Abstract
Acute Kidney Injury (AKI) is the main systemic complication and cause of death in viperid envenomation. Although there are hypotheses for the development of AKI, the mechanisms involved are still not established. The aim of this study was to evaluate the clinical-laboratorial-epidemiological factors associated with AKI in victims of Bothrops sp envenomation. This is an observational study carried out at the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado. AKI was defined according to the guidelines of the Acute Kidney Injury Network (AKIN). Among the 186 patients evaluated, AKI was observed in 24 (12.9%) after 48 hours of admission. Stage I was present in 17 (70.8%) patients, II in 3 (12.5%) and III in 4 (16.7%). Epidemiological characterization showed predominance of men, occurrence in rural areas, aged between 16–60 years, feet as the most affected anatomical region, and time to medical assistance less than 3 hours. Hypertension and diabetes were the comorbidities identified. Most of the accidents were classified as moderate, and clinical manifestations included severe pain, mild edema, local bleeding and headache. Laboratory results showed blood uncoagulability, hypofibrinogenemia, leukocytosis, increase of creatine kinase, and high lactate dehydrogenase levels. Multivariate analysis showed an association with high LDH levels [AOR = 1.01 (95% CI = 1.01–1.01, p<0.002)], local bleeding [AOR = 0.13 (95%CI = 0.027–0.59, p<0.009)], and the presence of comorbidities [AOR = 60.96 (95%CI = 9.69–383.30; p<0.000)]. Herein, laboratory markers such as high LDH levels along with local bleeding and comorbidities may aid in the diagnosis of AKI.
Introduction
Snakebite envenomation is a neglected public health problem in tropical countries of underdeveloped continents such as Africa, Asia, Latin America and Oceania [1–3]. Estimates suggest that about 1,800,000 cases and 94,000 deaths occur annually [2]. In Brazil, in 2015, data from the SINAN (national reporting platform) reported 15,454 envenomation cases with an estimated lethality rate of 0.4% [4]. In the state of Amazonas, an average of 1,500 snakebites are reported each year, resulting in an incidence rate of 52.8 cases per 100.000 inhabitants per year, where the proportion of lethality is higher than the national rate of 0.6% [5]. In the Amazon, the Bothrops genus is responsible for the highest proportion of snakebites [5–10]. The most important species is Bothrops atrox (common lancehead, Amazonian jararaca, jararaca and locally as surucucu) usually found in forested and populated areas [11].
The venom of this genus is composed of a complex mixture of peptides and biologically active proteins that induce a wide range of effects [12–14]. Local and/or systemic manifestations occur due to the tissue injury with subsequent inflammatory mediators release and changes in the coagulation and fibrinolytic system [15–19]. Victims of Bothrops atrox envenomation frequently present an intense acute inflammatory response [15] with pain, edema, flushing, bleeding, bruising and blistering at the bite site [20,21]. Systemic effects include headache, nausea, vomiting, spontaneous hemorrhage (gingival, nasal, digestive, hematuria, hematemesis), and more rarely disseminated intravascular coagulation and shock [22,23]. Complications occur due to clinical and environmental factors and include necrosis, secondary infection, Acute Kidney Injury (AKI), intracranial hemorrhage, compartment syndrome, amputations [24–27], and even infrequent situations such as abruptio placentae in pregnant women [22] and hepatic hematoma[28].
AKI is the most significant systemic complication and cause of death in envenomation by the Viperidae family, with Bothrops and Crotalus genera being the main cause [29–31]. The prevalence ranges from 10–29% [22,25,31,32] and 1.6–38.5% [31,33–37] respectively, depending on the causative species. AKI is 10 times more common in crotalic accidents than in bothropic, but the incidences are similar due to the greater number of accidents caused by the Bothrops genus [31,36]. Venom toxins have direct renal action, changing their structure [30,38–40] and physiology [41] due to capillary vulnerability. Moreover, Acute Tubular Necrosis (ATN) is the most common cause of AKI in snakebite envenomation [38,42,43]. Experimental studies associate this damage to factors such as serpent genus and geographic distribution, severity of the accident, time of exposure to venom [38,44], and inoculated amount of venom [30]. The pathogenesis of AKI has not yet been fully elucidated. However, a multifactorial origin is proposed [45] where some triggering factors would act in a combined or isolated manner [22,32,36,46]. Among these factors are hemodynamic disorders with bleeding or fibrin deposits in tubular structures, inflammatory processes, formation of immune complexes, and nephrotoxic action of venom [30,39,41]. The development of AKI following envenomation has been associated with the age of the individual, where children are more prone due to lower body surface area [25,44], age of the snake [47], venom composition and mechanism of action of the toxins [30,31], time elapsed between the accident and antivenom administration [29,32,35,44], and the presence of comorbidities such as diabetes mellitus and hypertension [43] have been associated with AKI in snakebite patients.
Distance to medical facilities becomes a major hindrance in the Amazon since victims need to travel long distances to healthcare services being prone to complications due to geographical peculiarities [26]. This dynamic results in hampered access to healthcare facilities therefore determining the use of traditional medicine as a treatment option [48]. The magnitude of the problem is expressed by underreporting of snakebites and development of complications [49], which can exemplify AKI. Besides, existing publications on the region only describe the frequency of the event, with no clinical studies especially on AKI [5,26,50]. Although studies point to hypotheses for the development of AKI, its mechanisms have not yet been fully established. Thus, the description of the renal injury pathogenesis based on clinical characteristics presented by patients could contribute to the clarification of the development of acute renal injury induced by envenomation. The aim of this study was to evaluate the clinical-laboratorial-epidemiological factors associated with AKI in victims of envenomation by Bothrops snakes.
Materials and methods
Study design
This is an observational study carried out from August 2014 to August 2016 at the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (FMT-HVD), a reference hospital for snakebite treatment in Manaus, capital city of Amazonas, Brazil, and the only institution in this city offering antivenom therapy. This study was approved by the Ethics Review Board of FMT-HVD (approval n° 602.907-0/2013). All participants signed a consent form after the explanation of the study aims. Under 18 years of age, the consent was signed by parents or guardians. The individual in this manuscript has given written informed consent (as outlined in PLOS consent form) to publish these case details.
Sample size calculation
Sample calculation was based on two studies conducted in the Amazon region, with an expected frequency of AKI after envenomation of 12% [5,51], at an 80% power and 5% of significance level. The minimum of 174 individuals was necessary adjusting by 15% of lost to follow-up.
Case selection
All patients included in the study were hospitalized due to the snakebite. Eligible patients were those diagnosed with clinical signs of envenomation by Bothrops genus. Pregnant women or individuals that underwent previous antivenom therapy in another hospital unit were not included in this study. According to the Brazilian Ministry of Health Guideline [12], envenomation by Bothrops species is classified as follows: I) mild envenomation, characterized by changes in clotting time or not, pain and edema involving one to two segments of the affected limb; II) moderate envenomation, with changes in clotting time or not, pain, evident edema involving three or more segments of the affected limb and local or systemic bleeding without hemodynamic repercussion; III) severe envenomation, that may present changes in clotting time, severe pain, severe hardened edema in the limb, severe hemorrhagic conditions with hemodynamic repercussion, compartment syndrome, and AKI. In addition to the signs of envenomation, patients should have at least two serum creatinine assessments, where the first creatinine test was performed prior to the antivenom therapy.
