Abstract
Purpose
AHOD0431 evaluated a response directed treatment paradigm, delivering minimal initial chemotherapy and low-dose radiation only for those not achieving CR, and a chemotherapy/low-dose radiation salvage regimen for protocol defined low-risk recurrence.
Patients and Methods
Age < 21 years with stage IA or IIA without bulk were eligible. We evaluated the strategy by determining the proportion receiving minimal chemotherapy alone, the proportion having a first or second remission without high dose chemotherapy with stem cell rescue or greater than 21 Gy involved field radiation therapy (IFRT) and overall survival (OS).
Results
278 subjects were eligible. At 4 years 49.0% received minimal chemotherapy and no radiation, 88.8% are in remission without high dose chemotherapy with stem cell rescue or greater than 21 Gy IFRT, and OS is 99.6%. Subjects with mixed cellularity (MC) histology have a 4 year event free survival (EFS) of 95.2%, significantly better than the 75.8% EFS for subjects with nodular sclerosis (NS) histology (p=0.008). Erythrocyte sedimentation rate (ESR) </= 20 and a negative FDG-PET after one cycle of chemotherapy (PET1) were associated with a favorable EFS outcome. The study was closed early when the use of radiation therapy exceeded the predefined monitoring boundary.
Conclusions
This limited chemotherapy response based approach was successful in patients who were PET1 negative, had MC histology or had low ESR. Evaluation of increased chemotherapy intensity or the integration of active new agents for nodular sclerosis histology patients with high ESR or who have a positive PET1 in this treatment paradigm is indicated.
Keywords: Pediatric Classical Hodgkin Lymphoma, PET response, Salvage Regimen, Mixed Cellularity Histology, Response Directed
Introduction
Treatment strategies for limited-stage classical Hodgkin lymphoma (cHL) in children and adolescents vary.1–3 Cure is reasonably expected in the great majority of children; however, the potential for long-term adverse sequelae of treatment remains significant.4,5 Event free survival (EFS) may be improved by globally intensifying initial therapy, but an alternative approach is to accept lower EFS and rely on higher dose salvage for those who fail. The former approach risks toxicity for the larger cohort; however the latter accepts lower EFS while minimizing toxic therapies with OS maintained by salvage approaches that may include radiation or high-dose chemotherapy with stem cell rescue. Given that EFS alone does not encompass the full value of initial therapy in cHL, our approach for limited-stage cHL utilized a low intensity initial chemotherapy regimen designed to limit cumulative exposure to chemotherapy agents associated with long term toxicities.
Response directed approaches tailor initial therapy in patients who demonstrate a favorable response to chemotherapy.6–8 No prior trial has paired limited upfront chemotherapy with a salvage regimen for subjects who recur in order to maintain OS while sparing many limited-stage patients upfront radiation therapy. The rationale for our approach was that many patients with limited-stage cHL who relapse with low disease burden after chemotherapy alone can be successfully re-treated with further chemotherapy and IFRT without the use of high dose chemotherapy/stem cell rescue or higher dose radiation therapy.9,10 Our objective was to investigate a response directed treatment paradigm delivering minimal initial chemotherapy, with low-dose radiation to those not achieving complete response (CR), and integrating a conventional chemotherapy/low-dose radiation salvage regimen for protocol defined low-risk recurrence. We also aimed to evaluate the role of PET1 response as a marker of chemosensitivity. To more fully understand the true risk and benefit of our algorithm, the analysis included the outcome of initial therapy, the outcome of therapy for protocol defined low-risk relapse, and overall survival.
Patients and Methods
Eligibility
Children, adolescents and young adults from ages 0 through 21 years were eligible for study participation. Subjects had newly diagnosed cHL, and met the following criteria: Ann Arbor Stage IA or IIA, without bulk disease. Bulk disease was defined as either a mediastinal mass > 1/3 the thoracic diameter on an upright PA chest x-ray or a continuous aggregate of nodal tissue that measures > 6 cm in the longest transverse diameter in any nodal area outside of the mediastinum.
