Abstract
Prolonged use of methamphetamine (meth) has been associated with episodic memory deficits in humans, and preclinical rat models of meth self-administration indicate the memory deficits are a consequence of meth use. Others have suggested that the meth-induced memory deficits may promote a cyclical pattern of drug use, abstinence, and relapse, although preclinical evidence for this relationship is somewhat lacking. The memory deficits in preclinical models manifest as a loss of novel object recognition (NOR) memory. These deficits occur one to two weeks after cessation of meth use and involve the perirhinal cortex, a parahippocampal region essential to NOR memory. We hypothesized that a loss of perirhinal cortex function contributes to both the NOR memory deficits and increased vulnerability to relapse in a novel-cue reinstatement model. To test this, we attempted to restore NOR memory in meth rats using an excitatory Gq-DREADD in perirhinal neurons. Activation of these neurons not only reversed the meth-induced deficit in NOR memory, but also restored novelty salience in a novel-cue reinstatement model. Thus, perirhinal cortex functionality contributes to both memory deficits in relapse in a long-access model of meth self-administration in rats, and chemogenetic restoration of perirhinal function restores memory and reduces relapse.
Methamphetamine (meth) abusers have episodic memory deficits that correlate with the extent of meth use (Simon et al. 2004; Scott et al. 2007; Dean et al. 2013), but to determine whether these memory deficits are a cause or consequence of meth abuse, it is necessary to use preclinical animal models. Long-access meth self-administration in rats induces recognition memory deficits measured in a novel object recognition (NOR) task (Reichel et al. 2011a,b; Scofield et al. 2015; Peters et al. 2016), in which rats must remember a familiar object to express a natural preference for a novel object. These memory deficits have been observed one to two weeks after abstinence from meth, and numerous findings point to perirhinal cortex dysfunction as a neurobiological substrate for these deficits. For instance, there is a loss of long-term depression (LTD) in perirhinal cortex slices after long-access meth (Scofield et al. 2015). This impaired LTD results, at least in part, from a loss of glutamate receptor expression (Reichel et al. 2011b; Scofield et al. 2015) and function (Peters et al. 2016) in perirhinal cortex. Collectively, these findings point to a meth-induced reduction in perirhinal cortex glutamatergic transmission.
There is a great deal of evidence indicating a critical role for the perirhinal cortex in NOR memory at the basal state, in drug-naive animals (Griffiths et al. 2008; Malkova et al. 2015). Pharmacological inactivation of the perirhinal cortex, or its disconnection from the prefrontal cortex (PFC), impairs NOR memory in drug-naive animals (Parker and Gaffan 1998; Winters et al. 2010). The perirhinal cortex also sends projections to the nucleus accumbens (NAc) (Christie et al. 1987; McIntyre et al. 1996), a target that is known to be an integral component of the meth relapse circuit (Rocha and Kalivas 2010). However, functional evidence supporting the relationship between the meth-induced memory deficits and relapse is lacking. Moreover, the neural circuitry underlying this relationship is also unknown. We recently demonstrated the importance of perirhinal cortex in a relapse setting involving novel cues, e.g., a novel-cue reinstatement paradigm (Peters et al. 2016). Restoring perirhinal glutamatergic transmission reduced the relapse index in this paradigm, a measure of the relative reinforcing potential of meth cues versus novel cues. Long-access, but not short-access, meth rats exhibit a NOR recognition memory deficit, and long-access rats exhibit a greater relapse index in the novel-cue reinstatement paradigm than short-access rats (Peters et al. 2016). This suggests a common neurobiological substrate may underlie both the recognition memory deficits and propensity to relapse after long-access meth.
Designer receptors exclusively activated by designer drugs (DREADDs) have been used to selectively activate (or deactivate) neurons in specific brain regions, cell types, and circuits to control numerous types of behaviors ranging from locomotion to relapse (Augur et al. 2016; Gomez et al. 2017). The inhibitory Gi-DREADD, for example, when expressed in insular cortex neurons, can restore meth-induced deficits in risky decision-making (Mizoguchi et al. 2015), and when specifically expressed in the anterior insular to central amygdala pathway can reduce relapse after voluntary abstinence (Venniro et al. 2017). In the voluntary abstinence model, animals choose to seek an alternate reward (e.g., sucrose) over the drug reward in a choice setting, an example of contingency management (Venniro et al. 2017). In our novel-cue reinstatement paradigm, short-access meth animals choose novel cues as frequently as they choose meth cues, but after long-access meth, they prefer meth cues (Peters et al. 2016). We view this as a failure in contingency management and hypothesize that it can be attributed to a deficit in perirhinal cortex function.
