Abstract
The prevalence of obesity-associated nonalcoholic fatty liver disease has significantly increased over the past decade and end stage liver disease secondary to nonalcoholic steatohepatitis has become one of the most common indications for liver transplantation. This both increases the demand for organs and decreases the availability of donor livers deemed suitable for transplantation. While in the past many steatotic livers were discarded due to concerns over enhanced susceptibility to ischemia reperfusion injury and organ failure, the discrepancy between supply and demand has resulted in increasing use of expanded criteria donor organs including steatotic livers. However, it remains controversial whether steatotic livers can be safely used for transplantation and how best to improve the performance of steatotic grafts. We aimed to evaluate the impact of diet-induced hepatic steatosis in a murine model of ischemia reperfusion injury. Using a diet high in trans-fat, fructose, and cholesterol (HTF-C diet) and a diet high in saturated fats, sucrose, and cholesterol (Western diet), we were able to establish models of mixed macrovesicular and microvesicular steatosis (HTF-C) and microvesicular steatosis (Western). We found that the presence of hepatic steatosis, whether it is predominantly macrovesicular or microvesicular, significantly worsens ischemia reperfusion injury as measured by plasma ALT levels and inflammatory cytokine concentration, and histological evaluation for necrosis. Additionally, we report on a novel finding in which hepatic ischemia reperfusion injury in the setting of steatosis results in the induction of the necroptosis factors, RIPK3, RIPK1, and MLKL. These data lay the groundwork for additional experimentation to test potential therapeutic approaches to limit ischemia reperfusion injury in steatotic livers by using a genetically tractable system.
Keywords: fatty liver, transplantation, graft injury, macrosteatosis, microsteatosis
Introduction
The worldwide prevalence of obesity has dramatically increased over the past two decades. In the United States, about a third of the adult population is considered obese (1) and worldwide, the prevalence is estimated to be about 13% (2). Obesity is associated with a range of cardiometabolic problems including coronary artery disease, insulin resistance, and nonalcoholic fatty liver disease (NAFLD)(3). As the prevalence of obesity has grown, there has been a concomitant increase in NAFLD, with a worldwide prevalence of about 20% (4). NAFLD encompasses a spectrum of liver disease ranging from simple steatosis to varying degrees of inflammation and fibrosis (nonalcoholic steatohepatitis; NASH). Individuals with NASH are then at increased risk of developing cirrhosis and hepatocellular carcinoma (5).
Over the past two decades the number of liver transplant recipients with a diagnosis of cirrhosis secondary to NASH has been increasing steadily (6). Furthermore, with successful treatment of Hepatitis C readily available, it is predicted that end stage liver disease secondary to NASH will become the most common indication for liver transplantation by 2020 (6). The higher prevalence of NASH increases the demand for organs while also placing a strain on the donor pool as many steatotic livers are deemed unsuitable for transplantation. One study found 5–15% of potential living donors have some degree of steatosis (7) and another study found 21% of cadaveric donors have at least moderate steatosis (8). A retrospective study found that 40% of discarded grafts were rejected due to the presence of steatosis (9). Due to the growing discrepancy between supply and demand, there has been increased use of marginal or expanded criteria donors such as older donor age, donation after cardiac death, and mild to moderate steatosis (10).
Steatotic livers are considered to be marginal donors by some transplant centers (10) due to concern for increased susceptibility to ischemia reperfusion (I/R) injury resulting in greater organ damage (11–18). It is thought that this results in inferior graft and patient survival as well as higher rates of initial poor function and primary nonfunction (19–21). While rodent studies have consistently shown worse liver injury in the setting of steatosis, these studies are limited by the use of genetic models of obesity and use of highly non-physiological diets such as the methionine choline deficient (MCD) diet (22). Furthermore, this may be controversial in humans as there are some reports that suggest no significant difference in complications (23) or patient and graft survival (24).
Unfortunately, much of the data in clinical studies used to support the idea that steatosis increases vulnerability to I/R injury are based on retrospective studies with variable definitions and degrees of steatosis. Some data suggest that macrovesicular steatosis, defined as the presence of a lipid droplet leading to displacement of the nucleus, is generally associated with poor outcomes and to be more injurious to the liver. However, microvesicular steatosis, defined as the presence of multiple lipid droplets without displacement of the nucleus, is thought to result in less liver injury during I/R (8, 17, 23, 25). The factors that drive these two types of steatosis are poorly understood and the concept that microvesicular steatosis is benign has not been examined in detail.
Herein, we used two different murine models of diet-induced obesity to create variable degrees of hepatic steatosis and inflammation to characterize its effects on hepatic I/R injury. Fortuitously, one high fat diet chosen for this study produced macrovesicular and microvesicular steatosis while the other produced predominantly microvesicular steatosis. Interestingly, we found that regardless of the high fat diet composition, the type of steatosis produced, or the degree of pre-existing inflammation, feeding mice these high fat diets that produced hepatic steatosis exacerbated liver injury. Interestingly, we also found that the presence of steatosis was associated with induction of necroptosis markers after I/R injury. Evaluating these mouse models also lays the groundwork for examining potential therapeutic interventions that could be tested to improve the function of marginal organs due to steatosis.
Results
Characterization of low fat control and high fat diet fed mice
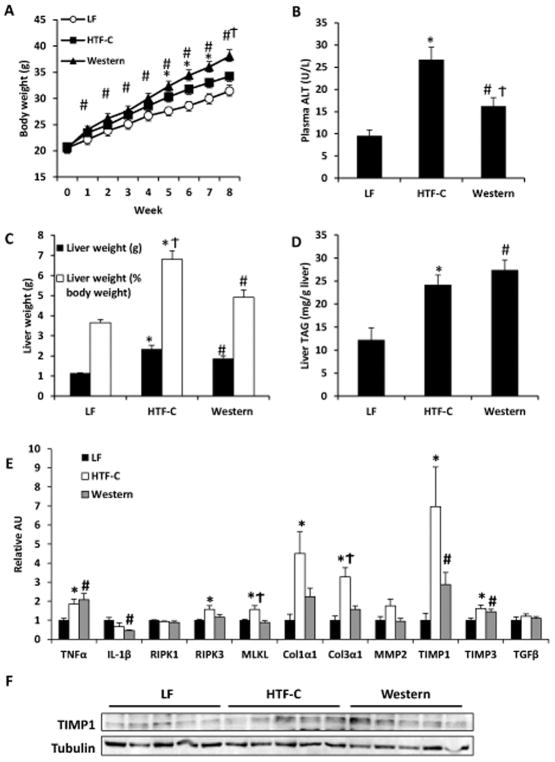
We first compared baseline characteristics of mice fed low fat or two distinct high fat diets for eight weeks. Mice on a low fat (LF) diet (70% carbohydrate, 20% protein, 10% fat) were compared to mice on a high fat diet (40% carbohydrate, 20% protein, 40% fat) enriched in trans-fatty acids, fructose, and cholesterol (2%; HTF-C) that was predicted to be the most injurious to the liver. Another group of mice were fed a high fat diet (42.7% carbohydrate, 15.2% protein, 42% fat) that was primarily saturated fatty acids and did not contain fructose with only 0.2% cholesterol (Western diet). As expected, animals on either high fat diets gained significantly more weight than animals fed the LF diet, but mice fed the Western diet also gained significantly more weight than the HTF-C diet-fed mice (Figure 1A).
