Abstract
Purpose
NRG Oncology/RTOG 1203 was designed to compare patient-reported acute toxicity and health-related quality of life during treatment with standard pelvic radiation or intensity-modulated radiation therapy (IMRT) in women with cervical and endometrial cancer.
Methods
Patients were randomly assigned to standard four-field radiation therapy (RT) or IMRT radiation treatment. The primary end point was change in patient-reported acute GI toxicity from baseline to the end of RT, measured with the bowel domain of the Expanded Prostate Cancer Index Composite (EPIC). Secondary end points included change in patient-reported urinary toxicity, change in GI toxicity measured with the Patient-Reported Outcome Common Terminology Criteria for Adverse Events, and quality of life measured with the Trial Outcome Index.
Results
From 2012 to 2015, 289 patients were enrolled, of whom 278 were eligible. Between baseline and end of RT, the mean EPIC bowel score declined 23.6 points in the standard RT group and 18.6 points in the IMRT group (P = .048), the mean EPIC urinary score declined 10.4 points in the standard RT group and 5.6 points in the IMRT group (P = .03), and the mean Trial Outcome Index score declined 12.8 points in the standard RT group and 8.8 points in the IMRT group (P = .06). At the end of RT, 51.9% of women who received standard RT and 33.7% who received IMRT reported frequent or almost constant diarrhea (P = .01), and more patients who received standard RT were taking antidiarrheal medications four or more times daily (20.4% v 7.8%; P = .04).
Conclusion
Pelvic IMRT was associated with significantly less GI and urinary toxicity than standard RT from the patient’s perspective.
INTRODUCTION
Estimates suggest that 13,240 women will be diagnosed with cervical cancer and 63,230 will be diagnosed with uterine cancer in the United States in 2018.1 Although multimodality therapy may be curative, postoperative radiation can cause significant morbidity, most notably GI.2-4 One prospective trial demonstrated that approximately 30% of patients who received surgery followed by standard four-field radiation therapy (RT) for endometrial cancer experienced significant acute diarrhea, which persisted in approximately 10% for up to 5 years after treatment.5 Treatment with concurrent chemotherapy with standard radiation yields even higher rates of acute GI toxicity.3
Pelvic RT for cervical and endometrial cancer requires treatment of the pelvic lymph nodes, which lie on the right and left sides of the pelvis, as well as the apex of the vagina. In patients who have undergone hysterectomy, small bowel fills the center of the pelvis between the pelvic lymph node targets. The standard technique for treating the pelvis is to use four beams coming from the anterior, posterior, and lateral aspects of the patient to treat a rectangular target that includes the bowel in the center of the pelvis, resulting in notable acute diarrhea. Intensity-modulated radiation therapy (IMRT) dose-calculation algorithms are used to shape the dose around target structures with multiple converging beams or arcs. This approach makes it possible to reduce the dose delivered to normal tissues, which are surrounded by target structures.
The dosimetric benefits and feasibility of pelvic IMRT are well documented. Pelvic IMRT delivers a lower dose to the small bowel and bladder than standard pelvic RT.6,7 Retrospective studies, and one small prospective investigation, have reported lower rates of physician-reported toxicity after IMRT than after standard RT.8,9 In addition, prospective studies have demonstrated that IMRT is feasible in the cooperative group setting.10 However, there have been few attempts to measure the impact of pelvic IMRT in terms of patient-reported toxicity. Here, the results of the first randomized trial, to our knowledge, designed to measure the impact of IMRT on patient-reported toxicity and quality of life are reported.
METHODS
Study Design
In this phase III multicenter randomized controlled trial, patients were stratified by dose (45 v 50.4 Gy), use of chemotherapy (yes v no), and disease site (cervix v endometrium), then randomly assigned 1:1 to receive either standard four-field pelvic RT or pelvic IMRT.11 Patients were treated to 45 Gy or 50.4 Gy on the basis of physician preference. Five cycles of cisplatin 40 mg/m2 weekly were administered at the physician’s discretion according to predefined pathologic criteria. The study protocol was approved by the institutional review board of each participating center and was registered in clinicaltrials.gov (ClinicalTrials.gov identifier: NCT01672892).
Eligibility
To be eligible for this study, women were required to have a confirmed histologic diagnosis of invasive cervical or endometrial cancer, indications for adjuvant RT after hysterectomy on the basis of pathologic risk factors (Data Supplement), and a Zubrod performance status of 0 to 2. Patients were excluded if they were unable to maintain a full bladder, required extended-field RT, had a history of inflammatory bowel disease, had evidence of active infection, or had previously received RT to the pelvis. All patients provided written informed consent.
