Abstract
Objective
The purpose of this investigation was to compare the effect of alprazolam and melatonin on oxidative stress, glicocalyx integrity and neurocognitive function in patients undergoing coronary artery bypass grafting (CABG).
Methods
Overall, 42 patients undergoing CABG were retrospectively included in this study. Blood samples which preserved at −70°C for a previous study were used for this study. The participants were divided into two groups. Patients in the Group A were administered alprazolam before the operation, whereas melatonin was used for premedication in the Group M. Blood samples were collected at three time points [T0: before anaesthesia induction, T1: admittance to intensive care unit (ICU), T2: 24 h after ICU admission], and oxidative stress parameters and glicocalyx integrity were evaluated. Furthermore, Mini-Mental State Examination was recorded to measure neurocognitive function.
Results
The total thiol levels which were measured as an antioxidant parameter were significantly higher, and free Hb values were significantly lower in the Group M compared to the Group A (p<0.05). No significant differences were found in order to oxidative stress parameter levels, extubation time, length of hospital stay, durations of cross-clamp, cardiopulmonary bypass and operation and Mini-Mental State Examination results between the two groups (p>0.05).
Conclusion
In light of positive effects on oxidatif stress parameters, melatonin may be considered as a good and safe premedication agent with its anxiolytic, antioxidant and minimal haemodynamic and respiratory effects.
Keywords: Oxidative stress, premedication, coronary artery bypass grafting
Introduction
Cardiovascular diseases associated with atherosclerosis are the most important cause of deaths worldwide (1). Coronary artery bypass grafting (CABG) surgery is an important treatment modality that is reported to be effective in the treatment of severe angina that cannot be treated with medical and percutaneous coronary intervention (2). In addition to non-physiological haemodynamic conditions that occur during CABG surgery, an apparent increase is observed in inflammatory response and oxidative stress biomarkers due to surgical trauma, cardiopulmonary bypass (CPB) and organ reperfusion injury (3–6). Oxygen free radicals, which develop as a result of this process, have an important role in the occurrence of cell damage; this cell damage has been found to be directly associated with postoperative complications and mortality (7, 8). Therefore, recent studies have focused on the use of antioxidant medications.
Benzodiazepines are the most popular drugs that are used as premedication for providing anxiolysis, amnesia and sedation. Alprazolam is a potent benzodiazepine with a low risk of drug abuse and addiction owing to the fast onset of its activation and short action time (9). Another drug that is popularly used as premedication is melatonin (N-acetyl-5-methoxy tryptamine). Melatonin is a hormone that is synthesised from tryptophan and secreted from the pituitary gland with a circadian rhythm. It easily passes through the cell membranes owing to its high lipophilicity and acts in all subcellular compartments including the mitochondria (10). It has sedative, anxiolytic, analgesia and anti-inflammatory features. It also functions as an effective antioxidant with its regulatory effect on antioxidant enzymes and as a free oxygen radical scavenger (11, 12).
Exposure to surgical stress leads to autonomic, visceral and immunological responses, thus causing oxidative stress and neurochemical and hormonal abnormalities. Various drugs can be used for premedication to suppress these responses. The superiority of anxiolytics over each other, which are used for premedication, has not been demonstrated. The aim of the present study was to compare the effects of alprazolam and melatonin, which are administered as premedication in open heart surgery, systemic oxidative stress, glycocalyx integrity and postoperative neurocognitive functions.
