Abstract
Traumatic brain injury has been associated with increased risk of Parkinson disease and parkinsonism, and parkinsonism and Lewy body disease (LBD) can occur with chronic traumatic encephalopathy (CTE). To test whether contact sports and CTE are associated with LBD, we compared deceased contact sports athletes (n = 269) to cohorts from the community (n = 164) and the Boston University Alzheimer disease (AD) Center (n = 261). Participants with CTE and LBD were more likely to have β-amyloid deposition, dementia, and parkinsonism than CTE alone (p < 0.05). Traditional and hierarchical clustering showed a similar pattern of LBD distribution in CTE compared to LBD alone that was most frequently neocortical, limbic, or brainstem. In the community-based cohort, years of contact sports play were associated with neocortical LBD (OR = 1.30 per year, p = 0.012), and in a pooled analysis a threshold of >8 years of play best predicted neocortical LBD (ROC analysis, OR = 6.24, 95% CI = 1.5–25, p = 0.011), adjusting for age, sex, and APOE ɛ4 allele status. Clinically, dementia was significantly associated with neocortical LBD, CTE stage, and AD; parkinsonism was associated with LBD pathology but not CTE stage. Contact sports participation may increase risk of developing neocortical LBD, and increased LBD frequency may partially explain extrapyramidal motor symptoms sometimes observed in CTE.
Keywords: American football, Chronic traumatic encephalopathy (CTE), Lewy body disease, Mild traumatic brain injury, Parkinson disease, Repetitive head impacts
INTRODUCTION
Mild traumatic brain injuries (TBI) sustained during contact athletics are increasingly recognized as a public health concern. An estimated 1.6–3.8 million concussions occur annually in the United States, with American football, hockey, soccer, and lacrosse accounting for the greatest incidence of sports-related concussions (1). Head impacts in contact sports that do not result in clinical symptoms (i.e. subconcussive injury) may still result in neuronal injury. In fact, years of play of a contact sport regardless of concussion history is associated with imaging abnormalities (2–5) and with the pathological stage of chronic traumatic encephalopathy (CTE) (6–9). Participants in contact sports may be at increased risk of developing neurodegenerative diseases in addition to CTE, such as amyotrophic lateral sclerosis (1,10), Parkinson disease, and parkinsonism (11). The severity and number of TBIs necessary for altering risk for neurodegenerative diseases are currently unknown.
Chronic traumatic encephalopathy is a neurodegenerative disease associated with repetitive head impacts (RHI), typically sustained through exposure to contact sports (6–9). Clinically, CTE is characterized by abnormalities in behavior (e.g. explosivity, impulsivity, physical and verbal violence, disinhibition), mood (e.g. hopelessness, suicidality, anxiety, and apathy), cognition (e.g. impairment in memory, executive function, attention and concentration, dementia), and motor function (e.g. ataxia, parkinsonism) (12). Distinguishing CTE clinically can be difficult because of its symptomatic overlap with other neurological diseases, including Alzheimer disease (AD) dementia and Parkinson disease (PD). In addition, co-morbid pathologies are common in CTE (8,13). Therefore, determining the pathologies that underlie the clinical heterogeneity in CTE is important for understanding pathogenesis and to aid clinical diagnosis and eventually target treatment.
Chronic traumatic encephalopathy is diagnosed neuropathologically by the accumulation of hyperphosphorylated and aggregated tau in neurons, astrocytes, and cell processes around small vessels and in the depths of the cerebral sulci (14). In American football players, the number of years of contact sports play significantly predicts the severity of tau pathology in the dorsolateral frontal cortex and the CTE stage (7,9). In addition, RHI may accelerate and alter the deposition and pathological progression of other neurodegenerative proteins. For example, individuals with a history of RHI and neuropathological diagnosis of CTE accumulate β-amyloid (Aβ) at a younger age and at an accelerated rate compared to a general autopsy cohort (8).
Traumatic brain injury has also been associated with the development of PD (15–19). In 2016, Crane et al found an association between TBI with loss of consciousness greater than 1 hour occurring before 25 years of age and increased risk of cortical Lewy bodies in pooled brain banks from community aging cohorts. They further found a clinical association between TBI with loss of consciousness and incident PD in 1 cohort and progression of parkinsonian signs in 2 others (11). In addition, retrospective analysis of a large veteran cohort found that a history of TBI, including mild TBI, was associated with increased risk of PD (19). To date, however, the role of RHI associated with contact sports and CTE in the development of Lewy bodies and parkinsonism is unknown.
The deposition of Lewy bodies within the central nervous system is regionally selective and appears to involve a limited number of stereotyped patterns (20,21). Lewy body disease (LBD) pathology can be classified as brainstem, limbic (transitional), neocortical (diffuse), or amygdala-predominant (21). This classification, however, may insufficiently describe the Lewy pathology for some individuals. Toledo et al, for example, assessed Lewy body pathology in 2 large cohorts of participants using hierarchical clustering to group pathological patterns and found additional patterns of deposition. Moreover, the presence of AD is associated with increased frequency and a distinct pattern of LBD, suggesting that coincident neurodegenerative disease can affect the development of LBD (22,23).
Both trauma and the presence of CTE pathology may influence α-synuclein deposition. Enhanced α-synuclein deposition may, in turn, explain the motor symptoms that sometimes occur in CTE. We hypothesized that participants with CTE have an altered regional distribution of LBD, that a history of exposure to RHI is associated with the development of neocortical LBD, and that participants with coincident CTE and LBD experience more severe clinical deterioration. We assessed the presence and distribution of Lewy body pathology in the brains of a total of 694 participants drawn from 3 distinct brain donation groups and tested associations between RHI, neuropathological diagnosis, and LBD.
MATERIALS AND METHODS
Participants
A total of 694 autopsy participants from 3 distinct brain donation groups were neuropathologically evaluated for neurodegenerative disease using previously published selection criteria and protocols (21). Of those, 269 were participants with a history of exposure to contact sports, including football, ice hockey, boxing, soccer, rugby, and martial arts at either the professional or the amateur level, recruited by the “Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE)” study at the Veteran’s Affairs-Boston University-Concussion Legacy Foundation (VA-BU-CLF) brain bank (UNITE group) (24). For a majority of brain donations, next-of-kin contacted the brain bank to donate near the time of death or following death. Other brain donors were referred by medical examiners, recruited by the Concussion Legacy Foundation, or agreed to donation during life through the Brain Donation Registry. Inclusion criteria were broad to optimize generalizability and only included a history of RHI exposure. A second group consisted of 261 participants from Boston University’s Alzheimer Disease Center (ADC) brain bank. The BU ADC enrolls and clinically follows participants with mild cognitive impairment, AD dementia, and normal cognition, where they undergo annual cognitive evaluations according to the National Alzheimer’s Disease Coordinating Center (NACC) protocol (25). A subset agrees to brain donation at the end of life; α-synuclein immunohistochemistry began routinely in 2003, and only participants who died since that time were included in this study. Finally, 164 participants were from the brain bank of the Framingham Heart Study (FHS), a community-based aging cohort that consists of the longitudinally followed participants and their offspring. FHS participants that had agreed to brain donation and whose next-of-kin had completed an extensive RHI and sports questionnaire identical to that used in the UNITE study (24) were included.
Consent for brain donation and research participation was provided by donor next-of-kin; institutional review board approval for brain donation was granted from both the Boston University Medical Center and the Edith Nourse Rogers Memorial Veterans Hospital, Bedford, MA. The Boston University Medical Center IRB also granted approval for postmortem clinical record review, neuropathological evaluation, and clinical interviews with donor family members.
