Abstract
Background & Aims
EUS-guided hepaticoenterostomy (EUS-HE) is usually reserved for palliation of malignant biliary obstruction after failed endoscopic retrograde cholangiography (ERC) or inaccessible biliary tree in surgically altered anatomy (SAA). We describe outcome of EUS-HE and antegrade therapy for benign biliary disease in patients with SAA.
Methods
Retrospective review of 20 consecutive patients with surgically altered anatomy and benign biliary obstruction who underwent EUS-HE performed by one endoscopist at a tertiary center over a three-year period.
Results
During the study period, 37 patients underwent EUS-HE, 24 for benign disease. Of these, 20 patients had SAA and were analyzed (15 females, mean age, 62 years). SAA consisted of 9 Roux-en-Y Gastric Bypass, 6 Roux-en-Y Hepaticojejunostomy, 2 Billroth II, and 3 Whipple. Indications for ERC were common bile duct stone (8), benign postsurgical stricture (7), chronic pancreatitis (3), inflammatory stricture (1) and treatment of bile leak (1). Five patients had previously failed balloon enteroscopy-assisted ERC. The approach was transgastric in 15 and transjejunal in 5. In all cases a branch of the left hepatic duct with a mean diameter of 7.8 mm was accessed. Median stent length was 80 mm with diameters of 8 or 10 mm. Antegrade, definitive endoscopic therapy via the HE was performed in 18 patients with an average of 2.7 procedures performed for resolution of stones and/or downstream strictures. HE stents were removed in 17 patients after a mean of 91 days without adverse events. Three patients experienced mild adverse events (one with postprocedural pancreatitis after placement of a 10F transpapillary stent, one with postprocedural abdominal pain, and one with postprocedural cholangitis) requiring hospitalization for less than 3 nights; no severe adverse events occurred. Average postprocedural hospital stay was 1.3 days. No deaths occurred during follow-up.
Conclusions
EUS-guided hepaticoenterostomy is safe and effective in the management of benign biliary obstruction in patients with surgically-altered anatomy. It creates a portal to allow definitive, antegrade therapy and is a viable alternative to other endoscopic methods in this patient population.
Keywords: Endoscopic ultrasound, biliary stricture, Roux-en-Y, choledocholithiasis, hepaticojejunostomy, hepaticogastrostomy, ERCP, ERC, Endoscopic Retrograde Cholangiography
1.0 Background
Endoscopic retrograde cholangiography (ERC) is well-established as the preferred non-operative modality for biliary drainage due to a high technical success rate and acceptable adverse event rate.1,2 In cases of failed ERC or when the biliary tree is inaccessible due to obstruction or surgically altered anatomy, percutaneous transhepatic biliary drainage (PTBD) is usually performed. Although PTBD is also an established drainage method, it has several notable disadvantages. These include risks of catheter dislodgement, bile leakage, bleeding, cholangitis, pain at the insertion site, and cosmetic issues.3–5 Therefore, PTBD is associated with a decrease in patient quality of life.6
Due to limitations of ERC and PTBD, therapeutic endoscopic ultrasound (EUS) has emerged as a promising modality for biliary drainage.7,8 EUS-guided choledochoduodenostomy and biliary rendezvous are now established as effective means for biliary drainage; however, their utility is of limited value in cases of altered anatomy, such as Roux-en-Y anastomosis, or in the setting of gastric and proximal duodenal obstruction. 9,10
EUS-hepaticoenterostomy (EUS-HE) is performed by puncturing a branch of the left intrahepatic duct and placing a biliary stent transmurally into the intrahepatic biliary system. EUS-hepaticogastrostomy was first described in 2003 by Burmester et al11 in 4 patients with malignant biliary obstruction who had failed standard ERC. Indications for EUS-HE have expanded to include failed ERC due to surgical anatomy or an inaccessible ampulla of Vater and may provide longer stent patency than EUS-guided choledochoduodenostomy. 12–15 Recent data suggest EUS-HE can allow for drainage of the right hepatic lobe.16,17
EUS-HE has almost exclusively been reserved for palliation of malignant biliary obstruction.19,20 Use of EUS-HE as an access point for treatment of benign biliary disease has been reported from outside the United States.21 The present study aims to describe the outcome of patients undergoing EUS-HE using a dedicated, non-foreshortening self-expandable metal biliary stent for benign biliary obstruction in patients with surgically-altered anatomy, as a means of providing access for definitive therapy.
