Abstract
Purpose
Patients with advanced cancer (ACPs) participating in phase I clinical trials inadequately understand many elements of informed consent (IC); however, the prevalence and impact of cognitive impairment has not been described.
Patients and Methods
ACPs enrolled onto phase I trials underwent neuropsychological assessment to evaluate cognitive functioning (CF) covering the following domains: memory (Hopkins Verbal Learning Test), executive functioning (Trail Making Test B), language (Boston Naming Test-Short Version and Controlled Oral Word Association Test), attention (Trail Making Test A and Wechsler Adult Intelligenence Scale-IV Digit Span), comprehension (Wechsler Adult Intelligence Scale-IV), and quality of life (Functional Assessment of Cancer Therapy–Cognitive Function). Structured interviews evaluated IC and decisional capacity. Psychological measures included distress (Hospital Anxiety Depression Scale) and depression (Beck Depression Inventory-II).
Results
One hundred eighteen ACPs on phase I trials were evaluated, with CF ranging from mild impairment to superior performance. Only 45% of ACPs recalled physician disclosure of the phase I trial purpose. The 50% of ACPs who correctly identified the phase I research purpose had greater CF compared with ACPs who did not, as revealed by the mean T scores for memory (37.2 ± 5.6 v 32.5 ± 5.1, respectively; P = .001), attention (29 ± 2.7 v 26.9 ± 2.4, respectively; P < .001), visual attention (35.2 ± 6.6 v 31.5 ± 6.2, respectively; P = .001), and executive function (38.9 ± 7.5 v 34 ± 7.1, respectively; P < .001). Older ACPs (≥ 60 years) were less likely to recall physician disclosure of phase I purpose than younger ACPs (30% v 70%, respectively; P = .02) and had measurable deficits in total memory (34.2 ± 5.0 v 37.3 ± 5.6, respectively; P = .002), attention (24.5 ± 2.6 v 28 ± 2.8, respectively; P < .001), and executive function (32.8 ± 7.3 v 36.4 ± 7.6, respectively; P = .01). Older ACPs, compared with younger ACPs, also had greater depression scores (10.6 ± 9.2 v 8.1 ± 5.2, respectively; P = .03) and lower quality-of-life scores (152 ± 29.6 v 167 ± 20, respectively; P = .03). After adjustment by age, no psychological or neuropsychological variable was further significantly associated with likelihood of purpose identification.
Conclusion
CF seems to play a role in ACP recall and comprehension of IC for early-phase clinical trials, especially among older ACPs.
INTRODUCTION
In clinical research, the informed consent (IC) process is viewed as a means by which research participants are protected from harm.1-5 An absolute requirement for adequate IC is intact decisional capacity, which includes the key element of comprehension of information provided during the IC process as well as appreciation, reasoning, and communication.6-9 In general, the IC process for clinical research participation begins with disclosure of important elements (eg, nature of research, alternatives, and risks and benefits of participation). In addition to information disclosure that is mindful of potential participants’ preferences for information, federal regulations governing human participant research require investigators to assist potential participants to understand this disclosed information to the greatest extent possible.10
In oncology, the IC process within the framework of phase I trials is especially significant because the primary scientific goal is to evaluate safety not efficacy, yet patients with advanced cancer (ACPs) often enroll onto trials with expectations of direct clinical benefit.11 As a result, ethical and clinical concerns exist about the decisional capacity of this population required for adequate IC. Prior research overwhelmingly indicates that ACPs have an inadequate measured understanding of phase I IC elements, including phase I trial research purpose as dose and toxicity determination, likelihood of therapeutic benefit, and alternatives to trial participation.4,11-30 Additional evidence suggests ACP understanding of phase I trials is influenced by overwhelming motivations for benefit and the IC process itself, including physician-investigator disclosure of the previously mentioned IC elements.4,11-16,25-30
Despite this evidence, ACP cognitive functioning (CF) and its effect on IC comprehension have never been formally evaluated. This is notwithstanding growing research demonstrating that patients with cancer experience mild, yet potentially clinically significant, cognitive impairment (CI) that is undetected without formal testing.5,8,9,31-33 Prior research reveals that CI in patients with cancer may be a result of several underlying factors.34-58 Since the early 1970s, CI has been associated with prior treatment effects (eg, chemotherapy, radiation). In patients with solid tumors, neuropsychological testing has revealed cognitive deficits involving attention, concentration, verbal and visual memory, and executive function.35-37 Multiple cognitive tasks (and neuropsychological measures) are associated with specific decisional capacity domains.39 For example, comprehension and understanding are associated with tasks of conceptualization and confrontation naming, executive functions, memory, and comprehension.39 Additional contributors strongly associated with CI known to impair decisional capacity include psychological distress, fatigue, sleep disturbances, opiate and other medication use, and biochemical manifestations of cancer (eg, hormonal fluctuations, cytokine deregulation).35-37,44,50,55-57 Moreover, as the incidence of CI increases with age, age-related impairments, and other comorbidities including working memory decline, concerns about CI and decisional capacity are further heightened in older ACPs.5,8,9,31-33,58,59
Given these concerns, our primary study objective was to formally describe CF related to decisional capacity in ACPs enrolling onto phase I trials. This study was designed to assess CF in ACPs on phase I trials using formal neuropsychological testing, including health-related quality of life (QOL) and ACP distress (depression and anxiety) impairing decision making, and to explore potential associations between age and ACP CF related to decisional capacity. We hypothesized that ACPs would report mild CI, diminished QOL, and mild distress as evaluated by formal neuropsychological and psychosocial assessment. We also hypothesized that poor CF, which may vary by age, would adversely impact and interfere with ACP IC understanding and reasoning related to phase I clinical trial decisions.
PATIENTS AND METHODS
Patients
Potential ACP participants recently providing IC for phase I trial participation were recruited from University of Chicago’s Developmental Therapeutics Clinic. Eligibility requirements for phase I trial enrollment included ACP ability to give IC as determined by phase I investigators, age ≥ 18 years, survival prognosis of ≤ 3 months, Karnofsky performance status ≥ 60%, and a documented diagnosis of advanced cancer proven to be refractory to standard therapy or for which no identifiable standard therapy exists.