Treatment Regimen and Response Criteria
Response determination was made by central imaging review after 1 and 3 cycles of chemotherapy. A negative FDG-PET corresponded to activity level at or below mediastinal blood pool as determined by visual inspection, while positive was defined as greater than mediastinal blood pool, as Deauville scoring was not customary at the time of the study. In cases where FDG-PET data provided to central review was insufficient to provide a reading, analysis was performed as per the institutional reading when available. Equivocal cases, when neither central nor institutional positive or negative readings were available, were analyzed as positive. Gallium scan, with negative defined as no residual detection of increased isotope uptake compared to background, was substituted for FDG-PET when the latter modality was not available. Complete response (CR) was defined by both anatomic reduction of at least 80% in the product of the perpendicular diameter (PPD) and FDG-PET negative after 3 chemotherapy cycles (PET3). No residual nodal mass could be greater than 2.0 cm in maximal transverse diameter, with the exception of the mediastinal mass, which could be larger than 2 cm provided that it demonstrated at least 80% reduction in the PPD. Partial response (PR) was defined by at least 50% reduction in the PPD of each of the areas of measurable disease, or return to normal nodal size, but not constituting a CR, regardless of FDG-PET response. Progressive disease (PD) was defined by at least 50% increase in the PPD of any of the involved nodes or nodal masses or new lesion(s) or progression of a nonmeasurable assessable disease site. Stable disease (SD) was defined by less than response PR but not PD. A low-risk relapse was defined as any recurrence that was stage IA or IIA without bulk disease occurring after achieving a CR with chemotherapy alone, regardless of time to relapse. Any other relapse was defined as a high-risk relapse.
The initial treatment regimen consisted of doxorubicin 25 mg/m2 on Day 1 and Day 2, vincristine 1.4 mg/m2 (max 2.8 mg) on Day 1 and Day 8, prednisone 20 mg/m2 twice a day on Days 1–7 and cyclophosphamide 600 mg/m2 on Day 1 and Day 2, given every 21 days with growth factor support for three cycles (AVPC). Those patients not in CR after three cycles received 21 Gy involved field radiation therapy (IFRT) in 1.5 Gy fractions over 14 sessions. Subjects that experienced a protocol defined low-risk relapse after initial chemotherapy alone were eligible for an integrated salvage regimen consisting of vinorelbine 25 mg/m2/dose on Day 1 and Day 5 and ifosfamide 3000 mg/m2/day continuous 24 hour infusion on Days 1–4 and given every 21 days with growth factor support for 2 cycles (VI) followed by dexamethasone 5 mg/m2 every 12 hours on Days 1 and Day 2, etoposide 100 mg/m2 every 12 hours on Days 1 and Day 2, cisplatin 90 mg/m2 Day 1, and cytarabine 3000 mg/m2 every 12 hours on Day 1 and Day 2, given every 21 days with growth factor support for two cycles (DECA). Salvage regimen subjects then received IFRT. IFRT was administered as 21 Gy in 1.5 Gy fractions over 14 sessions to areas defined as involved at initial presentation, as well as other areas involved at recurrence.
Statistical Considerations
Event free survival (EFS) was defined as the time from study entry to the first occurrence of disease relapse/progression, second malignancy, or death due to any cause; patients without report of such events were censored at the last contact. Overall survival (OS) was defined as time from study entry to death due to any cause; patients alive at last contact were censored.
Alternate endpoints of event free survival without radiation therapy (EFSnoRT) and Intensive Therapy Free Survival (ITFS) were evaluated in this trial of limited-stage cHL. EFSnoRT was defined from the time of study entry to a point of IFRT as prescribed by study or at treatment failure. An event for the endpoint of EFSnoRT was defined as: Less than CR after initial chemotherapy, any relapse following CR after initial chemotherapy, any second malignancy, or death from any cause. ITFS was defined as time from study entry to an indication for high dose chemotherapy/stem cell rescue (HSCT) or greater than 21 Gy IFRT. Events for ITFS were: Protocol defined high-risk relapse, any relapse after protocol directed IFRT (upfront or protocol prescribed salvage for protocol defined low-risk relapse in CR), any second malignancy, or death from any cause. Protocol defined low-risk relapses after CR were not considered failures for ITFS. Based on intention to treat principle, all patients with protocol defined low-risk relapse after CR were analyzed as if they received protocol salvage; their second relapses were included in ITFS.
The treatment strategy was intended to maintain an acceptable EFS rate while minimizing exposure to other than minimal chemotherapy (defined as EFSnoRT), and minimizing the need for intensive retrieval. Study targets included: 1) 4-year EFSnoRT of at least 65%; 2) 4-year ITFS of at least 95% (which limits exposure to intense therapies such as HSCT to under 5% of the treatment population); 3) 4-year EFS of at least 80%. The planned study sample size was 400 patients with a minimum of 2 years of follow-up on the last enrolled patient. The power was > 95% to detect a reduction in 4-year EFS rate from the null hypothesis of 0.80 to a rate of 0.74, a reduction in 4-year EFSnoRT rate from the null hypothesis of 0.65 to a rate of 0.58, and a reduction in 4-year ITFS rate from the null hypothesis of 0.95 to a rate of 0.91, based on one-sided test of the 4-year product limit estimate with 20% Type I error and as much as 20% censoring over the length of the study. Kaplan-Meier product-limit method was used to estimate these endpoints and OS, along with the Greenwood standard error estimates and 95% confidence intervals based on the log-log transformation. While survival curves were directly compared between groups with k-sample tests, multivariate Cox regressions were performed to verify the difference between the groups by including the possible covariates.
