Abstract
The prerequisites for responsible cannabis use are at the heart of current inquiries into cannabis decriminalization by policy makers as well as academic and nonacademic stakeholders at a global scale. Δ9-tetrahydrocannabinol (Δ9-THC), the prime psychoactive compound of the cannabis sativa, as well as cannabimimetics that resemble the pharmacological properties and psychological effects of Δ9-THC, lend themselves handsomely to the preclinical scrutiny of reward-related behavior because they carry marked translational value. Although a functional dichotomy of the psychological effects of Δ9-THC (rewarding versus aversive) has been abundantly reported in place conditioning (PC) paradigms, and might be best attributed to a dose-dependence of Δ9-THC, most PC studies with Δ9-THC feature no significant effects at all. Therefore, after decades of rigorous research, it still remains undetermined whether Δ9-THC generally exerts rewarding or aversive effects in rodents. Here, we set out to extrapolate the commonly alleged dose-dependence of the rewarding and aversive effects of Δ9-THC from the existing literature, at the behavioral pharmacological level of analysis. Specifically, our meta-analysis investigated: (i) the alleged bidirectional effects and dose-dependence of Δ9-THC in the PC test; (ii) methodological inconsistencies between PC studies; and (iii) other pharmacological studies on cannabinoids (i.e., dopamine release, anxiety, stress, conditioned taste aversion, catalepsy) to substantiate the validity of PC findings. Our findings suggest that: (i) Δ9-THC dose-dependently generates rewarding (1 mg/kg) and aversive (5 mg/kg) effects in PC; (ii) an inconsistent use of priming injections hampers a clear establishment of the rewarding effects of Δ9-THC in PC tests and might explain the seemingly contradictory plethora of nonsignificant THC studies in the PC test; and (iii) other pharmacological studies on Δ9-THC substantiate the dose-dependent biphasic effects of Δ9-THC in PC. A standardized experimental design would advance evidence-based practice in future PC studies with Δ9-THC and facilitate the pointed establishment of rewarding and aversive effects of the substance.
Pathological cannabis use has been recognized by current nosologies as the continued compulsive consumption of cannabis in spite of clinically relevant psychological (e.g., impaired goal-directed behavior, criminal activity) and social consequences (e.g., cessation of recreational activities with the family or peers, failures in significant life roles). Cannabis Use Disorder is incorporated into the dimensional category of Substance-Related and Addictive Disorders of the DSM-5 (2014), and Cannabis Related Disorders are an integral part of the Mental and Behavioral Disorders due to Psychoactive Substance Use category of the ICD-10 (2016).
The recently proposed causation-based Research Domain Criteria Project (RDoC, Insel et al. 2010) might accommodate transdiagnostic, neurobiological mechanisms underpinning the formation and maintenance of pathological cannabis use in the positive valence system. This domain spans the following constructs: approach motivation, initial responsiveness to reward attainment, sustained/longer-term responsiveness to reward attainment, reward learning, and habit. These constructs represent a temporal progression of reward processing and, in their abnormal form, might also underlie the development of reward dysfunction and ensuing obsessive substance use and addictive behaviors. This temporal course pays close attention to the hedonic response an organism initially elicits to the reward stimulus’ psychoactivity (“liking”), to the incentive value that motivates an increased likelihood of future engagement with the reward (“wanting”), and to related positive reinforcing properties of the reward and learning of reward-outcome contingencies that, in the long term, may contribute to the development of physical and psychological dependence, habitual, impulsive, and compulsive behavior, and withdrawal responses upon reward unavailability (Berridge et al. 2009; Baskin-Sommers and Foti 2015).
This review commences with a brief introduction into the endocannabinoid system (ECS) and its role in reward-related and addictive behaviors. We proceed by outlining the parameters of place conditioning (PC) paradigms. The primary objective of this review is to disentangle the dose-dependent effects of Δ9-THC in PC paradigms in terms of place preference (CPP), conditioned place aversion (CPA), and lack of effects.
The endocannabinoid system
The neuromodulatory ECS is the prime neurobiological system that is involved in the mediation of the rewarding and reinforcing effects of Δ9-THC (Mechoulam et al. 2014; Gould 2015). This system comprises the G-protein coupled cannabinoid receptors type 1 (CB1R), type 2 (CB2R), and GPR55, ionotropic TRPV1 receptor, their endogenous ligands (endocannabinoids), and the related synthesizing and degrading enzymes (Di Marzo et al. 2015; Morales and Reggio 2017; Zou and Kumar 2018). Δ9-THC shows a comparable affinity to both CB1Rs (Ki-values ranging from 5.05 to 80.3 nM) and CB2Rs (Ki-values ranging from 3.13 to 75.3 nM; Pertwee 2008), and may also bind to GPR55 (Brown 2007). Furthermore, Δ9-THC-associated aversive effects were recently linked not only to CB1R (Monory et al. 2007), but also to CB2Rs (Han et al. 2017).
Another category of cannabinoids that is of great translational interest, and which has received considerable attention in the last decade, is synthetic cannabinoids (Pintori et al. 2017). Cannabimimetics, a group of synthetic cannabinoids that echo the pharmacological properties and rewarding effects of Δ9-THC, are part of a recent wave of new psychoactive substances (NPS) that are frequently used for recreational purposes (Miliano et al. 2016). However, the consumption of cannabimimetics is associated with much higher health risks than natural cannabinoids (e.g., extended psychoactivity, more side effects; Fattore and Fratta 2011; Brents and Prather 2014). Some of the most prominent synthetic cannabinoids are R-(+)-WIN55212, (−)-CP55940, and HU-210 (HU), all three of which binding among others to CB1R. Studies on consequences of synthetic cannabinoids on PC are surprisingly scarce compared to Δ9-THC and will thus not be further considered by us.