Figure 1.
Baseline characteristics of mice fed LF control, HTF-C, or Western diet for eight weeks. [A] Body weight gain on respective diets. [B] Plasma ALT concentration. [C] Liver weight and liver weight expressed as percent of body weight. [D] Liver triglyceride content. Values are mean ± standard error of the mean. n=5 mice/group. [E] Hepatic expression of indicated genes encoding inflammatory cytokines, necroptosis factors, markers of stellate cell activation, and fibrosis in mice on diet for eight weeks. [F] Western blot analysis of TIMP1 and Col1α1 expression in mice fed LF, HTF-C, and Western diet for eight weeks. Values are mean ± standard error of the mean. n=5 mice/group. *p<0.05 LF vs HTF-C. #p<0.05 LF vs Western. †p<0.05 HTF-C vs Western. Abbreviations: ALT, alanine aminotransferase. TAG, triacylglycerol. AU, arbitrary units.
Before sacrifice, mice were fasted 4 h to normalize metabolic variables. Blood glucose concentrations at time of sacrifice were not significantly different among the different groups (LF 167.0 ± 4.9 mg/dl; HTF-C 163.8 ± 11.8 mg/dl; Western 169.8 ± 12.2 mg/dl). Western diet fed mice had higher plasma insulin concentrations compared to both LF and HTF-C diet-fed mice (LF 2114.2 ± 268.9 pg/ml; HTF-C 1867.0 ± 327.3 pg/ml; Western 3948.2 ± 709.8 pg/ml; Western diet p=0.042 versus other dietary groups). Both high fat diet groups had a higher baseline plasma ALT concentration versus LF control mice and HTF-C diet fed mice had a significantly higher plasma ALT concentration than Western diet fed mice (Figure 1B).
Both HTF-C and Western diet fed mice had a higher liver mass compared to LF control mice (Figure 1C). However, mice fed the HTF-C diet had a significantly higher liver weight as determined by percentage of body weight compared to Western diet fed mice (Figure 1C, 6.8% versus 4.9%, p=0.01). Both high fat diet groups had a higher hepatic triglyceride content than the LF control mice but they were not significantly different from each other (Figure 1D, p=0.33).
We then examined hepatic expression of genes encoding inflammatory cytokines, markers of liver injury, and stellate cell activation (Figure 1E). Both the HTF-C and Western diet fed mice had significantly elevated expression of the inflammatory cytokine, TNFα, and inhibitors of metalloproteinases, TIMP1 and TIMP3, compared to LF control mice. HTF-C diet fed mice also had higher expression of genes encoding fibrogenic matrix proteins, Col1α1 and Col3α1, than LF control mice but Western diet fed mice were not significantly different than LF control mice. Western blot analysis confirmed the increased expression of TIMP1 in HTF-C and Western diet-fed mice compared to LF controls (Figure 1F). HTF-C diet fed mice had a significantly higher expression of the necroptosis markers, RIPK3 and MLKL, than LF control mice and also had higher expression of MLKL versus Western diet fed mice.
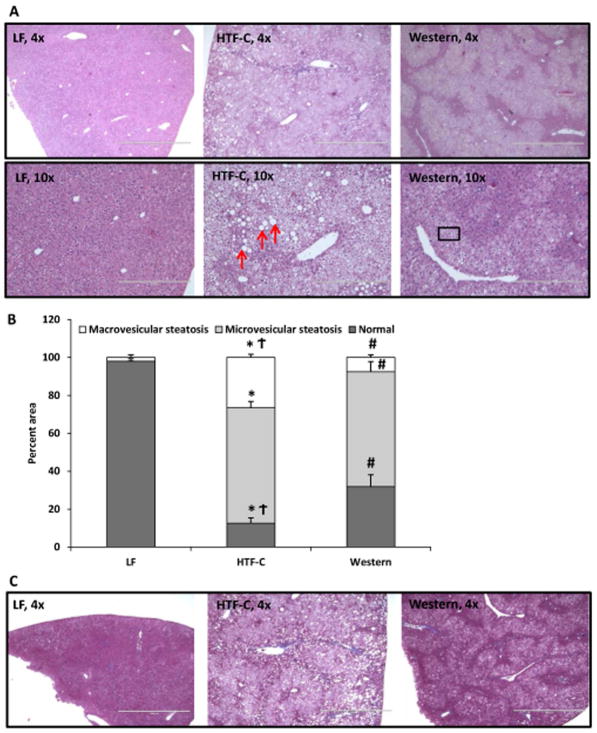
The pattern and characteristic of hepatic steatosis differs between the HTF-C diet and Western diet fed mice
An experienced liver pathologist (I.N.) examined histological specimens in detail and found that while both high fat diets result in hepatic steatosis, the degree and pattern of steatosis differed between the two diets (Figure 2A). Both high fat diet fed groups exhibited moderate to severe steatosis, defined as >30% steatosis (Figure 2B). HTF-C diet fed mouse livers exhibited marked macrovesicular steatosis (26.6%) and microvesicular steatosis (60.8%) (Figure 2B). In contrast, livers of Western diet fed mice exhibited predominantly microvesicular steatosis (60.4%) with a small percentage of macrovesicular steatosis (7.6%). Regardless of diet, none of the mice exhibited a significant degree of fibrosis as determined by trichrome stain (Figure 2C).
Figure 2.
The pattern and characteristic of hepatic steatosis differs between the HTF-C diet and Western diet fed mice. [A] Representative hematoxylin and eosin-stained liver sections from mice fed LF, HTF-C, and Western diet. [B] Percent macrovesicular steatosis versus microvesicular steatosis in liver specimens of mice fed LF, HTF-C, and Western diet. Red arrows indicate representative macrovesicular steatosis. Box indicates representative area of microvesicular steatosis. n=5 mice/group. [C] Representative Masson’s trichrome stain from mice fed LF, HTF-C, and Western diet fed mice. n=5 mice/group. Values are mean ± standard error of the mean. *p<0.05 LF vs HTF-C. #p<0.05 LF vs Western. †p<0.05 HTF-C vs Western.