Methods
The ethical committee of Acıbadem Mehmet Ali Aydınlar University (ATADEK-2018-1/11) approved the study. A total of 42 patients who underwent open heart surgery between February 2016 and April 2016 and administered alprazolam or melatonin for premedication were included in this retrospectively designed study. Plasma samples that had been previously extracted for a clinical research investigating postoperative cognitive functions and stored at −70°C in our clinic were used in the present study. Patients who qualified for the protocol of the previous study were divided into two groups. The melatonin group (Group M) (n=21) consisted of patients who were given a total of 6 mg melatonin, with 3 mg for 24 h before surgery and 3 mg in the morning of the operation day. On the other hand, the alprazolam group (Group A) (n=21) composed of patients who were orally given 0.5 mg alprazolam for 24 h before surgery. In the morning of the operation day, both groups were intramuscularly administered 0.06 mg kg−1 midazolam for 30 min before surgery. Blood samples extracted from the patients who were applied standard anaesthesia protocol for the evaluation of oxidative stress (T0: before induction, T1: at admission to the intensive care and T2: at postoperative 24 h) were analysed in terms of ischaemic modified albumin (IMA), advanced oxidative protein products (AOPPs), total thiol (T-SH), free haemoglobin (fHb) and sialic acid values. In order to assess the preoperative and postoperative neurocognitive states of the patients, the Mini-Mental State Examination was applied, and the obtained data were evaluated. Moreover, durations of intubation, cross-clamping, bypass, operation and hospitalisation were obtained from the files of the patients.
Protein oxidation
The modified form of the method developed by Hanasand et al. (13) was used for the measurement of AOPP levels with the spectrophotometric method. The concentration of AOPP was expressed as μmol L−1.
Total thiol
The spectrophotometric method was used for the measurement of T-SH levels. The concentration of T-SH was expressed as μmol L−1.
Free haemoglobin
The Harboe method (14) was used for the measurement of fHb levels. The concentration of fHb was expressed as g L−1.
Ischaemic modified albumin
The Bar-Or method (15) was used for the measurement of IMA levels. The concentration of IMA was expressed as ABS unit.
Sialic acid
The Sydow method (16) was used for the measurement of sialic acid levels. The concentration of sialic acid was expressed as mg mL−1.
Statistical analysis
The G-Power analysis software was used to determine sample size. The power of the study was found to be at least 80%. Blood sialic acid level was accepted as the primary outcome, and its percentage change was pre-determined at 20%. Data sets of the groups were expressed as mean±standard deviation after testing the convenience for normal distribution (Kolmogorov–Smirnov test). The repeated ANOVA test (post hoc Bonferroni) was used to compare the same group at different time points. The unpaired t-test was used to compare different groups at the same time point. GraphPad Prism 6.0 was used for statistical analysis. A p value <0.05 was accepted as statistically significant.
Results
There was no significant difference between the groups in terms of demographic data (Table 1). T-SH levels, which are markers of endogenous defence response against oxidative stress, were found to be significantly higher than basal levels in the melatonin group and in the alprazolam group at T1 time point (p<0.05) (Figure 1). FHb levels, which are parameters of oxidative stress, were higher at T1 time point than at T0 time point in both the alprazolam group and the melatonin group and were also lower in the melatonin group than in the alprazolam group at T1 time point (p<0.05) (Figure 2). There was no statistically significant difference between the groups at any time point in terms of sialic acid, AOPP and IMA levels. Furthermore, there was no significant difference between the groups with regard to the durations of intubation, hospitalisation, cross-clamping, bypass and operation and the results of the mini-mental test (MMT) (Table 2).
Table 1.
Demographic data
| Group A | Group M | p | |
|---|---|---|---|
| Age | 66.1±9.2 | 68.8±9.4 | 0.458 |
| Gender (F/M) | 8/13 | 8/13 | 1.000 |
| Height (cm) | 163.9±9.6 | 166.8±11.5 | 0.373 |
| Weight (kg) | 79.6±17.9 | 79.9±16.9 | 0.950 |
| BSA (m2) | 1.9±0.5 | 1.9±0.0 | 0.768 |
Values are given as mean±SD. BSA: body surface area; F: female; M: male
Figure 1.
