Abstract
Current clinicopathologic staging systems and serum biomarkers poorly discriminate tumor biology in hepatocellular carcinoma (HCC), with high recurrence rates following curative-intent surgical resection and liver transplantation (LT). Identification of accurate biomarkers for improved prognostication and treatment selection is a critical unmet need. We sought to develop a novel “liquid-biopsy” assay capable of detecting HCC circulating tumor cells (CTCs), and characterizing phenotypic subpopulations with prognostic significance. Utilizing HCC cell lines, a tissue microarray, and human blood samples, an antibody cocktail targeting the cell-surface markers asialoglycoprotein receptor (ASGPR), Glypican-3, and epithelial cell adhesion molecule (EpCAM) was optimized for HCC-CTC capture utilizing the NanoVelcro microfluidic assay. The ability of HCC-CTCs and vimentin(+)-CTCs (a subpopulation expressing an epithelial-to-mesenchymal phenotype) to accurately discriminate tumor stage, recurrence, progression, and overall survival was evaluated in a prospective study of 80 patients. Multimarker capture detected greater numbers of CTCs than any individual antibody alone for both cell line and patient samples (p<0.05). HCC-CTCs were identified in 59/61 patients (97%), and accurately discriminated HCC (median: 6 CTCs) and non-HCC patients (median: 1 CTC; AUROC=0.92, p<0.0001; sensitivity=84.2%, specificity=88.5%). Vimentin(+)-CTCs accurately discriminated early-stage, LT eligible patients (median: 0 CTCs) from locally advanced/metastatic, LT ineligible patients (median: 6 CTCs; AUROC=0.89, p<0.0001; sensitivity=87.1%, specificity=90.0%), and predicted overall survival for all patients (HR 2.21, p=0.001), and faster recurrence after curative-intent surgical or locoregional therapy in potentially curable early stage HCC (HR 3.14, p=0.002). In conclusion, we developed a novel multimarker CTC enrichment assay that detects HCC-CTCs with high efficiency and accuracy. A phenotypic subpopulation of vimentin(+)-CTCs appears to signify the presence of aggressive underlying disease and occult metastases, and may have important implications for treatment selection.
Keywords: Circulating Tumor Cell, Hepatocellular Carcinoma, Phenotype, Biomarker, Liquid Biopsy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second most common cause of cancer related death worldwide.1 Unfortunately, current clinicopathologic staging systems and serum biomarkers (e.g. alpha-fetoprotein, AFP) poorly discriminate both early-stage patients amenable to curative-intent surgical resection and liver transplantation (LT), where postoperative recurrence remains a significant challenge, and advanced-stage patients receiving chemotherapy, where predictors of response remain unavailable.2,3 Thus, the development of better biomarkers to aid in prognostication and treatment selection is an urgent, unmet need.
Circulating tumor cells (CTCs) are thought to originate from the primary tumor or metastatic sites, can be detected in the peripheral blood, and are implicated as a potential cause of post-surgical recurrence and metastases.4,5 While CTCs can serve as prognostic biomarkers in solid tumors, studies evaluating CTCs in HCC have found limited utility.6,7 One reason is that most CTC enrichment assays, including the FDA-approved CellSearch™ CTC assay, rely on the use of antibodies against the epithelial cell-surface marker EpCAM to “capture” CTCs by antigen-specific immunomagnetic separation from leukocytes. As only 20–35% of HCCs express EpCAM, methods based on EpCAM alone have resulted in low CTC detection rates and limited utility for HCC.8 Alternative CTC capture methods utilizing antibodies directed at hepatocyte-specific cell-surface markers7,9, CD45-depletion10, or microfluidic11 systems have all demonstrated increased efficiency in isolating HCC-CTCs. Furthermore, these non-EpCAM based methods allow for capture of distinct CTC subpopulations with more mesenchymal properties in HCC.9,12
The identification and significance of CTC subpopulations expressing a mesenchymal phenotype is an area of active investigation in many solid tumors due to their potential role in metastasis.13 Previous studies in HCC have demonstrated that epithelial-to-mesenchymal transition (EMT), associated with losing expression of cell-cell adhesion markers and gaining the migratory and invasive properties of a mesenchymal cell, is an important step in the metastatic cascade.14 Several studies in HCC have demonstrated that the overexpression of mesenchymal markers such as vimentin, an intermediate filament, is associated with more advanced tumors and worse prognosis.15,16 Thus, identifying CTCs that demonstrate an EMT phenotype holds promise for identifying patients likely to harbor aggressive underlying disease.
In this study, we investigated the use of a microfluidic, antibody-based CTC capture assay to efficiently capture HCC-CTCs and characterize CTC phenotypes of prognostic importance. Unlike existing technologies, the NanoVelcro CTC assay combines a microfluidic system with enhanced topographic interactions and CTC-capture antibody coated nanostructured substrates to allow for the efficient separation and capture of HCC-CTCs from background WBCs. The working principle of the NanoVelcro CTC Assay has been utilized for many solid tumors, including prostate cancer, melanoma, and pancreatic cancer.17,18 To optimize the use of the NanoVelcro Assay for detecting HCC-CTCs from patient blood, we investigated HCC CTC capture and immunostaining antibodies7,19 followed by validation of their efficacy using a HCC tissue microarray (TMA), HCC cell lines, and a pilot group of HCC patients. Subsequently, utilizing this optimized assay in a prospective study of patients with non-malignant liver disease (NMLD), HCC patients and healthy controls without HCC or other identified liver disease, we 1) identified, characterized, and enumerated HCC-CTCs and the subpopulation of vimentin(+)-CTCs, 2) evaluated their ability to discriminate between non-HCC and HCC patients, as well as early-stage and advanced-stage HCC patients, and 3) evaluated their prognostic utility for cancer progression, recurrence, and survival.