Procedure
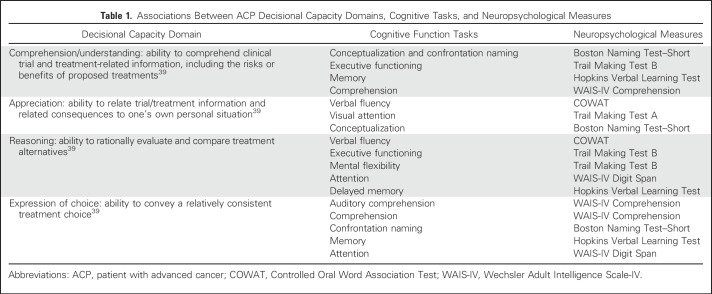
Institutional review board approval was obtained before study initiation. This prospective, original report of a consecutive ACP sample included neuropsychological assessments completed at one time point—10 days after ACP provision of consent for phase I trial participation (before receipt of investigational agent[s]). Once consent for this IC study was obtained, ACPs completed neuropsychological tests and the Functional Assessment of Cancer Therapy–Cognitive Function (FACT-COG), and IC and decisional capacity structured interview, and quantitative psychological measures (Hospital Anxiety and Depression Scale [HADS] and Beck Depression Inventory-II) in the clinic or infusion suite. One investigator (F.J.H.) trained in standardized administration of neuropsychological and psychological assessment, under the guidance of a neuropsychologist (E.R.L.), conducted testing and interviews. Neuropsychological testing assessed specific ACP cognitive tasks. Table 1 lists associations between ACP decisional capacity domains, cognitive tasks, and neuropsychological measures.39
Table 1.
Associations Between ACP Decisional Capacity Domains, Cognitive Tasks, and Neuropsychological Measures
Measures
Sociodemographic and clinical information was recorded per ACP self-report.
Neuropsychological tests.
Memory.
The Hopkins Verbal Learning Test–Revised (HVLT-R)60 is a test of immediate and delayed learning assessing episodic memory, learning, and retention. The task required ACPs to recall 12 words after examiner presentation over three trials. Raw score is the sum of words recalled over three trials. A second delayed recall score is the number of words recalled 20 to 25 minutes after the initial task.
Executive functioning.
The Trail Making Test B61 is a test of executive function, set shifting, inhibitory control, and flexibility. ACPs connected randomly distributed numbers and letters, alternating sequentially between both. Raw score was number of seconds needed for task completion.
Language.
The Boston Naming Test–Short62-65 is a picture test of confrontation naming and word retrieval in individuals with aphasia or language disturbance as a result of neurologic deficits. The test contains 15 line drawings graded in difficulty for ACPs to name the picture.
The Controlled Oral Word Association Test61,62,66,67 is an executive control measure assessing semantic and phonetic cues (eg, word retrieval, verbal initiation and fluency). ACPs recalled words beginning with the letter A within 60 seconds. Raw scores reflect number of all acceptable words.
Attention.
Wechsler Adult Intelligence Scale-IV Digit Span66 is a test of verbal working memory, sustained attention, and encoding. ACPs were read a number sequence and asked to recall it in exact order. Next, ACPs were read a number sequence and asked to recall it in reverse order. Raw score is the longest digit span recalled.
The Trail Making Test A61 is a test of visual scanning, graphomotor speed, and attention. ACPs were instructed to connect numbers, randomly distributed across the page, in sequence. Raw score was number of seconds needed for task completion.
Verbal comprehension.
Wechsler Adult Intelligence Scale-IV Comprehension61,62,66,67 is a measure of verbal comprehension, reasoning, and judgment. ACPs responded to questions based on understanding of general principles and social situations. Raw score was the number of questions answered correctly.
QOL.
The FACT-COG Version 235,68-72 is a health-related QOL measure with CF domains (perceived CI, cognition, QOL impact, and concerns from others), yielding the following four summary scores: CF, Impact on functional domain/interferences (IOF), impact on quality of life (QOL), and total. Responses rated on 5-point Likert scale (0 = never to 4 = several times a day) the frequency with which each statement occurred in the past week. Low scores indicate poor overall QOL.
Psychological measures.
The HADS73 is a 14-item distress scale with the following two subscales: seven items measure anxiety (eg, “Worrying thoughts go through my mind”) and seven items measure depression (eg, “I feel cheerful”). Responses are scored from 0 to 3 points, with subscale total scores of 0 to 21 points (score ranges: normal, 0 to 7; mild, 8 to 10; moderate, 11 to 14; and severe, 15 to 21).
The Beck Depression Inventory-II74 is a 21-item depression inventory measuring affective (eg, “I do not feel sad”) and somatic symptoms (eg, “My appetite is no worse than normal”). Responses are scored from 0 to 3 points (score ranges: minimal depression, 0 to 13; mild depression, 14 to 19; moderate depression, 20 to 28; and severe depression, 29 to 63).
Phase I IC structured interview.
Decisional capacity of the phase I clinical trial IC elements was assessed, as previously reported,14-16,75-78 including research purpose, trial alternatives, risk and benefits, and expectations of benefit. The interview included demographic, structured (yes or no), and open-ended questions. Two questions related to ACP recall and comprehension of the phase I research purpose were posed. First, ACPs were asked to recall physician disclosure of the phase I trial purpose (“When you enrolled, what did the physician tell you was the purpose of the investigational study?”). Second, ACP comprehension gained regarding phase I IC was evaluated (“As far as you know, what are the doctors and researchers trying to find out in the experimental study in which you are participating?”; Appendix, online only).
