Abstract
Abnormal early visual development can result in a constellation of neural and visual deficits collectively known as amblyopia. Among the many deficits, a common finding is that both saccadic and manual reaction times to targets presented to the amblyopic eye are substantially delayed when compared to the fellow eye or to normal eyes. Given the well-known deficits in contrast sensitivity in the amblyopic eye, a natural question is whether the prolonged reaction times are simply a consequence of reduced stimulus visibility. To address this question, in Experiment 1 we measure saccadic reaction times (RT) to perifoveal stimuli as a function of effective stimulus contrast (i.e., contrast scaled by the amblyopic eye's contrast threshold). We find that when sensory differences between the eyes are minimized, the asymptotic RTs of our anisometropic amblyopes were similar in the two eyes. However, our results suggest that some strabismic amblyopes have an irreducible delay at the asymptote. That is, even when the sensory differences of the stimulus were accounted for, these observers still had large interocular differences (on average, 77 ms) in saccadic reaction time. In Experiment 2, to assess the role of fixation on saccadic reaction time we compared reaction time with and without a foveal target (the “gap effect”). Our results suggest that, while removing the fixation target does indeed speed up reaction time in the amblyopic eye, the gap effect is similar in the two eyes. Therefore, the gap effect does not eliminate the irreducible delay in the amblyopic eye. Finally, in Experiment 3 we compared the interocular differences in saccadic and manual reaction times in the same observers. This allowed us to determine the relationship between the latencies in the two modalities. We found a strong correlation between the differences in saccadic and manual reaction times; however, the manual RT difference is about half that of saccadic RT, suggesting that there may be two separable effects on saccadic reaction time: (a) a central problem with directing actions to a target, related to disengagement of attention at the fovea, which results in delays in both saccadic and manual reaction times, and (b) a further delay in saccadic reaction times because of the motor refractory period from a previous saccade or microsaccade, made in an attempt to stabilize the amblyopic eye of strabismics.
Keywords: amblyopia, reaction time, saccadic eye movements, contrast sensitivity
Introduction
Abnormal early visual development can result in a constellation of neural and visual deficits collectively known as amblyopia. The most common causes of this neurodevelopmental abnormality in humans are strabismus (a turned eye) and anisometropia (unequal refractive error). Frequently occurring visual deficits include reduced visual acuity, contrast sensitivity, and stereopsis (McKee, Levi, & Movshon, 2003), but there are many other deficits in both spatial and temporal processing (for a recent review, see Hamm, Black, Dai, & Thompson, 2014).
One of the earliest reports of deficits in temporal processing was the finding that manual reaction times to targets presented to the amblyopic eye are substantially delayed when compared to the fellow eye or to normal eyes (Mackensen, 1958; von Noorden, l961; Levi, Harwerth, & Manny, 1979; Hamasaki & Flynn, 1981; Pianta & Kalloniatis, 1998; Roberts, Cymerman, Smith, Kiorpes, & Carrasco, 2016).
Delayed responses with the amblyopic eye are not limited to manual reaction time to centrally presented stimuli; saccadic responses to targets in the parafovea are also substantially delayed (Mackensen, 1958; Ciuffreda, Kenyon, & Stark, 1978a; Kenyon & Stark, 1978b; Niechwiej-Szwedo, Goltz, Chandrakumar, Hirji, & Wong, 2010; Niechwiej-Szwedo, Chandrakumar, Goltz, & Wong, 2012; McKee, Levi, Schor, & Movshon, 2016; Niechwiej-Szwedo, Goltz, Colpa, Chandrakumar, & Wong, 2017), as are responses in the delayed saccadic paradigm, which require both inhibitory control and maintained fixation on the target in order to detect the “go” signal given by the disappearance of the centrally fixated target (Perdziak, Witkowska, Gryncewicz, Przekoracka-Krawczyk, & Ober, 2014; Perdziak et al., 2016).
Neural response times are also delayed when viewing with the amblyopic eye. Visual evoked responses to fine gratings or checkerboards typically show both reduced amplitudes and increased latencies of the early components (Lombroso, Duffy, & Robb, 1969; Spekreijse, Khoe, & van der Tweel, 1972; Sokol & Bloom, 1973; Tuttle, 1973; Arden, Barnard, & Mushin, 1974; Yinon, Jakobovitz, & Auerbach, 1974; Levi, 1975; Levi & Harwerth, 1978a; Levi & Harwerth, 1978b; Sokol & Nadler, 1979; Manny & Levi, 1982; Bankó, Körtvélyes, Németh, Weiss, & Vidnyánszky, 2013; Bankó, Körtvélyes, Németh, & Vidnyánszky, 2014). Interestingly, a recent study found that amplitudes were reduced only with foveal stimulation but not with cortically scaled perifoveal stimulation, while responses were delayed and more variable at both the fovea and perifovea (Bankó et al., 2014).
What leads to increased saccadic reaction time?
Of the stimulus attributes that affect reaction time, the two relationships that have been most clearly described are contrast and luminance. These relationships are often modeled by Piéron's law (Piéron, 1914; Piéron, 1920; Piéron, 1952; see also Hughes & Kesley, 1984; Pins & Bonnet, 1996; Jaskowski & Sobieralska, 2004; Taylor, Carpenter, & Anderson, 2006). This law describes how, for a given scenario, we will respond slowly to stimuli that are at or near the threshold of our detection. From there, as the intensity of the stimulus increases, our reaction time decreases following a negative power function, until it reaches an asymptote.
Given the well-known deficits in contrast sensitivity in the amblyopic eye, a natural question is whether the prolonged reaction times are simply a consequence of reduced stimulus visibility. One advantage of measuring reaction time in amblyopic participants is that they can serve as their own control, thus eliminating the challenges associated with cognitive effects.
To address this question, we will measure saccadic reaction time to perifoveal stimuli as a function of effective stimulus contrast (i.e., contrast scaled by the amblyopic eye's contrast threshold). Thus, rather than plotting reaction time versus contrast in percent, we plot reaction versus contrast in threshold units (CTU), and use a modified version of Piéron's law described by Burr, Fiorentini, and Morrone (1998) to the reaction times measured in this study:
 |
where RT is the reaction time, α is the constant determining the steepness of the curve, C the stimulus contrast (in percent), Ct the observer's contrast threshold (in percent) and RTAsym is the reaction time asymptote. This function, which we will refer to as the Burr fit, goes to infinity at threshold and has a single estimate of contrast dependency (α), measured in ms, divided by log contrast expressed in threshold units. Because the Burr fit describes data already transformed into units of threshold, it has fewer degrees of freedom than the Piéron fit, and provides a better estimate of the asymptote.
Figure 1 illustrates several potential outcomes of manipulating α and RTAsym. The top panel shows three curves that are parallel over the entire range. The gray curve represents a normal or nonamblyopic eye. The blue curve represents the amblyopic eye with identical α and RTAsym—this is the prediction if the saccadic delay of the amblyopic eye is purely a consequence of the reduced visibility of the stimulus. Scaling the visibility by the contrast threshold, simply shifts the AE curve along the abscissa, resulting in superimposition of the curves of the two eyes. The red curve illustrates the amblyopic eye with identical α, but with RTAsym higher in the AE (i.e., an irreducible delay in the response of the amblyopic eye). The bottom panel shows that increasing or decreasing α, which determines the slope, with no change in RTAsym would result in the two curves converging at the asymptote. In both situations, manipulating contrast produced equivalent reaction times for high contrast stimuli.
Figure 1.
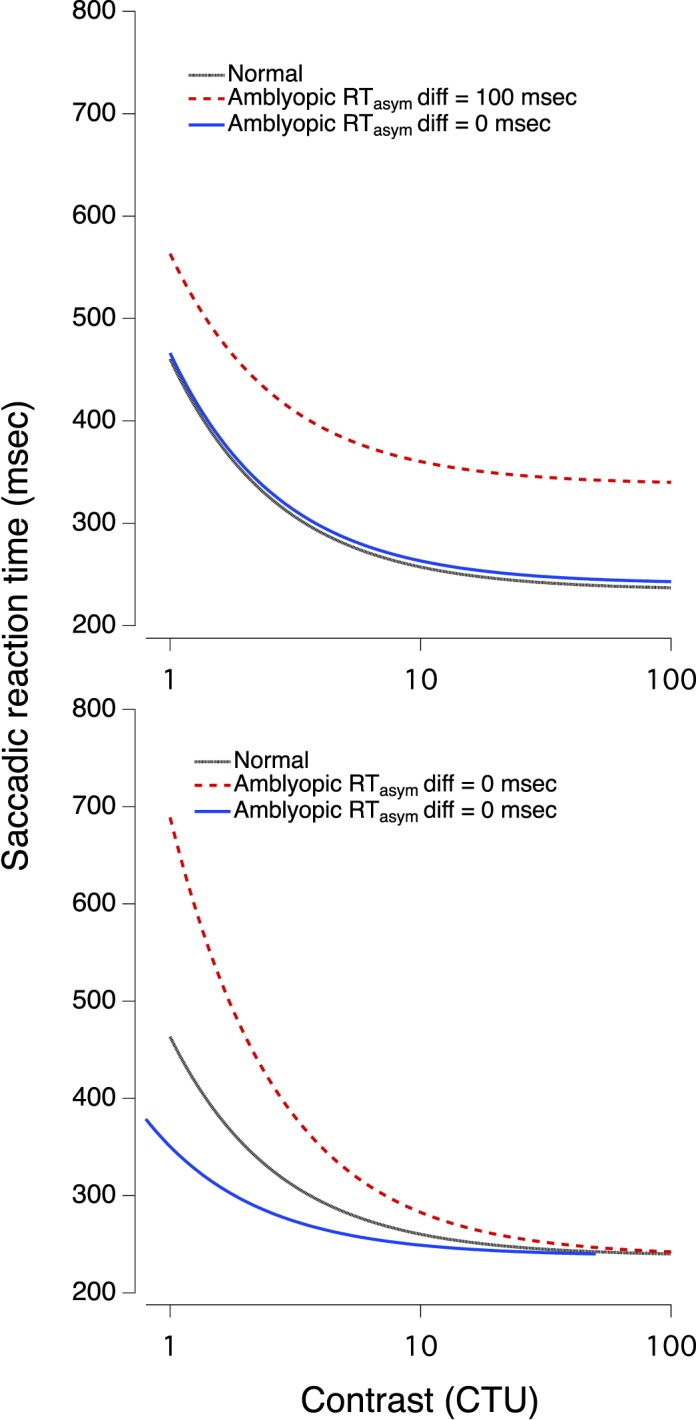
Hypothetical Burr fits, showing different predictions. See text for details.