Case definition of AKI
At least two measurements of creatinine levels were obtained from each patient during the study period. Acute kidney injury was defined according to the Acute Kidney Injury Network (AKIN) Guideline [52], as follows: Stage I, defined by an increase of >0.3 mg/dL or up to 199% of baseline creatinine levels; Stage II, defined by an increase of 200–299%; and Stage III, an increase in more than 300% in baseline creatinine or serum creatinine levels higher than 4.0 mg/dL with an abrupt elevation of at least 0.5 mg/dL. All patients diagnosed with AKI were referred to the institution’s nephrologist. Those classified as stage III AKI underwent renal replacement therapy.
Clinical and laboratorial parameters
Data collection included sociodemographic features such as sex, age (in years), anatomical region of bite, area of occurrence (rural or urban), work-related bite, time to medical assistance, clinical and laboratory characteristics (at the moment of the antivenom therapy-D0, first day-D1, second day-D2, third day-D3 and seventh day-D7 of hospitalization), including vital signs, severity classification (mild, moderate or severe), local and systemic manifestations, clinical outcome (discharge or death) and presence of comorbiditites declared by the patient.
Laboratory assays included enzyme immunoassay using monoclonal antibody anti-B. atrox on admission to assess antigenemia [53], whole blood counts, fibrinogen, serum levels of sodium, potassium, urea, creatinine, alanine transaminase (ALT), aspartate transaminase (AST), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), creatine kinase (CK). Urinalysis and clotting time were also performed.
Statistical analysis
A database was built using Epi Info 3.5.1™ (Center for Disease Control and Prevention, Atlanta, USA) forms fed by two independent investigators. Data analysis was carried out using the statistical package STATA v13 (Stata Corp, College Station, USA). Explanatory variables were grouped into hierarchical blocks [44,54]. The proximal block was composed of laboratory markers at admission, intermediate block by clinical findings at admission, and distal block by demographic and epidemiological variables. Univariate regression analysis was performed for each block individually. Variables with significance level of p<0.2 were included in the multivariate analysis per block. All variables with significance level of p<0.05 in the multivariate analysis per block were further included in the overall model (all blocks together). Crude Odds Ratio (OR) and Adjusted Odds Ratio (AOR) with their respective confidence intervals (CIs) were calculated for each hierarchical level and for the global model. The precision of the final model was assessed through Hosmer-Lemeshow goodness-of-fit test [30,45]. The significance level was considered for p<0.05.
Results
Patients’ characterization
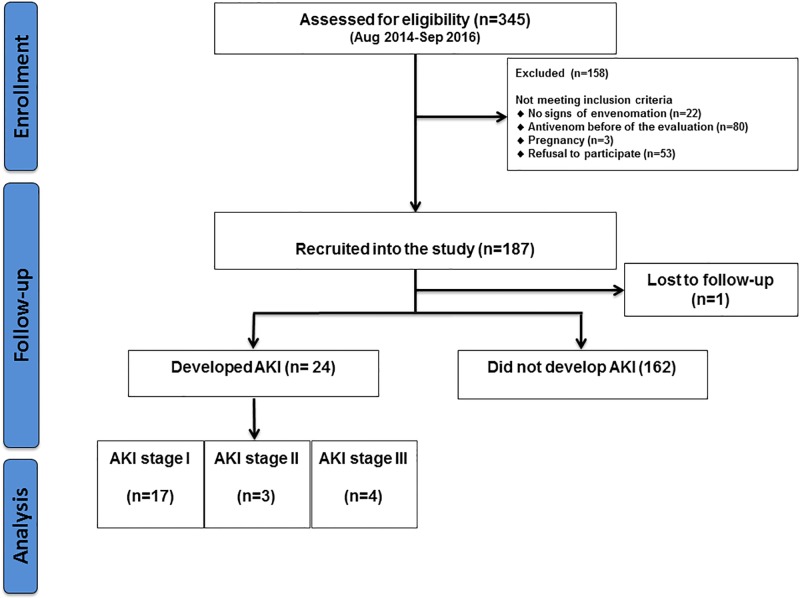
A total of 345 patients were assessed for eligibility in the study period. From the total, 187 met the inclusion criteria, with one patient lost to follow-up due to hospital evasion (Fig 1). Enzyme immunoassay confirmed the diagnosis of Bothrops sp. envenomation in all patients with complete follow-up.
Fig 1. Flowchart of inclusion of patients admitted at FMT-HVD following Bothrops snakebite.
Patients were mostly male (82.3%), from rural areas (87.1%) and aged between 16–60 years (82.8%). The main anatomical region bitten was foot (66.1%). Most bites were not related to work (59.7%). Time to medical assistance was mostly less than 3 hours (57.5%), the means this time was 4.5 hours and most accidents were moderate (49.0%). Mild and moderate cases were similarly distributed in patients with AKI. A tourniquet on the affected limb was made in 24.7%. No sociodemographic association was found with AKI (Table 1). No patient declared the usage of nonsteroidal anti-inflammatory drugs upon admission. Early adverse reactions to antivenom were observed in 28 patients (16.5%). No late adverse reactions were detected.
Table 1. Sociodemographic and clinical characteristics of patients with and without AKI admitted after snakebite.