MATERIALS AND METHODS
Antibody Selection for the Capture and Identification of HCC-CTCs
The capture of CTCs relies on the antigen-specific immobilization of CTCs on the NanoVelcro surface using antibodies directed against HCC cell surface markers. Immobilized CTCs are then identified and phenotyped based on their immunohistochemical staining characteristics (Supplemental Fig. 1). A wide selection of antibodies for both CTC capture, as well as CTC identification and phenotyping, were evaluated using an 8 HCC cell line panel as detailed in the supplemental methods. Antibodies demonstrating optimal staining and specificity across the cell line panel were then tested for suitability on a 114-patient HCC tissue microarray (TMA) generated from archived, resected HCC specimens at the University of California, Los Angeles.20 Antibodies against the cell-surface markers EpCAM (Cell Signaling, Danvers, MA), asialoglycoprotein-receptor (ASGPR; Abcam, Cambridge, UK), and glypican-3 (GPC-3; Santa Cruz Biotechnology, Santa Cruz, CA), as well as the cytoplasmic marker vimentin (Abcam), were used to stain the TMA (Supplemental Fig. 2). Staining intensity was assessed on a 4-point scale (none/weak/moderate/strong staining) by a single pathologist (S.W.F.), and tumors were considered to have stained positive if they displayed moderate or strong staining to any one of the antibodies tested (Supplemental Fig. 3). TMA staining results were summarized as shown in Fig. 1A.
Figure 1.
(A) A 114-tumor tissue microarray was used to test antibodies against markers of interest for the study. Percentage of tumors with moderate to strong staining for the given cell surface marker is shown and compared with the percentage of tumors staining for at least 1 of the 3 markers (Combined). (B) Capture efficiency of the NanoVelcro CTC capture assay using 8 HCC cell lines. Each individual antibody, as well as the multimarker combination of all 3 antibodies, was compared. The percentage of spiked cells that were captured and identified is reported. (C) Summary of capture efficiency data from (B) all 8 cell lines. The combination of all 3 antibodies demonstrated superior capture efficiency compared with the use of any single individual antibody alone. (D) Capture efficiency of NanoVelcro chips functionalized with each antibody individually as well as with the combination of all 3 antibodies for pilot study patient samples (n = 10). (E) Summary of capture efficiency data from (D) for each cell surface antibody demonstrating superior capture with the combination of all 3 antibodies (vs. each antibody alone, p<0.05). *–P<0.05; ***–P<0.001
Optimization of Multimarker CTC Capture
The NanoVelcro CTC assay utilizes a microfluidic chaotic mixer to enhance CTC interactions with the capture antibody coated nanosubstrate surface of the chip to enhance CTC capture rates (Supplemental Fig. 1A). Following CTC capture, CTCs are immunostained as outlined in our workflow (Supplemental Fig. 1B) and then identified and phenotyped using multi-color ICC and cytometric assessment (Supplemental Figs.4&5). While the performance of the NanoVelcro CTC assay has been reported for many solid tumors, we first validated the methodology for HCC using cell lines as previously described and detailed in the supplemental methods.21 All experiments were performed at the optimum flow rate of 1 mL per hour. (Supplemental Fig. 6A). Calibration experiments using both HCC cell lines (Fig. 1B&C) and HCC patient samples (Fig. 1D&E) were performed to compare the efficiency of the multimarker capture cocktail (EpCAM, ASGPR, GPC-3) to each antibody individually. Cell spiking experiments confirmed the capture efficiency of the assay for CTC concentrations as low as 10 cells in 2 mL of blood (Supplemental Fig. 6B). For calibration experiments, only 2 mL of blood was utilized; however, for clinical specimens all assays were run as parallel duplicate samples. Thus, CTC counts are recorded per 4 mL of blood except as noted otherwise.
Patient Recruitment and Blood Collection
Between April 2015 and September 2016, healthy controls, patients with non-malignant liver disease (NMLD – cirrhosis without HCC, adenoma, focal nodular hyperplasia), and patients with HCC were enrolled (IRB #14-001932). Inclusion criteria included pathologic or radiographic (LIRADS-5) diagnosis of HCC, with patients having synchronous or past (within 5 years) extrahepatic malignancies excluded from enrollment. A database of demographic and clinicopathologic information was maintained prospectively, with clinical staging assigned based on either pathologic (when available) or radiographic assessment (Milan criteria [MC]22 and University of California, San Francisco [UCSF]23 transplant criteria) (Supplemental Table 2) at study enrollment. Patients were categorized as early-stage if their tumors were within UCSF transplant criteria, locally-advanced if they were outside of UCSF but without extrahepatic disease, and metastatic if there was evidence of distant metastases. The modified Response Evaluation Criteria In Solid Tumors (mRECIST) guidelines were used to evaluate patient’s disease status as stable or progressing.24
Sample Processing, Chip Scanning and CTC Enumeration
Blood samples were processed as previously described and detailed in the supplemental methods using the parameters determined by the optimization experiments described above. Captured CTCs were imaged using immunocytochemistry (ICC), allowing for both cytometric and immunofluorescent identification parameters, and criteria for CTC identification were developed by a trained cytopathologist (M.S.). Our ICC and cytometric criteria for CTC identification were optimized for HCC-CTCs using the methods described in the supplemental methods and presented in Supplemental Figures 4&5. When analyzing the multi-channel ICC image, WBCs were defined as round/ovoid cells, DAPI+/CD45+/CK−, with size ≤ 6-μm; and HCC-CTCs are defined as round/ovoid cells, DAPI+/CD45−/CK+, with size ≥ 6-μm. Epithelial-to-mesenchymal phenotype, vimentin(+)-CTCs are the subpopulation of HCC-CTCs defined as round/ovoid events, DAPI+/CD45-/CK+/vimentin+, with size ≥ 6-μm. Any CD45 positivity greater than 2x background discounted a cell as being a CTC. CTCs were enumerated by the same blinded researcher (S.H.) and CTC counts are represented as a total count per 4-mL VB (Fig. 2B).