To test this hypothesis, we expressed an excitatory Gq-DREADD in perirhinal cortex neurons and examined the ability of its ligand, clozapine-N-oxide (CNO), to restore memory and reduce relapse in our novel-cue reinstatement paradigm after long-access meth. This allows us to test the role of perirhinal cortex in both behavioral paradigms in a within-subject manner, and thus examine the interdependency of these measures. We hypothesized that the meth-induced recognition memory deficits contribute to relapse in the novel-cue reinstatement paradigm, and that chemogenetic restoration of perirhinal function would both restore memory and reduce relapse. As novelty detection and novelty reward are critical to performance in both tasks, perirhinal cortex neurons may encode one or both of these processes. By artificially enhancing perirhinal functionality, it may be possible to restore novelty salience and its ability to act as an alternative reward in contingency management therapy.
Results
Meth self-administration
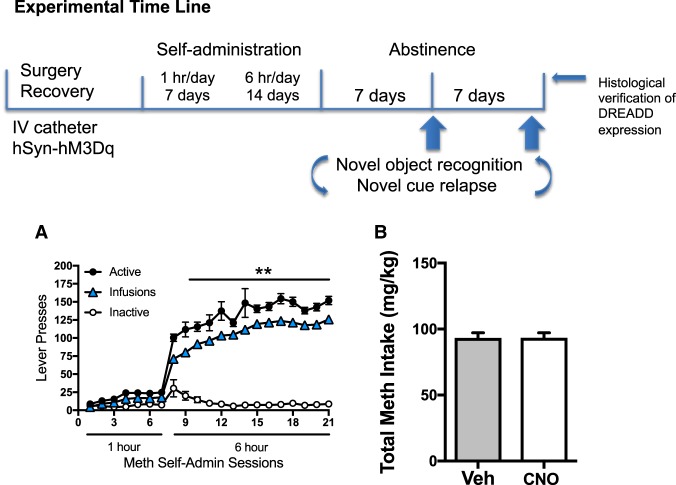
The experimental time line is depicted in Figure 1. Consistent with previous work from our laboratory and others, during the 21 d of meth self-administration (Fig. 1A), rats escalated the number of infusions they received over the course of the long access period (main effect of day: F(13,338) = 37.72, P < 0.001). Specifically, responding was greater on days 2 to 14 relative to day one of the long access protocol (Holm–Sidak's, P < 0.05). Consistently, active lever presses increased over time (main effect of day: F(13,338) = 1.9, P < 0.028) with days 7, 8, and 9 significantly above day one of long access. Total cumulative meth intake did not differ between rats that were assigned to vehicle or CNO groups (Fig. 1B).
Figure 1.
Long-access meth leads to escalation of meth intake. The experimental timeline is shown in the top panel. After recovering from surgical implantation of i.v. catheters and perirhinal delivery of the hSyn-hM3Dq, rats underwent long-access meth self-administration, followed by 1 wk of home cage abstinence. The order of object recognition memory and novel cue relapse tests was counterbalanced across subjects and separated by an additional week of home cage abstinence. Following these tests, brain tissue was histologically examined to verify HA-hM3Dq expression in perirhinal cortex. (A) The number of total active lever presses, those leading to a meth infusion, and inactive lever presses are shown over the course of daily meth self-administration sessions. (**) P < 0.01 comparing total active lever presses to those on the first 6-h meth session (indicative of escalation). (B) Total meth intake was equivalent between vehicle and CNO groups in this study.
Object recognition
The amount of time spent with and approaches to objects during the 3 min habituation session are presented in Table 1 along with locomotor activity counts. There were no group differences on any of these measures. On the test for recognition memory the two-way ANOVA revealed a significant interaction between object (novel versus familiar) and treatment (CNO versus vehicle) (Fig. 2A, F(1,24) = 9.55, P < 0.005). Follow up comparisons show that CNO increased the amount of time spent with the novel object whereas vehicle rats explored objects equally (Holm–Sidak's P < 0.05). There were no differences on the number of approaches to objects between groups (see Table 1). Direct comparisons between groups using the recognition index (time spent with novel object/time spent with both objects) showed that meth rats treated with CNO had significantly higher preference ratios than vehicle treated rats (Fig. 2B, t(24) = 3.27, P < 0.05). Importantly, no effects of CNO were observed in the absence of DREADD expression on this behavior (Supplemental Fig. 1). Similarly, a low dose of clozapine did not alter NOR memory in meth rats without DREADDs (Supplemental Fig. 1), which is worth noting given recent concerns that CNO back-conversion to clozapine accounts for DREADD activity (Michaelides et al. 2013).
