Abstract
RATIONALE
Stable isotope analysis integrates diet information over a time period specific to the type of tissue sampled. For metabolically active skin of free-ranging cetaceans, cells are generated at the basal layer of the skin and migrate outward until they eventually slough off, suggesting potential for a dietary time series.
METHODS
Skin samples from cetaceans were analyzed using continuous-flow elemental analyzer isotope ratio mass spectrometery (EA-IRMS). We used ANOVAs to compare the variability of δ13C and δ15N values within and among layers and columns (“cores”) of the skin of a fin, humpback, and sperm whale. We then used mixed-effects models to analyze isotopic variability among layers of 28 sperm whale skin samples, over the course of a season and among years.
RESULTS
We found layer to be a significant predictor of δ13C values in the sperm whale’s skin, and δ15N values the humpback whale’s skin. There was no evidence for significant differences in δ15N or δ13C values among cores for any species. Mixed effects models selected layer and day of the year as significant predictors of δ13C and δ15N values in sperm whale skin across individuals sampled during the summer months in the Gulf of Alaska.
CONCLUSIONS
These results suggest that skin samples from cetaceans may be subsampled to reflect diet during a narrower time period; specifically different layers of skin may contain a dietary time series. This underscores the importance of selecting an appropriate portion of skin to analyze based on the species and objectives of the study.
INTRODUCTION
Understanding changes in an animal’s diet and the temporal scale over which those changes occur can give valuable insight into habitat preferences, migration patterns, and potential for interactions with humans. Stable isotopes have become an increasingly important method for the study of marine mammal diets, trophic niche, movement patterns, physiology, historical ecosystem parameters, and other ecological studies 1–7. Stable carbon isotope ratios (δ13C values) are used as a proxy for determining foraging habitat because in marine systems, species in pelagic and offshore areas tend to have lower carbon isotope ratios than those in benthic and near-shore areas 8–11. Stable nitrogen isotope ratios (δ15N values) are an indicator of trophic position due to metabolic fractionation with each trophic level 12–18. Specifically δ15N values increase 2–4‰ per trophic level in marine mammals 4,19–22. For marine mammal species, the majority of analyses over recent years have focused on single-species niche breadth 23–27, comparative diet studies 1,3,28–31, and mixing models to determine diet composition 29,32. Unlike results of samples from stomach lavage or feces, stable isotope analysis of consumer tissues integrates diet over a longer time period than a single meal or day, and samples are more accessible from free-ranging cetaceans 33–35. However, key metabolic parameters allowing for precise interpretation of the temporal resolution of stable isotope results are often lacking, especially for metabolically active tissues. In addition, the variability of stable isotope ratios from different samples within a tissue is not well-established, although existing studies indicate that replicate samples of muscle tissue from individual fish and zooplankton produced variability within a single individual of <0.2‰, and a recent study with common and striped dolphins showed there were no significant differences among stable isotope ratios across various locations on the skin 36–39. This variability is essential context to appropriately interpret differences in stable isotope ratios observed among tissues or individuals.
To properly interpret stable isotope ratios of animal tissue, it is essential to account for the temporal dynamics of isotopic integration. Two aspects of integration are important to consider: diet-tissue incorporation rate and tissue turnover rate. Incorporation rate refers to the amount of time between prey ingestion and when the isotopic signature of that prey item enters the tissue being analyzed 40,41. This rate can be estimated in captive cetaceans through controlled feeding experiments, but is not well understood in free-ranging cetaceans, and depends on biochemical processes such as thermoregulation type, growth rate, reproductive status, and environmental stressors 21,22,33,40,42. Animal tissues can be categorized as metabolically active or inert. Metabolically active tissues have different isotopic incorporation rates, while interpretation of isotope patterns in metabolically inert tissues depends on tissue growth rates. Turnover rate refers to the rate of replacement of the tissue 43,44. Turnover rates are more widely studied in marine mammals, and can be low in metabolically active tissues such as liver or blood, or much higher in inert tissues such as whiskers, baleen, bone, and hair. Differences in turnover rate is one of the causes of variability in stable isotope signatures within and between tissues 33,45. Turnover rates in stable isotope analysis are often discussed in terms of the half-life of the tissue, or the time at which half of the tissue has been replaced 33,46,47. Inert tissues such as claws, baleen, and hair often preserve a dietary time series for individual marine mammals 7,23,27,30,48,49. In these tissues, diet-tissue incorporation occurs toward the basal layer of the tissue where new cells are generated, and the turnover rate is related to the growth rate of the tissue and thickness of the sample, with lower turnover rates for thinner sections 21,40,41,46. Controlled feeding experiments of captive animals have been used to study turnover rates for skin of smaller cetacean species (Table 1). Such experiments can only be performed on captive animals, excluding direct measurements for baleen whales.
Table 1.
Existing experiments addressing turnover rates of cetacean skin
| Species | Method | Estimated turnover rate (days, x̄ ± sd) | Type of turnover | Source |
|---|---|---|---|---|
| Blue whale (Balaenoptera musculus) | Free-ranging with known migratory diet shift | 163 ± 91 (δ15N) | Full isotopic incorporation | Busquets-Vass et al 41 |
| Bottlenose dolphin (Tursiops truncatus) | Labeled cells sub-epidermally | 73 | Full epidermal turnover | Hicks et al 58 |
| Bottlenose dolphin (Tursiops truncatus) | Controlled feeding | 17 ± 1.3 (δ15N) 22 ± 0.5 (δ13C) | Half life | Browning et al 22 |
| Bottlenose dolphin (Tursiops truncatus) | Controlled feeding | 180 ± 71 (δ15N)104 ± 35 (δ13C) | Full isotopic incorporation | Giménez et al 42 |
| Beluga (Delphinapterus leucas) | Labeled cells sub-epidermally | 70–75 | Full epidermal turnover | St. Aubin et al 59 |
Skin tissue (epidermis) is often used to study diet in free-ranging cetaceans because its collection is minimally invasive 2,29,34,50,51. Current stable isotope analysis of cetacean skin treats the skin as a homogenous tissue in terms of dietary information and uses a variety of methods to select the portion of skin used in analysis. Most common are analyses using the outer layer of skin as it sloughs off 52, homogenizing a small piece of skin cut from an undefined portion of a biopsy sample 2,35,53, extracting a section of skin from a stranded carcass 25,54, or a combination of these sampling methods 1,55.
Cetacean skin is divided into layers based on cellular structure, often referred to as the stratum basale/germinativum (inner), stratum spinosum (intermediate), and stratum externum (outer) 56,57. Cells in the stratum basale tend to be primarily large, tall epithelial cells that are well developed with many mitochondria, ribosomes, desmosomes, and lipid droplets. Cells in the stratum spinosum tend to have fewer mitochondria and ribosomes, but the same number of lipid droplets and desmosomes. This layer shows cells that are rounded or oval in shape, and they become flattened as they migrate out towards the stratum externum, where cells show keratinization and lipid droplets are densely packed. In the stratum externum the nucleus is often remnant, and sometimes completely intact, indicating that keratinization is not complete 56,57. Because epidermal cells are generated at the basal layer of skin (stratum basale) and migrate outward 58,59, there is the potential for a dietary time series to exist within cetacean skin. The basal layer of cetacean skin, adjacent to the dermis, is textured rather than flat 60. Small dermal papillae extend between a quarter to half-way up into the epidermis from the dermis 58–60. Hicks et al 58 labeled sub-epidermal cells in bottlenose dolphins to examine the rate of skin turnover. Labeled cells migrated first laterally out of the intruding papillae, and then vertically toward the outer layer of skin, before flaking off as the animal sloughed its skin (Figure 3 in 58). Labeled cells entering the skin from the tip of the dermal papillae in the stratum spinosum were able to move directly outward toward the surface in the stratum externum, with no lateral movement. Therefore, new cells may be found from the stratum basale to the furthest intrusion of the papillae in the stratum spinosum (Figure 3 in 58). This, combined with information on cellular differences between strata, suggests that epidermal cells get older on average in tissue further from the dermal-epidermal interface. Busquets-Vass et al41 hypothesized that due to this stratification within the skin, skin might contain a dietary time series. In stable isotope analysis of blue whale (Balaenoptera musculus) skin, Busquets-Vass et al found δ15N values to vary significantly between the inner (stratum basale) and outer (stratum externum) layers of skin in the California Current System, which corresponded temporally to differences in δ15N values of prey in two seasonal foraging grounds 41.
Among cetaceans in the Gulf of Alaska (GOA), there is a wide range of variability in diets, which vary considerably among individuals in some species, but are more homogenous in other species. Fin whales (Balaenoptera physalus) forage in both near-shore and offshore waters in the GOA. Fin whales worldwide have a fairly homogenous lower-trophic diet of euphausiid krill (Euphausiacea) and occasionally of small amounts of small schooling fish and copepods 19,61–65. Humpback whales (Megaptera novaeangliae) are generalist predators in coastal Southeast Alaskan waters, feeding on a varied diet of krill 66,67, herring 68–70 and other small schooling fish 71, that have a broad range of isotopic ratios 4,29. Sperm whales (Physeter macrocephalus) are the largest of the toothed whales, and are deep-diving offshore predators 72. While the current diet of sperm whales in the GOA is poorly understood, stomach content data from historical whaling shows that they feed on a variety of deep-sea fishes and squid 61,73–75. They are also known to remove sablefish (Anoplopoma fimbria) and halibut (Hippoglossus stenolepis) from commercial longline fishing gear in the GOA 76–79.
In this investigation, we aim to determine if layers of cetacean skin exhibit isotopically distinct signatures, which may be attributed to temporal shifts in diet. To address this objective we designed a pilot study consisting of two experiments. In the first experiment we tested for differences in δ13C and δ15N values both within and among layers of skin of three individual cetaceans: a stranded fin whale, a stranded humpback whale, and a free-ranging sperm whale. We hypothesized that subsamples from within the same layer of skin would be more isotopically similar than subsamples taken from different layers within the same full-thickness vertical core of the sample. More specifically, we hypothesized that layer would be a significant predictor of one or both stable isotope ratios in species with variable diets, while core would never be a significant predictor. This hypothesis rests on the assumptions that the cells within the same layer of skin were deposited closer to the same time on average than cells in the same core, and that the animal had undergone a detectable diet shift within the turnover time of the full skin thickness. For the humpback whale and sperm whale, two generalist foragers with diverse and seasonal diets, this was probably the case 61,66–71,73–75. For the fin whale, with a more homogenous diet, we expected there to be lower variability overall among layers 19,61–65. A second experiment measured the average differences among layers of a larger number of sperm whale skin samples, and tested if the differences between specific layers were consistent across individuals. We hypothesized that we would again find higher variability among layers in each sperm whale than would be expected from replicate samples within a single layer, but that there would not be consistent differences in stable isotope ratios among layers of skin across individual sperm whales. This hypothesis was based on the assumption that the sperm whales sampled might have individual differences in the timing and direction of diet shifts, the timing of seasonal migrations, and the timing of seasonal isotopic trends in the baseline of the food web relative to the sampling time.