Figure 2.
(A) Schematic depicting CTC identification via a 4-color ICC approach in conjunction with high-resolution fluorescent microscopy. Representative images of a HCC-CTC and the subpopulation of vimentin(+) CTCs shown at 400x magnification. The WBC staining pattern can be seen in the cell in the lower left corner of the HCC-CTC image. (B) CTC counts for both HCC CTCs and vimentin(+) CTCs for all patients enrolled in the study(n = 80). Patients are divided into groups based on staging criteria and sorted within groups based on total CTC count. Healthy indicates controls without HCC or other identified liver disease.
Statistical Methods
Continuous variables were summarized as medians and interquartile ranges (IQR) and compared using the Wilcoxon rank sum test, while categorical variables were summarized as frequencies and percentages and compared using the χ2 test/Fisher exact test. A two tailed p-value < 0.05 was considered to be statistically significant. Differences in staining efficiency and CTC capture for different markers were compared using the Wilcoxon matched-pairs signed rank test. CTC enumeration was reported per 4-mL of blood except as otherwise noted, and compared among groups utilizing the non-parametric Mann-Whitney U-test. Cutoff points for CTC enumeration was evaluated using Youden’s J statistic and using the cutoff finder web application.25 Diagnostic performance of CTCs was evaluated using receiver operating characteristic curves (ROCs) for determination of the area under the curve (AUROC) in addition to sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) calculations. Outlier analysis was performed with the Iterative Grubb’s method (α = 0.05). Outlier analysis did not result in any changes in the statistical interpretation of the results (data not shown).
Both CTC counts and AFP values had a skewed distribution and were log transformed. Post-transplant patients without a pre-transplant blood draw were not included in the survival analysis (n = 1). Overall survival (OS) was the time from initial blood draw (study enrollment) to last follow-up or death, with only death counting as an event. Progression-free survival (PFS) was evaluated only in the subset of advanced stage, incurable patients, with progression defined as measurable radiographic increase in tumor burden (measured by mRECIST), and measured from time of study enrollment to first radiographic progression or cancer-related death. Time to recurrence (TTR) was evaluated only in the subset of early stage, potentially curable patients undergoing curative intent surgical or locoregional therapy, where the initial post-treatment imaging showed no viable arterially enhancing lesions (irrespective of surgical resection, ablation, or in select cases, TACE treatment). Recurrence was defined as a new or recurrent radiographic lesion meeting OPTN criteria for HCC. Time to recurrence (TTR) of radiographically evident disease, progression-free (PFS), and overall survival (OS) were computed using Kaplan-Meier methods and compared by the log-rank (Mantel-Cox) test.
Univariate analysis of individual predictors of PFS and TTR was performed using the Fine and Gray competing risks Cox regression model to account for the competing risk of non-HCC related mortality. Factors identified as significant (p < 0.2) on univariate analysis were entered in to a multivariate competing risk Cox regression model to identify significant independent predictors of HCC progression and recurrence. Hazard ratios and 95% CIs were reported and statistical significance assessed based on non-overlapping 95% CIs. For multivariate analysis, final models for OS, PFS, and TTR included AFP, UCSF staging, and primary tumor size, in addition to CTC count for both HCC-CTCs and the subpopulation of vimentin(+) CTCs. The hazard ratios reported are per standard deviation increase (SD increase) of the CTC count on the log-scale. All statistical analyses and calculations were performed with the assistance of GraphPad Prism 7.0a (GraphPad Software, La Jolla, CA) and SAS (SAS Institute, Cary, NC), and follow the REporting recommendations for tumor MARKer prognostic studies (REMARK) guidelines.26
RESULTS
Antibody Selection for the Capture and Identification of HCC-CTCs
CTCs are first incubated with biotinylated antibody cocktail (EpCAM, ASGPR, GPC-3) and then “captured” by the NanoVelcro assay through the interaction of biotin on the CTCs with the streptavidin-coated surface of the NanoVelcro chips. To optimize CTC capture we utilized a TMA developed from 114 resected human HCC samples to screen the potential HCC-specific cell-surface capture markers ASGPR and GPC-3, in addition to the widely-utilized cell-surface marker EpCAM. Of the 114 tumors, 89 (78.9%) stained for at least 1/3 antibodies (ASGPR–65.8%, GPC-3–31.6%, EpCAM–7.9%, Fig. 1A). However, the combination of all 3 antibodies stained significantly more tumors than any single antibody alone for the entire TMA, with staining of any one or more of the antibodies considered positive (p < 0.001 for ASGPR alone and p<0.001 for EpCAM or GPC-3 alone).
Optimization of the NanoVelcro CTC Assay utilizing multimarker CTC Capture
Capture antibody optimization in HCC cell lines
Utilizing artificial blood samples consisting of 500 HCC cell line cells spiked in 2 mL normal blood samples, the CTC capture efficiency for each individual capture antibody (EpCAM, ASGPR, GPC-3) was compared to the combination of all 3 antibodies for 8 HCC cell lines (Fig. 1B). Use of the triple-antibody multimarker capture cocktail resulted in the highest CTC capture efficiency (> 80%), significantly greater than any single capture antibody alone (p=0.018 for EpCAM, p<0.001 for ASGPR and GPC-3, Fig. 1C).
Capture antibody optimization in human HCC patients
In a preliminary cohort of 10 patients with HCC, we evaluated the total CTCs enumerated using EpCAM alone, ASGPR alone, GPC-3 alone, and the triple multimarker antibody cocktail (Fig. 1D). Similar to our cell line results, we found that the combination of all 3 capture antibodies resulted in significantly greater CTCs captured compared with the use of any single antibody alone (vs. multimarker capture, p=0.041 for EpCAM, p=0.007 for ASGPR, and p=0.007 for GPC-3, Fig. 1E).