2.0 Patients and Methods
All adult patients (age ≥18 years) who underwent EUS-HE by one endoscopist at a large tertiary referral center between January 2015 and January 2018 were identified. Endoscopy reports, medical charts and relevant laboratory data were reviewed and recorded in accordance with Institutional Review Board protocol. Clinical and procedural data were collected, including etiology of biliary disease, indication for EUS-HE, endoscopic data (length and diameter of stent, anastomotic location, procedural findings), procedure-related adverse events, postprocedural symptoms, and clinical success, when available. The primary outcome was procedural success, which was defined as completion of EUS-guided biliary stent placement from left intrahepatic duct into the gastrointestinal tract. Adverse events were graded according to the American Society for Gastrointestinal Endoscopy lexicon.22
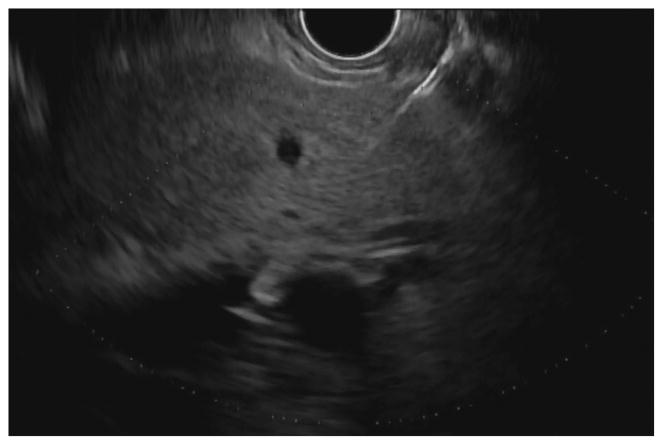
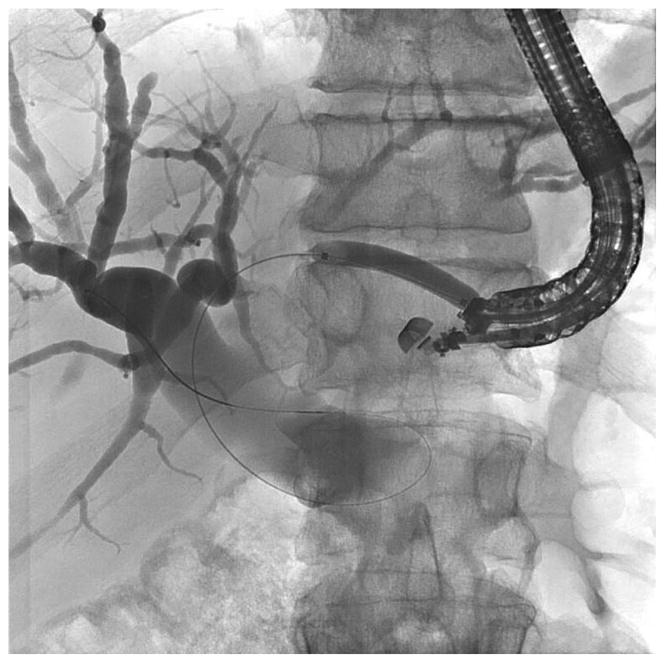
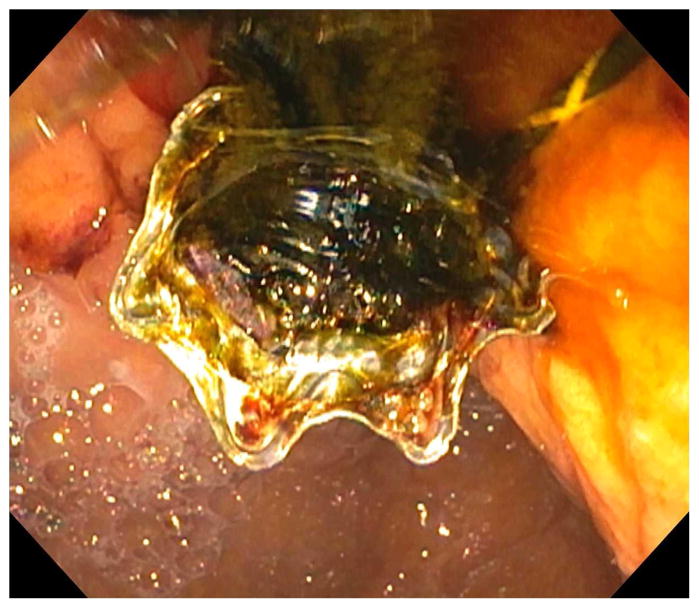
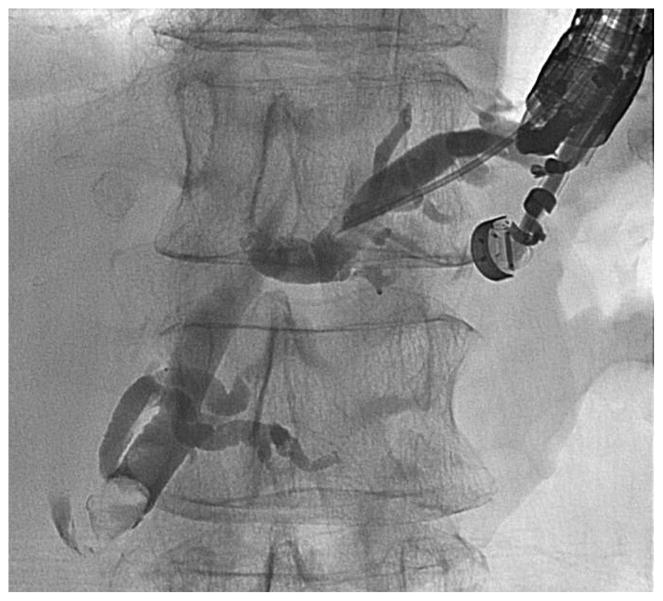
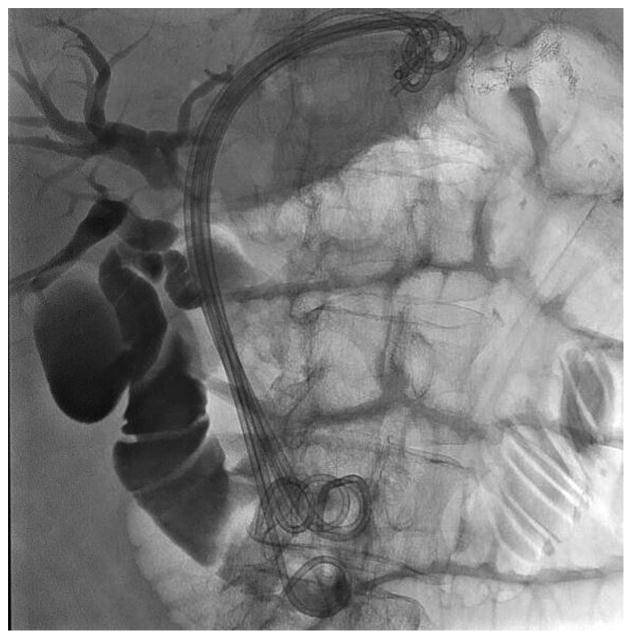
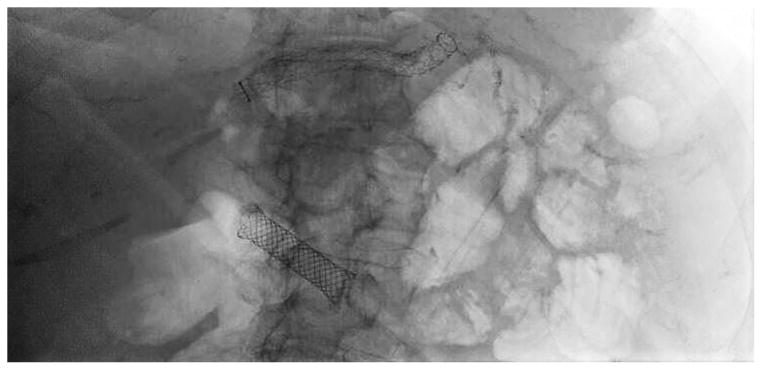
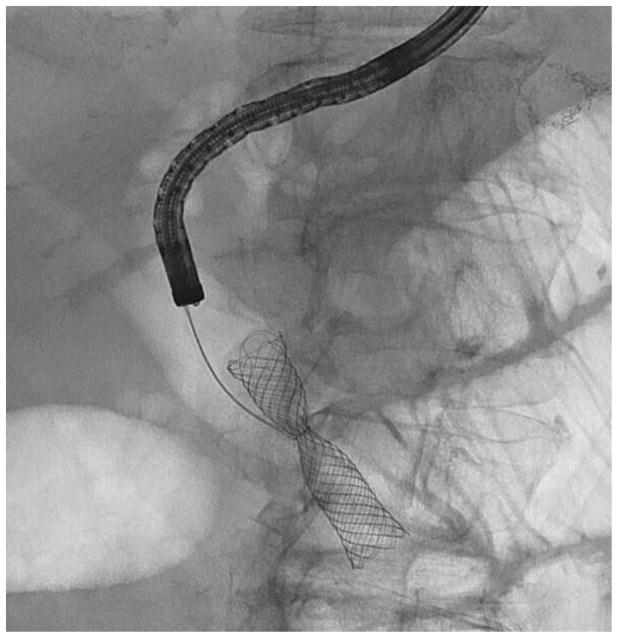
Before undertaking the procedure, all patients were informed of the risks, benefits, and alternatives of proceeding with EUS-guided transmural biliary drainage, and each provided written informed consent. General anesthesia and fluoroscopy were used in all cases. No patients received prophylactic antibiotics in our cohort. The technique of EUS-HE was performed as follows: a standard therapeutic channel oblique linear echoendoscope (GF-UCT180, Olympus America, Center Valley, Pa) was passed into the stomach or jejunum (five cases) to visualize the left lobe of the liver. Intrahepatic ducts of adequate caliber were identified with avoidance of intervening vessels as identified by Doppler. Ducts were not targeted based upon proximity to the periphery of the liver. A 19G needle (Expect, Boston Scientific, Marlborough, Ma) preloaded with water soluble contrast was used to puncture through the liver into the selected duct (Figure 1A) and entry was confirmed by contrast injection (Figure 1B). The needle was flushed with saline solution, and a 0.025-inch, 450-cm long hydrophilic-tipped guidewire (VisiGlide, Olympus) was passed antegrade into the biliary tree. The needle was withdrawn and the tract dilated (Figure 1C).
