Abstract
Purpose
To develop a real-time dynamic MRI method for comprehensive evaluation of velum movement during speech.
Methods
Dynamic MRI has been used to study velopharyngeal insufficiency (VPI) by imaging the movement of the velum during speech, because it can provide good anatomic details with no exposed radiation. To be able to comprehensively evaluate dynamic velum movement, a real-time spiral non-balanced SSFP sequence was developed with simultaneous dual-planar coverage and improved spatial and temporal resolution using a combination of parallel imaging and spatial and temporal compressed sensing to achieve 6x acceleration. New off-resonance correction and post-processing methods were also developed to reduce blurring and slice crosstalk.
Results
The method demonstrated good image quality for visualizing dynamic velum movement with reduced blurring and improved image homogeneity. Spatial resolution of 1.2*1.2 mm2 with 150 mm field-of-view (FOV) and temporal resolution of 20 frames-per-second with simultaneous dual-planar coverage was achieved.
Conclusions
This work describes a new technique for studying speech disorders using dual-planar accelerated spiral dynamic MRI.
Keywords: spiral, real-time velum imaging, parallel imaging, off-resonance correction
INTRODUCTION
The velopharyngeal mechanism, located between the oral and nasal cavities, is important for directing the flow of sound and air during speech. When the velum is at rest, air can flow out of the nasal cavity from the lungs. During most speech sounds, elevation and posterior retraction of the velum closes the velopharyngeal (VP) port – the passage between the nasal cavity and oral cavity. This closure necessarily prevents nasal air emission and directs airflow and voice through the oral cavity and out of the month. Incomplete closure of the VP port causes abnormal nasal airflow during speech and results in a speech impediment known as velopharyngeal insufficiency (VPI). VPI can be caused by a variety of disorders (structural, genetic, functional, or acquired) and is most commonly seen in children with a cleft palate repair. VPI is typically diagnosed by a trained clinician listening to the patient’s speech, but the instrumental evaluation of VPI has been generally limited to two undesirable options: nasal endoscopy and multi-planar video fluoroscopy. The major drawback of nasal endoscopy is the poor patient tolerance in very young children since it is very painful. Fluoroscopy requires significant patient cooperation and leads to radiation exposure (1–2).
Magnetic resonance imaging (MRI) has been used to study VPI because it provides the ability to analyze the anatomic details of the VP port in multiple arbitrary planes without radiation exposure. MRI of the velum before and after surgery has been performed for the evaluation of occult submucous cleft palate with an oblique coronal slice (3). Velum and nasopharyngeal wall modeling based on MRI and CT data has also been developed for the understanding of oral and nasopharyngeal functions during speech (4). However, in these methods, static MR images were acquired with very long scanning time and thus little or no dynamic information during speech could be obtained. This may lead to incomprehensive and/or inaccurate evaluation of the velum function.
Real-time MRI (RT-MRI) continuously acquires MR images of a dynamically evolving process and has emerged as a powerful tool to visualize the complex spatiotemporal coordination of upper airway structures including the tongue and velum during speech. A recent review (5) describes the current state-of-the-art protocols, including the choice of receiver coil, field strength, pulse sequences, k-space sampling strategy, audio acquisition and reconstruction, summarizing them in Table 1.
Radiofrequency (RF) spoiled gradient echo and balanced steady-state free precession (SSFP) sequences are two commonly used pulse sequences in RT-MRI of speech. The balanced SSFP sequence can improve the SNR, because the residual magnetization from a previous TR can be used in the next TR. However, it suffers from large areas of signal nulling or banding artifacts (Fig. 3 in (5)). A non-balanced SSFP sequence can utilize the residual TR without banding artifacts, because the uniform redistribution of spin phase angles prior the next RF-pulse, introduced by constant non-zero spoiler gradients, can smooth the position-dependent off-resonance frequencies by averaging over all values within a voxel. One goal of this study is to explore the use of a non-balanced SSFP sequence for RT-MRI of speech.
