Abstract
INTRODUCTION
We examined and compared plasma phospho-tau181 (pTau181) and total tau: 1) across the Alzheimer’s disease (AD) clinical spectrum; 2) in relation to brain amyloid (Aβ) PET, tau PET, and cortical thickness; and 3) as a screening tool for elevated brain Aβ.
METHODS
Participants included 172 cognitively unimpaired (CU), 57 mild cognitive impairment, and 40 AD dementia patients with concurrent Aβ PET(PiB), tau PET (AV1451), MRI, plasma total tau and pTau181.
RESULTS
Plasma total tau and pTau181 levels were higher in AD dementia patients compared to CU. Plasma pTau181 was more strongly associated with both Aβ and Tau PET. Plasma pTau181 was a more sensitive and specific predictor of elevated brain Aβ than total tau and was as good as, or better than, the combination of age and APOE.
DISCUSSION
Plasma pTau181 may have utility as a biomarker of AD pathophysiology and as a non-invasive screener for elevated brain Aβ.
Keywords: Plasma tau, Plasma phosphorylated tau, Amyloid PET, Tau PET, Alzheimer’s disease, Predicting brain amyloid
1. Introduction
Blood-based biomarkers of Alzheimer’s disease (AD) pathology (e.g., amyloid-beta [Aβ] or tau) will be essential for screening the general population, and in low/middle income countries, as the first step in a multistep process to determine which non-demented individuals are at greatest risk of AD dementia [1–3]. Because elevated brain Aβ is necessary for a diagnosis of AD dementia, and a requirement for some ongoing secondary prevention trials, a blood-based marker for predicting elevated brain Aβ would have great benefit. Several studies have examined either plasma or serum Aβ1–40 and Aβ1–42 peptides, but these measures have not consistently differed between AD dementia patients and cognitively unimpaired (CU) controls or were associated with cortical Aβ PET deposition [4,5].
Studies examining the clinical utility of plasma total tau have consistently reported that higher levels are associated with cognitive decline and risk of mild cognitive impairment (MCI) [6,7 ], but this relationship is independent of brain Aβ [7]. Phosphorylated-tau is thought to be more specific to AD pathogenesis than total tau [8]. Although blood measures of pTau have been difficult to measure to date due their low levels, recent studies have demonstrated it may be possible [9]. The goals of the present study using a novel assay for pTau were to: 1) examine and compare levels of plasma phospho-tau181 (pTau181) and plasma total tau by clinical diagnosis across the AD spectrum; 2) examine the associations between plasma pTau181 and total tau with Aβ PET, tau PET, and cortical thickness; and 3) determine the clinical utility of plasma pTau181 or total tau as a screening tool for elevated brain Aβ. Given the specificity of CSF pTau to AD pathophysiology [8], we hypothesized that plasma pTau181 would be a more precise marker than total tau for AD-specific patterns of Aβ PET, tau PET, and cortical thickness.
2. Methods
2.1. Participants
Mayo Clinic data was pooled from two sources: the Mayo Clinic Study of Aging (MCSA) and the Alzheimer’s Disease Research Center (ADRC). The MCSA is a population-based epidemiological cognitive aging study of Olmsted County, MN residents [10,11] who were initially sampled using the Rochester Epidemiology Project medical records linkage system. Beginning in 2004, the MCSA enrolled residents aged 70–89 years; in 2012 enrollment was extended to include residents aged 50 years and older. The ADRC recruits and follows selected patients initially seen in the referral behavioral neurology practice at Mayo Clinic. All CU in this study were enrolled in the MCSA. Those with MCI or AD dementia were enrolled in either the MCSA or the ADRC. For both studies, same day imaging of both Aβ and Tau PET began in 2016. The present analyses included the first individuals enrolled in the MCSA or ADRC with a diagnosis of CU, MCI, or AD and with Aβ PET, tau PET, MRI and blood (for total tau and pTau181 assays) at the same study visit. The study protocols were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. All participants provided written informed consent.
2.2. MCI and dementia diagnostic determination
For each participant, the clinical diagnosis was determined by a consensus committee including the neurologist, neuropsychologist, and the nurse who evaluated each participant. For MCSA participants, performance in a cognitive domain was compared with the age-adjusted scores of CU individuals previously obtained using Mayo’s Older American Normative Studies [12]. This approach relies on prior normative work and extensive experience with the measurement of cognitive abilities in an independent sample of subjects from the same population. Subjects with scores around 1.0 SD below the age-specific mean in the general population were considered for possible cognitive impairment. The operational definition of MCI was based on clinical judgment including a history from the patient and informant. Published criteria were used for the diagnosis: cognitive complaint, cognitive function not normal for age, essentially normal functional activities, no dementia [13]. A final decision about impairment in a cognitive domain was made after considering education, occupation, visual or hearing deficits, and reviewing all other participant information. The diagnosis of dementia [14] and AD dementia [15] were based on published criteria. Participants who performed in the normal range and did not meet criteria for MCI or dementia were deemed CU. Imaging was not considered in determining the clinical diagnosis.