Defining HCC-CTC Phenotypes
To ensure that our immunocytochemical staining of HCC-CTCs was specific for hepatocytes, selected patient samples were stained with CK as the primary epithelial marker, and co-stained with hepatocyte-specific markers AFP, arginase, and hep-par-1. AFP, arginase, and hep-par-1 staining was only noted in the CK+ cells and never in the CD45+ leukocytes, confirming the specificity of our ICC criteria for HCC-CTCs (Supplemental Fig. 5). Additionally, we discovered a subpopulation of vimentin(+) CTCs with an epithelial-to-mesenchymal phenotype. Based on these initial experiments, all prospective HCC patients enrolled in the study underwent enumeration of HCC-CTCs and the subpopulation of vimentin(+)-CTCs (Fig. 2).
Prospective Study Evaluating CTC Enumeration and Phenotype Utilizing Optimized Multimarker HCC NanoVelcro Assay
Of 84 patients approached for study participation, 80 patients underwent peripheral blood draw, CTC enumeration, and phenotyping using our optimized multimarker NanoVelcro HCC-CTC assay (Table 1). Four patients were excluded (2–refused informed consent, 1–synchronous cancer, 1– insufficient blood draw). Of the 80 enrolled patients, 61 had HCC, 11 had NMLD (7–cirrhosis without HCC, 4–benign liver lesions), and 8 were healthy controls (Fig. 3). Among patients with HCC, 31/61(50.8%) were early-stage (within UCSF transplant eligibility criteria),23 21/61(34.4%) were locally-advanced with extensive liver involvement, and 9/61(14.8%) were metastatic. Overall, 41% of enrolled patients received prior systemic or locoregional therapy (median 124 days, range 23 – 235 days prior to blood draw). Patients were followed for a median of 325 days after blood draw. Of the 61 patients with HCC, 28(45.9%) progressed and 20(32.8%) died. Patients with NMLD were followed for an equivalent amount of time. None of the NMLD or healthy control patients involved in the study developed HCC during follow-up.
Table 1.
Clinical, Laboratory, Radiologic, and Treatment Characteristics of the Prospective Cohort (n = 80).
| Characteristics | Data |
|---|---|
| Age, median (IQR) | 61 (53 – 68) |
| Female, n (%) | 23 (28.8) |
| Diagnosis, n (%) | |
| HCC | 61 (76) |
| Healthy | 8 (10) |
| Cirrhosis | 7 (9) |
| Adenoma | 4 (5) |
| Laboratory | |
| Physiologic MELD, median (IQR) | 8 (7 – 13) |
| Childs Class, n (%) | |
| A | 60 (75) |
| B | 9 (11.5) |
| C | 11 (13.8) |
| Most recent AFP, median (IQR) | 24 (5 – 790) |
| Maximum pre-draw AFP, median (IQR) | 38 (7 – 1148) |
| HCC Cause, n (%) | |
| HCV | 40 (65.6) |
| HBV | 11 (18) |
| NASH | 5 (8.2) |
| Other | 3 (4.9) |
| Unknown/non-cirrhotic | 2 (3.3) |
| Radiologic | |
| Maximum tumor diameter, median (IQR) | 4.6 (3.4 – 6.7) |
| Cumulative tumor diameter, median (IQR) | 6.2 (3.5 – 9.7) |
| Predraw treatment characteristics | |
| Any predraw treatment, n (% of all HCC) | 25 (41.0) |
| Early stage (within UCSF) | 8 (32.0) |
| Advanced stage (outside UCSF) | 17 (68.0) |
| No. of predraw treatments, n (% of treated pts) | |
| 1 | 12 (48) |
| 2 | 2 (8) |
| 3 | 5 (20) |
| 4+ | 6 (24) |
| Type of treatment, n (% of treated pts) | |
| Sorafenib | 10 (40) |
| Nivolumab | 4 (16) |
| Other systemic therapy | 1 (4) |
| Radioembolization (Y90) | 2 (8) |
| Transarterial chemoembolization (TACE) | 12 (48) |
| Thermal ablation | 9 (36) |
| Transplant criteria | |
| Within Milan criteria, n (%) | 23 (37.7) |
| Outside Milan, within UCSF criteria, n (%) | 8 (13.1) |
| Outside UCSF criteria/locoregional only, n (%) | 21 (34.4) |
| Metastatic disease, n (%) | 9 (14.8) |
| BCLC stage | |
| A | 11 (18) |
| B | 17 (27.9) |
| C | 26 (42.6) |
| D | 7 (11.5) |
IQR – interquartile range, HCC – hepatocellular carcinoma, MELD – Model for End-Stage Liver Disease, AFP – alphafetoprotein, HCV – hepatitis C virus, HBV – hepatitis B virus, NASH – Nonalcholic steatohepatitis, BCLC – Barcelona clinic liver cancer stage
Figure 3.
Diagnosis and staging flowchart for patients enrolled in the study as well as associated CTC counts listed as median(IQR) for HCC-CTCs and Vimentin(+) CTCs. *–Early Stage: patients with lesions within UCSF radiographic staging criteria; **–Locally Advanced: patients with lesions larger than UCSF criteria but without evidence of metastatic disease.
HCC-CTCs
HCC-CTCs were found in 59/61(96.7%) patients with HCC (median=6, range=0–23). Occasional patients with NMLD were found to have low numbers of CTCs (median:1, range:0–7), particularly those with inflammatory adenomas (Fig. 2B). A single CTC was found in 2/8(25%) healthy control patients, indicating a false positive. Among patients with HCC, CTC count correlated with stage, with a median of 3 CTCs in early stage patients (range:0–15), 9 CTCs in locally advanced HCC (range:0–14), and 12 CTCs in patients with metastatic HCC (range:2–23)(Fig. 3). Significantly more HCC-CTCs were found in patients with radiographic evidence of portal vein invasion (p = 0.001).