CR and EFS by ESR and C-reactive protein (CRP) were conducted using the Pearson’s Chi-squared tests and log-rank tests respectively. All analyses were conducted using STATA (College Station, TX: StataCorp LP).
Results
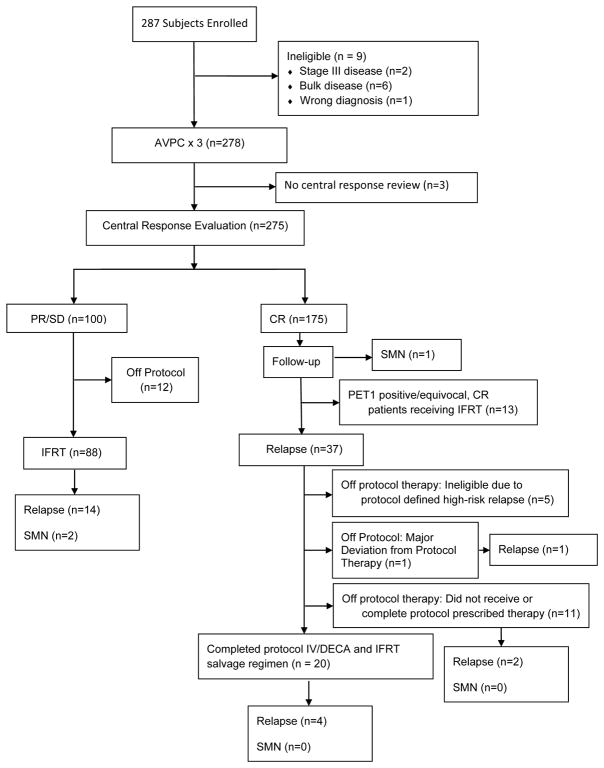
The study opened to enrollment starting 2/13/06 and enrolled 287 subjects of whom 9 were subsequently found to be ineligible; 278 subjects are the subject of this report (Figure 1). 22 subjects had Gallium scan as the sole nuclear medicine imaging modality to document response at end of 3 cycles of chemotherapy. In 7 cases the PET3 materials provided to central review were insufficient for response determination. 3 of these cases were equivocal and were analyzed as positive. On 12/4/2008, the study was temporarily closed to accrual because of a high risk of relapses among CR patients who were PET1 positive. A study recommendation was made to administer 21 Gy IFRT for all patients who had a positive or unknown PET1, unless they were more than 12 months of completing chemotherapy. The study was closed 4/3/2009 when the EFSnoRT rate dropped below the predefined goal. The median f/u is 77 months from enrollment. Patient characteristics are presented in Table 1.
Figure 1.
Flow diagram of the study
Table 1.
Patient Characteristics among eligible patients on AHOD0431
| N=278 | |
|---|---|
| Gender | |
|
| |
| male | 123 (44.2%) |
|
| |
| female | 155 (55.8%) |
|
| |
| Race | |
|
| |
| white | 217 (78.1%) |
|
| |
| black | 27 (9.7%) |
|
| |
| other | 8 (2.9%) |
|
| |
| missing | 26 (9.4%) |
|
| |
| Ethnicity | |
|
| |
| non-Hispanic | 222 (79.9%) |
|
| |
| Hispanic | 40 (14.4%) |
|
| |
| missing | 16 (5.8%) |
|
| |
| Stage | |
|
| |
| I | 67 (24.1%) |
|
| |
| II | 211 (75.9%) |
|
| |
| Age at enrollment | |
|
| |
| Median/range (years) | 15.3 (3.0–21.9) |
|
| |
| Pathology by central review | |
|
| |
| NS | 179 (64.4%) |
|
| |
| MC | 42 (15.1%) |
|
| |
| other/missing | 57 (20.5%) |
|
| |
| ESR | |
|
| |
| <=20 mm/hr | 143 (51.4%) |
|
| |
| >20 mm/hr | 134 (48.2%) |
|
| |
| missing | 1 (0.4%) |
|
| |
| CRP | |
|
| |
| <=2x Upper Limit | 173 (62.2%) |
|
| |
| >2x Upper Limit | 80 (28.8%) |
|
| |
| missing | 25 (9.0%) |
|
| |
| Number of Disease Sites | |
|
| |
| 1–2 | 183 (65.8%) |
|
| |
| 3+ | 92 (33.1%) |
|
| |
| missing | 3 (1.1%) |
Abbreviations: NS, Nodular Sclerosis; MC, Mixed Cellularity; ESR, Erythrocyte Sedimentation Rate; CRP, C-Reactive Protein; mm/hr, millimeter/hour; CR, Complete Remission; PR, Partial Remission; SD, Stable Disease
Toxicity
Treatment related toxicity was minimal during initial therapy. The most common toxicity was febrile neutropenia, occurring in 5.4% of subjects. Non-hematologic CTC 4.0 grade 3 or higher toxicities occurred in less than 5% of patients.