METHODS
Sample Acquisition: Experiment 1
Skin samples were acquired from a dead stranded fin whale, a dead stranded humpback whale, and a biopsy of a free-ranging sperm whale, all in the GOA (Table 2). Fin whale skin was acquired from a dead animal that arrived into the port of Seward, Alaska on the bulbous bow of a cruise ship on May 29, 2016 (NMFS Regional stranding ID 2016053). The animal was a sub-adult male, 50 feet in length, and in good body condition. The portion of skin taken for this analysis came from high on the animal’s dorsal flank, below the dorsal fin. Skin was also obtained from a dead humpback whale struck by a cruise ship near Juneau, Alaska on 27 July, 2010 (NMFS Regional stranding field ID 2010089). The whale was a non-pregnant female, measured 46 feet in length, and was reported to be in good body condition (blubber thickness 61.3 mm dorsolateral to dorsal fin). During the necropsy, scientists removed a section of skin and blubber from the dorsal surface approximately 10 cm by 7.5 cm, which was stored at -80° C. Skin was subsampled from this section, and measured 7 mm in thickness.
Table 2.
Types of samples used for experiments 1 and 2. F = fin whale, H = humpback whale, and S = sperm whale. Sample size is in parentheses.
| Stranded (Sample Size) | Live free ranging (Sample Size) | |
|---|---|---|
| Experiment 1 | F (1) H (1) | S (1) |
| Experiment 2 | - | S (28) |
Biopsy samples were collected from free-ranging sperm whales between 2003 and 2017 using a Barnett crossbow (150 lb draw weight), with bolts equipped with stainless steel tips measuring 40 mm in length and 7 mm in diameter, with a float for retrieval 50,51. Biopsy samples collect both skin and blubber, with skin being subsampled for this analysis. A single sample, collected from a male sperm whale in September 2015, was chosen for analysis of experiment 1 due to its large size, suitable for subsampling. The skin from the sperm whale sample measured 6 mm in depth, with a diameter of 7 mm.
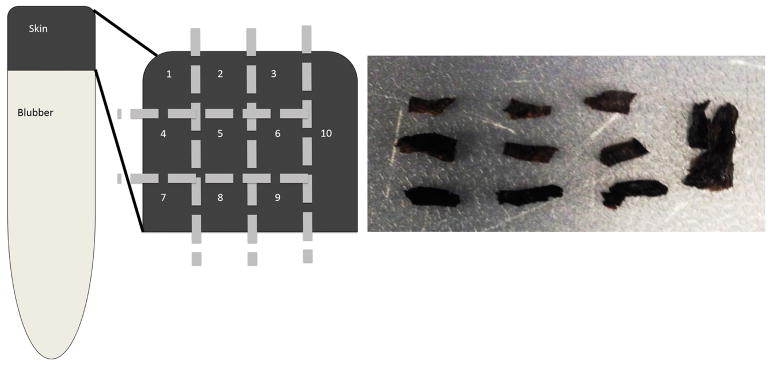
For stable isotope analysis, we subsampled a small 1-cm2 section of both the fin and the skin (full-depth) of the humpback whale, and the full skin sample of the sperm whale biopsy into 10 pieces (Figure 1). Each sample was cut into a 9 × 9 grid of three subsamples per row (laterally along sample), and three per column (vertically through depth of sample), and a tenth fully homogenized subsample (Figure 1). Our sub-sampling method produces multiple samples from the same layer, which allows for measures of variability and tests of significance. The lateral, or horizontal cuts, subset each core into “layers” assumed to correspond roughly to the three strata of skin mentioned earlier in this paper (Figure 1). We termed these layers “outer” referring to the stratum externum, the layer of skin adjacent to the whale’s environment (sample #s 1–3), “middle” referring to the intermediate layer of skin or stratum spinosum (sample #s 4 -6), and “inner” referring to the basal layer of skin or stratum basale (sample #s 7–9). Each layer was approximately 2 mm thick. The vertical columns of skin were cut transversal to the body, which we refer to as “cores” with each core containing samples 1, 4, and 7; 2, 5, and 8; and 3, 6, and 9 (Figure 1). These cuts were also made with each core cut at equal width relative to the full width of the sample. The final portion of the sample was a transversal cut including all layers, which we refer to as the “full” thickness sample, or sample 10.
Figure 1.
Left, schematic showing the methodology for sub-sampling a biopsy sample into 10 subsamples of skin to analyze differences within layers (1–3, 4–6, 7–9) and within cores (1,4,7 vs 2,5,8 vs 3,6,9). Right, photo showing sperm whale skin divided into 10 sections, as defined on the left.
Sample Acquisition: Experiment 2
Between 2003 and 2017 in the eastern GOA, skin from 28 free-ranging male sperm whales was collected via biopsy in the same manner as the sample from experiment 1 (Table 2). These samples were collected as part of the Southeast Alaska Sperm Whale Avoidance Project (SEASWAP, www.seaswap.info), which focuses on sperm whales that interact with longline fishing vessels in the eastern GOA, primarily targeting sablefish 76,78. From each of the samples a single column of skin was cut into subsamples corresponding to the three skin layers described above: “outer”, “middle”, and “inner”, parallel to the angle of the blubber-skin interface of each sample. All samples used in this experiment consisted of enough skin to cut into three layers each approximately 2.3 mm thick.
Sample Processing: Experiments 1 and 2
Lipid content and preservation method are known to affect the isotopic composition of tissues 80,81. All skin was stored prior to analysis at -80° C. Six tissue samples from sperm whale biopsies (experiment 2) were stored in a 20% saturation of dimethyl sulfoxide (DMSO). For both experiments, tissue was weighed, cut into small pieces to increase surface area for drying, and then oven-dried at 60°C for 24 hours. In addition to removing the influence of lipids in bulk tissues, extracting lipids can eliminate the effects of storage in DMSO tissues 35,55,82,83. However, lipid-extraction can alter the δ15N values of some species 84–86, although this has not been tested for sperm whales. Thus, we performed a small proof of concept study in which the 28 samples from experiment 2 were subsampled into two pieces with half analyzed without extracting lipids (NLE) and the other half with lipids extracted (LE). The differences between the extracted (LE) and non-extracted (NLE) δ15N values for each sample were analyzed using a one sample t-test for significance. The lipid-extracted samples did not have significantly different δ15N values from the non-extracted samples (t=−1.25, df=44, p=0.22). Meanwhile the δ13C values were significantly different between the LE and the NLE samples (t=−8.82, df=44, p<0.01), showing that the samples warranted lipid extraction. Finally, the C:N ratios of the non-lipid-extracted samples ranged from 3.3 to 4.7, ratios which were high enough to warrant lipid-extraction or correction 87. Thus, all the stable isotope ratios in this study were assessed using lipid-extracted samples.
For lipid-extraction, the dried tissue was soaked in 2 mL of a 2:1 solution of chloroform-methanol for 20 minutes in an ultrasonic bath to extract lipids 84,88,89. The lipid-filled solution was removed using disposable glass pipettes. This procedure was repeated three times 84,88,89. After lipid-extraction, the samples were rinsed with deionized water, again dried at 60°C for 12–24 hours, and re-weighed. The samples were then ground into a fine powder, and 0.2 to 0.4 mg aliquots were placed into tin capsules that were sent to the Alaska Stable Isotope Facility (ASIF) in Fairbanks, AK, USA for processing. At ASIF stable isotope data for bulk stable carbon (δ13C values) and nitrogen (δ15N values) isotope ratios was obtained using continuous-flow isotope ratio mass spectrometry (CFIRMS). This method utilizes a Flash 2000 elemental analyzer and a Conflo IV interfaced with a Delta VPlus isotope ratio mass spectrometer (all from Thermo Scientific, Bremen, Germany). Isotope composition was expressed as a ratio relative to an international standard, in delta notation (δ) as parts per thousand (‰):
| 1) |
where X is 13C or 15N, and R represents the respective abundance ratio of each isotope (e.g., 13C/12C or 15N/14N). Reference standards used were Vienna Pee-Dee Belemnite for carbon and atmospheric N2 for nitrogen. Analytical precision was ±0.2‰ for both δ13C and δ15N values.
Statistical Analysis: Experiment 1
For experiment 1 we used two-way analyses of variance (ANOVAs) to test for significant differences in the δ13C or δ15N values by layer and core (as a control). Layers were defined previously as a categorical independent variable with classes: inner, middle, and outer. Cores, also defined previously, were also a categorical independent variable with three classes. Cores were used as a control due to evidence that cetacean skin shows isotopic homogeneity across the body 39. This experiment aimed to examine the influence of the two different variables (layers and cores) on a continuous dependent variable (δ13C or δ15N values). This analysis was performed separately for each isotope ratio and separately on each of the three species. Models were run using the nine samples from each species (3 layer, by 3 core), omitting the full thickness-samples (#10s), as they do not include layers. The models were compared using the Akaike Information Criterion with a correction for low sample size (AICc values) 90. When significant differences among layers or cores were found, post-hoc tests (Tukey’s Honest Significance Differences) were used to determine which layers or cores differed significantly 91.
Statistical Analysis: Experiment 2
For experiment 2, the samples, due to smaller tissue sizes from biopsies, were cut into 3 layers from a single lipid-extracted column only. Observed differences in isotope ratios (δ13C and δ15N values) among layers were first examined visually by individual. Mixed effects models were then used to model isotope ratios as a function of layer and day of the year (DOY) as follows:
| 2) |
where yijk is the isotope ratio (δ13C or δ15N value) for individual whale i in layer of skin j and in year k, αj is a fixed intercept for layer j, ai represents a random intercept for each individual whale i, αk represents a random intercept for each year k, β1,j and β2,j are layer-specific coefficients that allow isotope ratios to change differently by layer over the course of the season (DOY) to capture potential lagged effects of diet changes across different layers, and the εij are residuals. The layer-specific coefficients β1,j and β2,j were estimated by including an interaction term between layer and DOY. Seasonal trends in isotope ratios were modeled as a quadratic function of DOY based on preliminary analyses that strongly suggested a curvilinear seasonal pattern. The random effects ai and ak, and residuals εij are assumed to be independent and normally distributed with mean zero and variances and σ2, respectively. The models were fitted using maximum likelihood or restricted maximum likelihood (REML) as implemented in package “nlme” for R version 3.3.1 92. Models were initially fitted using maximum likelihood estimation to allow for model selection using AICc values. Once the AICc-best model was identified, we re-estimated the final model using REML.