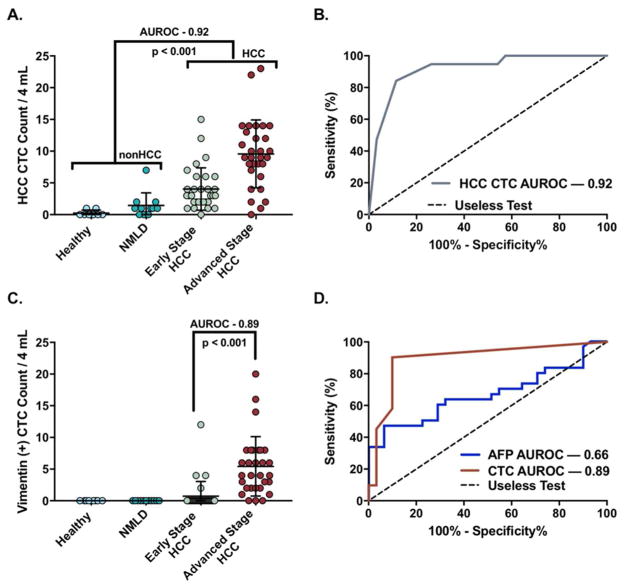
At the optimum cutoff of ≥2 CTCs/4 mL VB, HCC-CTC enumeration accurately discriminated among patients with and without HCC (NMLD or healthy controls) with a sensitivity=84.2%, specificity=88.5%, PPV=69.6%, NPV=94.7% (Fig. 4A) and an area under the ROC curve (AUROC) of 0.92 (95%CI = 0.86–0.99, p < 0.001), illustrating the ability of CTCs to discriminate patients with cancer from those without (Fig. 4B). A similar discriminatory performance was found when HCC patients were compared to NMLD alone (AUROC= 0.89).
Figure 4.
(A) CTC enumeration in patients with local or advanced HCC versus NMLD or healthy controls. Advanced stage patients include patients with locally advanced disease and metastatic disease (B) ROC curve for illustration of HCC CTC performance in the discrimination of HCC from NMLD or healthy controls. At a cutoff of ≥2 CTCs/4-mL VB, HCC CTC AUROC=0.92 (95%CI: 0.86–0.99, p<0.001). (C) Vimentin(+) CTC enumeration in patients with early or advanced stage HCC versus nonHCC patients. (D) Comparison of the performance of vimentin(+) CTCs and AFP in discriminating early, potentially curable, HCC from advanced stage HCC. At a cutoff of ≥1 vimentin(+) CTCs/4-mL VB, AUROC=0.89 (95%CI: 0.74–0.95, p<0.001) versus AFP (cutoff: ≥1600ng/mL), AUROC=0.66 (95%CI: 0.53–0.78, p=0.021).
Univariate Cox analysis of all HCC patients revealed that HCC-CTCs were associated with worse OS (HR:1.96, 95%CI: 1.12–3.42, p=0.018). In the subset of non-metastatic, potentially curable patients (n=30) who underwent locoregional therapy (resection, n=9; transplantation, n=5; RFA, n=11; or TACE, n=5), HCC-CTCs were associated with faster TTR (HR: 9.7, 95%CI: 2.08–45.19, p=0.004). In patients with incurable, locally advanced (n=15, diffuse infiltration or macrovascular invasion) or metastatic disease (n=8), HCC-CTCs were associated with worse PFS (HR: 2.09, 95%CI: 1.11–3.96, p=0.023). On multivariate analysis, including the covariates age, AFP, MELD score and tumor size, HCC-CTCs were again found to be significantly associated with PFS (HR: 2.09, 95%CI: 1.11–3.96, p=0.023) in the subset of advanced stage patients, but not TTR in potentially curable early stage patients or OS in all patients.
Vimentin(+)-CTCs
Vimentin(+) CTCs were found in 31(50.8%) patients with HCC (median:1, range:0–20) and never in patients with NMLD or healthy controls (Fig. 4C). Among HCC patients, the number of vimentin(+)-CTCs correlated with increasing tumor stage, with only 4/31(12.9%) early stage patients demonstrating vimentin(+)-CTCs (median:0, IQR:0–0, range:0–12), compared to 18/21(85.7%) locally advanced patients (median:4, IQR:2–6, range:0–8), and 9/9(100%) metastatic HCC patients (median:8, IQR:8–14, range:1–20). Significantly more vimentin(+)-CTCs were found in patient with radiographic evidence of portal vein invasion (p < 0.001).
At a cutoff of ≥1 CTC/4 mL VB, vimentin(+)-CTCs accurately discriminated transplant eligible HCC patients with early stage, potentially curable disease from transplant ineligible patients with locally advanced or metastatic HCC (Fig. 4C), with a sensitivity=87.1%, specificity=90.0%, PPV=90.0%, NPV=87.1%, and an AUROC of 0.89 (95%CI: 0.74–0.95, p<0.0001)(Fig. 4D), significantly superior to the AUROC of HCC-CTCs (AUROC 0.55; 95% CI: 0.47–0.63; p < 0.001) in discriminating early and advanced stage HCC. For additional comparisons, we performed the same analysis for AFP, the only widely available HCC biomarker. AFP discriminated early stage from advanced stage disease with a sensitivity=93.5%, specificity=46.7%, PPV=65.6%, NPV=65.5%, and AUROC of 0.66 (95% CI = 0.53 – 0.78, p = 0.021). Thus, vimentin(+)-CTCs were a significantly better predictor of advanced stage disease than AFP (DeLong’s-test, Z=2.6, p=0.009).