Toxicity during the VI salvage regimen (n=23) included a 17.4% incidence of febrile neutropenia, 13% grade 3 or higher vomiting, 13% infections, and 8.7% incidences of grade 3 or higher nausea and hypophosphatemia. The DECA portion of the salvage regimen (n=21) was associated with a 9.5% incidence of febrile neutropenia, and 9.5% incidences of grade 3 or greater hearing impairment, tinnitus, infections, vomiting and hypokalemia.
Outcomes
Following 3 cycles of AVPC chemotherapy CR was achieved in 175 subjects (64%), PR in 95 (34%) and SD in 5 (2%). Among the CR subjects, 13 who had completed chemotherapy or were near completion of chemotherapy received IFRT on the basis of the 12/4/08 study committee recommendation, including 2 patients without PET1 and 1 with equivocal PET1 per QARC central review. These patients were censored on the later date of the chemotherapy completion date or 12/4/2008 for the EFSnoRT analysis.
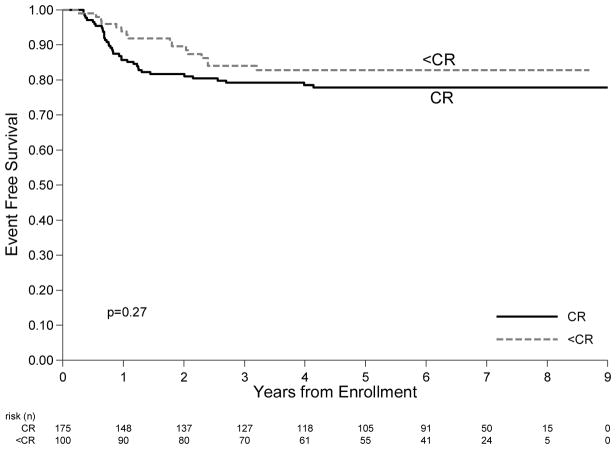
4 year EFS for the whole cohort is 79.9 % (95% CI: 74.6%–84.2%) (figure 2A). First events included 52 relapses and 3 SMNs. 4 year EFS for CR subjects was 77.5% (95% CI: 70.8%–83.4%) and for PR/SD subjects was 82.8% (95% CI: 73.4%–89.1%) (p=0.27) (figure 2B).
Figure 2.
A: Overall Survival for all patients. B: Event Free Survival by remission status after 3 courses of chemotherapy. Subjects that were PR received 21 Gy IFRT.
At 4 years EFSnoRT is 49.0% (95% CI: 42.8%–54.9%) (Supplemental figure 1A) A total of 137 events for the EFSnoRT endpoint were reported. Failures were due to lack of CR at the end of initial chemotherapy (n=100), relapse after initial CR (n=37) or SMN (n=1).
4 year OS is 99.6% (95% CI 97.0%–99.9%) (figure 2A). One death due to progressive disease occurred in a subject who was PR after chemotherapy and received RT per protocol but relapsed as Stage IV disease 7.5 months after enrollment.
Protocol directed salvage therapy outcomes and ITFS
The median time to first relapse among CR patients is 9.4 months (range 4.1–49.7 months). Among 31 subjects eligible for the salvage regimen of the study 11 did not receive protocol prescribed salvage. Stated reasons for not following protocol were: Study was open only to follow-up with the local IRB, treating physician preference and patient/family preference. Institutional query revealed that none of these patients received high dose chemotherapy with stem cell rescue as salvage for first relapse. 4 year ITFS is 88.8% (95% CI: 84.3%–92.1%) (Supplemental figure 1B). Combining the CR and PR/SD subjects, a total of 29 events occurred for ITFS. Failures for ITFS among CR subjects included: protocol defined high-risk relapses (n=5), second relapses (n=7) and SMN (n=1). Among the 100 PR/SD patients, 16 failures for ITFS (recurrences n=14, SMN n= 2) occurred.
Risk Factor Analysis at Initial Diagnosis
Predictors of CR and EFS among all patients and NS histology patients are presented in Table 2. ESR and CRP were not prognostic for MC histology patients. In multivariable analysis MC histology (HR 0.21; p=0.04) and ESR </= 20 (HR 0.41, p=0.03) remained significant predictors of EFS among all patients (Supplemental Table 1)
Table 2.