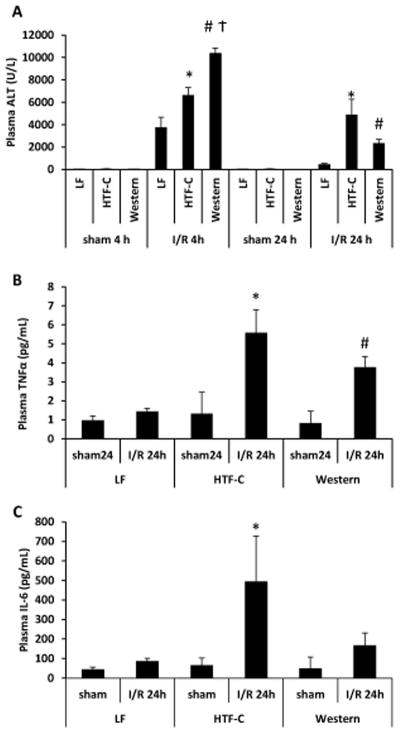
The presence of steatosis leads to increases in plasma ALT and inflammatory cytokine levels following I/R surgery
We next sought to characterize the response to I/R injury in mice fed these diets. As in the first experiment, after 8 weeks of high fat diet feeding, mice on the HTF-C and Western diet again gained significantly more weight and had higher hepatic triglyceride content than mice fed the LF control diet (Supplemental Figure 1A). Again, mice on the Western diet gained significantly more weight than mice on the HTF-C diet following eight weeks of diet, but in this cohort of mice, HTF-C diet fed mice had slightly, but significantly higher hepatic triglyceride content than Western diet fed mice (28.4 mg/g liver versus 24.6 mg/g liver, p=0.006) (Supplemental Figure 1B).
After both 4 and 24 h of reperfusion, plasma ALT levels were higher in both the HTF-C and Western diet fed mice compared to LF controls (Figure 3A). At 4 hours, Western diet fed mice had a significantly higher plasma ALT level than HTF-C diet fed mice (10,349 U/L versus 6,596 U/L, p<0.001), but at 24 hours there was no significant difference between the two high fat diet groups (2,330 U/L versus 4,858 U/L, p=0.10). No significant differences in plasma AST was detected among the dietary groups at 4 hours, but at 24 hours, HTF-C diet fed mice had significantly higher plasma AST levels than LF control mice (2497 U/L versus 1757 U/L, p=0.46) (Supplemental Figure 2). Sham operated mice did not have significantly different plasma AST or ALT levels among the three diet groups. We then evaluated plasma concentrations of the inflammatory cytokines, TNFα and IL-6. We found the HTF-C diet fed mice and the Western diet fed mice had higher levels of TNFα following I/R surgery compared to LF control mice (Figure 3B). There was no significant difference in the plasma concentrations of TNFα between HTF-C and Western diet fed mice (5.6 pg/mL versus 3.8 pg/mL, p=0.19). HTF-C diet fed mice also had higher plasma IL-6 concentrations compared to LF diet controls at 24 h after I/R surgery (Figure 3C).
Figure 3.
The presence of steatosis leads to increases in plasma ALT and TNFα following I/R surgery. [A] Plasma ALT concentration following sham or I/R surgery. Values are mean ± standard error of the mean. n=3 mice/sham surgery group. n=9 mice/4 h reperfusion I/R surgery. n=10 mice/24 h reperfusion I/R surgery. [B] Plasma TNFα concentration following sham or I/R surgery. Values are mean ± standard error of the mean. n=3 mice/sham surgery group. n=10 mice/24 h reperfusion I/R surgery. *p<0.05 LF vs HTF-C. #p<0.05 LF vs Western. †p<0.05 HTF-C vs Western. [C] Plasma IL-6 concentration following sham or I/R surgery. Values are mean ± standard error of the mean. n=3 mice/sham surgery group. n=8 mice/24 h reperfusion I/R surgery. *p<0.05 LF vs HTF-C. #p<0.05 LF vs Western.
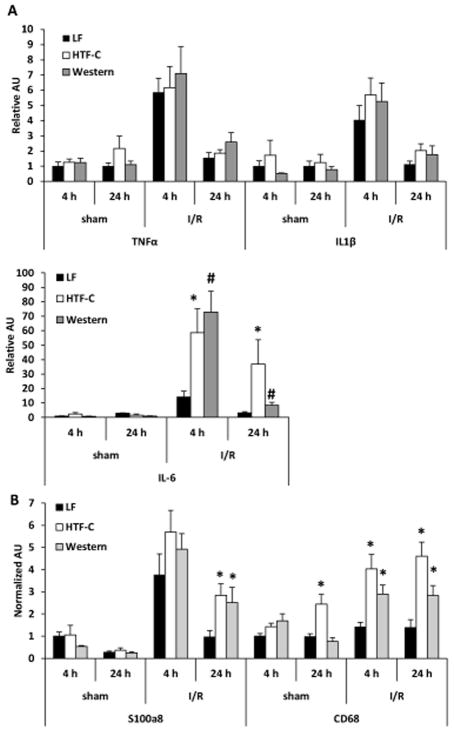
The presence of steatosis leads to increases in plasma ALT and inflammatory cytokine levels following I/R surgery
While plasma TNFα concentrations were increased in high fat diet mice compared to LF diet fed mice following I/R surgery, we did not find a significant difference in hepatic expression of TNFα or IL1β in an ischemic lobe of the liver (Figure 4A). Compared to LF controls, the expression of the gene encoding IL-6 was increased in HTF-C and Western diet fed mice following 4 hours and 24 hours of reperfusion after I/R surgery (Figure 4A). In addition, the expression of the neutrophil marker S100a8 and macrophage marker CD68 were increased in HTF-C and Western diet-fed mice compared to LF controls 24 h post-surgery (Figure 4B).
Figure 4.
The hepatic expression of inflammatory cytokines and markers of leukocyte infiltration following I/R surgery. [A] Hepatic expression of indicated genes encoding inflammatory cytokines following sham or I/R surgery. [B] Hepatic expression of indicated genes encoding markers of leukocyte infiltration following sham or I/R surgery. n=3 mice/sham group. n=9 mice/4 h reperfusion I/R surgery. n=10 mice/24 h reperfusion I/R surgery. Values are mean ± standard error of the mean. *p<0.05 LF vs HTF-C. #p<0.05 LF vs Western. †p<0.05 HTF-C vs Western. Abbreviations: AU, arbitrary units.