Clinical Assessment
For UNITE participants, RHI history, athletic or military service history, history of cognitive, mood, and behavior changes, and clinical status leading up to death were assessed via postmortem interviews with informants and through online surveys and medical record review as described previously (24). RHI exposure was defined by the total numbers of years a contact sport was played. All interviews were conducted by neurologists and neuropsychologists trained to assess for RHI exposure and neurodegenerative diseases, and all interviews were conducted blinded to the results of the neuropathological examination. To obtain additional clinical information, medical record review was performed (16). In FHS, an identical athletic history assessment to UNITE was performed with the donor’s next-of-kin. Athletic history was not available for BU ADC participants. For all studies, the presence of dementia was determined in a subset of participants by a panel of neurologists and neuropsychologists after review of medical and study records. A diagnosis of dementia was based on DSM-IV criteria. Within the ADC and UNITE, the presence or absence of parkinsonism was determined by a panel of neurologists and neuropsychologists during life (ADC) or after death (UNITE) following review of medical and study records.
Pathological Assessment
Neuropathological processing followed the procedures previously established for the VA-BU-CLF brain bank, which included a comprehensive analysis designed to screen for neurodegenerative conditions (24,26). The neuropathological diagnosis of CTE was made using the NINDS consensus criteria for CTE that include the presence of abnormal perivascular accumulations of hyperphosphorylated tau (ptau) in neurons, astrocytes, and cell processes in an irregular and patchy distribution concentrated at the depths of cortical sulci (14). The stage of CTE, graded from stage I to IV, was based on the extent and severity of the ptau pathology as previously described (7). The diagnosis for AD was made based on National Institute of Aging (NIA) Reagan criteria and included intermediate or high probability (27); a diagnosis of AD thus required sufficient pathology to meet at least the diagnostic threshold for classification as intermediate probability. Evaluation for diffuse and neuritic plaques was by 4G8 (BioLegend, San Diego, CA) and Bielschowsky silver staining, respectively, and characterized as being present or absent. The revised NIA-Alzheimer Association AD pathological criteria (21) were not applied due to the retrospective nature and size of the study groups. Diagnoses for frontotemporal lobar degeneration and LBD were made based on accepted pathological criteria (21,28).
Lewy bodies were assessed on LHE as well as with α-synuclein immunohistochemistry within the olfactory bulb and tract, medulla, midbrain, amygdala, entorhinal cortex, anterior cingulate gyrus, and dorsolateral frontal cortex. LBD pathology was classified as brainstem-predominant, limbic (transitional), neocortical (diffuse), or amygdala-predominant (21). In addition, participants with Lewy bodies limited to the olfactory bulb were classified as olfactory-predominant. Within each region, the degree of Lewy body pathology was semi-quantitatively assessed on a scale of 0–3. Regions in which no Lewy bodies were observed were scored “0”, 1–2 Lewy bodies were scored “1”, 3–4 Lewy bodies were scored “2”, and 5 or more Lewy bodies were scored “3” per 200× magnification.
Immunohistochemistry
Human tissue was fixed in periodate-lysine-paraformaldehyde, tissue blocks were paraffin-embedded, and sections were cut at 10 µm for immunohistochemistry. Antigen retrieval for α-synuclein and β-amyloid was performed with formic acid treatment for 2 minutes. Sections were incubated overnight at 4°C with antibodies to α-synuclein (rabbit polyclonal; Chemicon, Temecula, CA; 1:15,000), phosphorylated PHF-tau (AT8; Pierce Endogen, Rockford, IL; 1:2000), Aβ (4G8; BioLegend; 1:100,000), and pTDP-43 (pS409/410 mouse monoclonal; Cosmo Bio Co, Tokyo, Japan; 1:2000). Following 3 washes with phosphate-buffered saline (pH 7.4), sections were treated with biotinylated secondary antibody and labeled with a 3-amino-9-ethylcarbazol HRP substrate kit (Vector Laboratories, Burlingame, CA). The sections were then counterstained with Gill’s hematoxylin (Vector Laboratories H-3401) and subsequently coverslipped using Permount mounting medium (Thermo Scientific, Rockford, IL).
Quantitative ELISA Measurement of α-Synuclein, Aβ, and Tau
Frozen human frontal cortex primary gray matter was weighed and placed on dry ice. Freshly prepared, ice-cold 5 M Guanidine Hydrochloride in Tris-buffered saline (20 mM Tris-HCl, 150 mM NaCl, pH 7.4 TBS) containing 1:100 Halt protease inhibitor cocktail (Thermo Scientific) and 1:100 Phosphatase inhibitor cocktail 2 & 3 (Sigma, St. Louis, MO) was added to the brain tissue at 5:1 ratio and homogenized with Qiagen Tissue Lyser LT (Qiagen Inc., Germantown, MD) at 50 Hz for 5 minutes. The homogenate was then shaken (regular rocker) overnight at room temperature. The lysate was diluted with 1% Blocker A (Meso Scale Discovery (MSD) #R93BA-4, Rockville, MD) in wash buffer in accordance with the specific ELISA assays: 1:4000 for α-synuclein (MSD #K151TGD-2), 1:300 for total tau and tau phosphorylated at threonine 231 (pTau231; MSD #K15121D-2), and 1:4000 for β-amyloids 1-40 and 1-42 (MSD #K15200E-2). Samples were subsequently spun down at 17,000 g and 4°C for 15 minutes, and the supernatant was applied to the ELISA assays. A standard sandwich ELISA was used to measure tau phosphorylated at threonine 181 (pTau181), with the capturing antibody as AT270 against pTau181 (Thermo Scientific #MN1050) and a well-characterized T46 antibody against total tau as the detecting antibody (Thermo Scientific). Sulfo-tag conjugated anti-mouse secondary antibody (MSD) was used for signal detection by the MSD platform, and an MSD SECTOR S 600 Imager was used to measure analyte levels.
Microscopic Analysis of Tau Density
A tissue block from the dorsolateral frontal cortex was taken perpendicular to the superior frontal sulcus, embedded in paraffin, and cut at 10 μm. As it is often the earliest locus of CTE pathology, a tissue block taken from the dorsolateral frontal cortex is part of the standardized protocol used to assess for CTE (14). Because of its early involvement, tau pathology in the dorsolateral frontal cortex is a sensitive measure of CTE tau density, and increased tau burden in this region often heralds involvement of other brain regions (29). Slides immunostained for tau (AT8; Pierce Endogen) were scanned at 20× magnification with a Leica Aperio Scanscope (Leica Biosystems, Richmond, IL), and neurofibrillary tangle (NFT) density was determined as previously described (9). Briefly, using ImageScope (Leica Biosystems), the gray matter was highlighted from the pia to the boundary between the white and gray matters. Leica’s image analysis and automated counting software (Aperio nuclear algorithm, Version 9, Leica Biosystems) was calibrated for shape, size, and staining intensity to detect AT8-immunoreactive NFTs within the region of interest. Counts were normalized to the area measured and are presented as density within the analyzed region.
APOE Genotyping
DNA was extracted from brain tissue samples using a Qiagen QIAamp DNA extraction kit (Qiagen, Valencia, CA). Two single nucleotide polymorphisms (National Center for Biotechnology Information SNPs rs429358 and rs7412) were examined using TaqMan assays (Applied Biosystems, Foster City, CA). Allelic discrimination was automated using the manufacturer’s software. Positive controls, consisting of DNA for each APOE allele, were included on each plate and genotyped with restriction isotyping.