RESULTS
Experiment 1
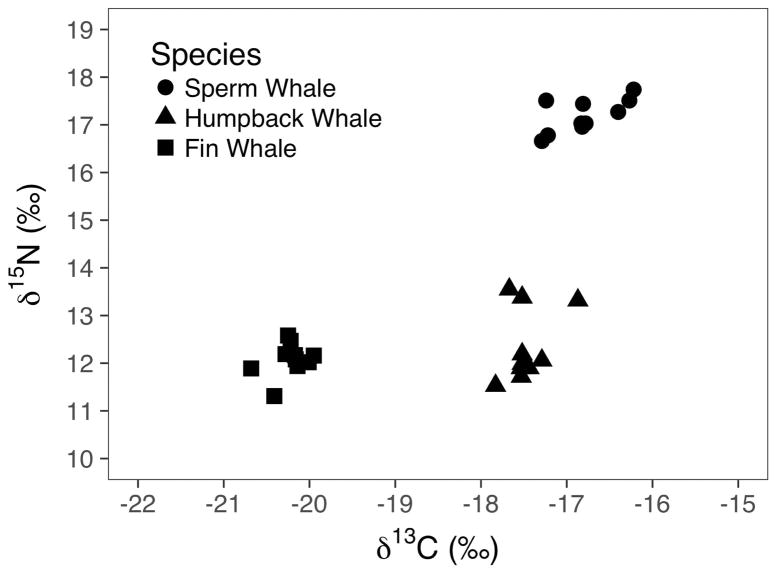
Stable carbon and nitrogen isotope ratios from the three cetacean species did not overlap in 2-D space: relative to the other species, the sperm whale had high δ15N and δ13C values, the fin whale had low δ15N and δ13C values, and the humpback whale had low δ15N values and high δ13C values (Table 3, Figure 2). Variability in δ15N values, as measured by their standard deviation across all skin subsamples, was more than twice as high in the humpback whale than in the other species (Table 3). The sperm whale had the highest variability in δ13C values (Table 3, Figure 2).
Table 3.
Means and standard deviations (sd) of δ13C and δ15N values and C:N ratios for each layer and overall of the three cetacean species sampled in experiment 1.
| Mean δ13C | s.d. δ13C | Mean δ15N | s.d. δ15N | Mean C:N | s.d. C:N | |
|---|---|---|---|---|---|---|
| Sperm whale | ||||||
| Outer | −17.2‰ | 0.2‰ | 17.2‰ | 0.4‰ | 3.6 | 0.1 |
| Middle | −17.0‰ | 0.3‰ | 16.9‰ | 0.2‰ | 3.4 | 0.2 |
| Inner | −16.2‰ | 0.1‰ | 17.5‰ | 0.2‰ | 3.1 | 0.1 |
| Overall | −16.8‰ | 0.5‰ | 17.2‰ | 0.4‰ | 3.4 | 0.2 |
|
| ||||||
| Humpback whale | ||||||
| Outer | −17.4‰ | 0.4‰ | 13.4‰ | 0.1‰ | 3.3 | 0.1 |
| Middle | −17.4‰ | 0.1‰ | 12.1‰ | 0.1‰ | 3.3 | <0.1 |
| Inner | −17.6‰ | 0.2‰ | 11.7‰ | 0.2‰ | 3.2 | <0.1 |
| Overall | −17.5‰ | 0.3‰ | 12.4‰ | 0.8‰ | 3.3 | 0.1 |
|
| ||||||
| Fin whale | ||||||
| Outer | −20.4‰ | 0.3‰ | 12.3‰ | 0.4‰ | 3.6 | 0.1 |
| Middle | −20.3‰ | 0.1‰ | 11.8‰ | 0.5‰ | 3.6 | 0.1 |
| Inner | −20.1‰ | 0.1‰ | 12.1‰ | 0.1‰ | 3.5 | 0.1 |
| Overall | −20.2‰ | 0.2‰ | 12.1‰ | 0.4‰ | 3.5 | 0.1 |
Figure 2.
Bi-plot showing carbon and nitrogen delta values of the 10 subsamples of each of the three species of whale sampled in this experiment.
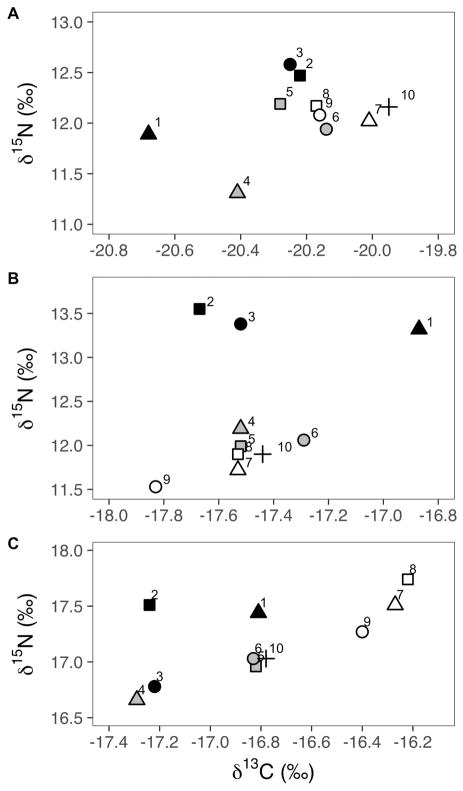
The fin whale samples did not differ significantly in δ15N or δ13C values among cores or layers (Table 4, Figure 3). Layer was a significant predictor for one stable isotope ratio each for both the sperm whale (δ13C value) and the humpback whale (δ15N value), while core was not significant for either species or isotope ratio. The humpback whale samples had significantly different δ15N values among layers (F2,6=122, p<0.001; Table 4, Figure 3) and all pairwise differences were significant (p=0.043, p<0.001, p<0.001 for inner-middle, middle-outer, and inner-outer comparisons, respectively). No significant differences were found in δ13C values among layers for the humpback skin (Table 4). The sperm whale sample had significantly different δ13C values among layers (F2,6=11.9, p=0.008; Table 4, Figure 3). Specifically, the inner layer of sperm whale skin had significantly higher δ13C values than both the middle (p=0.02) and the outer layers (p=0.01) (Table 4). No significant differences in δ15N values among layers were found in the sperm whale skin (Table 4).
Table 4.
Results from ANOVA tests for experiment 1 of stable isotope analysis of sperm whale, humpback whale, and fin whale cores and layers. Bold model results indicate significant relationships for each species and isotope ratio.
| Species | Factor | F-value | p-value |
|---|---|---|---|
|
| |||
| Sperm Whale | |||
| δ13C | Layer | F2,6=11.91 | p<0.01 |
| Core | F2,6=0.01 | p=0.99 | |
| Layer + Core | F4,4=4.06 | p=0.10 | |
|
|
|||
| δ15N | Layer | F2,6=3.44 | p=0.10 |
| Core | F2,6=0.72 | p=0.52 | |
| Layer + Core | F4,4=2.68 | p=0.18 | |
|
| |||
| Humpback Whale | |||
| δ13C | Layer | F2.6=0.79 | p=0.50 |
| Core | F2,6=0.87 | p=0.47 | |
| Layer + Core | F4,4=0.76 | p=0.60 | |
|
|
|||
| δ15N | Layer | F2,6=122.7; | p<0.01 |
| Core | F2,6=0.02 | p=0.98 | |
| Layer + Core | F4,4=60.14 | p=0.05 | |
|
| |||
| Fin Whale | |||
| δ13C | Layer | F2,6=1.79 | p=0.24 |
| Core | F2,6=0.69 | p=0.54 | |
| Layer + Core | F4,4=1.29 | p=0.41 | |
|
|
|||
| δ15N | Layer | F2,6=1.62 | p=0.27 |
| Core | F2,6=2.67 | p=0.15 | |
| Layer + Core | F4,4=4.59 | p=0.09 | |
Figure 3.
Bi-plot showing carbon and nitrogen delta values from each piece of tissue from the fin (A), humpback (B), and sperm (C) whale samples that were sub-sampled into 10 pieces (Figure 1). Layers are shaded the same with outer layer = black, middle layer = grey, and inner layer = white. Cores are indicated with shapes, from left to right = triangles, squares, and circles. Sample 10, the full sample, is shown in with a “+” symbol. Note differences in scale of x-axes.
Experiment 2
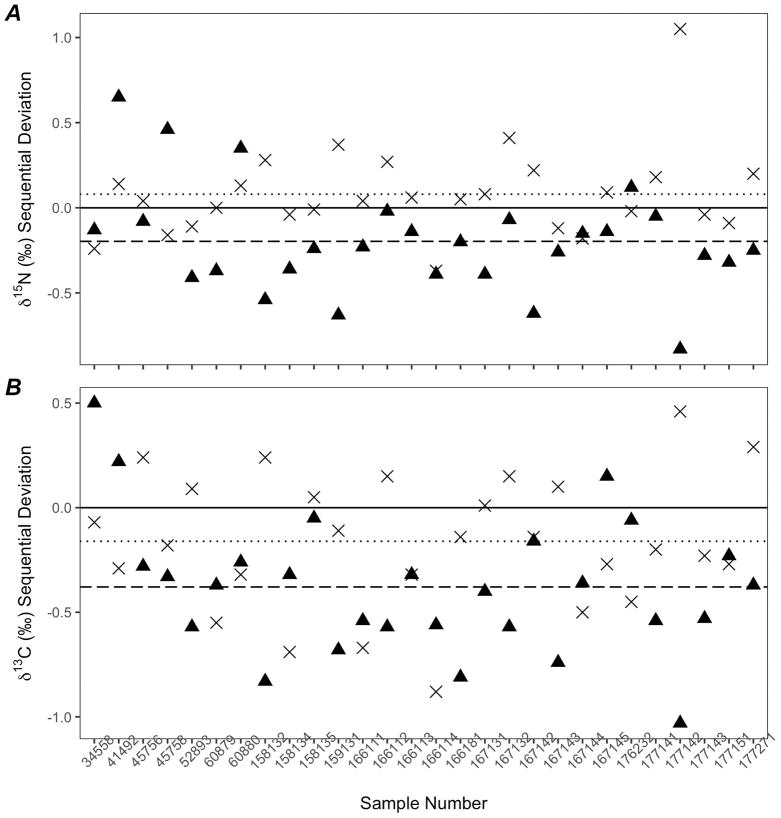
Among the 28 individual sperm whales, the stable isotope ratios showed broad ranges by layer. The range of δ15N values within an individual averaged 0.40±0.22‰ (min 0.1; max 1.1), while the range of δ13C values within an individual averaged 0.65±0.31‰ (min 0.1; max 1.0). Both the δ13C and the δ15N values decreased between the inner and middle layer on average, but these differences were highly variable across the 28 individual sperm whales (Figure 4). While δ15N values showed on average a 0.1‰ difference between the middle and outer layers, differences between the middle and inner layers were 0.2‰ (Figure 4A). For δ13C values, the outer layer decreased by an average of 0.4‰ from the middle layer (Figure 4B).