| Characteristics | Without AKI | With AKI | Total | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Sex | |||||
| Male | 132 | 81.5 | 21 | 87.5 | 82.3 |
| Female | 30 | 18.5 | 3 | 12.5 | 17.7 |
| Area of occurrence | |||||
| Rural | 141 | 87.0 | 21 | 87.5 | 87.1 |
| Urban | 21 | 13.0 | 3 | 12.5 | 12.9 |
| Age group in years | |||||
| 0–15 | 12 | 7.41 | 1 | 4.1 | 7.0 |
| 16–60 | 136 | 83.9 | 18 | 75.0 | 82.8 |
| >60 | 14 | 8.6 | 5 | 20.8 | 10.2 |
| Anatomical region of the injury | |||||
| Upper limbs | 1 | 0.6 | 1 | 4.2 | 1.1 |
| Lower limbs | 32 | 19.8 | 2 | 8.3 | 17.4 |
| Hand | 22 | 13.6 | 5 | 20.8 | 14.5 |
| Foot | 107 | 66.0 | 16 | 66.7 | 66.1 |
| Work-related accident | |||||
| No | 94 | 58.0 | 17 | 70.8 | 59.7 |
| Yes | 68 | 42.0 | 7 | 29.2 | 40.3 |
| Time to medical assistance (hours) | |||||
| 0–3 | 93 | 57.4 | 14 | 58.3 | 57.5 |
| 4–6 | 43 | 26.5 | 6 | 25.0 | 26.3 |
| 7–12 | 12 | 7.4 | 0 | 0.0 | 6.5 |
| 13–24 | 14 | 8.6 | 4 | 16.7 | 9.7 |
| Previous snakebite | |||||
| No | 136 | 83.9 | 24 | 100.0 | 86.0 |
| Yes | 26 | 16.1 | 0 | 0.0 | 14.0 |
| Accident classification | |||||
| Mild | 70 | 43.2 | 10 | 41.7 | 43.0 |
| Moderate | 81 | 50.0 | 10 | 41.7 | 49.0 |
| Severe | 11 | 6.8 | 4 | 16.6 | 8.1 |
| Used oral drugs | |||||
| No | 150 | 97.4 | 22 | 91.7 | 92.5 |
| Yes | 4 | 2.6 | 2 | 8.3 | 3.2 |
| Used tourniquets | |||||
| No | 120 | 74.1 | 20 | 83.3 | 75.3 |
| Yes | 42 | 25.9 | 4 | 16.7 | 24.7 |
Clinical characterization showed that the most frequent local manifestations were severe pain (46.2%) and mild edema (48.4%). Among the systemic signs, headache (26.9%), dizziness (14.5%), gingivorrhagia (8.6%), vomiting (7.0%) and nausea (8.1%) were the most common. Comorbities, such as hypertension and diabetes were associated with AKI (p<0.000). Complications namely in the form of necrosis (4.8%) and secondary infection (40.0%) were also identified. No patient presented hypovolemic shock syndrome. Local bleeding was associated with the development of AKI (p<0.001) (Table 2).
Table 2. Local/systemic signs, symptoms and complications of the patients with and without AKI admitted to the hospital after snakebite in Manaus, 2014 to 2016.
| Characteristics | Without AKI | With AKI | Total | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Local signs | |||||
| Pain | |||||
| No pain | 25 | 15.4 | 1 | 4.2 | 14.0 |
| Mild | 21 | 13.0 | 3 | 12.5 | 12.9 |
| Moderate | 44 | 27.2 | 6 | 25.0 | 26.9 |
| Severe | 72 | 44.4 | 14 | 58.3 | 46.2 |
| Swelling | |||||
| Mild (1–2 segments) | 79 | 48.8 | 11 | 45.8 | 48.4 |
| Moderate (3–4 segments) | 72 | 44.4 | 10 | 41.7 | 44.1 |
| Severe (≥ 5 segments) | 11 | 6.8 | 3 | 12.5 | 7.5 |
| Local Bleeding | |||||
| No | 89 | 54.9 | 10 | 41.67 | 53.2 |
| Yes | 73 | 45.1 | 14 | 58.33 | 46.8 |
| Systemic | |||||
| Headache | 38 | 23.5 | 12 | 50.0 | 26.9 |
| Dizziness | 25 | 15.4 | 2 | 8.3 | 14.5 |
| Gingival bleeding | 13 | 8.02 | 3 | 12.5 | 8.6 |
| Vomiting | 8 | 4.9 | 5 | 20.8 | 7.0 |
| Nausea | 13 | 8.0 | 2 | 8.3 | 8.1 |
| Comorbidities | |||||
| Hypertension | |||||
| Yes | 3 | 1.9 | 10 | 41.6 | 7.0 |
| No | 159 | 98.1 | 14 | 58.3 | 93.0 |
| Diabetes mellitus | |||||
| Yes | 1 | 0.6 | 2 | 8.3 | 1.6 |
| No | 161 | 99.4 | 22 | 91.7 | 98.4 |
| Complications | |||||
| Necrosis | 8 | 4.9 | 1 | 4.2 | 4.8 |
| Secondary infection | 63 | 38.9 | 11 | 45.8 | 40.0 |
Laboratory results revealed that patients with AKI had a slight thrombocytopenia and leukocytosis, increase in creatine kinase, as well as increase in lactate dehydrogenase. Clotting time was uncoagulable in 97.3%. The mean concentration of circulating venom was 44.42 ng/mL (Table 3). Among these parameters, high LDH levels (p<0.001) were associated with the development of AKI. The first blood evaluation occurred in the same time that the patient arrived in the hospital for assistance before administration of the antivenom.
Table 3. Laboratory results from patients with and without AKI admitted to the hospital after snakebite in Manaus from 2014 to 2016.
| Laboratory tests | Without AKI (n = 162) |
With AKI (n = 24) |
Total |
|---|---|---|---|
| Mean (±SD) | |||
| Antigenemia (ng/dL) | 54.01 (±52.39) | 34.83 (±32.55) | 44.42 (±42.47) |
| Fibrinogen level (mg/dL) | 243.73 (±137.34) | 201.54 (±128.73) | 222.64 (±133.3) |
| Platelets (x103/mm3) | 230.44 (±75.16) | 197.45 (±70.12) | 213.95 (±72.64) |
| Leukocytes (mm3) | 11.02 (±6.98) | 12.85 (±5.41) | 11.94 (±6.20) |
| Hemoglobin (g/dL) | 13.92 (±1.81) | 13.68 (±1.55) | 13.80 (±1.68) |
| Hematocrit (%) | 40.92 (±5.20) | 40.05 (±4.37) | 40.49 (±4.79) |
| K+ (mmol/L) | 3.89 (±0.37) | 4.09 (±0.53) | 3.99 (±0.9) |
| Na+ (mmol/L) | 139.28 (±5.23) | 135.75 (±8.03) | 138.52 (±6.63) |
| Lactate dehydrogenase (IU/L) | 330.19 (±95.44) | 465.13 (±260.34) | 397.66 (±177.89) |
| TGO/AST (IU/L) | 25.58 (±14.04) | 57.88 (±138.64) | 41.73 (±76.34) |
| TGP/ALT (IU/L) | 23.15 (±20.57) | 42.04 (±52.44) | 32.59 (±36.51) |
| Creatine Kinase (IU/L) | 189.07 (±223.92) | 286.66 (±342.69) | 237.87 (±283.31) |
Reference values: Leukocytes: 4.000–10.000/mm3; Fibrinogen: 200–400 mg/dL; Platelets: 130,000–400.000/mm3; Hemoglobin: 13.0–16.0 g/dL for males and 12.0–14.0 for females; Hematocrit: 40.0–52.0%; Lactate dehydrogenase: 211–423 IU/L; Creatinine: 0.5–1.2 mg/dL for adults and 0.3–1.0 mg/dL for children; Sodium: 135–145 mmol/L; Potassium: 3.6–5.2 mmol/L; Aspartate transaminase (AST): 2–38 IU/L; Alanine transaminase (ALT): 2–44 IU/L; Creatine phosphokinase: 24–190 IU/L.
Clinical outcomes and risk factors for AKI
Of the 186 patients in the study, 24 (12.9%) developed AKI after 48 hours of admission, and 162 (87.1%) did not develop such clinical condition. Stage I was observed in 17 (70.8%) patients, stage II in 3 (12.5%), and stage III in 4 (16.7%) patients (Fig 1). All patients who had a diagnosis of AKI were referred to the institution’s nephrologist. Those classified as stage III underwent dialysis. No patients had Chronic Kidney Disease (CKD) at admission ratified by the decrease in creatinine levels during follow-up thus confirming acuteness of disease. There were no deaths.