To improve spatial and temporal resolution, non-Cartesian k-space trajectories that have a higher sampling efficiency, such as spiral sampling, are often used for RT-MRI. Parallel imaging and compressed sensing can also be used to improve resolution by exploiting spatial and temporal redundancies in the data. These methods can be used separately or combined (6–12). However, off-resonance correction for spiral scanning when parallel and constrained reconstruction methods are used has not been well studied.
In addition, to obtain accurate evaluation of the velum movements, high spatial resolution (<1.5mm) and at least two views (sagittal and oblique coronal) are usually required. Although interleaved multi-planar real-time MRI acquisition has been applied in several studies (13–14), the spatial resolution was about 2mm and the dark band where multiple slices overlap due to reduced TR was not corrected, causing issues in accurate segmentation of velum.
In this study, we aim to address the problems outlined above and acquire dynamic image series of velum movements during speech with simultaneous dual-planar coverage and high spatial/temporal resolution using a real-time spiral non-balanced SSFP sequence. Parallel imaging and spatial and temporal compressed sensing reconstruction methods are used to achieve a 6x acceleration. A Chebyshev approximation based off-resonance correction method is incorporated into the reconstruction pipeline to reduce spiral blurring. The reconstructed image series from the two slices are then used for quantitative measurements of several characteristic variables of velum movements after a post-processing step to compensate for inter-slice crosstalk. The following sections describe these methods in detail.
METHODS
Sequence Design
In this application, a real-time spiral non-balanced SSFP sequence is used with constant spoiler gradients applied along the slice selection direction since it does not suffer from banding artifacts, especially around the air-tissue boundaries, compared with a balanced SSFP sequence (15).
The readout gradients of the spiral trajectory are designed using a linear variable density method (16–17) in which the sampling density linearly reduces from 1.2x at the center of k-space to 0.4x of the Nyquist sampling limit at the edge of k-space. The fully sampled spiral trajectory has 18 interleaves with 3.6 ms readout time per interleaf. To increase the temporal resolution of the dynamic image series, 6x undersampling is used such that each frame is reconstructed with only 3 interleaves of the entire k-space trajectory. Within each frame, the 3 interleaves are 120° apart from each other. The rotation angles between consecutive frames are varied and calculated using a bit-reversed order to minimize temporal correlations and thus reduce temporal blurring (18). The sampling trajectories are repeated with a period of 6 until the dynamic process is finished.
Combined Parallel Imaging and Spatial and Temporal Compressed Sensing
Since only 1/6 of k-space is covered in each frame, severe spatial and temporal aliasing artifacts will occur if only conventional gridding and FFT are used for image reconstruction. Therefore, spatial and temporal parallel imaging techniques and compressed sensing based methods are jointly used to reduce aliasing.
Spatial parallel imaging techniques take advantage of the multiple receiver coils used in the scan (19–21). SPIRiT (21) is a robust auto-calibrating parallel imaging technique that can be easily applied to spiral trajectories. The image reconstruction is performed as a minimization problem described as
| [1] |
in which y is the acquired k-space data based on the spiral k-space sampling pattern, D is the corresponding non-Cartesian Fourier transform operator in matrix form, and x is the image to be reconstructed. The second term represents the self-consistency of the k-space data from multiple receiver coils expressed in the image domain with a regularization parameter λ; G represents the SPIRiT kernel function and I is the identity matrix. G can be estimated from the low-resolution spiral field map data acquired before the beginning of the dynamic process to reduce motion artifacts.
In an image series for most dynamic applications, temporal redundancy exists because neighboring images are often highly correlated and/or the temporal frequencies of the dynamic process are sparse. Based on compressed sensing theory (22–23), aliasing from undersampled k-space data can be reduced by minimizing the l1 norm in a chosen sparse domain. In this application, since the tongue and velum movements are active and mostly non-repetitive, the temporal frequencies of the movements are not very sparse. However, since the tongue and velum movements are smooth and the remaining tissue in the field-of-view (FOV) remains mostly static, the temporal differences between neighboring frames are quite sparse. With this in mind, a temporal gradient term is added to Eq. [1] to further reduce aliasing (24). In addition, a spatial gradient (total variation) term can be also added to Eq. [1] due to the intrinsic spatial sparsity of medical images, particularly images of the velum (23). The resulting objective function of the image reconstruction is given as
| [2] |
in which D, x, y, G and and I have the same meaning as in Eq. [1] except that the temporal dimension is added. ∇t and ∇x are the gradient operators applied on the temporal and spatial dimension, respectively. α(r) and β are the corresponding regularization parameters.
