross BD, Weitekamp DP, Towards hyperpolarized (13)C-succinate imaging of brain cancer, Journal of magnetic resonance, 186 (2007) 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Golman K, Axelsson O, Jóhannesson H, Månsson S, Olofsson C, Petersson JS, Parahydrogen-induced polarization in imaging: Subsecond 13C angiography, Magnetic Resonance in Medicine, 46 (2001) 1–5. [DOI] [PubMed] [Google Scholar]
- [12].Bhattacharya P, Chekmenev EY, Reynolds WF, Wagner S, Zacharias N, Chan HR, Bunger R, Ross BD, Parahydrogen-induced polarization (PHIP) hyperpolarized MR receptor imaging in vivo: a pilot study of 13C imaging of atheroma in mice, NMR in biomedicine, 24 (2011) 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mewis RE, Atkinson KD, Cowley MJ, Duckett SB, Green GG, Green RA, Highton LA, Kilgour D, Lloyd LS, Lohman JA, Williamson DC, Probing signal amplification by reversible exchange using an NMR flow system, Magnetic resonance in chemistry : MRC, 52 (2014) 358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].van Weerdenburg BJ, Gloggler S, Eshuis N, Engwerda AH, Smits JM, de Gelder R, Appelt S, Wymenga SS, Tessari M, Feiters MC, Blumich B, Rutjes FP, Ligand effects of NHC-iridium catalysts for signal amplification by reversible exchange (SABRE), Chemical communications (Cambridge, England), 49 (2013) 7388–7390. [DOI] [PubMed] [Google Scholar]
- [15].Gloggler S, Muller R, Colell J, Emondts M, Dabrowski M, Blumich B, Appelt S, Para-hydrogen induced polarization of amino acids, peptides and deuterium-hydrogen gas, Phys Chem Chem Phys, 13 (2011) 13759–13764. [DOI] [PubMed] [Google Scholar]
- [16].Zeng HF, Xu JD, Gillen J, McMahon MT, Artemov D, Tyburn JM, Lohman JAB, Mewis RE, Atkinson KD, Green GGR, Duckett SB, van Zijl PCM, Optimization of SABRE for polarization of the tuberculosis drugs pyrazinamide and isoniazid, Journal of magnetic resonance, 237 (2013) 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hövener J-B, Schwaderlapp N, Borowiak R, Lickert T, Duckett SB, Mewis RE, Adams RW, Burns MJ, Highton LAR, Green GGR, Olaru A, Hennig J, von Elverfeldt D, Toward Biocompatible Nuclear Hyperpolarization Using Signal Amplification by Reversible Exchange: Quantitative in Situ Spectroscopy and High-Field Imaging, Analytical chemistry, 86 (2014) 1767–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Theis T, Ledbetter MP, Kervern G, Blanchard JW, Ganssle PJ, Butler MC, Shin HD, Budker D, Pines A, Zero-field NMR enhanced by parahydrogen in reversible exchange, J Am Chem Soc, 134 (2012) 3987–3990. [DOI] [PubMed] [Google Scholar]
- [19].Gong QX, Gordji-Nejad A, Blumich B, Appelt S, Trace Analysis by Low-Field NMR: Breaking the Sensitivity Limit, Analytical chemistry, 82 (2010) 7078–7082. [DOI] [PubMed] [Google Scholar]
- [20].Theis T, Ganssle P, Kervern G, Knappe S, Kitching J, Ledbetter M, Budker D, Pines A, Parahydrogen-enhanced zero-field nuclear magnetic resonance, Nature Physics, 7 (2011) 571–575. [Google Scholar]
- [21].Hövener J-B, Schwaderlapp N, Lickert T, Duckett SB, Mewis RE, Highton LAR, Kenny SM, Green GGR, Leibfritz D, Korvink JG, Hennig J, von Elverfeldt D, A hyperpolarized equilibrium for magnetic resonance, Nat Commun, 4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gloggler S, Emondts M, Colell J, Muller R, Blumich B, Appelt S, Selective drug trace detection with low-field NMR, Analyst, 136 (2011) 1566–1568. [DOI] [PubMed] [Google Scholar]
- [23].Barskiy DA, Kovtunov KV, Koptyug IV, He P, Groome KA, Best QA, Shi F, Goodson BM, Shchepin RV, Coffey AM, Waddell KW, Chekmenev EY, The feasibility of formation and kinetics of NMR signal amplification by reversible exchange (SABRE) at high magnetic field (9.4 T), J Am Chem Soc, 136 (2014) 3322–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pravdivtsev AN, Yurkovskaya AV, Lukzen NN, Vieth HM, Ivanov KL, Exploiting level anti-crossings (LACs) in the rotating frame for transferring spin hyperpolarization, Phys Chem Chem Phys, 16 (2014) 18707–18719. [DOI] [PubMed] [Google Scholar]
- [25].Franzoni MB, Graafen D, Buljubasich L, Schreiber LM, Spiess HW, Munnemann K, Hyperpolarized 1H long lived states originating from parahydrogen accessed by rf irradiation, Phys Chem Chem Phys, 15 (2013) 17233–17239. [DOI] [PubMed] [Google Scholar]
- [26].Kiryutin AS, Ivanov KL, Yurkovskaya AV, Vieth HM, Lukzen NN, Manipulating spin hyper-polarization by means of adiabatic switching of a spin-locking RF-field, Phys Chem Chem Phys, 15 (2013) 14248–14255. [DOI] [PubMed] [Google Scholar]
- [27].Ivanov KL, Pravdivtsev AN, Yurkovskaya AV, Vieth H-M, Kaptein R, The role of level anti-crossings in nuclear spin hyperpolarization, Progress in Nuclear Magnetic Resonance Spectroscopy, 81 (2014) 1–36. [DOI] [PubMed] [Google Scholar]
- [28].Theis T, Feng Y, Wu T, Warren WS, Composite and shaped pulses for efficient and robust pumping of disconnected eigenstates in magnetic resonance, J Chem Phys, 140 (2014) 014201. [DOI] [PubMed] [Google Scholar]
- [29].Claytor K, Theis T, Feng Y, Warren WS, Measuring Long-Lived 13C-Singlet State Lifetimes at Natural Abundance Journal of magnetic resonance, 239C (2014) 81–86. [DOI] [PubMed] [Google Scholar]
- [30].DeVience SJ, Walsworth RL, Rosen MS, Preparation of Nuclear Spin Singlet States Using Spin-Lock Induced Crossing, Phys. Rev. Lett, 111 (2013) 5. [DOI] [PubMed] [Google Scholar]
- [31].Feng Y, Theis T, Wu T-L, Kevin Claytor W Warren, Long-Lived Polarization Protected by Symmetry, journal of Chemical Physics, ((submitted)). [DOI] [PubMed] [Google Scholar]
- [32].Feng Y, Davis RM, Warren WS, Accessing long-lived nuclear singlet states between chemically equivalent spins without breaking symmetry, Nature Physics, 8 (2012) 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Claytor K, Theis T, Feng Y, Yu J, Gooden D, Warren W, Accessing long-lived disconnected spin-½ eigenstates through spins > ½, J Am Chem Soc, ((submitted)). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