In the block analysis, considering the proximal variables, after 48 hours of follow-up AKI had significant association with high lactate dehydrogenase levels [AOR = 1.01 (95%CI = 1.01–1.01); p<0.001)]. Among the intermediate variables only local bleeding showed association [AOR = 0.13 (95%CI = 0.04–0.45; p<0.001)]. As for the distal variables, comorbidities were also significantly associated [AOR = 37.85 (95%CI = 9.32–153.69; p<0.001)] (Table 4).
Table 4. Variables associated to acute renal failure in patients admitted to the hospital in Manaus from 2014 to 2016.
| Variables | Without AKI n (%) |
With AKI n (%) |
Crude OR (IC 95%) |
p | Adjusted OR (95%CI) |
p |
|---|---|---|---|---|---|---|
| Proximal variables | ||||||
| Antigenemia | ||||||
| Detectable | 135 (83.3) | 18 (75.0) | 0.99 (0.97–1.01) | 0.301 | ... | ... |
| Undetectable | 27 (16.7) | 6 (25.0) | 1 | |||
| Blood coagulability | ||||||
| Normal | 5 (3.1) | 0 (0.0) | 1 | |||
| Uncoagulable | 157 (96.9) | 24 (100.0) | 6.91 (1.98–24.10) | 0.002* | 3.51 (0.53–23.32) | 0.193 |
| Fibrinogen level | ||||||
| Normal | 116 (71.6) | 20 (83.3) | 1 | |||
| Abnormal | 46 (28.4) | 4 (16.7) | 0.99 (0.99–1.00) | 0.182 | ... | ... |
| Thrombocytopenia | ||||||
| Yes | 157 (96.9) | 24 (100.0) | 0.99 (0.99–1.00) | 0.042* | 0.99 (0.98–1.00) | 0.396 |
| No | 5 (3.1) | 0 (0.00) | 1 | |||
| Leukocytes | ||||||
| Normal | 96 (59.3) | 10 (41.7) | 1 | |||
| Abnormal | 66 (40.7) | 14 (58.3) | 1.03 (0.98–1.01) | 0.268 | ... | ... |
| Hemoglobin | ||||||
| Normal | 151 (93.2) | 23 (95.8) | 1 | |||
| Abnormal | 11 (6.8) | 1 (4.2) | 0.92 (0.71–1.20) | 0.547 | ... | ... |
| Hematocrit | ||||||
| Normal | 161 (99.4) | 24 (100.0) | 1 | |||
| Abnormal | 1 (0.6) | 0 (0.00) | 0.96 (0.88–1.06) | 0.432 | ... | ... |
| K+ | ||||||
| Normal | 161 (99.4) | 23 (95.8) | 1 | |||
| Abnormal | 1 (0.6) | 1 (4.2) | 3.48 (1.18–10.23) | 0.023* | 2.01 (0.36–11.29) | 0.424 |
| Na+ | ||||||
| Normal | 157 (96.9) | 24 (100.0) | 1 | |||
| Abnormal | 5 (3.1) | 0 (0.00) | 0.91 (0.85–0.98) | 0.009* | 0.92 (0.84–1.02) | 0.140 |
| LDH (U/L) | ||||||
| Normal | 135 (83.3) | 15 (62.5) | 1 | |||
| Abnormal | 27 (16.7) | 9 (37.5) | 1.01 (1.01–1.01) | 0.001* | 1.01 (1.01–1.01) | 0.012* |
| TGO/AST (IU/L) | ||||||
| Normal | 138 (85.2) | 17 (70.8) | 1 | |||
| Abnormal | 24 (14.8) | 7 (29.2) | 1.02 (0.99–1.04) | 0.197 | 0.99 (0.97–1.01) | 0.377 |
| TGP/ALT (IU/L) | ||||||
| Normal | 127 (78.4) | 16 (66.7) | 1 | |||
| Abnormal | 35 (21.6) | 8 (33.3) | 1.02 (1.00–1.03) | 0.015* | 1.01 (0.99–1.04) | 0.256 |
| CK- Creatine Kinase | ||||||
| Normal | 115 (71.0) | 13 (54.8) | 1 | |||
| Abnormal | 47 (29.0) | 11 (45.8) | 1.00 (1.00–1.00) | 0.081 | 1.00 (1.00–1.00) | 0.252 |
| Urine pH level | ||||||
| Normal | 122 (80.3) | 18 (75.0) | 1 | |||
| Abnormal | 30 (19.7) | 6 (25.0) | 0.58 (0.22–1.49) | 0.260 | ... | ... |
| Urine protein | ||||||
| No | 124 (80.5) | 17 (73.9) | 1 | |||
| Yes | 28 (19.5) | 6 (25.1) | 1.56 (0.56–4.32) | 0.389 | ... | ... |
| Urine hemoglobin | ||||||
| No | 138 (90.8) | 17 (73.9) | 1 | |||
| Yes | 14 (19.2) | 6 (25.1) | 3.48 (1.18–10.25) | 0.024* | 1.69 (0.26–10.89) | 0.580 |
| Urine red blood cells | ||||||
| No | 25 (32.9) | 5 (38.5) | 1 | |||
| Yes | 51 (67.1) | 8 (61.5) | 0.78 (0.23–2.65) | 0.695 | ... | ... |
| Intermediate variables | ||||||
| Local signs | ||||||
| Pain | ||||||
| No pain | 25 (15.4) | 1 (4.2) | 0.71 (0.41–1.25) | 0.244 | ... | ... |
| Mild | 21 (13.0) | 3 (12.5) | 0.66 (0.18–2.42) | 0.531 | ... | ... |
| Moderate | 44 (27.2) | 6 (25.0) | 0.31 (0.07–1.42) | 0.133 | ... | ... |
| Severe | 72 (44.4) | 14 (58.3) | 1.83 (0.18–18.6) | 0.608 | ... | ... |
| Swelling | ||||||
| Mild (1–2 segments) | 79 (48.8) | 11 (45.8) | 1 | |||
| Moderate (3–4 segments) | 72 (44.4) | 10 (41.7) | 0.88 (0.33–2.37) | 0.806 | ... | ... |
| Severe (≥5 segments) | 11 (6.8) | 3 (12.5) | 2.11 (0.68–6.49) | 0.191 | ... | ... |
| Local bleeding | ||||||
| Yes | 73 (45.1) | 14 (58.33) | 0.13 (0.04–0.45) | 0.001* | 0.13 (0.027–0.59) | 0.009* |
| No | 89 (54.9) | 10 (41.67) | 1 | |||
| Systemic | ||||||
| Dizziness | 25 (15.4) | 2 (8.3) | 0.49 (0.11–2.25) | 0.365 | ... | ... |
| Complications | ||||||
| Secondary infection | 63 (38.9) | 11 (45.8) | 1.96 (0.51–7.61) | 0.330 | ... | ... |
| Distal variables | ||||||
| Sex | ||||||
| Male | 132 (81.5) | 21 (87.5) | 1 | |||
| Female | 30 (18.5) | 3 (12.5) | 0.62 (0.17–2.24) | 0.475 | ... | ... |
| Area of occurrence | ||||||
| Rural | 141 (87.0) | 21 (87.5) | 0.97 (0.39–2.41) | 0.953 | ... | ... |
| Urban | 21 (13.0) | 3 (12.5) | 1 | |||
| Age group in years | ||||||
| 0–15 | 12 (7.4) | 1 (4.2) | 1 | |||
| 16–60 | 136 (84.0) | 18 (75.0) | 1.59 (0.19–12.95) | 0.666 | ... | ... |
| >60 | 14 (8.6) | 5 (20.8) | 4.29 (0.44–41.95) | 0.211 | ... | ... |
| Work-related accident | ||||||
| No | 94 (58.0) | 17 (70.8) | 0.57 (0.22–1.45) | 0.237 | ... | ... |
| Yes | 68 (42.0) | 7 (29.2) | 1 | |||
| Time to medical assistance (hours) | ||||||
| 0–3 | 12 (7.4) | 1 (4.2) | 1 | |||
| 4–6 | 30 (18.5) | 4 (16.7) | 0.93 (0.33–2.58) | 0.884 | ... | ... |
| 7–12 | 39 (24.1) | 3 (12.5) | ... | ... | ... | ... |
| 13–24 | 23 (14.2) | 4 (16.7) | 1.90 (0.55–6.59) | 0.313 | ... | ... |
| Accident classification | ||||||
| Mild | 70 (43.2) | 10 (41.7) | 1 | |||
