Abstract
Background/Objective
Hospitalized older patients are at increased risk of disability, which often includes restriction in community mobility. The study objective was to identify trajectories of recovery of community mobility among acutely ill older patients using the UAB Life-Space Assessment (LSA). Knowledge about recovery can inform therapies aimed toward preserving functional independence following hospitalization.
Design
Prospective observation cohort study.
Setting
Central Alabama, Birmingham VA Medical Center.
Participants
173 community-dwelling older adults, ≥ 65 years of age, hospitalized for non-surgical medical reasons.
Measurements
To determine how patients recover functional independence in community mobility after hospitalization, we collected LSA scores for the month before and monthly for six months after hospitalization (composite scores range from 0–120 with 120 reflecting completely unrestricted mobility).
Results
In the month after hospitalization, 92/173 (53%) patients had a clinically significant decrease in life-space mobility, while 42/173 (24%) were unchanged and 39/173 (23%) improved when compared to the month preceding hospitalization. Of patients with a life-space decrease, the majority recovered their pre-hospitalization mobility status during six months of follow-up, while 34% did not recover. Patients that decreased in life-space were hospitalized significantly longer (P=0.01) and, on average, had higher pre-hospital life-space scores (P=0.01) compared to patients that maintained or improved their baseline score.
Conclusion
A clinically significant loss of community mobility was common after hospitalization, but most patients recovered to pre-hospitalization mobility within six months of discharge. Research examining in-hospital and post-hospitalization interventions for allowing faster recovery of community mobility is needed.
Keywords: Life-space assessment, Mobility, Hospitalization, Older adult, Walking
INTRODUCTION
Hospitalization among older patients is associated with loss of functional independence, which often includes clinically significant restrictions in activities of daily living (ADL)1–9 and community mobility.10,11 Patient recovery from hospital-associated disability is an important investigative focus in geriatrics research.
Our understanding of post-hospitalization ADL recovery has been increasing for decades. Work by Covinsky et al. showed that among hospitalized older patients who develop ADL difficulty, only 29% recover to baseline function by discharge.3 Other studies showed that up to one-third of patients discharged with ADL disability recover to baseline function within 3 months of hospitalization, while for the majority of patients recovery can take as long as 12 months.5,6,12 Gill et al. showed that the likelihood for improvement or recovery of ADL function after hospitalization was most likely among non-frail patients with mild post-hospitalization disability.13 In sum, these findings clearly illustrate that recovery of independence in self-care after hospitalization is achievable for many but not all older patients.
There remains a gap in knowledge about post-hospital recovery of older adults. Post-hospitalization physical function encompasses a person’s ability to move about within his or her community as well as ADL ability. Prior evidence indicates that acute care medical patients, on average, experience a clinically meaningful decrease in mobility, as measured by the UAB Life-Space Assessment, with little recovery two years after discharge.10 Importantly, though, this study examined changes in mobility at the group-level and did not capture the magnitude of change in post-hospitalization mobility among individual patients. Therefore, the current investigation aims to determine the individual trajectories of post-hospitalization community mobility recovery among older patients. Based on findings showing that many older patients recover ADL function, we hypothesize that the majority of patients also recover community mobility in the months after discharge.
METHODS
Participants
200 patients, aged ≥65 years, admitted to the Birmingham Veterans Affairs Medical Center (BVAMC) medical wards between January 18, 2011–December 27, 2013 were recruited (Monday-Friday), enrolled, and followed for 6 months post-discharge. A trained research assistant interviewed physicians to identify patients eligible for participation. Inclusion criteria were age ≥65 years; not on isolation precautions; not imminently terminal (death expected within 30 days); English-speaking; and admitted for medical reasons only. Mini Cognitive Assessment (score < 3) and Confusion Assessment Method (> 0) were used to determine participants with dementia or delirium, respectively14, and for whom proxy consent was needed from appropriate next of kin per BVAMC’s institutional review board’s (IRB’s) protocol. Persons who can provide proxy consent in accordance with VA regulations: (1)Health-care agent, (2)Legal/special guardian, (3)Close adult relative, (4)Close friend. Written consent was obtained from all study participants regardless of cognitive status. Consent forms and study protocols were approved by the BVAMC IRB.