The presence of steatosis increases the extent of necrosis and macrophage infiltration following I/R surgery
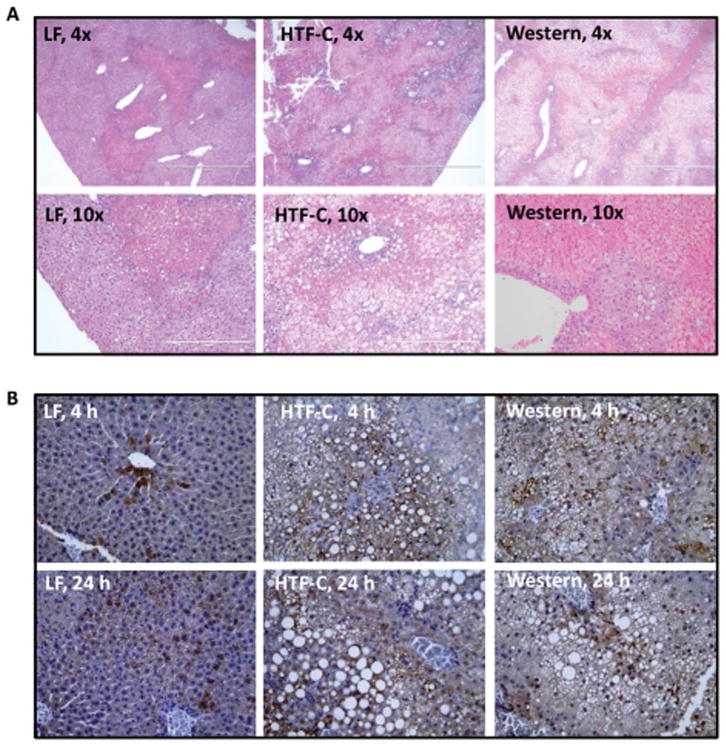
Following 4 hours of reperfusion, there is minimal histological evidence of necrosis in the liver specimens of all three groups (Supplemental Figure 3). However, at 24 hours of reperfusion, necrosis became histologically evident (Figure 5A). Compared to the LF diet fed mice, the HTF-C and Western diet fed mice had significantly more and larger areas of necrosis (10.0% versus 31.5%, p=0.01 and 10.0% versus 46.0%, p<0.001, respectively. While the Western diet fed mice trended towards increased areas of hepatic necrosis compared to the HTF-C fed mice, this was not statistically significant (46.0% versus 31.5%, p=0.17). Immunohistochemical staining for the macrophage marker CD68 demonstrated qualitatively that macrophage infiltration was increased 4 and 24 h after ischemia by HTF-C and Western diet feeding (Figure 5B).
Figure 5.
The presence of steatosis increases the extent of necrosis following I/R surgery. [A] Representative hematoxylin and eosin-stained liver sections from mice fed LF, HTF-C, or Western diet undergoing I/R surgery followed by 24 h reperfusion. [B] Representative CD68-stained liver sections from mice fed LF, HTF-C, or Western diet undergoing I/R surgery followed by 4 or 24 h reperfusion.
The presence of hepatic steatosis is associated with increased expression of necroptosis markers following I/R surgery
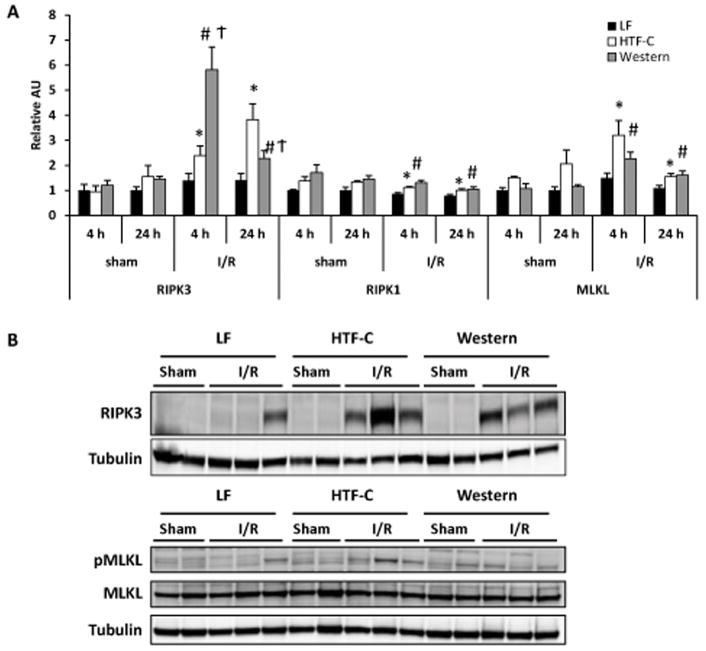
Interestingly, we found that hepatic expression of markers of necroptosis (RIPK1, RIPK3, and MLKL) were significantly elevated in the high fat diet fed mice compared to LF mice following hepatic I/R surgery (Figure 6A). We also found RIPK3 protein levels to be significantly increased in high fat diet fed mice at 24 hours compared to LF control mice (Figure 6B). Additionally, RIPK3 protein abundance was significantly higher in HTF-C diet fed mice compared to Western diet fed mice. The phosphorylation of the RIPK target MLKL also tended to be increased in HTF-C diet-fed mice after I/R surgery. Collectively, these data suggest that feeding mice these high fat diets that produce predominantly microvesicular or mixed macrovesicular and microvesicular steatosis in mice exacerbates I/R injury as assessed by transaminase and inflammatory cytokine levels, necrosis, and necroptotic signaling.
Figure 6.
The presence of hepatic steatosis is associated with increased expression of necroptosis markers following I/R surgery. [A] Hepatic gene expression of RIPK3, RIPK1, and MLKL in mice fed LF, HTF-C, or Western diet following sham or I/R surgery. [B] Western blots showing RIPK3 protein abundance and phosphorylation of the RIPK target MLKL in mice fed LF, HTF-C, or Western diet 24 h after sham or I/R surgery. Values are mean ± standard error of the mean. *p<0.05 LF vs HTF-C. #p<0.05 LF vs Western. †p<0.05 HTF-C vs Western. Abbreviations: AU, arbitrary units.
Discussion
In parallel with the growing obesity epidemic, there has also been an increase in the worldwide prevalence of NAFLD. Patients with NASH are more likely than the general population to develop end stage liver disease and this has become one of the leading causes of liver transplantation in the United States. This places a strain on the field of liver transplantation by both decreasing the availability of non-steatotic livers as well as increasing the demand for organs. The discrepancy between supply and demand has contributed to increased interest in the use of marginal donors such as steatotic livers. However, their widespread use is limited by uncertainty with respect to safety due to concerns regarding increased susceptibility to I/R injury in the setting of steatosis.
Clinical studies are often limited by their retrospective nature and differing definitions of steatosis. The clinical data have not been convincing as to whether steatosis, especially mild or microvesicular steatosis, results in increased I/R injury. Thus, multiple animal models have been used to study the impact of hepatic steatosis on I/R injury. The vast majority of these studies utilize either genetic models of steatosis or severe diets such as the MCD diet or the 60% high fat diet (HFD) (22). While genetic models of obesity, such as ob/ob mice, can reliably recreate macrovesicular steatosis, there is concern that hepatic steatosis in genetic models may be different from that associated with metabolic syndrome in humans and that leptin deficiency could have its own effects on ischemic outcomes (26). The MCD diet can reliably recreate findings consistent with NASH; however, it is not obesogenic and its systemic metabolic changes do not accurately reflect the metabolic derangements seen in humans affected by obesity (26, 27). Therefore, while rodent studies have generated fairly convincing data regarding the detrimental impact of hepatic steatosis on I/R injury, the specific shortcomings of the NAFLD models may limit their interpretation and applicability. Their artificial nature may also limit utility for testing potential therapeutics.