Interventions
All patients underwent computed tomography simulation for RT planning. For patients who received standard RT, four-field plans were defined according to description and images in the protocol. In both groups, normal tissues, including bowel bag, rectum, bladder, and bone marrow, were defined, and targets, including the nodal clinical target volume and vagina, were contoured. In the IMRT group, the vaginal contour was designed as an internal target volume using full and empty bladder scans to account for vaginal motion.12 In the standard RT group, an internal target volume was not used, because standard field borders provide a significant margin around the vagina.
In the standard RT group, the dose was prescribed to the isocenter using 6-MV or higher photon energies. Normalization to an isodose line between 97% and 100% and use of field-in-field technique to increase homogeneity were allowed. For IMRT treatment planning, the nodal clinical target volume and vaginal internal target volume were expanded by 7 mm to create a planning target volume.13 Dose was prescribed to the planning target volume using inverse planning approaches. IMRT, volumetric-modulated arc therapy, and tomotherapy were allowed. In both groups, patients were treated once a day, 5 days a week, with a daily fraction size of 1.8 Gy. In both groups, volumetric dose constraints were recommended for the target structures, and in the IMRT group, dose constraints were used for the normal tissues.
For quality assurance, the treatment plan was reviewed before the start of treatment of the first patient treated at each site in each group. Treatment plans for subsequent patients were reviewed after completion of treatment. Toxicity was managed according to the standard treatment approach for each site.
Assessments
Patients completed the Expanded Prostate Cancer Index Composite (EPIC), the Patient-Reported Outcomes–Common Terminology Criteria for Adverse Events (PRO-CTCAE), the Functional Assessment of Cancer Therapy instrument with cervix subscale (FACT-Cx), and EuroQOL’s EQ-5D. A separate consent question was required for participation in the PRO-CTCAE, FACT-Cx, and EQ-5D components. The EPIC is a patient-reported outcome questionnaire typically used with patients with prostate cancer.14 It is designed to evaluate bowel and urinary function both during and after irradiation of the pelvis. Only two of the four domains, bowel and urinary, were used in this study. The FACT-General (FACT-G) is a validated, 27-item measure of quality of life (QOL) in patients with cancer.15 The 27 items on the FACT-G are divided into four subscales, for physical, functional, social, and emotional well-being. The PRO-CTCAE was developed to characterize the frequency and severity of treatment toxicities and the extent to which these toxicities interfere with daily activities (Data Supplement).16 Physician-reported adverse events (AEs) were graded using the National Cancer Institute’s CTCAE version 4. Toxicity and QOL were evaluated before treatment, after 13 to 15 fractions (3 weeks), after 23 to 25 fractions (5 weeks), and 4 to 6 weeks and 1 and 2 years after completion of RT. To minimize the survey burden for patients, evaluations after 3 weeks of treatment included only the EPIC and PRO-CTCAE. EQ-5D results will be reported separately. Additional information regarding the PRO instruments, including scoring information, are included in the Data Supplement.
Study End Points
The primary end point was change in acute GI toxicity from baseline to 5 weeks measured with the bowel domain of the EPIC. Secondary end points included change in genitourinary toxicity from baseline to 5 weeks measured with the urinary domain of the EPIC, toxicity measured with the PRO-CTCAE, and QOL measured with the FACT-Cx. Validation of the EPIC in this patient population was a secondary end point and will be reported separately.
Statistical Analysis
NRG Oncology statisticians performed study analysis using SAS Version 9.4 of the SAS System for Windows. Using an effect size of 0.4 to represent the difference between arms in EPIC bowel domain at 5 weeks, a t test with one interim analysis, and a two-sided α of 0.05, a sample size of 225 was needed to achieve 85% statistical power. The sample size was increased by 20%, to 281, to account for anticipated attrition, given the use of a patient-reported outcome as the primary end point. The efficacy interim analysis used an O’Brien and Fleming boundary once 50% of evaluable patients reached 5 weeks and was tested at a significance level of .003, making the significance level for the final analysis .049 to preserve the overall type I error of 0.05. All eligible patients were included in the intent-to-treat analysis. Change scores were calculated as the score at follow-up minus the baseline score. For the EPIC and FACT-Cx, a negative change score indicates a decline in function. Continuous variables were compared between treatment groups using the t test or the Wilcoxon test if variables were not normally distributed. Categorical variables were compared between treatment groups using the χ2 test or Fisher’s exact test if sample sizes were small. A significance level of .05 was used for the EPIC bowel and urinary scores. A Bonferroni-adjusted α was used when testing subscales (0.0125 for the four FACT-G subscale scores, 0.0125 for the four EPIC urinary subscale scores, and 0.025 for the two EPIC bowel subscale scores). Patients with and without completed EPIC bowel assessments were compared at each time point. Linear fixed effects models using maximum likelihood were performed on the 5-week EPIC bowel and urinary scores adjusting for baseline score, treatment arm, stratification factors, age, race and Zubrod performance status.