Equating for contrast sensitivity may not produce the same asymptotic values (RTAsym) for the two eyes of some amblyopic observers, particularly for strabismic amblyopes. In a large-scale study, McKee et al. (2016) found prolonged saccadic latencies to perifoveal stimuli when fixating with the amblyopic eye, especially in strabismic amblyopes. They argued that deficits in contrast sensitivity could not explain the long average saccadic reaction time of the strabismic amblyopic eyes because the average contrast sensitivity of the strabismic amblyopes was essentially equal to that of the anisometropic amblyopes, but the average saccadic reaction time of the anisometropes was significantly shorter than that of the strabismics. However, this argument is based on indirect evidence. The current study will make detailed measurements of saccadic reaction time as a function of equivalent contrast in both strabismic and anisometropic amblyopes to determine if they differ.
What might account for an irreducible delay (i.e., an increased RTAsym), in saccadic reaction time in amblyopic observers? McKee et al. (2016) speculated that “the frequent microsaccades and the accompanying attentional shifts, made while strabismics struggle to maintain fixation with their amblyopic eyes, result in all types of reactions being irreducibly delayed” (p. 12).
Higher-level effects on reaction time: The gap effect
Saslow (1967) reported that when the fixation light was extinguished prior to the onset of the target light, the saccadic reaction time was much shorter. This unexpected finding was later termed the gap effect and sparked a great deal of behavioral and neurophysiological work in the subsequent 50 years.
There are many competing theories for mechanisms underlying the gap effect, reviewed in great depth by Jin and Reeves (2009). One likely component is the oculomotor readiness effect, suggested by Saslow in his original paper (1967): during fixation, the eye must continually work, using small saccades to return the gaze to the fixation point after small drifts. This active process could delay subsequent saccades, including those to the target. Removing the fixation spot reduces these fixational saccades, allowing for a faster response to the target. Further, as both large and small saccades, including fixational saccades, are linked to shifts in attention (Yuval-Greenberg, Merriam, & Heeger, 2014; Chen, Ignashchenkova, Thier, & Hafed, 2015), these movements would provide a mechanism by which spatial attention may be impaired in amblyopia.
In Experiment 2, we test the effect of removing the fixation target on saccadic reaction time in observers with amblyopia. By comparing reaction time with and without a foveal target, we can isolate the role of active fixation on saccadic reaction time, and compare differences in the gap effect for the amblyopic eye (AE) and non-amblyopic eye (NAE). If there is a larger gap effect in the AE than the NAE, it will show that fixation effort and accompanying microsaccades are an important part of the delay in responding with this eye.
Comparing manual and saccadic reaction times
Finally, in Experiment 3, we will measure manual reaction time in the same observers with the same targets used for measuring saccadic reaction time, again equating for differences in contrast sensitivity between the amblyopic and nonamblyopic eyes. If we find a high correlation between the delay in saccadic and manual RTs, that would suggest that there is a central problem with directing actions to targets presented to the amblyopic eye, perhaps a failure to attend to target onset.
In contrast to the large-scale study of McKee et al. (2016), here we report detailed measurements in a small group of well-characterized amblyopes with anisometropia and strabismus.
General methods and procedures
Study participants
The Research Subjects Review Boards at Smith-Kettlewell Eye Research Institute approved the study protocol. Informed consent was obtained from each participant and the study was conducted according to the tenets of the Declaration of Helsinki.
Eight adults (mean age: 54 ± 15, range 31–68 years; 5 men, 4 women) with unilateral amblyopia due to strabismus, anisometropia, or both completed at least one part of the study. In addition, in order to assess the role of strabismus per se, we also tested one subject with alternating strabismus without amblyopia (for subject details, see Supplementary Material). Two additional observers (both female, ages 63 and 75) with corrected to normal vision, served as neurotypical controls.
Participants were recruited through advertisements and local ophthalmologists. An experienced ophthalmologist provided an eye exam for each participant prior to enrolling. The inclusion criteria were: (a) age 18 years or older; (b) anisometropic amblyopia, strabismic amblyopia, or mixed amblyopia (i.e., anisometropic and strabismic); (c) interocular visual acuity difference of at least 0.2 logMAR; and (d) no history of eye surgery except to correct strabismus. Participants were excluded if they had any ocular pathological conditions (e.g., macular abnormalities) or nystagmus. All of our participants had visual acuities of 20/12–20/25+2 in the nonamblyopic eye. The retinal health of all participants was assessed as normal and cover tests were used to assess ocular alignment at both distance and near. The study took place at Smith-Kettlewell Eye Research Institute located in San Francisco, CA, and all participants were appropriately corrected for the experimental viewing conditions.
For this study, anisometropia was defined as >0.50 D difference in spherical equivalent refraction or 1.5 D difference in astigmatism in any meridian, between the two eyes (Wallace et al., 2011). Amblyopic subjects with anisometropia and an absence of manifest ocular deviation were classified as anisometropic amblyopes (aniso). Those with an ocular deviation (strabismus), as indicated by the cover test, were classified as strabismic (strab) irrespective of their refractive state, meaning that participants with both strabismus and anisometropia were classified as “strabismic.”
Procedure
Reaction time versus contrast functions were calculated for each eye of amblyopic and control participants following the sequence of events illustrated in Figure 2. Participants were instructed to fixate a white dot, while Gabor patches (with a standard deviation of ¾ of a cycle) were presented at 5° to the right or left of this dot. The participants were asked to respond by looking at the target (saccadic reaction time measurement) as quickly and accurately as possible.
Figure 2.
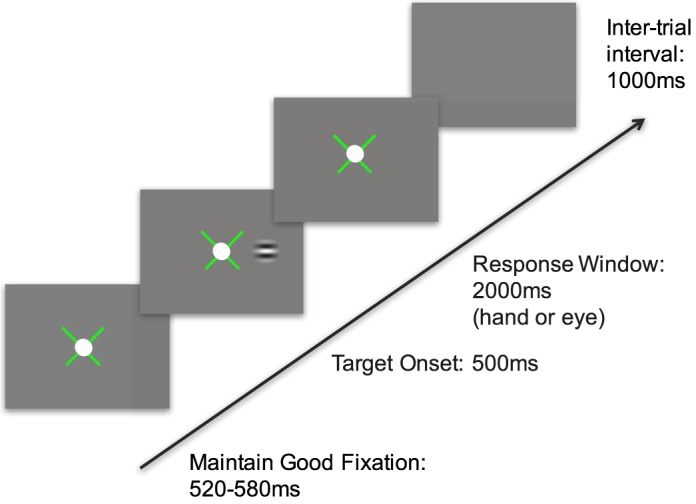
Procedure for Experiments 1 and 3. An x-shaped fixation guide appeared at the center of the monitor, with a 1° white fixation target placed on top. The fixation guide provided feedback, with green representing fixation within a 1°–2° window, and red noting that the fixation error was greater than this boundary. A red fixation guide paused the trials and reset the fixation timer. After ∼550 ms (± 30 ms) of proper fixation, a Gabor patch briefly appeared at 5° to the left or right, and participants had up to 2 s to respond by making a saccade (Experiment 1) to the target or by pressing the left or right arrow key specifying the side of target appearance (Experiment 3) as quickly and accurately as possible, followed by auditory feedback and a 1-s intertrial interval.
Stimulus parameters
The spatial frequencies of the Gabor targets were adjusted for each participant to allow reliable detection at mid to low contrast levels with the amblyopic eye. To find the appropriate stimulus properties, a cutoff frequency for the Gabor patch at 5° to the right or left of this dot was first determined in a yes/no procedure, in which the mean of three reversals was calculated (mean for all observers was 8 cycles per degree (cpd), range 2–14 cpd). Contrast thresholds, described below, were then measured for targets at a spatial frequency equal to half of this cutoff value.
Gabor patches were randomly presented to the left or right of fixation and threshold values for the nasal and temporal side of the amblyopic eye and nonamblyopic eye were measured independently. If one of the threshold values for this spatial frequency was greater than 20% (Michelson contrast), the spatial frequency was shifted lower, and the threshold values were remeasured. This was performed to ensure a sufficient range of stimulus intensities for each participant. Contrast thresholds were measured with the fellow eye using the same spatial frequency as the one used for the amblyopic eye.
For each eye, contrast thresholds were measured with two adaptive staircases, measuring the threshold for the left and right targets independently. Participants pressed the left or right arrow key, indicating the side at which the Gabor target appeared, and the contrast of the target increased or decreased logarithmically in a three-up, one-down manner. Each threshold block had 100 trials, with targets randomly appearing on the left or right side. To determine the final threshold for each side, the first reversal was discarded and the mean of the remaining reversals was calculated. If there were fewer than six reversals for one side, the contrast threshold measurement was rerun for that eye.
Experiment 1: Saccadic reaction time with equally visible stimuli in each eye
Methods
Saccadic reaction time measurements
After contrast thresholds were determined, participants completed one practice block of 10 trials with an easily detectable contrast level (contrast = 0.6). Subsequent blocks started with five practice trials at this level, then a green fixation dot was presented signifying the beginning of saccadic reaction time measurements. For the remainder of that block, there were 100 trials with Gabor patches presented at three of nine possible CTUs. These contrast threshold units were multiplied by the left and right threshold value to determine the raw contrast values measured in that block. There were at least two blocks per eye (for at least six total CTUs).
Setup and calibration.
Participants were seated 100 cm from a 21 in. ViewSonic G225f monitor (mean luminance of 30 cd/m2) in a dimly lit room, and positioned in a brow bar and chin rest such that the viewing eye was parallel to and gazing at the center of the screen (min luminance = 6 cd/m2, max luminance = 120 m2). The nonviewing eye was occluded by an eye patch. Eye movements were recorded with an EyeLink 1000, (SR Research, Mississauga, ON, Canada; 2,000 Hz monocular sampling rate and 0.25°–0.5° average accuracy when properly calibrated), mounted above the brow bar and aligned with the surface of the eye using a beam splitter.