Sociodemographic information
Patient sociodemographic information was collected at baseline (admission) including age, sex, and self-defined race.
Life-Space
Life-Space Assessment (LSA) is a self-reported measure of mobility inquiring about movements within one’s home to out-of-town during the four weeks prior to the assessment.10,15,16 The composite score is calculated based on distance traveled (such as within one’s room or town), frequency of movement, and independence (with/without assistive devices or personal help). Composite scores range from 0 to 120 points with higher scores indicating greater mobility. Previous analysis of test-retest reliability of LSA showed an intraclass correlation coefficient of 0.96.15 LSA is sensitive to change over time and has been used in previous studies among hospitalized older adults. LSA was completed at baseline (reflecting mobility in the four weeks pre-hospitalization) and during six monthly follow-up telephone interviews.
Description of Mobility Trajectories
Patients were classified into three groups based on a clinically significant change in LSA score between baseline and post-hospitalization-month 1 (PH1). Our previous work showed that a 5-point change in LSA score is clinically significant17, thus we classified patients into three groups: (1) Improved, increase ≥5 points, (2) Unchanged, fluctuate <5 points, (3) Decreased, decline ≥5 points. Decreased group patients were further differentiated into categories based on recovery to their pre-hospital LSA score by PH3 and PH6, or failure to recover by PH6. Recovery was defined as LSA score that was <5 points from baseline score. Twelve patients with baseline data, but missing data after PH1 were not included in the recovery trajectory proportions.
Transportation
Due to the importance of transportation in achieving higher life-space levels,16 we asked patients if their means of transportation changed between baseline and PH1.
Length of Stay
Hospital length of stay (LOS) was abstracted from patient charts at discharge.
Activities of Daily Living (ADL)
ADL function was assessed at several time points using the Katz Index of Independence in Activities of Daily Living (ADL).18 Patients self-reported whether they were independent, required some assistance, or required total assistance to complete each of the following basic ADLs: bathing, dressing, feeding, toileting, grooming, transferring, and walking. Summary scores were calculated by summing the individual ADL scores. Summary scores ranged from 7 (independent with all ADLs) to 21 (completely dependent with all ADLs).18
Depression
The Geriatric Depression Scale (GDS) short-form was used with a score >5 considered a positive screen for depression.17
Comorbidities
The Charlson Comorbidity index was completed by chart review at discharge.19
Re-hospitalizations
Re-hospitalizations during the six-month follow-up period were documented via self-report and VA hospital records.
Statistical analysis
Descriptive statistics were computed as mean (standard deviation, SD) for continuous variables and frequency (percent) for categorical variables. Welsh’s ANOVA for continuous variables and χ2 tests for categorical variables were used to determine significant differences between improved, unchanged, and decreased groups. Statistical significance was assigned when P < 0.05. Statistical analyses were completed with SAS software version 9.4.
RESULTS
Of the 200 patients enrolled, 3 withdrew after enrollment, 24 died during the 6-month study period, and 173 patients had complete life-space data at baseline (hospital admission) and post-hospitalization month-one (PH1). Of the 173 patients, mean age was 72.2 years (SD, 6.9), 20 (12%) were cognitively impaired requiring proxy consent, 37 (21%) self-identified as black, and 170 (98%) were male (Table 1). Common admitting diagnoses included congestive heart failure, chronic obstructive pulmonary disease, acute kidney infection, and pneumonia.
Table 1.