Table 1.
Time, approach, and locomotor activity during object recognition
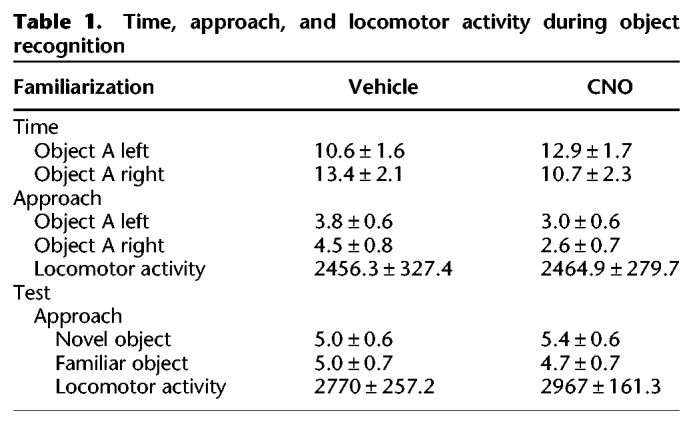
Figure 2.
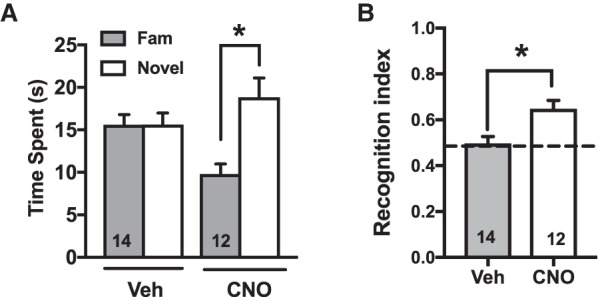
Chemogenetic activation of perirhinal cortex reverses meth-induced deficits in novel object recognition (NOR) memory. (A) Total time spent interacting with novel versus familiar objects on the NOR test is shown for vehicle versus CNO groups. (*) P < 0.01 comparing novel versus familiar. (B) A recognition index greater than 0.5 (dotted line) reflects the discrimination between novel and familiar objects on the NOR test (see main text for details). (*) P < 0.05 compared to a hypothetical mean of 0.5.
Novel cue responding
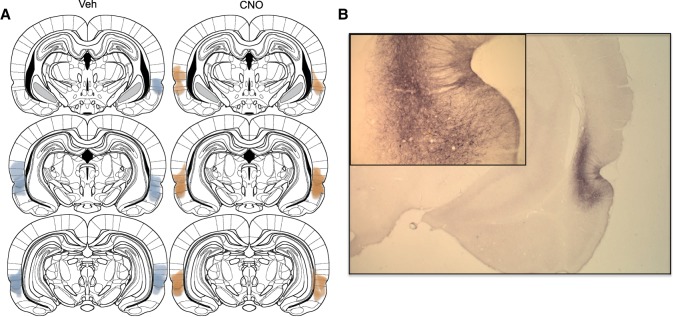
The number of lever presses on the drug-associated, novel, and inactive levers are depicted in Figure 3. Planned comparisons (Fig. 3A) between active and novel lever responding show that meth rats given vehicle responded more on the active/drug associated lever relative to the novel lever (t(48) = 2.84, P < 0.006). In contrast, CNO-treated rats responded equally on both levers (t(48) = 0.2, P > 0.05). Two-way ANOVA revealed a significant main effect of lever (F(2,48) = 15.94, P < 0.0001) and the interaction approached significance (F(2,48) = 2.5, P < 0.09). Direct comparisons between groups using the relapse index (novel lever responses/both lever responses) showed that meth rats treated with vehicle had significantly higher preference ratios than CNO-treated rats (Fig. 3B, t(24) = 1.9, P < 0.05). Histological verification of the Gq-DREADD expression was verified at the end of the experiment by immunohistochemical detection of the mCherry tag. The areas where expression was observed were marked for each animal and overlaid for graphical purposes (Fig. 4A). DREADD expression was centered around areas 35 and 36 of the perirhinal cortex, but neuropil staining extended into neighboring areas including the dorsally situated auditory association cortex (Te3), and the ventrally situated entorhinal cortex. A representative section depicting the immunohistochemical label is shown in Figure 4B.
