Abstract
This study investigated the role of BRAF mutation analysis in thyroid fine-needle aspiration (FNA) samples compared to ultrasonographic and cytological diagnoses. A total 316 patients underwent ultrasonography (US)-guided FNA with BRAFV600E mutation analysis to diagnose thyroid nodules. One hundred sixteen patients with insufficient US images (n = 6), follow-up loss (n = 43), or unknown final diagnosis (n = 67) were excluded from the study. Comparisons between US diagnoses, cytological diagnoses, and BRAF mutation analysis were performed. Of 200 thyroid nodules, there was US diagnosis with 1 false negative and 11 false positive cases, cytological diagnosis with 10 false negative and 2 false positive cases, and BRAFV600E mutation analysis with 19 false negative and 2 false positive cases. The sensitivity, specificity, positive and negative predictive values, and accuracy of BRAFV600E mutation analysis were 83.2%, 98.1%, 97.5%, 86.6%, and 91%, respectively. Of the 18 nodules with Bethesda category III, 9 were true positive, 6 were true negative, 3 was a false negative, and none were false positive on BRAF mutation analysis. In conclusion, we recommend that BRAFV600E mutation analysis only be performed for evaluating thyroid nodules with Bethesda category III, regardless of US diagnosis.
Introduction
Ultrasonography (US)-guided fine-needle-aspiration (FNA) is a simple and accurate tool for evaluating thyroid nodules [1]. With the rising incidence of thyroid cancer and improved nodule detection methods, more thyroid nodules are evaluated by US-guided FNA and cytology. However, these techniques have several limitations, such as operator dependency, different results according to nodular composition and size, false negative or positive cytological findings, and indeterminate cytology [1]. In cases of indeterminate cytology, there is a need for ancillary tests that may be used as adjuncts to thyroid cytology so that the additional information can be used to treat patients more effectively [2]. Genetic alterations have been shown to play a pathogenetic role in thyroid tumorigenesis, and several molecular markers have been investigated for their applicability to thyroid FNA [3, 4]. Among the several Raf kinase isoforms, the B type Raf kinase (BRAF) is the strongest activator of the downstream mitogen-activated extra-cellular signal regulated kinase signaling pathway [3, 4]. BRAFV600E mutations are associated with early tumorigenesis and aggressive behavior of papillary thyroid carcinoma (PTC), and are considered a specific marker of PTC [5, 6].
BRAFV600E is the most common type of BRAF mutation, and has been used as an adjunctive diagnostic tool for patients with thyroid nodules [7]. Several studies have shown that including BRAF mutation analysis with FNA significantly improves the diagnostic accuracy [7–9]. In particular, BRAF mutation analysis can be helpful for the diagnosis of thyroid nodules with atypia of undetermined significance on cytology [2, 9–11]. Additionally, BRAFV600E mutation analysis has a high positive predictive value (95.5–100.0%) for the diagnosis of malignancies in thyroid nodules with atypia of undetermined significance [2, 9–11]. Nevertheless, whether BRAFV600E analysis should be routinely used in clinical practice remains controversial. Numerous researchers have shown BRAFV600E mutation analysis to be an effective diagnostic approach for thyroid FNA samples, while others believe that its utility is limited owing to the low prevalence of BRAFV600E mutations in indeterminate nodules [2, 9–11]. In this study, we assessed the diagnostic role and characteristics of BRAFV600E mutation analysis in thyroid FNA compared to US diagnosis and cytology.