| Moderate | 81 (50.0) | 10 (41.7) | 0.86 (0.33–2.20) | 0.759 | ... | ... |
| Severe | 11 (6.8) | 4 (16.6) | 2.54 (0.67–9.55) | 0.166 | ... | ... |
| Used oral drugs | ||||||
| No | 4 (2.5) | 2 (8.3) | 1 | |||
| Yes | 150 (92.6) | 22 (91.7) | 1.97 (0.81–4.78) | 0.130 | ... | ... |
| Used tourniquets | ||||||
| No | 120 (74.1) | 20 (83.3) | 1 | |||
| Yes | 42 (25.9) | 4 (16.7) | 1.79 (0.75–4.26) | 0.189 | ... | ... |
| Comorbidities | ||||||
| No | 158 (97.5) | 14 (58.3) | 1 | |||
| Yes | 4 (2.47) | 10 (41.7) | 37.85 (9.32–153.69) | <0.001* | 60.96 (9.69–383.30) | <0.001* |
*The significance level was considered for p<0.05.
Reference values: Leukocytes: 4.000–10.000/mm3; Fibrinogen: 200–400 mg/dL; Platelets: 130,000–400.000/mm3; Hemoglobin: 13.0–16.0 g/dL for males and 12.0–14.0 for females; Hematocrit: 40.0–52.0%; Creatine phosphokinase: 24–190 IU/L; Creatine phosphokinase-MB: 2–25 IU/L; Lactate dehydrogenase: 211–423 IU/L; Creatinine: 0.5–1.2 mg/dL for adults and 0.3–1.0 mg/dL for children; Urea: 10–45 mg/dL; Sodium: 135–145 mmol/L; Potassium: 3.6–5.2 mmol/L; Aspartate transaminase: 2–38 IU/L; Alanine transaminase: 2–44 UI/L; Clotting time: 4–10 minutes; pH-urine: 5.5–7.0; Density-urine: 1025–1035.
Discussion
AKI is the main complication observed in snakebite envenomation [38]. It is estimated that between 1.6 to 38.5% of the victims develop AKI [36]. This wide variation in frequency is due to unclear establishment of the diagnostic criteria.
In this study, AKI was observed in 12.9% of cases, which is similar to previous studies in the Brazilian Amazon by Feitosa et al. and Souza et al. [5,50]. The development of AKI is an unestablished event [45] and, given that, there are proposals for some triggering events of hemodynamic, nephrotoxic and immunological nature [38]. As for circulatory abnormalities, hemorrhagic conditions, hypo or hypertension, vascular changes, intravascular hemolysis and disseminated intravascular coagulation (DIC) are described in the literature [29,43,45].
Vascular disorders occur through hemorrhagic and coagulant action induced by venom components of Bothrops snakes that promote endothelial injury by hemorrhagins [13] and consumption of coagulation factors [55]. In South America, this disorder has been caused by snakes of the Viperidae family [31,32]. In this study, changes were caused specifically by Bothrops snakes, also described in other papers on the Amazon region [5,6,49,56].
In human envenomation, it is has been shown that experimental models with venoms from B. jararaca, B. moojeni and B. alternatus, induced changes in tubular, glomerular and interstitial structures [39,41,57] indicating the action of venom toxins on the renal parenchyma occurs within a few hours [32]. However, the involvement of immune response in the AKI development still requires more experimental data. Its harmful effect may be caused by deposition of immunocomplexes, IgM and C3 molecules in mesangial areas characterizing glomerulonephritis.
Changes in the renal parenchyma reflect in the clinical presentation, defined and classified according to the International Society of Nephrology’s guideline of clinical practice (KDIGO) [52]. Diagnosis is based on serum creatinine and/or urinary volume and classified into three stages. In this study, stage I was presented by most patients (70.8%), while stage II occurred in 12.5% and stage III, which is indicative of renal replacement therapy (RRT), was present in 16.7% of patients. Due to hospital routine, it was not possible to obtain urinary volume measurement of all patients, which is a limitation of the study. The recommended treatment in these conditions is complementary, and it is necessary to adopt specific initial measures for envenomation and later for complications, supportive measures for hemodynamic disorders and specific actions for AKI [36].
Early and adequate administration of antivenom is crucial. Coagulation factors return to baseline levels between 6 to 24 hours after antivenom therapy [12,21,58]. This fact minimizes venom-induced actions, of circulatory and immunological order, that are related to renal disorder. The management of AKI ranges from the use of antidiuretics to increase urinary flow [59] to renal replacement therapy (RRT) such as peritoneal dialysis[26] and hemodialysis. In India, about 13.5% of the cases developed AKI, of which 48.3% needed dialysis [29]. It is important to note that the most common snakes that cause accidents in India are Daboia russellii and Echis carinatus that present venoms with hemotoxic and myotoxic actions [30,44].
In this study, there was no significant association between AKI and epidemiological aspects. These were similar to previous studies where it was seen a greater predominance of male individuals, living in rural areas, in productive age group, and having the feet as the main anatomical region reached [7,8,26]. This fact may be explained by the greater exposure to leisure and occupational activities [7].
Regarding clinical aspects, local inflammatory events such as pain and edema were the most commonly identified [5,21,26], venom toxins from Bothrops snakes present proteolytic action through inflammatory mediators that act by activating nociceptors and promoting changes in lymphatic vessels [16].