2.3. Imaging methods
Aβ PET imaging was performed with Pittsburgh Compound B (PiB) [16] and tau PET with AV1451 [17] on the same day. Participants also completed computed tomography for attenuation correction. Late uptake Aβ PET images were acquired from 40–60 minutes and tau PET from 80–100 minutes after injection. All PET images were analyzed with our in-house fully automated image processing pipeline [18], where image voxel values are extracted from automatically labeled regions of interest (ROIs) propagated from an MRI template. An Aβ positron emission tomography (PET) standardized update value ratio (SUVR) was formed from the voxel number weighted average of the median uptake in the prefrontal, orbitofrontal, parietal, temporal, anterior and posterior cingulate, and precuneus ROIs normalized to the cerebellar crus gray median. Based on previous work, elevated Aβ PET was defined as SUVR>1.42 [19]. Our primary tau PET ROI was the median uptake in the entorhinal cortex normalized to the cerebellar crus gray median. We focused on this ROI due to its sensitivity to Aβ PET among CU individuals [20]. A tau PET cut-point for the entorhinal cortex has not yet been validated so tau PET was only analyzed as a continuous variable. PET data were “sharpened” but not partial volume corrected. That is, voxels whose probability of being CSF was greater than the probability of being gray or white matter were excluded from PET ROI measures. MRI was performed on one of two 3T GE systems. The MRI measure was a FreeSurfer (v5.3)-derived AD-signature meta-ROI composed of the surface-area weighted average of the mean cortical thickness in the following individual ROIs: entorhinal, inferior temporal, middle temporal, and fusiform.
2.4. Plasma total tau
Blood was collected in-clinic after an overnight fast. The blood was centrifuged, aliquoted, and stored at −80°C. Plasma total tau was measured on the Quanterix Simoa-HD1 Platform, as described previously [21]. Briefly, samples were thawed on wet ice, centrifuged at 500xg for 5 minutes at 4C, diluted 1:8 in kit sample buffer, and analyzed according to the kit protocol on the Simoa-HD1 [21].
2.5. Plasma pTau181
The pTau181 assay was designed to measure pTau181 in plasma and was optimized to measure disease-related differences through the selection of monoclonal antibodies (mAb) used in the assay. Selection of the mAb pair provided a unique combination of sensitivity and selectivity for the tau forms in plasma that are different between CU and AD subjects. Briefly, the assay was performed on a streptavidin small spot plate using the Meso Scale Discovery (MSD) platform. Biotinylated-AT270 was used as a capture antibody (anti-pT181 Tau antibody, Thermo Fisher, catalog number: MN1050) and SULFO-TAG-LRL (anti-tau mAb developed by Lilly Research Laboratory) for the detector. Antibodies were conjugated with Sulfo-NHS-Biotin (Thermo Scientific, catalog number: 21327) or MSD GOLD SULFO-TAG NHS-Ester (Meso Scale Discovery, catalog number: R91AO) according to the manufacturer’s protocol. The assay was calibrated using recombinant tau (4R2N, NCBI tau v2) protein that was phosphorylated in vitro using a reaction with glycogen synthase kinase-3β (GSK3β) and characterized by mass spectrometry [22,23]. The same sample used for the plasma total tau assay was thawed again for use in pTau181 assay. The sample was thawed on wet ice, centrifuged at 500xg for 5 minutes and diluted 1:2.5 in Diluent 35 (Meso Scale Discovery, catalog #: R50AE) with the addition of HBR1 to a concentration of 200 μg/mL (Scantibodies Inc, catalog #: 3KC533).
2.6. Assessment of covariates
Participant demographics (e.g., sex, age, years of education) were ascertained during the in-person interview at the in-clinic exam. APOE genotyping was performed from a blood draw taken at the in-clinic exam.
2.7. Statistical analyses
ANOVA and Chi-square tests were used to examine group (e.g., CU, MCI, AD) differences. Spearman’s rho was used to measure the correlation between variables. The distribution of both plasma pTau181 and total tau was right skewed so the variables were log transformed prior to regression analyses. Linear regression was used to examine the relationship between each of the tau measures and continuous neuroimaging measures. Logistic regression was used when examining dichotomous neuroimaging outcomes (i.e., Aβ PET>1.42; cortical thickness<2.67mm). All models were adjusted for age, sex, and APOE.
We examined and compared the predictive value of plasma pTau181 and total tau for elevated Aβ PET and abnormal cortical thickness using areas under the receiver operating characteristic curve (AUROC). As mentioned earlier, a tau PET cut-point in the entorhinal cortex has not yet been validated so these analyses could not be performed. To determine the utility of the tau measures in a variety of clinical populations, we separately determined AUROC for all participants, non-demented participants, MCI only, and CU only. Age and the APOE ε4 allele are the strongest predictors of elevated brain Aβ and AD dementia. Therefore, for comparison purposes, we provided the AUROC for age alone, presence of an APOE ε4 allele alone, age and APOE ε4 allele, each plasma tau measure alone, and each plasma tau measure and age and APOE ε4 allele. Finally, to assess whether the predictive value of the tau measures differed by APOE, we stratified by the presence of an ε4 allele. The Liu method was used to identify cut-off values that maximized sensitivity and specificity [24]. Areas under ROC curves were compared using an algorithm suggested by DeLong, DeLong, and Clarke-Pearson (1988) [25]. All analyses were completed using Stata Version 13.0 (StataCorp, College Station, TX).