Statistical Methodology
Statistical analysis was performed with SPSS version 20.0 (IBM Corp, Armonk, NY), Prism v6 (GraphPad Software, La Jolla, CA), and SAS version 9.4 (SAS Institute Inc., Cary, NC). A chi-square test for proportions was used to assess differences between cohorts with respect to demographic and neuropathological characteristics and between CTE pathology groups with respect to clinical and exposure measures and frequency of LBD type. As age is a known driver of neurodegenerative pathology and no participants age <50 years old were found to have Lewy bodies or AD, comparisons between CTE pathology groups were restricted to participants with age at death >50 years in Table 2. We used a 2-sample chi-square test to assess the frequency of Lewy body pathology between brain bank groups and between groups with coincident pathology, using Bonferroni correction to account for multiple comparisons. To evaluate levels of α-synuclein, Aβ1-40, Aβ1-42, pTau231, pTau181, and the density of NFTs between groups, we applied an ANOVA with post-hoc Bonferroni multiple comparisons testing. Although variation in these measures is not uniformly normal, ANOVA is robust to the non-normality of errors (30).
TABLE 2.
Clinical and Exposure Measures Between CTE Pathology Groups
| CTE | CTE-LBD | CTE-AD-LBD | p Value | |
|---|---|---|---|---|
| Sample size (n) | 86 | 36 | 17 | – |
| Age of First Exposure | 12.6 (0.3) | 12.2 (0.5) | 12.2 (0.9) | 0.777 |
| Years of Exposure | 16.5 (0.7) | 15.9 (1.1) | 15.7 (2.3) | 0.856 |
| Age at Death | 70.1 (1.1) | 73.5 (1.6) | 77.2 (2.6) | 0.020 |
| CTE Symptom Duration | 19.1 (1.8) | 14.9 (1.7) | 23.9 (7.2) | 0.169 |
| Symptom Latency | 38.7 (1.8) | 43.8 (2.3) | 35.1 (6.5) | 0.226 |
| CTE Stage | 3.13 (0.10) | 3.00 (0.17) | 3.44 (0.30) | 0.350 |
| Diffuse Aβ Plaques | 47.8% | 82.8% | 100% | <0.001*,†,‡ |
| Neuritic Aβ Plaques | 34.3% | 58.6% | 100% | <0.001*,‡,§ |
| Dementia | 61.4% | 91.4% | 100% | <0.001*,†,‡ |
| Parkinsonism | 19.5% | 60.0% | 30.8% | <0.001*,† |
| APOE ɛ4 presence ± (%) | 28/53 (34.6%) | 15/19 (44.1%) | 7/8 (46.7%) | 0.495* |
Numbers are presented as mean (SEM); subjects <50 years-old excluded.
χ2 test for proportions.
p < 0.05 for comparison between CTE and CTE-LBD.
p < 0.05 for comparison between CTE and CTE-AD-LBD.
p < 0.05 for comparison between CTE-LBD and CTE-AD-LBD.
We used unbiased hierarchical clustering to assess the similarity between participants in their regional distribution of Lewy body pathology. Using this method, all participants with LBD semiquantitative scores in the selected regions of interest, irrespective of cohort or pathology coincident with LBD, were compared to one another; participants with greater similarity in regional distribution of Lewy bodies aligned (i.e. clustered) more closely, and participants branched increasingly farther apart from one another had increasing dissimilarity in their regional Lewy body patterning. Binary logistic regression analyses were applied to test associations between predictors (age, sex, Apolipoprotein E (APOE) ɛ4, contact sports exposure, and pathology) and the outcomes of neocortical Lewy body pathology, dementia, and parkinsonism. Pooled analyses were performed controlling for cohort and the interaction between cohort and contact sports play where indicated.
RESULTS
Participant groups varied by demographics, RHI exposure, and pathology at death (Table 1). On average, UNITE participants were considerably younger with a greater proportion of males than the FHS or ADC cohorts. As expected, participants in the UNITE group also had a significantly greater number of years of exposure to contact sports compared to the FHS cohort (contact sports history was not available for ADC). Percentages listed in Table 1 reflect the percent of individuals within each cohort with pathology that meets the threshold diagnostic criteria for each disease. The frequency of pathology varied between cohorts as expected with the greatest percentage of pathological control (no significant pathology identified) participants in the FHS cohort, the greatest percentage of participants with CTE pathology in the UNITE cohort, and the greatest percentage of participants with a diagnosis of AD in the ADC cohort. Across groups, all participants with CTE had a history of contact sports play. The frequency of LBD was significantly different between cohorts with the greatest frequency in the ADC cohort (Table 1). Although many (n = 86) had CTE, no participants <50 years old had LBD. Therefore, participants with age of death >50 years old with CTE were stratified by the presence of Lewy body and AD pathology in Table 2. Between CTE, CTE-LBD, and CTE-AD-LBD there were no significant differences in the age they first started playing contact sports (age of first exposure), years of exposure to RHI, CTE symptom duration, duration between age of first exposure to contact sports and symptom onset (symptom latency), or CTE stage; age at death did significantly differ between groups (p = 0.020). CTE-LBD and CTE-AD-LBD participants were significantly more likely to have diffuse Aβ plaques and dementia than CTE participants without LBD (p < 0.05), and CTE-AD-LBD participants were significantly more likely to have neuritic Aβ plaques and parkinsonism than CTE participants (p < 0.05; Table 2).
TABLE 1.
Demographic and Neuropathological Characteristics of Study Groups
| FHS | UNITE | ADC | p Value | |
|---|---|---|---|---|
| Sample size (n) | 164 | 269 | 261 | — |
| Age at Death, yrs (SEM) | 87.2 (0.8) | 57.4 (1.3) | 83.3 (0.7) | <0.001,‖,‖|,‖‖ |
| Range | 57–105 | 14–98 | 34–111 | |
| Sex, male/female (%) | 71/93 (43.3) | 268/1 (99.6) | 174/87 (66.7) | <0.001¶,‖,‖|,‖‖ |
| Contact sports exposure* | ||||
| Yes/no (%) | 19/145 (11.6) | 269/0 (100) | – | <0.001¶ |
| Football, n (%) | 9 (47.7) | 227 (84.4) | ||
| Hockey, n (%) | 9 (47.7) | 14 (5.2) | ||
| Other†, n (%) | 1 (5.3) | 28 (10.4) | ||
| Total years, mean (SEM) | 6.6 (0.9) | 15.1 (0.4)‡ | <0.001 | |
| Contact sports exposure >8 years, n (%) | 2 (1.2) | 189 (86.7)‡ | <0.001¶,‖ | |
| LBD, any, n (%) | 51 (31.1) | 54 (20.2) | 115 (44.1) | <0.001¶,‖,‖|,‖‖ |
| LBD, neocortical, n (%) | 12 (7.3) | 18 (6.7) | 34 (13.0) | 0.028¶,‖| |
| AD, n (%) | 66 (40.2) | 36 (13.2) | 187 (72.2) | <0.001¶‖,‖|,‖‖ |
| CTE, n (%) | 1 (0.6) | 217 (81.3) | 7 (3.2)§ | <0.001¶,‖,‖| |
| FTLD, n (%) | 18 (11.0) | 13 (4.9) | 32 (12.4) | 0.008¶,‖| |
| Control, n (%) | 45 (27.4) | 49 (18.2) | 16 (6.1) | <0.001¶,‖|,‖‖ |
| APOE ɛ4 presence ± (%) | 36/116 (23.7) | 87/164 (34.7) | 35/32 (52.2) | <0.001¶,‖|,‖‖ |
Control participants did not meet threshold for major pathological diagnosis. Mean years of exposure are for those subjects with contact sports exposure > 0. Contact sports exposure data was not available for ADC participants.