Baseline Patient Characteristics Based on Initial Change in LSA Score
| Characteristic | All (N=173) |
Decreased (N=92) |
Unchanged (N=42) |
Improved (N=39) |
P Value |
|---|---|---|---|---|---|
| Age, y, mean (SD) | 72.2 (6.9) | 72.1 (7.2) | 71.8 (6.5) | 72.8 (6.5) | 0.8 |
| Male, No. (%) | 170 (98.3) | 91 (98.9) | 41 (97.6) | 38 (97.4) | 0.7 |
| Black, No. (%) | 44 (25.4) | 15 (16.5) | 14 (33.3) | 15 (20.5) | 0.09 |
| Mean LOS, d (SD) | 3.7 (3.2) | 4.0 (3.2) | 3.9 (4.2) | 2.8 (1.4) | 0.01** |
| ADL 2-week PTA, mean (SD) | 7.5 (1.4) | 7.4 (1.2) | 7.3 (0.7) | 7.8 (2.1) | 0.3 |
| Geriatric Depression Scale, mean (SD) | 4.3 (3.0) | 4.4 (3.1) | 4.3 (3.0) | 4.3 (2.8) | 0.9 |
| Charlson Comorbidity Index, mean (SD) | 3.5 (2.3) | 3.3 (2.3) | 3.6 (2.5) | 3.6 (2.2) | 0.8 |
| No Change in Driving Status, No. (%) | 143 (82.7) | 76 (82.6) | 37 (88.1) | 30 (76.6) | 0.4 |
| LSA PTA 1-month, mean (SD) [range] | 58.4 (25.0) [3, 120] | 63.3 (22.4) [13, 120] | 57.7 (25.0) [3, 102] | 47.8 (28.1) [4, 110] | 0.01** |
| LSA Post-hospitalization 1-month, mean (SD) [range] | 51.0 (25.2) [6, 120] | 44.0 (21.5) [6, 100] | 58.2 (25.2) [6, 102] | 60.0 (28.9) [15, 120] | 0.0007*** |
Study patient baseline characteristics according to change in life-space assessment (LSA) score between baseline and post-hospitalization month 1. Improved group: ≥ 5 point increase; Unchanged group: fluctuate < 5 points; Decreased group: ≥ 5 point decline. LOS = length of stay; ADL = activities of daily living; PTA = prior to admission; LSA = life-space assessment. Welsh’s ANOVA analysis was completed comparing the Decreased, Unchanged, and Improved groups.
P < 0.05;
P < 0.01;
P < 0.001.
The baseline characteristics of the three groups (Decreased, Unchanged, or Improved) are presented in Table 1. Statistically significant group differences were observed in mean LOS. On average, patients that decreased in LSA score had the longest LOS while those that improved had the shortest. Mean baseline LSA scores were significantly different between the three groups. Patients in the Decreased group were admitted with an average LSA score of 63 points (SD, 22), while those in the Unchanged or Improved groups were admitted with an average score of 58 points (SD, 25) and 48 points (SD, 28), respectively. Of the 173 study patients, 171 patients had complete ADL data. We found that 25/171 (15%) had not returned to their baseline ADL status by PH1.
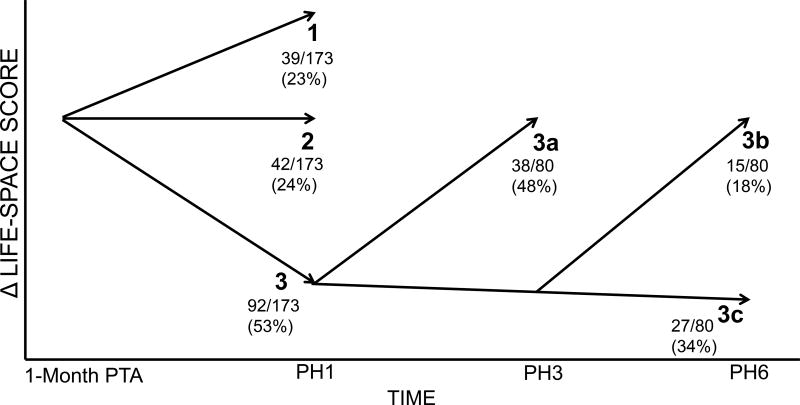
Figure compares LSA scores at baseline to PH1; 39/173 patients (22%; Trajectory 1) improved with an average 12.5 point (SD, 6.3) increase, 42/173 patients (25%; Trajectory 2) were unchanged with an average 0.5 point (SD, 2.2) increase, and 92/173 patients (53%; Trajectory 3) decreased in LSA score with an average 20 point (SD, 12.2) decline.