3. Results
3.1. Demographic, imaging, and plasma tau characteristics by group
Participants included 172 CU, 57 MCI, and 40 AD dementia. The AD dementia patients had a slightly lower mean age compared to the CU and MCI participants but there were no differences between the groups with regards to sex or education. As expected, clinical severity was associated with higher Aβ SUVR, higher tau PET entorhinal cortex SUVR, and lower cortical thickness in an AD-signature ROI (Table 1). Among all participants, the mean (SD) plasma level of total tau was 7.72 (8.51) pg/ml (median 5.79 pg/ml), and for pTau181 was 6.08 (2.30) pg/ml (median 5.55 pg/ml). Plasma total tau and pTau181 were modestly correlated (Spearman rho = 0.286, P < .001). AD dementia patients had significantly higher mean levels of total tau compared to MCI (P =.029) or CU participants (P < .001), but there was no difference between the CU and MCI participants. AD dementia participants also had higher mean levels of pTau181 compared to CU (P < .001), but not MCI (P =.251) participants. MCI had higher levels of pTau181 compared to CU, but the results did not reach statistical significance (P =.060).
Table 1.
Participant characteristics, mean (IQR) or N (%), by clinical diagnosis
| CU (N = 172) | MCI (N = 57) | AD (N = 40) | |||
|---|---|---|---|---|---|
| Mean (SD)/N(%) | Mean (SD)/N(%) | Mean (SD)/N(%) | F value | P value* | |
| Age | 71.9 (9.5) | 71.4 (10.7) | 67.7 (9.2) | 3.08 | .048 |
| Male | 119 (69.2%) | 45 (79.0%) | 23 (57.5%) | .077 | |
| Education | 15.2 (2.5) | 15.0 (3.3) | 15.1 (2.8) | 0.16 | .850 |
| ≥1 APOE ε4 | 50 (29.1%) | 20 (35.1%) | 28 (70.0%) | < .001 | |
| Aβ PET SUVR | 1.5 (0.4) | 1.8 (0.6) | 2.4 (0.4) | 76.24 | < .001 |
| Aβ PET>1.42 SUVR | 72 (41.9%) | 28 (49.1%) | 38 (95.0%) | < .001 | |
| Tau PET entorhinal cortex | 1.1 (0.1) | 1.2 (0.2) | 1.8 (0.3) | 177.84 | < .001 |
| Cortical thickness | 2.7 (0.1) | 2.6 (0.2) | 2.3 (0.2) | 75.73 | < .001 |
| Cortical thickness<2.67mm | 68 (39.5%) | 35 (61.4%) | 37/39 (94.9%) | < .001 | |
| Plasma phospho-tau 181 | 6.4 (6.4) | 9.0 (13.9) | 11.6 (4.1) | 7.08 | < .001 |
| Plasma total tau | 5.9 (1.9) | 5.9 (2.8) | 7.2 (2.8) | 5.44 | < .001 |
Abbreviations: AD, Alzheimer’s disease dementia; Aβ, amyloid-beta; APOE, apolipoprotein E; CU, cognitively unimpaired; IQR, interquartile range; MCI, mild cognitive impairment; PET, positron emission tomography; SUVR, standard uptake value ratio .
Analyses of group differences included chi-square tests for dichotomous variables and ANOVA for continuous variables.
3.2. Associations of plasma tau measures with Aβ PET, tau PET, and cortical thickness
Across the total study population, higher plasma tau and pTau181 levels were associated with all neuroimaging measures of Aβ PET, tau PET, and cortical thickness in an AD-signature region after adjustment for age, sex, and APOE (Table 2). However, only higher plasma pTau181 levels, and not total tau, were associated with higher Aβ PET SUVR within each clinical diagnostic group (CU, MCI, AD). Dichotomizing Aβ PET SUVR>1.42, each log unit increase in plasma pTau181 was associated with a 2.8-fold (95% confidence interval (CI) 1.15–7.05) and a 5.7-fold increased odds (95% CI 0.86–38.16) of elevated brain Aβ among CU and MCI, respectively. Higher pTau181 was not significantly associated with higher tau PET entorhinal cortex SUVR among CU participants, but was associated with higher tau entorhinal cortex PET SUVR among both MCI and AD dementia participants. Plasma pTau181 was not significantly associated with cortical thickness in an AD-signature region. In contrast to plasma pTau181, total tau was associated with cortical thickness among both the CU and MCI participants but not with Aβ or tau PET.
Table 2.