Univariate analysis of all HCC patients revealed that vimentin(+)-CTCs were highly associated with inferior OS (HR: 2.21, 95%CI: 1.38–3.52, p=0.001) (Fig. 5A). Perhaps more notably, CTCs were able to discriminate outcomes in the subset of potentially curable patients undergoing locoregional therapy (resection, n=9; transplantation, n=5; RFA, n=11, and TACE, n=5). In this subset of 30 patients who radiographically demonstrated no residual disease following treatment, the presence of any pre-treatment vimentin(+)-CTCs were associated with faster TTR (HR:3.14, 95%CI: 1.50–6.57, p=0.002)(Fig. 5B). Similarly, in the subset of incurable locally advanced or metastatic patients (n=23), vimentin(+)-CTCs were predictive of worse PFS (HR:1.81, 95%CI: 1.02–3.22, p=0.043)(Fig. 5C). On multivariate analysis, vimentin(+)-CTCs were found to be significantly associated with OS (HR:2.21, 95%CI: 1.38–3.56, p=0.001) in all patients, PFS (HR:2.16, 95%CI: 1.33–4.42, p=0.002) in the subset of advanced stage, incurable patients, and with a trend towards faster TTR (HR:2.45, 95%CI: 0.91–6.57, p=0.076) in the subset of early stage, potentially curable patients undergoing surgical or locoregional therapy.
Figure 5.

(A) Overall survival at the optimized cutoff of ≥1 vimentin(+) CTC, all HCC patients. (B) Time to recurrence following potentially curative locoregional therapy at the optimized cutoff of ≥1 vimentin(+) CTC (n=30). (C) Progression-free survival at the optimized cutoff of ≥4 vimentin(+) CTCs, for patients with locally advanced or metastatic disease not amenable to potentially curative locoregional therapy (n=23).
Potential Utility of HCC-CTCs
The value of HCC-CTCs and vimentin(+)-CTCs as biomarkers goes beyond initial prognosis, presenting potential utility for both longitudinal disease monitoring as well as appropriate treatment selection. 11 patients in the study underwent serial blood draws and CTC enumeration over the course of treatment, with disappearance of CTCs following successful tumor resection and ablation, and a subsequent reappearance of CTCs prior to clinical recurrence (data not shown). One such example is illustrated in Fig. 6A. The patient was a 63-year-old gentleman with compensated cirrhosis and a 5.8 cm arterially enhancing biopsy-proven HCC. His pre-resection blood draw revealed 8 vimentin(+)-CTCs (14 total HCC-CTCs). He underwent a partial right hepatectomy which revealed a 5.8 cm dominant lesion with a subcentimeter satellite lesion with microvascular invasion. CTC enumeration at 1 and 2 months post-resection revealed no CTCs, with MRI imaging revealing no evidence of recurrence. However, his 3rd post-resection blood draw revealed 6 vimentin(+)-CTCs (12 HCC-CTCs), with subsequent MRI demonstrating a segment 8 HCC recurrence.
Figure 6.
Demonstration of the potential utility of the HCC-CTC Assay (A) Disease monitoring after surgical resection. Pre-surgical blood draw revealed 8 vimentin(+) CTCs (14 total HCC-CTCs), with subsequent CTC enumeration revealing no CTCs at 1 and 2 months following resection of a 5.8 cm HCC, consistent with surveillance MRI imaging revealing no evidence of recurrence. A 3rd post-resection blood draw revealed 6 vimentin(+) CTCs (12 total HCC-CTCs), presaging the subsequent MRI demonstration of a segment 8 HCC recurrence. (B) Vimentin(+) CTCs are highly predictive of earlier recurrence following potentially curative locoregional therapy, and appear to be a surrogate for patients who have tumors with aggressive underlying tumor biology. This patient had a solitary 4cm right hepatic lobe HCC without evidence of metastases. Despite being radiographically staged as an early stage patient, she was found to have 12 vimentin(+) CTCs (15 HCC-CTCs total) prior to any treatment. Despite successful locoregional therapy with TACE, she soon developed multifocal HCC on her 1-month post-procedure scan with rapid development of metastatic lung nodules at 3-months post-procedure.
The presence of vimentin(+)-CTCs consistently portended faster time to recurrence following locoregional treatment (Fig. 5D), with an example illustrated in Fig. 6B. The patient had a solitary 4cm right hepatic lobe HCC without evidence of metastases, but despite being radiographically staged as an early stage patient, was found to have 12 vimentin(+) CTCs (15 HCC-CTCs). Despite successful locoregional therapy with TACE, she was found to have multifocal HCC on her 1 month post-procedure scan with rapid development of metastatic lung nodules at 3 months’ post-procedure.
DISCUSSION
The limitations of existing clinicopathologic staging systems of HCC is evident in the high recurrence rate following locoregional therapies or curative-intent surgical interventions such as resection or transplantation. CTCs are emerging as a promising biomarker for several cancer types, but their application to HCC has been limited when utilizing existing assays that rely on epithelial cell-surface markers alone for CTC capture. In this study, we present the development of a novel multimarker CTC capture platform that allows for the identification, enumeration, and analysis of HCC-CTCs with high sensitivity and specificity. Furthermore, we identify a phenotypic subpopulation of vimentin(+)-CTCs, which are highly associated with advanced or metastatic HCC, increased recurrence after potentially curative therapy, and inferior progression-free and overall survival.