Cannabinoids and reward
Recent articles that reviewed the putative mechanisms linking cannabinoids to addictive behaviors have emphasized the genetic (López-Moreno et al. 2012; Szutorisz and Hurd 2018), molecular (Solinas et al. 2008; Panlilio et al. 2013; D'Addario et al. 2014), and cellular (Sidhpura and Parsons 2011; Covey et al. 2015; Parsons and Hurd 2015; Busquets-Garcia et al. 2018) levels of analysis, and have specified the involvement of the ECS in drug, food, social, and sexual reward processing (Solinas et al. 2008; Fattore et al. 2010a; Tarragon and Moreno 2018), with drug intake being prioritized over natural rewards in drug addictions (Hyman et al. 2006; Edwards and Koob 2010). Taking a behavioral pharmacological slant on cannabinoids and addictive behavior, further reviews have synthesized cross-species evidence for the existence of rewarding (“liking”) and reinforcing (“wanting”) effects of cannabinoids and their pharmacological efficiency (Tanda and Goldberg 2003; Murray et al. 2007; Haney 2008; Panlilio et al. 2010). As for the reinforcing properties of cannabinoids, reviews have delineated the unidirectional and reciprocal modulatory relationship of cannabinoids with substances of abuse (Maldonado et al. 2006; Serrano and Parsons 2011; Johnson and Lovinger 2016; Sloan et al. 2017) such as nicotine (Gamaleddin et al. 2015; Scherma et al. 2016), opioids (Cooper and Haney 2009), ethanol/alcohol (López-Moreno et al. 2010; Pava and Woodward 2012; Kleczkowska et al. 2015), and psychostimulants like cocaine (Hayase 2017) and amphetamines (Wiskerke et al. 2008; Su and Zhao 2017). While analyses of cannabinoid modulation of the reinforcing effects of substances of abuse have unearthed a crucial role of the ECS (Tzschentke 2007; Wills and Parker 2016), the defining action of cannabinoid agonists is of utmost interest to current inquiries into responsible cannabis consumption by policy makers and academic and nonacademic stakeholders. Moreover, most of the listed articles contain summarized data ascertained in self-administration paradigms that rest on distinct operant learning mechanisms and advance our knowledge of the reinforcing effects (i.e.,“wanting”) of cannabinoids. Yet, other behavioral assays are at hand to exclusively tackle the rewarding effects of CB1R agonists, and Δ9-THC specifically, based on Pavlovian learning. These assays are valuable tools that aid the development of a mechanistic explanation of the initial responsiveness to Δ9-THC reward attainment (i.e., “liking”). The most prominent of such paradigms is the Place Conditioning test (PC; Mackintosh 1974; Bardo and Bevins 2000; Bevins and Cunningham 2006). The PC has ubiquitously yielded a CB1R- and dose-dependence of rewarding biphasic (relaxation versus anxiety/paranoia) conditioned effects (Cooper and Haney 2008, 2009; Sanchis-Segura and Spanagel 2006; Tzschentke 2007; Murray and Bevins 2010), thereby hinting at the particular suitability of this test for cannabinoid pharmacological behavioral research.
Conditioned place preference and avoidance
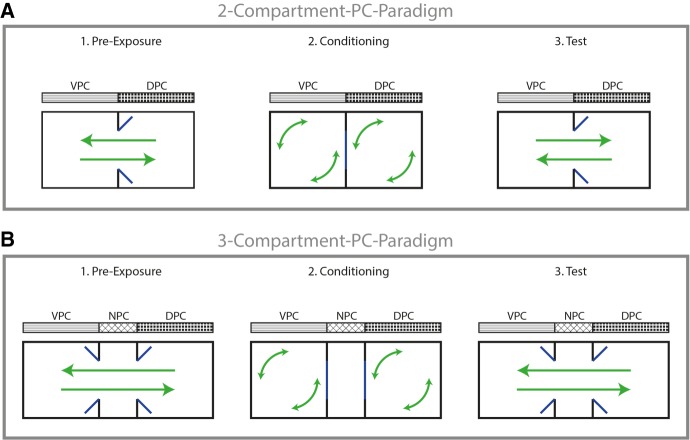
The PC paradigm is the most popular form of conditioned preference procedures and can operationalize rewarding and aversive effects of a substance. In this test, an animal—typically a rodent—is exposed to a behavioral apparatus in which it encounters two or three compartments, separated by removable doors (Tzschentke 2007). Variations in apparatus design complicate conclusive result interpretation, and partially explain differences in outcome studies in PC paradigms (Murray and Bevins 2010). We will succinctly introduce some of the fundamental PC experimental variables to familiarize the “uninformed” reader (cf. Fig. 1).
Figure 1.
Schematic illustration of the PC paradigm. (A) 2-compartment apparatus. (B) 3-compartment apparatus. Conditioned place preference (CPP) is established by either significantly more time spent in the drug-paired compartment (DPC) after conditioning than before (i.e., test versus preexp. DPC) or significantly more time spent in the DPC than in the vehicle-paired compartment (VPC) during test (i.e., DPC versus VPC). Conditioned place aversion (CPA) is operationalized by either significantly less time spent in the DPC after conditioning than before (i.e., test versus preexp. DPC) or significantly less time spent in the DPC than in the VPC during test (i.e., DPC versus VPC). In a three-compartment setting, the time spent in the nonpaired compartment (NPC) is in most studies not included into the measurement of a CPP or CPA.
Compartments
In a two-compartment setting, each of the equally sized chambers accommodates distinctive environmental cues—of visual, tactile, and/or olfactory nature—to provide the animal with a unique perception of each area. In drug-induced PC, a substance/drug (unconditioned stimulus) is associated with one compartment (i.e., the drug-paired compartment, DPC, conditioned stimulus) and a vehicle is paired with the other compartment (vehicle-paired compartment, VPC). In three-compartment settings, the chambers at either end are slightly larger than the concentric box (nonpaired compartment), which remains as a neutral place of retreat, and only the two side compartments are used for drug- and vehicle-pairings.
Procedural designs
In an unbiased design the administered drug is associated with a compartment that is arbitrarily chosen by the experimenter, irrespective of any innate preference of the animal for one of the two compartments. In a biased design, an initial preference for either of the two conditioning compartments represents a crucial variable (Sanchis-Segura and Spanagel 2006), and the drug is either paired with the preferred or nonpreferred compartment. Several studies have concluded that unbiased procedures and balanced apparatus construction is crucial, specifically for cannabinoid-related PC research (Cunningham et al. 2003; Roma and Riley 2005; Murray and Bevins 2010).
Phases
The entire PC procedure is divided into three distinct phases: preexposure, conditioning, and testing. During preexposure, the animal is placed in the nonpaired compartment (NPC) of the three-compartment apparatus or at the transition between the DPC and VPC in the two-compartment apparatus, and can gain free access to all compartments. Consequently, rodents get accustomed to the environmental cues associated with the compartments (no drugs being administered), the time spent in the DPC and VPC is measured, and an initial preference for the compartments is tested. In the conditioning phase, rodents are administered the drug or vehicle before being introduced into the drug-paired or the VPC for 30 min. These pairings of drug and vehicle with the different compartments are repeated in an alternating fashion over several days (trials) to augment the conditioning process. Some studies administer an additional drug injection 1 or 2 d before starting with the conditioning procedure to provide a preexperience of the drug effects (drug priming), which is supposed to ameliorate possible aversive responses to the drug. In the test phase, the development of an approach or avoidance of the DPC is tested in a drug-free state. Analogous to the preexposure, the rodents are placed in the center of the apparatus.
Quantification methods
A CPP of the administered substance is established when the animal spends either significantly more time in the DPC after conditioning than before (i.e., test versus preexp. DPC) or spends significantly more time in the DPC than in the VPC during test (i.e., DPC versus VPC). A conditioned place aversion (CPA) is established when the animal spends either significantly less time in the DPC after conditioning than before (i.e. test versus preexp. DPC) or spends significantly less time spent in the DPC than in the VPC during test (i.e., DPC versus VPC) (Tzschentke 2007). In a three-compartment setting, the time spent in the NPC is in most studies not included into the measurement of CPP or CPA.