Associations between plasma pTau181 or total tau and continuous and dichotomous neuroimaging measures of Aβ PET, tau PET, and cortical thickness in an AD-signature region
| Log Plasma phospho-tau 181 | Log Plasma total tau | |||
|---|---|---|---|---|
| b or OR (95% CI) | P | b or OR (95% CI) | P | |
| All (N=269) | ||||
| Aβ PET | 0.23 (0.18, 0.28) | <0.001 | 0.12 (0.03, 0.22) | 0.010 |
| Aβ PET >1.42 SUVR | 7.13 (3.27, 15.54) | <0.001 | 2.83 (1.12, 7.13) | 0.027 |
| Tau PET entorhinal cortex | 0.19 (0.14, 0.23) | <0.001 | 0.16 (0.08, 0.23) | <0.001 |
| Cortical thickness | −0.15 (−0.19, −0.11) | <0.001 | −0.20 (−0.27, −0.13) | <0.001 |
| Cortical thickness <2.67mm | 3.39 (1.77, 6.48) | <0.001 | 6.31 (2.35, 16.89) | <0.001 |
| CU (N=172) | ||||
| Aβ PET | 0.10 (0.03, 0.17) | 0.003 | 0.004 (−0.09, 0.10) | 0.926 |
| Aβ PET >1.42 SUVR | 2.84 (1.15, 7.045) | 0.024 | 1.94 (0.61, 6.16) | 0.262 |
| Tau PET entorhinal cortex | 0.02 (−0.02, 0.07) | 0.251 | 0.04 (−0.03, 0.11) | 0.287 |
| Cortical thickness | −0.02 (−0.06, 0.02) | 0.368 | −0.05 (−0.11, 0.01) | 0.090 |
| Cortical thickness <2.67mm | 1.01 (0.47, 2.20) | 0.973 | 3.43 (1.01, 11.61) | 0.047 |
| MCI (N=57) | ||||
| Aβ PET | 0.17 (0.06, 0.27) | 0.003 | 0.001 (−0.22, 0.22) | 0.993 |
| Aβ PET >1.42 SUVR | 5.72 (0.86, 38.16) | 0.071 | 1.52 (0.19, 12.41) | 0.696 |
| Tau PET entorhinal cortex | 0.13 (0.06, 0.20) | <0.001 | 0.16 (−0.03, 0.35) | 0.100 |
| Cortical thickness | −0.07 (−0.14, 0.01) | 0.072 | −0.20 (−0.33, −0.07) | 0.003 |
| Cortical thickness <2.67mm | 4.47 (0.68, 29.54) | 0.120 | 1.80 (0.27, 12.11) | 0.546 |
| AD (N=40) | ||||
| Aβ PET | 0.20 (0.06, 0.34) | 0.005 | 0.10 (−0.10, 0.30) | 0.300 |
| Aβ PET >1.42 SUVR* | ||||
| Tau PET entorhinal cortex | 0.18 (0.06, 0.30) | 0.013 | 0.07 (−0.22, 0.36) | 0.609 |
| Cortical thickness | −0.14 (−0.32, 0.05) | 0.147 | −0.21 (−0.45, 0.03) | 0.086 |
| Cortical thickness <2.67mm* | ||||
Abbreviations: AD, Alzheimer’s disease dementia; Aβ, amyloid-beta; CI, confidence interval; CU, cognitively unimpaired; MCI, mild cognitive impairment; OR, odds ratio; PET, positron emission tomography; SUVR, standardized uptake value ratio. All models adjusted for age, sex, and APOE.
Dichotomous models could not be run because 38 of the 40 had elevated Aβ PET and 37 of 40 had reduced cortical thickness.
3.3. Plasma tau measures by clinical diagnosis and elevated brain Aβ PET
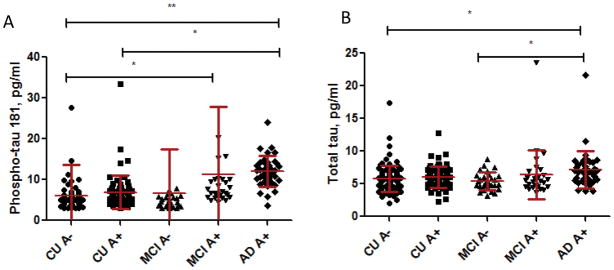
Given the associations between pTau181 and Aβ PET, we next examined mean differences in the plasma tau measures by both clinical diagnosis and abnormal Aβ PET (Table 3, Fig. 1). Two of the clinically diagnosed AD dementia subjects were not found to have elevated Aβ PET and, thus, were excluded from this analysis.
Table 3.
Participant characteristics by clinical diagnosis and elevated brain Aβ PET
| CU A- (N = 100) | CU A+ (N = 72) | MCI A- (N = 29) | MCI A+ (N = 28) | AD A+ (N = 38) | |||
|---|---|---|---|---|---|---|---|
| Mean (SD)/N(%) | Mean (SD)/N(%) | Mean (SD)/N(%) | Mean (SD)/N(%) | Mean (SD)/N(%) | F value | P value | |
| Plasma phospho-tau 181 | 6.0 (7.6) | 6.9 (4.1) | 6.7 (10.8) | 11.3 (16.5) | 12.0 (3.8) | 5.11 | < .001 |
| Plasma total tau | 5.8 (2.0) | 6.0 (1.7) | 5.4 (1.3) | 6.4 (3.7) | 7.2 (2.9) | 3.45 | .009 |
| Age | 70.4 (9.8) | 74.0 (8.8) | 66.7 (9.3) | 76.3 (10.0) | 67.2 (9.2) | 7.14 | < .001 |
| Male | 72 (72.0%) | 47 (65.3%) | 27 (93.1%) | 18 (64.3%) | 22 (57.9%) | .023 | |
| Education | 15.4 (2.4) | 15.0 (2.7) | 15.0 (3.6) | 14.9 (2.9) | 15.1 (2.8) | 0.34 | .853 |
| APOE ε4 | 16 (16.0%) | 34 (47.2%) | 5 (17.2%) | 15 (53.6%) | 28 (73.7%) | < .001 | |
| Aβ PET SUVR | 1.3 (0.1) | 1.8 (0.4) | 1.3 (0.1) | 2.2 (0.5) | 2.5 (0.4) | 146.82 | < .001 |
| Aβ PET>1.42 SUVR | 0 (0%) | 72 (100%) | 0 (0%) | 28 (100%) | 38 (100%) | < .001 | |
| Tau PET entorhinal cortex | 1.1 (0.1) | 1.1 (0.1) | 1.1 (0.1) | 1.3 (0.3) | 1.8 (0.3) | 131.50 | < .001 |
| Cortical thickness | 2.7 (0.1)) | 2.7 (0.2) | 2.7 (0.2) | 2.6 (0.2) | 2.3 (0.2) | 40.23 | < .001 |
| Cortical thickness<2.67mm | 34 (34.0%) | 34 (47.2%) | 13 (44.9%) | 22 (78.6%) | 35 (94.6%) | < .001 |
Abbreviations: AD, Alzheimer’s disease dementia; Aβ, amyloid-beta; APOE, apolipoprotein E; CU, cognitively unimpaired; IQR, interquartile range; MCI, mild cognitive impairment; PET, positron emission tomography; SUVR, standardized uptake value ratio.