The majority of patients presented blood uncoagulability (97.3%). The pathophysiological mechanism of hemostatic disorders relies on the consumption of coagulation factors and pro-coagulant activation, as well as thrombin-like enzymes [60]. According to the guidelines of the Brazilian Ministry of Health, clotting time is not a diagnostic criterion for the definition of envenomation [12]. Studies indicate 23 to 43.1% of patients who are victims of accidents in the Amazon region present blood uncoagulability, varying from case to case [5,7,26]. In addition, thrombocytopenia is a result of platelet aggregation inhibition by serine proteases [60]. Another parameter related to hemostatic changes induced by the venom was the presence of hemorrhagic conditions, also showed by Athappan et al. [29].
In this study, most patients who developed AKI had comorbidities namely hypertension and diabetes. It is known that such comorbidities lead to increased risk for the development of this condition[51], as a result of long-term damage to target organs such as heart and kidneys. Also, the difference in serum lactate dehydrogenase levels among patients with AKI was significant. This is a marker of cell injury as shown by Castro et al. in experimental models of B. jararaca-induced tubular injury [39].
In the multivariate analysis, changes in lactate dehydrogenase levels followed by local bleeding and presence of comorbidities, were independently associated with the onset of AKI. This data was shown by Aye et al. where it was observed that the administration of antivenom more than 2 hours from bite, leukocytosis, DIVC, rhabdomyolysis, hyponatremia and microscopic hematuria were factors associated with the clinical picture of AKI [61]. In a retrospective study, independent factors associated with AKI at admission were the presence of hemorrhagic changes and long hospital stay [32].
Thrombocytopenia and liver enzyme release were shown to be statistically significant in those events according to Naqvi et al. [62]. In India, a prospective observational study found that patients who took longer to medical assistance and presented hypotension, albuminuria, anemia, changes in bleeding time, prothrombin time and total bilirubin serum levels, developed AKI [63].
This study had limitations. First, it may underestimate the real prevalence of AKI since only snakebites that took no longer than 24 hours to hospital admission were included in this study. Besides, it was not possible to perform urine volume measurement and calculate creatinine clearance during the study. Imaging tests would also be a good tool to complement diagnosis although not used in this study. For logistical reasons, it was not possible to follow-up participants at the end of the study either personally or by telephone, to identify the chronicity of the case.
Conclusion
Acute renal failure was an important systemic complication observed in patients envenomed by Bothrops snakes. This study may aid in understanding some of the toxin-induced changes in the human body. The associated risk factors for AKI were high lactate dehydrogenase levels followed by local bleeding and the presence of comorbidities, majorly hypertension, suggesting that these laboratory and clinical aspects may aid in the diagnosis, management, clinical evolution and possibly reduce the frequency of these events in those victims. Further prospective studies are needed in order to elucidate the pathogenesis of AKI and to identify the different factors involved.
Supporting information
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was supported by Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) Decision 287/2013.
References
- 1.Warrell D. Guidelines for the management of snake-bites. 2010.
- 2.Kasturiratne A, Wickremasinghe AR, Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med. 2008;5: 1591–1604. 10.1371/journal.pmed.0050218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chippaux J-P. Guidelines for the production, control and regulation of snake antivenom immunoglobulins. [Internet]. Biologie aujourd’hui. 2010. 10.1051/jbio/2009043 [DOI] [PubMed] [Google Scholar]
- 4.Brasil. Ministério da Saúde. Sistema de Informação de Agravos de Notificação—SINAN. In: Ministério da Saúde [Internet]. 2016 [cited 12 Nov 2016]. http://portalsaude.saude.gov.br/images/pdf/2016/janeiro/20/1-Casos-Ofidismo-2000-2015.pdf
- 5.Feitosa EL, Sampaio VS, Salinas JL, Queiroz AM, Silva IM, Gomes AA, et al. Older Age and Time to Medical Assistance Are Associated with Severity and Mortality of Snakebites in the Brazilian Amazon: A Case-Control Study. PLoS One. 2015;10: e0132237 10.1371/journal.pone.0132237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima ACSF, Campos CEC, Ribeiro JR. Epidemiological profile of snake poisoning accidents in the State of Amapá. Rev Soc Bras Med Trop. SBMT; 2009;42: 329–335. 10.1590/S0037-86822009000300017 [DOI] [PubMed] [Google Scholar]
- 7.Moreno E, Queiroz-Andrade M, Lira-da-silva RM, Tavares-Neto J. Clinical and epidemiological characteristics of snakebites in Rio Branco, Acre. Rev Soc Bras Med Trop. 2005;38: 15–21. [DOI] [PubMed] [Google Scholar]
- 8.Nascimento SP. Epidemiological characteristics of snakebites in the State of Roraima, Brazil, 1992–1998. Cad Saude Publica. 2000;16: 271–276. Available: http://www.scielosp.org/pdf/csp/v16n1/1589.pdf [DOI] [PubMed] [Google Scholar]
- 9.Mourão De Moura V, Veras Mourão RH, Dos-Santos MC. Snake bites in northern Brazil and the use of plant species as an alternative and complementary treatment serotherapy. Sci Amaz. 2015;1: 73–84. [Google Scholar]
- 10.Wen FH, Monteiro WM, Silva AMM, Tambourgi DV., da Silva IM, Sampaio VS, et al. Snakebites and Scorpion Stings in the Brazilian Amazon: Identifying Research Priorities for a Largely Neglected Problem. PLoS Negl Trop Dis. 2015;9: e0003701 10.1371/journal.pntd.0003701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernarde PS. Serpentes Peçonhentas e Acidentes Ofídicos no Brasil. São Paulo: Anolis Books; 2014. [Google Scholar]
- 12.Brasil. Fundação Nacional de Saúde. Manual de Diagnóstico e Tratamento de Acidentes por Animais Peçonhentos. Brasília: Fundação Nacional de Saúde; 2001.