Materials and methods
Study population
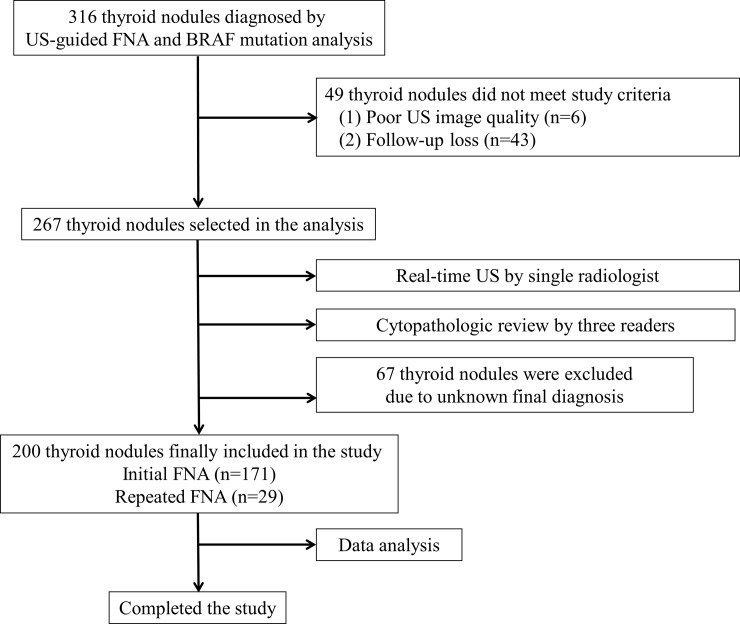
This retrospective study was approved by the Busan Paik Hospital institutional review board (IRB 2016-10-0168); the requirement for informed consent was waived. A single radiologist (who had performed >500 US-guided FNAs/year over 11 years) conducted US-guided FNA to diagnose 316 thyroid nodules (mean size, 12.9 ± 10.1 mm; range, 2.9–52.1 mm) in 316 patients (258 women, 58 men; mean age, 49.8 years; age range, 9–86 years) between January 2013 and December 2015. Among them, there were 146 subcentimetric thyroid nodules. The reasons of US-FNA for these subcentimetric nodules included suspicious US features (n = 95), the presence of contralateral thyroid malignancy (n = 8), and patients’ requests (n = 43). Of the 316 thyroid nodules, 6 nodules with unclear US images and 43 that were lost to follow-up were excluded from the study (Fig 1).
Fig 1. Diagram illustrating the enrollment of subjects using the study’s algorithm.
Thyroid US and nodule classification
Real-time thyroid US was performed by the same radiologist, using a high-resolution US instrument (iU 22; Philips Medical Systems, Bothell, WA, USA) equipped with a 12–15 MHz linear probe. For thyroid nodules, benign US features included an ovoid shape, isoechogenicity, a smooth margin, and peripheral vascularity; borderline US features of thyroid nodules included hypoechogenicity, centrally predominant vascularity, and a solid, round configuration; and malignant US features included marked hypoechogenicity, a spiculated margin, microcalcifications, and a taller-than-wide shape [12, 13]. US diagnosis for each thyroid nodule was classified into 1 of 5 categories based on the real-time US examination results: “benign” (3 or more benign US features and no malignant or borderline US features), “probably benign” (1 or 2 benign US features and no malignant or borderline US features), “indeterminate” (1 or more borderline US features and no malignant US features), “suspicious for malignancy” (1 malignant US feature, regardless of benign or borderline US features), or “malignant” (2 or more malignant US features) [12, 13]. In the calculation of diagnostic accuracy in US diagnosis, “benign” and “probably benign” categories were classified as benign, whereas “suspicious for malignancy” and “malignant” categories were classified as malignancy, but “indeterminate” US category cases were excluded.
US-guided FNA and cytological diagnosis
US-guided FNA was performed immediately after thyroid US by the same radiologist using a 1-needle puncture, no aspiration device, and no local anesthesia [14]. For each sample, a smear was prepared on 4–6 slides using the flipping-extraction technique; samples were fixed in 95% ethanol and examined after Papanicolaou staining. The cytological analysis was categorized as follows: inadequate (Bethesda category I), benign (Bethesda category II), atypia of undetermined significance or follicular lesion of undetermined significance (Bethesda category III), suspicious for a follicular neoplasm (Bethesda category IV), suspicious for malignancy (Bethesda category V), or malignant (Bethesda category VI) [15]. Three cytopathologists with different levels of experience (13, 14, and 20 years) who were blinded to the US-based diagnoses performed the cytological diagnoses. In the calculation of diagnostic accuracy in cytological diagnosis, Bethesda category I and II were classified as benign, whereas Bethesda category V and VI were classified as malignancy, but “indeterminate” cytology cases (Bethesda category III and IV) were excluded.