RESULTS
Patients
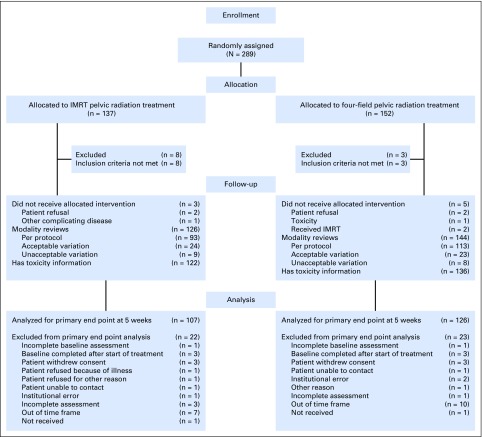
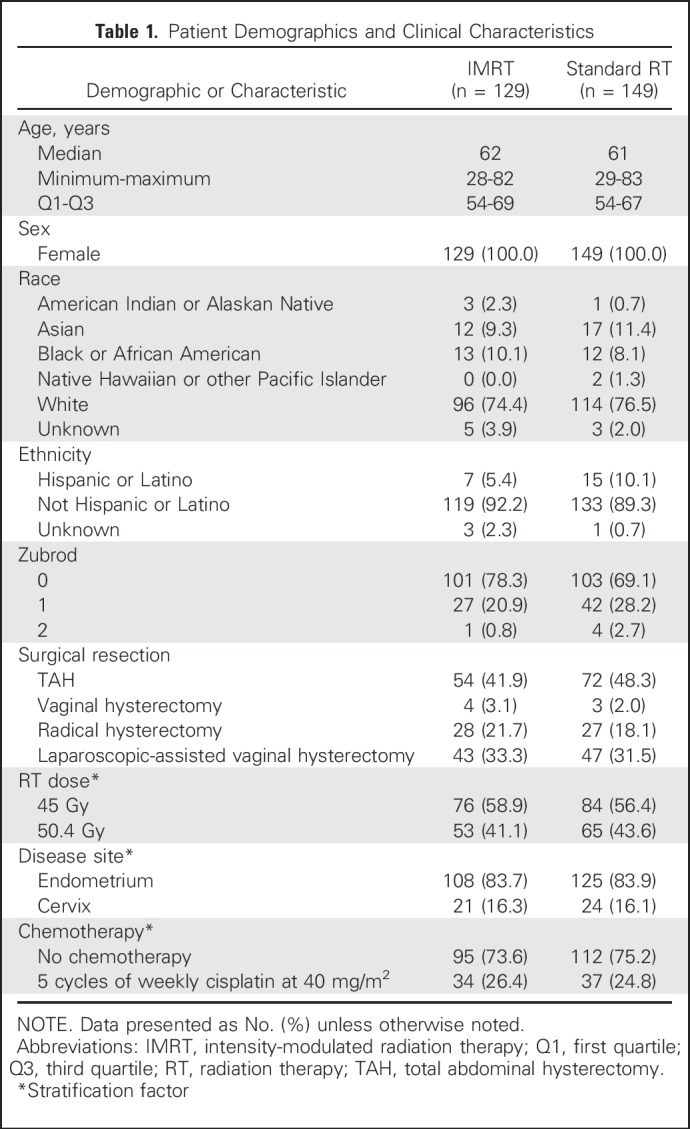
Between November 2012 and August 2015, 289 patients were enrolled; 11 were ineligible because either they did not meet specified protocol criteria regarding disease features or because the date of diagnosis or pretreatment evaluation information was not available. Of the 278 eligible patients, 129 were randomly assigned to IMRT, and 149 were randomly assigned to standard RT (Fig 1). There were no significant differences in pretreatment characteristics (Table 1). Seventy-one patients planned to receive chemotherapy (Table 1). Of the 68 patients who actually received chemotherapy (two of these patients withdrew consent), 92.6% were treated per protocol or with an acceptable deviation. For RT reviews, > 90% of patients were treated per protocol or with an acceptable deviation.