Statistical Analysis
All data were analyzed using Stata 13.0 statistical software (Stata, College Station, TX).79 Demographics were summarized using frequencies, percentages, medians and means, ranges, and standard deviations. To examine age differences as a result of expected increase in CI with advancing age, summary statistics were tabulated for the total population and dichotomized on the basis of population median into two groups (age < 60 and age ≥ 60 years), and characteristics were compared between groups using the t test, Fisher’s exact test, or χ2 test. Distinct multivariable logistic regression models were applied for each psychological and neuropsychological measure adjusted by demographic variables. For neuropsychological tests, T scores were calculated using mean raw score, with overall mean T scores, standard deviations, and ranges. t tests were completed to detect differences between group scores. T scores range from 20 (profound deficit) to 80 (very superior performance) with CF classifications as follows: very superior, 70 to 80; superior, 64 to 69; high average, 58 to 63; average, 43 to 57; low average 37 to 42; borderline, 30 to 36; impaired, 28 to 29; mild, 27.2 to 27.9; moderate, 26 to 27; severe, 24 to 25; and profound, < 24. The statistical significance threshold was set at P < .05.
Phase I IC Content Analysis
This was an iterative process of qualitative analysis of ACP response to structured interview inquiry as recorded within each decisional capacity domain for phase I IC. Responses were read by investigators (F.J.H. and H.S.N.) and coded using a consensus process of whether ACPs understood phase I IC and purpose. Responses involving dosage, toxicity, or dosage and toxicity were considered correct. Other responses (eg, cure) were considered incorrect. One investigator (C.K.D.) resolved discrepancies. Investigators systematically identified recurrent themes and generated a category list for responses using the constant comparison method to confirm conceptual development. Coded responses were summarized as proportions to specific questions, enabling subsequent quantitative analyses to identify associations between variables of interest.
RESULTS
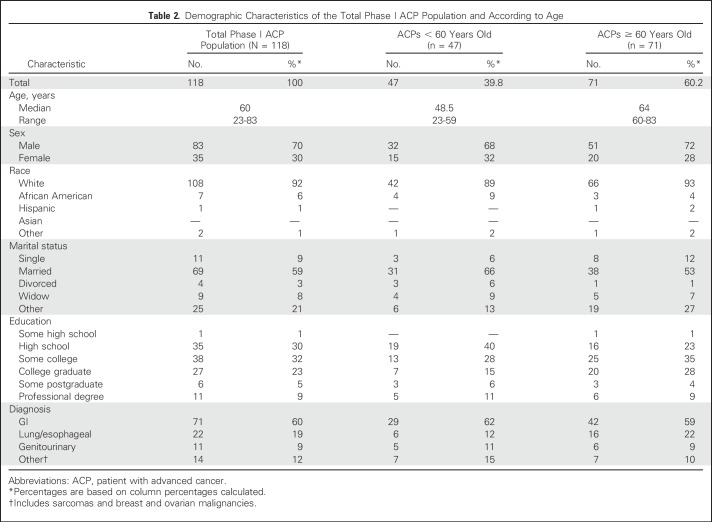
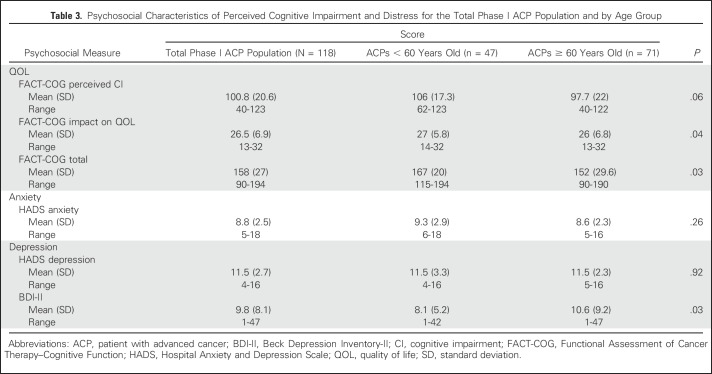
A consecutive sample of 251 ACPs enrolled onto phase I trials from the University of Chicago’s Developmental Therapeutics Clinic were approached for study participation. A total of 133 patients did not complete full assessment with survey as a result of unexpected toxicity or fatigue. No significant differences in demographics were found between noncompleters and completers with the exception of ACP age (56 ± 11.4 years v 61 ± 9.7, respectively; P = .02). Noncompleters were younger and disenrolled early from trial participation. No ACP refused study participation. A final sample of 118 ACPs (47%) consented and completed full assessment with survey. Table 2 lists the demographics for the total population and the age groups. In the total population, the median age was 60 years (range, 23 to 83 years), 70% were male, 92% were white, 37% were college or professional graduates, and 60% had a GI malignancy. ACPs reported mild anxiety, moderate depression (HADS), and poor total FACT-COG QOL (Table 3).
Table 2.
Demographic Characteristics of the Total Phase I ACP Population and According to Age
Table 3.
Psychosocial Characteristics of Perceived Cognitive Impairment and Distress for the Total Phase I ACP Population and by Age Group
Neuropsychological Outcomes
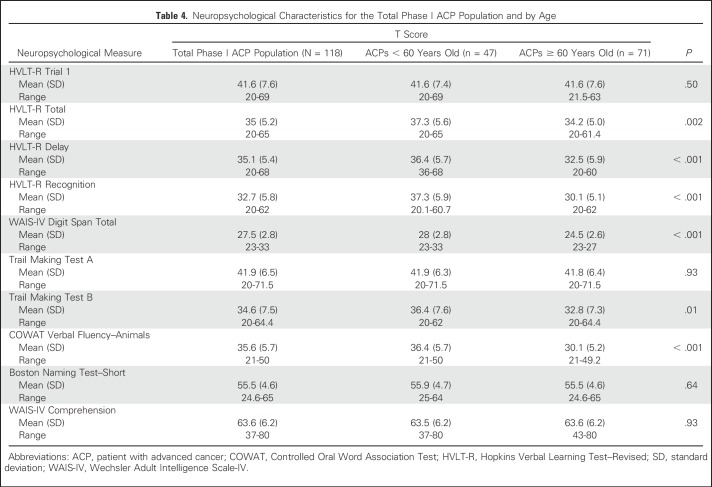
ACP neuropsychological T scores for CF are listed in Table 4. Overall, phase I ACP CF ranged from mild impairment to superior performance (27.5 ± 2.8 to 63.6 ± 6.2). ACPs experienced borderline impairment in CF for memory, executive function, and verbal fluency. ACPs exhibited low average attention, average language, and superior comprehension.