Note that the parameter α is a function of spatial position r since, as mentioned before, some areas in the FOV experience more temporal changes than others; therefore, smaller penalties on the temporal gradient will be applied in these areas. The dynamic level at each position r can be estimated from low spatial resolution training images (25) or from a reconstructed image series with a spatially uniform α using Eq. [2]. In the second approach, the estimated α(r) is fed back to Eq. [2] to improve the temporal sharpness by re-distributing the penalties on the temporal sparsity. In this application, the second approach is used and the parameter α(r) is estimated with the following equation:
| [3] |
in which S(r) is the estimated variance of the image series at location r.
Eq. [2] is solved using a two-step iterative non-linear conjugate gradient method. In the first step, Eq. [2] is minimized with a spatially constant α(r). The initial image series are reconstructed with a view sharing approach to minimize spatial aliasing. In the second step the resulting images from the first step are fed to Eq. [2] as the initial image series together with the estimated α(r) using Eq. [3]. The reconstruction is performed offline in Matlab.
Off-Resonance Correction
A spiral trajectory is more sensitive to off-resonance effects than a Cartesian trajectory; therefore, off-resonance correction is usually required to reduce blurring. In most applications, a linear correction method based on a low-resolution B0 field map can reduce most of the blurring when the spiral readout is not too long (28). However, in this application, the substantial air-tissue boundaries can break the linear assumption and thus need non-linear corrections. A fast conjugate phase reconstruction method based on a Chebyshev approximation has been developed to correct for the non-linear off-resonance due to B0 inhomogeneity and concomitant gradients with fully sampled k-space data (26–27). A brief description of the method is as follows: a low-resolution B0 field map is first acquired with two single-shot spirals at different TEs (27); a high-resolution B0 field map is then calculated with a local minimum phase requirement based on the low-resolution B0 field map; Chebyshev demodulated images are then reconstructed/calculated based on a Chebyshev polynomial and the final image is combined using a Chebyshev approximation so that each pixel is demodulated at its corresponding local off-resonance frequency, derived from the high-resolution B0 field map and the calculated concomitant gradient field map, as expressed in the following equations:
| [4] |
in which ck(Δw(r)) is the Chebyshev coefficient as a function of local off-resonance frequency Δw(r). Ik(r) is the kth order Chebyshev demodulated image calculated from
| [5] |
with the total readout length τ and the density compensation function W(r) From Eq. [5], the 0th order Chebyshev demodulated image I0(r) is actually the reconstructed image with conventional gridding and FFT operations.
In this application, the Chebyshev demodulated images Ik(r) cannot be directly calculated from Eq. [5] since the k-space is highly undersampled. However, as introduced in the previous section, I0(r) can be obtained using the combined parallel imaging and spatial and temporal compressed sensing with undersampled k-space data. To obtain higher order Chebyshev images Ik(r) (k > 0 ) using Eq. [5], the missing k-space data is inversely gridded using the resulting I0(r). Finally, the corrected image m(r) is obtained using Eq. [4]. A flowchart of the algorithm is included as Supporting Figure S1.