Dose-dependence of the rewarding effects of Δ9-THC
Behavioral pharmacological studies in rodents traditionally ascertain the efficiency of cannabinoids through assessment of the tetrad, which comprises (i) decreased locomotion (e.g., open-field test, holeboard), (ii) decreased body temperature, (iii) increased analgesia (e.g., hot plate or tail immersion test), and (iv) increased catalepsy (e.g., bar test). Other than for the tetrad (Monory et al. 2007), tests of fear and anxiety have revealed a biphasic nature of Δ9-THC pharmacological action. Low doses of CB1R agonists (≤1 mg/kg) tend to induce anxiolytic and hence potentially rewarding effects (Moreira and Wotjak 2010), whereas high doses (>1 mg–10 mg/kg) and pharmacological blockage of CB1R (Marsicano et al. 2002) engender anxiogenic (Viveros et al. 2005; Patel and Hillard 2006; Micale et al. 2013) and psychotogenic (Bhattacharyya et al. 2009, 2010) effects.
PC studies on highly addictive drugs, such as psychostimulants and opiates (e.g., heroin), clearly reveal the rewarding properties of these drugs (marked establishment of CPPs). It also seems to be well-established that natural and synthetic cannabinoids produce CB1R-dependent CPA in high doses and CPP in lower doses (Murray et al. 2007; Murray and Bevins 2010; Wills and Parker 2016; Pintori et al. 2017). However, even though the bidirectional effects of Δ9-THC on PC might be ascribed to a dose-dependence, what still remains apparently inconclusive is that most studies with Δ9-THC feature no significant effects on PC at all.
Lead questions
The principal question of this study revolves around the generality of the rewarding or aversive effects of Δ9-THC in rodent PC studies and the reasons for the frequently observed inefficacy of treatment. Specifically, our meta-analysis: (i) weighs the evidence for the hypothesized bidirectional effects (rewarding versus aversive) and dose-dependence of Δ9-THC, in the PC paradigm, (ii) contains an examination of methodological inconsistencies between studies, and (iii) relates the findings from PC experiments to other pharmacological studies on cannabinoid pharmacology (i.e., dopamine release, anxiety, stress, conditioned taste aversion, catalepsy).
Materials and Methods
Inclusion/exclusion criteria for the systematic review
The selection of studies in the present analysis is aimed at shifting the focus of current review activities to the most relevant translational research on cannabinoids and reward-related behavior. Therefore, we have targeted PC studies (two- or three-compartment PC apparatus) that solely used exogenous cannabinoid administration (without any pre-, co-, or post-treatment with any other substances) via intraperitoneal (i.p.) and intravenous (i.v.) injections in rodents (mice and rats), with food and water ad libitum. Food intake and energy balance are known to modulate the metabolism of endocannabinoids (Matias and Di Marzo 2006; Cristino et al. 2014), which is why research with deprivational protocols has been excluded. Studies on other substances have also evinced that food restriction appears to exert crucial, long-lasting influences on PC procedures which even persist in mice re-fed ad libitum (Cabib et al. 2000). Furthermore, a pulmonary route of administration makes use of an intriguing approach, given that humans prevalently consume cannabis through inhalation (i.e., by smoking). However, compared to i.p. or i.v. injections, the pulmonary uptake of vaporized substances produces qualitatively different effects on behavior, most presumably due to pharmacokinetic differences (Manwell et al. 2014). Moreover, due to the lack of evidence about plasma concentration that results from pulmonary administration protocols, a comparison of dose-response-relationships with i.p. and s.c. treatment is practically impossible. Therefore, such studies were also discounted in the present article. Furthermore, as PC studies with Δ9-THC almost exclusively resort to male rodents and only few assessed age-dependencies, we did not factor in sex and age as methodological traits. The systemic literature search was conducted using several databases, including Web of Science, Scopus, and Pubmed. Search terms encompassed: Δ9-THC, THC, cannabinoid receptor type 1, CB1R, cannabinoids, cannabis, reward, addiction, behavior, dose, dosage, CPP, CPA, and PC.
All selected PC studies are listed in the Supplemental Material (Supplemental Table 1), juxtaposing different methodological traits concerning test animals (species, strain) and procedural aspects of PC (drug compound, dose, priming, PC design [biased, unbiased], number of compartments, PC quantification method). The total amount of PC experiments conducted with Δ9-THC and considered in this article encompasses 73 assessments. When referring to individual PC experiments and their results, it must be noted that each group of animals, which was exposed to a different treatment or procedure, was counted separately—including the groups belonging to the same study.
Methodology of the meta-analysis
Here, dose-dependent differences of Δ9-THC were first tested between the selected PC assessments, establishing either a CPP, CPA, or no significant effects (NEs). Second, upon discovering that significant differences in dose dependence could only be established by comparing CPAs with CPPs and CPAs with NEs, it remained questionable why there are no significant differences between CPPs and NEs. To further examine the high cooccurrence of CPPs and NEs at similar dose-ranges, procedural parameters other than the dose of Δ9-THC were tested for differences. To this end, five methodological parameters were more closely scrutinized: (rat-)strain, drug priming, PC design (biased versus unbiased), PC quantification method, and number of compartments. Kruskal–Wallis test followed by Dunn's multiple comparison tests were applied for dose-dependence comparisons (CPPs versus CPAs versus NEs) and the χ2 test for procedural differences (CPPs versus NEs). Third, to further substantiate that Δ9-THC exerts positive internal states at low doses and negative internal states at high doses, the dose-dependence of CPPs and CPAs were compared with animal studies assessing other Δ9-THC behavioral and physiological traits. To this end, studies on Δ9-THC-induced stress (corticosterone secretion), catalepsy, conditioned taste aversion, and anxiety (anxiogenic-like effects) were selected to substantiate causality between CPA findings and high doses of Δ9-THC. Conversely, studies on the anxiolytic-like effects of Δ9-THC, on Δ9-THC-induced dopamine release in the mesocorticolimbic system, and on Δ9-THC self-administration (SA) were consulted to conceivably corroborate a causal link between CPP and low doses of Δ9-THC. Wilcoxon signed rank test (one-sided question) were used for dose-dependent comparisons (PC versus other Δ9-THC pharmacological effects). Statistical significance was accepted if P < 0.05.
Results
Dose-range
The dose range of Δ9-THC in all considered PC experiments extends from a minimal concentration of 0.01 mg/kg to a maximal concentration of 20 mg/kg. Altogether 45 PC assessments with Δ9-THC administration were conducted with rats (62%) and 28 with mice (38%). The highest amount of PC experiments for both mice and rats results in the dose range of 0 mg/kg < dose ≤ 1 mg/kg (>50%), followed by the dose range of 1 mg/kg < dose ≤ 5 mg/kg (>35%), and only very few studies at concentrations higher than 5 mg/kg (Fig. 2A).
Figure 2.