Histology, ESR and CRP as predictors of outcome among limited-stage patients: All histology and restricted to nodular sclerosis.
| CR (%) | p Value | 4-yr EFS CR no RT | N | p Value | 4-yr EFS PR w RT | N | p Value | 4-yr EFS | N | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All histology | |||||||||||
| Histology | 0.35 | 0.009 | 0.3 | 0.008 | |||||||
|
| |||||||||||
| MC | 29 (69.1%) | 96.6% | 29 | 92.3% | 13 | 95.2% | 42 | ||||
|
| |||||||||||
| NS | 108 (61.4) | 72.6% | 98 | 78.9% | 61 | 75.8% | 179 | ||||
|
| |||||||||||
| ESR | 0.18 | 0.009 | 0.86 | 0.02 | |||||||
|
| |||||||||||
| <=20 | 96 (67.6) | 85.0% | 89 | 82.4% | 39 | 85.1% | 143 | ||||
|
| |||||||||||
| >20 | 79 (59.9) | 68.4% | 73 | 82.9% | 48 | 75.1% | 134 | ||||
|
| |||||||||||
| CRP | 0.015 | 0.01 | 0.44 | 0.02 | |||||||
|
| |||||||||||
| <=2x Uln | 120 (70.2) | 82.8% | 111 | 87.1% | 41 | 84.6% | 173 | ||||
|
| |||||||||||
| >2x Uln | 43 (54.4) | 61.7% | 40 | 79.0% | 34 | 70.6% | 80 | ||||
| Nodular Sclerosis | |||||||||||
|
| |||||||||||
| ESR | 0.96 | 0.007 | 0.92 | 0.01 | |||||||
|
| |||||||||||
| <=20 | 52 (61.9) | 85.9% | 46 | 79.6% | 28 | 84.0% | 85 | ||||
|
| |||||||||||
| >20 | 56 (61.5) | 61.4% | 52 | 80.7% | 32 | 69.4% | 93 | ||||
|
| |||||||||||
| CRP | 0.084 | 0.03 | 0.91 | 0.12 | |||||||
|
| |||||||||||
| <=2x Uln | 69 (67.7) | 80.2% | 62 | 80.9% | 28 | 80.9% | 104 | ||||
|
| |||||||||||
| >2x Uln | 33 (54.1) | 56.8% | 31 | 80.0% | 26 | 68.3% | 62 | ||||
Columns correspond to the rate for CR, EFS among subjects that achieved a CR and received no IFRT, EFS among subjects that achieved PR and received protocol IFRT and overall EFS.
Abbreviations: CR, Complete Remission; EFS, Event Free Survival; RT, Radiation Therapy; PR, Partial Remission; ESR, Erythrocyte Sedimentation Rate; CRP, C-Reactive Protein; Uln, Upper Limit of Institutional Normal Range.
Outcomes by centrally reviewed histology
221 Subjects had centrally reviewed histology available. Subjects that did not have centrally reviewed histology did not differ from the rest of the study population by gender, ethnicity, disease stage, ESR, CRP, or number of disease sites. Patients without central histology review had a lower percentage of White and a higher percentage of Unknown race (p=0.04) (Supplemental Table 2). Mixed cellularity histology (MC) was present in 19% (n=42) of enrolled subjects for whom centrally reviewed pathology was available. Compared to patients with NS histology, MC patients were more likely to be male (p < 0.0001), Hispanic ethnicity (p=0.02), have stage I disease (p< 0.0001), have lower end of CRP (p=0.03) and have less than 3 sites of disease (p=0.02) (Supplemental Table 3). 4 year EFS among MC subjects was 95.2% (95% CI: 82.3%–98.8%), significantly better than the 4 year EFS of 75.8% (95% CI: 68.5%–81.6%) among subjects with nodular sclerosis histology (NS) (p=0.008) (Figure 3A). This superiority remains significant in a multivariable cox regression model (HR=0.21, p=0.038) (Supplemental Table 1). However, there was no difference in CR rates after initial chemotherapy by histology (69.1% MC versus 61.4% NS, p=0.35). The 2 relapses among the 42 subjects with MC histology included one relapse in a subject achieving CR with initial chemotherapy and another in a subject with PR who received IFRT.