Figure 3.
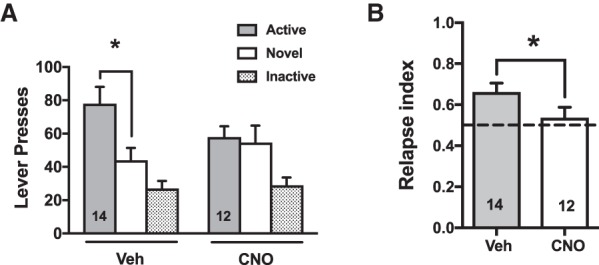
Chemogenetic activation of perirhinal cortex reverses meth-induced preference for meth cues in a reinstatement model involving novelty choice. (A) Total number of active lever presses (delivering meth cues) versus novel lever presses (delivering novel cues), as well as inactive lever presses, are shown for vehicle versus CNO groups on the novel cue relapse test. (*) P < 0.05 comparing active versus novel. (B) A relapse index greater than 0.5 (dotted line) reflects a preference to respond for meth cues in this model (see main text for details). (*) P < 0.05 comparing vehicle versus CNO groups.
Figure 4.
Histological verification of the Gq-DREADD expression in perirhinal cortex. (A) The extent of all detectable neuropil and cell body immunohistochemical detection of the mCherry tag on the DREADD is shown for vehicle versus CNO groups separately, across the anteroposterior extent of the perirhinal cortex. (B) A representative photomicrograph of the mCherry expression in the perirhinal cortex is shown.
Discussion
The present study validates the therapeutic efficacy of using targeted chemogenetics to reverse meth-induced deficits in recognition memory and reduce relapse. Chemogenetic activation of the perirhinal cortex was effective on both these measures, lending support to the notion that meth-induced recognition memory deficits may predispose to relapse, thus perpetuating the cycle of meth use and abuse. Notably, these memory deficits are only observed after long-access (not short-access) meth (Rogers et al. 2008; Reichel et al. 2011b), and thus may reflect a stage of substance abuse, beyond recreational use. For this past study, we used a relapse model that incorporated a choice between novel versus meth cues and found notable differences between long- and short-access meth, with long-access rats exhibiting a higher relapse index, indicative of a preference to respond for meth cues versus novel ones (Peters et al. 2016). The common role of perirhinal cortex across recognition memory and relapse in this model may reflect the role of this region in novelty discrimination and/or salience. Since the basolateral amygdala (BLA) is noted for its regulatory role in relapse for drug conditioned cues (See 2002), it may provide a critical input to perirhinal cortex to permit discrimination between conditioned versus novel cues.
Deficits in object recognition memory, as measured using a simple two-object task like the one used herein, are generally thought to reflect an impairment in novelty discrimination. The perirhinal cortex plays an essential role in this process, as early as the time of initial object exploration (e.g., familiarization phase). At this time, sensory information about the objects is relayed to perirhinal cortex through higher-order association cortices, and LTD occurs at perirhinal synapses (Griffiths et al. 2008; Massey et al. 2008). This LTD is thought to be the neural substrate encoding the familiarity of encountered objects (Banks et al. 2014). Thus, upon subsequent encounter of a novel object versus familiar object (e.g., test phase), novelty discrimination occurs within perirhinal synapses, with those encoding the novel object presumably now undergoing LTD for the first time, whereas those encoding the familiar object already existing in a depressed state (Griffiths et al. 2008). The memory for the familiar object is therefore encoded and retrieved by this perirhinal plasticity. Similarly, the perirhinal cortex in humans exhibits multivoxel pattern similarity using fMRI across encoding and retrieval phases of episodic memory (Tompary et al. 2016).