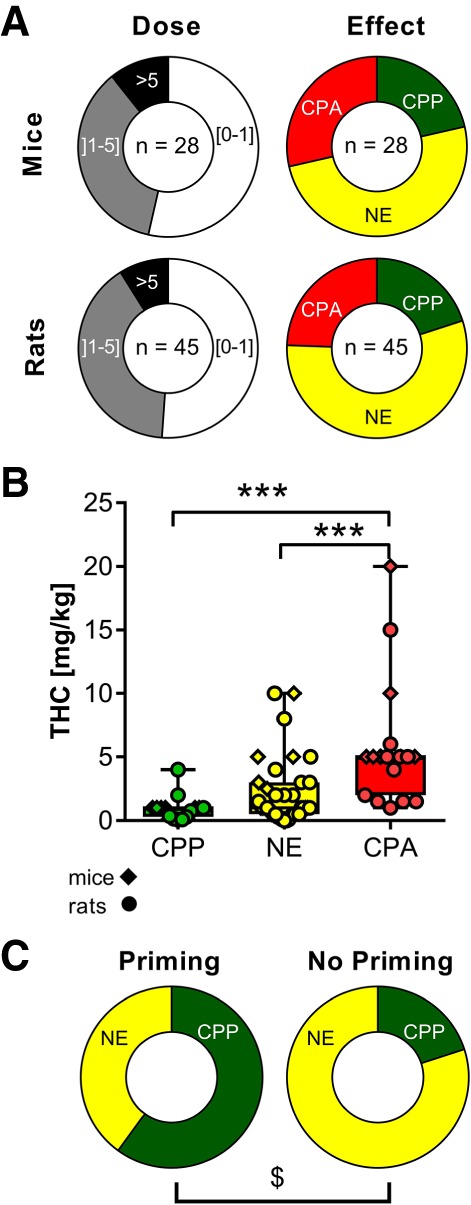
Prevalence and dose-dependency of CPP, CPA, and nonsignificant effects (NE) with Δ9-THC. (A) The majority of studies has been performed with doses ≤1 mg/kg, and failed to reveal any effect at all (NE). (B) Dose-dependent distribution of CPPs, aversions (CPA), and no significant effects (NE) with Δ9-THC [KW(3) = 24.12, (***) P < 0.0001, Kruskal–Wallis, followed by Dunn's multiple post hoc test]. (C) Studies with drug priming show a bias toward the development of CPP, whereas studies without drug priming often fail to reveal any effect [χ2(1) = 6.352, ($) P = 0.0117].
Efficacy of treatment
By far the majority of studies (>50%) failed to reveal any effect of Δ9-THC at all (NE; Fig. 2A), and only a minority reported the occurrence of CPP or CPA (∼25%, each; Fig. 2A).
Parametric analysis of CPP
In mice, all experiments were conducted by using an unbiased CPP design with an apparatus consisting of three compartments, except for one study with an unstated design. In rats, CPP was established with an unbiased design as well (except one study not stating the design). Only the number of compartments differs with five experiments using a two-compartment and three experiments using a three-compartment apparatus. To estimate CPP, the time spent in the DPC during preexposure and the test-phase (test versus preexp. DPC) was compared in all mouse assays (100%) and in six with rats (67%). The prevailingly used strains in mice were CD-1 (67%), and in rats Long-Evans (33%) and Wistar (44%).
Parametric analysis of CPA
Virtually no priming injections were administered in both mice and rats. Δ9-THC conditioning with mice was undertaken by predominantly using an unbiased design. Likewise, an unbiased design was mostly used with rats (82%). A three-compartment apparatus was prevailingly used with mice (88%). In contrast, four assessments with three compartments (36%) and seven with two compartments (64%) were performed in rats. In mice, CPA was predominantly determined by comparing the time spent in the compartment during preexposure and test phase (test versus preexp. DPC; 88%). On the contrary, both test versus preexp. DPC and DPC versus VPC test comparisons were performed in rats. The used rat strains were Wistar, Long–Evans, Sprague–Dawley, and Lister Hooded. In mice, C57BL/6J, F4, and F5 generation back-crosses to C57BL/6 and 1:1 hybrids of 129/SV and C57BL/6J were used in addition to CD-1 mice.
Parametric analysis of experiments with no significant effects (NEs)
Except for a few biased assessments with rats (28%), most studies in mice and rats used the unbiased design. Again the three-compartment setup predominated studies in mice, but was applied only in half of the rat studies. Data were analyzed by comparing the time spent in the DPC during preexposure and test phase in the majority of studies. The strains used in these nonsuccessful experiments in rats were mainly Sprague–Dawley (40%) and Wistar (44%), and CD-1 in mice (64%).
Δ9-THC dose-dependence of CPPs, CPAs, and NEs
A dose-dependent distribution of PC experiments is illustrated separately for CPPs, NEs, and CPAs in Figure 2B. CPPs were established with doses ranging from 0.075 to 4 mg/kg (mice: 1 mg/kg; rats: 0.075–4 mg/kg). CPAs, on the other hand, were implemented at doses reaching from 1 to 20 mg/kg (mice: 5–20 mg/kg; rats: 1–15 mg/kg). Nonsuccessful experiments range from 0.01 to 10 mg/kg (mice: 0.3–10 mg/kg; rats: 0.01–10 mg/kg). The median values for CPAs are 5 mg/kg compared to 1 mg/kg for CPPs and NEs. We tested for systematic dose effects in CPPs, NEs, and CPAs, and obtained significant group differences (KW(3) = 24.12, P < 0.0001), indicating that animals treated with higher doses of Δ9-THC more often developed CPA than CPP or NE (Fig. 2B).
Comparison of methodological implementations between CPPs and NEs
As a dose-related comparison did not reveal significant group differences between CPPs and NEs, we compared other procedural measures such as rat strains, drug priming, PC designs, PC quantifications, and the number of compartments between experiments resulting either in CPP or NE. No other procedural traits were significantly different between CPPs and NEs, except for priming injections (priming: χ2(1) = 6.352, P = 0.0117; cf. Fig. 2C).
Dose-dependence of appetitive versus aversive effects of Δ9-THC in other animal studies
To investigate the putative causal link between the dose ranges of Δ9-THC and the establishment of a CPP or CPA, PC findings with Δ9-THC were dose-dependently compared with other pharmacological effects of Δ9-THC (i.e., increase in dopamine release, anxiolytic-/anxiogenic-like effects, corticosterone secretion, conditioned taste aversion, and catalepsy). The studies on these pharmacological effects are listed, along with their findings, in Supplemental Table 2. The dose-dependent comparison of the aforementioned results with PC findings on Δ9-THC is illustrated in Figure 3. Dopamine release and anxiolytic-like effects induced by Δ9-THC seemed appropriate to be taken as indicators for “pleasant” effects, given that the release of dopamine in the mesolimbic system is generally acknowledged to play a crucial role in reward and reinforcement, while the anxiolytic-like effects provide an alleviation of fear and anxiety. On the other hand, we referred to traits such as Δ9-THC-induced anxiogenic-like effects, corticosterone secretion, conditioned taste aversion, and catalepsy as indicators for “unpleasant” effects.
Figure 3.