Experimental Setup
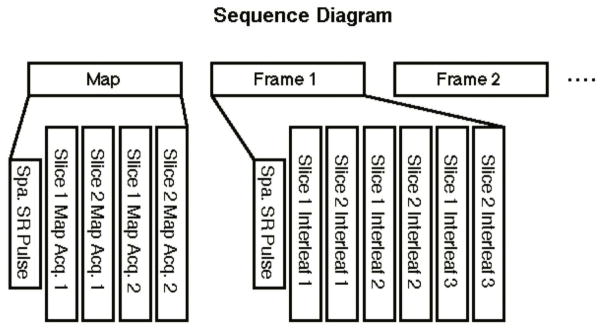
As described in the introduction, for a comprehensive evaluation of velum movements, a mid-sagittal slice was first chosen, and then a perpendicular oblique-coronal slice was selected and rotated to be approximately parallel to the velum movement direction and in the plane of the principal soft palate muscle, the levator veli palatine. This was similar to the plane acquired in (29). Acquisitions of the two slices were interleaved between every TR. Since TR is much shorter (~7ms) than the time span of one frame (~50ms), the two slices can be treated as being acquired simultaneously. Spatial saturation pulses were applied superior and inferior to the imaging slices for a reduced FOV to achieve higher spatial resolution. However, instead of being played for every TR and every slice, the saturation pulses were played only once for each frame and were shared by the two slices to reduce scan time and improve temporal resolution. This is achievable because the time spanned by each frame is much shorter than the T1 values in the saturated regions. Figure 1 shows the sequence diagram. The low-resolution B0 field map was acquired once in the beginning of the sequence.
Figure 1.
Sequence diagram for real-time spiral GRE sequence with dual-planar coverage. The low-resolution field map is acquired before the dynamic process. The spatial saturation pulses were played once for each frame.
The experiments were performed on a Siemens Avanto 1.5 T scanner equipped with a 4-channel head coil and a 2-channel neck coil. Healthy volunteers with informed consent were scanned while being asked to repeat specific phonetic sounds and sentences. The sequence parameters were: TR: 6.96 ms, TE: 0.78 ms, flip angle: 20°, field-of-view (FOV): 150*150 mm2, slice thickness: 8 mm, spatial resolution: 1.2*1.2 mm2, temporal resolution: 20 frames-per-second (fps).
Post Processing
Dark Band Correction
Since the data acquisition of these two slices is interleaved, the effective TR for most regions in a single slice is actually 2*TR, except for the intersected region of the two slices; therefore, there will be a dark band in the images of both slices due to lower signal magnitude. This may affect the accuracy of velum boundary depiction and thus needs correction. In practice, the positions of the two dark bands are pre-calculated from the prescribed slice positions and the corresponding decay ratios are estimated from the mean image intensities of the neighboring bands so that the dark bands can be compensated accordingly.
Velum Movement Analysis
For each frame of the dynamic image series, the velum and posterior pharyngeal wall were outlined from the mid-sagittal slice and the nasopharyngeal and oropharyngeal openings were outlined from the oblique-coronal slice using Osirix image processing software. The contours were then exported into Matlab to calculate the following variables of interest for biomechanics: contact length (length of contact between velum and posterior pharyngeal wall), contact distance (from the C1–C2 vertebral disc to the center of the velum contact), minimum distance between velum and posterior pharyngeal wall (PPW), oropharyngeal area and nasopharyngeal area.
RESULTS
Off-Resonance Correction
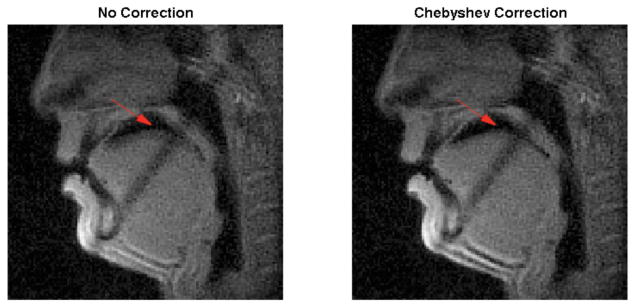
Figure 2 shows the effect of the Chebyshev approximation based off-resonance correction method in the reconstructed images. The air-tissue boundaries around the oropharygeal cavity and the velum indicated by the red arrow are greatly sharpened with the Chebyshev correction method (right) as compared with no off-resonance correction (left). This facilitates the boundary depiction and improves the accuracy of the quantitative measurements.
Figure 2.
Comparison of the reconstructed images without (left) and with (right) Chebyshev approximation based off-resonance correction. The boundaries of the oropharyngeal cavity and the velum indicted by the red arrow are greatly sharpened with the proposed method. No dark band correction is applied in this step.