Figure 1.
EUS-guided hepaticogastrostomy in a patient with an inaccessible ampulla. A: EUS image showing 19G needle passed into a dilated left intrahepatic branch; B: Radiographic image showing contrast injection into the biliary tree; C: Radiographic image showing balloon dilation of tract; D–E: Radiographic and endoscopic image immediately after stent deployment.
Early in the experience attempts were made to pass a biliary dilating balloon (Hurricane RX, Boston Scientific) directly over the wire. If it did not successfully pass, electrocautery was used (2 patients) and consisted of passage of a standard biliary needle knife over the wire followed by balloon dilation of the tract. Later in the experience a tapered 5-4-3F catheter (Contour ERCP cannula, Boston Scientific) was routinely passed over the wire to dilate the tract to allow passage of the dilating balloon and to avoid the use of electrocautery. Balloon dilation was initiated from well within the left intrahepatic duct and continued sequentially toward the endoscope tip and across the gastric or jejunal wall. A commercially available, fully covered, non-foreshortening, self-expandable metal biliary stent (VIABIL Biliary Endoprosthesis, W.L. Gore & Associates, Flagstaff, Ariz) was placed across the hepaticoenterostomy under fluoroscopic and endoscopic visualization (Figure 1D–E). The stent length chosen was ≥2 cm the measured distance from the transducer to the punctured duct. Four-cm-long stents were used sparingly and only when peripheral ducts were punctured. In most cases a plastic biliary stent was passed through the SEMS to provide anchoring, to cross the papilla in the presence of bile duct stones, potentially minimize bile reflux symptoms, treat downstream anastomotic strictures, and provide drainage of the right hepatic lobe when hilar strictures were present.
Statistical analyses were performed using Stata version 15.1 (StataCorp, Tex). All continuous variables are expressed as mean ± standard deviation and categorical variables are expressed as proportions (%). The Student t-test was used to compare continuous measures, and 2-tailed Fisher exact test was used to compare differences in proportions between groups. Because of a small number of events, we did not perform a logistic regression analysis. A P value < .05 was considered statistically significant.