Fig 1.
CONSORT diagram. The number of participants in the primary end point analysis equals the number of participants assigned to the treatment group minus ineligible patients and the number excluded from the analysis. IMRT, intensity-modulated radiation therapy.
Table 1.
Patient Demographics and Clinical Characteristics
Patient-Reported GI Toxicity
Detailed results regarding validation of the EPIC in this population will be reported separately. Briefly, use of the EPIC bowel and urinary domains in this patient population was validated, because the results showed sensitivity to treatment, acceptable reliability for the domains and subscales, internal consistency, and agreement with both the FACT-Cx instrument and PRO-CTCAE items. The percentage of patients responding with a high score to each EPIC question is provided in Data Supplement.
The EPIC was completed by 96.7% of eligible patients at baseline, 88.1% at week 3 of RT, and 86.6% at week 5 of RT. There were no differences in pretreatment characteristics between patients who completed the EPIC compared with those who did not at weeks 3 and 5 of RT (Data Supplement). There were no baseline differences in bowel summary or subscale scores between the IMRT and standard RT groups. In both groups, mean patient-reported bowel function declined over the course of treatment (Fig 2A). The mean decreases in the EPIC bowel summary and subscale scores between baseline and both 3 weeks and 5 weeks, the primary end point, were significantly greater for patients in the standard RT group than for patients in the IMRT group (Fig 2A). The effect size at 5 weeks was 0.26 standard deviations (SDs). The standard group experienced a larger mean decline from baseline to 3 weeks as compared with the IMRT group (−18.6 v −14.0, respectively; P = .048). This difference was maintained at 5 weeks, in which the standard group had a larger mean decline in bowel score than the IMRT group (−23.6 v −18.6, respectively; P = .048) and persisted after adjusting for other variables in a model (estimate, −6.30; SD, 2.42; P = .01; Data Supplement). The differences were greatest in the bowel function subscale between the standard and IMRT groups (−21.0 v −14.5, respectively; P = .02). There was no significant difference in mean score in the bowel bother subscale between the standard and IMRT groups.
Fig 2.
Expanded Prostate Cancer Index Composite (EPIC) assessment of toxicity. Changes in EPIC (A) bowel, and (B) urinary summary scores between baseline and week 3 of radiation therapy (RT), week 5 of RT, and 4 to 6 weeks after completion of RT. Higher scores reflect better function so that greater declines are seen in patients with increased burden of symptoms. Error bars represent 95% CIs. (*) Statistically significant difference. IMRT, intensity-modulated radiation therapy.
In response to the PRO-CTCAE, during week 5 of RT, 51.9% of patients in the standard RT group as compared with 33.7% of patients in the IMRT group reported experiencing diarrhea frequently or almost constantly (Fig 3; P = .01). Furthermore, 9.3% of patients in the standard RT group, but only 1.1% of patients in the IMRT group, reported having fecal incontinence frequently or almost constantly (P = .01). This symptom interfered with daily function quite a bit or very much for 12.9% of patients in the standard RT group and 4.4% of patients in the IMRT group (P = .04). At week 5, 20.4% of women in the standard RT group were using antidiarrheal medications four or more times daily, compared with 7.8% of women in the IMRT group (P = .04).
Fig 3.
Patient-Reported Outcomes–Common Terminology Criteria for Adverse Events scores after 5 weeks of radiation treatment. High toxicity scores were considered as selection of level 4 or 5 responses with each question on a 5-point scale. These level 5 and 5 responses for each question were the following: [1] frequently or almost constantly; [2] quite a bit or very much; [3] severe or very severe. (*) Statistically significant difference. IMRT, intensity-modulated radiation therapy; RT, radiation therapy.