DNA extraction and BRAFV600E mutation analysis
After US-guided FNA for cytology, aspiration was repeated for BRAFV600E mutation analysis. Genomic DNA was extracted from obtained aspirates using the QIAamp DNA extraction kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. T1799A transversion was detected using the Anyplex BRAFV600E real-time detection system (Seegene Inc., Seoul, Korea). Genomic DNA (5 μL) was added to the 15 μL reaction mixture (2 μL of 10× BRAF Oligo Mix, 3 μL of 8-methoxypsoralen solution, and 10 μL of 2× Anyplex PCR Master Mix [Seegene Inc., Seoul, Korea]). Real-time polymerase chain reaction was performed using a CFX96 real-time polymerase chain reaction system (Bio-Rad, Hercules, CA, USA) with the following conditions: 15 min at 95°C, followed by 15 cycles of 15 s at 95°C and 30 s at 60°C, and then by 35 cycles of 30 s at 95°C and 32 s at 60°C. For real-time polymerase chain reaction, the cycle threshold was defined as the number of cycles required for the fluorescent signal to exceed the threshold. The cycle threshold values of the target and internal control were <33 and 30, respectively. Each run contained both a positive and negative control. In the calculation of diagnostic accuracy in BRAF mutation analysis, negatives were classified as benign, whereas positives were classified as malignancy.
Final diagnosis
In our hospital, thyroid surgery is performed as follows: (1) malignant cytology (Bethesda category V and VI), (2) positive BRAF mutation analysis, (3) indeterminate cytology (Bethesda category III and IV) but suspicious US diagnosis (“suspicious for malignancy” and “malignant” categories), and (4) patient request. On the other hand, patients with nodules that were diagnosed as benign on both US and cytology received follow-up by thyroid US instead of undergoing thyroid surgery or repeated US-guided FNA. The final diagnoses of the thyroid nodules were determined as follows: (1) when surgery was performed, diagnosis was according to the histopathological results; (2) when nonsurgical thyroid nodules in the “benign” or “probably benign” US categories exhibited benign cytological results and no suspicious features on follow-up US, they were considered benign; and (3) when nonsurgical thyroid nodules in the “indeterminate,” “suspicious for malignancy,” or “malignant” US categories exhibited benign cytological results on both initial and repeated US-guided FNAs, they were considered benign.
Statistical analysis
The data were tested for normal distribution by employing the Kolmogorov-Smirnov test. The age at the time of diagnosis and sizes of thyroid nodules are expressed as means ± standard deviations. Mean differences in age and sizes of thyroid nodules between the 2 groups (BRAF mutation-positive vs. BRAF mutation-negative) were compared using the independent t-test. Group comparisons of categorical variables related to US diagnoses, FNA results, and final diagnoses were performed using the χ2 test or, for small cell values, the Fisher’s exact test. The diagnostic indices (sensitivity, specificity, positive and negative predictive values, and accuracy) of BRAF mutation analyses were also calculated. All statistical analyses were performed using the IBM SPSS Statistics 19.0 software package (SPSS, Chicago, IL, USA). A P-value <0.05 was considered statistically significant.
Results
Of the 267 thyroid nodules (mean diameter, 12.8 ± 9.7 mm; range, 2.9–52.1 mm) in 267 patients (223 women, 44 men; mean age, 52.1 ± 12.1 years; age range, 18–84 years), 67 (mean diameter, 9.8 ± 6.7 mm; range, 2.9–37.6 mm) were excluded because of an unknown final diagnosis (Fig 1). Ultimately, 200 thyroid nodules (mean diameter, 13.8 ± 10.3 mm; range, 3.0–52.1 mm) in 200 patients (167 women, 33 men; mean age, 51.5 ± 12.7 years; age range, 18–79 years) were included, representing 171 (85.5%) initial and 29 (24.5%) repeated FNAs. The locations of the thyroid nodules included the right thyroid lobe (n = 108), left thyroid lobe (n = 85), and isthmus (n = 7). The final diagnoses of the 200 thyroid nodules were determined as follows: (1) histopathological analysis (n = 106); (2) benign diagnoses according to US and cytology, with no US follow-up (n = 59); and (3) indeterminate or malignant on US, but benign cytological results based on both initial and repeated US-guided FNAs (n = 35). There were 95 PTCs, 1 follicular thyroid carcinoma, 2 medullary thyroid carcinomas, 3 follicular adenomas, 5 nodular hyperplasia, and 94 non-surgical benign nodules. The nodule size was significantly larger in the BRAF mutation-negative group (14.8 mm) than in the BRAF mutation-positive group (9.1 mm) (p < 0.0001), whereas there was no significant difference in patient age (p = 0.146).