Figure 4.
Twenty-eight sperm whale skin samples showing sequential differences in δ15N (A) and δ13C (B) values between layers. Differences between the middle and inner layer (▲) are on average (dashed line) greater than differences between the middle and outer layers (×, dotted line).
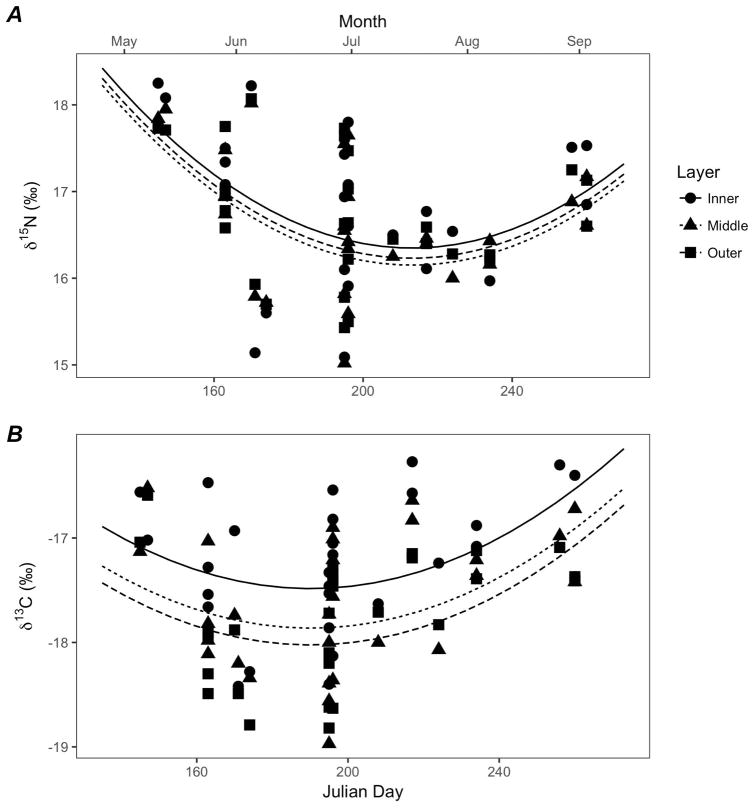
The AICc-best model for δ15N values included layer and day of the year (DOY) as predictors, without an interaction term (Table 5). A likelihood ratio test determined that the random effect of year did not significantly improve the fit of the model (L.ratio=5.68, p=1); hence it was not included in the final model, thereby effectively pooling data across years. The model suggests that δ15N values in all three layers decreased sharply on average through the first part of the season to a minimum in mid-summer, before increasing again towards the end of the season (Figure 5A). Random whale-specific intercepts suggested that the variability in mean δ15N values among individual whales (σa=0.63‰) was much larger than the residual variability within individuals (σ=0.21‰) as evident in a large spread around the mean seasonal trend. The best-fit model indicated that layer and day of the year explained 92% of the variability in δ15N values (R2=0.92). Although the best model included differences in mean δ15N values among layers, Tukey post-hoc tests showed no significant pairwise differences between layers (Middle-Inner t= −1.07, p=0.53; Outer-Inner, t= −0.64, p=0.80; Outer-Middle, t=0.43, p=0.90).
Table 5.
Results from linear mixed effects model selection for Experiment 2, identifying variables that influence δ15N and δ13C values in layers of skin of 28 sperm whales. Table shows the top five models for each isotope ratio. “DOY” refers to the day of the year variable, which is also included in the model as a quadratic term, and is allowed to interact with the layer variable. The symbol “+” indicates the factor variable is included in the model. Weights refer to AICc weights from each model.
| Model Rank | DOY2 | Layer | DOY* Layer | df | logLik | ΔAICc | Weight | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| δ15N | 1 | + | + | 8 | −36.05 | 0 | 0.81 | |
| 2 | + | 6 | −40.36 | 3.81 | 0.12 | |||
| 3 | + | 6 | −41.47 | 6.02 | 0.04 | |||
| 4 | + | + | + | 12 | −34.35 | 7.09 | 0.02 | |
| 5 | 4 | −45.79 | 10.10 | 0.01 | ||||
|
| ||||||||
| δ13C | 1 | + | + | 8 | −37.21 | 0 | 0.75 | |
| 2 | + | 6 | −40.76 | 2.28 | 0.24 | |||
| 3 | + | + | + | 12 | −36.52 | 9.10 | 0.01 | |
| 4 | + | 6 | −60.28 | 41.32 | 0 | |||
| 5 | 4 | −63.84 | 43.85 | 0 | ||||
Figure 5.
Observed sperm whale δ15N (A) and δ13C (B) values for each layer with respect to Julian day of the year, and predicted means for each layer of skin (solid line = inner, dotted line = middle, dashed line = outer) based on best-fit models.
For δ13C values, the best model chosen using AICc selection included layer and DOY as predictors (Table 5). As with δ15N values, the random effect of year did not significantly improve the fit of the model (L.ratio=1.25e-12, p=1) and this was excluded from the final model. Coefficients for all the top-ranked models indicated that the δ13C values decreased significantly from the inner layer of skin to the outer layer (Figure 5B). The R2 value for the best-fit model (R2=0.86) suggested that layer and day of the year could explain 86% of the variance in stable isotope ratios. The random variability in mean δ13C values among individuals (σa=0.50‰) was much larger than the residual variability within individuals (σ=0.25‰). Based on the reduced model, pairwise differences were significant between the inner and middle layer (t= −2.49, p=0.03) and between the inner and outer layers (t= −3.55, p=0.001), but not between the middle and outer layers (t= −1.06, p=0.54).
DISCUSSION
The results from experiment 1 provide preliminary support to the hypothesis of a dietary time series in layers of the skin of cetaceans 41, specifically in humpback and sperm whales. We found significant differences in stable isotope ratios among different layers but not among cores in both sperm and humpback whales, which are known for their isotopically diverse diets 61,66–71,73–75. Furthermore, the stable isotope ratios did not differ among layers or cores in the skin of the fin whale, consistent with our expectations for a species which is thought to have a less variable diet 19,61–65. These results suggest that subsampling only the inner layer of cetacean tissue could provide a means for assessing recent diets, better suited for interpretation with observational feeding studies and comparative studies where prey is sampled as well.
While the ANOVA results found that cores were not included in the best-fit model, there was variability in stable isotope ratios across layers (Figure 3). However, while systematic in the differences among layers, the variability within a layer (across cores) was minimal and random. Furthermore, all but two of the ranges in δ13C and δ15N values within a layer were under 0.5‰, which was lower than the range of stable isotope ratios measured across 11 different skin positions in common and striped dolphins 39. Other causes of variability within a layer, including the two outlier layers with high variability, may be related to limitations in accuracy of cutting layers when sampling the skin. Increasing our sample size for each species would probably smooth out that variability. We have included subsampling within layers (cores) to serve as a control because we do not expect stable isotope ratios to vary systematically by core (we do have this expectation of values by layer). However, it is no surprise that there would be some random variation, which is what makes core a useful control, and thus the low variability in the stable isotope ratios of cores found in this study justifies the ANOVA results of non-significance, implying heterogeneity.
The stable isotope ratios in experiment 1 were within or near the range of what is to be expected from each of these species 1,2,19,53. The lack of significant differences between cores or layers in the fin whale indicates that the whale did not undergo a marked diet-shift during the turnover time of the skin. This was expected given the fairly homogenous diet of this species 19,53,61. The fin whale’s δ13C values were surprisingly low, 1–2‰ lower than the δ13C values observed from other studies of fin whales 19,53,65. However, two of these studies were conducted at either a much lower latitude 53 or a combination of a lower latitude and different ocean basin 19, both of which can significantly affect δ13C values 93–96. In particular, higher latitude foraging is known to result in lower (more negative) δ13C values in the North Pacific Ocean 93,94,96. Witteveen and Wynne 65 found δ13C values of near-shore fin whales in a similar region to this study to be on average 1.5‰ higher than found in this study. Given similar latitude in the same ocean basin, this suggests that the fin whale in this study was foraging further offshore. Stable isotope studies of zooplankton in Prince William Sound and the central GOA have shown strong patterns of lower δ13C values offshore 97, though seasonal and annual differences and fluctuations do exist. This whale was struck by a cruise ship transiting the GOA, whereas the samples from Witteveen and Wynne were collected near-shore close to Kodiak Island, perhaps resulting in higher δ13C values 65.
The difference in stable nitrogen isotope ratios between the inner and outer layers of the humpback whale could indicate diet shifts from higher trophic level herring in the spring to lower trophic level krill in the summer before it died. Humpback whales in Southeast Alaska eat a mixed diet of herring, krill, and other forage fish 66–69,98, and have a lower trophic level diet overall than those in other areas of Alaska 2. The humpback whale sampled in this analysis was found dead on July 28, 2010, and while deposition rates are unknown for this species, incorporation of stable isotopes from prey into tissue has been estimated in other cetacean species to occur between 2.5 and 6 months 41,42,59. Herring spawn in Southeast Alaska in the spring, forming dense aggregations that attract large numbers of humpback whales as they return from their low-latitude breeding areas 70,98. The outer layer of our sample, representing the least recent diet, shows the highest δ15N value, which is consistent with that of a whale foraging primarily on herring or other schooling fish such as eulachon or pollock 29. Krill are the most common humpback whale prey in Southeast Alaska and have their highest energy content in late summer and fall 67, which could explain the low δ15N values in the inner layer of our sample that reflects prey consumption during the summer months.