FHS: Framingham Heart Study; UNITE: Understanding Neurologic Injury and Traumatic Encephalopathy; ADC: Alzheimer’s Disease Center; LBD: Lewy body disease; AD: Alzheimer disease; CTE: Chronic Traumatic Encephalopathy; FTLD: Frontotemporal Lobar Degeneration.
sport type defined as sport played at highest level.
includes boxing, rugby, mixed martial arts, and soccer.
out of 218 with documented exposure years.
out of 222 evaluated.
χ2 test for proportions.
p-value < 0.05 between FHS and UNITE.
p < 0.05 between UNITE and ADC.
p < 0.05 between FHS and ADC groups.
Association Between RHI Exposure and Neocortical LBD
Because the physical strain following mild TBI and the tau pathology of CTE are both primarily cortical (7,31), we hypothesized that LBD associated with RHI would likewise be cortical. To test the hypothesis that the number of years of exposure to RHI was associated with the development of neocortical LBD, a binary logistic regression was performed on FHS and UNITE participants with both APOE genotype and data on contact sports play. In each model, we included age, sex, APOE ɛ4 allele frequency, cohort, and an interaction term between cohort and contact sports play interaction for the calculation of cohort-specific odds ratio (Table 3). There was sufficient overlap in age at death between groups to justify including age in the model (Supplementary DataFigure S1). Neocortical LBD was predicted by years of exposure to contact sports with an FHS specific OR = 1.30 (p = 0.012) and a UNITE specific OR = 1.15 (p = 0.013). A pooled analysis of the FHS and UNITE brain banks showed that years of exposure to contact sports (OR = 1.07, p = 0.014) and age at death (OR = 1.05, p = 0.005) significantly predicted the development of neocortical LBD, adjusting for APOE ɛ4 and sex.
TABLE 3.
Contact Sports Play as a Predictor of Neocortical Lewy Body Disease in the FHS, UNITE, and Combined Brain Banks
| FHS (n = 149) |
UNITE (n = 204) |
Combined (n = 353) |
||||
|---|---|---|---|---|---|---|
| Predictors | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Years of contact sports play | 1.30 (1.06–1.60) | 0.012 | 1.15 (1.03–1.28) | 0.013 | 1.07 (1.01–1.13) | 0.014 |
| Contact sports play >8 years | 40.0 (1.75–912) | 0.028 | 1.71 (0.20–14.9) | 0.627 | 6.24 (1.53–25.4) | 0.011 |
FHS: Framingham Heart Study; UNITE: Understanding Neurologic Injury and Traumatic Encephalopathy; participants limited to those with APOE genotype data. Cohort-specific and pooled analyses adjusting for age, sex, and APOE ɛ4.
To investigate the strength of the association between contact sports and LBD, sensitivity analyses were performed. Because the mean years of contact sports exposure was much higher in UNITE (15.1 years) and the effect of contact sports play was greater in FHS than UNITE, we hypothesized that there may be a threshold lower level of contact sports exposure. A receiver operating curve analysis showed the highest C-statistic at a threshold of 8 years of contact sports play. When this threshold (>8 years vs <8 years of contact sports play) was used as a binary variable for exposure in the logistic regression model, neocortical LBD was significantly associated with higher exposure (>8 years) of contact sports play (OR = 40.0, p = 0.028) in the FHS cohort, but not in the UNITE group (OR = 1.71, p = 0.627), adjusting for age, sex, and APOE ɛ4. However, the confidence intervals are wide, likely due to sparse data. A pooled analysis demonstrated that contact sports play >8 years (OR = 6.24, p = 0.011) and age (OR = 1.06, p = 0.003) were significantly associated with the development of neocortical LBD, adjusting for sex and APOE ɛ4 (Table 3). To test whether the associations were present when only American football was considered, we performed the pooled analysis on football players and participants without contact sports history and obtained similar results. Separate analyses for brainstem, limbic, and amygdala-predominant LBD did not reveal any significant associations with years of contact sports play (data not shown).
Associations Between Coincident Pathology and Lewy Body Distribution
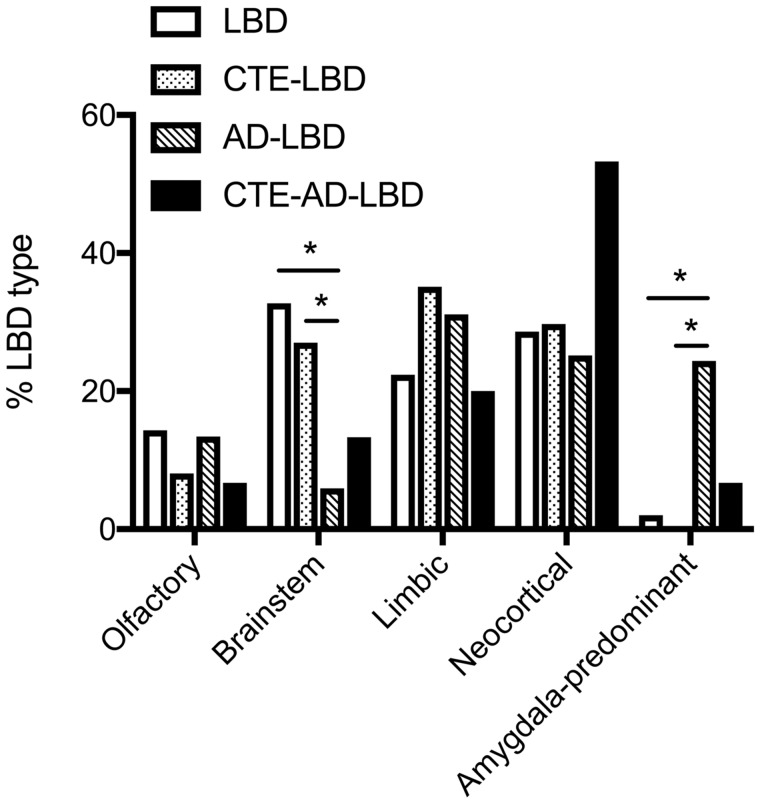
We next compared the regional distribution of Lewy bodies between pathological subject groups. Comparing participants with LBD, CTE-LBD, AD-LBD, and CTE-AD-LBD, we found that the pattern of Lewy body deposition proportioned significantly differently between pathological groups (χ2 = 45.6, p < 0.001) such that participants with LBD or CTE-LBD had a greater frequency of brainstem LBD and a lower frequency of amygdala-predominant LBD than AD-LBD (p < 0.05). Although participants with CTE-AD-LBD had a nominally greater frequency of neocortical LBD (53%) than the other groups, no significant difference was observed after Bonferroni multiple comparison correction (χ2 = 4.1, p = 0.129 for adjusted comparison to AD-LBD; Fig. 1).
FIGURE 1.