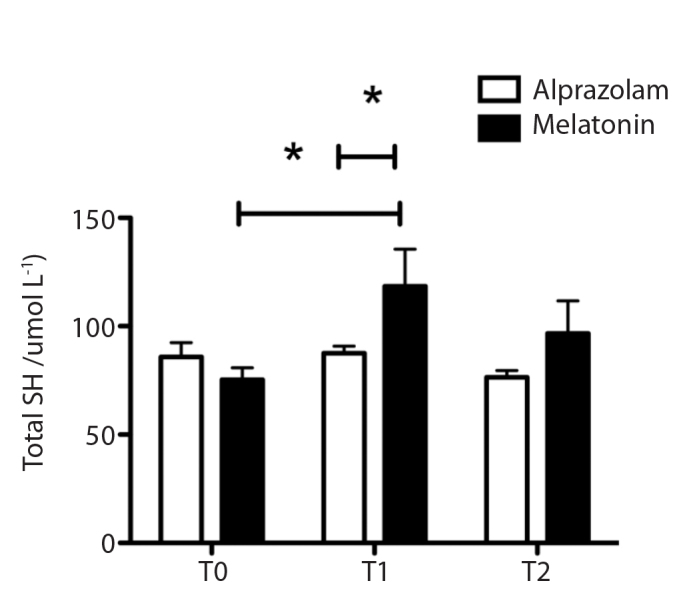
T-SH levels at time points
Figure 2.
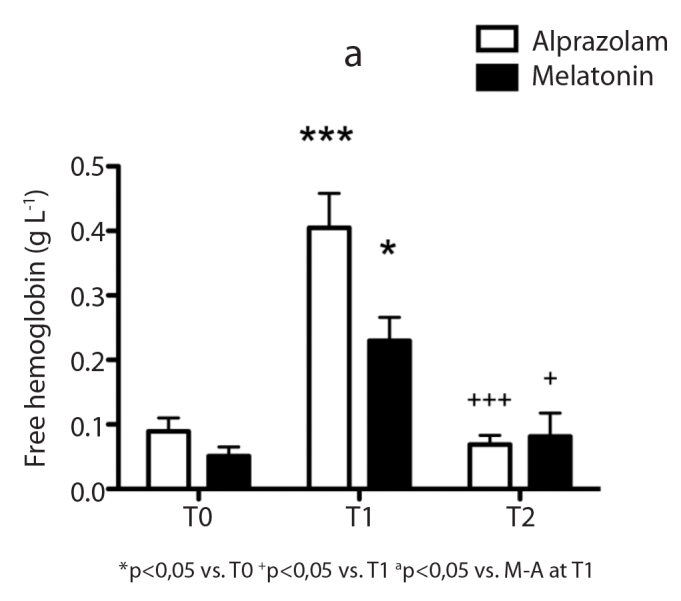
Free haemoglobin levels at time points
Table 2.
Statistical evaluation of parameters for the two groups
| Group A | Group M | p | |
|---|---|---|---|
| Duration of intubation (min) | 591,2±243,2 | 587,9±223,0 | 0,963 |
| Duration of stay at the intensive care (h) | 28,3±12,4 | 29,4±12,1 | 0,774 |
| Duration of hospitalisation (day) | 9,0±4,8 | 10,6±3,7 | 0,214 |
| Duration of cross-clamping (min) | 47,3±15,8 | 56,6±25,4 | 0,167 |
| Duration of CPB (min) | 82±24,4 | 134,7±184,5 | 0,202 |
| Duration of operation (min) | 209,4±29,8 | 213±38,0 | 0,767 |
| Preoperative MMT | 24,4±3,2 | 23,0±4,7 | 0,292 |
| MMT at discharge from the intensive care | 23,3±3,9 | 22,6±4,3 | 0,598 |
| MMT at discharge from the hospital | 23,8±3,4 | 23,4±5,4 | 0,796 |
Values are given as mean±SD. CPB: cardiopulmonary bypass; MMT: minimental test
Discussion
Melatonin is a good anxiolytic that possesses anti-inflammatory and analgesia properties, has a minimal effect on haemodynamics and respiration and reduces oxidant damage (11, 12). Oxidative stress observed during and after coronary artery bypass surgery is a condition that can cause harmful results to the patient. Myocardial ischaemia–reperfusion injury (17, 18), contact of the blood with non-physiological surfaces during CPB (19) and anxiety are the factors that contribute to the occurrence of oxidative stress. It is known that local homoeostasis is impaired as a result of ischaemia–reperfusion injury, and cell death occurs with apoptosis and necrosis, leading to many clinical problems (20). Therefore, the use of premedication agents, which are known to decrease oxidant damage, and the monitorisation of oxidative stress/antioxidant mechanism markers can be positive for the patient. While many markers are used in the literature, the parameters of AOPP, sialic acid, IMA and fHb levels, which we evaluated in our study, are quite valuable for the demonstration of oxidative stress. On the other hand, T-SH level also provides useful data as a major determinant of the antioxidant defence mechanism.