Prior to each block, eye position was calibrated using a 5-point calibration display (center and the four corners). After calibration, participants were instructed to maintain fixation on a bright white dot (1°, 120 cd/m2) that was displayed on a gray background (30 cd/m2). A red or green crosshair surrounding the fixation dot provided feedback regarding fixation status. Participants were required to maintain good fixation (within a window of 1°) for 550 ms (± 30 ms) prior to target onset. In a few cases, the window had to be increased to 2°.
Data analysis.
At least 10 saccadic reaction time measurements (mean 35, range 11–76) were used to calculate each data point on the saccadic reaction time curves. The large range primarily reflects the number of trials that had to be discarded because the RT was too fast or too slow (especially near threshold), and the random number of trials of a certain condition in each block. The contrasts were distributed from approximately 1 CTU (contrast detection threshold) to 9 CTU. A minimum of six contrast levels (maximum nine) were used to fit the reaction time function described below.
For each contrast level, correct responses were averaged to find the mean and standard error of the reaction time. Saccadic response times below 120 ms and above 800 ms were eliminated to reduce the effect of express saccades, guesses, and exploring the screen to find a target. Mean response times and standard errors were plotted as a function of contrast in threshold units (CTU), and the data were fit with the Burr function (Equation 1) using Igortm (Wavemetrics, Lake Oswego, OR).
Results
Saccadic reaction times
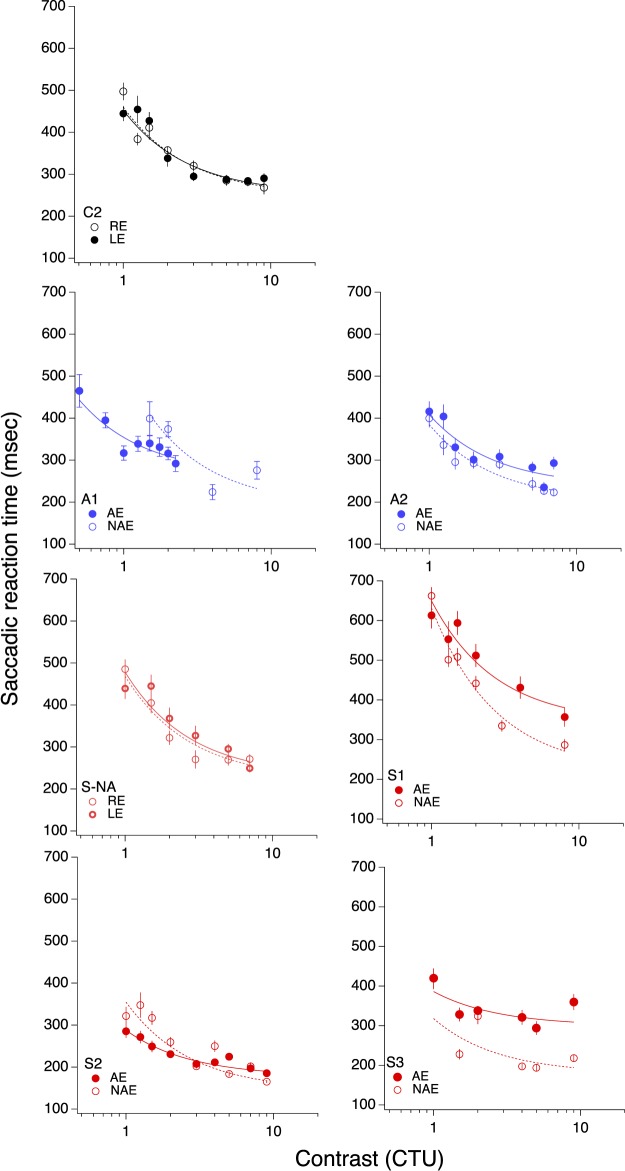
Saccadic latencies as a function of contrast threshold units are plotted in Figure 3 for each eye of each participant (one age-matched control in black, two anisometropic in blue, one strabismic but not amblyopic, and three strabismic amblyopes in red) and fit with a Burr function.
Figure 3.
Saccadic reaction time as function of contrast threshold unit. The solid symbol is the amblyopic (or nondominant) eye; the open symbol is the nonamblyopic (or dominant) eye.
The coefficient values for the slope and asymptote from the fits are reported in for each patient, along with the reduced chi-square value (i.e., chi-square divided by degrees of freedom, ν, where ν = n − m (i.e., the number of observations n minus the number of fitted parameters m. The same values were calculated for the control participants, and the dominant eye (DE) and nondominant eye (NDE) reported in Table 1.
Table 1.
Burr fit coefficients: saccadic reaction time. The number of degrees of freedom 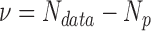 , and the reduced chi-square is given by
, and the reduced chi-square is given by 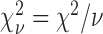 . Asterisks indicate cases where the two eyes' coefficients differ by more than the 95% confidence interval.
. Asterisks indicate cases where the two eyes' coefficients differ by more than the 95% confidence interval.
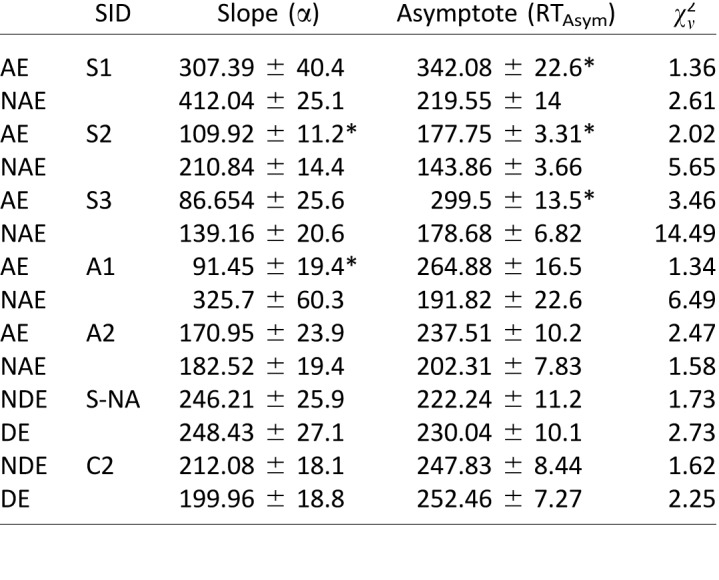
For the strabismic amblyopes (in red), asymptotic response times made with the AE were significantly slower than with the NAE, as the difference in RTAsym values is greater than the 95% confidence interval (CI) for the two fitted points. By comparison, the differences between the two eyes in anisometropic amblyopes (blue) and controls (black) were not significant.
Further, for two of the patients (one strabismic and one anisometropic), the slope parameter of the AE fell outside of the 95% CI of the NAE, with a shallower slope in the amblyopic eye. This shows that not only are the asymptotic reaction times different between the two eyes, but that these RT differences are apparent across the entire shape of the function.
Both coefficients were remarkably similar between the two eyes in the neurotypical control observer (C2), showing that the Burr fits can accurately describe the data and that in normal observers there is no difference in slope or asymptote between the two eyes. Interestingly, the fits were also very similar in the two eyes of the strabismic observer with no amblyopia (S-NA), suggesting that strabismus per se is not responsible for the prolonged saccadic RTs. Evidence of the goodness of fit can be seen in the reduced chi-square values, with only two of the 14 functions having a reduced chi-square value of greater than 6.
Accuracy
In general, targets near threshold were detectable above chance and quickly plateaued to ceiling by the middle of the reaction time function. Unfortunately, when measuring the saccadic reaction times, the eye tracker periodically lost the eye during the block, causing the trial to be recorded as incorrect when the subject did indeed make the correct eye movement. In these instances, the participants usually reported seeing the targets and this was noted and corrected. Still, this left some error in the accuracy measurements for these subjects, and this error varied from trial to trial, block to block, and eye to eye.
Accuracy values for the same contrast values were measured more robustly for manual reaction times (described below) and were, on average 86% ± 2%. It is unlikely that accuracy would be much different with the eye movement response, and in the a few cases where the eye tracker consistently tracked the eye for an entire 100-trial block, participants had 80%–100% accuracy.
Relationship between reaction time difference and visual acuity
As can be seen in Figure 4, there is a strong correlation between the loss in visual acuity and the magnitude of the saccadic reaction time difference between eyes (r = 0.79). This finding is remarkably similar to the robust relationship reported by McKee et al. (2016) and shown by the dots in Figure 4, where they found a correlation of r = 0.75 with 393 abnormal observers (p ≤ 0.001).
Figure 4.
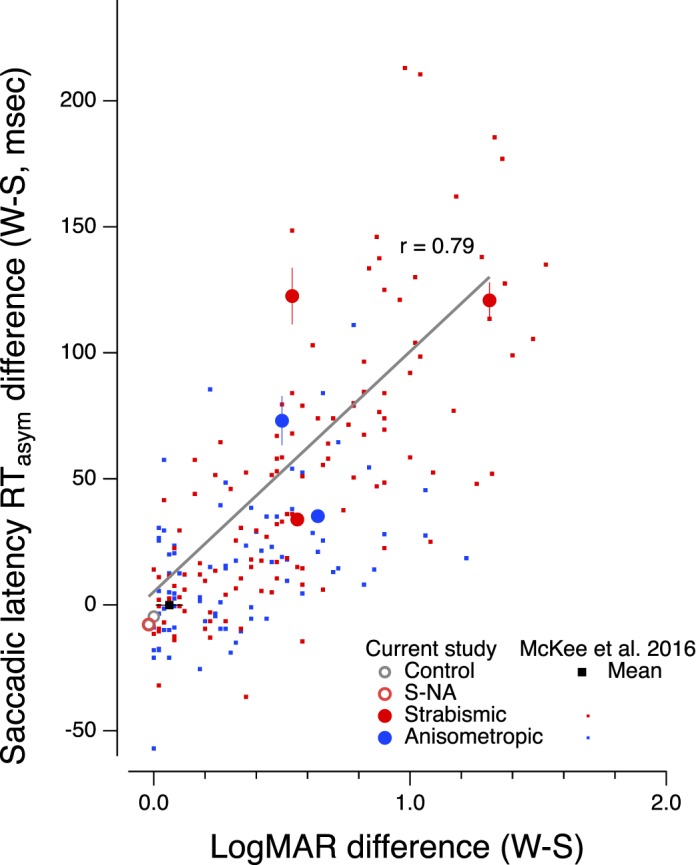
Reaction time difference (amblyopic or nondominant eye − dominant eye) versus LogMAR difference (amblyopic or nondominant eye − dominant eye). Large symbols are from the current study. Small symbols are from McKee et al. (2016). The solid black square is the normal control mean from McKee et al. (2016); the open gray circle is a neurotypical control observer from the current study, and the unfilled red circle is the nonamblyopic strabismic observer. Solid red and blue circles are individual data for strabismic and anisometropic amblyopes, respectively.