Patient-Reported Urinary Toxicity
Urinary function declined over the course of treatment in both treatment groups. At baseline, the mean EPIC urinary summary score was higher for patients in the standard RT group than for patients in the IMRT group (88.5 [SD, 14.4] v 86.2 [SD, 13.3]; P = .03) with significant differences in both the urinary function and urinary incontinence subscales. The mean decreases in the EPIC urinary summary score between baseline and weeks 3 and 5 were greater for patients in the standard RT group than for patients in the IMRT group (−6.0 [SD, 14.5] v −2.5 [SD, 11.3]; P = .04 for 3 weeks and −10.4 [SD, 17.5] v −5.6 [SD, 15.3]; P = .03 for 5 weeks; Fig 2B). Modeling showed a significant treatment arm effect in favor of the IMRT arm for EPIC urinary score at 5 weeks (estimate, −4.59; SD, 2.19; P = .04; Data Supplement).
Physician-Reported Toxicity
Eight patients did not receive any protocol treatment and were excluded from the physician-reported toxicity assessment. There were no grade 5 AEs and only one grade 4 AE reported in the standard RT group related to treatment (other reproductive system and breast disorders). Both groups had similar rates of grade 3 and 4 AEs related to protocol treatment: 16.4% in the IMRT group and 11.0% in the standard group (Data Supplement; P = .28). Rates were also similar between the IMRT and standard RT groups for acute grade 2 and higher GI AEs (26.2% v 22.1%; P = .43).
QOL
On the FACT-Cx, the mean score on the additional concerns relevant to cervical cancer subscale showed a decline of 4.9 points (SD, 6.5) in the standard RT group versus 2.7 points (SD, 6.1) in the IMRT group (P = .015; Table 2). There were no significant differences between the two groups in the FACT-G subscale scores or mean Trial Outcome Index score (Table 2). Descriptive statistics for the additional concerns relevant to the cervical cancer subscale are located in the Data Supplement.
Table 2.
Change in FACT-Cx Scores 5 Weeks From the Start of RT

DISCUSSION
In this randomized trial, pelvic IMRT resulted in less impact on bowel function during treatment than standard pelvic RT. Similarly, treatment with IMRT resulted in less impact on urinary function during treatment. QOL metrics demonstrated that patients treated with IMRT also had a smaller decline in physical function and additional treatment-related concerns during the course of RT. Responses on the PRO-CTCAE were consistent with EPIC, showing a greater frequency of diarrhea in the standard RT group than in the IMRT group.
This study was developed to focus on diarrhea, the most common toxicity experienced by patients during pelvic radiation. The PORTEC-2 study established that patients receiving pelvic radiation were more likely to experience diarrhea that affected their quality of life, requiring that they remain close to a toilet, resulting in a lower level of social functioning.5 After 7 years, approximately 8% of women had chronic diarrhea and 11% still experienced clinically significant fecal leakage, as compared with 1% and 3% of patients treated with vaginal cuff brachytherapy, respectively.17 In our study, acute bowel and urinary toxicity had improved by 4 to 6 weeks after completion of RT in both arms of the study, but longer-term analysis of patient-reported outcomes after IMRT or standard RT will be needed to determine if IMRT also affects chronic toxicity from pelvic radiation treatment.
Patient-reported measures of toxicity are often discordant with physician-reported toxicity, revealing more serious toxicity than providers reported.16,18 In a randomized trial of patients assessed with patient-reported measures of toxicity during chemotherapy, clinical incorporation of PROs improved health-related QOL and increased the number of cycles of chemotherapy received.19 Given the sensitivity of PROs at detecting clinically significant toxicity during pelvic RT and the potential for medical and dietary interventions to improve these symptoms, routine incorporation of PROs during RT may be an effective approach to reduce toxicity during radiation treatment.
Demonstrating the value of IMRT is especially important, given the significant additional costs and resources needed for IMRT. The use of IMRT has significantly expanded in the United States over the past decade. An analysis of the National Cancer Database demonstrated that the use of IMRT for postoperative RT in patients with uterine cancer increased from 1.9% of patients in 2004 to 32.4% of patients in 2012.20 Models analyzing the cost effectiveness of IMRT suggest IMRT becomes increasingly cost effective in the years after treatment.21 However, generation of cost-effectiveness models is highly sensitive to the estimates of toxicity between groups of patients. The results reported herein should provide more accurate information to be used in a forthcoming cost-effectiveness analysis.