3.0 Results
During the study period, 37 patients underwent EUS-HE, 24 for benign disease. Of these, 20 patients had surgically altered upper gastrointestinal or biliary anatomy and were analyzed; technical success in this group was 100%. There were 15 females, (median age, 62 years). and postsurgical anatomy consisted of 15 Roux-en-Y (9 gastric bypass, 6 hepaticojejunostomy), 2 Billroth II gastrectomy, 3 Whipple. Twelve were outpatients at the time of EUS-HE and 5 had previously failed balloon enteroscopy-assisted ERC. Indications for the procedure were common bile duct stone (8), benign postsurgical stricture (7), benign stricture secondary to chronic pancreatitis (3), inflammatory stricture (1), and bile leak (1). Patient demographic data are shown in Table 1.
Table 1.
Patient demographics
| EUS-hepaticoenterostomy (n=20) | ||
| Mean age (SD) | 62.0 | 16.3 |
| Females, n (%) | 15 | 75.0% |
| Surgically altered anatomy | ||
| • Roux-en-Y gastric bypass | 9 | |
| • Roux-en-Y hepaticogastrostomy | 6 | |
| • Billroth II | 2 | |
| • Whipple | 3 | |
| Prior unsuccessful ERCP (%) | 5 | 25.0% |
| Reason for prior unsuccessful ERCP | ||
| • Unable to recognize papilla | 2 | |
| • Unable to advance wire beyond stricture | 3 | |
| Indication for ERCP | ||
| • Common bile duct stone | 8 | |
| • Benign postsurgical stricture | 7 | |
| • Benign stricture secondary to chronic pancreatitis | 3 | |
| • Inflammatory stricture | 1 | |
| • Bile leak | 1 | |
3.1 Procedure
The approach was transgastric in 15 patients and transjejunal in 5. The tract was dilated before stent deployment via tapered catheters and standard biliary balloon dilators (mean dilation diameter 4.5 mm) followed by stent placement. Stent diameters were 8 mm (15 patients) or 10 mm (5 patients) with lengths of 40 mm (one patient), 60 mm (4 patients), 80 mm (12 patients), and 100mm (3 patients). One patient required overlapping stents of 40 mm and 100 mm because there was concern the initial stent (40 mm) was too short, with insufficient length within the stomach to prevent migration into the peritoneum. In all cases, the stent was deployed into a branch of the left hepatic duct; the average duct diameter was 7.8 mm. Of 12 outpatient procedures, 1 patient was admitted for abdominal pain after the procedure and the other 11 remained as outpatients. Inpatients were hospitalized for a mean of 1.3 days (SD ± 1.6). Three patients experienced mild adverse events (one with postprocedural pancreatitis after placement of a 10F transpapillary stent, one with aforementioned postprocedural abdominal pain, and one with postprocedural cholangitis) requiring hospitalization for less than 3 nights. Procedural and outcomes data are shown in Table 2.
Table 2.
Procedural and Outcomes Data
| EUS-hepaticoenterostomy (n=20) | ||
| Mean procedure time, minutes (SD) | 129.9 | 44.4 |
| Proximal puncture site | ||
| • Stomach | 15 | |
| • Jejunum | 5 | |
| Cases requiring electrocautery | 2 | |
| Mean size of left hepatic duct, mm (SD) | 7.8 | 4.9 |
| Mean dilation diameter before stent deployment (SD) | 4.5 | 1.1 |
| Cases requiring 2 overlapping stents | 1 | |
| Median stent length, mm | 80 | |
| Median stent diameter, mm | 8 | |
| ERC and Antegrade Therapy | ||
| Cases with ERC performed through EUS-HG (%) | 18 | 90% |
| Stricture present on ERC | 7 | |
| Mean number of procedures to resolution of condition (SD) | 2.7 | 0.9 |
| Clinical Outcome | ||
| Outpatient cases, n (%) | 12 | 60.0% |
| Outpatients discharged to home, n (%) | 11 | 91.7% |
| Mean length of hospital stay after procedure, days (SD) | 1.3 | 1.6 |
| Mean decrease in bilirubin after procedure, mg/dL(SD) | 1.3 | 4.3 |
| Patients with unplanned surgical intervention, n (%) | 0 | 0% |
| Adverse events (Severity Grade) | ||
| • Postprocedural pancreatitis (Mild) | 1 | 5.0% |
| • Postprocedural abdominal pain (Mild) | 1 | 5.0% |
| • Postprocedural cholangitis (Mild) | 1 | 5.0% |
| Patients with eventual stent removal, n (%) | 17 | 85.0% |
| Mean length of time stent left in place, days (SD) | 91.2 | 96.4 |
| Median length of follow-up, days (Range) | 122 | (24 - 528) |
| Patients with clinical success, n (%) | 20 | 100% |
| Deaths during follow-up period | 0 | 0% |
In 18 patients (90%), antegrade endoscopic biliary therapy was subsequently performed through the HE by using standard duodenoscopes (Olympus) and cholangioscopes (Spy DS, Boston Scientific), and in some cases antegrade direct per-oral cholangioscopy using a 5.4 mm upper endoscope (Olympus) for stone clearance and/or treatment of downstream strictures (Figures 2 and 3). Stone clearance was performed using balloon sphincteroplasty, cholangioscopy, and antegrade sweeping, with successful clearance in all. Patients who underwent antegrade therapy had a mean of 2.7 procedures for resolution of condition. In 2 patients with distal common bile duct strictures, a fully covered stent was placed antegrade across the stricture at the time of HG whereby an additional fully covered stent was placed through the ampulla of Vater, anatomically separate from the HG stent. The distal stents were subsequently retrieved endoscopically using a small-caliber (5.4 mm) forward-viewing endoscope passed through the HG tract after removal of HG stents. (Figure 4).