Interestingly, we did not find a significant relationship between the interocular contrast threshold ratio and reaction time difference for saccadic latencies. This finding is important in that it shows that there is no residual effect of contrast sensitivity in this study. By normalizing for contrast threshold, we can effectively remove this factor from the RT differences that were found in earlier studies.
The main result of Experiment 1 suggests that, at least for some amblyopic observers, saccadic reaction times are prolonged, even when the signal strength in the two eyes is equated. In the next experiment, we investigate the role of active fixation in this irreducible delay. As previously noted, when the eye is trying to maintain fixation, small eye movements may delay RTs, but we were unable to monitor these small measurements. Thus, it is possible that the AE requires greater effort to maintain attention and fixation and that this results in the delayed RT in our strabismic amblyopes. Removing the fixation spot prior to target onset should eliminate or reduce fixational effort, and may potentially eliminate the irreducible delay.
Experiment 2: The gap effect on RT
Methods and procedures
The procedure was nearly identical to that of Experiment 1, but instead of changing the contrast of the Gabor patch on each trial, the temporal gap between the offset of the fixation spot and the onset of the Gabor target was randomly chosen from one of six possibilities: an overlap where the fixation target persisted throughout the trial; a 0 ms condition where the target appeared simultaneously with the extinguishing of the fixation target; and 100 ms, 200 ms, 300 ms, and 400 ms gap conditions (Figure 5). Additionally, unlike Aim 1, in which the spatial frequency of the Gabor patch was adjusted for each participant, everyone in Aim 2 had a Gabor patch of 4 c/deg presented at full (98%) contrast.
Figure 5.
Procedure for Experiment 2. An x-shaped fixation guide appeared at the center of the monitor, with a 1° white fixation target placed on top. The fixation guide provided feedback, with green representing fixation within a 1° window, and red noting that the fixation error was greater than this boundary. A red fixation guide paused the trials and reset the fixation timer. After ∼550 ms (± 30 ms) of proper fixation, one of six potential gap durations occurred. After the gap period, a Gabor patch briefly appeared at 5° to the left or right, and participants had up to 2 s to respond by making a saccade toward the target, followed by auditory feedback and a 1-s intertrial interval.
Analysis
Reaction time measurements (mean: 22, min 9: max = 51) were averaged for the baseline value; min, mean, and max trial numbers for each gap duration were similar to those in the baseline condition. Response times below 120 ms and above 800 ms were eliminated to reduce the effect of express saccades and random guesses. Correct responses were averaged to find the mean and standard error of the reaction time.
Study participants
A subset of five (n = 5) adults (mean age: 54 ± 15, range 31–68 years) with unilateral amblyopia and two of the normally sighted control observers from Experiment 1 also completed Experiment 2. Subject identification codes have been maintained from the previous study. Note that subject S5 participated in the Experiment 2, but not in Experiment 1.
Results
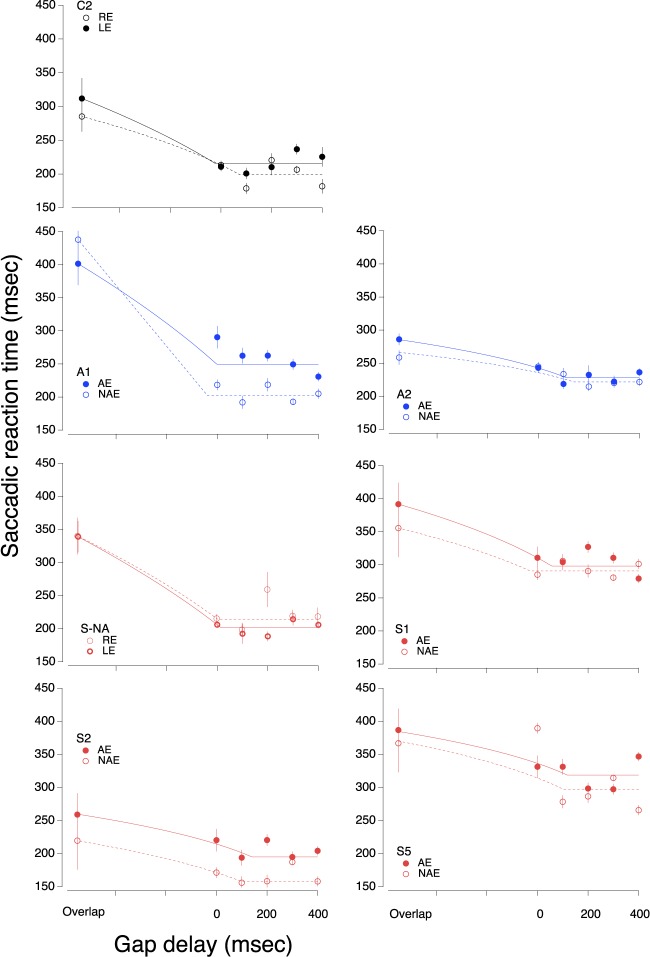
Saccadic reaction times are plotted as a function of gap delay in Figure 6 for each eye of each participant (three strabismic in red, two anisometropic in blue, and two each eye of each participant [three strabismic in red, two anisometropic in blue, and two age-matched controls in black]).
Figure 6.
Saccadic reaction time as function of gap duration. The solid symbol is the amblyopic eye or nondominant eye; the open symbol is the nonamblyopic eye or dominant eye. The lines fit to the data represent a 2-line fit of the form SL = SLgap × (GD/SLover) × GD (for GD< SLover) + n × GD (for GD ≥ SLover), where SL = saccadic reaction time; SLgap is the asymptotic value with a gap; SLover is the saccadic reaction time with overlap, GD is the gap delay, and N is the slope of line 1. Note that the sloping line segments do not appear straight because the overlap condition is plotted at an arbitrary negative delay.
We fit the data with a 2-line fit (see Figure 6 legend for details), a sloped line constrained by the overlap condition, and a horizontal line (slope = 0) fit to the asymptotic gap conditions, and calculated the gap effect as the difference between the measured overlap condition, and the fit to the asymptotic gap conditions (we find similar results taking the difference between the overlap condition and mean reaction time at each gap delay). We note that while some previous studies report a U-shaped pattern in the gap effect (e.g., Braun & Breitmeyer, 1988), we did not find this, even in our neurotypical observers. Braun and Breitmeyer (1988) measured the gap effect at nine different temporal offsets, ranging from −500 ms to 500 ms, compared to the six offsets (−100 ms to 400 ms) in the current study; it is possible that we are limited by the number of temporal offsets measured. It is important to note, however, that our control observers had mean gap effect sizes at the 200 ms delay that were very similar to those reported by Braun and Breitmeyer (1988), as well as many other studies.
We note that the reaction times for the overlap condition are generally longer than those measured for the highest contrast in Experiment 1 for the same observers. This observation argues that uncertainty about whether the fixation stimulus will disappear adds to the delay when it remains visible throughout the target sequence.
To assess if there were significant interocular differences in gap size, paired t tests were run to compare the gap effect for the five durations in the AE versus NAE. The results of the t tests are presented in Table 2.
Table 2.
Interocular gap size t test values (AE vs. NAE). * indicates p < 0.05.

For strabismic amblyopes, removing the fixation target does speed saccadic reaction time in the amblyopic eye, but not anymore so than the nonamblyopic eye. For example, the saccadic reaction time of S2 is slower in the AE than the NAE, but the gap size in both eyes is more or less the same. A similar pattern was observed, for the controls, although there was little to no difference in the raw reaction times, unlike several of the strabismic amblyopes.
The two anisometropic amblyopes did have significant differences in the gap effect between the two eyes; intriguingly, however, they were in opposite directions. A2 had a shallow gap effect for both eyes, but because the amblyopic eye was slower in the overlap condition, the gap effect was slightly, but significantly, larger in the AE than the NAE. In this one patient, it is interesting to note that by removing the fixation target prior to target onset, the RT difference between the two eyes was eliminated. In A1 however, the gap effect for the AE was significantly smaller than in the NAE. It's unclear what might drive these differences in anisometropic amblyopes, given our small sample size.
To further quantify the gap effect, we calculated the average gap effect for the five durations to determine one mean gap effect value for each eye. These results are plotted in Figure 7 (top panel), with the mean gap size in the NAE or dominant eye on the abscissa and the mean gap size for the AE or nondominant eye on the ordinate. All but participant A1 had a slightly larger gap effect in the AE or nondominant eye than the NAE or dominant eye.
Figure 7.