Lewy body disease type is altered in participants with CTE (CTE-LBD) compared to AD (AD-LBD). The distribution of LBD type within each pathological group is shown. A chi-squared test between participants with Lewy body disease (LBD), CTE with LBD (CTE-LBD), AD with LBD (AD-LBD), and CTE with AD and LBD (CTE-AD-LBD) shows a significantly different distribution of LBD type between pathological groups (χ2 = 45.6, p < 0.001). Participants with LBD alone or with CTE-LBD have significantly more brainstem LBD and significantly less amygdala-predominant LBD than AD-LBD (*p < 0.05 adjusted with the Bonferroni method for multiple comparisons). CTE-AD-LBD participants have a greater frequency of neocortical LBD than the other groups, but this difference did not achieve significance (χ2 = 4.1, p = 0.129 for adjusted comparison to AD-LBD).
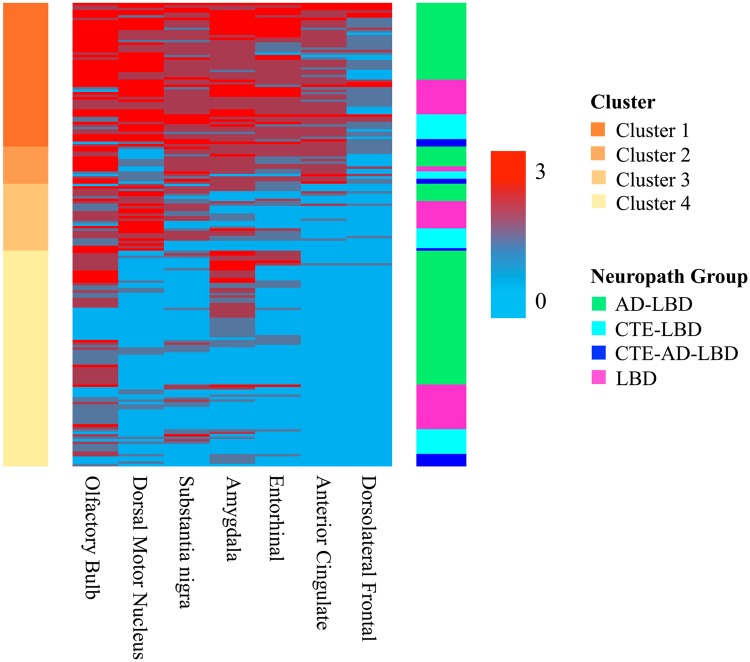
To further test whether pathology was associated with a unique distribution of Lewy bodies, we performed unbiased hierarchical clustering on all participants with LBD. A similar analysis was recently performed on LBD participants with and without AD and yielded 4 main clusters that roughly corresponded with LBD type (22). Our data likewise generated 4 distinct clusters (Fig. 2). Cluster 1 featured the greatest LBD burden and was largely comprised of participants with Lewy bodies in all regions studied, including the neocortex. In Cluster 2, LBD was found prominently in the olfactory bulb, substantia nigra, amygdala, entorhinal cortex, and anterior cingulate with relative sparing of the dorsal motor nucleus of the vagus nerve. Cluster 3 was comprised of participants with Lewy body pathology concentrated in the olfactory bulb, dorsal motor nucleus of the vagus nerve, and substantia nigra with little pathology in the amygdala, entorhinal cortex, anterior cingulate, or dorsolateral frontal cortex. In Cluster 4, LBD was largely restricted to the olfactory bulb and amygdala. Each cluster was named to reflect the pathological patterning of Lewy bodies within that cluster: Cluster 1 was labeled “neocortical,” Cluster 2 “limbic transitional,” Cluster 3 “brainstem,” and Cluster 4 “olfactory bulb/amygdala.”
FIGURE 2.
Regional distribution of Lewy body deposition by unsupervised cluster analysis. A cluster heatmap shows the Lewy body pathology distribution within clusters. The column on the left indicates the cluster identity, and the column on the right represents the pathological diagnoses. The color scale indicates the semiquantitative severity of Lewy body pathology.
The distribution of pathological groups was compared between clusters (Table 4). No significant differences were observed in regional distribution of Lewy bodies between LBD participants and CTE-LBD (χ2 = 1.08, p = 0.781). Participants with AD-LBD had a significantly different distribution of LBD from CTE-LBD (χ2 = 9.69, p = 0.021) and LBD only (χ2 = 9.40, p = 0.024) such that a greater proportion of participants with AD-LBD clustered into the olfactory bulb/amygdala cluster in comparison to either control-LBD or CTE-LBD. Participants with CTE-AD-LBD often grouped in the olfactory/amygdala cluster but were not significantly different from LBD (χ2 = 3.41, p = 0.332), AD-LBD (χ2 = 1.38, p = 0.710), or CTE-LBD (χ2 = 2.01, p = 0.570). LBD-only participants most commonly clustered into either the neocortical (31%) or the olfactory bulb/amygdala (40%) clusters with lesser representation in the limbic (5%) and brainstem (24%) clusters. Participants with either AD-LBD or CTE-AD-LBD had greatest representation in the olfactory bulb/amygdala cluster, accounting for 54% and 46% of participants in each pathology group, respectively. Only 8% and 7% of AD-LBD participants clustered into the limbic and brainstem clusters, respectively, as did 18% and 9% of participants with CTE-AD-LBD. The frequency of CTE-LBD in the neocortical and olfactory bulb/amygdala clusters was equivalent at 32% each, with 10% and 26% in the limbic and brainstem clusters, respectively. Examined within each cluster, participants with CTE-LBD have nearly uniformly a lower average age at death than participants in the other pathology groups. Overall, while LBD and CTE-LBD clustered similarly to one another, they clustered distinctly from AD-LBD.
TABLE 4.
Distribution and Mean Age of Unsupervised Clusters for Each Pathological Group
| Cluster | LBD |
CTE-LBD |
AD-LBD |
CTE-AD-LBD |
||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Age | n (%) | Age | n (%) | Age | n (%) | Age | |
| 1 (neocortical) | 14 (31) | 82.1 ± 2.2 | 10 (32) | 75.6 ± 2.4 | 31 (31) | 85.7 ± 2.0 | 3 (27) | 77.3 ± 4.5 |
| 2 (limbic) | 2 (5) | 79.0 ± 3.0 | 3 (10) | 75.7 ± 1.8 | 8 (8) | 82.0 ± 1.9 | 2 (18) | 82.0 ± 2.0 |
| 3 (brainstem) | 11 (24) | 88.1 ± 2.5 | 8 (26) | 70.3 ± 4.2 | 7 (7) | 89.6 ± 2.0 | 1 (9) | 97.9 |
| 4 (olfactory/ amygdala) | 18 (40) | 87.6 ± 2.1 | 10 (32) | 72.1 ± 4.1 | 54 (54) | 84.0 ± 1.3 | 5 (46) | 70.6 ± 1.9 |
Age is presented as mean ± SEM. Percentages are given for within each pathological group.