Dynamic Velum Images and Analysis
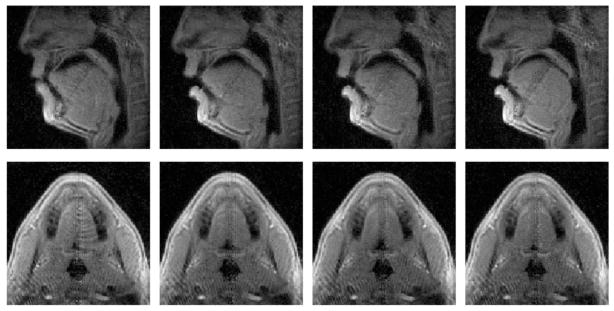
Figure 3 shows 4 frames of both slices indicating the velum movements when the volunteer pronounces a given sentence, reconstructed with the proposed method. The signal decay at the dark bands from both slices corresponding to the intersection area is compensated using the method introduced before. The aliasing due to undersampling is also suppressed to a minimum level with our proposed reconstruction method. Corresponding video files are included as Supporting Videos S1 and S2.
Figure 3.
Reconstructed images at 4 time points of both slices are shown indicating the different locations of the velum during pronunciations. Top row: mid-sagittal slice, bottom row: oblique coronal slice. The dark bands are largely eliminated in both slices, making the velum much more homogeneous. Aliasing artifacts are also minimized using the proposed reconstruction method with a 6x undersampling.
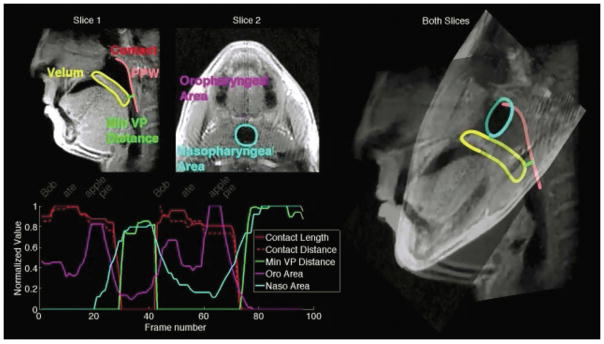
Figure 4 shows a snapshot of the measurement results of the variables of interest recorded while the sentence “Bob ate apple pie” is pronounced twice by the volunteer. The two slices are overlapped with their actual orientations (right) to more clearly show the oropharygeal and nasopharygeal cavities and boundaries of the velum and PPW. The dynamic curves of the variables of interest as indicated on the top left are plotted against the frame number on the bottom left with the time for each word well aligned.
Figure 4.
A snapshot of the measurement results of the variables of interest recorded while the sentence “Bob ate apple pie” is pronounced twice by the volunteer. Top left: locations of the variables of interest; bottom left: time-varying quantities of interest against the frame number with the time for each word well aligned. The right portion of the figure shows the 3-D orientation of the two slices.
DISCUSSION
We have presented preliminary results of dynamic velum movement analysis using dual-planar dynamic MRI with a customized spiral non-balanced SSFP sequence and combined parallel imaging and spatial and temporal compressed sensing spatial reconstruction techniques. The two slices were carefully chosen to gain more comprehensive information of the velum movements and were acquired simultaneously to eliminate errors from separate measurements.
The spiral sequence was optimized for this application. The number of spiral interleaves per frame is chosen to be 3 and spaced 120° apart to maximize the in-frame coverage of k-space. This spiral trajectory is also advantageous in terms of compressed sensing, since the aliasing is spread out across the entire k-space and noise coherence is minimized. The rotation angle through-frame was chosen with a bit-reversed method with a repetition period of 6. This supported a view sharing reconstruction method to provide an initial image series with minimum spatial aliasing in Eq. [2]. This also reduced the temporal coherence such that temporal blurring can be minimized with the proposed temporal parallel imaging method. The golden-ratio method, which rotates the in-frame interleaves by a golden angle for each new frame, was also implemented and tested. However, since it cannot cover the entire k-space with shared views, the spatial aliasing level of the output images with Eq. [2] was much higher compared with the bit-reversed method used in this study.