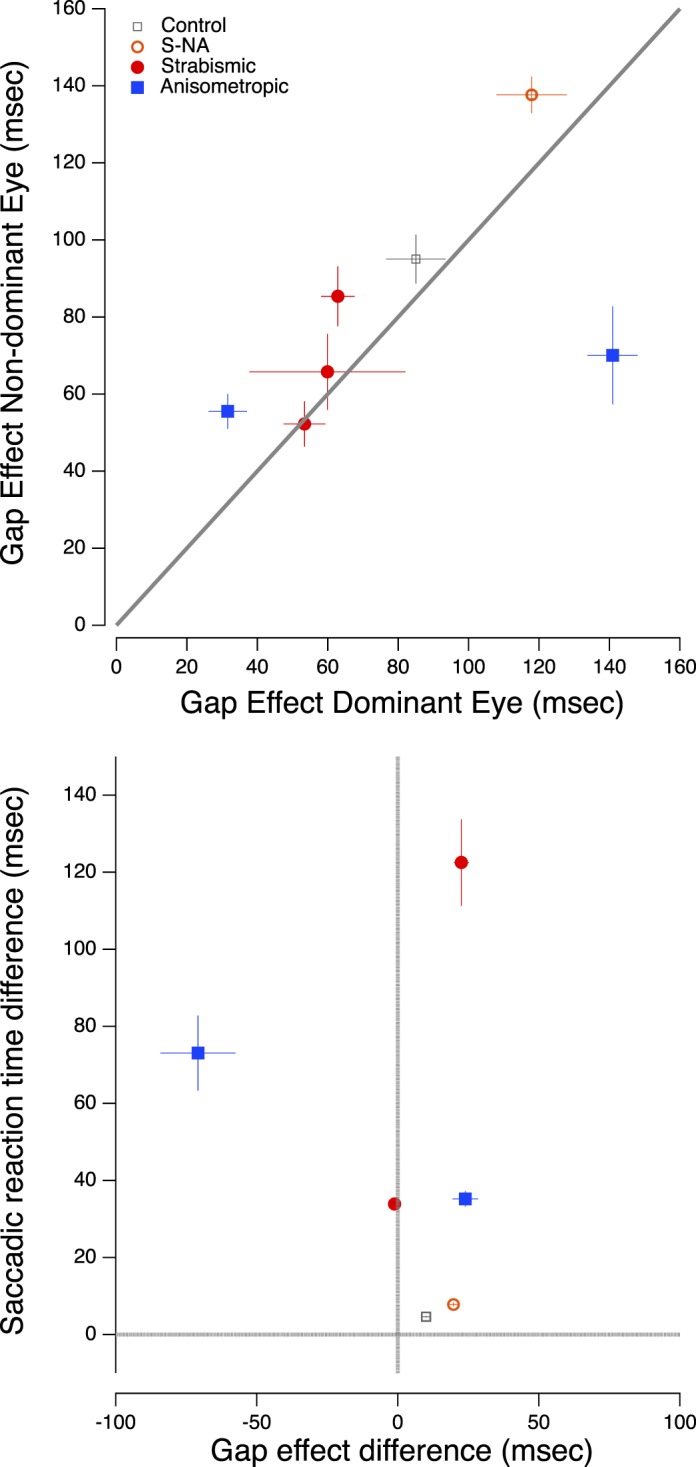
(Top) Mean gap effect in the amblyopic and nonamblyopic eyes. (Bottom) Saccadic reaction time difference between the two eyes (i.e., nondominant − dominant) versus the gap effect difference (i.e., nondominant − dominant). The vertical and horizontal lines represent no interocular difference in gap effect and no interocular difference in saccadic reaction time, respectively. As noted above, subject S5 participated in the Experiment 2, but not in Experiment 1.
A repeated measures ANOVA was run to compare the within-subject means to the between-subjects means. This analysis suggested that overall there was much more variation between subjects (F = 11.68, p < 0.01) than between the two eyes of the observers (F = 0.94, p = 0.37).
The gap effect does not eliminate the irreducible delay
If the gap effect were to account for the irreducible delay seen in the previous study, it would need to be approximately equal to the size of the saccadic reaction time difference between the two eyes. The bottom panel of Figure 7 plots the saccadic reaction time difference between the two eyes (i.e., nondominant − dominant) on the ordinate versus the gap effect difference (i.e., nondominant − dominant) on the abscissa. This figure makes two interesting points: (a) with the exception of A2, who did indeed have a relatively small SL difference (35 ms) and gap effect difference (24 ms) that were approximately equal, all other subjects do not have similar gap effect and SL differences. This is driven mainly by the fact that for most subjects the gap effect difference is very small. This plot also clearly illustrates the extent of the outlier, A1, who only had a moderate difference in SL between eyes, but a large gap effect difference. For this subject, removing the fixation target increases the irreducible delay almost two-fold. (b) While three of the four amblyopes showed modest interocular differences in the gap effect, compared with the control observers all four had very large differences in saccadic reaction time, with the AE greatly delayed relative to the NAE.
Experiment 3: Manual reaction time with equally visible stimuli in the two eyes
While many previous studies have shown that manual reaction times to targets presented to the amblyopic eye are substantially delayed when compared to the fellow eye or to normal eyes (Mackensen, 1958; von Noorden, 1961; Levi et al., 1979; Hamasaki & Flynn, 1981), none has compared manual and saccadic reaction times with identical stimuli in the same observers (we note, however, that Niechwiej-Szwedo et al. [2017] did compare saccadic and reaching RTs in the same observers with identical stimuli). In order to assess the generality of the irreducible delay, in Experiment 3 we measure manual reaction time in the same observers.
Methods and procedures
The observers, stimuli, methods and procedures were identical to those described above, except that participants were asked to respond by pressing the left or right arrow key specifying the side of target appearance (manual reaction time measurement) as quickly and accurately as possible. Manual reaction time functions were calculated for each eye of amblyopic and control participants following the sequence of events illustrated in Figure 2 (mean number of trials per condition ≈ 40; range 26–102). Participants were instructed to fixate a white dot, while Gabor targets were presented at 5° to the right or left of this dot.
Results
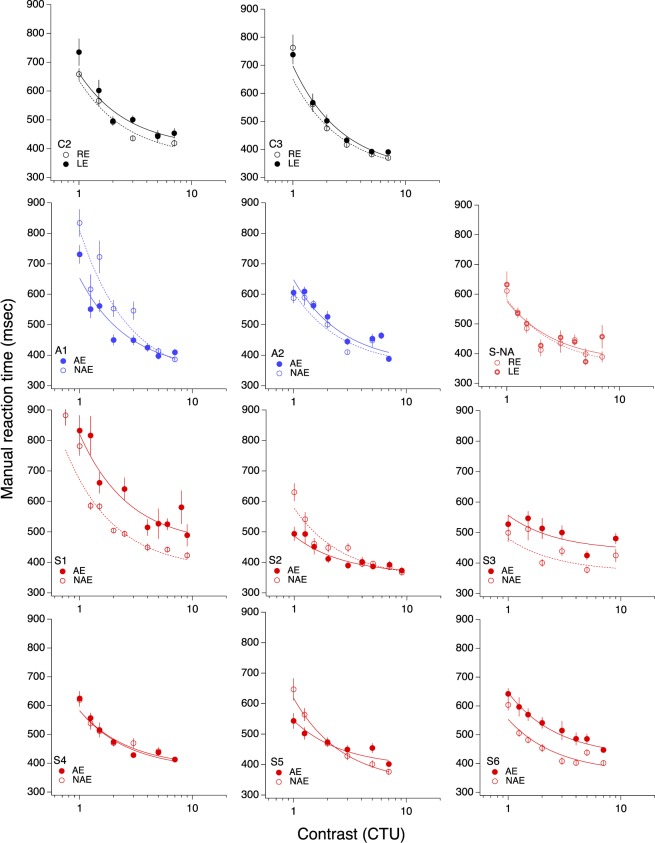
The manual reaction time functions, while not identical, are largely similar to the saccadic reaction time functions (Figure 8 and Table 3). As can be seen in Figure 9, it is important to note that the interocular differences in saccadic and manual reaction time are strongly correlated (r = 0.96; r = 0.98 if the correlation is restricted to the amblyopic observers only), suggesting the possibility that there may be a central problem with directing actions to a target, perhaps a failure to attend to target onset. We note that the manual reaction time difference is about half the saccadic reaction time difference (the data fall above the unity line), which suggests that there may be two elements slowing saccadic reaction time (a saccadic refractory period as implied by our variability analysis, below, plus some central problem shared by both eyes and hands).
Figure 8.
Manual reaction time as function of contrast (in threshold units). The solid symbol is the amblyopic (or nondominant) eye; the open symbol is the nonamblyopic (or dominant) eye.
Table 3.
Burr fit coefficients: manual reaction time. The number of degrees of freedom 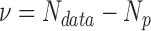 , and the reduced chi-square is given by
, and the reduced chi-square is given by 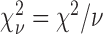 . * indicate cases in which the difference in parameter values for the two eyes exceeds the 95% confidence interval.
. * indicate cases in which the difference in parameter values for the two eyes exceeds the 95% confidence interval.
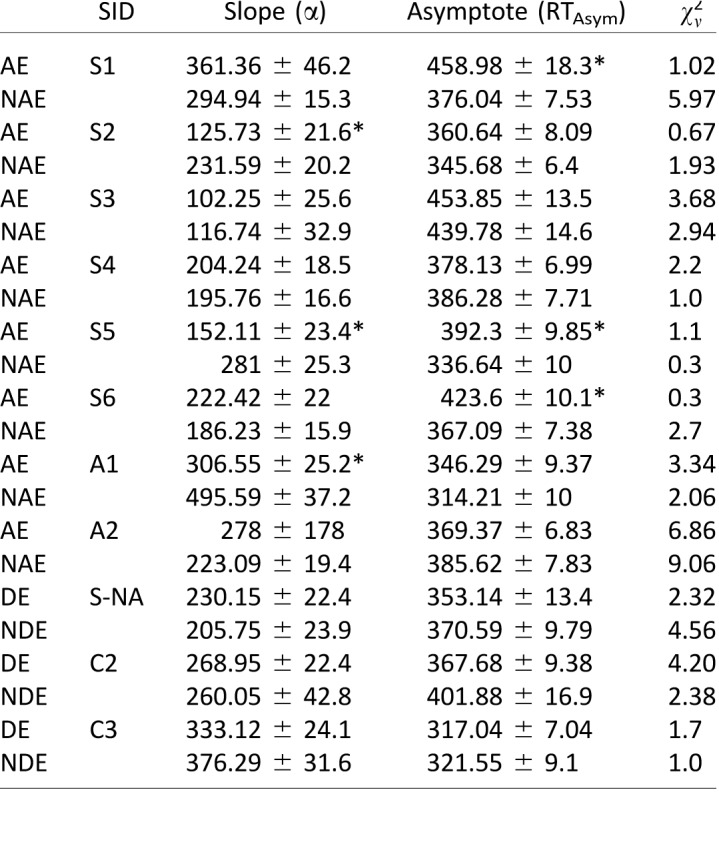
Figure 9.