LBD-Associated Alterations in Neurodegenerative Proteins
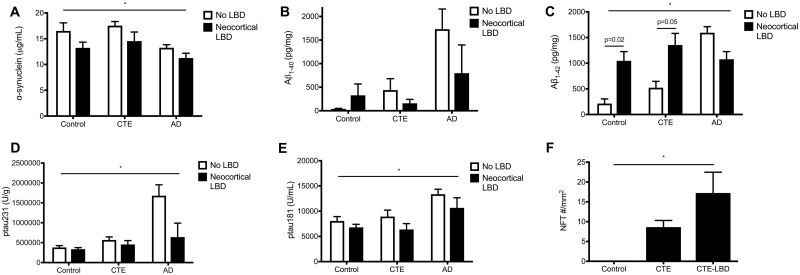
Quantitative ELISA was used to measure α-synuclein, Aβ1-40, Aβ1-42, pTau231, and pTau181 in the dorsolateral frontal cortex of control, AD, and CTE participants without LBD and with neocortical LBD. Levels of α-synuclein varied significantly by pathology (p = 0.025) and by the presence of neocortical LBD (p = 0.018, ANOVA) such that the presence of neocortical LBD was associated with reduced total α-synuclein in all pathology groups (Fig. 3A). As expected, AD participants had the most Aβ1-40; however, no significant difference was found in Aβ1-40 levels between participants without LBD and those with neocortical LBD within any of the pathological groups (Fig. 3B). Levels of Aβ1-42 varied significantly by pathology (p = 0.009) and by the presence of neocortical LBD (p = 0.040, ANOVA), and Sidak post-hoc analyses demonstrated that participants with neocortical LBD had significantly higher levels compared to participants without LBD in the control (p = 0.02) and CTE (p = 0.05) pathology groups. In contrast, participants with neocortical LBD and coincident AD had a non-significant decrease in concentration of Aβ1-42 in comparison to participants with AD but without LBD (Fig. 3C). Levels of pTau231 were different between pathological groups (p = 0.032, ANOVA) with increased levels in AD, but did not significantly differ with the presence of LBD (Fig. 3D). Similarly, levels of pTau181 were different between pathological groups with increased levels in AD (p = 0.041, ANOVA) but not by LBD (Fig. 3E). On the other hand, when the density of AT8-immunopositive NFTs was determined for control, CTE, and CTE-LBD participants, NFT density was increased in CTE and increased further in CTE with neocortical LBD (p = 0.038, ANOVA). Multiple comparison testing (Dunnett) showed increased NFT density in CTE-LBD compared to control participants (p = 0.035; Fig. 3F).
FIGURE 3.
Comparison of neurodegenerative protein density between pathological groups. Between participants with neocortical LBD and without LBD: (A) α-synuclein levels varied significantly between pathological groups (control, CTE, or AD; p = 0.025) and by the presence of neocortical LBD (p = 0.018, ANOVA). (B) Aβ1-40 levels were not significantly different by pathological group or neocortical LBD. (C) Aβ1-42 levels varied significantly by pathology (p = 0.009) and by the presence of neocortical LBD (p = 0.040, ANOVA). Multiple comparison testing (Bonferroni) showed significantly increased Aβ1-42 in neocortical LBD compared to participants without LBD for both controls (p = 0.02) and CTE (p = 0.05). (D) Levels of pTau231 were different between pathological groups with increased levels in AD (p = 0.032) but did not differ with LBD. (E) Levels of pTau181 (U/mL) were different between pathological groups with increased levels in AD (p = 0.041) but did not differ with LBD. (F) The density of neurofibrillary tangles (NFT) by AT8 immunostaining was increased in CTE and further increased in CTE with neocortical LBD compared to controls (p = 0.038, ANOVA).
Clinical Associations With Pathology Groups
We tested the relative roles of AD, LBD, and CTE pathology as predictors of dementia or parkinsonism. All FHS and UNITE participants with neocortical LBD had dementia, and all FHS and ADC participants with CTE had dementia; therefore, these variables were not included in the model of dementia within these cohorts. A binary logistic regression demonstrated that AD was significantly associated with dementia in the FHS (OR = 5.95, p < 0.001) and ADC (OR = 33.4, p < 0.001) studies, adjusting for age and sex. In UNITE, dementia was significantly associated with CTE stage (OR = 1.41 per stage, p = 0.019), adjusting for age at death (OR = 1.09, p < 0.001), sex, and AD. In a pooled analysis, dementia was significantly associated with neocortical LBD (OR = 12.1, p < 0.001), AD (OR = 9.69, p < 0.001), and CTE stage (OR = 1.65 per stage, p < 0.001), adjusting for age at death (OR = 1.05, p < 0.001), sex, and cohort (Table 5).
TABLE 5.
Predictors of Dementia in the FHS, UNITE, ADC, and Combined Brain Banks
| Predictors | FHS* (n = 164) |
ADC* (n = 167) |
UNITE* (n = 251) |
Combined† (n = 582) |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| LBD, neocortical‡ | – | – | 1.37 (0.28–6.70) | 0.699 | – | – | 12.1 (3.38–43.5) | <0.001 |
| AD | 5.95 (2.89–12.3) | <0.001 | 33.4 (10.0–111) | <0.001 | 2.41 (0.75–7.79) | 0.142 | 9.69 (5.57–16.8) | <0.001 |
| CTE stage (0-IV)‡ | – | – | – | – | 1.41 (1.06–1.88) | 0.019 | 1.65 (1.38–1.98) | <0.001 |
FHS: Framingham Heart Study; UNITE: Understanding Neurologic Injury and Traumatic Encephalopathy; ADC: Alzheimer’s Disease Center.
Adjusting for age and sex.
Adjusting for age, sex, and cohort.
All participants with neocortical LBD in FHS and UNITE and with CTE in FHS and ADC had dementia, and therefore these variables were not included in the model for those cohorts (-).
The presence of parkinsonism was assessed in a subset of the ADC and UNITE participants. Binary logistic regression analysis showed that in the ADC cohort, parkinsonism tended to be associated with LBD (any; OR = 2.11, p = 0.094), adjusting for age, sex, and AD. In UNITE, parkinsonism was associated with LBD (OR = 3.44, p = 0.001), but not CTE stage, adjusting for age and AD. In a pooled analysis, parkinsonism was again significantly associated with LBD (OR = 2.82, p < 0.001), but not CTE or AD, adjusting for age, sex, and cohort (Table 6). Overall, this suggests that extrapyramidal motor symptoms are predicted by LBD and not CTE pathology.
TABLE 6.
Predictors of Parkinsonism in the ADC, UNITE, and Combined Brain Banks
| Predictors | ADC* (n = 104) |
UNITE* (n = 246) |
Combined† (n = 350) |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| LBD, any | 2.11 (0.88–5.06) | 0.094 | 3.44 (1.68–7.06) | 0.001 | 2.82 (1.63–4.89) | <0.001 |
| CTE stage (0–IV) | – | – | 0.92 (0.69–1.22) | 0.550 | 0.83 (0.65–1.07) | 0.152 |
| AD | 0.64 (0.25–1.65) | 0.356 | 1.36 (0.58–3.17) | 0.478 | 0.94 (0.50–1.77) | 0.848 |
ADC: Alzheimer’s Disease Center; UNITE: Understanding Neurologic Injury and Traumatic Encephalopathy.
Adjusting for age and sex.
Adjusting for age, sex, and cohort.
DISCUSSION
We examined the clinical and pathological relationships between RHI exposure, CTE, and LBD within a group of deceased athletes with RHI exposure (UNITE), a community-based aging cohort (FHS), and a cohort enriched for AD from the BU ADC. The number of years of exposure to RHI through contact sports play was associated with the development of neocortical LBD in the FHS cohort, the UNITE cohort, and in a pooled analysis controlling for age, sex, and APOE ɛ4 allele status. Among participants with CTE and LBD, the distribution of Lewy bodies was most likely to be limbic, neocortical, or brainstem, while among participants with co-morbid AD and LBD, the distribution of Lewy bodies was most likely to be limbic, neocortical, or amygdala-predominant. Among participants with CTE, neocortical LBD was associated with increased Aβ1-42 and increased tau pathology. Clinically, across studies dementia was significantly associated with neocortical LBD, CTE stage, AD, and age at death. Parkinsonism was significantly associated with LBD and age at death, but not CTE stage. Overall, the increased frequency of LBD following RHI exposure may partially explain the extrapyramidal motor symptoms sometimes observed in CTE.