Figure 2.
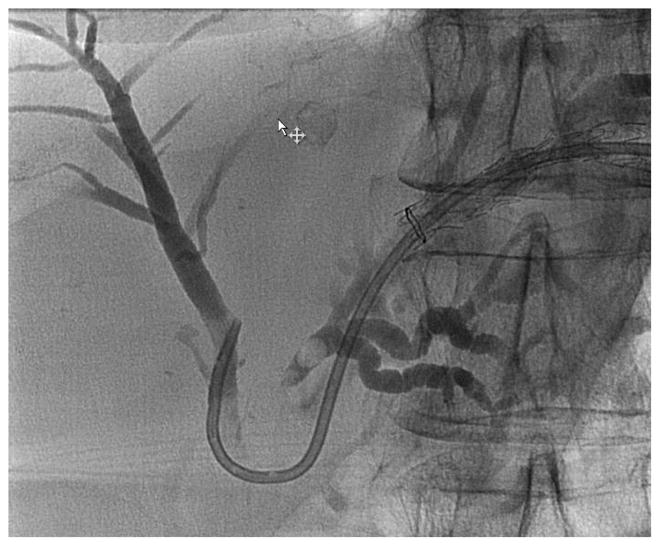
EUS-guided hepaticogastrostomy in a patient with remote surgical hepaticojejunostomy (HJ) after bile duct injury during laparoscopic cholecystectomy. Attempted single balloon retrograde cholangiopancreatography failed.
A: Radiographic image showing contrast injection into the biliary tree through a 19G needle. An obstructing stone is seen proximal to the HJ; B: Radiographic image showing hepaticogastrostomy stent in place. Through the HG stent a 7Fr plastic stent was placed into the right system across the bifurcation after balloon dilation. The patient subsequently underwent antegrade large bore plastic stent placement and antegrade stone removal followed by eventual removal of all stents. She remains well more than 18 months later.
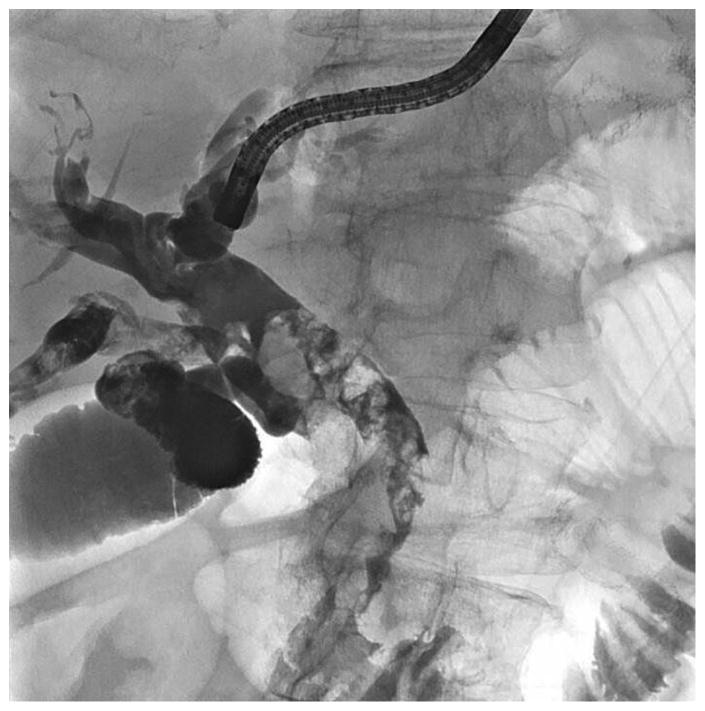
Figure 3.
Endoscopic treatment of stones and downstream stricture in patient with intact papilla. A: Cholangiogram via the HG showing extensive stone burden; B: Cholangiographic image of antegrade balloon dilation of stricture; C: Cholangiogram showing stone clearance but with stricture: D: Placement of multiple plastic stents via the HG to dilate stricture.
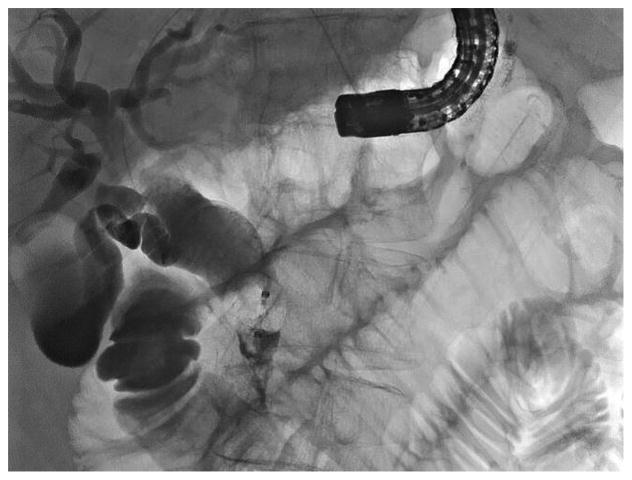
Figure 4.
Scout radiograph at time of fully covered stent removal from HG showing both HG and distal CBD stents. B: Radiographic view showing small caliber upper endoscope inserted through HG tract after HG stent removal; the distal stent is ensnared and was withdrawn proximally.
The EUS-HE stent was eventually removed in 17 patients without difficulty using a standard polypectomy snare or grasping device after a mean duration of 91 days (SD ± 96) and without adverse events. Two patients are awaiting antegrade treatment of their biliary disease and stent removal and one frail, elderly patient declined stent removal; these patients were not censored from analysis and there were no patients lost to follow-up. No deaths occurred during the follow-up period and all patients went on to have clinical success.