Analyses of group differences included chi-square tests for dichotomous variables and ANOVA for continuous variables.
Fig. 1.
(A) Mean (SD) plasma phospho-tau 181, and (B) mean (SD) total tau by clinical diagnosis and elevated Aβ PET. Abbreviations: AD, Alzheimer’s disease dementia; CU, cognitively unimpaired; MCI, mild cognitive impairment. For illustrative purposes only, the following outliers were not included: (A) 1 CU at 75.7 pg/ml and 2 MCI at 62.4 and 93.3 pg/ml; (B) none. *P < .05; **P < .01
The AD dementia A+ group had higher mean pTau181 levels compared to both the CU A− (P =.002) and CU A+ (P =.025) groups. The MCI A+ group also had higher mean levels than the CU A− group (P =.033). There was no difference between the CU A+ and A− groups or the CU and MCI A+ groups. For total tau, the only significant group difference was higher mean levels for the AD dementia A+ group compared to the CU A− group (P =.016) and the MCI A− group (P =.015).
3.4. Correlation between plasma tau measures and tau PET by clinical diagnosis and elevated brain Aβ PET
Plasma pTau181 was more strongly correlated with higher tau PET SUVR in the entorhinal cortex among participants with elevated brain Aβ PET compared to those without (Table 4). The strength of the correlation between plasma pTau181 and tau PET increased with increasing disease severity, but was not found among the AD dementia A+. In comparison to plasma pTau181, the correlations between plasma total tau and tau PET were lower and only significant among the CU A−. Correlations of plasma pTau181 and total tau with all 47 tau PET regions are shown in Supplementary Table 1 and Supplementary Table 2.
Table 4.
Correlation between plasma pTau181 or total tau with tau PET entorhinal cortex SUVR by clinical diagnosis and elevated Aβ PET
| Clinical diagnosis and Aβ PET status* | Phospho-tau 181 | Total tau | ||
|---|---|---|---|---|
| Spearman’s rho | P value | Spearman’s rho | P value | |
| CU A− | −0.057 | 0.573 | 0.238 | 0.017 |
| CU A+ | 0.287 | 0.015 | 0.069 | 0.563 |
| MCI A− | 0.284 | 0.136 | 0.212 | 0.270 |
| MCI A+ | 0.437 | 0.020 | 0.104 | 0.598 |
| AD A+ | 0.125 | 0.456 | 0.159 | 0.341 |
| All A− | 0.018 | 0.835 | 0.228 | 0.009 |
| All A+ | 0.580 | <0.001 | 0.194 | 0.022 |
Abbreviations: AD, Alzheimer’s disease dementia; Aβ, amyloid-beta; CU, cognitively unimpaired; MCI, mild cognitive impairment; PET, positron emission tomography; SUVR, standardized uptake value ratio.
A+ = Aβ PET SUVR>1.42.
3.5. Accuracy of the plasma tau measures for elevated Aβ PET
Within each group, plasma pTau181 was a better predictor of elevated Aβ PET compared to total tau (P < .01), age (P < .05), or APOE (P < .05) alone (Table 5). Plasma pTau181 was also as good as the combined predictive value of age and APOE. In APOE stratified analyses, the AUROC for pTau181, but not total tau, was higher than age (all P < .05) for both ε4 carriers and non-carriers.
Table 5.