US and cytological diagnoses as well as BRAFV600E mutation analyses of the 200 thyroid nodules are summarized in Table 1, along with their final diagnostic results. Of the benign US group (n = 82), 2 benign thyroid nodules were positive on BRAF mutation analysis, whereas they showed Bethesda category II on cytology. In the indeterminate US group (n = 20), no nodules were positive on BRAF mutation analysis. Two malignant thyroid nodules with indeterminate US diagnoses and negative BRAF mutation analysis were diagnosed as PTC and follicular thyroid carcinoma after thyroid surgery, respectively. In the malignant US group (n = 98), 17 malignant nodules were negative on BRAF mutation analysis (15 PTCs and 2 medullary thyroid carcinomas). Among these cases, 11 showed malignant cytology, the remainders had Bethesda category I (n = 1), II (n = 2), and III (n = 3). In the benign cytology group (Bethesda category I or II, n = 104), 4 malignant nodules were negative on BRAF mutation analysis (3 PTCs and 1 follicular thyroid carcinoma) and 2 benign nodules were positive on BRAF mutation analysis (1 nodular hyperplasia and 1 non-surgical benign nodule). In the indeterminate cytology group (n = 18), 12 nodules were surgically removed because of positive BRAF mutation analysis (n = 9) and malignant US diagnosis (n = 3). In the malignant cytology group (Bethesda category V or VI, n = 78), there were 12 malignant nodules with negative BRAF mutation analysis (10 PTCs and 2 medullary thyroid carcinomas). As for the diagnostic indices of BRAF mutation analyses for the 200 thyroid nodules, BRAFV600E mutation analysis revealed 79 true positive, 2 false positive, 103 true negative, and 16 false negative cases; the sensitivity, specificity, positive and negative predictive values, and accuracy were 83.2%, 98.1%, 97.5%, 86.6%, and 91%, respectively. When medullary and follicular thyroid carcinoma cases were excluded, the sensitivity, specificity, positive and negative predictive values, and accuracy of BRAF mutation analysis were 83.2%, 98.0%, 97.5%, 86.2%, and 90.9%, respectively.
Table 1. Ultrasonographic diagnoses, cytological diagnoses, and BRAF mutation analyses of 200 thyroid nodules according to final results.
| BRAF mutation analysis | ||
|---|---|---|
| Negative (n = 119, 59.5%) | Positive (n = 81, 40.5%) | |
| US diagnosis | ||
| Benign (n = 44, 22%) |
NH (1), NSBN (41); (n = 42, 21%) |
NH (1), NSBN (1); (n = 2, 1%) |
| Probably benign (n = 38, 19%) |
NH (2), FA (2), NSBN (33); (n = 37, 18.5%) |
PTC (1); (n = 1, 0.5%) |
| Indeterminate (n = 20, 10%) |
PTC (1), FTC (1), FA (1), NSBN (9); (n = 12, 6%) |
PTC (8); (n = 8, 4%) |
| Suspicious for malignancy (n = 57, 28.5%) |
PTC (10), MTC (1), NH (1), NSBN (10); (n = 22, 11%) |
PTC (35); (n = 35, 17.5%) |
| Malignant (n = 41, 20.5%) |
PTC (5), MTC (1); (n = 6, 3%) |
PTC (35); (n = 35, 17.5%) |
|
Cytological diagnosis (Bethesda category) |
||
| I (n = 5, 2.5%) | PTC (1), NSBN (3); (n = 4, 2%) |
PTC (1); (n = 1, 0.5%) |
| II (n = 99, 49.5%) | PTC (2), FTC (1), FA (3), NH (2), NSBN (84); (n = 92, 46%) |
PTC (5), NH (1), NSBN (1); (n = 7, 3.5%) |
| III (n = 18, 9%) | PTC (3), NSBN (6); (n = 9, 4.5%) |
PTC (9); (n = 9, 4.5%) |
| IV (n = 0, 0%) | 0 | 0 |
| V (n = 25, 12.5%) | PTC (3), NH (2); (n = 5, 2.5%) |
PTC (20); (n = 20, 10%) |
| VI (n = 53, 26.5%) | PTC (7), MTC (2); (n = 9, 4.5%) |
PTC (44); (n = 44, 22%) |
Note.—Numbers in parentheses are prevalence of each item. US, ultrasonography; NH, nodular hyperplasia; NSBN, Non-surgical benign nodules, which were finally determined by repeated fine-needle aspiration cytology or fine-needle aspiration cytology and follow-up ultrasonographic findings. FA, follicular adenoma; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma.