4.0 Discussion
ERC in patients with surgically altered anatomy of the upper gastrointestinal tract and/or biliary tree is often challenging or impossible. Until recently, there were limited endoscopic therapeutic options available for biliary drainage and PTBD was the mainstay of therapy. With the advent of EUS-guided procedures, internal biliary drainage is possible in cases where ERC would otherwise not be an option. There are limited data on the safety and efficacy of EUS-hepaticoenterostomy in resolving biliary obstruction, and almost exclusively from patients with malignant disease.23–25 Imai et al described EUS-hepaticogastrostomy in 37 patients with malignant biliary obstruction performed at a tertiary medical center in Japan with a 97.6% technical success rate. The stent used in this study (Zeo Stent; ZEON Medical, Tokyo, Japan) is only available in limited markets. A second study of 41 patients, 95% of whom had malignant disease by De Cassan et al from France reported a technical success rate of 90.2%. They used a stent (Giobor stent, Taewoong Medical, Gyeonggi-do, South Korea), which is also limited in availability. Other studies evaluating the use of EUS-hepaticogastrostomy have been composed either entirely or almost entirely of malignant disease. Further, the stents used are typically available in limited markets, hindering adoption of the procedure inside the United States and other regions without access to this equipment.
The present study describes the largest retrospective case series of EUS-HE in patients with surgically altered anatomy and benign biliary obstruction. Also included in this case series we report five cases of EUS-guided hepaticojejunostomy (EUS-HJ). EUS-HJ, may be the preferred approach in patients with reduced stomach size such as in partial gastrectomy and Roux-en-Y gastric bypass. Limitations to the present study include a single-center experience with the potential for selection bias and uncertain generalizability to other centers.
The endoscopic approach to the biliary tree in patients with surgically-altered anatomy and benign biliary disease often involves the use of long length endoscopes, including balloon assisted enteroscopes. Disadvantages include increased procedure duration, low technical success rates and a reduced toolset for therapeutic intervention.26 In a study by Khashab et al of patients with surgical anatomy and biliary obstruction, technical success was 65.3% in enteroscopy-assisted ERCP and 98% in EUS-guided biliary drainage.27 Alternately, a laparoscopic approach, whereby the duodenoscope is introduced transabdominally through a trocar placed into the excluded stomach with a surgeon’s assistance is complicated by the coordination of multiple specialty providers, need to sterilize equipment and often low-quality fluoroscopy available within the operating room.28 Further, this approach is complicated by a relatively high rate of abscess formation at the access site.29
When determining a patient’s candidacy for EUS-HE, several factors should be considered. Currently we recommend that it be reserved for patients in whom standard ERC failed or was deemed impossible due to anatomic constraints; this may change with increasing experience. Second, the left hepatic duct (or another portion of the bile duct) must be within reach of the endosonographer’s needle and allow placement of a stent; this is typically no greater than 10cm from the gastric or jejunal wall. A stent of sufficient length should be chosen at the outset to prevent stent maldeployment and internal migration into the peritoneum, and subsequent need for rescue maneuvers. In the event that the stent does not bridge the gastrointestinal wall, a second stent can be placed coaxially within the original stent to bridge the distance, assuming wire access is maintained. Finally, the success rate for EUS-HE will be higher if the intrahepatic ducts are sufficiently dilated in caliber in order to serve as a suitable target for needle puncture and stent placement. In our study, the mean left hepatic duct size was 7.6mm (SD ± 5.0).
EUS-guided HE allowed treatment of benign biliary diseases similar to what is done by interventional radiologists when percutaneous transhepatic therapy is undertaken.30 We used the hepaticoenterostomy as an access point for clearance of choledocho- and hepatico-lithiasis as well as treatment of downstream strictures, when present. Stones were cleared after balloon dilation of native papillae and single-operator cholangioscopy or antegrade direct per-oral cholangioscopy, the latter performed with small-caliber upper endoscopes.31 Had percutaneous therapy been undertaken these patients may have been burdened with an external drain for an extended period; recent data demonstrate patients overwhelmingly prefer internal biliary drainage when given the option.32
Outside of the United States, especially designed self-expandable metal stents for the creation of hepaticoenteric anastomoses are available. We used a commercially available, fully covered self-expandable biliary stent that is FDA-approved for palliation of malignant biliary strictures. Important characteristics of this stent include non-foreshortening during and after deployment, lack of movement of the stent during deployment as the constraining material is unraveled and prevents the need for counter maneuvers, and the presence of anti-migration fins. We did not experience limitations to lack of dedicated EUS devices. Wire shearing did not occur because the wire used was 0.025-inch, and within a 19-gauge needle there is adequate space around the wire. Additionally, the coating of the wire used is unique to other wires so that shearing of the wire jacket did not occur.
Of note, it is well known that PTBD in patients with cholangitis carries a risk of sepsis immediately after the procedure from transient bacteremia due to the needle’s course through the highly vascular hepatic tissue.33 This risk also pertains to EUS-HE for the same reason. Although we did not routinely administer prophylactic antibiotics in this cohort, some of our patients with malignant biliary obstruction have developed cholangitis after EUS-HE (unpublished). This coupled with a guideline on percutaneous transhepatic cholangiographic procedures that recommends all patients be treated with appropriate prophylactic antibiotics to minimize septic adverse events34 leads us to also recommend that all patients undergoing EUS-HE receive prophylactic antibiotics, especially those with bacterobilia from prior biliary instrumentation (surgical, endoscopic, or percutaneous). The duration of antibiotic therapy postprocedurally should be individualized.