Predictive accuracy of plasma pTau181 and total tau for elevated Aβ PET
| All (CU+MCI+AD) (N=269) | All Non-demented (CU+MCI)(N=229) | CU only (N=172) | MCI only (N=57) | |
|---|---|---|---|---|
| AUCROC (95% CI) | AUCROC (95% CI) | AUCROC (95% CI) | AUCROC (95% CI) | |
| All | ||||
| Age | 0.582 (0.514, 0.650) | 0.643 (0.572, 0.715) | 0.597 (0.512, 0.682) | 0.777 (0.653, 0.902) |
| * Age, APOE ε4− | 0.610 (0.521, 0.699) | 0.649 (0.558, 0.739) | 0.586 (0.475, 0.696) | 0.840 (0.712, 0.968) |
| * Age, APOE ε4+ | 0.614 (0.477, 0.752) | 0.681 (0.543, 0.820) | 0.670 (0.494, 0.846) | 0.720 (0.486, 0.954) |
| ≥1 APOE ε4 allele | 0.699 (0.647, 0.751) | 0.664 (0.605, 0.722) | 0.656 (0.588, 0.724) | 0.682 (0.564, 0.799) |
| Age + ≥1 APOE ε4 allele | 0.750 (0.691, 0.808) | 0.747 (0.683, 0.811) | 0.709 (0.629, 0.790) | 0.839 (0.737, 0.940) |
| Plasma Phospho-tau 181 | 0.803 (0.749, 0.856) | 0.750 (0.685, 0.814) | 0.704 (0.624, 0.785) | 0.852 (0.752, 0.952) |
| Plasma Total tau | 0.598 (0.531, 0.666) | 0.564 (0.489, 0.639) | 0.566 (0.479, 0.653) | 0.565 (0.414, 0.717) |
| * APOE ε4− | ||||
| Plasma Phospho-tau 181 | 0.737 (0.656, 0.817) | 0.691 (0.601, 0.781) | 0.653 (0.541, 0.766) | 0.772 (0.622, 0.923) |
| Plasma Total tau | 0.571 (0.479, 0.663) | 0.558 (0.460, 0.656) | 0.563 (0.450, 0.675) | 0.542 (0.329, 0.754) |
| * APOE ε4+ | ||||
| Plasma Phospho-tau 181 | 0.860 (0.761, 0.958) | 0.821 (0.705, 0.937) | 0.765 (0.608, 0.922) | 0.960 (0.871, 1.000) |
| Plasma Total tau | 0.698 (0.575, 0.821) | 0.636 (0.492, 0.779) | 0.656 (0.490, 0.823) | 0.547 (0.226, 0.868) |
Abbreviations: Aβ, amyloid-beta; APOE, apolipoprotein E; AUROC, area under the receiver operator characteristic curve; CI, confidence interval; CU, cognitively unimpaired; MCI, mild cognitive impairment; PET, positron emission tomography.
There were 171 participants without an APOE ε4 allele; 159 were non-demented, including 122 CU and 37 MCI. There were 98 participants with an APOE ε4 allele; 70 were non-demented, including 50 CU and 20 MCI.
4. Discussion
The present results demonstrate that plasma pTau181 and total tau are differentially associated with neuroimaging measures of AD pathology. Both plasma tau and pTau181 levels were elevated in AD dementia patients compared to CU. However across the diagnostic groups, pTau181 was consistently associated with both Aβ and Tau PET whereas total tau was associated with cortical thickness. Further, when examining the utility of plasma pTau181 and total tau for predicting elevated brain Aβ, pTau181 was the most accurate predictor and was as good as, or better than, the combination of age and APOE. In APOE-stratified analyses, pTau181 was a better predictor of elevated brain Aβ compared to age alone. Together, these results highlight the potential use of plasma pTau181 as a non-invasive blood-based screener of AD pathophysiology and for identifying individuals at greatest risk of AD dementia in the general population or for secondary AD prevention trials.
Although blood-based biomarkers of AD pathophysiology have the advantage over CSF or neuroimaging measures with regards to feasibility at the population-level, cost, and invasiveness, the field has been hampered by lack of reproducibility and clinical utilization. Indeed, across multiple cohorts and assays blood-based measures of Aβ1–40 or Aβ1–42 have not been consistently associated with the clinical diagnosis and prognosis of AD dementia, or with cortical Aβ PET deposition [4,5]. A recent publication using stable isotope labeling kinetics reported that plasma Aβ1–42 concentration correlated with the CSF Aβ42/Aβ40 ratio and had good accuracy for predicting the sensitivity and specificity of elevated brain Aβ [26] but additional studies are needed to validate and longitudinally examine this new blood-based measure.
In contrast to blood Aβ measures, studies examining the clinical utility of plasma total tau have been consistent. Across four cohorts and two independent laboratories, participants with MCI or AD dementia had higher plasma total tau levels compared to CU participants. However, there was considerable overlap between groups and no significant differences between CU and MCI [6,21,27]. Longitudinally, higher levels of plasma total tau have been associated with cognitive decline and risk of MCI [6,7 ], but this relationship was independent of brain Aβ [7]. Thus, plasma total tau could be a useful prognostic marker for cognitive decline but it is not specific to the AD pathophysiological process.
The total tau results of the present study, using a different population than previously published [7,21], are consistent. First, plasma total tau levels were higher among AD dementia patients compared to MCI or CU. Second, the difference between MCI and CU was not statistically significant and there was substantial overlap between all of the groups. Third, plasma total tau was more strongly associated with cortical thickness, albeit in an AD-signature region, than with Aβ or Tau PET. Taken together, these results further demonstrate that plasma total tau may be a useful marker of general cognitive decline or neurodegeneration, but is not specific to AD pathophysiology.
While both CSF total tau and pTau are elevated in prodromal AD and AD dementia [4,28], total tau (but not pTau) is also elevated in traumatic brain injury, stroke, and Creutzfeldt-Jakob disease [29–32]. Thus, CSF pTau is thought to be more specific for the pathophysiological state associated with the accumulation of AD-type tau pathology [8]. Based on this reasoning, the new A/T/N research criteria consider elevated CSF pTau indicative of accumulation of AD-like tau, “T”. In contrast, elevated CSF total tau is considered indicative of active neuronal injury, “N” [33]. Our findings, but now in plasma, similarly corroborate this distinction. Our measure of plasma pTau181 was associated with both Aβ and Tau PET, whereas plasma total tau was only associated with cortical thickness in an AD signature region.