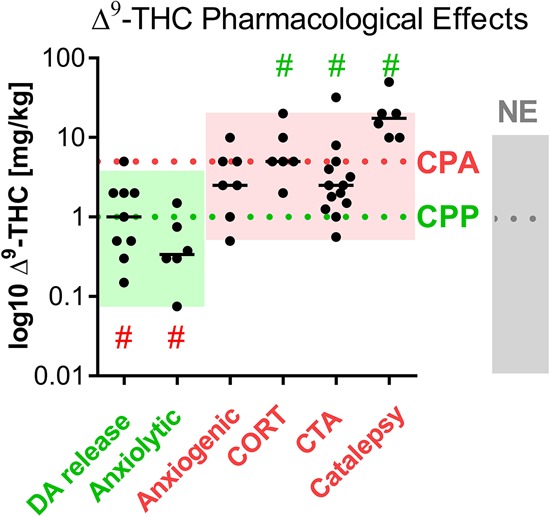
Other dose-dependent appetitive (green) and aversive (red) effects of Δ9-THC. Results of individual experiments/ groups are plotted as black dots (median values as black lines); statistics: Wilcoxon signed rank test (one-sided question), P < 0.05 versus median of CPA and CPP, respectively. Median values of CPPs (1 mg/kg), CPAs (5 mg/kg), and NEs (1 mg/kg; cf. Fig. 2B) are incorporated as green, red, and gray dashed lines, respectively; dose ranges of CPPs, CPAs, and NEs are illustrated in the respective (yet fainter) colors. # (red) p < 0.05 versus median of CPA, # (green) p < 0.05 versus median of CPP (Wilcoxon signed test).
The dose-ranges of dopamine release (0.15 mg/kg ≤ dose ≤ 5 mg/kg) and anxiolytic-like effects (0.075 mg/kg ≤ dose ≤ 1.5 mg/kg) tendentially corresponded to the dose range of CPPs (0.075 mg/kg ≤ dose ≤ 4 mg/kg). In contrast, the dose ranges of anxiogenic-like effects (0.5 mg/kg ≤ dose ≤ 10 mg/kg), corticosterone secretion (2 mg/kg ≤ dose ≤ 20 mg/kg), conditioned taste aversion (0.56 mg/kg ≤ dose ≤ 32 mg/kg) and catalepsy (10 mg/kg ≤ dose ≤ 50 mg/kg) overlapped rather with the dose-range of CPAs (1 mg/kg ≤ dose ≤ 20 mg/kg) than with CPPs (0.075 mg/kg ≤ dose ≤ 4 mg/kg; Fig. 3). However, both the dose ranges of dopamine release and anxiolytic-like effects on one hand and the dose ranges of anxiogenic-like effects, corticosterone secretion, and conditioned taste aversion on the other overlapped with the dose-range of NEs (Fig. 3).
In relation to the median of CPPs (1 mg/kg) and NEs (1 mg/kg), the medians of anxiogenic-like effects (2.5 mg/kg), corticosterone secretion (5 mg/kg), conditioned taste aversion (2.5 mg/kg), and catalepsy (17.5 mg/kg) lay above it. As opposed to this, the median of anxiolytic-like effects (0.34 mg/kg) lay below it and that of dopamine release (1 mg/kg) equaled it. Taking instead the median of CPAs as a reference point (5 mg/kg), it is notable that the median of corticosterone secretion equaled it. Furthermore, the only median that lay above the median of CPAs is that of catalepsy (Fig. 3). To test for significant difference between the dose-dependency of CPPs and CPAs and the dose-dependency of the other pharmacological effects of Δ9-THC, a Wilcoxon signed rank test (one-sided question) was applied. While on one hand the test revealed that the doses of Δ9-THC for dopamine release and anxiolytic-like effects were significantly different from the median dose of Δ9-THC to establish CPAs (5 mg/kg), it evinced on the other hand that the doses of Δ9-THC for corticosterone secretion, conditioned taste aversion, and catalepsy (but not for anxiogenic-like effects) were significantly different from the median dose of Δ9-THC to establish CPPs (1 mg/kg) (Fig. 3).
Discussion
Despite the considerable progress made in the neurosciences to study the mechanistic underpinnings of the pharmacology of cannabinoids, studies on Δ9-THC continue to provide seemingly inconclusive findings, which hamper an explanation of cannabis reward, not to mention cannabis addiction. Here, we sought to investigate the effects of Δ9-THC on the PC procedure to sharpen our knowledge of the contended involvement of exogenous cannabinoids in reward-related processes (specifically the “liking” domain) that are allegedly affiliated to drug addiction. Data substantiated (i) the dose-dependence of the bidirectional effects of Δ9-THC on PC (CPPs at low doses [1 mg/kg] versus CPAs at high doses [5 mg/kg]), (ii) identified significant methodological inconsistencies between CPPs and NEs with Δ9-THC, specifically regarding the application of priming injections, and (iii) elucidated the conformity of PC findings with other pharmacological effects of Δ9-THC (dopamine release, anxiety, stress as indexed by increased corticosterone secretion, conditioned taste aversion, and catalepsy), thus exhaustively corroborating the validity of CPPs and CPAs as a dose-dependent measure for the rewarding and aversive effects of Δ9-THC.
Dose-dependence
Considering that cannabis is expected to have highly rewarding properties, it is surprising that slightly more studies reported the occurrence of CPA rather than CPP. The dose-dependent juxtaposition of CPPs, CPAs, and NEs clearly demonstrated that the dose ranges of NEs markedly overlap with the dose ranges of CPPs and, in part, also CPAs. When testing for significant group differences, it was possible to reveal that animals treated with higher doses of Δ9-THC more often developed a CPA than a CPP or NE. This finding is in accordance with common conjectures, stating that high doses of Δ9-THC elicit aversive effects (Murray et al. 2007; Murray and Bevins 2010). In line with the evidence that Δ9-THC exerts bidirectional effects in a dose-dependent manner (Valjent et al. 2002; Murray et al. 2007; Murray and Bevins 2010; Rey et al. 2012), post-hoc group comparisons yielded significant differences in dose range between CPPs (occurring rather at low doses Δ9-THC) and CPAs (occurring rather at high doses Δ9-THC). However, when comparing these two groups with the dose range of NEs, only CPAs could be set apart, while no significant difference in dose-dependence between CPPs and NEs was found. This is unexpected considering that the PC paradigm has entrenched itself as an adequate procedure to assess both rewarding and aversive effects of drugs (Sanchis-Segura and Spanagel 2006; Tzschentke 2007; Murray and Bevins 2010). Furthermore, given that cannabis is such a highly desired recreational drug and that even self-administration studies have unambiguously shown that squirrel monkeys readily self-administer Δ9-THC (Tanda et al. 2000; Justinova et al. 2003, 2004), it seems hardly comprehensible why the rewarding effects of Δ9-THC are rather tricky to unveil in PC studies with rodents. However, the high incidence of NEs and the overlapping dose range with CPP is conform with the assumption that Δ9-THC has only weak reinforcing properties, at least in rodents, compared to other psychotropic drugs (Justinova et al. 2005). The situation might be different, if an experiment would be performed with stressed animals in an anxiety-like state, where low doses of Δ9-THC may ameliorate the negative affect (Riebe et al. 2012; Micale et al. 2013; Bedse et al. 2017; Patel et al. 2017) and, if combined with a distinct test compartment, might favor the development of CPP. The possibility that Δ9-THC interferes with cognitive processes, and thus hinders the establishment of CPP is rather unlikely, given the occurrence of memory-dependent CPA at higher doses.