Table 4.
Neuropsychological Characteristics for the Total Phase I ACP Population and by Age
ACP Phase I IC Recall and Comprehension
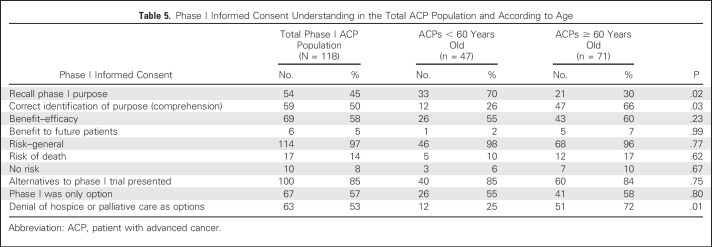
Table 5 lists ACP IC understanding of phase I trials according to age. Only 45% of ACPs recalled disclosure of dosage or toxicity as the primary phase I research purpose. In total, 50% of ACPs correctly identified dosage as the phase I purpose.
Table 5.
Phase I Informed Consent Understanding in the Total ACP Population and According to Age
Associations Between Neuropsychological Outcomes, ACP Phase I IC Recall, and Comprehension
ACPs who recalled the physician disclosure of the trial purpose as dosage had better CF than ACPs who failed to recall purpose, as measured by HVLT-R delayed memory (41 ± 6.7 v 32.1 ± 5.4, respectively; P = .01), recognition (38.5 ± 5.7 v 21 ± 4.1, respectively; P = .001), and digit span attention (31 ± 2.8 v 27.9 ± 2.4, respectively; P < .001). Regarding trial comprehension, ACPs who identified purpose had better CF than ACPs who failed to identify purpose, as assessed by HVLT-R delayed memory (37.2 ± 5.6 v 32.5 ± 5.1, respectively; P = .001); digit span attention (29 ± 2.7 v 26.9 ± 2.4, respectively; P < .001), Trail Making Test A visual attention (35.2 ± 6.6 v 31.5 ± 6.2, respectively; P = .001), and Trail Making Test B mental flexibility (38.9 ± 7.5 v 34 ± 7.1, respectively; P < .001).
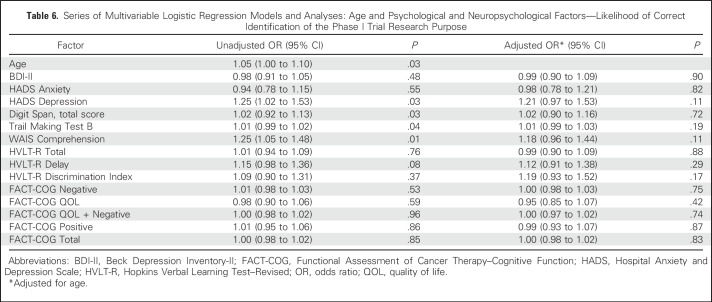
Highly educated ACPs were more likely to identify trial purpose compared with less educated ACPs (53% v 27%, respectively; P = .03). Female sex was associated with greater likelihood of purpose identification (70% v 47% for men; P = .04). After adjustment by age, no psychological or neuropsychological variable was further significantly associated with likelihood of purpose identification (Table 6).
Table 6.
Series of Multivariable Logistic Regression Models and Analyses: Age and Psychological and Neuropsychological Factors—Likelihood of Correct Identification of the Phase I Trial Research Purpose
Neuropsychological Outcomes and ACP Comprehension of Phase I IC According to Age
Age is well known to affect performance on neuropsychological measures. As expected, several, but not all, measures indicate significant differences between age groups. Older ACPs performed poorly compared with younger ACPs on memory, attention, executive function, and verbal fluency tasks (Table 4). They also had greater depression and poorer total QOL (Table 3). Older ACPs were less likely to recall purpose compared with younger ACPs (30% v 70%, respectively; P = .02), yet more likely to correctly identify trial purpose (66% v 26%, respectively; P = .03) and to deny that supportive care was presented (72% v 25%, respectively; P = .01; Table 5).
DISCUSSION
The key foundation of IC is the presence of intact decisional capacity. This study examined the essential IC elements for phase I trials and their relationship to ACP decisional capacity and CF using formal neuropsychological testing. On the basis of tests of memory (recall and recognition), verbal fluency, and executive functioning, we found evidence of borderline CI in ACPs enrolled onto phase I trials. Our data reveal that CF may play a role in ACP recall and comprehension of IC for phase I clinical trials. Other factors, including demographics, psychological distress, and QOL, may also be associated with this recall and comprehension.
Neuropsychological testing detected evidence of CI in ACPs involving encoding, retrieval, and recognition of consent information, as well as attention and executive functioning required for decision making. These data suggest that some ACPs may not be fully equipped to process information necessary to provide adequate IC. CI is prevalent in ACPs59 but was particularly salient in older ACPs, who experienced more significant deficits in memory, language, attention, and executive functioning and were less likely to recall physician disclosure of trial purpose. This is consistent with research revealing that recall decreases with age, yet is dependent on quantity of information provided; when more information is discussed, older ACPs experience significant challenges remembering this information.80 Therefore, physician communication of phase I IC should be tailored to engage visual and cognitive abilities of older ACPs in order to enhance understanding given that many ACPs cognitively receive and process trial content differently. Oncologists must enhance this IC process using communication practices including corrected feedback (repetition of information and categorizing complex information), provision of pictorial representations, and teachback (checking understanding;eg, “We discussed a great deal today. Can you tell me in your own words what is the purpose of a Phase I clinical trial?”) where ACPs engage in cognitive tasks (eg, naming, executive function, memory, comprehension). Indeed, it is not quantity of information alone but also quality of IC that is critical in this communication.