Of the 200 thyroid nodules, 171 (mean diameter, 14.2 ± 10.4 mm; range, 3.0–52.1 mm) had no previous FNA history; the US diagnoses, cytological diagnoses, and BRAFV600E mutation analyses of these thyroid nodules that underwent initial US-guided FNA are summarized in Table 2 according to their final results. In the benign US diagnosis group (n = 69), 2 nodules (2.9%) were positive on BRAF mutation analysis, whereas they were Bethesda category II on cytology. In the malignant US diagnosis group (n = 86), 13 nodules were false negative on BRAF mutation analysis (13 PTCs). Among these false negative cases, 10 showed malignant cytology, whereas the remainders were Bethesda category II (n = 2) and III (n = 2). In the benign cytology group (n = 87), 2 benign nodules (1 nodular hyperplasia and 1 non-surgical benign nodule) were false positive and 3 malignant nodules (2 PTCs and 1 follicular thyroid carcinoma) were false negative on BRAF mutation analysis. In the malignant cytology group (n = 70), 10 malignant nodules (10 PTCs) were false negative on BRAF mutation analysis.
Table 2. Ultrasonographic diagnoses, cytological diagnoses, and BRAF mutation analyses of 171 thyroid nodules with the initial fine-needle aspiration according to final results.
| BRAF mutation analysis | ||
|---|---|---|
| Negative (n = 95, 55.6%) | Positive (n = 76, 44.4%) | |
| US diagnosis | ||
| Benign (n = 39, 22.8%) |
NSBN (37); (n = 37, 21.6%) |
NH (1), NSBN (1); (n = 2, 1.2%) |
| Probably benign (n = 30, 17.5%) |
FA (1), NH (1), NSBN (27); (n = 29, 17.0%) |
PTC (1); (n = 1, 0.6%) |
| Indeterminate (n = 16, 9.4%) |
PTC (1), FTC (1), FA (1), NSBN (6); (n = 9, 3.5%) |
PTC (7); (n = 7, 4.1%) |
| Suspicious for malignancy (n = 50, 29.2%) |
PTC (10), NH (1), NSBN (5); (n = 16, 9.4%) |
PTC (34); (n = 34, 19.9%) |
| Malignant (n = 36, 21.1%) |
PTC (3), MTC (1); (n = 4, 2.3%) |
PTC (32); (n = 32, 18.7%) |
| Cytological diagnosis (Bethesda category) | ||
| I (n = 3, 1.8%) | NSBN (2); (n = 2, 1.2%) |
PTC (1); (n = 1, 0.6%) |
| II (n = 84, 49.1%) | PTC (2), FTC (1), FA (2), NH (2), NSBN (70); (n = 77, 45.0%) |
PTC (5), NH (1), NSBN (1); (n = 7, 4.1%) |
| III (n = 14, 8.2%) | PTC (2), NSBN (3); (n = 5, 2.9%) |
PTC (9); (n = 9, 3.5%) |
| IV (n = 0, 0%) | 0 | 0 |
| V (n = 21, 12.3%) | PTC (3); (n = 3, 1.8%) |
PTC (18); (n = 18, 10.5%) |
| VI (n = 49, 28.7%) | PTC (7), MTC (1); (n = 8, %) |
PTC (41); (n = 41, 24.0%) |
Note.—Numbers in parentheses are prevalence of each item. US, ultrasonography; NSBN, Non-surgical benign nodules, which were finally determined by repeated fine-needle aspiration cytology or fine-needle aspiration cytology and follow-up ultrasonographic findings. NH, nodular hyperplasia; FA, follicular adenoma; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma.