Figure.
Theoretical model representing changes in Life-Space Assessment (LSA) score over time from pre-hospitalization (one month prior to hospital admission; baseline) to 6 months following hospital discharge. The 173 patients with complete data at baseline and PH1 were categorized into three trajectories. Trajectory 1: patients who increased ≥ 5 points; Trajectory 2: patients who fluctuated < 5 points; Trajectory 3: patients who decreased ≥ 5 points. Of the 92 patients in Trajectory 3, 80 patients had complete data at PH3 and PH6 and were further differentiated into sub-trajectories. Trajectory 3a: patients who recovered to their baseline LSA score (<5 points of baseline) by PH3; Trajectory 3b: patients who recovered to baseline LSA score by PH6; Trajectory 3c: patients who did not recover to baseline LSA score by PH6. PTA = prior to admission; PH = post-hospitalization month.
Figure also illustrates mobility recovery among patients in the Decreased group. Of the 92 patients that decreased, 80 patients had complete post-hospitalization data. Thirty-eight of these patients (48%; Trajectory 3a) recovered within 3 months post-discharge, and an additional 15 (18%; Trajectory 3b) recovered within 6 months. In total, 53/80 patients (66%) recovered to their within 5 points of their pre-hospitalization LSA score by PH6. The remainder, 27/80 patients (34%; Trajectory 3c), did not recover during 6 months of follow-up. There was no significant difference in re-hospitalizations during the follow-up period between patients that recovered by PH3 and PH6 and patients that did not recover (data not shown).
DISCUSSION
This study illustrates that among a cohort of older hospitalized patients, the majority experienced a clinically significant decrease in community mobility (as measured by the LSA) in the first month after hospitalization. Among patients who experienced mobility decline, approximately two-thirds recovered to baseline mobility within six months.
Contrary to our prior work using growth curve modeling, which showed, on average, patients decreased in community mobility and never recovered over multiple years after hospitalization;10 we show that more than half of patients experienced a decrease in mobility, and that among the patients that recover, most recover quickly post-discharge. While one-third of the sample did not recover mobility at any point during the follow-up period, 85% of patients recovered baseline ADL function within one-month of discharge. The proportion of patients who experienced sustained ADL disability in this study is lower compared to prior studies, which noted that 30% of patients failed to recover ADL function in the months following hospitalization.5,6 These results suggest that even if patients recover ADL function they may remain disabled in mobility in the months following hospitalization.
We observed that the patients who decreased in mobility from baseline to PH1 had a pronounced mobility restriction with an average LSA score decrease of 20 points. An example of a 20-point decrease is an older adult who previously reported going into town 1–3 times/week without assistance (LSA score, 63), but who now goes into town 1–3 times/week with assistance of another person (LSA score, 43). While most of these patients recovered mobility within six months of hospitalization, they experienced a profound, albeit temporary, loss of mobility with potentially significant consequences including reduced quality of life, and increased risk of institutionalization and death.20–22 Family may also be negatively affected by having to provide increased informal care23.
A major difference between patients with improved mobility in the first month after hospitalization and those with decreased mobility was baseline mobility status. Patients who improved had more restricted pre-hospitalization life-space (LSA score, 46), while patients who decreased were considerably less restricted in mobility before hospitalization (LSA score, 63). Reasons for this disparity are not clear. We found no significant group differences in comorbidities, cognition or depressive symptoms at admission, ADL function two weeks prior to admission, or age. One possible explanation is that patients with improved LSA score experienced a slowly worsening course of disease in the month pre-admission resulting in restricted mobility demonstrated by low LSA scores. The patients with decreased post-hospital mobility may have had less restricted mobility in the month pre-admission due to sudden onset of illness resulting in rapid hospitalization and increased LOS. Future studies need to assess admission acuity of illness to determine its impact on pre-hospitalization LSA scores.