- 13.Gutiérrez J, Rucavado A, Escalante T, Díaz C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon. 2005;45: 997–1011. 10.1016/j.toxicon.2005.02.029 [DOI] [PubMed] [Google Scholar]
- 14.Sousa LF, Nicolau CA, Peixoto PS, Bernardoni JL, Oliveira SS, Portes-Junior JA, et al. Comparison of Phylogeny, Venom Composition and Neutralization by Antivenom in Diverse Species of Bothrops Complex. PLoS Negl Trop Dis. 2013;7: e2442 10.1371/journal.pntd.0002442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreira V, Dos-Santos MC, Nascimento NG, da Silva HB, Fernandes CM, D’Império Lima MR, et al. Local inflammatory events induced by Bothrops atrox snake venom and the release of distinct classes of inflammatory mediators. Toxicon. Elsevier Ltd; 2012;60: 12–20. 10.1016/j.toxicon.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 16.Teixeira C, Cury Y, Moreira V, Picolo G, Chaves F. Inflammation induced by Bothrops asper venom. Toxicon. Elsevier Ltd; 2009;54: 988–997. 10.1016/j.toxicon.2009.05.026 [DOI] [PubMed] [Google Scholar]
- 17.De Oliveira RB, Ribeiro LA, Jorge MT. Risk factors associated with coagulation abnormalities in Bothrops envenoming. Rev Soc Bras Med Trop. 2003;36: 657–663. [PubMed] [Google Scholar]
- 18.Harshavardhana H, Pasha I, Prabhu N, Amira, Ravi P. Snake bite induced coagulopathy: A study of clinical profile and predictors of poor outcome. Int J Sci Study. 2014;2: 2–5. Available: http://www.ijss-sn.com/uploads/2/0/1/5/20153321/ijss_apr-01.pdf [Google Scholar]
- 19.Kamiguti AS, Cardoso JLC, Theakston RDG, Sano-Martins IS, Hutton RA, Rugman FP, et al. Coagulopathy and Haemorrhage in Human Victims of Bothrops jararaca envonoming in Brazil. Toxicon. 1991;29: 961–972. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira FN, Brito MT, Morais ICO, Fook SML, Albuquerque HN. Accidents caused by Bothrops and Bothropoides in the State of Paraiba: epidemiological and clinical aspects. Rev da Soc Bras Med Trop. 2010;43: 662–667. [DOI] [PubMed] [Google Scholar]
- 21.Cardoso JLC, Fan HW, França FOS, Jorge MT, Leite RP, Nishioka SA, et al. Randomized comparative trial of three antivenoms in the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in São Paulo, Brazil. Q J Med. 1993;85: 315–325. [PubMed] [Google Scholar]
- 22.Otero R, Gutiérrez J, Beatriz Mesa M, Duque E, Rodríguez O, Luis Arango J, et al. Complications of Bothrops, Porthidium, and Bothriechis snakebites in Colombia. A clinical and epidemiological study of 39 cases attended in a university hospital. Toxicon. 2002;40: 1107–1114. Available: http://www.ncbi.nlm.nih.gov/pubmed/12165312 [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro LA, Jorge MT. Acidente por serpentes do gênero Bothrops: série de 3.139 casos. Rev Soc Bras Med Trop. 1997;30: 475–480. [DOI] [PubMed] [Google Scholar]
- 24.Milani Júnior R, Jorge M, de Campos F, Martins F, Bousso A, Cardoso J, et al. Snake bites by the jararacuçu (Bothrops jararacussu): clinicopathological studies of 29 proven cases in São Paulo State, Brazil. QJM. 1997;90: 523–34. [DOI] [PubMed] [Google Scholar]
- 25.Bucaretchi F, Herrera SRF, Hyslop S, Bacarat ECE, Vieira RJ. Snakebites by Bothrops spp in children in Campinas, São Paulo, Brazil. Rev Inst Med Trop São Paulo. 2001;43: 329–333. [DOI] [PubMed] [Google Scholar]
- 26.Borges CC, Sadahiro M, Dos-Santos MC. Epidemiological and clinical aspects of snake accidentes in the municipalities of the State of Amazonas, Brazil. Rev Soc Bras Med Trop. 1999;32: 637–646. Available: http://www.scielo.br/pdf/rsbmt/v32n6/0860.pdf [PubMed] [Google Scholar]
- 27.Valle LA, Silva D da FR, Magalhães PH, Mattos PA, Leal JA. Bilateral amputation of inferior extremities due to serious Bothrops accident: a case report. Arq Med Hosp Fac Cienc Med St Casa São Paulo. 2008;53: 81–84. [Google Scholar]
- 28.Cunha FC, Heerdt M, Torrez PPQ, de França FOS, Dal Molin GZ, Battisti R, et al. First report of hepatic hematoma after presumed bothrops envenomation. Rev Soc Bras Med Trop. 2015;48: 633–635. 10.1590/0037-8682-0084-2015 [DOI] [PubMed] [Google Scholar]
- 29.Athappan G, Balaji M, Navaneethan U, Thirumalikolundusubramanian P. Acute Renal Failure in Snake Envenomation: A Large Prospective Study. Saudi J Kidney Dis Transplant. 2008;19: 404–410. [PubMed] [Google Scholar]
- 30.Kanjanabuch T, Sitprija V. Snakebite Nephrotoxicity in Asia. Semin Nephrol. 2008;28: 363–372. 10.1016/j.semnephrol.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 31.Pinho FMO, Yu L, Burdmann EA. Snakebite-Induced Acute Kidney Injury in Latin America. Semin Nephrol. 2008;28: 354–362. 10.1016/j.semnephrol.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 32.Albuquerque PLMM, Silva GB, Jacinto CN, Lima JB, Lima CB, Amaral YS, et al. Acute kidney injury after snakebite accident treated in a Brazilian tertiary care centre. Nephrology. 2014;19: 764–70. 10.1111/nep.12327 [DOI] [PubMed] [Google Scholar]
- 33.Pinho FMO, Zanetta DMT, Burdmann EA. Acute renal failure after Crotalus durissus snakebite: a prospective survey on 100 patients. Kidney Int. 2005;67: 659–667. 10.1111/j.1523-1755.2005.67122.x [DOI] [PubMed] [Google Scholar]
- 34.Bucaretchi F, Herrera SRF, Hyslop S, Baracat ECE, Vieira RJ. Snakebites by Crotalus durissus ssp in children in Campinas, São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2002;44: 133–138. 10.1590/S0036-46652002000300004 [DOI] [PubMed] [Google Scholar]
- 35.Cupo P, Azevedo M, Hering S. Acidente crotálico na infância: aspectos clínicos, laboratoriais, epidemiológicos e abordagem terapêutica. Rev Soc Bras Med Trop. 1991;24: 87–96. [DOI] [PubMed] [Google Scholar]
- 36.Albuquerque PLMM, Jacinto CN, Silva GB Jr, Lima JB, do Veras MSB, Daher EF. Acute kidney injury caused by Crotalus and Bothrops snake venom: a review of epidemiology, clinical manifestations and treatment. Rev Inst Med Trop Sao Paulo. 2013;55: 295–301. 10.1590/S0036-46652013000500001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silveira P, Nishioka S. South American rattlesnake bite in a Brazilian teaching hospital. Clinical and epidemiological study of 87 cases, with analysis of factors predictive of renal failure. Trans R Soc Trop Med Hyg. 1992;86: 562–4. [DOI] [PubMed] [Google Scholar]