Additional verification of the potential utility of plasma pTau181 was that its relationship with Aβ and tau PET followed the typical AD pathological progression. As demonstrated in Table 4, the correlation between plasma pTau181 and Tau PET entorhinal cortex SUVR was only present in A+ groups, and was stronger in MCI A+ compared to CU A+. The lack of correlation within the AD A+ group is likely due to the complexity in AV1451 binding. In our recent work, AV1451 preferentially bound to mature intracellular tangles and not extracellular “ghost” tangles seen later in the disease process [34]. This may have contributed to an insufficient range to see correlations in the AD A+ group. The correlation of plasma pTau181 with Aβ adds additional evidence to the amyloid cascade hypothesis and places the detection of abnormal tau on PET earlier in the trajectory of the disease, but still after onset of abnormal Aβ PET. This can be attributed to plasma pTau181 being more sensitive than Tau PET to detect the presence of NFT pathology and may allow for earlier intervention of tau targeting therapeutics. Additional studies will need to assess temporal changes in both Tau PET and plasma pTau181 and to determine which measure is more sensitive to treatment-induced changes.
Plasma pTau181 not only showed a stronger association with brain Aβ PET, but also had good sensitivity and specificity for predicting elevated brain Aβ across the clinical severity of the disease. To date, the strongest predictors of elevated brain Aβ are age and the APOE ε4 allele. To be a useful non-invasive screener, pTau181 should be as good as or better than the predictive value of the combination of age and APOE. Further, it should also enhance prediction among individuals who are APOE ε4- because there are currently no ways of identifying risk of elevated brain Aβ among these individuals other than age. In this study, plasma pTau181 was as good of a predictor of elevated brain Aβ, if not better, than the combination of age and APOE. Further, among both APOE ε4 carriers and non-carriers, plasma pTau181 was a significantly better predictor of brain Aβ than age across all diagnoses. Thus, pTau181 enhances the predictive value of elevated brain Aβ for both ε4 carriers and non-carriers. This is an important result given the urgent need to identify, in a cost-effective manner, which non-demented individuals have elevated brain Aβ.
There are multiple strengths to the study including the large sample size, well-characterized participants, and availability of same day Aβ and Tau PET imaging. However, the age of our AD group was relatively young compared to the average late-onset AD population. Given the lack of replication of most blood-based biomarkers, validation in another cohort is needed. Indeed, , It is encouraging that our plasma pTau181 and total tau results are aligned with CSF findings, that plasma pTau181 is more associated with the AD pathophysiological progress, and that our plasma total tau results are consistent with previous studies. To further develop plasma pTau181 into a clinically useful biomarker, future research will need to assess intra-individual variability, identify sample collection procedures or participant characteristics that may affect pTau181 levels, determine the prognostic value of plasma pTau181 for clinical progression and the serial relationship between change in pTau181 to change in Tau PET, and identify the best context of use [1–3].
Supplementary Material
Supplementary Table 1. Correlations between plasma phospho-Tau 181 and Tau PET Regions of Interest (ROIs).
Supplementary Table 2. Correlations between plasma total tau and Tau PET Regions of Interest (ROIs).
RESEARCH IN CONTEXT.
Systematic review: We reviewed the literature using traditional (e.g., PubMed) resources. CSF phosphorylated tau has utility as a prognostic/diagnostic Alzheimer’s disease (AD) biomarker. However, studies have not examined the utility of plasma phosphorylated tau 181 (pTau181) or compared it to plasma total tau.
Interpretation: Both plasma pTau181 and total tau increased with AD clinical severity. Plasma pTau181 was more strongly associated with amyloid- and tau-PET. In contrast, total tau was more strongly associated with cortical thickness. Plasma pTau181 was as good as or better than age and APOE in predicting elevated brain amyloid. These results suggest that plasma pTau181 may have utility as a marker of AD pathology and as a potential first-line screener in the population for AD pathology.
Future directions: Validation of these results in other populations is needed. Future research should also determine the factors affecting pTau181 levels and its best context of use.
Highlights.
Plasma total tau and phosphorylated tau 181 (pTau181) increased with AD severity
Plasma pTau181, but not total tau, was higher among those with elevated brain Aβ
Plasma pTau181 was associated with both Aβ and Tau PET; total tau was associated with cortical thickness
Plasma pTau181 was a more sensitive and specific predictor of elevated brain Aβ than total tau
Plasma pTau181 was as good as, or better than, age and APOE alone in predicting brain Aβ
Acknowledgments
Funding: This study was supported by funding from the National Institutes of Health/National Institute on Aging grants U01 AG006786, R01 AG011378, R01 AG041851, P50 AG16574, and R01 AG049704, the GHR Foundation, and was made possible by the Rochester Epidemiology Project (R01 AG034676). The sample preparation and analysis was carried out by Kolby L. Janzen of Advanced Testing Laboratories. We would like to greatly thank AVID Radiopharmaceuticals, Inc., for their support in supplying AV-1451 precursor, chemistry production advice and oversight, and FDA regulatory cross-filing permission and documentation needed for this work.