The perirhinal cortex is heavily interconnected with numerous brain regions known to play a critical role in NOR memory and/or relapse (Kealy and Commins 2011). While neither the PFC, nor the hippocampus, are required for NOR memory in the simple two-object task used here, they undoubtedly communicate with the perirhinal cortex during encoding and retrieval of NOR memory under normal conditions. Further, the PFC is critical for reinstatement in preclinical self-administration models of relapse for most drugs of abuse including meth (Rocha and Kalivas 2010). Chemogenetic activation of the perirhinal cortex would be expected to enhance glutamatergic output to the PFC, thereby enhancing relapse if the primary targets are principal pyramidal neurons. Currently it is unknown whether the perirhinal cortex favors principal neurons over cortical interneurons, which are preferentially targeted by the ventral hippocampus (Sotres-Bayon et al. 2012). Thus, enhancing output through this pathway could either promote or reduce relapse, but the specific neuroanatomy is unknown, nor is the specific role of this projection in reinstatement behavior.
Activity in NAc neurons is necessary for both cued and meth-primed reinstatement of meth seeking (Rocha and Kalivas 2010), and both the perirhinal cortex and PFC provide a glutamatergic input to the NAc (Christie et al. 1987; Brog et al. 1993). The PFC input to the core drives the reinstatement of cocaine seeking (McFarland et al. 2003), and PFC activity is necessary for the reinstatement of both cocaine and meth seeking (McFarland and Kalivas 2001; Rocha and Kalivas 2010). Cocaine induces opposing adaptations in ventral hippocampal versus PFC inputs to the NAc, suggesting these pathways may exert opposing influences on NAc output to regulate relapse (Pascoli et al. 2014). Little is known about the role of perirhinal inputs to the NAc in the reinstatement of drug seeking; although it is possible this pathway, like the ventral hippocampal inputs, may oppose the PFC's relapse-promoting input to NAc neurons. This would be consistent with our results that activating perirhinal cortex (including its accumbens projections) reduced relapse in our model. The NAc also plays a role in novelty choice behavior and is critical for novelty-induced place preference (Pierce et al. 1990). However, it is unknown whether this behavioral response to novelty is processed by the same NAc neurons involved in the reinstatement of drug-seeking behavior.
The BLA, like the PFC, provides a glutamatergic input to the NAc that has been shown to drive relapse, particularly reinstatement elicited by conditioned cues (See et al. 2003; Di Ciano and Everitt 2004). The perirhinal cortex has heavy reciprocal connections with the BLA and has been proposed to act as a gateway between the amygdala and the hippocampal-parahippocampal network (Koganezawa et al. 2008). BLA input generally facilitates information propagation from perirhinal to entorhinal cortex, and into the hippocampus, supporting the notion that the perirhinal cortex may integrate emotional and sensory information before it is conveyed to the hippocampus (Kajiwara et al. 2003). Interestingly, novel cues were found to be as effective at triggering reinstatement as cocaine conditioned cues, and both types of cues activated a common neural circuit, measured by Fos expression (Bastle et al. 2012). Given that our relapse model incorporates competition between novel cues and conditioned meth cues, the BLA could provide a critical input to the perirhinal cortex conveying the emotional significance of the meth cues, allowing the competition between meth and novel cues within the perirhinal cortex to be computed at a synaptic level before the outcome is relayed to the hippocampus.
In sum, the perirhinal cortex is in a key anatomical position to regulate both NOR memory and relapse, and chemogenetic activation of perirhinal neurons reversed meth-induced deficits in NOR memory and reduced relapse in a reinstatement model involving choice between novel versus meth cues. Future studies should determine whether the perirhinal cortex regulates relapse to meth cues independent of novelty competition. Current evidence suggests that perirhinal cortex neurons may be in a depressed state after long-access meth and restoring their activity via numerous mechanisms restores NOR memory and reduces relapse (Scofield et al. 2015; Peters et al. 2016). Thus, interventions aimed at reversing meth-induced neuroadaptations in perirhinal cortex may break the cyclical pattern of meth abuse, cognitive deficits, and relapse. Additional studies are needed to decipher the specific neural circuitry by which perirhinal cortex exerts its therapeutic influence on cognition and relapse, which will undoubtedly facilitate novel treatment strategies for meth addiction.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Harlan) weighing 250–275 g on arrival were used for these experiments and were individually housed on a reversed 12:12 light–dark cycle (6 a.m. light on). All experiments were conducted during the rats’ dark cycle. Food (standard rat chow) and water were available ad libitum, except during self-administration, when rats were food-restricted to 20–30 g of chow per day. Procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council) and approved by the IACUC of the Medical University of South Carolina.