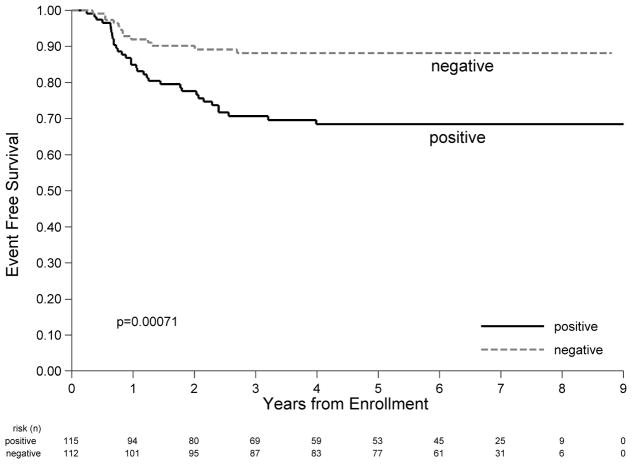
Figure 3.
A: EFS by Histology. B: EFS by very early response as determined by FDG-PET after 1 course of chemotherapy.
PET1 results
PET1 was evaluable for 227 subjects. There was no difference in demographics, histology, stage, CRP or number of disease sites between those without a PET1 compared to those without a PET1. However those without a PET1 were more likely to have ESR > 20 (p=0.05) (Supplemental Table 4). Among 227 subjects with PET1, 49% were PET1 negative. In 8 cases the PET1 materials provided to central review were insufficient for response determination. One of these cases was equivocal and was analyzed as positive; the remainder were analyzed as per institutional report. PET1 positive subjects did not differ from PET1 negative subjects by demographics, disease histology or number of disease sites. However, PET1 positive patients were more likely to be Stage II (p=0.0034), have ESR > 20 (p<0.0001), and have an elevated CRP at presentation (p=0.044) (Supplemental Table 5). 4 year EFS was significantly higher at 88.2% (95% CI: 80.5%–93.0%) for PET1 negative subjects, and 68.5% (95% CI: 58.7%–76.4%) for PET1 positive/equivocal subjects (p=0.0007) (Figure 3B). Compared with PET1 positive/equivocal patients, the PET1 negative population still have significantly better performance in terms of EFS after the demographics and baseline characteristics are considered in a multivariable cox regression model (HR 0.39; p=0.012) (Supplemental Table 6). Among subjects that achieved a CR after 3 cycles of chemotherapy and did not receive IFRT, the 4 year EFS was 84.9% (95% CI: 74.9%–91.1%) for PET1 negative (n=81) versus 59.6% (95% CI: 44.1%–72.0%) for PET1 positive/equivocal (n=48) (p=0.001). Among subjects that were PR after 3 cycles of chemotherapy and received IFRT, the 4 year EFS for PET1 negative (n=25) vs. PET1 positive/equivocal (n=51) were 96.0% (95% CI: 74.8%–99.4%) vs. 69.5% (95% CI: 53.8%–80.7%) respectively (p=0.015).
Second malignant neoplasms
There have been 3 second malignant neoplasms. Two secondary AML cases occurred: One 4 years after completing chemotherapy only in a subject in CR, and the other 2.8 years following completion of IFRT in a PR subject. Thyroid carcinoma occurred in a patient with cervical node HL 2 years after completing IFRT based on PR status. There have been no SMNs in patients receiving the salvage regimen. There have been no deaths due to SMN.
Discussion
We evaluated anatomic and FDG-PET response after three cycles of AVPC chemotherapy as a marker of sufficiency of treatment, and the role of very early FDG-PET response as an indicator of chemosensitivity. The paradigm allowed us to successfully treat almost half of our subjects with only 3 cycles of AVPC chemotherapy. with an OS of 99.6%. Patients with MC histology also had high EFS and OS with this approach. Incorporating a uniform salvage regimen for protocol defined low-risk recurrences after chemotherapy only, in an effort to minimize high dose chemotherapy with stem cell rescue procedures or greater than 21 Gy IFRT, yielded an ITFS of 88.8%. In addition we demonstrate that despite a lower EFS than contemporary trials for limited stage cHL the substantial majority were able to avoid high dose chemotherapy with stem cell rescue procedures. 2,3 Extended follow-up will be required to fully assess the value of this strategy with regard to late effects, including the consequences of the use of 21 Gy IFRT in almost half of patients.
While limited to stage I/IIA non-bulky disease, our study included subjects with high ESR, high CRP, and multiple sites of disease, factors that have previously been associated with a less favorable outcome.11 Unfavorable prognostic indicators are expected to correlate with outcome in the setting of a reduced intensity regimen.12 Despite the inclusion of subjects with unfavorable risk criteria the 4 year OS is excellent and the ITFS, although below protocol target, was 88.8%. Our data suggest that patients with limited stage non-bulky NS histology with ESR </= 20 and those with PET1 negativity and patients with MC histology could be successfully treated with a minimal initial chemotherapy approach, supporting a risk and response based approach in this group.