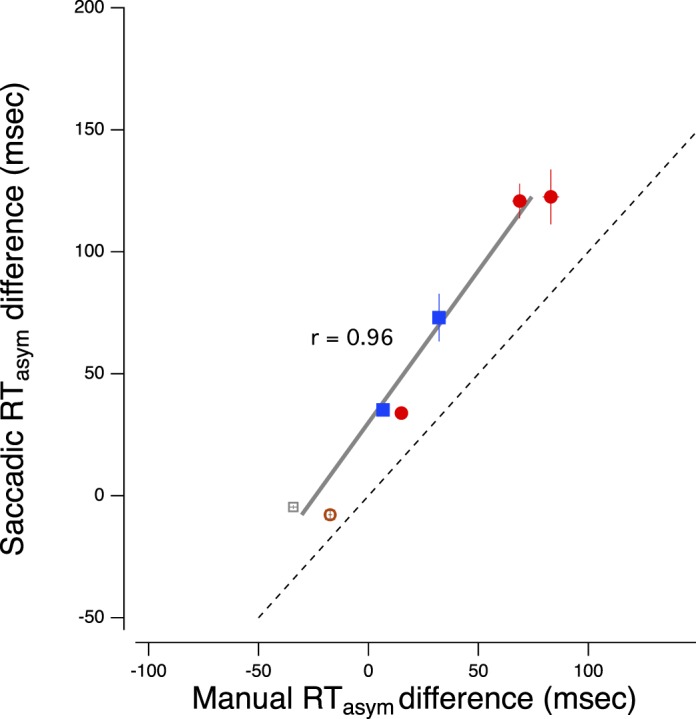
Saccadic asymptotic reaction time difference (nondominant or amblyopic − dominant) versus manual asymptotic reaction time difference (nondominant or amblyopic − dominant). The solid gray line is the best fitting linear regression. The dashed line is the unity line.
Parallel versus intersecting Burr functions
While the Burr functions in Figures 3 and 8 for control observers are almost completely superimposed, showing that the reaction-time difference between their two eyes is nearly null for all contrasts, we find different patterns in the amblyopic observers. In the Supplementary Material we ask whether delays in the amblyopic eyes RTs can be understood by shifting the Burr fit of the fellow eye, and whether a shift is both necessary and sufficient.
Variability in reaction times
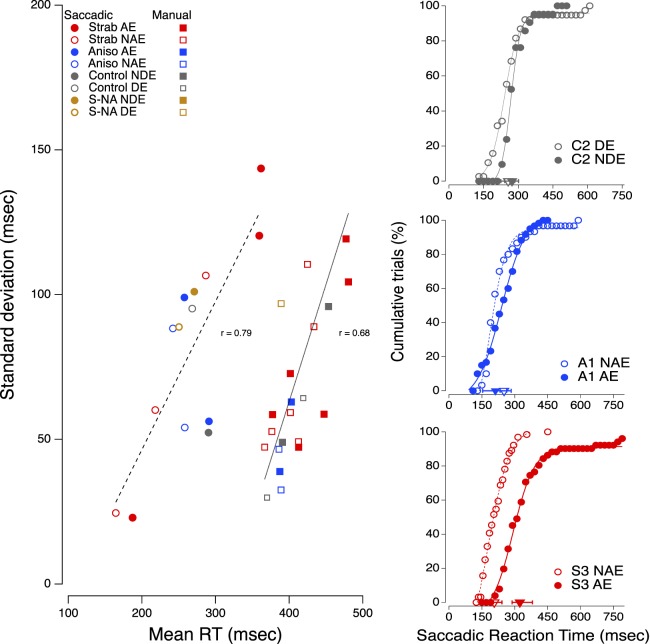
Short reaction times should be less variable than long reaction times, if only because there is a lower bound set by the time needed to initiate a motor movement. On the left of Figure 10, we have plotted the standard deviation as a function of mean reaction time for the asymptotic saccadic and manual reaction times (highest contrast). Clearly, the means and standard deviations are highly correlated for both types of movement; the longer the reaction time, the more variable the onset of the response. All reaction time models predict increasing variability with increasing reaction time. For example, in diffusion models, such as the model described by Palmer, Huk, and Shadlen (2005), choice reaction time is determined by the accumulation of evidence favoring one choice over the other—a fluctuating path towards a decision threshold, which is modulated over time by the sensory noise associated with each possibility. Reaction times are longer for stimuli presented near threshold, because the difference between the signal (in our case, a target presented on the left) and the noise (no target on the right) is small and thus, fluctuations in noise can pull the decision back and forth between the two choices, producing a longer time to reach the decision threshold. This random noise necessarily varies from trial-to-trial, so longer reaction times are also more variable. Stimuli far above the sensory threshold should produce fast reaction times with little variation, but other sources of internal noise that are not related to signal strength, such as inattentiveness to target onset, could produce longer, more variable reaction times even for strong signals.
Figure 10.
(Left). Standard deviation as a function of mean reaction time for the asymptotic saccadic and manual reaction times (highest contrast). (Right) The cumulative percentage of trials as a function of saccadic reaction time for each eye of one neurotypical observer (top panel), one anisometropic amblyope (middle panel) and one of the two strabismic observers with long saccadic reaction time (bottom panel). The triangles on the bottom of each graph show the median reaction time and the interquartile range for each cumulative function.
One explanation for the longer reaction times associated with amblyopia is that the sensory input from the amblyopic eye has intrinsically more noise than normal (Levi, Klein, & Chen, 2007; Levi et al., 2008), and correspondingly greater variability from trial-to-trial. By equating the intrinsic contrast (signal strength) of the stimuli presented to the amblyopic eye to that of the nonamblyopic eye, we removed one source of the additional noise in the amblyopic eye. Indeed, after equating for contrast threshold differences, there were no significant interocular differences in the asymptotes for saccadic or manual reaction times in any of our anisometropic amblyopes (Tables 1 and 3). Equating contrast also removed the interocular difference in asymptotic saccadic reaction time for one strabismic amblyope (Table 1), as well the interocular difference in manual reaction times for three of the six strabismic amblyopes (Table 3).
What additional source of noise accounts for the extraordinarily long saccadic reaction times of the remaining two strabismic observers? The mean saccadic times (red filled circles in Figure 10) of their amblyopic eyes are so long that they hover almost over the centroid of manual reaction times below them. On the right side of Figure 10, we have plotted the cumulative percentage of trials as a function of saccadic reaction time for each eye of one normal observer (top panel), one anisometropic amblyope (middle panel) and one of the two strabismic observers with long saccadic reaction times (bottom panel). The triangles on the bottom of each graph show the median reaction time and the interquartile range for each cumulative function. These measurements were made with targets presented at high contrast, so the saccadic reaction time curves for the normal observer are steep with little variation. The curves for the anisometropic amblyope resemble the normal curves; the median values for the two eyes are almost identical and the interquartile range is similar to the normal range. The curve for the nonamblyopic eye (NAE) of the strabismic amblyope resembles the normal curves in both median and range, but the curve for the amblyopic eye barely overlaps the curve for the NAE. Intriguingly, the times for the amblyopic eye fall within the normal range on about 40% of trials, which suggests that this eye can initiate saccades with normal reaction time. The puzzling data are the 20% of trials where the times exceed 450 ms.
We speculate that the constant drift of the amblyopic eyes observed in many strabismic observers may account for the long variable times of these two strabismics (Schor & Hallmark, 1978; Chung, Kumar, Li, & Levi, 2015). To counter the drift, strabismic amblyopes make many corrective saccades. Thus, on many trials, these observers may have just initiated a corrective saccade when the test target appears. The refractive period of these corrective saccades could delay the initiation of a subsequent saccade by as much as 500 ms (Otero-Millan, Troncoso, Macknik, Serrano-Pedraza, & Martinez-Conde, 2008). While this speculation addresses prolonged saccadic reaction time, it does not explain the very high correlation between saccadic and manual reaction time (Figure 9), nor why the interocular difference in saccadic reaction time is roughly twice that of manual reaction time. We shall consider these issues in the Discussion.
Discussion
The goal of the first experiment was to determine if sensory differences could account for the delayed response time in amblyopic patients. In other words, if the effective stimulus strength were equated, would persons with amblyopia still be slower when responding with their amblyopic eye? Would the results be different between the strabismic and anisometropic amblyopes?
To determine this, we calculated the contrast threshold of the stimuli for each eye, measured the saccadic reaction time at several intensities that were multiples of this threshold, and fit these data with a Burr function. While the asymptotic RTs of our anisometropic amblyopes were similar in the two eyes, our results suggest that some strabismic amblyopes have an irreducible delay at the asymptote. That is, even when the sensory differences of the stimulus were accounted for, these observers still had a large interocular difference (average of 77 ms) in saccadic reaction time.
These findings replicate and extend previous results. Levi and colleagues (1979) measured manual response time as a function of contrast and found a significant difference between the eyes of strabismic patients. However, it is difficult to make strong conclusions regarding interocular differences from their data, given the fact that the target (physical) contrast levels were the same for both eyes. This design resulted in an effective stimulus strength for the AE that was weaker than the NAE at each contrast level. When replotted as a function of contrast threshold units, the reaction time functions for the two eyes were almost superimposed, except at asymptote where a significant difference remained (McKee et al., 2016).
Pianta and Kalloniatis (1998), also measured manual reaction time differences in amblyopic observers and equated the contrast between the two eyes to account for sensitivity differences. They report no RT differences in all four of their anisometropic subjects, two of which were amblyopic by standard clinical criteria. While our two anisometropic amblyopes also follow this pattern, our results highlight why it's important not to generalize the previous findings to the entire patient group, as we find a much different pattern in strabismic amblyopes.
As was described in several other studies (e.g., Ciuffreda et al., 1978a; Ciuffreda et al., 1978b; McKee et al., 2016) that measured saccadic reaction time in patients with amblyopia, there was considerable variability among patients, which shows the need to report individual results. In studies where only the mean results are reported (e.g., Niechwiej-Szwedo et al., 2017), the effect can be blurred, as some patients can have large differences between the two eyes (upwards of 100 ms), while some have no significant difference.