In the present study, we chose two different high fat diet models that produce many features of fatty liver disease to further evaluate the impact of hepatic steatosis on I/R injury. As expected, mice fed either a HTF-C diet or Western diet gained significantly more weight and had a higher hepatic triglyceride content than mice fed a LF control diet. However, the histological evaluation of liver specimens from mice fed HTF-C versus Western diet revealed qualitative differences in the pattern and distribution of steatosis. The regulation of lipid droplet size is complex. In adipocytes, much more is known regarding lipid droplet formation and size (28). While it is known that different pathologic states in the liver are more prone to macrovesicular steatosis or microvesicular steatosis (29), the factors that regulate this process are poorly understood. It is possible that some component of the HTF-C diet, such as the trans-fat, cholesterol, or fructose, promotes the formation of larger lipid droplets. Herein, the HTF-C diet resulted in marked macrovesicular and microvesicular steatosis while the Western diet created predominantly microvesicular steatosis. We found that the presence of pre-existing inflammation and macrovesicular steatosis predictably and significantly worsened I/R injury. We also found the presence of microvesicular steatosis was associated with poor tolerance to I/R injury. While some clinical studies (8, 23, 25) have advocated for the safe use of livers with microvesicular steatosis, other clinical studies express concern regarding the use of livers with microvesicular steatosis (30, 31). The discrepancy in data is likely secondary to variable degrees of microvesicular steatosis (ranging from minimal to severe) as well as variability in interpretation of histological samples and differences in clinical variables of donors and recipients.
Our findings and other rodent studies suggest caution should be applied when concluding that microvesicular steatosis is benign in the context of I/R surgery (17, 32, 33). Selzner and colleagues reported lower tolerance to ischemia reperfusion injury in mice with microvesciular steatosis using a choline deficient (CD) diet (17). However, it was not reported whether their use of the CD diet resulted in obesity or metabolic derangements and while plasma AST concentration was significantly higher in CD mice compared to lean controls, the extent of necrosis trended towards an increase, but was not statistically different (17). In another study by Llacuna and colleagues, the authors used a high cholesterol diet to induce microvesciular steatosis (33); however, this did not result in increased hepatic triglyceride content. It is possible that cholesterol accumulation results in increased sensitivity to I/R injury (33). Lastly, Kato and colleagues report poor tolerance to ischemia reperfusion after 5 or 8 weeks on a 60% HFD, which induced microvesicular and macrovesicular steatosis, respectively (32). These previous studies are in general agreement with the present study using two high fat diets that result in increased hepatic triglyceride content and exacerbated I/R injury in mice.
While the Western diet fed mice had small areas of macrovesicular steatosis, this represented less than 10% of the liver while microvesicular steatosis represented 60.4% of the liver. One study noted that the presence of mild macrovesicular steatosis (0–20% steatosis) had comparable long term outcomes and plasma parameters as donor livers with no steatosis, while the presence of moderate macrovesicular steatosis (20–50%) had significantly higher peak ALT concentrations following transplantation (34). Hence, our use of the Western diet generates a consistent model of hepatic microvesicular steatosis and thus provides an accurate representation of microvesicular steatosis and its tolerance to I/R injury. We prefer this model to the HTF-C or other high fat/high fructose diets to dissect mechanism and test potential therapies. While not perfect, this diet contains a more physiologic fat, cholesterol, and fructose composition and produces obesity and insulin resistance compared to other diets currently in use.
We identified several parameters that were biomarkers for enhanced I/R injury in steatotic livers, including the established marker alanine transaminase. Plasma ALT levels were significantly higher in both groups of high fat diet fed mice compared to LF control mice at all reperfusion time points. This is congruent with previous clinical studies (19, 20, 24) as well as rodent studies (13, 14, 35–38). While plasma AST levels were not different among the three groups at 4 hours of reperfusion following I/R surgery, ALT is much more specific for hepatocellular injury as AST rise can be attributed to cardiac injury, skeletal injury, and/or hemolysis (39–41). Consistent with previous studies of hepatic steatosis and I/R injury (14, 37), we were also able to demonstrate higher plasma TNFα and IL-6 concentrations in high fat diet fed mice after I/R, which is consistent with an overall pro-inflammatory state. This supports the theory that steatotic livers may be more susceptible to I/R injury due to overproduction of inflammatory cytokines. Hepatic IL-6 expression was increased in the high fat diet fed mice following I/R surgery, but we did not detect any differences in hepatic expression of TNFα or IL1β following I/R surgery among the three different diets. The disconnect between TNFα concentrations in circulation and tissue expression suggests that post-transcriptional mechanisms affect the concentration in circulation. Given that TNFα cleavage by matrix metalloproteases is well known to be a regulated step that controls its release (42) and that the presence of soluble receptors in blood can affect the half-life of the circulating cytokine (43), it is potentially not surprising that mRNA does not track with protein. It is also possible, that other tissues are contributing to the TNFα in circulation.
Although I/R injury is largely unavoidable in most liver related surgeries, the mechanism by which it leads to graft dysfunction is not fully understood. While the initial ischemic insult can result in direct cell injury and death, the bulk of the injury occurs during the reperfusion phase in which a sustained proinflammatory response results in a robust generation of inflammatory cytokines and reactive oxygen species (44). We report a novel and interesting finding in which the HTF-C and Western diet fed mice had higher levels of necroptosis markers, RIPK3, RIPK1, and MLKL, compared to LF control mice following I/R surgery. The role of necroptosis in liver injury has been examined in both rodent models as well as clinical studies (45, 46). RIPK3 expression has been found to be elevated in mouse models of liver injury (47) as well as human subjects with liver disease (48). To our knowledge, we are not aware of other studies showing increased expression of necroptosis markers in the setting of hepatic steatosis and I/R injury. Additionally, recent studies in hepatic (49) and cardiac I/R injury (45, 50) have also demonstrated an elevation in RIPK3 and attenuation of injury utilizing necroptosis inhibitors. While RIPK3 may represent an excellent biomarker for abnormal or exaggerated I/R injury, its exact role in these models is not yet fully elucidated. For instance, in a recent publication, Roychowdry and colleagues demonstrate increased susceptibility to high fat diet induced liver injury in RIPK3 knock out mice (RIPK3−/−)(51), while two previous publications using the MCD diet have shown RIPK3−/− to be protective (47, 48). RIPK3 deficiency may also be protective in the context of ethanol-induced liver injury (52). It is possible that the differences may be secondary to experimental paradigm or dietary or mouse strain differences (46, 53). In the future, further in vitro and in vivo studies utilizing genetic knockouts and small molecule inhibitors will be needed to elucidate the role of RIPK3, RIPK1, and MLKL in hepatic I/R injury in the setting of hepatic steatosis and determine whether targeting necroptotic signaling is a potentially viable strategy for reducing I/R injury.