In association with increased oxidative stress level developing despite developments in anaesthesia and postoperative care in cases of open heart surgery, research for a treatment modality that will decrease oxidant response and improve antioxidant defence mechanism is becoming more popular every day. Melatonin is a molecule that is used for this purpose, that can take oxidative stress under control over different mechanisms and the effectiveness of which has been demonstrated in many studies (11, 21, 22). This molecule creates the desired effect as a free oxygen radical scavenger by performing antioxidant enzyme induction and pro-oxidant enzyme inhibition and increasing the effectiveness of mitochondrial oxidative phosphorylation (23). The antioxidant characteristic of alprazolam has also been emphasised in some studies. However, these studies have been conducted mostly on animals, and only one study has investigated the antioxidant characteristic of alprazolam in humans, but its effect could not be demonstrated (24). In our study, an apparent increase in T-SH levels, which showed endogenous response to oxidative stress, and a decrease in fHb levels, which were an oxidant damage marker, were observed in the melatonin group. Compared to the baseline values, increased fHb levels at T1 time point were evaluated to be associated with surgical stress. On the other hand, there was no significant difference at any time point for other oxidative stress markers. In a study performed on patients undergoing CABG, which supports our results, it was demonstrated that the use of melatonin leads to the activation of ‘nuclear factor-like 2’, the main regulatory molecule of the antioxidant defence system, and can be used owing to its positive effects on ischaemia–reperfusion damage (25).
Neuropsychiatric disorders, such as anxiety, delirium, cognitive dysfunction and sleep disturbance, are the important causes of morbidities that cannot be prevented despite developments in surgery and anaesthesia and that can affect long-term quality of life after CABG. The effects of drugs used for premedication are not clear. However, some studies have revealed a relationship between melatonin secretion and delirium, intensive care psychosis and behavioural and sleep disorders, and it has been observed that melatonin is an endogenous regulator for sleep pattern in patients with cardiac bypass (26, 27). There are some studies that showed that the sensitivity of the use of only MMT is low for the demonstration of cognitive dysfunction after heart surgery (28), and it is also known that some factors, such as educational level and age, affect the test results. In our study, MMT was used to evaluate neurocognitive functions, but there was no significant difference between the two groups. We believe that the application of MMT and the evaluation of its results might be insufficient for obtaining data related to neurocognitive functions because our study was designed retrospectively, and it was impossible to determine patient population.
The present study has some limitations. One was the low number of patients. The retrospective design of the study was a restrictive factor for the determination of patient population. Moreover, data on the dose of melatonin for premedication are inadequate, and the dose required to reduce surgery-associated oxidative stress has been found to be 10 mg kg−1 (29, 30). The dose of melatonin used in our study was quite low. It might have been insufficient, and other oxidative stress parameters might have been unaffected due to this reason. Another issue is that melatonin level is influenced by various factors. Benzodiazepines, opioids and beta blockers are known to affect melatonin secretion (26, 27), but their effects on exogenous melatonin are unknown. Therefore, these drugs that are frequently used in patients undergoing CABG have a confusing effect on the interpretation of study results.
Conclusion
Melatonin is a good agent for premedication considering its anxiolytic and antioxidant features and its safe effects on haemodynamics and respiration. It is known that the secretion of melatonin decreases in patients with coronary artery disease (31), and even this condition can form a basis for the use of melatonin for premedication. Since it can contribute to long-term quality of life and decreased morbidity, we believe that further studies should be performed on more patients at different doses.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Acibadem Mehmet Ali Aydinlar University (ATADEK-2018-1/11).