ACP CI may be further exacerbated by underlying psychological distress, as older ACPs tended to report more depressive symptoms. In addition, in the older and total ACP groups, depression may have impacted appropriate recall and comprehension of the phase I trial purpose. These results support prior evidence indicating that ACPs’ realistic expectations of outcome are associated with psychological morbidity.14,81,82 Also, older ACPs had significantly poorer QOL, as indicated by FACT-COG. Given the terminal status of phase I ACPs, cognitive-related issues coupled with disease progression suggest this is a vulnerable population whose ability to provide IC is questionable in many cases, particularly among older patients. The CI discovered could further be expected to worsen ACP emotional, social, and physical well-being in the long term.35,36 ACPs who are neurologically robust are more likely to recall and understand IC information as provided by a physician-investigator during trial discussions.
Several limitations of our study should be noted. It is statistically difficult to control for individual differences in consent forms from multiple trials. However, although the phase I consent form is standardized to include key IC elements as mandated by the institutional review board and federal regulations, the consent form is only one piece of a complicated, rigorous IC process involving, first, formal oncologist’s disclosure of key phase I IC elements to the ACP, followed by oncologist-ACP dialogue involving the nature of IC, concluded by stringent review and signing of the form with an opportunity for the ACP to gain clarity. Our goal was not to analyze the impact of the consent form only but all aspects related to the IC process, such as understanding and communication, as defined by multiple cognitive tasks, potentially affected by potential CI. Next, formal control groups were not included. Healthy, age-matched people or ACPs receiving outpatient chemotherapy should be considered. We desired to describe whether CI was present in this selected ACP group and to examine its effect on phase I IC recall and comprehension. A second limitation involved the one-time CF assessment at initiation of phase I trial enrollment. Longitudinal study assessing ACP CF during the trial would provide meaningful data. An additional limitation includes significant selection bias (118 of 251 ACPs enrolled onto phase I trials completed assessment). It may be that ACPs who completed the study were healthier with better CF compared with those unable to complete the study. Finally, reporting on only two outcomes strongly reduces the impact of study results. However, we examined all four domains of decisional capacity, finding only comprehension to be associated with demographic variables of interest. Data were skewed for additional domains (eg, appreciation [“Do you feel you had the option to refuse study?”; 100% responded “yes”]). Similar results were found for choice and reasoning. We focused on recall, although not a capacity domain, as a significant cognitive task specific to memory and representative in all domains. In addition, any differences in neuropsychological performance are only limited to statistical significance. With regard to any associations, especially between phase I IC decisional capacity and neuropsychological outcomes, we recognize that any explanation is speculative. However, these associations do provide hypotheses for further study.
Future research must determine the significance of CI and the impact of comorbidities on ACP IC comprehension for phase I clinical trials. The underlying causes of CI and contributors (eg, distress) are likely multifactoral.34-59,83,84 Educators and caregivers should become involved in the IC process. Potential ACP barriers to understanding accurate information involve studying oncologists and patients during the IC process. Brief cognitive measures (eg, Montreal Cognitive Assessment) might provide options for determining which ACPs need additional IC communication. Moreover, longitudinal study of ACPs throughout phase I trial enrollment and beyond (hospice) should assess decisional capacity. Finally, application of pharmacologic (eg, modafinil, methylphenidate), clinical (eg, cognitive rehabilitation, biofeedback, brief cognitive-behavioral therapy), and communication support tools and/or interventions (corrected feedback or teachback) should be considered to address CI.85-101 Such interventions will assist ACPs with coping, QOL, and decision making.
Appendix
Structured Interview Questions Assessing Comprehension of Elements of Informed Consent by Patients With Advanced Cancer
Understanding and comprehension.
What is the purpose of this investigational study? (When you enrolled, what did the physician tell you the purpose of the study was?)
What alternatives were present to you in addition to joining the investigational study?
Can you recall the side effects that were presented to you during the explanation of this experimental protocol? Can you name a couple?
Appreciation.
Why do you think the physician talked to you about joining the study today?
Did you feel like you had the option to refuse to be in the investigational study?
If no, why did you feel like you did not have the option to refuse to be in the investigational study?
Do you feel like you can withdraw at any time?
Interview Questions Assessing Elements of Informed Consent
Reasoning.
What were the benefits of choosing to participate in this study?
What were the risks of participating in this study?
Communication.
Did you have the opportunity to ask the physician questions?
What questions did you ask that were not answered?
What information did you receive about the drug trial you are in that you didn’t understand?
Interview Questions Assessing Elements of Informed Consent
-
Was any nonexperimental therapy discussed with you before you made a decision to participate in the study at the University of Chicago?
–If yes, what kind of therapy was discussed?
Was the possibility of no chemotherapy discussed with you as an option?
Was the possibility of care that would only relieve symptoms but would not have any chance of destroying your cancer discussed with you?
As far as you know, what are the doctors and researchers trying to find out in the experimental study in which you are participating?
-
Of the following choices, which one best states the research purpose of the investigational drug study you are in?
I don’t know the purpose
To determine the side effects and the right dose of the drug
To determine if the drug can cure my cancer
To determine if the drug can destroy or shrink my cancer
Footnotes
Supported by National Institutes of Health Grant No. RO1 CA 087605-01A1 (C.K.D.) and the Greenwall Foundation Program for Bioethics (C.K.D. and G.A.S.)
AUTHOR CONTRIBUTIONS
Conception and design: Fay J. Hlubocky, Greg A. Sachs, Christopher K. Daugherty
Financial support: Christopher K. Daugherty
Administrative support: Fay J. Hlubocky, Greg A. Sachs, Christopher K. Daugherty
Provision of study materials or patients: Mark J. Ratain
Collection and assembly of data: Fay J. Hlubocky, Christopher K. Daugherty
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Do Patients With Advanced Cancer Have the Ability to Make Informed Decisions for Participation in Phase I Clinical Trials?