Footnotes
Conflict of Interest Disclosures: Dr. Mielke served as a consultant to Eli Lilly and Lysosomal Therapeutics, Inc., and receives research support from the National Institutes of Health (R01 AG49704, P50 AG44170, U01 AG06786 RF1 AG55151), Department of Defense (W81XWH-15-1), and unrestricted research grants from Biogen, Roche, and Lundbeck. Mr. Hagen has no disclosures. Drs. Xu, Chai, Airey and Dage are employees of Eli Lilly. Dr Lowe consults for Bayer Schering Pharma, Piramal Life Sciences and Merck Research and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals and the NIH (NIA, NCI). Drs. Machulda receives research support from the National Institutes of Health (U01 AG006786). Dr. Roberts receives research support from the National Institutes of Health (U01 AG006786) and an unrestricted research grant from F. Hoffman-La Roche. Dr. Knopman served as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRx Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer’s Disease Cooperative Study; and receives research support from the NIH (R01 AG011378, P50 AG016574, U01 AG006786, AG029550, AG032306, and U01 HL096917). Dr. Jack has provided consulting services for Eli Lilly. He receives research funding from the National Institutes of Health (R01 AG011378, U01 HL096917, U01 AG024904, RO1 AG041851, R01 AG037551, R01 AG043392, and U01 AG006786), and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. Dr. Petersen serves on scientific advisory boards for Roche, Inc., Merck, Inc., Biogen, Inc., and Genentech, Inc.; receives royalties from the publication of Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the National Institutes of Health (P50 AG016574, U01 AG006786, U01 AG024904, and R01 AG011378).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Bryant SE, Mielke MM, Rissman RA, Lista S, Vanderstichele H, Zetterberg H, et al. Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement. 2017;13:45–58. doi: 10.1016/j.jalz.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–28. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–63. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 4.Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–84. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 5.Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1:371–80. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, et al. Plasma tau in Alzheimer disease. Neurology. 2016;87:1827–35. doi: 10.1212/WNL.0000000000003246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mielke MM, Hagen CE, Wennberg AMV, Airey DC, Savica R, Knopman DS, et al. Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the Mayo Clinic Study on Aging. JAMA Neurol. 2017;74:1073–80. doi: 10.1001/jamaneurol.2017.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–13. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 9.Tatebe H, Kasai T, Ohmichi T, Kishi Y, Kakeya T, Waragai M, et al. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol Neurodegener. 2017;12:63. doi: 10.1186/s13024-017-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–9. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, et al. Mayo’s Older Americans Normative Studies: WAIS-R norms for ages 56 to 97. Clin Neuropsychol. 1992;6:1–30. [Google Scholar]
- 13.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- 15.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz AJ, Yu P, Miller BB, Shcherbinin S, Dickson J, Navitsky M, et al. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain. 2016;139:1539–50. doi: 10.1093/brain/aww023. [DOI] [PubMed] [Google Scholar]
- 18.Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR., Jr Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26:600–8. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack CR, Jr, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 2017;13:205–16. doi: 10.1016/j.jalz.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vemuri P, Lowe VJ, Knopman DS, Senjem ML, Kemp BJ, Schwarz CG, et al. Tau-PET uptake: regional variation in average SUVR and impact of amyloid deposition. Alzheimers Dement. 2017;6:21–30. doi: 10.1016/j.dadm.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dage JL, Wennberg AM, Airey DC, Hagen CE, Knopman DS, Machulda MM, et al. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016;12:1226–34. doi: 10.1016/j.jalz.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rankin CA, Sun Q, Gamblin TC. Tau phosphorylation by GSK-3beta promotes tangle-like filament morphology. Mol Neurodegener. 2007;2:12. doi: 10.1186/1750-1326-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. J Neurochem. 2000;74:1587–95. doi: 10.1046/j.1471-4159.2000.0741587.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu X. Classification accuracy and cut point selection. Stat Med. 2012;31:2676–86. doi: 10.1002/sim.4509. [DOI] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 26.Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13:841–9. doi: 10.1016/j.jalz.2017.06.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, et al. Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther. 2013;5:9. doi: 10.1186/alzrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmqvist S, Zetterberg H, Mattsson N, Johansson P, Minthon L, Blennow K, et al. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 2015;85:1240–9. doi: 10.1212/WNL.0000000000001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E, et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297:187–90. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- 30.Ost M, Nylen K, Csajbok L, Ohrfelt AO, Tullberg M, Wikkelso C, et al. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology. 2006;67:1600–4. doi: 10.1212/01.wnl.0000242732.06714.0f. [DOI] [PubMed] [Google Scholar]
- 31.Skillback T, Rosen C, Asztely F, Mattsson N, Blennow K, Zetterberg H. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: results from the Swedish Mortality Registry. JAMA Neurol. 2014;71:476–83. doi: 10.1001/jamaneurol.2013.6455. [DOI] [PubMed] [Google Scholar]
- 32.Buerger K, Otto M, Teipel SJ, Zinkowski R, Blennow K, DeBernardis J, et al. Dissociation between CSF total tau and tau protein phosphorylated at threonine 231 in Creutzfeldt-Jakob disease. Neurobiol Aging. 2006;27:10–5. doi: 10.1016/j.neurobiolaging.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–47. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4:58. doi: 10.1186/s40478-016-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Correlations between plasma phospho-Tau 181 and Tau PET Regions of Interest (ROIs).
Supplementary Table 2. Correlations between plasma total tau and Tau PET Regions of Interest (ROIs).