A unique strength of our study was the utilization of a multimarker capture antibody cocktail that allowed for the detection of CTCs across all HCC stages with high sensitivity and specificity. The majority of current CTC enrichment platforms rely on antibodies to the cell-surface marker EpCAM alone, potentially limiting their utility in HCC where expression of EpCAM is reported in only 20–35% of tumors.8 ASGPR, a transmembrane cell-surface protein highly expressed in well-differentiated HCCs, yielded high CTC capture rates in previous HCC studies, and was used in our antibody cocktail.7,9,19 Based on our TMA studies, we also included GPC-3, since its expression has been associated with the presence of poorly-differentiated HCC,27 arguably the most important subset of tumors to be detected given their poor prognosis. This unique three-marker antibody cocktail allowed us to detect CTCs from 96.7% of all patients with HCC, compared to 20–50% captured using EpCAM alone.6,28–30
Arguably our most important finding was the identification of the subpopulation of vimentin(+)-CTCs and their association with more advanced stage disease, faster HCC progression and inferior survival for all patients and the subset of potentially curable patients undergoing locoregional treatments(Fig. 5A&C). Vimentin is an intermediate filament ubiquitously expressed in normal mesenchymal cells and is involved in cellular processes including stress resistance and structural integrity.31 Vimentin overexpression is widely regarded as the canonical marker of EMT in epithelial cancers 31, and tumors expressing vimentin demonstrate accelerated tumor growth and increased invasiveness. In HCC, vimentin overexpression in the primary tumor has been linked to more aggressive tumor biology, inferior survival outcomes, and the establishment of the tumor initiating capacity critical for metastases.14,15
The importance of vimentin overexpression in CTCs as a marker of metastatic potential has been established previously in several cancer types, but the utility of vimentin for HCC-CTCs has only recently been considered.13,32–34 Our finding that vimentin(+)-CTCs were found almost exclusively in patients with advanced stage HCC corroborates previous reports in breast and prostate cancer that found the presence of vimentin(+)-CTCs as a marker of metastatic disease and worse outcomes.35 Given that cytokeratin-positive epithelial cells have been found in the blood of patients with inflammatory gastrointestinal disease such as inflammatory bowel disease and cirrhosis we feel that the use of additional markers is important to ensure that no benign epithelial cells are being mis-identified as CTCs.36 This is one reason we believe that vimentin(+)-CTCs may hold more clinical relevance for HCC, where the vast majority of patients have underlying liver cirrhosis. Furthermore, we demonstrated that vimentin(+)-CTCs vastly outperform the only existing serum biomarker, AFP, for both HCC staging and prognosis (Fig. 4D). In particular, our finding that vimentin(+)-CTCs are highly predictive of earlier recurrence and worse survival in otherwise indistinguishable early stage patients undergoing locoregional or surgical therapy highlights their potential utility as a biomarker for liver transplant candidate selection. Hopefully, the use of vimentin(+)-CTCs as an adjunct biomarker may help overcome the limitation of existing clinicopathologic staging systems in distinguishing cancer-specific outcomes within the subset of potentially curable patients.
Our study builds upon a number of recent publications highlighting the potential utility of CTCs, and CTC phenotyping in particular. Wang et al used fluorescent in situ hybridization of mesenchymal and epithelial mRNA transcripts in CTCs to demonstrate that the presence of 1 or more mesenchymal CTCs was associated with early recurrence after curative-intent resection.32 Similarly and using the same CTC capture system, Chen et al., found that CTCs can discriminate between patients with metastatic and non-metastatic disease, and that significantly more mesenchymal CTC were found in patients with metastatic disease and higher BCLC stage.34 These recent studies, along with our current findings, highlight the potential of mesenchymal CTCs, such as vimentin(+)-CTCs, as a clinical biomarker for patients with HCC that may play an important role in prognostication and treatment selection.
We believe that the value of HCC-CTCs as a tool for both longitudinal monitoring of disease and identification of patients with aggressive underlying disease is apparent. Given the ease and repeatability of the non-invasive HCC-CTC assay, the reliable serial monitoring of a patient’s response to treatment would be possible. In patients undergoing surgical resection and ablation, total HCC-CTCs dropped significantly after treatment, but increased in the subset of patients who subsequently proved to have recurrence on follow up imaging (Fig. 6). In addition, Vimentin(+)-CTCs were highly predictive of recurrence and progression in the subset of potentially curable patients undergoing successful locoregional therapy who initially demonstrated no evidence of disease on post-treatment imaging. This highlights the potential role of vimentin(+)-CTCs for the selection of patients undergoing curative liver transplantation, where the identification of patients likely to have poor outcomes is a critical, unmet need to avoid the loss of scarce donor allografts. We plan to further investigate these potential roles with ongoing recruitment, enumeration, and phenotyping of HCC-CTCs in early stage patients undergoing curative intent treatment.
In conclusion, we developed a HCC multimarker antibody-based CTC capture assay that allows for the identification of HCC-CTCs as well as a distinct subpopulation of vimentin(+)-CTCs. We first optimized and validated the assay’s functionality for capturing HCC-CTCs using both spiked cell line and clinical samples. In a subsequent prospective study of 80 patients (one of the largest HCC-CTC studies to date), 61 of whom had HCC, our assay allowed for detection of HCC-CTCs from nearly all patients with HCC, with highly accurate discrimination between patients with HCC and those with NMLD or healthy controls. Most importantly, a phenotypic subpopulation of EMT-type, vimentin(+)-CTCs allowed for accurate discrimination of patients with early stage, potentially curable HCC from those with advanced stage, incurable disease. The presence of any vimentin(+)-CTCs was associated with earlier recurrence and inferior progression free survival in the subset of patients undergoing curative intent locoregional therapy. Our early results indicate that the novel NanoVelcro CTC assay is effective for HCC-CTC capture and phenotyping, with vimentin(+)-CTCs showing great promise for identifying early stage patients with occult aggressive disease. Validation of our findings in a larger prospective cohort of patients may allow for the adoption of an HCC-CTC assay as an important biomarker in this deadly malignancy.
Supplementary Material
Acknowledgments
Financial Support:
V.G.A. was supported by an American Surgical Association Foundation Fellowship award.
List of Abbreviations
- AFP
alpha-fetoprotein
- ASGPR
asialoglycoprotein receptor
- AUROC
area under the receiver operator curve
- CK
cytokeratin
- CTC
circulating tumor cell
- DAPI
4′,6-diamidino-2-phenylindole
- EMT
epithelial-to-mesenchymal transition
- EpCAM
epithelial cell adhesion molecule
- GPC-3
glypican-3; MC
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- ICC
immunocytochemistry
- IQR
interquartile range
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- MC
Milan criteria
- mRECIST
modified Response Evaluation Criteria In Solid Tumors
- NMLD
non-malignant liver disease
- NASH
nonalcoholic steatohepatitis
- NPV
negative predictive value
- OS
overall survival
- PFS
progression-free survival
- PPV
positive predictive value
- RFA
radio-frequency ablation
- RFS
recurrence-free survival
- TACE
transcatheter arterial chemoembolization
- TMA
tissue microarray
- TTR
time to recurrence
- UCSF
University of California, San Francisco
- VIM
vimentin
Footnotes
Conflicts of Interest:
Hsian-Rong Tseng: (i) The intellectual property that is associated with this study (NanoVelcro assay) has been licensed to CytoLumina Technologies Corp.; and (ii) Hsian-Rong Tseng has his financial interests in CytoLumina Technologies Corp. given his role as one of the company’s co-founders. All other authors have nothing to disclose.