The aim of the second experiment was to compare saccadic reaction time with and without a foveal fixation target to isolate the role of fixation on saccadic timing in the amblyopic population. While removing the fixation target did indeed speed up reaction time in the amblyopic eye, the gap effect was similar in the two eyes. Therefore, the gap effect does not eliminate the irreducible delay in the amblyopic eye. Additionally, the gap effect in the NAE of the strabismic observers (and one anisometropic amblyope) was smaller than in either eye of the normally sighted control subjects (see Figure 8), although this effect did not reach statistical significance. While the magnitude of the gap effect in the AE is related to the acuity in that eye, the interocular difference in the gap effect is not significantly correlated with either the interocular difference in acuity or contrast sensitivity. Interestingly, while the increased RT appears to be mainly monocular in nature for the saccadic reaction time (Experiment 1) and manual reaction time (Experiment 3), there may also be a component of fixation that is impaired in both eyes (Bedell & Flom, 1985; Chung et al., 2015), which may explain why the gap effect is smaller in both eyes of our strabismic amblyopes.
Our hypothesis is that fixation instability in the amblyopic eye is driving the increased reaction time for both saccadic and manual movements, producing the very high correlation between the two reaction times shown in Figure 9. Thus, at first glance, the results of the gap experiment may seem counterintuitive. Given that the fixation stimulus has been extinguished, one might suppose that strabismic amblyopes would make fewer microsaccades. However, Siepmann, Reinhard, and Herzau (2006) showed that the fixation stability of some strabismic amblyopes is modulated by recognition effort (the lower the effort, the greater the instability), so that even in the absence of the fixation stimulus, the amblyopic eye may drift and saccade. Indeed, Schor and Hallmark (1978) observed large drifts and saccades when their strabismic observers attempted to maintain fixation in darkness with their amblyopic eyes. Note that our observers were required to maintain fixation within a small window (see methods) before target onset or the trial was terminated, so they were undoubtedly attempting to maintain fixation.
According to the attentional explanation of the gap effect, attention must disengage from the fixation target in the overlap condition before moving and re-engaging at the peripheral target. For gap trials, attention is already disengaged, allowing for a faster response to the saccadic target. We note that for our neurotypical observers, removing the fixation target (0 gap) substantially reduces the saccadic reaction time. Indeed, at gap = 0, the saccadic reaction time is not statistically different from that at gap = 200 ms. We speculate that the normal observers may not make many microsaccades, so the main effect of turning off fixation is to disengage attention. This result is also true of most of the amblyopes, except S5, so we think disengagement of attention may be a general phenomenon. However, if, as we suspect, the amblyopic eye of strabismics continues its pattern of drift and corrective saccades, even in the absence of a fixation point, these corrective saccades would still engage attention during the gap. We further note that fixation instability in amblyopia is correlated with acuity loss (Chung et al., 2015), which might explain the correlation between reaction time and acuity (Figure 4). In a recent study, Roberts et al. (2016) manipulated both voluntary and involuntary attention in adults with amblyopia, using contrast scaled stimuli, and found that despite low-level deficits, deployment of covert spatial attention remained intact, consistent with Sharma, Levi, and Klein (2000). Deployment of attention may be normal, but if at the onset of the test target, attention is engaged elsewhere due to corrective saccades, reaction time will be slowed.
It would be interesting to see if the gap effect increases with visual training that has been shown to improve visual function in amblyopia. For example, a study by Li, Ngo, and Levi (2015) provided evidence that patients with amblyopia who played video games improved on an attentional blink measure, a similar task in that it involves both temporal processing and foveal attention.
Summary and conclusions
In summary, we show that in some strabismic amblyopes there is still an irreducible delay in both saccadic and manual reaction times when sensory differences between the two eyes are carefully controlled, and that the gap effect does not eliminate the irreducible RT delay. Intriguingly, the gap effect is reduced in both eyes of some amblyopic patients, adding to the growing evidence of abnormalities in the NAE of some amblyopic patients (Bedell & Flom, 1985; Hou, Pettet, & Norcia, 2008; Chung et al., 2015; Meier, Sum, & Giaschi, 2016; Meier & Giaschi, 2017) and consistent with the notion that amblyopia may reflect a generalized neurological problem (Atkinson, 2017), rather than being confined to just one eye. Finally, the strong correlation between the interocular difference in saccadic and manual reaction times supports the conclusion of two separable effects on saccadic reaction time: (a) a central problem with directing actions to a target, perhaps a failure to attend to target onset, due to an impaired ability to disengage spatial attention, which results in delays in both saccadic and manual reaction times, and (b) a further delay in saccadic reaction times because of the motor refractory period from a previous saccade or microsaccade, made in an attempt to stabilize the amblyopic eye of strabismics. Future studies need careful measurements of eye movements in amblyopic patients while they are doing a task to determine whether abnormal fixational eye movements might account, at least in part, for the sluggish performance with the AE of strabismic amblyopes.
Supplementary Material
Acknowledgments
This study was supported by a research grant R01EY020976 from the National Eye Institute and by a Fellowship from the Smith-Kettlewell Eye Research Institute. We acknowledge Saeideh Ghahghaei's technical assistance and advice in setting up the experimental system for this study.
Commercial relationships: none.
Corresponding author: Dennis Levi.
Email: dlevi@berkeley.edu.
Address: School of Optometry and the Helen Wills Neuroscience Institute, University of California, Berkeley, CA, USA.
Contributor Information
Christina Gambacorta, Email: cgambacorta@apple.com.
Jian Ding, Email: jian.ding@berkeley.edu.
Suzanne P. McKee, Email: suzannemckee4@gmail.com.
Dennis M. Levi, Email: dlevi@berkeley.edu.
References
- Arden G. B, Barnard W. M, Mushin A. S. Visually evoked responses in amblyopia. British Journal of Opthalmology. (1974);58:183–192. doi: 10.1136/bjo.58.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. The Davida Teller Award Lecture, 2016: Visual brain development: A review of dorsal stream vulnerability—motion, mathematics, amblyopia, actions, and attention. Journal of Vision, (2017);17(3):1–24.:26. doi: 10.1167/17.3.26. https://doi.org/1167/17.3.26 PubMed] [ Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankó E. M, Körtvélyes J, Németh J, Vidnyánszky Z. Amblyopic deficit beyond the fovea: Delayed and variable single-trial ERP response latencies, but unaltered amplitudes. Investigative Ophthalmology & Visual Science. (2014);55(2):1109–1117. doi: 10.1167/iovs.13-13507. [DOI] [PubMed] [Google Scholar]
- Bankó E. M, Körtvélyes J, Németh J, Weiss B, Vidnyánszky Z. Amblyopic deficits in the timing and strength of visual cortical responses to faces. Cortex. (2013);49(4):1013–1024. doi: 10.1016/j.cortex.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Bedell H. E, Flom M. C. Bilateral oculomotor abnormalities in strabismic amblyopes: Evidence for a common central mechanism. Documenta Ophthalmologica. (1985);59:309–321. doi: 10.1007/BF00159166. [DOI] [PubMed] [Google Scholar]
- Braun D, Breitmeyer B. G. Relationship between directed visual attention and saccadic reaction times. Experimental Brain Research. (1988);73(3):546–552. doi: 10.1007/BF00406613. [DOI] [PubMed] [Google Scholar]
- Burr D. C, Fiorentini A, Morrone C. Reaction time to motion onset of luminance and chromatic gratings is determined by perceived speed. Vision Research. (1998);38(23):3681–3690. doi: 10.1016/s0042-6989(98)00056-x. [DOI] [PubMed] [Google Scholar]
- Chen C.-Y, Ignashchenkova A, Thier P, Hafed Z. M. Neuronal response gain enhancement prior to microsaccades. Current Biology. (2015);25(16):2065–2074. doi: 10.1016/j.cub.2015.06.022. [DOI] [PubMed] [Google Scholar]
- Chung S. T, Kumar G, Li R. W, Levi D. M. Characteristics of fixational eye movements in amblyopia: Limitations on fixation stability and acuity? Vision Research. (2015);114:87–99. doi: 10.1016/j.visres.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda K. J, Kenyon R. V, Stark L. Increased saccadic latencies in amblyopic eyes. Investigative Ophthalmology & Visual Science. (1978a);17(7):697–702. [PubMed] [Google Scholar]
- Ciuffreda K. J, Kenyon R. V, Stark L. Processing delays in amblyopic eyes: Evidence from saccadic latencies. Optometry & Vision Science. (1978b);55(3):187–196. doi: 10.1097/00006324-197803000-00008. [DOI] [PubMed] [Google Scholar]
- Hamasaki D. I, Flynn J. T. Amblyopic eyes have longer reaction times. Investigative Ophthalmology & Visual Science. (1981);21(6):846–853. [PubMed] [Google Scholar]
- Hamm L. M, Black J, Dai S, Thompson B. Global processing in amblyopia: A review. Frontiers in Psychology. (2014);5:583. doi: 10.3389/fpsyg.2014.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C, Pettet M. W, Norcia A. M. Abnormalities of coherent motion processing in strabismic amblyopia: Visual-evoked potential measurements. Journal of Vision. (2008);8(4):1–12.:2. doi: 10.1167/8.4.2. https://doi.org/1167/8.4.2 PubMed] [ Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes H. C, Kelsey J. V. Response-dependent effects on near-threshold detection performance: Saccades versus manual responses. Attention, Perception, & Psychophysics. (1984);35(6):543–546. doi: 10.3758/bf03205950. [DOI] [PubMed] [Google Scholar]