A study strength is use of the LSA, given the ease of administration and effectiveness in detecting change in community mobility over-time. LSA captures the domain of participation. An additional strength is that a portion of the study population had cognitive impairment and/or dementia contributing to the generalizability of our results.
A limitation of this study is the use of Veterans Affairs hospital patients who may not provide an accurate representation of all older patients.24,25 Our study sample was mostly male, and evidence supports that women live longer than men and compose a larger segment of the older adult population.26 Another limitation is the recovery time course used in the study. It is possible that patients recovered mobility after the study period. However, previous work in ADLs suggests that little recovery occurs after six months.5
Overall, the current investigation illustrates that older patients hospitalized for medical conditions are at risk for loss of community mobility. While recovery will likely occur for many patients, implementing methods for accelerating the recovery process or for preventing a community mobility decline entirely could be beneficial in getting patients back to pre-hospitalization functional independence. Evidence suggests that increasing mobility during hospitalization through provision of mobility aides, or system-based interventions, prevents hospital-associated mobility disability.11,27 Additionally, interventions that target other modifiable factors known to impact life-space, such as physical function and cognition, may also reduce or prevent hospitalization-associated mobility restriction.28–30 Future work needs to identify patients at greatest risk, so targeted in-hospital and post-acute interventions can be provided to mitigate negative effects of hospitalization on community mobility.
Impact Statement.
We certify that this work is novel. Studies examining post-hospitalization recovery of function in older adults has largely focused on activities of daily living. These works have illustrated that patient recovery is variable ranging from quick recovery to poor recovery resulting in long-term disability after hospitalization. Evidence presented herein aims to build on existing knowledge of post-hospitalization recovery by describing changes in another essential component of functional independence, community mobility. We show that over half of older patients experience a clinically significant restriction of mobility in the month after hospitalization, but that most of these patients recover within 6 months of discharge. Our findings suggest that loss of community mobility and subsequent recovery is likely, and may also indicate that programs in the hospital and during post-acute care aimed at mobility could allow a speedier recovery.
Acknowledgments
The authors would like to thank Project Manager Tatiana Brecht for her role in data collection and project coordination (Affiliation: Department of Medicine, University of Alabama at Birmingham, Birmingham, AL; Funding Source: VA Rehabilitation Research & Development Merit Review Grant E7036R).
Conflicts of Interests: Grants received: C.J. Brown (VA Rehabilitation R&D, National Institute on Aging).
Sponsor’s Role: The “Trajectory of Recovery of Life-Space Mobility after Hospitalization” was funded by a VA Rehabilitation R&D Scientific Merit award to Dr. Brown. The funding agency had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: C. Loyd: Data analysis and interpretation, drafting and critically revising manuscript for content. T.M. Beasley: Statistical analysis, critical revision of manuscript for content. R.S. Miltner, D. Clark, and B. King: Critical revision of manuscript for content. C.J. Brown: Obtaining funding, study concept and design, analysis and interpretation of data, and drafting and critically revising manuscript for content.