The 29 thyroid nodules that had undergone two or more US-guided FNA sessions (mean diameter, 11.8 ± 9.7 mm; range, 4.1–48.0 mm) are summarized in Table 3. Among them, 15 nodules (51.7%) had Bethesda category III on cytology after the initial US-guided FNA, including 4 PTCs, 1 follicular adenoma, 1 nodular hyperplasia, and 9 non-surgical benign nodules. Of the 4 PTCs, 3 were BRAFV600E-mutation positive and 1 was mutation-negative. No false positive cases were found in this group following BRAF mutation analysis.
Table 3. Ultrasonographic diagnoses, cytological diagnoses, and BRAF mutation analyses of 29 thyroid nodules with the repeated fine-needle aspiration according to final results.
| BRAF mutation analysis | ||
|---|---|---|
| Negative (n = 24) | Positive (n = 5) | |
| US diagnosis | ||
| Benign (n = 5) | NH (1), NSBN (4) | 0 |
| Probably benign (n = 8) | FA (1), NH (1), NSBN (6) | 0 |
| Indeterminate (n = 4) | NSBN (3) | PTC (1) |
| Suspicious for malignancy (n = 7) | MTC (1), NSBN (5) | PTC (1) |
| Malignant (n = 5) | PTC (2) | PTC (3) |
|
Cytological diagnosis (Bethesda category) |
||
| I (n = 2) | PTC (1), NSBN (1) | 0 |
| II (n = 15) | FA (1), NSBN (14) | 0 |
| III (n = 4) | PTC (1), NSBN (3) | 0 |
| IV (n = 0) | 0 | 0 |
| V (n = 4) | NH (2) | PTC (2) |
| VI (n = 4) | MTC (1) | PTC (3) |
Note.—Numbers in parentheses are prevalence of each item. US, ultrasonography; NH, nodular hyperplasia; NSBN, Non-surgical benign nodules, which were finally determined by repeated fine-needle aspiration cytology or fine-needle aspiration cytology and follow-up ultrasonographic findings. FA, follicular adenoma; MTC, medullary thyroid carcinoma; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma.
Discussion
BRAF mutation analysis has been used to improve the diagnostic accuracy in thyroid FNA. However, the rate of BRAF mutation positivity ranges from 29% to 69% in previous studies [6, 9, 15]. To date, the routine application of BRAF mutation analysis is not recommended because of its cost and questionable diagnostic utility [6]. In the literature, BRAF mutation analysis is recommended on thyroid nodules with indeterminate cytology [6, 10]. Our results concurred with this guideline. Of the 15 repeated-FNA nodules that were Bethesda category III in the initial US-guided FNA, 3 PTCs were surgically excised because of BRAFV600E mutation positivity.
US diagnosis is considered useful for evaluating thyroid nodules [1, 12]. Moreover, US-guided FNA has been used for the diagnosis of thyroid nodules with suspicious US features [1, 13]. Thus, both US diagnosis and US-guided FNA appear to be sufficient for the evaluation and management of thyroid nodules. However, these methods are unhelpful for thyroid nodules with malignant US features and benign cytology or for thyroid nodules with indeterminate cytology [1, 15, 16]. Hence, BRAFV600E mutation analysis was introduced, which several studies found to be more effective in thyroid nodules with suspicious US features than in those with benign US features [17–19]. In the malignant US group in our study, BRAFV600E mutation analysis exhibited no false positives, whereas false negatives on BRAF mutation analysis were found in 15 PTCs and 2 medullary thyroid carcinomas. Of the benign US group (n = 82), 4 nodules were false positive on BRAFV600E mutation analysis (showing benign cytology), whereas there were no false negative cases. Thus, the routine application of BRAFV600E mutation analysis is not recommended in thyroid FNA.