Although we achieved an in-plane spatial resolution of 1.2 mm2, this is calculated based on the extent of k-space and the FOV. The undersampled regions near the edge of k-space in the linear variable density design may cause the actual resolution, calculated using the full-width-half-maximum method, to be a bit lower. Other factors may also contribute to resolution loss, including the iterative reconstruction and temporal blurring. The spatial resolution was verified with a static phantom (results not shown) with an effective resolution at about 1.4 mm2.
In most model-based reconstruction methods, parameters need to be tuned to yield optimal results. We have three parameters in our final reconstruction, whose values were empirically chosen as λ = 0.01, α = 1e – 6, β = 1e – 6. A comprehensive study to compare images reconstructed at different weights was not performed; however, we did find the resulting images to be overly blocky when the spatial and temporal total variation weights α and β were larger than 1e – 5.
As is generally known, the spiral trajectory is much more sensitive to off-resonance effects than the Cartesian trajectory, in that off-resonance results in image blurring when using spirals, rather than geometric distortion. Many off-resonance correction methods have been developed for fully sampled spiral trajectories. In this study, we chose to use the Chebyshev approximation method due to its speed and robustness. We also extended this method to work with spatial and temporal parallel reconstruction techniques when an undersampled spiral trajectory is used. In fact, this extension is not limited to the reconstruction method introduced in this paper. With compressed sensing methods, sparsity in a specific domain is exploited to reduce aliasing. In contrast, the blurring happens due to the convolution of the original image with the point-spread function (PSF) of the spiral trajectory, which will have a wider main lobe when off-resonance is present. In practice, the convolution will generally not affect the sparsity level of the original image since the PSF is mostly local. Therefore, the resulting image from the compressed sensing reconstruction is the original image convolved with the PSF kernel and thus can be deblurred using various deblurring methods as if it were directly reconstructed from fully sampled k-space.
In traditional MRI sequences, slices are mostly parallel so that no intersection exists between two slices. In this application, due to the special requirements of the slice locations, the two slices will overlap within a band. This may be detrimental for post processing, as the dark band is precisely across the image features we are interested in. Here we developed a simple compensation method to address this problem. Since the ultimate goal of this study was to provide a tool to describe the velum movement, and not to pursue accurate contrast, we did not further evaluate the accuracy of this method as it relates to true image contrast.
In this study, the dynamic information of velum movements during speech is presented as time-varying quantities of each measurement of interest. The overall goal of this work is to harness this information to advance therapeutic techniques for the treatment of VPI and other speech disorders. No direct image-based metric was calculated to evaluate aliasing and temporal blurring; instead, we performed an evaluation of the effects of the image quality on the image segmentation that will be used to guide therapy. Feedback from the segmentation process showed that except for the frames with the fastest velum movement, the segmentation quality of all other frames was modest to very good, yielding accurate quantification overall. Future intra- and inter-observer studies will be performed to calculate errors in the quantification. Furthermore, the clinical application of each variable of interest to evaluating overall velum function is beyond the scope of this paper, as well as how each variable is specifically related to VPI. Further study in collaboration with biomechanics experts, speech therapists, and surgeons will apply the data from our imaging method to clinical practice. Furthermore, the utilization of the imaging method is not limited to VPI studies. The spatial and temporal resolution and coverage is chosen to satisfy the needs of accurately quantifying the velum movements. In many other applications, the requirements may be different, but the sequence parameters can be re-optimized accordingly for best results with few changes to the data acquisition and reconstruction method introduced in this paper.
In conclusion, a new imaging method was developed for dynamic velum movement analysis. An accelerated dynamic spiral non-balanced SSFP sequence and imaging protocol were implemented for simultaneous dual-planar coverage. The combined parallel imaging and spatial and temporal compressed sensing techniques and Chebyshev approximation based off-resonance correction method were developed to reduce aliasing from the undersampled k-space data and blurring from the spiral trajectory. Image post-processing methods were applied to reduce dark band due to slice crosstalk and achieve more quantitative information for biomechanics analysis. This study establishes an important advancement for studying speech disorders using dynamic MRI.
Supplementary Material
Supporting Figure S1. Flowchart of the proposed off-resonance correction algorithm to reduce spatial blurring. The off-resonance correction is performed after the model-based reconstruction.