- Jaśkowski P, Sobieralska K. Effect of stimulus intensity on manual and saccadic reaction time. Attention, Perception, & Psychophysics. (2004);66(4):535–544. doi: 10.3758/bf03194899. [DOI] [PubMed] [Google Scholar]
- Jin Z, Reeves A. Attentional release in the saccadic gap effect. Vision Research. (2009);49(16):2045–2055. doi: 10.1016/j.visres.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Levi D. M. Patterned and unpatterned visual evoked response in strabismic and anisometropic amblyopia. American Journal of Optometry and Physiological Optics. (1975);52:445–464. doi: 10.1097/00006324-197507000-00002. [DOI] [PubMed] [Google Scholar]
- Levi D. M, Harwerth R. S. Contrast evoked potentials in strabismic and anisometropic amblyopia. Investigative Ophthalmology & Visual Science. (1978a);17(6):571–575. [PubMed] [Google Scholar]
- Levi D. M, Harwerth R. S. A sensory mechanism for amblyopia: Electrophysiological studies. Investigative Ophthalmology & Visual Science. (1978b);55:163–171. doi: 10.1097/00006324-197803000-00005. [DOI] [PubMed] [Google Scholar]
- Levi D. M, Harwerth R. S, Manny R. E. Suprathreshold spatial frequency detection and binocular interaction in strabismic and anisometropic amblyopia. Investigative Ophthalmology & Visual Science. (1979);18(7):714–725. [PubMed] [Google Scholar]
- Levi D. M, Klein S. A, Chen I. The response of the amblyopic visual system to noise. Vision Research. (2007);47(19):2531–2542. doi: 10.1016/j.visres.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D. M, Klein S. A, Chen I. What limits performance in the amblyopic visual system: Seeing signals in noise with an amblyopic brain. Journal of Vision. (2008);8(4):1–23.:1. doi: 10.1167/8.4.1. PubMed Article https://doi.org/10.1167/8.4.1 PubMed ] [ PubMed ] [ Article. [DOI] [PubMed] [Google Scholar]
- Li R. W, Ngo C. V, Levi D. M. Relieving the attentional blink in the amblyopic brain with video games. Scientific Reports. (2015);5:8483. doi: 10.1038/srep08483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso C. T, Duffy F. H, Robb R. M. Selective suppression of cerebral evoked potentials to patterned light in amblyopia ex anopsia. Electroencephalography and Clinical Neurophysiology. (1969);27:238–247. doi: 10.1016/0013-4694(69)90052-2. [DOI] [PubMed] [Google Scholar]
- Mackensen G. Reaktionszeitmessungen bei Amblyopia. Albrecht von Graefes Archiv fur Ophthalmologie. (1958);159:636–642. [PubMed] [Google Scholar]
- Manny R. E, Levi D. M. Psychophysical investigations of the temporal modulation sensitivity function in amblyopia: Uniform field flicker. Investigations in Ophthalmology and Visual Science. (1982);22:515–524. [PubMed] [Google Scholar]
- McKee S. P, Levi D. M, Movshon J. A. The pattern of visual deficits in amblyopia. Journal of Vision. (2003);3(5):380–405.:5. doi: 10.1167/3.5.5. PubMed Article https://doi.org/10.1167/3.5.5 PubMed ] [ PubMed ] [ Article. [DOI] [PubMed] [Google Scholar]
- McKee S. P, Levi D. M, Schor C. M, Movshon J. A. Saccadic latency in amblyopia. Journal of Vision. (2016);16(5):1–15.:3. doi: 10.1167/16.5.3. PubMed Article https://doi.org/10.1167/16.5.3 PubMed ] [ PubMed ] [ Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier K, Giaschi D. Unilateral amblyopia affects two eyes: Fellow eye deficits in amblyopia. Investigative Ophthalmology & Visual Science. (2017);58(3):1779–1800. doi: 10.1167/iovs.16-20964. [DOI] [PubMed] [Google Scholar]
- Meier K, Sum B, Giaschi D. Global motion perception in children with amblyopia as a function of spatial and temporal stimulus parameters. Vision Research. (2016);127:18–27. doi: 10.1016/j.visres.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Chandrakumar M, Goltz H. C, Wong A. M. Effects of strabismic amblyopia and strabismus without amblyopia on visuomotor behavior, I: Saccadic eye movements. Investigative Ophthalmology & Visual Science. (2012);53(12):7458–7468. doi: 10.1167/iovs.12-10550. [DOI] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Goltz H. C, Chandrakumar M, Hirji Z. A, Wong A. M. Effects of anisometropic amblyopia on visuomotor behavior, I: Saccadic eye movements. Investigative Ophthalmology & Visual Science. (2010);51(12):6348–6354. doi: 10.1167/iovs.10-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Goltz H. C, Colpa L, Chandrakumar M, Wong A. M. Effects of reduced acuity and stereoacuity on saccades and reaching movements in adults with amblyopia and strabismus. Investigative Ophthalmology & Visual Science. (2017);58(2):914–921. doi: 10.1167/iovs.16-20727. [DOI] [PubMed] [Google Scholar]
- Otero-Millan J, Troncoso X. G, Macknik S. L, Serrano-Pedraza I, Martinez-Conde S. Saccades and microsaccades during visual fixation, exploration, and search: Foundations for a common saccadic generator. Journal of Vision. (2008);8(14):1–18.:21. doi: 10.1167/8.14.21. PubMed Article https://doi.org/10.1167/8.14.21 PubMed ] [ PubMed ] [ Article. [DOI] [PubMed] [Google Scholar]
- Palmer J, Huk A. C, Shadlen M. N. The effect of stimulus strength on the speed and accuracy of a perceptual decision. Journal of Vision. (2005);5(5):376–404.:1. doi: 10.1167/5.5.1. PubMed Article https://doi.org/10.1167/5.5.1 PubMed ] [ PubMed ] [ Article. [DOI] [PubMed] [Google Scholar]
- Perdziak M, Witkowska D, Gryncewicz W, Przekoracka-Krawczyk A, Ober J. The amblyopic eye in subjects with anisometropia show increased saccadic reaction time in the delayed saccade task. Frontiers in Integrative Neuroscience. (2014);8(77):1–13. doi: 10.3389/fnint.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdziak M, Witkowska D, Gryncewicz W, Przekoracka-Krawczyk A, Ober J. Not only amblyopic but also dominant eye in subjects with strabismus show increased saccadic latency. Journal of Vision. (2016);16(10):1–11.:12. doi: 10.1167/16.10.12. PubMed Article https://doi.org/10.1167/16.10.12 PubMed ] [ PubMed ] [ Article. [DOI] [PubMed] [Google Scholar]
- Pianta M. J, Kalloniatis M. Characteristics of anisometropic suppression: Simple reaction time measurements. Attention, Perception, & Psychophysics. (1998);60(3):491–502. doi: 10.3758/bf03206869. [DOI] [PubMed] [Google Scholar]
- Piéron H. II. Recherches sur les lois de variation des temps de latence sensorielle en fonction des intensités excitatrices. L'Année Psychologique. (1914);20(1):17–96. [Google Scholar]
- Piéron H. III. Nouvelles recherches sur l'analyse du temps de latence sensorielle et sur la loi qui relie ce temps à l'intensité d'excitation. L'Année Psychologique. (1920);22(1):58–142. [Google Scholar]
- Piéron H. The sensations: Their functions, processes, and mechanisms. London: Frederick Muller Ltd; (1952). [Google Scholar]
- Pins D, Bonnet C. On the relation between stimulus intensity and processing time: Piéron's law and choice reaction time. Attention, Perception, & Psychophysics. (1996);58(3):390–400. doi: 10.3758/bf03206815. [DOI] [PubMed] [Google Scholar]
- Roberts M, Cymerman R, Smith R. T, Kiorpes L, Carrasco M. Covert spatial attention is functionally intact in amblyopic human adults. Journal of Vision. (2016);16(15):1–19.:30. doi: 10.1167/16.15.30. PubMed Article https://doi.org/10.1167/16.15.30 PubMed ] [ PubMed ] [ Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslow M. G. Effects of components of displacement-step stimuli upon reaction time for saccadic eye movement. Journal of the Optical Society of America. (1967);57(8):1024–1029. doi: 10.1364/josa.57.001024. [DOI] [PubMed] [Google Scholar]
- Schor C, Hallmark W. Slow control of eye position in strabismic amblyopia. Investigations in Ophthalmology & Vision Science. (1978);17(6):577–581. [PubMed] [Google Scholar]
- Sharma V, Levi D. M, Klein S. A. Undercounting features and missing features: evidence for a high-level deficit in strabismic amblyopia. Nature Neuroscience. (2000);3(5):496–501. doi: 10.1038/74872. [DOI] [PubMed] [Google Scholar]
- Siepmann K, Reinhard J, Herzau V. The locus of fixation in strabismic amblyopia changes with increasing effort of recognition as assessed by scanning laser ophthalmoscope. Acta Ophthalmologica Scandinavica. (2006);84(1):124–129. doi: 10.1111/j.1600-0420.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- Sokol S, Bloom B. Visually evoked cortical responses of amblyopes to a spatially alternating stimulus. Investigative Ophthalmology. (1973);12(12):936–939. [PubMed] [Google Scholar]
- Sokol S, Nadler D. Simultaneous electroretinograms and visually evoked potentials from adult amblyopes in response to a pattern stimulus. Investigations in Ophthalmology & Vision Science. (1979);18(8):848–855. [PubMed] [Google Scholar]
- Spekreijse H, Khoe L. H, van der Tweel L. H. A case of amblyopia, electrophysiology and psychophysics of luminance and contrast. The Visual System: Proceedings of the 9th ISCERG Symposium. New York; Plenum: (1972). (Eds.) [DOI] [PubMed] [Google Scholar]
- Taylor M. J, Carpenter R. H, Anderson A. J. A noisy transform predicts saccadic and manual reaction times to changes in contrast. Journal of Physiology. (2006);573(Pt. 3):741–751. doi: 10.1113/jphysiol.2006.105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle D. R. Electrophysiological studies of functional amblyopia utilizing pattern reversal techniques. (Unpublished doctoral dissertation), University of Louisville; Kentucky: (1973). [Google Scholar]
- von Noorden G. K. Reaction time in normal and amblyopic eyes. Archives of Ophthalmology. (1961);66:695–701. doi: 10.1001/archopht.1960.01840010819002. [DOI] [PubMed] [Google Scholar]
- Wallace D. K, Lazar E. L, Melia M, Birch E. E, Holmes J. M, Hopkins K. B, Kraker R. T, Kulp M. T, Pang Y, Repka M. X, Tamkins S. M, Weise K. K, Pediatric Eye Disease Investigator G. Stereoacuity in children with anisometropic amblyopia. Journal of American Association for Pediatric Ophthalmology and Strabismus. (2011);15(5):455–461.:21. doi: 10.1016/j.jaapos.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yinon U, Jakobovitz L, Auerbach E. The visual evoked response to stationary checkerboard patterns in children with strabismic amblyopia. Investigative Ophthalmology & Visual Science. (1974);13(4):293–296. [PubMed] [Google Scholar]
- Yuval-Greenberg S, Merriam E. P, Heeger D. J. Spontaneous microsaccades reflect shifts in covert attention. Journal of Neuroscience. (2014);34(41):13693–13700. doi: 10.1523/JNEUROSCI.0582-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.