In case of discordance between the US and cytological diagnoses of thyroid nodules, cytological diagnoses take priority [20]. However, in the management of thyroid nodules that have benign cytology but are positive on BRAF mutation analysis, thyroidectomy should be considered in nodules that have 2 or more malignant US features [21]. In our study, 2 false positive BRAFV600E mutation cases were benign on US and cytology, while 16 false negative BRAFV600E mutation cases were malignant on US or cytology. Thus, a combination of US diagnosis, cytological diagnoses, and BRAFV600E mutation analysis may be required for a reliable diagnosis.
BRAF mutation analysis may be effective for the initial US-guided FNA of thyroid nodules with suspicious US features [18]. However, the routine use of BRAF mutation analysis may not be cost-effective in clinical practice. In our study, 85.5% of the nodules (171/200) had undergone initial FNA. In the benign US diagnosis group (n = 69), there were 2 false positive cases on BRAFV600E mutation analysis; these cases showed benign cytology. In the benign cytology group (n = 87), there were 2 false positive cases on BRAFV600E mutation analysis. Such false positive results may prevent patients from receiving the appropriate treatment; therefore, the routine use of BRAFV600E mutation analysis at the time of the initial FNA of the thyroid nodules is unlikely to be helpful.
There are several limitations to this study. First, not all thyroid nodules were surgically confirmed. Ninety-five non-surgical benign nodules with only repeated US-guided FNA or US follow-up were included. Second, we excluded 67 non-surgical thyroid nodules from the calculation of the diagnostic index; this may have resulted in selection bias. Third, of the 200 thyroid nodules investigated, there were no cases with Bethesda category IV. Fourth, the PTC subtypes were not evaluated. Finally, three cytopathologists with different levels of experience in cytological analysis interpreted the FNA slides; however, we did not investigate interobserver variability.
Conclusions
BRAFV600E mutation analysis is not particularly helpful in cases of benign US diagnosis. Furthermore, BRAFV600E mutation analysis at the time of the initial FNA of thyroid nodules may not be of benefit because of the lack of cost-effectiveness in clinical practice. Therefore, we suggest that BRAFV600E mutation analysis should be performed only for evaluating thyroid nodules with Bethesda category III, regardless of US diagnosis.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeong SH, Hong HS, Lee EH, Cha JG, Park JS, Kwak JJ. Outcome of thyroid nodules characterized as atypia of undetermined significance or follicular lesion of undetermined significance and correlation with Ultrasound features and BRAF(V600E) mutation analysis. Am J Roentgenol. 2013;201(6):W854–860. 10.2214/AJR.12.9901 [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer. 2007;110(1):38–46. 10.1002/cncr.22754 [DOI] [PubMed] [Google Scholar]
- 4.Ohori NP, Singhal R, Nikiforova MN, Yip L, Schoedel KE, Coyne C, et al. BRAF mutation detection in indeterminate thyroid cytology specimens: underlying cytologic, molecular, and pathologic characteristics of papillary thyroid carcinoma. Cancer Cytopathol. 2013;121(4):197–205. 10.1002/cncy.21229 [DOI] [PubMed] [Google Scholar]
- 5.Park HJ, Moon JH, Yom CK, Kim KH, Choi JY, Choi SI, et al. Thyroid "atypia of undetermined significance" with nuclear atypia has high rates of malignancy and BRAF mutation. Cancer Cytopathol. 2014;122(7):512–520. 10.1002/cncy.21411 [DOI] [PubMed] [Google Scholar]
- 6.Di Benedetto GD. Thyroid fine-needle aspiration: the relevance of BRAF mutation testing. Endocrine. 2014;47(2):351–353. 10.1007/s12020-014-0222-1 [DOI] [PubMed] [Google Scholar]