The sperm whale’s higher δ15N values than the fin and humpback whales reflect its higher trophic position (Table 3, Figure 2). The sperm whale’s higher δ13C values can be attributed to foraging on higher trophic level species found further offshore than the two baleen whales (Table 3, Figure 2). An increase in the δ13C value of the inner layer of sperm whale skin of just over 1‰ represents a trophic level difference and could indicate a recent shift to more benthic prey or higher-latitude prey 9,94,99,100. Higher-latitude feeding tends to result in higher δ13C values in sperm whale skin in the southern hemisphere 52, which would be consistent with the inner layer of sperm whale skin in this study if the whale had been a recent arrival to Alaskan waters 94,99,101. Conversely higher latitude has shown decreased δ13C values in other studies, which would not be supported by this data 94. Foraging on larger squid and foraging closer to shore results in higher δ13C values in sperm whale tissue 94. Finally, even if the whale had been foraging in the same Alaskan waters for an extended period of time, seasonal shifts in the isotopic baseline of the ecosystem could be responsible for the trophic level shift between layers 6,11,100. The lack of significant differences in the δ15N values of this individual could simply indicate that the trophic position of sperm whale prey between different foraging areas or during the timeline of the skin was not significantly different. There have been no comprehensive stable isotope analyses of prey species of sperm whales in the GOA to confirm this, but the δ15N values found in this study are similar to those measured from adult male sperm whales in the Gulf of California 1.
The specific dietary time period represented by each layer of skin remains unknown, and probably varies between species and regions. A recent study with blue whales has estimated full isotopic incorporation (turnover) in nitrogen isotopes (δ15N values) of the largest cetacean and baleen whale to be 163±91 (mean±SD) days 41. For odontocete species, the most comprehensive controlled feeding experiments have used bottlenose dolphins as a subject, one of which found 95% turnover to be achieved in 104±35 days for δ13C values and 180±71 days for δ15N values 42. The results from these two studies are surprising given that they estimated similar isotopic incorporation rates despite vast differences in size of the two cetaceans represented, the blue whale and the bottlenose dolphin 41,42. For δ15N values alone, assuming that new skin is formed and sloughed at a constant rate, each layer (strata) of skin could be estimated to represent 54±52.5 or 68.7±49 days of dietary information in the blue whale and bottlenose dolphin studies, respectively, and applied to other (e.g. sperm whale) species. It must be noted too that water temperature probably has an effect on epidermal turnover. Studies have shown that both killer whales and beluga whales use seasonal migration to or through warmer waters to stimulate epidermal molt and skin sloughing 59,102. The isotopic incorporation rates studied recently in blue whales in two different foraging regions found very different isotopic incorporation rates between the regions (84±5 days for Gulf of California versus 242±44 days for the California Current) 41. The authors hypothesized that cooler water temperatures in the California Current ecosystem could have slowed turnover rates in that region compared with the warmer water of the Gulf of California.
For experiment 2, layers of skin from 28 individual sperm whales showed similar patterns to those found in the single sperm whale from experiment 1. However, experiment 2 also showed a surprising consistency in the patterns of isotopic differences among layers, highlighting the need for more research. The range of δ15N values among layers in an individual averaged 0.4‰ (min=0.1‰; max=1.1‰), while the range of δ13C values among layers averaged 0.7‰ (min=0.1‰; max=1.5‰). Given the variability of 0.1‰ found in replicate skin samples from a single humpback whale35, these findings indicate that sperm whale skin reveals differences in isotopic ratios among layers that cannot be explained by within-tissue variability alone nor by the precision of the mass spectrometer.
The presence of trends in stable isotope ratios among layers of different individuals found in mixed effects modeling of experiment 2 was not expected (Figures 4 and 5). In spite of high variability among individuals, the inner layer of skin generally had higher δ15N and δ13C values than other layers throughout the summer season (Figure 5) with the pattern being more pronounced in the δ13C values. This pattern persisted over time, across a significant seasonal trend where δ15N values dropped mid-summer and increased again in late summer (Figure 5A) and δ13C values showed a slight decrease earlier in the summer and then increased in late summer (Figure 5B). Had these animals all come to Alaska around the same time period in late spring, we would have expected the later-season samples to have outer and middle layers trending toward the inner layer (and inner layers of the early-season samples) as the full thickness of the skin began to represent their Alaskan foraging ground habitats. Similarly, if all animals had been experiencing the same seasonal shift in diet, we would again have expected later season samples to have outer and middle layers trending toward the inner layer. Finally, if animals had all been foraging in different areas prior to coming to Alaskan waters, or if they had been eating variable diets, we might have expected layers to all exhibit differences individually, but we would not expect those differences to carry the same trend, in that the value in the inner layer of skin was consistently higher across multiple individuals taken over a 4 month time period. Instead the trend the we see is that, no matter when they were sampled (May through September), most sperm whales had higher δ13C values in their inner layer than in their outer layer.
There are a few potential reasons for why we might see a trend in layers of sperm whale skin with consistently higher δ13C values in the inner layer of skin across multiple individuals. It is possible that the turnover rate in sperm whales at high latitudes is extremely long, as Busquets-Vass et al 41 hypothesized for blue whales, and each layer of skin represents a longer time period than the estimated 54–68 days mentioned above. Given Busquets-Vass et al’s 41 estimation of 242±44 days for isotopic incorporation in the California Current region (the northernmost region of their study), each layer would represent 80±25 days, probably more for whales inhabiting even colder waters in the GOA. Indeed, evidence suggests that sperm whales slough large amounts of skin in Gulf of California waters 103, while anecdotal evidence from the authors’ 15-year history of field research is that sloughed skin is rarely if ever seen in GOA waters. We propose that isotopic incorporation rates of sperm whale skin slow as they move to higher latitudes. With our range of sampling dates spanning from late May to mid-September (115 days total), our samples could conceivably represent one season (summer). Thus, the inner layer of skin may represent a shift in diet, baseline isotope ratios, or large-scale movement for each whale individually to higher δ13C values than in their other layers. While each individual whale has its own “niche” of diet preference, the higher isotopic ratios in the inner layers may represent a large-scale, seasonal baseline shift from winter/spring to summer/fall signatures that affected all individuals regardless of sampling date.
Although the inner layer of skin may represent a longer time period spanning an entire summer, or nearly four months time, the specific causes of higher δ13C values in the inner layer of skin remain inconclusive. Studies on plankton and squid have shown that δ13C values generally decrease with increasing latitude 93,101, although they may slightly increase again at latitudes north of 40°N 94. This pattern is opposite to what we would expect if the inner layer of our samples represented the result of a migration from warmer equatorial waters to Alaskan waters in the summer. It must be noted that sperm whales have a higher trophic level than species in these studies; thus, the inner layer would not be an ideal tissue to infer baseline shifts because trophic discrimination probably obscures changes in baseline patterns. In addition, while male sperm whales are generally thought to move to GOA waters in the spring and summer months, no pattern exists for sperm whale departures from Alaskan waters 104, suggesting that arrivals are similarly non-patterned. Shifting prey species between regions could also explain changes in stable isotope ratios, as sperm whales in the eastern GOA are known to consume more deep sea fish than squid, unlike other regions off the western coast of North America where squid is the primary diet item 1,61,73,74,105. While the stable isotope ratios of prey in the eastern GOA are largely unknown, the δ13C values of squid in the California Current region increase with size 1 and are generally higher than those of deep sea fish in that region 11. This pattern is again opposite to what would be expected if the inner layer of skin represented diet from the GOA versus diet from lower latitude habitats. Together, these potential isotopic shifts that would result in opposite patterns in the layers of sperm whale skin from what was observed in this study signal that there is much still to be learned about how stable isotopes move through ecological systems and are incorporated into consumer tissues.
The observed consistent stable isotope gradient among individuals could also be due to improperly extracted lipids or structural differences among layers, but we consider these explanations to be unlikely. Differences in lipids among different layers of the skin may not be properly accounted for in the analyses. C:N ratios can be an indicator of carbon content, and are used as a metric for lipid extraction in studies where high lipid content is inherent to the tissue. 82,106 We are confident that our lipid-extraction methods were not the cause of the trends observed in δ13C values between layers. We used standard lipid-extraction techniques 84,88,89, and found δ13C values to vary significantly between non-lipid-extracted and lipid-extracted values. There was also no trend in δ13C values when plotted against the C:N ratios of our lipid-extracted samples. Opinion in the literature varies when discussing the C:N ratios of sufficiently lipid-extracted tissues with appropriate values between 3.5 and 4, and our values ranged from 2.9 to 3.8 falling within the range of other studies 21,81,82,87. In addition, if lipid-extraction were not complete, we would expect the inner layer, nearest to the blubber, to have lower δ13C values than other layers, rather than higher. Furthermore, differences between layers were not completely consistent across individuals (Figure 4B), including some individuals with lower δ13C values in the inner layer. These differences among individuals suggest that the patterns that we are seeing are not due to structural differences in the layers of skin that might affect isotope ratios.
Within the consistent pattern of higher delta values in the inner layer of sperm whale skin, there was also a significant seasonal gradient for both δ13C and δ15N values. The interpretation of this seasonal gradient across the sampling period of May to September also depends on isotopic incorporation rates, and the temporal snapshot of each layer. A slower incorporation rate as suggested above, with the inner layer representing a 4-month period, would potentially indicate a lagged dietary shift, where the inner layer of skin is responding to foraging changes some time before they appear in the skin. Rather than a single factor driving the patterns of change over time that were observed, it is more likely a combination of seasonal and regional diet shifts, changes in isotopic baselines, and more nuanced movement behaviors that influence the stable isotope ratios observed in this study.
The differences in seasonal trends among layers indicate that some trends may also be masked by the high variability among individual whales and by the uneven distribution of samples across the season. The nature of the opportunistic sampling for sperm whale tissues resulted in the earliest sampling date of late May, and the latest sampling date of mid-September, representing 3.5 months or around ¼ of a year. Within this relatively short sampling season, the majority of samples were collected in July (Figure 5). With a turnover rate in high latitudes that is potentially upwards of 242 days (2/3 of a year), sampling whales early in the season (March and April) as well as later in the season (October and November), and re-sampling the same individuals over the course of a season would shed more light on the specific seasonal drivers of diet for these animals. In addition, increased knowledge of prey characteristics and availability in these habitats would be useful to better understand sperm whale foraging habits in this region.