There are several limitations to the work presented herein. A potential weakness in our study is the absence of female mice in the study design. In the future, it will be useful to use both female and male mice in order to detect possible sex differences in the response to high fat diet or to I/R injury. Decreasing the length of time that mice are maintained on the studied high fat diets may produce less steatosis and allow for investigation of milder forms of steatosis in I/R injury. Shorter duration on Western diet may also eliminate the small percentage of macrovesicular steatosis and create pure a microvesicular steatosis model. Future studies may also benefit from studying longer reperfusion time points to address long-term survival and outcomes related to liver function and hepatic regeneration. It will also be interesting to use this model system to evaluate the effectiveness of potential therapeutic interventions on these outcomes. Additionally, as always, findings in rodent studies should be applied with caution to the clinical setting. Nevertheless, these data suggest that steatotic livers with microvesicular steatosis due to a fairly physiologic (42% fat) diet are more susceptible to I/R injury and that use of these organs for transplantation may be harmful. Further investigation into the pathogenic mechanisms may allow us to find new therapeutic targets in the future.
In summary, we have successfully used two different models of diet-induced hepatic steatosis to assess its impact on hepatic I/R injury. We have shown that the presence of steatosis exacerbates I/R injury as measured by plasma ALT, inflammatory cytokines, and necroptosis markers. Furthermore, histological evaluation demonstrates more extensive necrotic injury in the presence of hepatic steatosis following I/R injury. Through the use of two different diets producing mixed macrovesicular and microvesicular versus predominantly microvesicular hepatic steatosis, we were able to assess whether or not the response to I/R injury may be different depending on the pattern of steatosis. This distinction will be a valuable tool for future studies evaluating differences in mechanism of injury in the setting of microvesicular steatosis and mixed steatosis.
Materials and Methods
Animal study design
Male 5 week old C57BL/6J mice were purchased from Jackson laboratory (Bar Harbor, ME). They were allowed to acclimate for one week on standard chow diet. They were then placed on a low fat control diet (LF) (Research Diets Inc. D12450B), high trans-fat and cholesterol diet (HTF-C) (D09100301 Research Diets Inc.), or a high saturated fat “western” diet (Western) (Envigo TD 88137). Body weight was checked weekly. Mice were maintained on diet for 8 weeks prior to surgery. All animal studies were approved by the Institutional Animal Use and Care Committee of Washington University School of Medicine and comply with the Guide for the Care and Use of Laboratory Animals as outlined by the National Academy of Sciences.
Hepatic ischemia-reperfusion (I/R) surgery
Hepatic ischemia was induced using a 70% ischemia model as previously described (54). Briefly, mice were anesthetized using isoflurane inhalation. Sustained release buprenorphine and bupivacaine were used for analgesia. A midline laparotomy was performed to expose abdominal contents. Hepatic ischemia was induced by cross clamping the hepatic artery, portal vein, and bile duct distal to the branch point to the right lateral lobe to induce ischemia to the median and left lobes. The clamp was released after 60 minutes followed by 4 hours or 24 hours of reperfusion. Mice undergoing sham surgery underwent a midline laparotomy and were maintained under anesthesia for 60 minutes without cross clamping of the hepatic artery and portal vein. Mice were allowed to recover for either 4 hours or 24 hours. At those specified times, mice were euthanized and plasma and liver samples were collected. A portion of the left lobe was preserved in 10% neutral buffered formalin for histological evaluation. The remaining liver samples were immediately frozen in liquid nitrogen and then stored at −80°C for future analysis. Blood samples were collected from the inferior vena cava at the time of sacrifice and plasma collected by centrifugation.
Plasma parameters
Tail blood glucose was measured by using a One-Touch Ultra glucometer (LifeScan). Plasma insulin was quantified by an immunoassay (Singulex, Alameda, CA) by the Core Laboratory for Clinical Studies at Washington University. Plasma alanine transaminase (ALT) and aspartate transaminase (AST) were measured using commercially available colorimetric kinetic assays (Teco Diagnostics, Anaheim, CA) according the manufacturer’s instructions. Plasma TNFα level was measured using a commercially available ELISA kit (R&D systems, Minneapolis, MN). Plasma levels of IL-6 were determined, per manufacturer’s protocol, with a commercially available magnetic bead suspension assay (MilliporeSigma, Billerica, MA) using the Luminex 200 analyzer (Luminex Corp., Austin, TX).
Triglyceride assay
A portion of the caudate lobe (non-ischemic) was obtained at time of sacrifice. Liver samples were then homogenized in saline followed by addition of 1% sodium deoxycholate to solubilize lipids. Hepatic triglyceride content was measured using a commercially available enzymatic assay (Thermo Fisher Scientific).
mRNA isolation and quantitative RT-PCR
Total liver mRNA was extracted with RNA Bee (Isotex Diagnostics, Friendswood, TX) based on manufacturer’s instructions. Complementary DNA was synthesized using a commercially available high capacity reverse transcription kit (Applied Biosystems, Foster City, CA). Quantitative real time PCR was performed with Power SYBR Green with the use of an ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster City, CA). Arbitrary units of mRNA of the genes of interest were corrected to 36B4 expression. Primers were obtained through Integrated DNA Technologies (Coralville, IA). The primer sequences used in this study are presented in Supplemental Table 1.
Western blot analysis
For western blot analysis, liver protein extracts were obtained from the liver using the following buffer: 15mM NaCl, 20mM Tris, 1mM ethylene diamine tetraacetic acid, 0.2% NP-40, 10% glycerol. Lysates were normalized to protein concentration, denatured, and run on Criterion TGX precast PAGE gels (BioRad, Hercules, CA) and transferred onto polyvinylidene fluoride membranes. Antibodies to RIPK3 (1:1,000; ProSci (#2283), Poway, CA), TIMP1 (1,1000; Abcam (#ab38978), Cambridge, MA), PhosphoS345-MLKL (1,1000; Abcam (#ab196436), Cambridge, MA), MLKL (1,1000; Sigma-Aldrich (#SAB1302339), St. Louis, MO) and tubulin (1:1000; Sigma-Aldrich (#T5168), St. Louis, MO) were used according to the manufacturers’ instructions. Membranes were probed with an antirabbit IRDye 800CW (1:10,000; LI-COR) secondary antibody and an antimouse IRDye 700CW secondary antibody (1:10,000; LI-COR). Blots were developed on an infrared Odyssey developer (LI-COR).
Histopathology and Immunohistochemistry
A portion of the left lateral lobe was harvested at the time of sacrifice and placed in 10% neutral buffered formalin. Formalin-fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin and/or Masson’s trichrome stain. A liver pathologist (I.N.) blinded to the conditions assessed the presence of steatosis and necrosis in the liver specimens. A portion of the left lateral lobe was harvested at the time of sacrifice and placed in 10% neutral buffered formalin. Formalin-fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) and/or Masson’s trichrome stain. The H&E slides were reviewed by a liver pathologist (I.N.) in a blinded fashion. Steatosis was assessed as microvesicular and macrovesicular. Microvesicular steatosis was defined as presence of numerous, small fat vacuoles with a centrally placed hepatocyte nucleus, whereas large droplet was defined as large or small vacuoles of fat that displaces the hepatocyte nuclei (55). The grade of steatosis and necrosis were assessed as percent area of involvement per slide. The fibrosis stage was assessed based on NASH CRN criteria (56).