Head injuries can occur with a variety of mechanisms and severity. A recent study demonstrated that a history of a single TBI with loss of consciousness (LOC) >1 hour was associated with increased risk of incident PD and a single TBI with LOC of any duration was associated with the progression of parkinsonian signs (11). Pathologically, a single TBI with LOC >1 hour was associated with an increased risk of Lewy bodies in the frontal and temporal cortical regions. In contrast, head impacts experienced in contact sports are typically repetitive, less severe, and without LOC. In fact, the number of years of play of contact sports, not concussion history, best predicts CTE stage and the severity of tau pathology in the frontal cortex (6,7,9). However, similar to the findings of TBI with LOC in other brain donation cohorts, we found that years of exposure to RHI through contact sports play was significantly associated with an increase in the odds of having neocortical LBD.
Interestingly, effects were different between a community-based aging population (FHS) and the UNITE cohort. All participants in the UNITE cohort had a history of RHI, potentially diminishing its effect due to uniform exposure and range restriction (32), while relatively few FHS participants had this history. That years of RHI exposure had a greater association with neocortical LBD in FHS than in UNITE suggests that the threshold of RHI exposure necessary to increase the risk of neocortical LBD is lower than the typical exposure duration in UNITE (∼15 years). Receiver operating curve analysis showed the highest C-statistic at a threshold of 8 years of contact sports play, and binary logistic regression for pooled FHS and UNITE groups demonstrated that athletes with higher exposure (>8 years) of contact sports play were significantly more likely to have neocortical LBD than study participants with less exposure when adjusting for age, APOE ɛ4, and sex.
Patterns of Lewy Body Deposition
Contrary to our initial hypothesis, the pattern of LBD deposition was not significantly different between LBD-CTE and LBD without CTE or AD. LBD-CTE participants were significantly more likely to have brainstem LBD and less likely to have amygdala-predominant LBD than AD with LBD. Similar results were found when we performed unbiased hierarchical clustering to examine the regional distribution of Lewy body pathology in LBD, AD, and CTE. We found clusters analogous to those found in a previous analysis of LBD and AD (22) and that roughly corresponded to neocortical, limbic transitional, brainstem, and olfactory bulb/amygdala. Thus, Lewy body pathology appears to involve similar regions in CTE as in LBD alone. It remains to be determined, however, whether regions not evaluated in this study (e.g. other cortical regions and mammillary bodies) are specifically affected in CTE. In addition, because our clustering analysis did not account for variance in age, the equivalent Lewy body distribution that we observe between clusters is only descriptive of the endpoint, not the progression. Indeed, within each cluster, participants with CTE-LBD are nearly uniformly younger at death compared to participants with other pathology.
LBD and Pathology-Related Changes in Neurodegenerative Proteins
Levels of neurodegenerative proteins were significantly altered in frontal cortex in the presence of AD, CTE, or neocortical LBD pathology. α-synuclein is normally abundant in the presynaptic compartment (33,34), and we found decreased α-synuclein levels in participants with neocortical LBD, which may reflect synaptic dysfunction or loss (35–37). Previous studies have shown that CSF α-synuclein levels are diminished in participants with LBD compared to controls (38,39). Participants with neocortical LBD and with coincident neocortical LBD and CTE showed a marked increase in Aβ1-42 in comparison to control and CTE participants without neocortical LBD, respectively. In AD, there was no significant difference in Aβ1-42 levels with or without LBD, perhaps due to a ceiling effect from already high levels or because neocortical LBD lowers the threshold of AD pathology necessary to progress towards dementia and death (40). Overall, these data confirm previous reports demonstrating associations between LBD and increased Aβ (41).
The severity of tau pathology in the dorsolateral frontal cortex varied by pathological group but not by the presence of neocortical LBD. Quantitation of AT8-positive NFTs demonstrated significantly increased NFT density in CTE and CTE-LBD compared to controls. Levels of tau phosphorylated at sites 231 or 181 showed increased levels in AD but no significant difference between groups with or without neocortical LBD.
Associations With Clinical Outcomes
Recently, studies of a large clinical veterans cohort showed an association between mild TBI and both dementia and PD (42,43). Here we show that the pathologies present at death were significant predictors of dementia or parkinsonism. In a pooled analysis, dementia was significantly associated with neocortical LBD and stage of CTE in addition to AD pathology and age at death. Parkinsonism was significantly associated with LBD of any type but not with CTE stage. These results suggest that the previously reported association between CTE and aberrant extrapyramidal motor function (12,44,45) may be due to co-morbid LBD pathology.
It remains to be determined what pathophysiologic changes underlie other motor aberrancies (e.g. ataxia) observed in CTE that differ from the extrapyramidal motor symptoms studied here. For example, it has been noted that the frequency of motor features in professional boxers exceeds that observed in professional football players, which may be the result of more severe cerebellar pathology (12). Further study into how the biomechanics of head trauma and the associated pathologies differ between sports may offer further explanation for the broad array of motor symptoms that can be observed in CTE. In addition, although our recent study did not show an association with age of first exposure to contact sports and the development of LBD, the age of first exposure may modify the presentation of symptoms (46).
Limitations
There are several limitations inherent to our study population and methodology. The athlete participants for the UNITE study were largely self-selected or referred by next-of-kin after death and do not represent all individuals who play contact sports or sustain RHI. Although the FHS brain donation cohort was recruited from the larger FHS community-based study, autopsy-based selection bias is still present, as evidenced by the high rate of neurodegenerative disease. However, the FHS cohort is likely more representative of contact sports play in the general population. The 3 cohorts varied significantly in mean age at death and sex frequency, which we attempted to adjust for in our regression analyses. Although the 3 cohorts were distinct, data was carefully harmonized. Neuropathological evaluation was performed for all 3 studies by the same experienced neuropathologists using standard research protocols, and dementia and parkinsonism diagnoses were obtained by expert clinicians using research-quality guidelines. Methods for determination of RHI exposure were standardized across FHS and UNITE; however, they depended on retrospective review and may have introduced bias. Confidence intervals on the odds ratio were wide for the 8 years of play threshold due to sparse data, which can inflate estimates, and therefore the specific ORs are likely not accurate (47). Future studies incorporating more participants with neocortical LBD and contact sports play will be necessary to more accurately determine the best threshold and risk of contact sports participation. In addition, studies utilizing prospective clinical data are necessary to confirm and expand associations with RHI and the clinical timeline of symptoms.
Conclusions
Lewy body distribution was not significantly altered by co-morbid CTE pathology; however, participants with CTE-LBD had a younger mean age of death than LBD alone. We found the number of years an individual was exposed to RHI through contact sports was associated with the development of neocortical LBD, and LBD, in turn, was associated with parkinsonism and dementia. In pooled analyses, stage of CTE was independently associated with the presence of dementia, but not parkinsonism, when controlling for LBD, suggesting that co-morbid LBD largely accounts for extrapyramidal motor symptoms in CTE.
Supplementary Material
ACKNOWLEDGMENTS
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We gratefully acknowledge the use of resources and facilities at the Edith Nourse Rogers Memorial Veterans Hospital (Bedford, MA) as well as all the individuals whose participation and contributions made this work possible.