CONCLUSION
In light of these experiments and findings of variable stable isotope ratios among layers of cetacean skin, we suggest that this pilot study provides conditional evidence that cetacean skin shows a dietary time series. Directed sampling of specific layers of skin across a population may allow for more nuanced analysis in dietary trends of whales. However, we also contend that attributing trends in the observed stable isotope ratios to specific ecological systems is more nuanced than may have been previously thought. Above all, this study supports the argument that in stable isotope analysis of cetacean skin it is important to select a portion of skin to analyze based on the species and objectives of the study. Due to structure of skin, there may be a significant temporal gradient between the inner and outer layers of skin. For studies focused on diet in species where foraging preference may change over time or the species may migrate, the inner layer of skin may give the most precise information to compare with prey and ecosystem baselines or in mixing models to assess prey contribution. However, for studies focusing on general isotopic niche and trophic level calculations, a full-thickness homogenized sample of skin may provide a more appropriate long-term average of diet composition for the species. Collection of sloughed skin samples may be useful as an opportunistic sampling method for species that have a more general diet, do not exhibit prey switching, and/or do not migrate. Most importantly, further research with cetacean skin should seek to expand on this research to examine specifically how stable isotopes move through the tissue, and at what temporal rates. Overall, it is imperative that researchers communicate the portion of skin used in the study to allow the scientific community to better assess conclusions taken from isotope studies.
Supplementary Material
Acknowledgments
We would like to thank Kate Savage, John Moran, Johanna Vollenweider, and Russ Andrews for assistance acquiring skin samples and necropsy information on the humpback and fin whales, and allowing us to use a small portion for analysis. Russ Andrews, Nellie Werner, John Calambokidis, and Greg Schorr performed biopsy sampling of sperm whales. All biopsy samples from 2003–2016 were collected under NMFS Permit #14122, and samples from 2016 and 2017 were collected under NMFS Permit #18529, both issued to Jan Straley. Kelly Robertson at Southwest Fisheries Science Center provided archival of 2003–2010 samples, and shipped them between locations. Mat Wooller provided initial feedback on the study with his “ten well-chosen samples” exercise as part of his Stable Isotope Techniques in Environmental Research class. Kate Hauch assisted with stable isotope preparation of humpback and sperm whale samples. Finally, we would like to thank Jennifer Cedarleaf for archival and management of historical sperm whale sample data at the Cetacean Human Interaction Lab in Sitka. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers UL1GM118991, TL4GM118992, or RL5GM118990. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Ruiz-Cooley RI, Gendron D, Aguíñiga S, Mesnick S, Carriquiry JD. Trophic relationships between sperm whales and jumbo squid using stable isotopes of C and N. Mar Ecol Prog Ser. 2004;277:275–283. doi: 10.3354/meps277275. [DOI] [Google Scholar]
- 2.Witteveen BH, Worthy GAJ, Wynne KM, Roth JD. Population structure of North Pacific humpback whales on their feeding grounds revealed by stable carbon and nitrogen isotope ratios. Mar Ecol Prog Ser. 2009;379:299–310. doi: 10.3354/meps07900. [DOI] [Google Scholar]
- 3.Witteveen BH, Worthy GaJ, Roth JD. Tracing migratory movements of breeding North Pacific humpback whales using stable isotope analysis. Mar Ecol Prog Ser. 2009;393:173–183. doi: 10.3354/meps08231. [DOI] [Google Scholar]
- 4.Witteveen BH, Worthy GaJ, Wynne KM, Hirons AC, Andrews AG, Markel RW. Trophic levels of North Pacific Humpback whales (Megaptera novaeangliae) through analysis of stable isotopes: Implications on prey and resource quality. Aquat Mamm. 2011;37(2):101–110. doi: 10.1578/AM.37.2.2011.101. [DOI] [Google Scholar]
- 5.Bailleul F, Authier M, Ducatez S, et al. Looking at the unseen: Combining animal bio-logging and stable isotopes to reveal a shift in the ecological niche of a deep diving predator. Ecography (Cop) 2010;33(4):709–719. doi: 10.1111/j.1600-0587.2009.06034.x. [DOI] [Google Scholar]
- 6.Newsome SD, Etnier MA, Kurle CM, Waldbauer JR, Chamberlain CP, Koch PL. Historic decline in primary productivity in western Gulf of Alaska and eastern Bering Sea: Isotopic analysis of northern fur seal teeth. Mar Ecol Prog Ser. 2007;332:211–224. doi: 10.3354/meps332211. [DOI] [Google Scholar]
- 7.Hobson KA, Schell DM, Renouf D, Noseworthy E. Stable carbon and nitrogen isotopic fractionation between diet and tissue of captive seals: implications for dietary reconstruction involving marine mammals. Can J Fish Aquat Sci. 1996;53:528–533. doi: 10.1139/z00-008. [DOI] [Google Scholar]
- 8.Boutton TW. Stable Carbon Isotope Ratios of Natural Materials: II. Atmospheric, Terrestrial, Marine, and Freshwater Environments. In: Coleman D, Fry B, editors. Carbon Isotope Techniques. San Diego, CA: Academic Press; 1991. pp. 173–185. [Google Scholar]
- 9.Cherel Y, Hobson KA. Geographical variation in carbon stable isotope signatures of marine predators: A tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser. 2007;329:281–287. doi: 10.3354/meps329281. [DOI] [Google Scholar]
- 10.Hobson KA, Piattt JF, Pitocchelli J. Using Stable Isotopes to Determine Seabird Trophic relationships. J Anim Ecol. 1994;63(4):786–798. [Google Scholar]
- 11.Miller TW, Brodeur RD, Rau G, Omori K. Prey dominance shapes trophic structure of the northern California Current pelagic food web: Evidence from stable isotopes and diet analysis. Mar Ecol Prog Ser. 2010;420:15–26. doi: 10.3354/meps08876. [DOI] [Google Scholar]
- 12.Checkley DM, Entzeroth LC. Elemental and isotopic fractionation of carbon and nitrogen by marine, planktonic copepods and implications to the marine nitrogen cycle. J Plankton Res. 1985;7(4):553–568. doi: 10.1093/plankt/7.4.553. [DOI] [Google Scholar]
- 13.Checkley DM, Miller CA. Nitrogen isotope fractionation by oceanic zooplankton. Deep Sea Res Part A, Oceanogr Res Pap. 1989;36(10):1449–1456. doi: 10.1016/0198-0149(89)90050-2. [DOI] [Google Scholar]
- 14.Gaebler OH, Vitti TG, Vukmirovich R. Isotope effects in metabolism of 14N and 15N from unlabled dietary proteins. Can J Biochem. 1966;44(1966) doi: 10.1139/o66-142. [DOI] [PubMed] [Google Scholar]
- 15.Hobson KA, Welch HE. Determination of trophic relationships within a high Arctic marine food web using δ13C and δ15N analysis. Mar Ecol Prog Ser. 1992;84(1):9–18. doi: 10.3354/meps084009. [DOI] [Google Scholar]
- 16.Minagawa M, Wada E. Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta. 1984;48(5):1135–1140. doi: 10.1016/0016-7037(84)90204-7. [DOI] [Google Scholar]
- 17.Steele KW, Daniel RM. Fractionation of nitrogen isotopes by animals: a further complication to the use of variations in the natural abundance of 15N for tracer studies. J Agric Sci. 1978;90:7–9. doi: 10.1017/S002185960004853X. [DOI] [Google Scholar]
- 18.Peterson BJ, Fry B. Stable isotopes in ecosystem studies. Annu Rev Ecol Syst. 1987;18:293–320. doi: 10.1146/annurev.es.18.110187.001453. [DOI] [Google Scholar]
- 19.Borrell A, Abad-Oliva N, Gõmez-Campos E, Giménez J, Aguilar A. Discrimination of stable isotopes in fin whale tissues and application to diet assessment in cetaceans. Rapid Commun Mass Spectrom. 2012;26(14):1596–1602. doi: 10.1002/rcm.6267. [DOI] [PubMed] [Google Scholar]
- 20.DeNiro MJ, Epstein S. Influence of Diet on the Distribution of Nitrogen Isotopes in Animals. Geochim Cosmochim Acta. 1981;45(3):341–351. doi: 10.1016/0016-7037(81)90244-1. [DOI] [Google Scholar]
- 21.Newsome SD, Clementz MT, Koch PL. Using stable isotope biogeochemistry to study marine mammal ecology. Mar Mammal Sci. 2010;26(3):509–572. doi: 10.1111/j.1748-7692.2009.00354.x. [DOI] [Google Scholar]
- 22.Browning NE, Dold C, Fan IJ, Worthy GaJ. Isotope turnover rates and diet-tissue discrimination in skin of ex situ bottlenose dolphins (Tursiops truncatus) J Exp Biol. 2014;217(2):214–221. doi: 10.1242/jeb.093963. [DOI] [PubMed] [Google Scholar]
- 23.Best PB, Schell D. Stable Isotopes in southern right whale (Eubalaena australis) baleen as indicators of seasonal movements, feeding and growth. Mar Biol. 1996;124:483–494. [Google Scholar]
- 24.Hooker SK, Iverson SJ, Ostrom P, Smith SC. Diet of northern bottlenose whales inferred from fatty-acid and stable-isotope analyses of biopsy samples. Can J Zool. 2001;79(8):1442–1454. doi: 10.1139/z01-096. [DOI] [Google Scholar]
- 25.Monteiro S, Ferreira M, Vingada JV, López A, Brownlow A, Méndez-fernandez P. Application of stable isotopes to assess the feeding ecology of long-finned pilot whale (Globicephala melas) in the Northeast Atlantic Ocean. J Exp Mar Bio Ecol. 2015;465:56–63. doi: 10.1016/j.jembe.2015.01.007. [DOI] [Google Scholar]