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Fay J. Hlubocky
No relationship to disclose
Greg A. Sachs
Honoraria: CVS Health
Eric R. Larson
No relationship to disclose
Halla S. Nimeiri
Employment: AbbVie
Stock or Other Ownership: AbbVie
David Cella
Stock or Other Ownership: FACIT.org
Consulting or Advisory Role: AbbVie, Bayer, GlaxoSmithKline, Pfizer
Research Funding: Novartis (Inst), Genentech (Inst), Ipsen (Inst), Bayer (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline, Bayer
Kristen E. Wroblewski
No relationship to disclose
Mark J. Ratain
Stock or Other Ownership: Biscayne Pharmaceuticals
Consulting or Advisory Role: AbbVie, Biscayne Pharmaceuticals, Cyclacel, Shionogi Pharma, Amgen
Research Funding: AbbVie (Inst), Dicerna (Inst)
Patents, Royalties, Other Intellectual Property: Royalties related to UGT1A1 genotyping for irinotecan, royalties related to UGT1A1 genotyping for irinotecan (Inst), pending patent related to a genomic prescribing system, pending patent related to a genomic prescribing system (Inst)
Expert Testimony: Multiple generic companies
Jeffrey M. Peppercorn
Employment: GlaxoSmithKline (I)
Stock or Other Ownership: GlaxoSmithKline (I)
Research Funding: Pfizer
Christopher K. Daugherty
Consulting or Advisory Role: Daiichi Sankyo
REFERENCES
- 1.Faden RR, Beauchamp TL: A History and Theory of Informed Consent. New York, NY, Oxford University Press, 1986 [PubMed] [Google Scholar]
- 2.Beauchamp TL, Childress JF: Principles of Bioethics (ed 4). New York, NY, Oxford University Press, 1994 [Google Scholar]
- 3.Daugherty CK: Impact of therapeutic research on informed consent and the ethics of clinical trials: A medical oncology perspective. J Clin Oncol 17:1601-1617, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Berghmans R: Capacity and consent. Curr Opin Psychol 14:491-499, 2001 [Google Scholar]
- 5.Casarett DJ, Karlawish JH, Hirschman KB: Identifying ambulatory cancer patients at risk of impaired capacity to consent to research. J Pain Symptom Manage 26:615-624, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Jonsen AR, Seigler M, Winslade WJ: Clinical Ethics (ed 4). New York, NY, McGraw-Hill, 1998 [Google Scholar]
- 7.Grisso T, Appelbaum PS: The MacArthur Treatment Competence Study. III: Abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav 19:149-174, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Appelbaum PS, Grisso T: Assessing patients’ capacities to consent to treatment. N Engl J Med 319:1635-1638, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Hougham GW, Sachs GA, Danner D, et al. : Empirical research on informed consent with the cognitively impaired. IRB 25:S26-S32, 2003. (suppl 25) [PubMed] [Google Scholar]
- 10.Department of Health and Human Services : Federal Common Rule, 1991, Title 45, CFR, Code of Federal Regulations, Part 46. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html
- 11.Meropol NJ, Weinfurt KP, Burnett CB, et al. : Perceptions of patients and physicians regarding phase I cancer clinical trials: Implications for physician-patient communication. J Clin Oncol 21:2589-2596, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Willems Y, Sessa C: Informing patients about phase I trials: How should it be done? Acta Oncol 28:106-107, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Tomamichel M, Sessa C, Herzig S, et al. : Informed consent for phase I studies: Evaluation of quantity and quality of information provided to patients. Ann Oncol 6:363-369, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Daugherty C, Ratain MJ, Grochowski E, et al. : Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol 13:1062-1072, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Daugherty CK, Ratain MJ, Minami H, et al. : Study of cohort-specific consent and patient control in phase I cancer trials. J Clin Oncol 16:2305-2312, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Daugherty CK, Banik DM, Janish L, et al. : Quantitative analysis of ethical issues in phase I trials: A survey interview of 144 advanced cancer patients. IRB 22:6-14, 2000 [PubMed] [Google Scholar]
- 17.Yoder LH, O’Rourke TJ, Ethyre A, et al. : Expectations and experiences of patients with cancer participating in phase I clinical trials. Onc Nurs Forum 24:891-896, 1997 [PubMed] [Google Scholar]
- 18.Itoh K, Sasaki Y, Fujii H, et al. : Patients in phase I trials of anti-cancer agents in Japan: Motivation, comprehension and expectations. Br J Cancer 76:107-113, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng JD, Hitt J, Koczwara B, et al. : Impact of quality of life on patient expectations regarding phase I clinical trials. J Clin Oncol 18:421-428, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Joffe S, Cook EF, Cleary PD, et al. : Quality of informed consent in cancer clinical trials: A cross-sectional survey. Lancet 358:1772-1777, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Rodenhuis S, van den Heuvel WJ, Annyas AA, et al. : Patient motivation and informed consent in a phase I study of an anticancer agent. Eur J Cancer Clin Oncol 20:457-462, 1984 [DOI] [PubMed] [Google Scholar]
- 22.Roberts TG, Jr, Goulart BH, Squitieri L, et al. : Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA 292:2130-2140, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Horstmann E, McCabe MS, Grochow L, et al. : Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med 352:895-904, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Weber JS, Levit LA, Adamson PC, et al. : American Society of Clinical Oncology policy statement update: The critical role of phase I trials in cancer research and treatment. J Clin Oncol 33:278-284, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox AC, Fallowfield LJ, Jenkins VA: Communication and informed consent in phase 1 trials: A review of the literature. Support Care Cancer 14:303-309, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kass N, Taylor H, Fogarty L, et al. : Purpose and benefits of early phase cancer trials: What do oncologists say? What do patients hear? J Empir Res Hum Res Ethics 3:57-68, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins V, Solis-Trapala I, Langridge C, et al. : What oncologists believe they said and what patients believe they heard: An analysis of phase I trial discussions. J Clin Oncol 29:61-68, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Brown R, Bylund CL, Siminoff LA, et al. : Seeking informed consent to phase I cancer clinical trials: Identifying oncologists’ communication strategies. Psychooncology 20:361-368, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Jenkins VA, Anderson JL, Fallowfield LJ: Communication and informed consent in phase 1 trials: A review of