Several randomized trials have compared physician-reported toxicity for patients treated with IMRT and standard RT. A randomized trial enrolling 331 women in Canada22 compared IMRT to standard RT for women with breast cancer. The study reported a significantly higher rate of moist desquamation among women receiving standard RT, and patients who developed moist desquamation were more likely to have pain and decreased QOL. In another randomized controlled trial, men with localized prostate cancer were treated with hypofractionated IMRT or standard RT. The rate of grade 2 or higher genitourinary toxicity was higher in the standard RT group than in the IMRT group (12.3% v 2.7%; P = .02).23 A third randomized trial compared standard RT with IMRT for patients with head and neck cancer. This study reported a lower incidence of grade 2 or higher xerostomia after IMRT than after standard RT (24% v 53%; P = .024).24 Each of these studies used physician-reported assessment of toxicity as the primary end point of the study, rather than patient-reported toxicity, as was used in this study.
In summary, the results from the first randomized trial, to our knowledge, comparing the impact of pelvic IMRT and standard pelvic RT on acute patient-reported toxicity have herein been reported. GI and genitourinary toxicity were higher in the standard RT group at the end of treatment, and these differences were consistent across multiple patient-reported measures of toxicity. Long-term evaluation of patients is ongoing to determine if these differences persist over time and to compare recurrence rates between the two groups.
ACKNOWLEDGMENT
The human investigations were performed after approval by a local Human Investigations Committee and in accordance with an assurance filed with and approved by the Department of Health and Human Services.
Footnotes
Supported by National Cancer Institute Grants No. U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology Statistics and Data Management Center), and UG1CA189867 (National Cancer Institute Community Oncology Research Program).
Clinical trial information: NCT01672892.
AUTHOR CONTRIBUTIONS
Conception and design: Ann H. Klopp, Karen M. Gil, Lari Wenzel, Kent Gifford, David K. Gaffney, William Small Jr, Stephanie L. Pugh, Lisa A. Kachnic, Deborah W. Bruner
Provision of study materials or patients: Shannon N. Westin, Spencer Thompson
Collection and assembly of data: Ann H. Klopp, Anamaria R. Yeung, Shannon N. Westin, William Small Jr, Spencer Thompson, Desiree E. Doncals, Amy Chang, Vijayananda Kundapur, Michael L. Haas, Yong Bae Kim, Catherine L. Ferguson, Stephanie L. Pugh, Deborah W. Bruner
Data analysis and interpretation: Ann H. Klopp, Anamaria R. Yeung, Snehal Deshmukh, Karen M. Gil, Lari Wenzel, Shannon N. Westin, David K. Gaffney, William Small Jr, Guilherme H.C. Cantuaria, Brian P. Yaremko, Dasarahally S. Mohan, Yong Bae Kim, Stephanie L. Pugh, Lisa A. Kachnic, Deborah W. Bruner
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patient-Reported Toxicity During Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology–RTOG 1203
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Ann H. Klopp
No relationship to disclose
Anamaria R. Yeung
No relationship to disclose
Snehal Deshmukh
No relationship to disclose
Karen M. Gil
No relationship to disclose
Lari Wenzel
Consulting or Advisory Role: Array BioPharma
Shannon N. Westin
Consulting or Advisory Role: Roche, AstraZeneca, Ovation Sciences, Medivation, Genentech, Vermillion, Casdin Capital, Medscape, Clovis Oncology, Watermark Research Partners, Gerson Lehrman Group, Vaniam Group, Bio Ascend, Tesaro
Research Funding: AstraZeneca, Novartis, Merck, Biomarin Pharmaceutical, GlaxoSmithKline, Karyopharm Therapeutics (I), Celgene (I), Critical Outcome Technologies, Bayer, Tesaro, Kite Pharma (I)
Kent Gifford
No relationship to disclose
David K. Gaffney
No relationship to disclose
William Small Jr
Honoraria: Carl Zeiss Meditec
Consulting or Advisory Role: Varian Medical Systems
Speakers' Bureau: Carl Zeiss Meditec
Research Funding: Carl Zeiss Meditec
Travel, Accommodations, Expenses: Carl Zeiss Meditec
Spencer Thompson
Travel, Accommodations, Expenses: Isoray
Desiree E. Doncals
No relationship to disclose
Guilherme H.C. Cantuaria
No relationship to disclose
Brian P. Yaremko
No relationship to disclose
Amy Chang
No relationship to disclose
Vijayananda Kundapur
No relationship to disclose
Dasarahally S. Mohan
Employment: The Permante Medical Group
Michael L. Haas
Consulting or Advisory Role: Novocure
Yong Bae Kim
No relationship to disclose
Catherine L. Ferguson
No relationship to disclose
Stephanie L. Pugh
Research Funding: Millennium (Inst)
Lisa A. Kachnic
Honoraria: Epic, Insys Therapeutics
Patents, Royalties, Other Intellectual Property: UpToDate
Deborah W. Bruner
No relationship to disclose
REFERENCES
- 1.Noone AM, Howlader N, Krapcho M, et al. (eds): SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/ [Google Scholar]