Procedural differences between CPPs and NEs with Δ9-THC
We also assessed whether the failure to induce CPP at lower doses (0 mg/kg < dose ≤ 1 mg/kg) relates to procedural differences in studies reporting CPP versus NE. Among the many parameters considered, only drug priming injections seem to favor CPPs versus NEs. Pretreatment with a drug prior to PC (priming) is an acknowledged way to habituate animals to the injection procedure and effects of the drug. This is expected to diminish possible aversive effects, which might occur in drug-naïve animals (Valjent and Maldonado 2000; Quinn et al. 2008). Some PC studies also clearly showed that priming injections can play a critical role in avoiding NEs. For instance, in a 1 mg/kg Δ9-THC PC study (Valjent and Maldonado 2000), two separate PC assessments were implemented: Animals not pretreated with the drug before the assessment (no priming) showed no significant effects on PC, whereas priming resulted in a CPP, suggesting that there appeared to be a higher susceptibility in animals with a drug preexperience to develop a significant behavioral response (CPP) to low doses of Δ9-THC, than in naïve animals. The exact molecular underpinnings of drug priming are not clear. They may include intracellular signaling processes (Cannich et al. 2004) and receptor desensitization/internalization (e.g., Dudok et al. 2015).
PC versus other pharmacological effects of Δ9-THC
Leaving all nonsignificant PC findings aside, CPPs was clearly ascribed to low doses of Δ9-THC (median: 1 mg/kg) and CPAs to high doses of Δ9-THC (median: 5 mg/kg). When other behavioral and neurochemical readouts are considered, there is a remarkable overlap in the dose-response relationship with appetitive (e.g., dopamine release in the mesolimbic system, anxiolytic effects), respectively aversive consequences of Δ9-THC treatment (e.g., anxiogenic effects, increased corticosterone secretion, conditioned taste aversion). The reduction in locomotor activity and the incidence of catalepsy (Monory et al. 2007; Viñals et al. 2015) at high Δ9-THC concentrations may not only cause discomfort to the animals, but also interfere with information gathering and, thus, learning processes.
If the biphasic consequences of CB1R agonists on anxiety-like behavior (Rey et al. 2012) can be generalized to other behavioral tasks, low doses of Δ9-THC may promote CPP via CB1R on cortical glutamatergic neurons, whereas high doses induce CPA via CB1R on GABAergic neurons. Hence, CB1R on different neuronal populations hold the balance between appetitive and aversive behavior. It is conceivable that drug priming may shift this balance toward appetitive behavior, likely by differentially affecting CB1R signaling on GABAergic versus glutamatergic neurons. Future studies should use cell-type specific CB1knockout mice to validate those assumptions.
Procedural design to pointedly establish a CPP or a CPA
Overall, we can say that the PC paradigm is a valid method to assess the dose-dependent biphasic nature of Δ9-THC, even though it remains rather tricky to unveil the rewarding effects of Δ9-THC. This intricacy may, in part, be ascribed to the rather weak rewarding and reinforcing effects of Δ9-THC. However, the comparability of PC findings and thus their evaluation are also significantly hampered by the differences in PC methodology. Thus, it is crucial that future PC studies prevailingly resort to standardized and entrenched procedures.
In light of our findings, we suggest a procedural design to pointedly establish both a CPP as well as CPA. We limit our suggestions to the methodological traits evaluated in the meta-analysis alongside with a few other traits, which have proven to be suitable parameters in past studies (Box 1).
Box 1. Prototypic experimental PC design to pointedly study CPP vs. CPA with Δ9-THC in mice.
Experimental subjects
Adult male C57BL/6 mice (n ≥ 8).
Place conditioning
On basis of the unbiased design with a three-compartment apparatus over the course of eight consecutive days (treatment with Δ9-THC on uneven and with vehicle on even days; length of conditioning session: 30 min, with the animals confined either to the drug-paired or the vehicle-paired compartment), preceded by a preexposure session (15 min; 24 h before starting with PC) and followed by the test session (15 min; 24 h after finishing with PC) with free exploration of three compartments. CPP or CPA is assessed by comparing the time spent in the drug-paired compartment during the test session vs. preexposure.
Treatment
Mice will be treated either with 1 mg/kg Δ9-THC (CPP) or 5 mg/kg Δ9-THC (CPA) i.p. before placement into the drug-paired compartment. In CPP, mice are primed with 1 mg/kg Δ9-THC before starting with the PC in order to accustom them to potential side effects of the treatment.
Sex and age
Specific knowledge about the sex-dependence of the rewarding effects of Δ9-THC is sketchy, given that PC studies with Δ9-THC have been conducted almost exclusively with male rodents (Hempel et al. 2017). This rather ungrounded negligence of sex differentiation is an issue that needs to be addressed in future PC studies. In fact, sex-differences in cannabis use have become a rather current topic: A growing number of women has been reported to use cannabis more frequently and with a higher propensity to develop cannabis use disorder than men (Cooper and Craft 2018). This might be interrelated with findings showing that women use cannabis at a faster rate, that they exhibit a higher sensitivity to its reinforcing effects, and that they develop more severe withdrawal symptoms than men (Marusich et al. 2014; Cooper and Craft 2018).
Preclinical studies have yielded sex-dependent differences in CB1R density and G-protein activation (Rubino and Parolaro 2011; Cooper and Craft 2018). Furthermore, it has been specified that female animals self-administer synthetic cannabinoids faster and at higher rates than males and are more susceptible to drug and cue-induced reinstatement (Fattore et al. 2007, 2010b; Hempel et al. 2017). The implications of these findings are not yet entirely clear. Albeit, sex might be an important factor involved in the increased sensitivity to the rewarding as well as reinforcing properties of cannabinoids (Fattore et al. 2007, 2010b; Hempel et al. 2017) and the development of stronger withdrawal symptoms in females (Harte-Hargrove and Dow-Edwards 2012). However, more studies investigating the putative sexual dimorphism of cannabinoid reward and reinforcement are needed on both physiological and behavioral levels, in order to uncover the underlying mechanisms.
The need of further investigations applies likewise to the age-dependence of the rewarding effects of Δ9-THC. Even though few in number, the existing studies clearly show a trend of adult rodents perceiving the effects Δ9-THC as more aversive than adolescents (Schramm-Sapyta et al. 2007; Quinn et al. 2008; Pandolfo et al. 2009). Cannabis use and the development of cannabis use disorder are more frequent in adolescent users relative to middle-aged and older adults (Haug et al. 2017). Thus, it would be reasonable to assess the relation between the afore-described trend, observed in preclinical studies, with human behavior more in depth. For this, more physiological and behavioral studies on the relation between age and cannabis reward are required.