At our center, the approach to managing biliary disease in patients with RYGB anatomy depends upon need for acute intervention, such as in the case of acute cholangitis, where urgent biliary decompression is required. In these instances, we proceed with EUS-HE, when the intrahepatic ducts can be accessed in order to obtain source control and resolve cholestasis. In other instances, such choledocholithiasis without evidence of infection or in patients with acute recurrent pancreatitis, we typically perform EUS-directed transgastric ERCP (EDGE). This approach was first described in 2014 by Kedia et al and involves the creation of a fistulous tract by placing a lumen-apposing metal stent between either the jejunum or gastric pouch and the excluded stomach.35 After allowing the tract to mature, a duodenoscope is advanced through the stent, into the excluded stomach, duodenum and ampulla. Once it is decided that no further interventions will be required, the stent is removed.
In summary, the present study shows the feasibility of using EUS-guided hepaticoenterostomy for access to the biliary tree in patients with surgically altered anatomy. This approach, using a commercially available self-expandable biliary stent, is safe and efficacious in the management of benign obstruction in this patient population. We believe such an approach will be preferred over percutaneous therapy; however, further studies are needed to determine optimal patient selection and procedural technique. As experience accrues with EUS-HE and techniques become refined, one could envision head-to-head trials with ERC as primary therapy for bile duct obstruction, especially in patients with surgically altered anatomy.
Acknowledgments
Funding declaration: Dr. James receives research and training support in part by a grant from the NIH (T32DK007634).
Acronyms
- ERC
endoscopic retrograde cholangiography
- EUS
endoscopic ultrasound
- EUS-HE
endoscopic ultrasound-guided hepaticoenterostomy
- PTBD
percutaneous transhepatic biliary drainage
- SAA
surgically altered anatomy
Footnotes
Conflicts of interest: Dr. Baron is a consultant and speaker for Boston Scientific, W.L. Gore, Cook Endoscopy and Olympus America
Contributions: Dr. James collected data, analysed data, authored key portions of the manuscript and edited key portions of the manuscript. Dr. Fan collected and analysed data. Dr. Baron authored key portions of the manuscript, edited key portions of the manuscript and selected key images for figure creation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5.0 References
- 1.Baron TH, Grimm IS, Swanstrom LL. Interventional Approaches to Gallbladder Disease. N Engl J Med. 2015;373:357–65. doi: 10.1056/NEJMra1411372. [DOI] [PubMed] [Google Scholar]
- 2.Williams EJ, Taylor S, Fairclough P, et al. BSG Audit of ERCP. Are we meeting the standards set for endoscopy? Results of a large-scale prospective survey of endoscopic retrograde cholangio-pancreatograph practice. Gut. 2007;56:821–9. doi: 10.1136/gut.2006.097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrasco CH, Zornoza J, Bechtel WJ. Malignant biliary obstruction: complications of percutaneous biliary drainage. Radiology. 1984;152:343–6. doi: 10.1148/radiology.152.2.6739796. [DOI] [PubMed] [Google Scholar]
- 4.Oh HC, Lee SK, Lee TY, et al. Analysis of percutaneous transhepatic cholangioscopy-related complications and the risk factors for those complications. Endoscopy. 2007;39:731–6. doi: 10.1055/s-2007-966577. [DOI] [PubMed] [Google Scholar]
- 5.Winick AB, Waybill PN, Venbrux AC. Complications of percutaneous transhepatic biliary interventions. Tech Vasc Interv Radiol. 2001;4:200–6. doi: 10.1016/s1089-2516(01)90026-5. [DOI] [PubMed] [Google Scholar]
- 6.Sharaiha RZ, Kumta NA, Desai AP, et al. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg Endosc. 2016;30:5500–5505. doi: 10.1007/s00464-016-4913-y. [DOI] [PubMed] [Google Scholar]
- 7.Khashab MA, Valeshabad AK, Afghani E, et al. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557–65. doi: 10.1007/s10620-014-3300-6. [DOI] [PubMed] [Google Scholar]
- 8.Park DH, Song TJ, Eum J, et al. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos) Gastrointest Endosc. 2010;71:413–9. doi: 10.1016/j.gie.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Hara K, Yamao K, Niwa Y, et al. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol. 2011;106:1239–45. doi: 10.1038/ajg.2011.84. [DOI] [PubMed] [Google Scholar]
- 10.Park DH, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276–84. doi: 10.1016/j.gie.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 11.Burmester E, Niehaus J, Leineweber T, Huetteroth T. EUS-cholangio-drainage of the bile duct: report of 4 cases. Gastrointest Endosc. 2003;57:246–51. doi: 10.1067/mge.2003.85. [DOI] [PubMed] [Google Scholar]
- 12.Bories E, Caillol F, Pesenti C, Giovannini M. Short-term results after hepaticogastrostomy guided by echo-endoscopy: Monocentric retrospective study. Endosc Ultrasound. 2014;3(Suppl 1):S14. [PMC free article] [PubMed] [Google Scholar]
- 13.Ogura T, Chiba Y, Masuda D, et al. Comparison of the clinical impact of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for bile duct obstruction with duodenal obstruction. Endoscopy. 2016;48:156–63. doi: 10.1055/s-0034-1392859. [DOI] [PubMed] [Google Scholar]