References
- 1.Lamont CT, Sampson S, Matthias R, et al. The outcome of hospitalization for acute illness in the elderly. J Am Geriatr Soc. 1983;31(5):282–288. doi: 10.1111/j.1532-5415.1983.tb04872.x. [DOI] [PubMed] [Google Scholar]
- 2.McVey LJ, Becker PM, Saltz CC, et al. Effect of a geriatric consultation team on functional status of elderly hospitalized patients. A randomized, controlled clinical trial. Ann Intern Med. 1989;110(1):79–84. doi: 10.7326/0003-4819-110-1-79. [DOI] [PubMed] [Google Scholar]
- 3.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 4.Gill TM, Allore HG, Holford TR, et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292(17):2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 5.Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med. 1996;156(6):645–652. [PubMed] [Google Scholar]
- 7.Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270. doi: 10.1111/j.1532-5415.2004.52354.x. [DOI] [PubMed] [Google Scholar]
- 8.Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6(11):e26951. doi: 10.1371/journal.pone.0026951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: "She was probably able to ambulate, but I'm not sure". JAMA. 2011;306(16):1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 10.Brown CJ, Roth DL, Allman RM, et al. Trajectories of life-space mobility after hospitalization. Ann Intern Med. 2009;150(6):372–378. doi: 10.7326/0003-4819-150-6-200903170-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown CJ, Foley KT, Lowman JD, Jr, et al. Comparison of Posthospitalization Function and Community Mobility in Hospital Mobility Program and Usual Care Patients: A Randomized Clinical Trial. JAMA Intern Med. 2016;176(7):921–927. doi: 10.1001/jamainternmed.2016.1870. [DOI] [PubMed] [Google Scholar]
- 12.Sager MA, Rudberg MA. Functional decline associated with hospitalization for acute illness. Clin Geriatr Med. 1998;14(4):669–679. [PubMed] [Google Scholar]
- 13.Gill TM, Allore HG, Gahbauer EA, et al. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919–1928. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 15.Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51(11):1610–1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 16.Peel C, Sawyer Baker P, Roth DL, et al. Assessing mobility in older adults: the UAB Study of Aging Life-Space Assessment. Phys Ther. 2005;85(10):1008–1119. [PubMed] [Google Scholar]
- 17.Brown CJ, Kennedy RE, Lo AX, et al. Impact of Emergency Department Visits and Hospitalization on Mobility Among Community-Dwelling Older Adults. Am J Med. 2016;129(10):1124 e1129–1124 e1115. doi: 10.1016/j.amjmed.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz S, Ford AB, Moskowitz RW, et al. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Sheppard KD, Sawyer P, Ritchie CS, et al. Life-space mobility predicts nursing home admission over 6 years. J Aging Health. 2013;25(6):907–920. doi: 10.1177/0898264313497507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentley JP, Brown CJ, McGwin G, Jr, et al. Functional status, life-space mobility, and quality of life: a longitudinal mediation analysis. Qual Life Res. 2013;22(7):1621–1632. doi: 10.1007/s11136-012-0315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy RE, Sawyer P, Williams CP, et al. Life-Space Mobility Change Predicts 6-Month Mortality. J Am Geriatr Soc. 2017;65(4):833–838. doi: 10.1111/jgs.14738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemper P. The use of formal and informal home care by the disabled elderly. Health Serv Res. 1992;27(4):421–451. [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan RO, Teal CR, Reddy SG, et al. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5 Pt 2):1573–1583. doi: 10.1111/j.1475-6773.2005.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- 26.Austad SN. Why women live longer than men: sex differences in longevity. Gend Med. 2006;3(2):79–92. doi: 10.1016/s1550-8579(06)80198-1. [DOI] [PubMed] [Google Scholar]
- 27.King BJ, Steege LM, Winsor K, et al. Getting Patients Walking: A Pilot Study of Mobilizing Older Adult Patients via a Nurse-Driven Intervention. J Am Geriatr Soc. 2016;64(10):2088–2094. doi: 10.1111/jgs.14364. [DOI] [PubMed] [Google Scholar]
- 28.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 29.Landefeld CS, Palmer RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338–1344. doi: 10.1056/NEJM199505183322006. [DOI] [PubMed] [Google Scholar]
- 30.Counsell SR, Holder CM, Liebenauer LL, et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572–1581. doi: 10.1111/j.1532-5415.2000.tb03866.x. [DOI] [PubMed] [Google Scholar]