Intravenous catheter surgery
Rats were anesthetized with ketamine (57 mg/kg, i.p.), xylazine (8.7 mg/kg, i.p.), and equithesin (0.7 mL/kg, i.p.). Ketorolac (2.0 mg/kg, i.p.) and cefazolin (200 mg/kg, s.c.) were administered preoperatively for analgesia and to protect against post-surgical pain and infection, respectively. A silastic catheter was inserted into the right jugular vein and was passed subcutaneously over the shoulder, exiting between the scapula via a small incision. This end of the catheter was attached to a cannula with an external port for i.v. drug delivery. During self-administration, rats received an infusion (0.1 mL) of saline before each session to verify catheter patency. After each session, catheters were flushed with cefazolin (10 mg/0.1 mL), followed by 0.05 mL of taurolidine-citrate catheter locking solution to maintain catheter patency.
Virus microinjection and immunohistochemical detection
Under the same plane of anesthesia the Gq-DREADD (AAV2-hSyn-HA-hM3D(Gq)-IRES mCitrine, Bryan Roth, University of North Carolina, Chapel Hill, NC) was microinjected into the perirhinal cortex (coordinates measured from bregma at the skull surface, angled 10° laterally, as follows: anterior–posterior (AP) −4.8 mm, medial-lateral (ML) −5 mm, DV −7.5mm). Gq-DREADDs were allowed 3 wk to reach maximal expression levels before CNO testing began. AAV was microinjected in a volume of 0.75 µL/side (1012 IU/mL) at a rate of 0.1 µL/min, allowing 10 min for diffusion.
Following the last test session, rats were transcardially perfused with 10% buffered formalin, and brains were removed for immunohistochemistry on free-floating (40 µm) sections. Tissue was treated with peroxidase, then blocked with 2% normal donkey serum in PBS. Sections were incubated overnight at 4°C in primary antibody: mouse anti-hemagglutinin (HA) (1:1000; Covance #MMS101-P, RRID: AB_2314672). The secondary antibody was biotin-SP conjugated donkey anti-mouse IgG (1:500; Jackson ImmunoResearch Laboratories). The signal was amplified with an avidin-biotin complex (1:500), then reacted with diaminobenzidine (in 5% nickel). Tissue was mounted onto slides, dehydrated, cover-slipped, and examined under a microscope to visualize the Gq-DREADD.
Meth self-administration
Behavioral chambers (30 × 20 × 20 cm3, Med Associates) were housed inside sound-attenuating cubicles containing a houselight, a fan, two retractable levers, a drug-delivery arm attached to a swivel, and a spring leash that enclosed the tubing for drug delivery. Tygon tubing was connected to a 10 mL meth syringe fitted to an infusion pump. Fans provided white noise and ventilation. At the beginning of each session, the house light turned on and levers extended, signaling meth availability. Responding on the active lever delivered a 2 sec infusion of methamphetamine hydrochloride (Sigma; dissolved in sterile saline; 20 mg/50 mL/infusion) followed by an unsignaled 20 sec timeout period where responding was without consequence. A white stimulus light positioned above the active lever was used to signal each meth infusion. Responding on the inactive lever was without consequence. At the end of the program, the house light turned off and levers retracted. Self-administration started 5 d after surgery. Rats were placed in the chambers for daily 1 h sessions on an FR1 schedule of reinforcement. After 7 d, session length was increased to 6 h (long access) over the last 2 wk of self-administration. Rats were then placed into abstinence for 7 d. Half the rats were tested for novel object recognition on day 7 of abstinence and half the rats tested for novel cue reinstatement. One week later the tests were switched. Treatment groups (CNO versus vehicle) did not change between tests.
Novel cue relapse test
Novel cue responding was conducted as previously described (Peters et al. 2016). In brief, a novel lever and white stimulus light were positioned on the opposite wall from the active lever. The light was covered with pieces of Velcro on the top and bottom, leaving only a small slit for the light to disperse. Responding on the novel lever resulted in illumination of the novel cue light (15 sec duration; FR1; 20 sec timeout). To minimize associations between the visual cues during the test, placement of the novel cue + lever was on the opposite wall relative to the active and inactive levers. In total, the novel and active cues differed in intensity, visual and tactile features, and spatial location in the chamber. Vehicle (DMSO) or CNO (10 mg/kg, ip) was administered 30 before the test session and responding on all levers was recorded over the 2 h session.