Informed Consent: Due to the retrospective design of the study, informed consent was not taken.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - F.T., H.U., I.G.; Design - F.T., M.G.C., U.A.; Supervision - F.T., M.G.C., U.A.; Resources - M.T., H.U., G.K., K.V.; Materials - M.T., I.G.; Data Collection and/or Processing - M.G.C., H.U., I.G., M.T.; Analysis and/or Interpretation - M.G.C., M.T., G.K., K.V.; Literature Search - M.T., H.U., G.K., K.V.; Writing Manuscript - F.T., M.G.C.; Critical Review -F.T., M.G.C., U.A.; Other - U.A., H.U., M.T., G.K., K.V.
Conflict of Interest: Authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ruff CT, Braunwald E. The evolving epidemiology of acute coronary syndromes. Nat Rev Cardiol. 2011;8:140–7. doi: 10.1038/nrcardio.2010.199. [DOI] [PubMed] [Google Scholar]
- 2.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2012;143:4–34. doi: 10.1016/j.jtcvs.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflamattory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72. doi: 10.1161/01.CIR.0000034509.14906.AE. [DOI] [PubMed] [Google Scholar]
- 4.Meng QH, Zhu S, Sohn N, Mycyk T, Shaw SA, Dalshaug G, et al. Release of cardiac biochemical and inflammatory markers in patients on cardiopulmonary bypass undergoing coronary artery bypass grafting. J Card Surg. 2008;23:681–7. doi: 10.1111/j.1540-8191.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 5.Schulze MB, Rimm EB, Li T, Rifai N, Stampfer MJ, Hu FB. C-reactive protein and incident cardiovascular events among men with diabetes. Diabetes Care. 2004;27:889–94. doi: 10.2337/diacare.27.4.889. [DOI] [PubMed] [Google Scholar]
- 6.Del Aguila LF, Claffey KP, Kirwan JP. TNF-alpha impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am J Physiol. 1992;276:849–55. doi: 10.1152/ajpendo.1999.276.5.E849. [DOI] [PubMed] [Google Scholar]
- 7.Quaniers JM, Leruth J, Alber a, Limet RR, Defraigne JO. Comparision of inflammatory responses after off-pump and on-pump coronary surgery using surface modifying additives circuit. Ann Thorac Surg. 2006;81:1683–90. doi: 10.1016/j.athoracsur.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 8.Hennein HA, Ebba H, Rodriguez JL, Merrick SH, Keith FM, Bronstein MH, et al. Relationship of the proinflammatory cytokines to myocardial ischemia and dysfunction after uncomplicated coronary revascularization. Thorac Cardiovasc Surg. 1994;108:626–35. [PubMed] [Google Scholar]
- 9.Verster JC, Volkerts ER. Clinical pharmacology, clinical efficacy, and behavioral toxicity of alprazolam: A review of the literature. CNS Drug Rev. 2004;10:45–76. doi: 10.1111/j.1527-3458.2004.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menendez-Pelaez A, Reiter RJ. Distribution of melatonin in mammalian tissues: the relative importance of nuclear versus cytosolic localization. J Pineal Res. 1993;15:59–69. doi: 10.1111/j.1600-079X.1993.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 11.Reiter J, Tan DX, Manchester LC. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001;34:247–56. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- 12.Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, et al. Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res. 2003;34:75–8. doi: 10.1034/j.1600-079X.2003.02111.x. [DOI] [PubMed] [Google Scholar]
- 13.Hanasand M, Omdal R, Norheim KB. Improved detection of advanced oxidation protein products in plasma. Clin Chim Acta. 2012;413:901–6. doi: 10.1016/j.cca.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 14.Harboe M. A method for determination of hemoglobin in plasma by near-ultraviolet spectrophotometry. Scand J Clin Lab Invest. 1959;11:66–70. doi: 10.3109/00365515909060410. [DOI] [PubMed] [Google Scholar]