References
- 1.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agopian VG, et al. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416–427. doi: 10.1016/j.jamcollsurg.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072–2079. doi: 10.1158/1078-0432.CCR-13-0547. [DOI] [PubMed] [Google Scholar]
- 4.Baccelli I, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 5.Court CM, et al. Reality of Single Circulating Tumor Cell Sequencing for Molecular Diagnostics in Pancreatic Cancer. The Journal of molecular diagnostics: JMD. 2016;18:688–696. doi: 10.1016/j.jmoldx.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan JL, Yang YF, Yuan CH, Chen H, Wang FB. Circulating Tumor Cells for Predicting the Prognostic of Patients with Hepatocellular Carcinoma: A Meta Analysis. Cell Physiol Biochem. 2015;37:629–640. doi: 10.1159/000430382. [DOI] [PubMed] [Google Scholar]
- 7.Xu W, et al. Isolation of circulating tumor cells in patients with hepatocellular carcinoma using a novel cell separation strategy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:3783–3793. doi: 10.1158/1078-0432.CCR-10-0498. [DOI] [PubMed] [Google Scholar]
- 8.de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. The Journal of pathology. 1999;188:201–206. doi: 10.1002/(SICI)1096-9896(199906)188:2<201::AID-PATH339>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Li YM, et al. Epithelial-mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis. 2013;4:e831. doi: 10.1038/cddis.2013.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nel I, et al. Individual profiling of circulating tumor cell composition and therapeutic outcome in patients with hepatocellular carcinoma. Translational oncology. 2013;6:420–428. doi: 10.1593/tlo.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan ST, et al. Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: a prospective study. Annals of Surgery. 2011;254:569–576. doi: 10.1097/SLA.0b013e3182300a1d. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, et al. Biofunctionalized magnetic nanospheres-based cell sorting strategy for efficient isolation, detection and subtype analyses of heterogeneous circulating hepatocellular carcinoma cells. Biosensors & bioelectronics. 2016;85:633–640. doi: 10.1016/j.bios.2016.05.071. [DOI] [PubMed] [Google Scholar]
- 13.Yu M, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65:798–808. doi: 10.1016/j.jhep.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Hu L, et al. Association of Vimentin overexpression and hepatocellular carcinoma metastasis. Oncogene. 2004;23:298–302. doi: 10.1038/sj.onc.1206483. [DOI] [PubMed] [Google Scholar]
- 16.Yang MH, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- 17.Chen JF, et al. Subclassification of prostate cancer circulating tumor cells by nuclear size reveals very small nuclear circulating tumor cells in patients with visceral metastases. Cancer. 2015 doi: 10.1002/cncr.29455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin M, et al. Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Accounts of chemical research. 2014;47:2941–2950. doi: 10.1021/ar5001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, et al. Detection of circulating tumor cells in hepatocellular carcinoma using antibodies against asialoglycoprotein receptor, carbamoyl phosphate synthetase 1 and pan-cytokeratin. PloS one. 2014;9:e96185. doi: 10.1371/journal.pone.0096185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buzzanco A, et al. Digital quantitation of HCC-associated stem cell markers and protein quality control factors using tissue arrays of human liver sections. Exp Mol Pathol. 2014;97:399–410. doi: 10.1016/j.yexmp.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ankeny JS, et al. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br J Cancer. 2016;114:1367–1375. doi: 10.1038/bjc.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzaferro V, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 23.Yao FY, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 24.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budczies J, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McShane LM, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) Journal of the National Cancer Institute. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 27.Libbrecht L, et al. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol. 2006;30:1405–1411. doi: 10.1097/01.pas.0000213323.97294.9a. [DOI] [PubMed] [Google Scholar]
- 28.Schulze K, et al. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. International journal of cancer. Journal international du cancer. 2013;133:2165–2171. doi: 10.1002/ijc.28230. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Lorencio MI, et al. Comparison of Two Types of Liquid Biopsies in Patients With Hepatocellular Carcinoma Awaiting Orthotopic Liver Transplantation. Transplant Proc. 2015;47:2639–2642. doi: 10.1016/j.transproceed.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Ogle LF, et al. Imagestream detection and characterisation of circulating tumour cells - A liquid biopsy for hepatocellular carcinoma? J Hepatol. 2016;65:305–313. doi: 10.1016/j.jhep.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, et al. Correlation Between Postoperative Early Recurrence of Hepatocellular Carcinoma and Mesenchymal Circulating Tumor Cells in Peripheral Blood. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2017 doi: 10.1007/s11605-017-3619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HM, et al. Cell-surface major vault protein promotes cancer progression through harboring mesenchymal and intermediate circulating tumor cells in hepatocellular carcinomas. Scientific reports. 2017;7:13201. doi: 10.1038/s41598-017-13501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Cao SW, Cai Z, Zheng L, Wang Q. Epithelial-mesenchymal transition phenotypes of circulating tumor cells correlate with the clinical stages and cancer metastasis in hepatocellular carcinoma patients. Cancer Biomark. 2017;20:487–498. doi: 10.3233/CBM-170315. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong AJ, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9:997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pantel K, et al. Circulating epithelial cells in patients with benign colon diseases. Clin Chem. 2012;58:936–940. doi: 10.1373/clinchem.2011.175570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.