Novel object recognition
Novel object recognition memory testing was performed as previously described (Reichel et al. 2011b; Peters et al., 2016; Scofield et al. 2015). In brief, rats were habituated to the test apparatus twice for 5 min without objects. On abstinence day 7 or 14 (see Experimental Procedures), rats explored two identical objects for 3 min. Immediately after this familiarization session, vehicle (DMSO) or CNO (10 mg/kg, ip) was administered. 90 min later rats were placed back on the apparatus with an object from the familiarization phase and a novel object for 3 min. Data was recorded and scored using Ethovision XT 8.0 (Noldus). Objects consisted of combinations of a PVC pipe (6.4 × 3.8 cm2), a light bulb (8.9 cm), and a plastic bottle (Reichel et al. 2011b; Scofield et al. 2015; Peters et al. 2016).
Statistical analyses
Meth intake (mg/kg) and active lever responding were the primary dependent measures for self-administration and were analyzed with a one-way repeated measures analysis of variance (ANOVA) over the 14 d of long access. Time spent, approaches, and motor activity were the dependent variables during object recognition familiarization and testing. Time spent and approaches were analyzed with two-way mixed factors ANOVA with object (novel versus familiar) as within subjects variable and treatment group (CNO versus veh) as a between subjects variable. Additionally, data were converted to a recognition index (novel object exploration/novel object + familiar object exploration) to demonstrate that object recognition occurred for each group by comparisons of the recognition index to a hypothetical mean of 0.5 using independent t-tests. A recognition index of 0.5 indicates equal time spent exploring both objects, greater than 0.5 indicates more exploration of the novel object, and less than 0.5 indicates more exploration of the familiar object. Based on our previous work with the novel cue task we specifically hypothesized that active lever responding would differ from the novel lever only in vehicle-treated animals but that CNO would normalize responding to both levers. To test this hypothesis, we conducted planned comparisons between active and novel lever responding for the vehicle and CNO groups with independent t-tests. We also converted data to a Relapse Index to reflect the within-subject choice for the meth cue + lever versus the novel cue + novel lever. The formula parallels that used to calculate the recognition index for novel object recognition: (Active Lever Presses/Active + Novel Lever presses). Thus, a relapse index above 0.50 indicates relatively higher responding for the meth-conditioned cue versus the novel cue, and an index of 0.50 indicates indifference for these cues. Statistical analysis was conducted with the alpha set at 0.05, family wise error was controlled for with Holm–Sidak's corrections and all data are expressed as the mean ± SEM.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Drug Addiction research grant DA033049 to C.M.R. and DA038235 and DA044524 to J.P. We thank Shannon Ghee and Carole Berini for technical support. The CNO was generously provided by the NIDA Drug Supply Program.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.046797.117.
References
- Augur IF, Wyckoff AR, Aston-Jones G, Kalivas PW, Peters J. 2016. Chemogenetic activation of an extinction neural circuit reduces cue-induced reinstatement of cocaine seeking. J Neurosci 36: 10174–10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks PJ, Warburton EC, Brown MW, Bashir ZI. 2014. Mechanisms of synaptic plasticity and recognition memory in the perirhinal cortex. Prog Mol Biol Transl Sci 122: 193–209. [DOI] [PubMed] [Google Scholar]
- Bastle RM, Kufahl PR, Turk MN, Weber SM, Pentkowski NS, Thiel KJ, Neisewander JL. 2012. Novel cues reinstate cocaine-seeking behavior and induce Fos protein expression as effectively as conditioned cues. Neuropsychopharmacology 37: 2109–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. 1993. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338: 255–278. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Summers RJ, Stephenson JA, Cook CJ, Beart PM. 1987. Excitatory amino acid projections to the nucleus accumbens septi in the rat: a retrograde transport study utilizing D[3H]aspartate and [3H]GABA. Neuroscience 22: 425–439. [DOI] [PubMed] [Google Scholar]
- Dean AC, Groman SM, Morales AM, London ED. 2013. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 38: 259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. 2004. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci 24: 7167–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, et al. 2017. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357: 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S, Scott H, Glover C, Bienemann A, Ghorbel MT, Uney J, Brown MW, Warburton EC, Bashir ZI. 2008. Expression of long-term depression underlies visual recognition memory. Neuron 58: 186–194. [DOI] [PubMed] [Google Scholar]