- 15.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia- a preliminary report. J Emerg Med. 2000;19:311–5. doi: 10.1016/S0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 16.Sydow G. A simplified quick method for determination of sialic acid in serum. Biomed Biochim Acta. 1985;44:1721–3. [PubMed] [Google Scholar]
- 17.Grech ED, Dodd NJF, Jackson MJ, Marrison WL, Faragher EB, Ramsdale DR. Evidence for free radical generation after primary percutaneous transluminal coronary angioplasty recanalization in acute myocardial infarction. Am J Cardiol. 1996;77:122–7. doi: 10.1016/S0002-9149(96)90580-9. [DOI] [PubMed] [Google Scholar]
- 18.Kato K, Shao Q, Elimban V, Lukas A, Dhalla NS. Mechanism of depression in cardiac sarcolemmal Na+ - K+ -ATPase by hypochlorous acid. Am J Physiol. 1998;275:826–31. doi: 10.1152/ajpcell.1998.275.3.C826. [DOI] [PubMed] [Google Scholar]
- 19.Eiselt J, Racek J, Opatrny K., Jr Paired filtration dialysis and free radicals. Cas Lek Cesk. 2001;140:238–41. [PubMed] [Google Scholar]
- 20.Aivaditi C, Vourliotakis G, Georgopoulos S. Oxidative stress during abdominal aortic aneurysm repair biomarkers and antioxidant’s protective effect: a review. Eur Rev Med Pharmacol Sci. 2011;15:245–52. [PubMed] [Google Scholar]
- 21.Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, Livrea MA. The chemistry of melatonin’s interaction with reactive species. J Pineal Res. 2003;34:1–10. doi: 10.1034/j.1600-079X.2003.02112.x. [DOI] [PubMed] [Google Scholar]
- 22.Hardeland R, Poeggeler B, Niebergall R, Zelosko V. Oxidation of melatonin by carbonate radicals and chemiluminescence emitted during pyrrole ring cleavage. J Pineal Res. 2003;34:17–25. doi: 10.1034/j.1600-079X.2003.02941.x. [DOI] [PubMed] [Google Scholar]
- 23.Reiter RJ, Tan X, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress: a review. J Biomed Sci. 2000;7:444–58. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 24.Güner Can M, Ilgaz Koçyiğit Ö, Aksu U, Özer A, Toraman F. Effect of Alprazolam on Redox Status in Renal Transplantation Donors and Recipients. Ann Transplant. 2017;22:354–60. doi: 10.12659/AOT.903695. [DOI] [PubMed] [Google Scholar]
- 25.Haghjooy Javanmard S, Ziaei A, Ziaei S, Ziaei E, Mirmohammad-Sadeghi M. The effect of preoperative melatonin on nuclear erythroid 2-related factor 2 activation in patients undergoing coronary artery bypass grafting surgery. Oxid Med Cell Longev. 2013 doi: 10.1155/2013/676829. ID: 676829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin YQ, Luo AL, Guo XY, Li LH, Huang YG. Postoperative neuropsychological change and its underlying mechanism in patients undergoing coronary artery bypass grafting. Chin Med J (Engl) 2007;120:1951–7. [PubMed] [Google Scholar]
- 27.Yin YQ, Luo AL, Guo XY, Li LH, Ren HZ, Ye TH, et al. Perioperative melatonin circadian secretion in patients undergoing coronary artery bypass grafting surgery. Zhonghua Yixue Zazhi. 2004;84:456–9. [PubMed] [Google Scholar]
- 28.Burker EJ, Blumenthal JA, Feldman M, Thyrum E, Mahanna E, White W, et al. The Mini Mental State Exam as a predictor of neuropsychological functioning after cardiac surgery. Int J Psychiatry Med. 1995;25:263–76. doi: 10.2190/VDMB-RJV7-M7UK-YYKG. [DOI] [PubMed] [Google Scholar]
- 29.Gitto E, Romeo C, Reiter RJ, Impellizzeri P, Pesce S, Basile M, et al. Melatonin reduces oxidative stress in surgical neonates. J Pediatr Surg. 2004;39:184–9. doi: 10.1016/j.jpedsurg.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 30.De Seabra MLV, Bignotto M, Pinto LR, Tufik S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J Pineal Res. 2000;29:193–200. doi: 10.1034/j.1600-0633.2002.290401.x. [DOI] [PubMed] [Google Scholar]
- 31.Guo X, Kuzumi E, Charman SC, Vuylsteke A. Perioperative melatonin secretion in patients undergoing coronary artery bypass grafting. Anesth and Analg. 2002;94:1085–91. doi: 10.1097/00000539-200205000-00006. [DOI] [PubMed] [Google Scholar]