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Clinical Sciences Research and Development Merit Award (I01-CX001038); Veterans Affairs Biorepository (BX002466); Alzheimer’s Association (NIRG-305779, NIRG-362697); National Institute of Aging (RF1AG054156, R56AG057768, K23AG046377, R01AG016495, R01AG008122, R01AG033040); National Institute of Neurological Disorders and Stroke (U01NS086659, F32NS096803, R01NS017950); National Institute of Aging Boston University AD Center (P30AG13846; supplement 0572063345-5); Department of Defense Peer Reviewed Alzheimer’s Research Program (PRARP #13267017); National Heart, Lung, and Blood Institute, Framingham Heart Study (N01-HC-25195, HHSN268201500001I); Concussion Legacy Foundation. IM receives funding as a Fonds de Recherche du Québec—Santé (FRQS) postdoctoral scholar. This work was also supported by unrestricted gifts from the Andlinger Foundation and WWE.
The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Guerriero RM, Proctor MR, Mannix R, et al. Epidemiology, trends, assessment and management of sport-related concussion in United States high schools. Curr Opin Pediatr 2012;24:696–701 [DOI] [PubMed] [Google Scholar]
- 2. Singh R, Meier TB, Kuplicki R, et al. Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA 2014;311:1883–8 [DOI] [PubMed] [Google Scholar]
- 3. Koerte IK, Lin AP, Muehlmann M, et al. Altered neurochemistry in former professional soccer players without a history of concussion. J Neurotrauma 2015;32:1287–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poole VN, Abbas K, Shenk TE, et al. MR spectroscopic evidence of brain injury in the non-diagnosed collision sport athlete. Dev Neuropsychol 2014;39:459–73 [DOI] [PubMed] [Google Scholar]
- 5. Nauman EA, Breedlove KM, Breedlove EL, et al. Post-season neurophysiological deficits assessed by ImPACT and fMRI in athletes competing in American football. Dev Neuropsychol 2015;40:85–91 [DOI] [PubMed] [Google Scholar]
- 6. Stein TD, Alvarez VE, McKee AC.. Concussion in chronic traumatic encephalopathy. Curr Pain Headache Rep 2015;19:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136:43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stein TD, Montenigro PH, Alvarez VE, et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol 2015;130:21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cherry JD, Tripodis Y, Alvarez VE, et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun 2016;4:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lehman EJ, Hein MJ, Baron SL, et al. Neurodegenerative causes of death among retired National Football League players. Neurology 2012;79:1970–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crane PK, Gibbons LE, Dams-O'Connor K, et al. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol 2016;73:1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: Literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther 2014;6:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 2017;318:360–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016;131:75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gardner RC, Burke JF, Nettiksimmons J, et al. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol 2015;77:987–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: A meta-analysis. J Neurosurg 2016;124:511–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee P-C, Bordelon Y, Bronstein J, et al. Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology 2012;79:2061–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jafari S, Etminan M, Aminzadeh F, et al. Head injury and risk of Parkinson disease: A systematic review and meta-analysis. Mov Disord 2013;28:1222–9 [DOI] [PubMed] [Google Scholar]
- 19. Gardner RC, Byers AL, Barnes DE, et al. Mild TBI and risk of Parkinson disease. Neurology 2018;90:e1771–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211 [DOI] [PubMed] [Google Scholar]
- 21. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 2011;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toledo JB, Gopal P, Raible K, et al. Pathological α-synuclein distribution in subjects with coincident Alzheimer’s and Lewy body pathology. Acta Neuropathol 2016;131:393–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uchikado H, Lin W-L, DeLucia MW, et al. Alzheimer disease with amygdala Lewy bodies: A distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol 2006;65:685–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mez J, Solomon TM, Daneshvar DH, et al. Assessing clinicopathological correlation in chronic traumatic encephalopathy: Rationale and methods for the UNITE study. Alzheimers Res Ther 2015;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: The uniform data set. Alzheimer Dis Assoc Disord 2007;21:249–58 [DOI] [PubMed] [Google Scholar]
- 26. Vonsattel J-PG, Del Amaya MP, Keller CE.. Twenty-first century brain banking. Processing brains for research: The Columbia University methods. Acta Neuropathol 2008;115:509–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hyman BT, Trojanowski JQ.. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 1997;56:1095–7 [DOI] [PubMed] [Google Scholar]
- 28. Mackenzie IRA, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol 2010;119:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Darby A, Adams JW, Babcock K, et al. Detection of CTE in autopsy cohorts using restricted cortical sampling. J Neuropathol Exp Neurol 2015;74:588–639 [Google Scholar]
- 30. Pearson ES. The analysis of variance in cases of non-normal variation. Biometrika 1931;23:114–33 [Google Scholar]
- 31. Ghajari M, Hellyer PJ, Sharp DJ.. Computational modelling of traumatic brain injury predicts the location of chronic traumatic encephalopathy pathology. Brain 2017;140:333–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rose G. Sick individuals and sick populations. Int J Epidemiol 2001;30:427–32 [DOI] [PubMed] [Google Scholar]
- 33. Burré J, Sharma M, Tsetsenis T, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010;329:1663–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Logan T, Bendor J, Toupin C, et al. α-Synuclein promotes dilation of the exocytotic fusion pore. Nat Neurosci 2017;20:681–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vallortigara J, Whitfield D, Quelch W, et al. Decreased levels of VAMP2 and monomeric alpha-synuclein correlate with duration of dementia. J Alzheimers Dis 2016;50:101–10 [DOI] [PubMed] [Google Scholar]
- 36. Mukaetova-Ladinska EB, Andras A, Milne J, et al. Synaptic proteins and choline acetyltransferase loss in visual cortex in dementia with Lewy bodies. J Neuropathol Exp Neurol 2013;72:53–60 [DOI] [PubMed] [Google Scholar]
- 37. Campbell BCV, Li Q-X, Culvenor JG, et al. Accumulation of insoluble α-synuclein in dementia with lewy bodies. Neurobiol Dis 2000;7:192–200 [DOI] [PubMed] [Google Scholar]
- 38. Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, et al. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: A cohort study. Lancet Neurol 2011;10:230–40 [DOI] [PubMed] [Google Scholar]
- 39. Toledo JB, Korff A, Shaw LM, et al. CSF α-synuclein improves diagnostic and prognostic performance of CSF tau and Aβ in Alzheimer’s disease. Acta Neuropathol 2013;126:683–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Postupna N, Keene CD, Crane PK, et al. Cerebral cortical Aβ42 and PHF-τ in 325 consecutive brain autopsies stratified by diagnosis, location, and APOE. J Neuropathol Exp Neurol 2015;74:100–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colom-Cadena M, Grau-Rivera O, Planellas L, et al. Regional overlap of pathologies in lewy body disorders. J Neuropathol Exp Neurol 2017;76:216–24 [DOI] [PubMed] [Google Scholar]
- 42. Barnes DE, Byers AL, Gardner RC, et al. Association of mild traumatic brain injury with and without loss of consciousness with dementia in US Military veterans. JAMA Neurol 2018. doi: 10.1001/jamaneurol.2018.0815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gardner RC, Burke JF, Nettiksimmons J, et al. Dementia risk after traumatic brain injury vs nonbrain trauma: The role of age and severity. JAMA Neurol 2014;71:14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013;81:1122–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mawdsley C, Ferguson FR.. Neurological disease in boxers. Lancet 1963;2:795–801 [DOI] [PubMed] [Google Scholar]
- 46. Alosco ML, Mez J, Tripodis Y, et al. Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann Neurol 2018;83:886–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Greenland S, Mansournia MA, Altman DG.. Sparse data bias: A problem hiding in plain sight. BMJ 2016;352:i1981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.