- Kajiwara R, Takashima I, Mimura Y, Witter MP, Iijima T. 2003. Amygdala input promotes spread of excitatory neural activity from perirhinal cortex to the entorhinal-hippocampal circuit. J Neurophysiol 89: 2176–2184. [DOI] [PubMed] [Google Scholar]
- Kealy J, Commins S. 2011. The rat perirhinal cortex: a review of anatomy, physiology, plasticity, and function. Prog Neurobiol 93: 522–548. [DOI] [PubMed] [Google Scholar]
- Koganezawa N, Taguchi A, Tominaga T, Ohara S, Tsutsui K, Witter MP, Iijima T. 2008. Significance of the deep layers of entorhinal cortex for transfer of both perirhinal and amygdala inputs to the hippocampus. Neurosci Res 61: 172–181. [DOI] [PubMed] [Google Scholar]
- Malkova L, Forcelli PA, Wellman LL, Dybdal D, Dubach MF, Gale K. 2015. Blockade of glutamatergic transmission in perirhinal cortex impairs object recognition memory in macaques. J Neurosci 35: 5043–5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Phythian D, Narduzzo K, Warburton EC, Brown MW, Bashir ZI. 2008. Learning-specific changes in long-term depression in adult perirhinal cortex. J Neurosci 28: 7548–7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. 2001. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21: 8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. 2003. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23: 3531–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre DC, Kelly ME, Staines WA. 1996. Efferent projections of the anterior perirhinal cortex in the rat. J Comp Neurol 369: 302–318. [DOI] [PubMed] [Google Scholar]
- Michaelides M, Anderson SA, Ananth M, Smirnov D, Thanos PK, Neumaier JF, Wang GJ, Volkow ND, Hurd YL. 2013. Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks. J Clin Invest 123: 5342–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi H, Katahira K, Inutsuka A, Fukumoto K, Nakamura A, Wang T, Nagai T, Sato J, Sawada M, Ohira H, et al. 2015. Insular neural system controls decision-making in healthy and methamphetamine-treated rats. Proc Natl Acad Sci 112: E3930–E3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A, Gaffan D. 1998. Interaction of frontal and perirhinal cortices in visual object recognition memory in monkeys. Eur J Neurosci 10: 3044–3057. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O'Connor EC, Lüscher C. 2014. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509: 459–464. [DOI] [PubMed] [Google Scholar]
- Peters J, Scofield MD, Ghee SM, Heinsbroek JA, Reichel CM. 2016. Perirhinal cortex mGlu5 receptor activation reduces relapse to methamphetamine seeking by restoring novelty salience. Neuropsychopharmacology 41: 1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Crawford CA, Nonneman AJ, Mattingly BA, Bardo MT. 1990. Effect of forebrain dopamine depletion on novelty-induced place preference behavior in rats. Pharmacol Biochem Behav 36: 321–325. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE. 2011a. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology 62: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. 2011b. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology 36: 782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. 2010. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci 31: 903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. 2008. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology 199: 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Trantham-Davidson H, Schwendt M, Leong KC, Peters J, See RE, Reichel CM. 2015. Failure to recognize novelty after extended methamphetamine self-administration results from loss of long-term depression in the perirhinal cortex. Neuropsychopharmacology 40: 2526–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. 2007. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17: 275–297. [DOI] [PubMed] [Google Scholar]
- See RE. 2002. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav 71: 517–529. [DOI] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. 2003. Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci 985: 294–307. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dacey J, Glynn S, Rawson R, Ling W. 2004. The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat 27: 59–66. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. 2012. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76: 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompary A, Duncan K, Davachi L. 2016. High-resolution investigation of memory-specific reinstatement in the hippocampus and perirhinal cortex. Hippocampus 26: 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, Cifani C, Marchant NJ, Yizhar O, Bossert JM, et al. 2017. The anterior insular cortex→central amygdala glutamatergic pathway is critical to relapse after contingency management. Neuron 96: 414–427 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bartko SJ, Saksida LM, Bussey TJ. 2010. Muscimol, AP5, or scopolamine infused into perirhinal cortex impairs two-choice visual discrimination learning in rats. Neurobiol Learn Mem 93: 221–228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.