the literature from January 2005 to July 2009. Support Care Cancer 18:1115-1121, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Pentz RD, White M, Harvey RD, et al. : Therapeutic misconception, misestimation, and optimism in participants enrolled in phase 1 trials. Cancer 118:4571-4578, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dale W, Hougham GW, Hill EK, et al. : High interest in screening and treatment for mild cognitive impairment in older adults: A pilot study. J Am Geriatr Soc 54:1388-1394, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Bial AK, Schilsky RL, Sachs GA: Evaluation of cognition in cancer patients: Special focus on the elderly. Crit Rev Oncol Hematol 60:242-255, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Hurria A, Rosen C, Hudis C, et al. : Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: A pilot prospective longitudinal study. J Am Geriatr Soc 54:925-931, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Tannock IF, Ahles TA, Ganz PA, et al. : Cognitive impairment associated with chemotherapy for cancer: Report of a workshop. J Clin Oncol 22:2233-2239, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Vardy J, Wong K, Yi QL, et al. : Assessing cognitive function in cancer patients. Support Care Cancer 14:1111-1118, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Vardy J, Tannock I: Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol 63:183-202, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Vardy J, Rourke S, Tannock IF: Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. J Clin Oncol 25:2455-2463, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Ahles TA, Saykin AJ: Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer 7:192-201, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moye J, Marson DC: Assessment of decision-making capacity in older adults: An emerging area of practice and research. J Gerontol B Psychol Sci Soc Sci 62:P3-P11, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Vardy J, Wefel JS, Ahles T, et al. : Cancer and cancer-therapy related cognitive dysfunction: An international perspective from the Venice cognitive workshop. Ann Oncol 19:623-629, 2008 [DOI] [PubMed] [Google Scholar]
- 41.McDonald BC, Conroy SK, Ahles TA, et al. : Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Res Treat 123:819-828, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wefel JS, Vardy J, Ahles T, et al. : International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 12:703-708, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Janelsins MC, Kohli S, Mohile SG, et al. : An update on cancer- and chemotherapy-related cognitive dysfunction: Current status. Semin Oncol 38:431-438, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fardell JE, Vardy J, Johnston IN, et al. : Chemotherapy and cognitive impairment: Treatment options. Clin Pharmacol Ther 90:366-376, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Weiss HD, Walker MD, Wiernik PH: Neurotoxicity of commonly used antineoplastic agents (first of two parts). N Engl J Med 291:75-81, 1974 [DOI] [PubMed] [Google Scholar]
- 46.Weiss HD, Walker MD, Wiernik PH: Neurotoxicity of commonly used antineoplastic agents (second of two parts). N Engl J Med 291:127-133, 1974 [DOI] [PubMed] [Google Scholar]
- 47.Kaasa S, Olsnes BT, Thorud E, et al. : Reduced short-term neuropsychological performance in patients with nonsmall-cell lung cancer treated with cisplatin and etoposide. Antibiot Chemother (1971) 41:226-231, 1988 [DOI] [PubMed] [Google Scholar]
- 48.Meyers CA, Abbruzzese JL: Cognitive functioning in cancer patients: Effect of previous treatment. Neurology 42:434-436, 1992 [DOI] [PubMed] [Google Scholar]
- 49.Cimprich B, So H, Ronis DL, et al. : Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology 14:70-78, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Pusztai L, Mendoza TR, Reuben JM, et al. : Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 25:94-102, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Schagen SB, Hamburger HL, Muller MJ, et al. : Neurophysiological evaluation of late effects of adjuvant high-dose chemotherapy on cognitive function. J Neurooncol 51:159-165, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Tope DM, Ahles TA, Silberfarb PM: Psycho-oncology: Psychological well-being as one component of quality of life. Psychother Psychosom 60:129-147, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Cimprich B: Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nurs 22:185-194, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Saykin AJ, Ahles TA, McDonald BC: Mechanisms of chemotherapy-induced cognitive disorders: Neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry 8:201-216, 2003 [PubMed] [Google Scholar]
- 55.Iconomou G, Mega V, Koutras A, et al. : Prospective assessment of emotional distress, cognitive function, and quality of life in patients with cancer treated with chemotherapy. Cancer 101:404-411, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Silberfarb PM, Philibert D, Levine PM: Psychosocial aspects of neoplastic disease: II. Affective and cognitive effects of chemotherapy in cancer patients. Am J Psychiatry 137:597-601, 1980 [DOI] [PubMed] [Google Scholar]
- 57.Lee BN, Dantzer R, Langley KE, et al. : A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation 11:279-292, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Ahles TA, Saykin AJ, McDonald BC, et al. : Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J Clin Oncol 28:4434-4440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burton CZ, Twamley EW, Lee LC, et al. : Undetected cognitive impairment and decision-making capacity in patients receiving hospice care. Am J Geriatr Psychiatry 20:306-316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benedict RHB, Schretlen D, Groninger L, et al. : Hopkins verbal learning test revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 12:43-55, 1998 [Google Scholar]
- 61.Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, AZ, Neuropsychology Press, 1985 [Google Scholar]
- 62.Spreen O, Strauss E: A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, NY, Oxford University Press, 1991 [Google Scholar]
- 63.Lacy MA, Gore PA, Pliskin NH, et al. : Verbal fluency task equivalence. Clin Neuropsychol 10:305-308, 1996 [Google Scholar]
- 64.Gladsjo JA, Schuman CC, Evans JD, et al. : Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment 6:147-178, 1999 [DOI] [PubMed] [Google Scholar]
- 65.del Toro CM, Bislick LP, Comer M, et al. : Development of a short form of the Boston naming test for individuals with aphasia. J Speech Lang Hear Res 54:1089-1100, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Weschler D: Manual for the Wechsler Adult Intelligence Scale (ed 3). New York, NY, The Psychological Corporation, 1997 [Google Scholar]