Formalin-fixed and paraffin embedded tissues were also sectioned and stained for CD68 using DAB immune-peroxidase staining. Anti-CD68 antibody (Abcam, ab125212, 1:500) was incubated overnight at 4°C. After washing with PBS-Tween20, Anti-Rabbit secondary antibody (Jackson Lab, 111-064-144, 1:800) was applied for 1 hour at room temperature. After washing with PBS-Tween20, slides were incubated with streptavidin-horse radish peroxidase (SA-HRP) (Jackson Lab, 011-030-084, 1:,1000), and developed with Betazoid DAB for 3 minutes (Biocare, BDB2004), and counterstained with Hematoxylin.
Statistical analysis
Statistical comparisons were made using a t-test. All data are presented as mean+/− standard error of the mean with statistical significance defined as a p≤0.05. Equality of variances and normality of the data was ascertained through Levene’s test and Kolmogorov-Smirnov tests, respectively. Data from luminex analysis were analyzed via nonparametric analysis of variance Kruskal-Wallis test. When the omnibus test was satisfied, the Mann-Whitney U post-hoc test was used to look for individual diet differences. An alpha level of 0.05 was considered significant.
Supplementary Material
Acknowledgments
Grants and financial support:
Work in the authors’ lab was supported by NIH grants R01 DK078187 and R01 DK104735 to BNF and several core laboratories of Washington University (P30 DK052574; P30 DK20579; P30 DK56341). K.H.H.L is a Pediatric Gastroenterology Training Grant Fellow supported by T32 DK0077653. A.M.H is supported by the American Diabetes Association Innovative Basic Science Award 1-17-IBS-109. K.S.M is supported by NIH grant K99 HL136658. C.J.W is supported by NIH R01 HL107594.
Abbreviations
- ALT
alanine transaminase
- CD
choline deficient
- HFD
high fat diet
- HTF-C
high trans-fat, fructose, cholesterol
- I/R
ischemia reperfusion
- LF
low fat
- MLKL
mixed-lineage kinase domain-like
- MCD
methionine choline deficient
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- RIPK
receptor interacting protein kinase
Footnotes
Potential conflicts of interest: Nothing to report.
References
- 1.2017 Mar 17; https://www.cdc.gov/obesity/data/prevalence-maps.html.
- 2.2017 Mar 17; http://www.who.int/mediacentre/factsheets/fs311/en/. http://www.who.int/mediacentre/factsheets/fs311/en/
- 3.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, Bugianesi E, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080. doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 6.Angulo P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl. 2006;12:523–534. doi: 10.1002/lt.20738. [DOI] [PubMed] [Google Scholar]
- 7.Rinella ME, Alonso E, Rao S, Whitington P, Fryer J, Abecassis M, Superina R, et al. Body mass index as a predictor of hepatic steatosis in living liver donors. Liver Transpl. 2001;7:409–414. doi: 10.1053/jlts.2001.23787. [DOI] [PubMed] [Google Scholar]
- 8.Urena MA, Ruiz-Delgado FC, Gonzalez EM, Segurola CL, Romero CJ, Garcia IG, Gonzalez-Pinto I, et al. Assessing risk of the use of livers with macro and microsteatosis in a liver transplant program. Transplant Proc. 1998;30:3288–3291. doi: 10.1016/s0041-1345(98)01033-1. [DOI] [PubMed] [Google Scholar]
- 9.Escartin A, Castro E, Dopazo C, Bueno J, Bilbao I, Margarit C. Analysis of discarded livers for transplantation. Transplant Proc. 2005;37:3859–3860. doi: 10.1016/j.transproceed.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 10.Feng S, Lai JC. Expanded criteria donors. Clin Liver Dis. 2014;18:633–649. doi: 10.1016/j.cld.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selzner N, Selzner M, Jochum W, Clavien PA. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol. 2003;39:55–61. doi: 10.1016/s0168-8278(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 12.Serafin A, Rosello-Catafau J, Prats N, Xaus C, Gelpi E, Peralta C. Ischemic preconditioning increases the tolerance of Fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002;161:587–601. doi: 10.1016/S0002-9440(10)64214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–1288. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 14.Anderson CD, Upadhya G, Conzen KD, Jia J, Brunt EM, Tiriveedhi V, Xie Y, et al. Endoplasmic reticulum stress is a mediator of posttransplant injury in severely steatotic liver allografts. Liver Transpl. 2011;17:189–200. doi: 10.1002/lt.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teramoto K, Bowers JL, Kruskal JB, Hara J, Iwai T, Endo M, Clouse ME. In vivo microscopic observation of fatty liver grafts after reperfusion. Transplant Proc. 1994;26:2391. [PubMed] [Google Scholar]
- 16.Teramoto K, Bowers JL, Kruskal JB, Clouse ME. Hepatic microcirculatory changes after reperfusion in fatty and normal liver transplantation in the rat. Transplantation. 1993;56:1076–1082. doi: 10.1097/00007890-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Selzner N, Selzner M, Jochum W, Amann-Vesti B, Graf R, Clavien PA. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J Hepatol. 2006;44:694–701. doi: 10.1016/j.jhep.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 18.Reiniers MJ, van Golen RF, van Gulik TM, Heger M. Reactive oxygen and nitrogen species in steatotic hepatocytes: a molecular perspective on the pathophysiology of ischemia-reperfusion injury in the fatty liver. Antioxid Redox Signal. 2014;21:1119–1142. doi: 10.1089/ars.2013.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ploeg RJ, D’Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, et al. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Marsman WA, Wiesner RH, Rodriguez L, Batts KP, Porayko MK, Hay JE, Gores GJ, et al. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation. 1996;62:1246–1251. doi: 10.1097/00007890-199611150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Verran D, Kusyk T, Painter D, Fisher J, Koorey D, Strasser S, Stewart G, et al. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl. 2003;9:500–505. doi: 10.1053/jlts.2003.50099. [DOI] [PubMed] [Google Scholar]
- 22.Chu MJ, Hickey AJ, Phillips AR, Bartlett AS. The impact of hepatic steatosis on hepatic ischemia-reperfusion injury in experimental studies: a systematic review. Biomed Res Int. 2013;2013:192029. doi: 10.1155/2013/192029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fishbein TM, Fiel MI, Emre S, Cubukcu O, Guy SR, Schwartz ME, Miller CM, et al. Use of livers with microvesicular fat safely expands the donor pool. Transplantation. 1997;64:248–251. doi: 10.1097/00007890-199707270-00012. [DOI] [PubMed] [Google Scholar]
- 24.McCormack L, Petrowsky H, Jochum W, Mullhaupt B, Weber M, Clavien PA. Use of severely steatotic grafts in liver transplantation: a matched case-control study. Ann Surg. 2007;246:940–946. doi: 10.1097/SLA.0b013e31815c2a3f. discussion 946–948. [DOI] [PubMed] [Google Scholar]