Conclusions
Δ9-THC holds the potential to induce both CPP and CPA, depending on the dose. The high incidence of negative findings, however, speaks against strong reinforcing properties of Δ9-THC and might result from competing appetitive versus aversive consequences of the treatment, which outbalance each other. Drug priming seems to shift the balance toward appetitive consequences, since it favors the occurrence of CPP. Future studies should consider animals of both sexes at different developmental stages (e.g., adolescence versus adulthood) and internal states (e.g., stressed versus nonstressed, food-restricted versus ad libitum fed) to more closely resemble the situations in humans. At the same time, more effort has to be done to disentangle the rewarding properties of Δ9-THC in terms of “liking” versus “wanting” (e.g., by using self-administration paradigms).
Supplementary Material
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.046870.117.
References
- Bardo MT, Bevins RA. 2000. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology 153: 31–43. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Foti D. 2015. Abnormal reward functioning across substance use disorders and major depressive disorder: considering reward as a transdiagnostic mechanism. Int J Psychophysiol 98: 227–239. [DOI] [PubMed] [Google Scholar]
- Bedse G, Hartley ND, Neale E, Gaulden AD, Patrick TA, Kingsley PJ, Uddin MJ, Plath N, Marnett LJ, Patel S. 2017. Functional redundancy between canonical endocannabinoid signaling systems in the modulation of anxiety. Biol Psych 82: 488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. 2009. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol 9: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Cunningham CL. 2006. Place conditioning: a methodological analysis. In Tasks and techniques: a sampling of the methodologies for the investigation of animal learning, behavior and cognition (ed. Anderson MJ), pp. 99–110. Nova Science Publishers, Inc., New York. [Google Scholar]
- Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O'Carroll C, Allen P, Seal ML, Fletcher PC, Crippa JA. 2009. Modulation of mediotemporal and ventrostriatal function in humans by Δ9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psych 66: 442–451. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, O'Carroll CM, Seal M, Allen P, et al. 2010. Opposite effects of Δ9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 35: 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Prather PL. 2014. The K2/Spice phenomenon: emergence, identification, legislation and metabolic characterization of synthetic cannabinoids in herbal incense products. Drug Metab Rev 46: 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ. 2007. Novel cannabinoid receptors. Br J Pharmacol 152: 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-Garcia A, Bains J, Marsicano G. 2018. CB1 receptor signaling in the brain: extracting specificity from ubiquity. Neuropsychopharmacology 43: 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Orsini C, Le Moal M, Piazza PV. 2000. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science 289: 463–465. [DOI] [PubMed] [Google Scholar]
- Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G. 2004. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem 11: 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Craft RM. 2018. Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacol 43: 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. 2008. Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor. Addict Biol 13: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. 2009. Actions of Δ9-tetrahydrocannabinol in cannabis: relation to use, abuse, dependence. Int Rev Psych 21: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Wenzel JM, Cheer JF. 2015. Cannabinoid modulation of drug reward and the implications of marijuana legalization. Brain Res 1628: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L, Becker T, Di Marzo V. 2014. Endocannabinoids and energy homeostasis: an update. Biofactors 40: 389–397. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. 2003. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology 170: 409–422. [DOI] [PubMed] [Google Scholar]
- D'Addario C, Micioni Di Bonaventura M, Pucci M, Romano A, Gaetani S, Ciccocioppo R, Maccarrone M. 2014. Endocannabinoid signaling and food addiction. Neurosci Biobehav Rev 47: 203–224. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Stella N, Zimmer A. 2015. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci 16: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudok B, Barna L, Ledri M, Szabó SI, Szabadits E, Pintér B, Woodhams SG, Henstridge CM, Balla GY, Nyilas R, et al. 2015. Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat Neurosci 18: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF. 2010. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol 5: 393–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fratta W. 2011. Beyond THC: the new generation of cannabinoid designer drugs. Front Behav Neurosci 5: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. 2007. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol 152: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Melis M, Fadda P, Pistis M, Fratta W. 2010. The endocannabinoid system and nondrug rewarding behaviours. Exp Neurol 224: 23–36. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Fadda P, Fratta W. 2010. Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol 160: 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaleddin IH, Trigo JM, Gueye AB, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B. 2015. Role of the endogenous cannabinoid system in nicotine addiction: novel insights. Front Psychiatry 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J. 2015. The cannabis crop. Nature 525: S2–S3. [DOI] [PubMed] [Google Scholar]
- Han X, He Y, Bi GH, Zhang HY, Song R, Liu QR, Egan JM, Gardner EL, Li J, Xi ZX. 2017. CB1 receptor activation on VgluT2-expressing glutamatergic neurons underlies Δ9-tetrahydrocannabinol (Δ9-THC)-induced aversive effects in mice. Sci Rep 7: 12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M. 2008. Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addict Biol 14: 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Dow-Edwards DL. 2012. Withdrawal from THC during adolescence: sex differences in locomotor activity and anxiety. Behav Brain Res 231: 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug NA, Padula CB, Sottile JE, Vandrey R, Heinz AJ, Bonn-Miller MO. 2017. Cannabis use patterns and motives: a comparison of younger, middle-aged, and older medical cannabis dispensary patients. Addict Behav 72: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase T. 2017. Epigenetic mechanisms associated with addiction-related behavioural effects of nicotine and/or cocaine. Behav Pharmacol 28: 493–511. [DOI] [PubMed] [Google Scholar]
- Hempel BJ, Wakeford AG, Nelson KH, Clasen MM, Woloshchuk CJ, Riley AL. 2017. An assessment of sex differences in Δ9-tetrahydrocannabinol (THC) taste and place conditioning. Pharmacol Biochem Behav 153: 69–75. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. 2006. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29: 565–598. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 167: 748–751. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Lovinger DM. 2016. Presynaptic G protein-coupled receptors: gatekeepers of addiction? Front Cell Neurosci 10: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. 2003. Self-administration of Δ9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology 169: 135–140. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Munzar P, Goldberg SR. 2004. The opioid antagonist naltrexone reduces the reinforcing effects of Δ9-tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology 173: 186–194. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Goldberg SR, Heishman SJ, Tanda G. 2005. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav 81: 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowska P, Smaga I, Filip M, Bujalska-Zadrozny M. 2015. Cannabinoid ligands and alcohol addiction: a promising therapeutic tool or a humbug? Neurotox Res 29: 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Moreno J, Lopez-Jimenez A, Gorriti M, De Fonseca F. 2010. Functional interactions between endogenous cannabinoid and opioid systems: focus on alcohol, genetics and drug-addicted behaviors. Curr Drug Targets 11: 406–428. [DOI] [PubMed] [Google Scholar]