While our EFS differs from earlier studies, the proportion of subjects that were successfully treated without radiation therapy is higher than most contemporary limited-stage pediatric trials when analysis is limited to classical HL.2,3 We have identified high ESR, NS histology and PET1 positivity as poor prognostic features in the setting of this minimal chemotherapy approach, and that potentially justify augmentation of therapy. Although IFRT in these patients may improve the overall EFS rate, some of this cohort may be treatable without IFRT if the intensity or duration of chemotherapy is increased following the PET1 response. This may also be a population for consideration of novel targeted agents or immune modulating therapy. Our results concur with a preliminary report of the EuroNet-PHL-C1 Study suggesting that high ESR as well as bulk disease are adverse prognostic indicators within their TG1 cohort (Stage IA/IB-IIA).13 Further evaluation of regimens with limited long term toxicities or the integration of novel or targeted agents to minimize the need for RT in patients with NS histology with these prognostic factors should be considered.
Therapy for recurrent Hodgkin lymphoma often involves high-dose chemotherapy with autologous stem cell rescue, sometimes in association with additional radiation therapy to selected regions.9,10 A unique aspect of the current study was the inclusion of a standardized salvage regimen for protocol defined low-risk recurrence after chemotherapy alone. We considered this retrieval regimen of IV/DECA with 21 Gy low-dose IFRT to be a preferable alternative to autologous HSCT. However, cumulative doses of chemotherapy have potential to confer long term risks, particularly related to fertility in males and females.14 In all, 88.2% of subjects were effectively treated without high dose chemotherapy or more than 21 Gy IFRT, demonstrating the utility of a combined modality salvage regimen for recurrent disease after limited initial chemotherapy. Although 5 patients who recurred after initial chemotherapy alone had protocol defined high-risk relapses, the current OS for these patients is 100%.
In the current trial PET1 was a significant predictor of EFS following AVPC chemotherapy alone, using a conservative definition of favorable response of metabolic activity less than or equal to mediastinal blood pool, now equated to a Deauville score of 2. Early FDG-PET response has previously been demonstrated to be prognostic in a variety of studies primarily in adults; typically after two or three cycles of chemotherapy and in a variety of risk strata.15,16 However, very early PET scanning after one cycle in limited-stage disease in children and adolescents has not previously been reported. The implications for dose modification based on this observation remain to be explored, with the potential for minimization of therapy for favorable very early responders, or augmentation of therapy for those without early metabolic response.7 The potential to identify candidates for therapy augmentation after the first cycle of chemotherapy may allow for maximal therapeutic intervention without substantially prolonging therapy duration or adding to cumulative doses of cardiotoxic or gonadotoxic agents.
Notably, in contrast to prior North American or European cohorts, this study cohort had a high rate of MC cases and demonstrates excellent EFS with 3 cycles of AVPC and response based IFRT in patients with MC histology. MC disease is more common in younger children and children of Hispanic ethnicity. This difference in response of MC disease compared to NS disease has not been previously reported in limited-stage pediatric cHL. While lymphocyte predominant disease is now treated as a separate entity, recent trials in pediatric cHL have not stratified MC histology patients differently by risk or treatment pathways. This new observation should prompt further investigation regarding the biological differences of limited-stage MC HL, its association with EBV disease, and MC specific treatment regimens.
In conclusion, AHOD0431 suggests a potential strategy for limiting chemotherapy in limited-stage Hodgkin lymphoma patients with NS histology with low ESR and with MC histology using very early PET1 response based modification of therapy and an integrated conventional chemo-radiotherapy salvage regimen for protocol defined low-risk relapse. Prognostic factors identified herein may lead to better tailoring of therapy beyond stage. We demonstrate the principle and utility of paired salvage regimens following upfront low-dose chemotherapy, which allows sparing of the majority of patients potentially unnecessary treatment intensity. Our novel endpoints, EFSnoRT and ITFS, are a paradigm to facilitate analysis of trials focused on reduction of therapy where EFS alone is inadequate. Future directions include exploration for augmentation of therapy for subjects that have high ESR or unfavorable response to the first cycle of chemotherapy, incorporation of MC histology into treatment stratification for limited-stage Hodgkin lymphoma, and optimization of salvage regimens for recurrences after minimal initial therapy in limited-stage Hodgkin lymphoma.
Supplementary Material
Acknowledgments
Supported by National Cancer Institute Grants U10 CA98543 to the Children’s Oncology Group Chair, Statistics & Data Center Grant U10CA098413, NCTN Operations Center Grant U10CA180886 and NCTN Statistics & Data Center Grant U10CA180899. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
The authors wish to acknowledge Dr. James A Nachman, who was instrumental in the development of this study concept prior to his untimely death. Additionally Drs. John Thomson and Cherie Dunphy are acknowledged for their contribution to the central review of planned radiation fields and pathology respectively.