- 25.Han S, Ko JS, Kwon G, Park C, Lee S, Kim J, Kim G, et al. Effect of pure microsteatosis on transplant outcomes after living donor liver transplantation: a matched case-control study. Liver Transpl. 2014;20:473–482. doi: 10.1002/lt.23824. [DOI] [PubMed] [Google Scholar]
- 26.Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8:35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 27.London RM, George J. Pathogenesis of NASH: animal models. Clin Liver Dis. 2007;11:55–74. viii. doi: 10.1016/j.cld.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Thiam AR, Farese RV, Jr, Walther TC. The biophysics and cell biology of lipid droplets. Nat Rev Mol Cell Biol. 2013;14:775–786. doi: 10.1038/nrm3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunt EM. Pathology of fatty liver disease. Mod Pathol. 2007;20(Suppl 1):S40–48. doi: 10.1038/modpathol.3800680. [DOI] [PubMed] [Google Scholar]
- 30.Cieslak B, Lewandowski Z, Urban M, Ziarkiewicz-Wroblewska B, Krawczyk M. Microvesicular liver graft steatosis as a risk factor of initial poor function in relation to suboptimal donor parameters. Transplant Proc. 2009;41:2985–2988. doi: 10.1016/j.transproceed.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Noujaim HM, de Ville de Goyet J, Montero EF, Ribeiro CM, Capellozzi VL, Crescentini F, Casagrande M, et al. Expanding postmortem donor pool using steatotic liver grafts: a new look. Transplantation. 2009;87:919–925. doi: 10.1097/TP.0b013e31819b3f76. [DOI] [PubMed] [Google Scholar]
- 32.Kato H, Kuriyama N, Duarte S, Clavien PA, Busuttil RW, Coito AJ. MMP-9 deficiency shelters endothelial PECAM-1 expression and enhances regeneration of steatotic livers after ischemia and reperfusion injury. J Hepatol. 2014;60:1032–1039. doi: 10.1016/j.jhep.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llacuna L, Fernandez A, Montfort CV, Matias N, Martinez L, Caballero F, Rimola A, et al. Targeting cholesterol at different levels in the mevalonate pathway protects fatty liver against ischemia-reperfusion injury. J Hepatol. 2011;54:1002–1010. doi: 10.1016/j.jhep.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 34.Soejima Y, Shimada M, Suehiro T, Kishikawa K, Yoshizumi T, Hashimoto K, Minagawa R, et al. Use of steatotic graft in living-donor liver transplantation. Transplantation. 2003;76:344–348. doi: 10.1097/01.TP.0000071205.52835.A4. [DOI] [PubMed] [Google Scholar]
- 35.Ellett JD, Evans ZP, Atkinson C, Schmidt MG, Schnellmann RG, Chavin KD. Toll-like receptor 4 is a key mediator of murine steatotic liver warm ischemia/reperfusion injury. Liver Transpl. 2009;15:1101–1109. doi: 10.1002/lt.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Badry AM, Moritz W, Contaldo C, Tian Y, Graf R, Clavien PA. Prevention of reperfusion injury and microcirculatory failure in macrosteatotic mouse liver by omega-3 fatty acids. Hepatology. 2007;45:855–863. doi: 10.1002/hep.21625. [DOI] [PubMed] [Google Scholar]
- 37.He S, Atkinson C, Evans Z, Ellett JD, Southwood M, Elvington A, Chavin KD, et al. A role for complement in the enhanced susceptibility of steatotic livers to ischemia and reperfusion injury. J Immunol. 2009;183:4764–4772. doi: 10.4049/jimmunol.0900550. [DOI] [PubMed] [Google Scholar]
- 38.Evans ZP, Palanisamy AP, Sutter AG, Ellett JD, Ramshesh VK, Attaway H, Schmidt MG, et al. Mitochondrial uncoupling protein-2 deficiency protects steatotic mouse hepatocytes from hypoxia/reoxygenation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G336–342. doi: 10.1152/ajpgi.00049.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172:367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC Public Policy Committee of the American Association for the Study of Liver D. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 41.Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367–1384. doi: 10.1053/gast.2002.36061. [DOI] [PubMed] [Google Scholar]
- 42.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 43.Wee S, Pascual M, Eason JD, Schoenfeld DA, Phelan J, Boskovic S, Blosch C, et al. Biological effects and fate of a soluble, dimeric, 80-kDa tumor necrosis factor receptor in renal transplant recipients who receive OKT3 therapy. Transplantation. 1997;63:570–577. doi: 10.1097/00007890-199702270-00015. [DOI] [PubMed] [Google Scholar]
- 44.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, Gautheron J, et al. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res. 2014;103:206–216. doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- 46.Dara L, Liu ZX, Kaplowitz N. Questions and controversies: the role of necroptosis in liver disease. Cell Death Discov. 2016;2:16089. doi: 10.1038/cddiscovery.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, Koppe C, Kreggenwinkel K, et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med. 2014;6:1062–1074. doi: 10.15252/emmm.201403856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Afonso MB, Rodrigues PM, Carvalho T, Caridade M, Borralho P, Cortez-Pinto H, Castro RE, et al. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clin Sci (Lond) 2015;129:721–739. doi: 10.1042/CS20140732. [DOI] [PubMed] [Google Scholar]
- 49.Hong JM, Kim SJ, Lee SM. Role of necroptosis in autophagy signaling during hepatic ischemia and reperfusion. Toxicol Appl Pharmacol. 2016;308:1–10. doi: 10.1016/j.taap.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22:175–182. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- 51.Roychowdhury S, McCullough RL, Sanz-Garcia C, Saikia P, Alkhouri N, Matloob A, Pollard KA, et al. Receptor interacting protein 3 protects mice from high-fat diet-induced liver injury. Hepatology. 2016;64:1518–1533. doi: 10.1002/hep.28676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roychowdhury S, Chiang DJ, Mandal P, McMullen MR, Liu X, Cohen JI, Pollard J, et al. Inhibition of apoptosis protects mice from ethanol-mediated acceleration of early markers of CCl4 -induced fibrosis but not steatosis or inflammation. Alcohol Clin Exp Res. 2012;36:1139–1147. doi: 10.1111/j.1530-0277.2011.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rendina-Ruedy E, Hembree KD, Sasaki A, Davis MR, Lightfoot SA, Clarke SL, Lucas EA, et al. A Comparative Study of the Metabolic and Skeletal Response of C57BL/6J and C57BL/6N Mice in a Diet-Induced Model of Type 2 Diabetes. J Nutr Metab. 2015;2015:758080. doi: 10.1155/2015/758080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abe Y, Hines IN, Zibari G, Pavlick K, Gray L, Kitagawa Y, Grisham MB. Mouse model of liver ischemia and reperfusion injury: method for studying reactive oxygen and nitrogen metabolites in vivo. Free Radic Biol Med. 2009;46:1–7. doi: 10.1016/j.freeradbiomed.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lefkowitch JH. Scheuer’s Liver Biopsy Interpretation. (9) 2016;Chapter 7:101–106. [Google Scholar]
- 56.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.