- 26.Fleming AH, Clark CT, Calambokidis J, Barlow J. Humpback whale diets respond to variance in ocean climate and ecosystem conditions in the California Current. Glob Chang Biol. 2015:1214–1224. doi: 10.1111/gcb.13171. [DOI] [PubMed] [Google Scholar]
- 27.Newsome SD, Tinker MT, Monson DH, et al. Using stable isotopes to investigate individual diet specialization in California sea otters (Enhydra lutris nereis) Ecology. 2009;90(4):961–974. doi: 10.1890/07-1812.1. [DOI] [PubMed] [Google Scholar]
- 28.Wright DL, Witteveen B, Wynne K, Horstmann-Dehn L. Evidence of two subaggregations of humpback whales on the Kodiak, Alaska, feeding ground revealed from stable isotope analysis. Mar Mammal Sci. 2015;31(4):1378–1400. doi: 10.1111/mms.12227. [DOI] [Google Scholar]
- 29.Witteveen BH, Worthy GAJ, Foy RJ, Wynne KM. Modeling the diet of humpback whales: An approach using stable carbon and nitrogen isotopes in a Bayesian mixing model. Mar Mammal Sci. 2012;28(3):E233–E250. doi: 10.1111/j.1748-7692.2011.00508.x. [DOI] [Google Scholar]
- 30.Newsome SD, Etnier Ma, Gifford-Gonzalez D, et al. The shifting baseline of northern fur seal ecology in the northeast Pacific Ocean. Proc Natl Acad Sci U S A. 2007;104(23):9709–9714. doi: 10.1073/pnas.0610986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hobson KA, Sease JL, Merrick RL, Piatt JF. Investigating Trophic Relationships of Pinnipeds in Alaska and Washington using Stable Isotope Ratios of Nitrogen and Carbon. Mar Mammal Sci. 1997;13(1):114–132. [Google Scholar]
- 32.Bjorkland RH, Pearson SF, Jeffries SJ, Lance MM, Acevedo-gutiérrez A, Ward EJ. Stable isotope mixing models elucidate sex and size effects on the diet of a generalist marine predator. Mar Ecol Prog Ser. 2015;526:213–225. doi: 10.3354/meps11230. [DOI] [Google Scholar]
- 33.Tieszen LL, Boutton TW, Tesdahl KG, Slade NA. Fractionation and Turnover of Stable Carbon Isotopes in Animal Tissues : Implications for δ 13 C Analysis of Diet. Oecologia. 1983;57(1):32–37. doi: 10.1007/BF00379558. [DOI] [PubMed] [Google Scholar]
- 34.Bowen WD, Iverson SJ. Methods of estimating marine mammal diets: A review of validation experiments and sources of bias and uncertainty. Mar Mammal Sci. 2013;29(4):719–754. doi: 10.1111/j.1748-7692.2012.00604.x. [DOI] [Google Scholar]
- 35.Todd S, Ostrom P, Lien J, Abrajano J. Use of Biopsy Samples of Humpback Whale (Megaptera novaeangliae) Skin for Stable Isotope (d13C) Determination. J Northwest Atl Fish Sci. 1997;22:71–76. [Google Scholar]
- 36.Ingram T, Matthews B, Harrod C, Stephens T, Grey J, Markel R. Lipid extraction has little effect on the δ15N of aquatic consumers. Limnol Oceanogr Methods. 2007 Apr;5:338–343. doi: 10.4319/lom.2007.5.338. 2016. [DOI] [Google Scholar]
- 37.Vander Zanden MJ, Rasmussen JB. Variation in δ15N and δ13C trophic fractionation: Implications for aquatic food web studies. Limnol Oceanogr. 2001;46(8):2061–2066. doi: 10.4319/lo.2001.46.8.2061. [DOI] [Google Scholar]
- 38.Hannides CCS, Popp BN, Landry MR, Graham BS. Quantification of zooplankton trophic position in the North Pacific Subtropical Gyre using stable nitrogen isotopes. Limnol Oceanogr. 2009;54(1):50–61. doi: 10.4319/lo.2009.54.1.0050. [DOI] [Google Scholar]
- 39.Arregui M, Josa M, Aguilar A, Borrell A. Isotopic homogeneity throughout the skin in small cetaceans. Rapid Commun Mass Spectrom. 2017;31(18):1551–1557. doi: 10.1002/rcm.7936. [DOI] [PubMed] [Google Scholar]
- 40.Thomas SM, Crowther TW. Predicting rates of isotopic turnover across the animal kingdom: A synthesis of existing data. J Anim Ecol. 2015;84(3):861–870. doi: 10.1111/1365-2656.12326. [DOI] [PubMed] [Google Scholar]
- 41.Busquets-Vass G, Newsome SD, Calambokidis J, et al. Estimating Blue Whale Skin Isotopic Incorporation Rates and Baleen Growth Rates: Implications for Assessing Diet and Movement Patterns in Mysticetes. PLoS One. 2017;12(5):e0177880. doi: 10.1371/journal.pone.0177880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giménez J, Ramírez F, Almunia J, Forero MG, de Stephanis R. From the pool to the sea: Applicable isotope turnover rates and diet to skin discrimination factors for bottlenose dolphins (Tursiops truncatus) J Exp Mar Bio Ecol. 2016;475:54–61. doi: 10.1016/j.jembe.2015.11.001. [DOI] [Google Scholar]
- 43.Zilversmit DB, Entenman C, Fishler MC. On the calculation of “turnover time” and “turnover rate” from experiments involving the use of labeling agents. J Gen Physiol. 1943;26(3):325–331. doi: 10.1085/jgp.26.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reiner JM. The Study of Metabolic Turnover Rates by Means of Istopic Tracers. Arch Biochem Biophys. 1953;46(1):53–79. doi: 10.1016/0003-9861(53)90170-2. [DOI] [PubMed] [Google Scholar]
- 45.Hobson KA, Clark RG. Assessing Avian Diets Using Stable Isotopes I: Turnover of 13 C in Tissues. Condor. 1992;94(1):181–188. doi: 10.2307/1368807. [DOI] [Google Scholar]
- 46.Vander Zanden MJ, Clayton MK, Moody EK, Solomon CT, Weidel BC. Stable isotope turnover and half-life in animal tissues: A literature synthesis. PLoS One. 2015;10(1):1–16. doi: 10.1371/journal.pone.0116182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caut S, Laran S, Garcia-Hartmann E, Das K. Stable isotopes of captive cetaceans (killer whales and bottlenose dolphins) J Exp Biol. 2011;214:538–545. doi: 10.1242/jeb.045104. [DOI] [PubMed] [Google Scholar]
- 48.Hobson KA, Schell DM. Stable carbon and nitrogen isotope patterns in baleen from eastern Arctic bowhead whales ( Balaena mysticetus ) Can J Fish Aquat Sci. 1998;55:2601–2607. [Google Scholar]
- 49.Hobson KA, Sease JL. Stable Isotope Analyses of Tooth Annuli Reveal Temporal Dietary Records : an Example Using Steller Sea Lions. Mar Mammal Sci. 1998;14(1):116–129. doi: 10.1111/j.1748-7692.1998.tb00694.x. [DOI] [Google Scholar]
- 50.Lambertsen RH. A Biopsy System for Large Whales and Its Use for Cytogenetics. J Mammal. 1987;68(2):443–445. [Google Scholar]
- 51.Winn HE, Bischoff WL, Taruski AG. Cytological Sexing of Cetacea. Mar Biol. 1973;23:343–346. [Google Scholar]
- 52.Marcoux M, Whitehead H, Rendell L. Sperm whale feeding variation by location,year, social group and clan: evidence from stable isotopes. Mar Ecol Progess Ser. 2007;333:309–314. doi: 10.3354/meps333309. [DOI] [Google Scholar]
- 53.Gendron D, Aguíñiga S, Carriquiry JD. δ15N and δ13C in skin biopsy samples: a note on their applicability for examining the relative trophic level in three rorqual species. J Cetacean Res Manag. 2001;3(1):1–4. [Google Scholar]
- 54.Abend AG, Smith TD. Differences in stable isotope ratios of carbon and nitrogen between long-finned pilot whales (Globicephala melas) and their primary prey in the western north Atlantic. ICES J Mar Sci. 1997;54:500–503. doi: 10.1006/jmsc.1996.0192. [DOI] [Google Scholar]
- 55.Ryan C, McHugh B, Trueman CN, Harrod C, Berrow SD, O’Connor I. Accounting for the effects of lipids in stable isotope (d13C and d15N values) analysis of skin and blubber of balaenopterid whales. Rapid Commun Mass Spectrom. 2012;26(23):2745–2754. doi: 10.1002/rcm.6394. [DOI] [PubMed] [Google Scholar]
- 56.Geraci J, St Aubin D, Hicks B. The epidermis of odontocetes: a view from within. In: Bryden M, Harrison R, editors. Research on Dolphins, Part 1. Oxford: Clarendon Press; 1986. pp. 3–21. [Google Scholar]
- 57.Reeb D, Best PB, Kidson SH. Structure of the Integument of Southern Right Whales, Eubalaena australis. Anat Rec. 2007;290:596–613. doi: 10.1002/ar.20535. [DOI] [PubMed] [Google Scholar]
- 58.Hicks BD, St Aubin DJ, Geraci JR, Brown WR. Epidermal Growth in the Bottlenose Dolphin, Tursiops truncatus. J Invest Dermatol. 1985;85(1):60–63. doi: 10.1111/1523-1747.ep12275348. [DOI] [PubMed] [Google Scholar]
- 59.StAubin DJ, Smith TG, Geraci JR. Seasonal epidermal molt in beluga whales, Delphinapterus leucas. Can J Zool. 1990;68(2):359–367. doi: 10.1139/z90-051. [DOI] [Google Scholar]
- 60.Giacometti L. The Skin of the Whale (Balaenoptera physalus) Anat Rec. 1967;159:69–75. doi: 10.1002/ar.1091590110. [DOI] [PubMed] [Google Scholar]
- 61.Flinn RD, Trites AW, Gregr EJ, Perry RI. Diets of Fin, Sei, and Sperm Whales in British Columbia: an Analysis of Commercial Whaling Records. Mar Mammal Sci. 2002;18(3):663–679. [Google Scholar]
- 62.Pauly D, Trites AW, Capuli E, Christensen V. Diet composition and trophic levels of marine mammals. ICES J Mar Sci. 1998;55:467–481. doi: 10.1006/jmsc.1997.0280. [DOI] [Google Scholar]
- 63.Mizroch SA, Rice DW, Breiwick JM. The fin whale, Balaenoptera physalus. Mar Fish Rev. 1984;46(4):20–24. [Google Scholar]
- 64.Tershy BR. Body Size, Diet, Habitat Use, and Social Behavior of Balaenoptera Whales in the Gulf of California. J Mammal. 1992;73(3):477–486. [Google Scholar]
- 65.Witteveen BH, Wynne KM. Trophic niche partitioning and diet composition of sympatric fin (Balaenoptera physalus) and humpback whales (Megaptera novaeangliae) in the Gulf of Alaska revealed through stable isotope analysis. Mar Mammal Sci. 2016;32(4):1319–1339. doi: 10.1111/mms.12333. [DOI] [Google Scholar]