Footnotes
Conflict of interest: None
Author Contributions:
FG Keller: conceptualization, data curation, investigation, methodology, project administration, writing original draft and writing review and editing
Sharon M Castellino: conceptualization, project administration, writing review and editing
Lu Chen: data curation, formal analysis, methodology, writing original draft and writing review and editing
Qinglin Pei: data curation, formal analysis, writing review and editing
Stephan D Voss: project administration, writing review
Kathleen M McCarten: project administration, writing review and editing
Stacy L Senn: project administration
Allen Buxton: data curation, formal analysis
Rizvan Bush: data curation, formal analysis
Louis S Constine: conceptualization, project administration, writing review and editing
Cindy L Schwartz: conceptualization, methodology, writing review and editing.
References
- 1.Donaldson SS, Link MP, Weinstein HJ, et al. Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin’s disease. J Clin Oncol. 2007;25:332–337. doi: 10.1200/JCO.2006.08.4772. [DOI] [PubMed] [Google Scholar]
- 2.Mauz-Körholz C, Hasenclever D, Dörffel W, et al. Procarbazine-Free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: The GOPH-HD-2002 Study. J Clin Oncol. 2010;28:3680–3686. doi: 10.1200/JCO.2009.26.9381. [DOI] [PubMed] [Google Scholar]
- 3.Metzger ML, Weinstein HJ, Hudson MM, et al. Association between radiotherapy vs no radiotherapy based on early response to VAMP chemotherapy and survival among children with favorable risk Hodgkin lymphoma. JAMA. 2012;307:2609–2616. doi: 10.1001/jama.2012.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellino SM, Geiger AC, Mertens AC, et al. Morbidity and mortality of long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien MM, Donaldson SS, Balise RR, et al. Second malignant neoplasms in survivors of pediatric Hodgkin’s lymphoma treated with low-dose radiation and chemotherapy. J Clin Oncol. 2010;28:1232–1239. doi: 10.1200/JCO.2009.24.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz CL, Constine LS, Doojduen V, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood. 2009;114:2051–2059. doi: 10.1182/blood-2008-10-184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk Hodgkin lymphoma: a report for the Children’s Oncology Group Study AHOD0031. J Clin Oncol. 2014;32:3651–3658. doi: 10.1200/JCO.2013.52.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dörffel W, Rühl U, Lüders H, et al. Treatment of children and adolescents with Hodgkin lymphoma without radiotherapy for patients in complete remission after chemotherapy: Final results of the multinational trial GPOH-HD95. J Clin Oncol. 2013;31:1562–1568. doi: 10.1200/JCO.2012.45.3266. [DOI] [PubMed] [Google Scholar]
- 9.Harker-Murray PD, Drachtman RA, Hodgson DC, et al. Stratification of Treatment Intensity in Relapsed Pediatric Hodgkin Lymphoma. Pediatr Blood Cancer. 2014;61:579–586. doi: 10.1002/pbc.24851. [DOI] [PubMed] [Google Scholar]
- 10.Schellong G, Dörffel W, Claviez A, et al. Salvage therapy of progressive and recurrent Hodgkin’s disease: Results from a multicenter study of the pediatric DAL/GPOH-HD study group. J Clin Oncol. 2005;23:6181–6189. doi: 10.1200/JCO.2005.07.930. [DOI] [PubMed] [Google Scholar]
- 11.Haase R, Vilser C, Mauz-Korholz C, et al. Evaluation of the prognostic meaning of C-reactive protein (CRP) in children and adolescents with classical Hodgkin’s lymphoma (HL) Klin Padiatr. 2012;224:377–381. doi: 10.1055/s-0032-1323824. [DOI] [PubMed] [Google Scholar]
- 12.Hasenclever D. The disappearance of prognostic factors in Hodgkin’s disease. Ann Oncol. 2002;13(Supplement 1):75–78. doi: 10.1093/annonc/13.s1.75. [DOI] [PubMed] [Google Scholar]
- 13.Landman-Parker J, Wallace H, Hasenclever D, et al. First international inter-group study for classical Hodgkin lymphoma in children and adolescents: EuroNet-PHL-C1. Report of the latest interim analysis. Haematologica. 2016;101(Supplement 5):35. [Google Scholar]
- 14.Green DM, Nolan VG, Goodman PJ, et al. The Cyclophosphamide Equivalent Dose as an Approach for Quantifying Alkylating Agent Exposure. A Report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallamini A, Hutchings M, Rigacci L, et al. Early Interim 2-[18F]Fluoro-2 Deoxy-D-Glucose Positron Emission Tomography is prognostically superior to International Prognostic Score in advanced-stage Hodgkin’s lymphoma: A report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–3752. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 16.Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predict treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52–59. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.