- 38.Sitprija V. Snakebite nephropathy. Nephrology. 2006;11: 442–448. 10.1111/j.1440-1797.2006.00599.x [DOI] [PubMed] [Google Scholar]
- 39.de Castro I, Burdmann E, Seguro A, Yu L. Bothrops venom induces direct renal tubular injury: role for lipid peroxidation and prevention by antivenom. Toxicon. 2004;43: 833–9. 10.1016/j.toxicon.2004.03.015 [DOI] [PubMed] [Google Scholar]
- 40.Machado Braga MD, Costa Martins AM, Alves CD, de Menezes DB, Martins RD, Ferreira Barbosa PS, et al. Purification and renal effects of phospholipase A2 isolated from Bothrops insularis venom. Toxicon. 2008;51: 181–190. 10.1016/j.toxicon.2007.08.017 [DOI] [PubMed] [Google Scholar]
- 41.Barbosa PSF, Havt A, Facó PEG, Sousa TM, Bezerra ISAM, Fonteles MC, et al. Renal toxicity of Bothrops moojeni snake venom and its main myotoxins. Toxicon. 2002;40: 1427–1435. 10.1016/S0041-0101(02)00156-3 [DOI] [PubMed] [Google Scholar]
- 42.Cruz LS, Vargas R, Lopes AA. Snakebite envenomation and death in the developing world. Ethn Dis. 2009;19(1 Suppl): S1-42-6. [PubMed] [Google Scholar]
- 43.Waikhom R, Sircar D, Patil K, Bennikal M, Gupta S Das, Pandey R. Long-Term Renal Outcome of Snake Bite and Acute Kidney Injury: A Single-Center Experience. Ren Fail. 2012;34: 271–274. 10.3109/0886022X.2011.647297 [DOI] [PubMed] [Google Scholar]
- 44.Danis R, Ozmen S, Celen MK, Akin D, Ayaz C, Yazanel O. Snakebite-induced acute kidney injury: data from Southeast Anatolia. Ren Fail. 2008;30: 51–55. 10.1080/08860220701742021 [DOI] [PubMed] [Google Scholar]
- 45.Chugh K, Pal Y, Chakravarty R, Datta B, Mehta R, Sakhuja V, et al. Acute renal failure following poisonous snakebite. Am J Kidney Dis. 1984;IV: 30–38. 10.1016/S0272-6386(84)80023-2 [DOI] [PubMed] [Google Scholar]
- 46.Rodrigues Sgrignolli L, Florido Mendes GE, Carlos CP, Burdmann E a. Acute kidney injury caused by bothrops snake venom. Nephron Clin Pract. 2011;119: c131–c137. 10.1159/000324228 [DOI] [PubMed] [Google Scholar]
- 47.Pinho FM, Burdmann EA. Fatal cerebral hemorrhage and acute renal failure after young Bothrops jararacussu snake bite. Ren Fail. 2001;23: 269–77. [DOI] [PubMed] [Google Scholar]
- 48.Moura VM, Mourão RHV. Aspectos do Ofidismo no Brasil e Plantas Medicinais utilizadas como complemento à soroterapia. Sci Amaz. 2012;1: 17–26. [Google Scholar]
- 49.Waldez F, Vogt RC. Ecological and epidemiological aspects of snakebites in riverside communities of the lower Purus River, Amazonas, Brazil. Acta Amaz. Instituto Nacional de Pesquisas da Amazônia; 2009;39: 681–692. 10.1590/S0044-59672009000300025 [DOI] [Google Scholar]
- 50.Souza ARB. Snakebite by Bothrops atrox (Lin. 1758) in the State of Amazonas—Brazil: Study of 212 cases with identified snake. Rev Patol Trop. 2002;31: 267–268. Available: http://www.revistas.ufg.br/index.php/iptsp/article/viewFile/14573/9140 [Google Scholar]
- 51.Anathhanam S, Lewington AJP. Acute kidney injury. J R Coll Physicians Edinb. 2013;43: 323–329. [DOI] [PubMed] [Google Scholar]
- 52.Kellum J a, Lameire N, Aspelin P, Barsoum RS, Burdmann E a, Goldstein SL, et al. KDIGO Clinical Practice Guideline for Acute Kidney Injury [Internet]. Kidney international supplements. 2012. [Google Scholar]
- 53.Colombini M. Reatividade antigênica cruzada entre os venenos de Bothrops atrox e Lachesis muta muta e desenvolvimento de um teste imunoenzimático diferencial para acidentes causados por essas serpentes. Universidade de São Paulo. 2003.
- 54.Victora CG, Huttly SR, Fuchs SC, Olinto MT. The role of conceptual frameworks in epidemiological analysis: A Hierarchical Approach. Int J Epidemiol. 1997;26: 224–227. 10.1093/ije/26.1.224 [DOI] [PubMed] [Google Scholar]
- 55.Sano-Martins IS, Fan HW, Castro SCB, Tomy SC, França FOS, Jorge MT, et al. Reliability of the simple 20 minute whole blood clotting test (WBCT20) as an indicator of low plasma fibrinogen concentration in patients envenomed by Bothrops snakes. Toxicon. 1994;32: 1045–1050. 10.1016/0041-0101(94)90388-3 [DOI] [PubMed] [Google Scholar]
- 56.Pierini SV, Warrell DA, de Paulo A, Theakston RD. High incidence of bites and stings by snakes and other animals among rubber tappers and Amazonian Indians of the Juruá Valley, Acre State, Brazil. Toxicon. 1996;34: 225–236. [DOI] [PubMed] [Google Scholar]
- 57.Nogueira FA Jr. Avaliação dos efeitos citotóxicos e renais promovidos pelo veneno da serpente Bothrops alternatus [Internet]. Universidade Federal do Ceará; 2017. http://www.repositorio.ufc.br/bitstream/riufc/21899/1/2017_dis_fanjúnior.pdf [Google Scholar]
- 58.de Pardal PP O, Souza SM, Monteiro MR de C da C, Fan HW, Cardoso JLC, França FOS, et al. Clinical trial of two antivenoms for the treatment of Bothrops and Lachesis bites in the north eastern Amazon region of Brazil. Trans R Soc Trop Med Hyg. 2004;98: 28–42. Available: http://www.ncbi.nlm.nih.gov/pubmed/14702836 [DOI] [PubMed] [Google Scholar]
- 59.Macedo E, Bouchard J, Metha R. Prevention and Nondialytic Management of Acute Kidney Injury Comprehensive clinical Nephrology. 4th ed New York: Elsevier Saunders; 2010. pp. 830–842. [Google Scholar]
- 60.Sano-Martins I, Santoro M. Distúrbios hemostáticos em envenenamentos por animais peçonhentos no Brasil Animais Peçonhentos do Basil—Biologia, Clínica e terapêutica dos Acidentes. 2nd ed São Paulo: Sarvier; 2009. pp. 331–351. [Google Scholar]
- 61.Aye K, Thanachartwet V, Soe C, Desakorn V, Thwin K, Chamnanchanunt S, et al. Clinical and laboratory parameters associated with acute kidney injury in patients with snakebite envenomation: a prospective observational study from Myanmar. BMC Nephrol. BMC Nephrology; 2017;18: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naqvi R. Snake-bite-induced Acute Kidney Injury. J Coll Physicians Surg Pakistan. 2016;26: 517–520. [PubMed] [Google Scholar]
- 63.Dharod M V, Patil TB, Deshpande AS, Gulhane R V, Patil MB, Bansod Y V. Clinical Predictors of Acute Kidney Injury Following Snake Bite Envenomation. N Am J Med Sci. 2013;5: 594–599. 10.4103/1947-2714.120795 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.