- López-Moreno JA, Echeverry-Alzate V, Bühler K. 2012. The genetic basis of the endocannabinoid system and drug addiction in humans. J Psychopharmacology 26: 133–143. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. 1974. The psychology of animal learning. Academic Press, London. [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. 2006. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29: 225–232. [DOI] [PubMed] [Google Scholar]
- Manwell LA, Charchoglyan A, Brewer D, Matthews BA, Heipel H, Mallet PE. 2014. A vapourized Δ9-tetrahydrocannabinol (Δ9-THC) delivery system part I: development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents. J Pharmacol Toxicol Met 70: 120–127. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, et al. 2002. The endogenous cannabinoid system controls extinction of aversive memories. Nature 418: 530. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Antonazzo KR, Craft RM, Wiley JL. 2014. Evaluation of sex differences in cannabinoid dependence. Drug Alcohol Depend 137: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Di Marzo V. 2006. Endocannabinoid synthesis and degradation, and their regulation in the framework of energy balance. J Endocrinol Invest 29: 15–16. [PubMed] [Google Scholar]
- Mechoulam R, Hanus LO, Pertwee R, Howlett AC. 2014. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci 15: 757–764. [DOI] [PubMed] [Google Scholar]
- Micale V, Di Marzo V, Sulcova A, Wotjak CT, Drago F. 2013. Endocannabinoid system and mood disorders: priming a target for new therapies. Pharmacol Ther 138: 18–37. [DOI] [PubMed] [Google Scholar]
- Miliano C, Serpelloni G, Rimondo C, Mereu M, Marti M, De Luca MA. 2016. Neuropharmacology of new psychoactive substances (NPS): focus on the rewarding and reinforcing properties of cannabimimetics and amphetamine-like stimulants. Front Neurosci 10: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schütz G, Wotjak CT, Lutz B, Marsicano G. 2007. Genetic dissection of behavioural and autonomic effects of Δ9-tetrahydrocannabinol in mice. PLoS Biol 5: e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales P, Reggio PH. 2017. An update on non-CB1, non-CB2 cannabinoid related G-protein-coupled receptors. Cannabis Cannabinoid Res 2: 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Wotjak CT. 2010. Cannabinoids and anxiety. Curr Top Behav Neurosci 2: 429–450. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. 2010. Cannabinoid conditioned reward and aversion: behavioral and neural processes. ACS Chem Neurosci 1: 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Morrison PD, Henquet C, Forti MD. 2007. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci 8: 885–895. [DOI] [PubMed] [Google Scholar]
- Pandolfo P, Vendruscolo LF, Sordi R, Takahashi RN. 2009. Cannabinoid-induced conditioned place preference in the spontaneously hypertensive rat—an animal model of attention deficit hyperactivity disorder. Psychopharmacology 205: 319–326. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Goldberg SR. 2010. Animal models of cannabinoid reward. Br J Pharmacol 160: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Goldberg SR. 2013. Inhibition of FAAH and activation of PPAR: new approaches to the treatment of cognitive dysfunction and drug addiction. Pharmacol Ther 138: 84–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Hurd YL. 2015. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci 16: 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. 2006. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther 318: 304–311. [DOI] [PubMed] [Google Scholar]
- Patel S, Hill MN, Cheer JF, Wotjak CT, Holmes A. 2017. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev 76: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ, Woodward JJ. 2012. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol 46: 185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. 2008. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol 153: 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintori N, Loi B, Mereu M. 2017. Synthetic cannabinoids: the hidden side of Spice drugs. Behav Pharmacol 28: 409–419. [DOI] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, Thompson MR, Dawson B, Mallet PE, Kashem MA, et al. 2008. Adolescent rats find repeated Δ9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology 33: 1113–1126. [DOI] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros M, Lutz B. 2012. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABAB receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology 37: 2624–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe CJ, Pamplona FA, Kamprath K, Wotjak CT. 2012. Fear relief—toward a new conceptual frame work and what endocannabinoids gotta do with it. Neuroscience 204: 159–185. [DOI] [PubMed] [Google Scholar]
- Roma PG, Riley AL. 2005. Apparatus bias and the use of light and texture in place conditioning. Pharmacol Biochem Behav 82: 163–169. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. 2011. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front Behav Neurosci 5: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. 2006. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol 11: 2–38. [DOI] [PubMed] [Google Scholar]
- Scherma M, Muntoni AL, Melis M, Fattore L, Fadda P, Fratta W, Pistis M. 2016. Interactions between the endocannabinoid and nicotinic cholinergic systems: preclinical evidence and therapeutic perspectives. Psychopharmacology 233: 1765–1777. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. 2007. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology 191: 867–877. [DOI] [PubMed] [Google Scholar]
- Serrano A, Parsons LH. 2011. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Ther 132: 215–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhpura N, Parsons LH. 2011. Endocannabinoid-mediated synaptic plasticity and addiction-related behavior. Neuropharmacology 61: 1070–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan ME, Gowin JL, Ramchandani VA, Hurd YL, Le Foll B. 2017. The endocannabinoid system as a target for addiction treatment: trials and tribulations. Neuropharmacology 124: 73–83. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D. 2008. The endocannabinoid system in brain reward processes. Br J Pharmacol 154: 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Zhao M. 2017. Endocannabinoid mechanism in amphetamine-type stimulant use disorders: A short review. J Clin Neurosci 46: 9–12. [DOI] [PubMed] [Google Scholar]
- Szutorisz H, Hurd YL. 2018. High times for cannabis: epigenetic imprint and its legacy on brain and behavior. Neurosci Biobehav Rev 85: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. 2003. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms: a review of recent preclinical data. Psychopharmacology 169: 115–134. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. 2000. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci 3: 1073–1074. [DOI] [PubMed] [Google Scholar]
- Tarragon E, Moreno JJ. 2018. Role of endocannabinoids on sweet taste perception, food preference, and obesity-related disorders. Chem Senses 43: 3–16. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. 2007. Review on CPP: measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12: 227–462. [DOI] [PubMed] [Google Scholar]
- Valjent E, Maldonado R. 2000. A behavioural model to reveal place preference to Δ9-tetrahydrocannabinol in mice. Psychopharmacology 147: 436–438. [DOI] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson M, Caboche J, Maldonado R. 2002. Behavioural and biochemical evidence for interactions between Δ9-tetrahydrocannabinol and nicotine. Br J Pharmacol 135: 564–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñals X, Moreno E, Lanfumey L, Cordomi A, Pastor A, de La Torre R, Gasperini P, Navarro G, Howell LA, Pardo L, et al. 2015. Cognitive impairment induced by Δ9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS Biol 13: e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. 2005. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav 81: 331–342. [DOI] [PubMed] [Google Scholar]
- Wills KL, Parker LA. 2016. Effect of pharmacological modulation of the endocannabinoid system on opiate withdrawal: a review of the preclinical animal literature. Front Pharmacol 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerke J, Pattij T, Schoffelmeer AN, De Vries TJ. 2008. The role of CB1 receptors in psychostimulant addiction. Addict Biol 13: 225–238. [DOI] [PubMed] [Google Scholar]
- Zou S, Kumar U. 2018. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci 19: E833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.