- 66.Nowacek DP, Friedlaender AS, Halpin PN, et al. Super-aggregations of krill and humpback whales in Wilhelmina bay, Antarctic Peninsula. PLoS One. 2011;6(4):2–6. doi: 10.1371/journal.pone.0019173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szabo A. Immature euphausiids do not appear to be prey for humpback whales (Megaptera novaeangliae) during spring and summer in Southeast Alaska. Mar Mammal Sci. 2015;31(2):677–687. doi: 10.1111/mms.12183. [DOI] [Google Scholar]
- 68.Rice SD, Moran JR, Straley JM, Boswell KM, Heintz RA. Significance of Whale Predation on Natural Mortality Rate of Pacific Herring in Prince William Sound. 2010 [Google Scholar]
- 69.Boswell KM, Rieucau G, Vollenweider JJ, et al. Are spatial and temporal patterns in Lynn Canal overwintering Pacific herring related to top predator activity? Can J Fish Aquat Sci. 2016;73(9):1307–1318. doi: 10.1139/cjfas-2015-0192. [DOI] [Google Scholar]
- 70.Straley JM, Moran JR, Boswell KM, et al. IN PRESS Seasonal presence and potential influence of foraging humpback whales on Pacific herring populations wintering in the Gulf of Alaska. Deep Res Part II. 2017;147:173–186. doi: 10.1016/j.dsr2.2017.08.008. [DOI] [Google Scholar]
- 71.Witteveen BH, Foy RJ, Wynne KM, Tremblay Y. Investigation of foraging habits and prey selection by humpback whales (Megaptera novaeangliae) using acoustic tags and concurrent fish surveys. Mar Mammal Sci. 2008;24(3):516–534. doi: 10.1111/j.1748-7692.2008.00193.x. [DOI] [Google Scholar]
- 72.Whitehead H. Sperm Whales. Chicago: The University of Chicago Press; 2003. [Google Scholar]
- 73.Kawakami T. A review of sperm whale food. Sci Rep Whales Res Inst. 1980;32:199–218. [Google Scholar]
- 74.Okutani T, Nemoto T. Squids as the food of sperm whales in the Bering Sea and Alaskan Gulf. Sci Rep Whales Res Inst. 1964;18:111–121. [Google Scholar]
- 75.Clarke MR, MacLeod N. Cephalopod remains from sperm whales caught off Western Canada. Mar Biol. 1980;59(4):241–246. [Google Scholar]
- 76.Sigler MF, Lunsford CR, Straley JM, Liddle JB. Sperm whale depredation of sablefish longline gear in the northeast Pacific Ocean. Mar Mammal Sci. 2008;24(1):16–27. doi: 10.1111/j.1748-7692.2007.00149.x. [DOI] [Google Scholar]
- 77.Hill PS, Laake JL, Mitchell E. Results of a pilot program to document interactions between sperm whales and longline vessels in Alaskan waters. NOAA Tech Memo. 1999:42. NMFS-AFSC-(November) [Google Scholar]
- 78.Straley J, O’Connell V, Liddle J, et al. Southeast Alaska Sperm Whale Avoidance Project (SEASWAP): a successful collaboration among scientists and industry to study depredation in Alaskan waters. ICES J Mar Sci. 2015;72(5):1598–1609. doi: 10.1093/icesjms/fsv090. [DOI] [Google Scholar]
- 79.Peterson MJ, Carothers C. Whale interactions with Alaskan sablefish and Pacific halibut fisheries: Surveying fishermen perception, changing fishing practices and mitigation. Mar Policy. 2013;42:315–324. doi: 10.1016/j.marpol.2013.04.001. [DOI] [Google Scholar]
- 80.DeNiro MJ, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta. 1978;42(5):495–506. doi: 10.1038/317806a0. [DOI] [Google Scholar]
- 81.McConnaughey T, McRoy CP. Food-Web structure and the fractionation of Carbon isotopes in the bering sea. Mar Biol. 1979;53(3):257–262. doi: 10.1007/BF00952434. [DOI] [Google Scholar]
- 82.Lesage V, Morin Y, Rioux È, Pomerleau C, Ferguson SH, Pelletier É. Stable isotopes and trace elements as indicators of diet and habitat use in cetaceans: Predicting errors related to preservation, lipid extraction, and lipid normalization. Mar Ecol Prog Ser. 2010;419:249–265. doi: 10.3354/meps08825. [DOI] [Google Scholar]
- 83.Newsome SD, Chivers SJ, Berman Kowalewski M. The influence of lipid-extraction and long-term DMSO preservation on carbon (δ13C) and nitrogen (δ15N) isotope values in cetacean skin. Mar Mammal Sci. 2017 doi: 10.1111/mms.12454. [DOI] [Google Scholar]
- 84.Sweeting CJ, Polunin NVC, Jennings S. Effects of chemical lipid extraction and arithmetic lipid correction on stable isotope ratios of fish tissues. Rapid Commun Mass Spectrom. 2006;20(4):595–601. doi: 10.1002/rcm.2347. [DOI] [PubMed] [Google Scholar]
- 85.Murry Ba, Farrell JM, Teece Ma, Smyntek PM. Effect of lipid extraction on the interpretation of fish community trophic relationships determined by stable carbon and nitrogen isotopes. Can J Fish Aquat Sci. 2006;63(10):2167–2172. doi: 10.1139/f06-116. [DOI] [Google Scholar]
- 86.Sotiropoulos MA, Tonn WM, Wassenaar LI. Effects of lipid extraction on stable carbon and nitrogen isotope analyses of fish tissues: Potential consequences for food web studies. Ecol Freshw Fish. 2004;13(3):155–160. doi: 10.1111/j.1600-0633.2004.00056.x. [DOI] [Google Scholar]
- 87.Post DM, Layman Ca, Arrington DA, Takimoto G, Quattrochi J, Montaña CG. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia. 2007;152(1):179–189. doi: 10.1007/s00442-006-0630-x. [DOI] [PubMed] [Google Scholar]
- 88.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226(1):497–509. doi: 10.1007/s10858-011-9570-9. [DOI] [PubMed] [Google Scholar]
- 89.Logan JM, Lutcavage ME. A comparison of carbon and nitrogen stable isotope ratios of fish tissues following lipid extractions with non-polar and traditional chloroform/methanol solvent systems. Rapid Commun Mass Spectrom. 2008;22(7):1081–1086. doi: 10.1002/rcm. [DOI] [PubMed] [Google Scholar]
- 90.Burnham KP, Anderwon DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2. New York: Springer-Verlag; 2002. [Google Scholar]
- 91.Zar JH. Biostatistical Analysis. 2. Englewood Cliffs, NJ, NJ: Prentice-Hall, Inc; 1984. [Google Scholar]
- 92.R Core Team. R: A language and environment for statistical comuting. R Foundation for Statistical Computing; Vienna, Australia: 2016. [Google Scholar]
- 93.Goericke R, Fry B. Variations of marine plankton δ13C with latitude, temperature, and dissolved CO2 in the world ocean. Global Biogeochem Cycles. 1994;8(1):85–90. [Google Scholar]
- 94.Ruiz-Cooley RI, Gerrodette T. Tracking large-scale latitudinal patterns of δ13C and δ15N along the E Pacific using epi-mesopelagic squid as indicators. Ecosphere. 2012;3(7):1–17. doi: 10.1890/ES12-00094.1. [DOI] [Google Scholar]
- 95.Navarro J, Coll M, Somes CJ, Olson RJ. Trophic niche of squids: Insights from isotopic data in marine systems worldwide. Deep Res Part II Top Stud Oceanogr. 2013;95:93–102. doi: 10.1016/j.dsr2.2013.01.031. [DOI] [Google Scholar]
- 96.Graham BS, Koch PL, Newsome SD, McMahon KW, Aurioles D. Using Isoscapes to Trace the Movements and Foraging Behavior of Top Predators in Oceanic Systems. In: West JB, et al., editors. Isoscapes: Understanding Movement, Pattern, and Process on Earth Through Isotope Mapping. Springer Science + Business Media; 2010. pp. 299–318. [DOI] [Google Scholar]
- 97.Kline TC. Characterization of carbon and nitrogen stable isotope gradients in the northern Gulf of Alaska using terminal feed stage copepodite-V Neocalanus cristatus. Deep Res Part II. 2009;56(24):2537–2552. doi: 10.1016/j.dsr2.2009.03.004. [DOI] [Google Scholar]
- 98.Heintz RA, Moran J, Vollenweider JJ, et al. Humpback whale predation and the case for top-down control of local herring populations in the Gulf of Alaska. AFSC Q report, Res Featur. 2010 Nov-Dec;:1–6. http://www.afsc.noaa.gov/Quarterly/ond2010/ond2010featurelead.htm.
- 99.Takai N, Onaka S, Yatsu A, Kidokoro H, Sakamoto W. Geographical variations in carbon and nitrogen stable isotope ratios in squid. J Mar Biol Assoc United Kingdom. 2000;80:675–684. [Google Scholar]
- 100.Fry B. Stable Isotope Ecology. New York, NY: Springer; 2006. [Google Scholar]
- 101.Rau GH, Sweeney RE, Kaplan IR. Plankton 13C:12C ratio changes with latitude: differences between northern and southern oceans. Deep Res. 1982;29(8A):1035–1039. [Google Scholar]
- 102.Durban JW, Pitman RL. Antarctic killer whales make rapid, round-trip movements to subtropical waters: evidence for physiological maintenance migrations? Biol Lett. 2012;8(2):274–277. doi: 10.1098/rsbl.2011.0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Díaz-Gamboa RE, Gendron D, Busquets-Vass G. Isotopic niche width differentiation between common bottlenose dolphin ecotypes and sperm whales in the Gulf of California. Mar Mammal Sci. 2017;34(2):440–457. doi: 10.1111/mms.12465. [DOI] [Google Scholar]
- 104.Straley J, Schorr G, Thode AM, et al. Depredating sperm whales in the Gulf of Alaska: local habitat use and long distance movements across putative population boundaries. Endanger Species Res. 2014;24(2):125–135. doi: 10.3354/esr00595. [DOI] [Google Scholar]
- 105.Harvey JT, Friend T, McHuron EA. Cephalopod remains from stomachs of sperm whales (Physeter macrocephalus) that mass-stranded along the Oregon coast. Mar Mammal Sci. 2014;30(2):609–625. doi: 10.1111/mms.12063. [DOI] [Google Scholar]
- 106.Logan JM, Jardine TD, Miller TJ, Bunn SE, Cunjak RA, Lutcavage ME. Lipid corrections in carbon and nitrogen stable isotope analyses: Comparison of chemical extraction and modelling methods. J Anim Ecol. 2008;77(4):838–846. doi: 10.1111/j.1365-2656.2008.01394.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.