- 7.Moon WJ, Choi N, Choi JW, Kim SK, Hwang TS. BRAF mutation analysis and sonography as adjuncts to fine-needle aspiration cytology of papillary thyroid carcinoma: their relationships and roles. Am J Roentgenol. 2012;198(3):668–674. 10.2214/AJR.11.7185 [DOI] [PubMed] [Google Scholar]
- 8.Zatelli MC, Trasforini G, Leoni S, Frigato G, Buratto M, Tagliati F, et al. BRAF V600E mutation analysis increases diagnostic accuracy for papillary thyroid carcinoma in fine-needle aspiration biopsies. Eur J Endocrinol. 2009;161(3):467–473. 10.1530/EJE-09-0353 [DOI] [PubMed] [Google Scholar]
- 9.Kim SW, Lee JI, Kim JW, Ki CS, Oh YL, Choi YL, et al. BRAFV600E mutation analysis in fine-needle aspiration cytology specimens for evaluation of thyroid nodule: a large series in a BRAFV600E-prevalent population. J Clin Endocrinol Metab. 2010;95(8):3693–3700. 10.1210/jc.2009-2795 [DOI] [PubMed] [Google Scholar]
- 10.Hyeon J, Ahn S, Shin JH, Oh YL. The prediction of malignant risk in the category "atypia of undetermined significance/follicular lesion of undetermined significance" of the Bethesda System for Reporting Thyroid Cytopathology using subcategorization and BRAF mutation results. Cancer Cytopathol. 2014;122(5):368–376. 10.1002/cncy.21396 [DOI] [PubMed] [Google Scholar]
- 11.Su X, Jiang X, Xu X, Wang W, Teng X, Shao A, et al. Diagnostic value of BRAF(V600E)-mutation analysis in fine-needle aspiration of thyroid nodules: a meta-analysis. OncoTargets Ther. 2016;9:2495–2509. 10.2147/OTT.S101800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YJ, Kim DW, Park YM, Park HK, Jung SJ, Kim DH, et al. Comparison of sonographic and cytological diagnoses of solid thyroid nodules: emphasis on discordant cases. Diagn Cytopathol. 2015;43(12):953–958. 10.1002/dc.23363 [DOI] [PubMed] [Google Scholar]
- 13.Kim DW, Park JS, In HS, Choo HJ, Ryu JH, Jung SJ. Ultrasound-based diagnostic classification for solid and partially cystic thyroid nodules. Am J Neuroradiol. 2012;33(6):1144–1149. 10.3174/ajnr.A2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DW, Choo HJ, Park JS, Lee EJ, Kim SH, Jung SJ, et al. Ultrasonography-guided fine-needle aspiration cytology for thyroid nodules: an emphasis on one-sampling and biopsy techniques. Diagn Cytopathol. 2012;40(Suppl 1):E48–54. 10.1002/dc.21669 [DOI] [PubMed] [Google Scholar]
- 15.Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009;19(11):1159–1165. 10.1089/thy.2009.0274 [DOI] [PubMed] [Google Scholar]
- 16.Puxeddu E, Filetti S. BRAF mutation assessment in papillary thyroid cancer: are we ready to use it in clinical practice? Endocrine. 2014:45(3):341–343. 10.1007/s12020-013-0139-0 [DOI] [PubMed] [Google Scholar]
- 17.Kim DW. Benign lesions that mimic thyroid malignancy on ultrasound. Can Assoc Radiol J. 2015;66(1):79–85. 10.1016/j.carj.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 18.Nam SY, Han BK, Ko EY, Kang SS, Hahn SY, Hwang J, et al. BRAF V600E mutation analysis of thyroid nodules needle aspirates in relation to their ultrasonographic classification: a potential guide for selection of samples for molecular analysis. Thyroid. 2010;20(3):273–279. 10.1089/thy.2009.0226 [DOI] [PubMed] [Google Scholar]
- 19.Moon HJ, Kim EK, Chung WY, Choi JR, Yoon JH, Kwak JY. Diagnostic value of BRAFV600E mutation analysis of thyroid nodules according to ultrasonographic features and the time of aspiration. Ann Surg Oncol. 2011;18(3):792–799. 10.1245/s10434-010-1354-z [DOI] [PubMed] [Google Scholar]
- 20.Koh J, Choi JR, Han KH, Kim E, Yoon JH, Moon HJ, et al. Proper indication of BRAF(V600E) mutation testing in fine-needle aspirates of thyroid nodules. PLoS ONE. 2013;8(5):e64505 10.1371/journal.pone.0064505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SY, Kim EK, Kwak JY, Moon HJ, Yoon JH. What to do with thyroid nodules showing benign cytology and BRAFV600E mutation? A study based on clinical and radiologic features using a high sensitive analytic method. Surgery. 2015;157(2):354–361. 10.1016/j.surg.2014.09.003 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.