- 67.Reitan RM, Davison LA: Clinical Neuropsychology: Current Status and Applications. Washington, DC, Hemisphere Publishing, 1974 [Google Scholar]
- 68.Lai JS, Butt Z, Wagner L, et al. : Evaluating the dimensionality of perceived cognitive function. J Pain Symptom Manage 37:982-995, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wagner LI, Cella D, Sweet J, et al. : Chemotherapy-related cognitive deficits: A qualitative examination of patients and providers. Ann Behav Med 25:S56, 2003 [Google Scholar]
- 70.Cella DF, Tulsky DS, Gray G, et al. : The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol 11:570-579, 1993 [DOI] [PubMed] [Google Scholar]
- 71.Webster K, Cella D, Yost K: The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: Properties, applications, and interpretation. Health Qual Life Outcomes 1:79, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagner LI, Sweet J, Butt Z, et al. : Measuring patient self-reported cognitive function: Development of the Functional Assessment of Cancer Therapy–Cognitive Function Instrument. J Support Oncol 7:W32-W39, 2009 [Google Scholar]
- 73.Zigmond AS, Snaith RP: The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 67:361-370, 1983 [DOI] [PubMed] [Google Scholar]
- 74.Beck AT, Ward C, Mendelson M: An inventory for measuring depression. Arch Gen Psychiatry 4:561-571, 1961 [DOI] [PubMed] [Google Scholar]
- 75.Daugherty CK, Fitchett G, Murphy PE, et al. : Trusting God and medicine: Spirituality in advanced cancer patients volunteering for clinical trials of experimental agents. Psychooncology 14:135-146, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Helft PR, Hlubocky F, Wen M, et al. : Associations among awareness of prognosis, hopefulness, and coping in patients with advanced cancer participating in phase I clinical trials. Support Care Cancer 11:644-651, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Hlubocky FJ, Ratain MJ, Wen M, et al. : Complementary and alternative medicine among advanced cancer patients enrolled on phase I trials: A study of prognosis, quality of life, and preferences for decision making. J Clin Oncol 25:548-554, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Llewellyn-Thomas HA, McGreal MJ, Thiel EC, et al. : Patients’ willingness to enter clinical trials: Measuring the association with perceived benefit and preference for decision participation. Soc Sci Med 32:35-42, 1991 [DOI] [PubMed] [Google Scholar]
- 79.StataCorp : Stata Statistical Software: Release 13. College Station, TX, StataCorp LP, 2013 [Google Scholar]
- 80.Jansen J, Butow PN, van Weert JC, et al. : Does age really matter? Recall of information presented to newly referred patients with cancer. J Clin Oncol 26:5450-5457, 2008 [DOI] [PubMed] [Google Scholar]
- 81.Cohen L, de Moor C, Amato RJ: The association between treatment-specific optimism and depressive symptomatology in patients enrolled in a phase I cancer clinical trial. Cancer 91:1949-1955, 2001 [DOI] [PubMed] [Google Scholar]
- 82.Taylor SE, Kemeny ME, Reed GM, et al. : Psychological resources, positive illusions, and health. Am Psychol 55:99-109, 2000 [DOI] [PubMed] [Google Scholar]
- 83.Selnes OA, Gottesman RF: Neuropsychological outcomes after coronary artery bypass grafting. J Int Neuropsychol Soc 16:221-226, 2010 [DOI] [PubMed] [Google Scholar]
- 84.Weiss B: Chemobrain: A translational challenge for neurotoxicity . Neurotoxicity 29:891-898, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eslinger PJ: Neuropsychological Interventions: Clinical Research and Practice. New York, NY, Guilford Press, 2002 [Google Scholar]
- 86.Sohlberg MM, Mateer CA: Introduction to Cognitive Rehabilitation: Theory and Practice. New York, NY, Guilford Press, 1989 [Google Scholar]
- 87.Sohlberg MM, Mateer CA: Cognitive Rehabilitation: An Integrative Neuropsychological Approach. New York, NY, Guilford Press, 2001 [Google Scholar]
- 88.Cicerone KD, Dahlberg C, Kalmar K, et al. : Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Arch Phys Med Rehabil 81:1596-1615, 2000 [DOI] [PubMed] [Google Scholar]
- 89.Carney N, Chesnut RM, Maynard H, et al. : Effect of cognitive rehabilitation on outcomes for persons with traumatic brain injury: A systematic review. J Head Trauma Rehabil 14:277-307, 1999 [DOI] [PubMed] [Google Scholar]
- 90.Lundorff LE, Jønsson BH, Sjøgren P: Modafinil for attentional and psychomotor dysfunction in advanced cancer: A double-blind, randomised, cross-over trial. Palliat Med 23:731-738, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Mar Fan HG, Clemons M, Xu W, et al. : A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer 16:577-583, 2008 [DOI] [PubMed] [Google Scholar]
- 92.Meyers CA, Weitzner MA, Valentine AD, et al. : Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. J Clin Oncol 16:2522-2527, 1998 [DOI] [PubMed] [Google Scholar]
- 93.Ferguson RJ, Ahles TA, Saykin AJ, et al. : Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology 16:772-777, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferguson RJ, McDonald BC, Rocque MA, et al. : Development of CBT for chemotherapy-related cognitive change: Results of a waitlist control trial. Psychooncology 21:176-186, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vardy JL, Dhillon HM, Pond GR, et al. : Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal, controlled study. J Clin Oncol 33:4085-4092, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vardy J, Dhillon HM, Pond GR, et al. : Cognitive function and fatigue after diagnosis of colorectal cancer. Ann Oncol 25:2404-2412, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Asher A, Myers JS: The effect of cancer treatment on cognitive function. Clin Adv Hematol Oncol 13:441-450, 2015 [PubMed] [Google Scholar]
- 98.Weiss BD: Health Literacy and Patient Safety: Help Patients Understand: Manual for Clinicians, (ed 2). Washington, DC, American Medical Association Foundation and American Medical Association, 2007 [Google Scholar]
- 99.Davis TC, Williams MV, Marin E, et al. : Health literacy and cancer communication. CA Cancer J Clin 52:134-149, 2002 [DOI] [PubMed] [Google Scholar]
- 100.Nishimura A, Carey J, Erwin PJ, et al. : Improving understanding in the research informed consent process: A systematic review of 54 interventions tested in randomized control trials. BMC Med Ethics 14:28, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taub HA, Kline GE, Baker MT: The elderly and informed consent: Effects of vocabulary level and corrected feedback. Exp Aging Res 7:137-146, 1981 [DOI] [PubMed] [Google Scholar]