- 2.Rotman M, Sedlis A, Piedmonte MR, et al. : A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: Follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys 65:169-176, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Peters WA, III, Liu PY, Barrett RJ, II, et al. : Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 18:1606-1613, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Keys HM, Roberts JA, Brunetto VL, et al. : A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 92:744-751, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Nout RA, Putter H, Jürgenliemk-Schulz IM, et al. : Five-year quality of life of endometrial cancer patients treated in the randomised Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur J Cancer 48:1638-1648, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Roeske JC, Lujan A, Rotmensch J, et al. : Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys 48:1613-1621, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Ahamad A, D’Souza W, Salehpour M, et al. : Intensity-modulated radiation therapy after hysterectomy: Comparison with conventional treatment and sensitivity of the normal-tissue-sparing effect to margin size. Int J Radiat Oncol Biol Phys 62:1117-1124, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Hasselle MD, Rose BS, Kochanski JD, et al. : Clinical outcomes of intensity-modulated pelvic radiation therapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 80:1436-1445, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Gandhi AK, Sharma DN, Rath GK, et al. : Early clinical outcomes and toxicity of intensity modulated versus conventional pelvic radiation therapy for locally advanced cervix carcinoma: A prospective randomized study. Int J Radiat Oncol Biol Phys 87:542-548, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Jhingran A, Winter K, Portelance L, et al. : A phase II study of intensity modulated radiation therapy to the pelvis for postoperative patients with endometrial carcinoma: Radiation therapy oncology group trial 0418. Int J Radiat Oncol Biol Phys 84:e23-e28, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Zelen M: The randomization and stratification of patients to clinical trials. J Chronic Dis 27:365-375, 1974 [DOI] [PubMed] [Google Scholar]
- 12.Jhingran A, Salehpour M, Sam M, et al. : Vaginal motion and bladder and rectal volumes during pelvic intensity-modulated radiation therapy after hysterectomy. Int J Radiat Oncol Biol Phys 82:256-262, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Lim K, Small W, Jr, Portelance L, et al. : Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys 79:348-355, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Wei JT, Dunn RL, Litwin MS, et al. : Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 56:899-905, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Cella DF, Tulsky DS, Gray G, et al. : The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol 11:570-579, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Basch E, Dueck AC, Rogak LJ, et al. : Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol 3:1043-1050, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Boer SM, Nout RA, Jürgenliemk-Schulz IM, et al. : Long-term impact of endometrial cancer diagnosis and treatment on health-related quality of life and cancer survivorship: Results from the randomized PORTEC-2 trial. Int J Radiat Oncol Biol Phys 93:797-809, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Xiao C, Polomano R, Bruner DW: Comparison between patient-reported and clinician-observed symptoms in oncology. Cancer Nurs 36:E1-E16, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Basch E, Deal AM, Kris MG, et al. : Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 34:557-565, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osborn V, Schwartz D, Lee YC, et al. : Patterns of care of IMRT usage in postoperative management of uterine cancer. Gynecol Oncol 144:130-135, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Chen LA, Kim J, Boucher K, et al. : Toxicity and cost-effectiveness analysis of intensity modulated radiation therapy versus 3-dimensional conformal radiation therapy for postoperative treatment of gynecologic cancers. Gynecol Oncol 136:521-528, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Pignol JP, Olivotto I, Rakovitch E, et al. : A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol 26:2085-2092, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Viani GA, Viana BS, Martin JE, et al. : Intensity-modulated radiotherapy reduces toxicity with similar biochemical control compared with 3-dimensional conformal radiotherapy for prostate cancer: A randomized clinical trial. Cancer 122:2004-2011, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Ghosh-Laskar S, Yathiraj PH, Dutta D, et al. : Prospective randomized controlled trial to compare 3-dimensional conformal radiotherapy to intensity-modulated radiotherapy in head and neck squamous cell carcinoma: Long-term results. Head Neck 38:E1481-E1487, 2016. (suppl 1) [DOI] [PubMed] [Google Scholar]