- 14.Uemura RS, Khan MA, Otoch JP, Kahaleh M, Montero EF, Artifon ELA. EUS-guided Choledochoduodenostomy Versus Hepaticogastrostomy: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2018;52:123–130. doi: 10.1097/MCG.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 15.Khan MA, Khan S, Khan AR, et al. Endoscopic Ultrasound Guided Biliary Drainage Comes of Age: a Systematic Review and Meta-Analysis. Gastrointestinal Endoscopy. 2015;81:AB421. [Google Scholar]
- 16.Park SJ, Choi JH, Park DH, et al. Expanding indication: EUS-guided hepaticoduodenostomy for isolated right intrahepatic duct obstruction (with video) Gastrointest Endosc. 2013;78:374–80. doi: 10.1016/j.gie.2013.04.183. [DOI] [PubMed] [Google Scholar]
- 17.James TW, Grimm IS, Baron TH. Hilar cholangiocarcinoma diagnosed by endoscopic ultrasound-guided hepaticogastrostomy and cholangioscopy. Endoscopy. 2017;49:E260–E261. doi: 10.1055/s-0043-115895. [DOI] [PubMed] [Google Scholar]
- 18.Ogura T, Yamamoto K, Sano T, et al. Stent length is impact factor associated with stent patency in endoscopic ultrasound-guided hepaticogastrostomy. J Gastroenterol Hepatol. 2015;30:1748–52. doi: 10.1111/jgh.13021. [DOI] [PubMed] [Google Scholar]
- 19.Almadi M, Khashab M, Dhir V. Endoscopic ultrasonography-guided biliary drainage: A meta-analysis of publications with fifty or more patients. Endoscopic Ultrasound. 2017;6:7. [Google Scholar]
- 20.van Geenen EJM, Siersema PD. Stent migration into the abdominal cavity after EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2018;87:617–618. doi: 10.1016/j.gie.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Iwashita T, Nakai Y, Hara K, Isayama H, Itoi T, Park DH. Endoscopic ultrasound-guided antegrade treatment of bile duct stone in patients with surgically altered anatomy: a multicenter retrospective cohort study. J Hepatobiliary Pancreat Sci. 2016;23:227–33. doi: 10.1002/jhbp.329. [DOI] [PubMed] [Google Scholar]
- 22.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Imai H, Takenaka M, Omoto S, et al. Utility of Endoscopic Ultrasound-Guided Hepaticogastrostomy with Antegrade Stenting for Malignant Biliary Obstruction after Failed Endoscopic Retrograde Cholangiopancreatography. Oncology. 2017;93(Suppl 1):69–75. doi: 10.1159/000481233. [DOI] [PubMed] [Google Scholar]
- 24.De Cassan C, Bories E, Pesenti C, et al. Use of partially covered and uncovered metallic prosthesis for endoscopic ultrasound-guided hepaticogastrostomy: Results of a retrospective monocentric study. Endosc Ultrasound. 2017;6:329–335. doi: 10.4103/2303-9027.209869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee TH, Choi JH, Park do H, et al. Similar Efficacies of Endoscopic Ultrasound-guided Transmural and Percutaneous Drainage for Malignant Distal Biliary Obstruction. Clin Gastroenterol Hepatol. 2016;14:1011–1019. doi: 10.1016/j.cgh.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 26.Shah RJ, Smolkin M, Yen R, et al. A multicenter, U.S. experience of single-balloon, double-balloon, and rotational overtube-assisted enteroscopy ERCP in patients with surgically altered pancreaticobiliary anatomy (with video) Gastrointest Endosc. 2013;77:593–600. doi: 10.1016/j.gie.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Khashab MA, El Zein MH, Sharzehi K, et al. EUS-guided biliary drainage or enteroscopy-assisted ERCP in patients with surgical anatomy and biliary obstruction: an international comparative study. Endosc Int Open. 2016;4:E1322–E1327. doi: 10.1055/s-0042-110790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brockmeyer JR, Grover BT, Kallies KJ, Kothari SN. Management of biliary symptoms after bariatric surgery. Am J Surg. 2015;210:1010–6. doi: 10.1016/j.amjsurg.2015.07.003. discussion 1016–7. [DOI] [PubMed] [Google Scholar]
- 29.Grimes KL, Maciel VH, Mata W, Arevalo G, Singh K, Arregui ME. Complications of laparoscopic transgastric ERCP in patients with Roux-en-Y gastric bypass. Surg Endosc. 2015;29:1753–9. doi: 10.1007/s00464-014-3901-3. [DOI] [PubMed] [Google Scholar]
- 30.Miranda-García P, Gonzalez JM, Tellechea JI, Culetto A, Barthet M. EUS hepaticogastrostomy for bilioenteric anastomotic strictures: a permanent access for repeated ambulatory dilations? Results from a pilot study. Endosc Int Open. 2016;4:E461–5. doi: 10.1055/s-0042-103241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosmer A, Abdelfatah MM, Law R, Baron TH. Endoscopic ultrasound-guided hepaticogastrostomy and antegrade clearance of biliary lithiasis in patients with surgically-altered anatomy. Endosc Int Open. 2018;6:E127–E130. doi: 10.1055/s-0043-123188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam K, Kim DU, Lee TH, et al. Patient perception and preference of EUS-guided drainage over percutaneous drainage when endoscopic transpapillary biliary drainage fails: An international multicenter survey. Endosc Ultrasound. 2018;7:48–55. doi: 10.4103/eus.eus_100_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark CD, Picus D, Dunagan WC. Bloodstream infections after interventional procedures in the biliary tract. Radiology. 1994;191:495–9. doi: 10.1148/radiology.191.2.8153328. [DOI] [PubMed] [Google Scholar]
- 34.Saad WEA, Wallace MJ, Wojak JC, Kundu S, Cardella JF. Quality Improvement Guidelines for Percutaneous Transhepatic Cholangiography, Biliary Drainage, and Percutaneous Cholecystostomy. J Vasc Interv Radiol. 2010;21(6):789–795. doi: 10.1016/j.jvir.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Kedia P, Sharaiha RZ, Kumta NA, Kahaleh M. Internal EUS-directed transgastric ERCP (EDGE): game over. Gastroenterology. 2014;147:566–8. doi: 10.1053/j.gastro.2014.05.045. [DOI] [PubMed] [Google Scholar]