Supporting Video S1. Video of the first slice from the data shown in Fig. 3. This video shows the velum movements when a volunteer pronounces a given sentence, acquired and reconstructed with the proposed method.
Supporting Video S2. Video of the second slice from the data shown in Fig. 3. This video shows the velum movements when a volunteer pronounces a given sentence, acquired and reconstructed with the proposed method.
Acknowledgments
NIH R21 EB022309, Siemens Medical Solutions
References
- 1.Pigott RW. An analysis of the strengths and weaknesses of encoscopic and radiological investigations of velopharyngeal incompetence based on a 20 year experience of simultaneous recording. Br J Plast Surg. 2002;55(1):32–4. doi: 10.1054/bjps.2001.3732. [DOI] [PubMed] [Google Scholar]
- 2.Sinclair SW, Davies DM, Bracka A. Comparative reliability of nasal pharyngoscopy and videofluorography in the assessment of velopharyngeal incompetence. Br J Plast Surg. 1982;35(2):113–7. doi: 10.1016/0007-1226(82)90146-1. [DOI] [PubMed] [Google Scholar]
- 3.Kuehn DP, Ettema SL, Goldwasser MS, Barkmeier JC, Wachtel JM. Magnetic resonance imaging in the evaluation of occult submucous cleft palate. Cleft Palate Craniofac J. 2001;38(5):421–31. doi: 10.1597/1545-1569_2001_038_0421_mriite_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 4.Serrurier A, Badin P. A three-dimensional articulatory model of the velum and nasopharyngeal wall based on MRI and CT data. J Acoust Soc Am. 2008;123(4):2335–455. doi: 10.1121/1.2875111. [DOI] [PubMed] [Google Scholar]
- 5.Lingala SG, Sutton BP, Miquel ME, Nayak KS. Recommendations for real-time speech MRI. J Magn Reson Imaging. 2016;43:28–44. doi: 10.1002/jmri.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton BP, Conway C, Bae Y, Brinegar C, Liang ZP, Kuehn DP. Dynamic imaging of speech and swallowing with MRI. Conf Proc IEEE Eng Med Biol Soc. 2009;2009(1):6651–4. doi: 10.1109/IEMBS.2009.5332869. [DOI] [PubMed] [Google Scholar]
- 7.Fu M, Christodoulou AG, Naber AT, Kuehn DP, Liang ZP, Sutton BP. High-frame-rate multislice speech imaging with sparse sampling of (k,t)-space. Proc. 20th Ann. Meeting of ISMRM; Melbourne, Austrialia. 2012; p. 12. [Google Scholar]
- 8.Kim YC, Narayanan SS, Nayak KS. Flexible retrospective selection of temporal resolution in real-time speech MRI using a golden-ratio spiral view order. Magn Reson Med. 2011;65(5):1365–71. doi: 10.1002/mrm.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niebergall A, Zhang S, Kunay E, Keydana G, Job M, Uecker M, Frahm J. Real-time MRI of speaking at a resolution of 33 ms: undersampled radial FLASH with nonlinear inverse reconstruction. Magn Reson Med. 2013;69(2):477–85. doi: 10.1002/mrm.24276. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Block KT, Frahm J. Magnetic resonance imaging in real time: Advances using radial FLASH. J Magn Reson Imaging. 2010;31(1):101–9. doi: 10.1002/jmri.21987. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Olthoff A, Frahm J. Real-time magnetic resonance imaging of normal swallowing. J Magn Reson Imaging. 2012;35(6):1372–9. doi: 10.1002/jmri.23591. [DOI] [PubMed] [Google Scholar]
- 12.Burdumy M, Traser L, Richter B, Echternach M, Korvink JG, Hennig J, Zaitsev M. Acceleration of MRI of the vocal tract provides additional insight into articulator modifications. J Magn Reson Imaging. 2015;42(4):925–35. doi: 10.1002/jmri.24857. [DOI] [PubMed] [Google Scholar]
- 13.Kim YC, Proctor MI, Narayanan SS, Nayak KS. Improved imaging of lingual articulation using real-time multislice MRI. J Magn Reson Imaging. 2012;35(4):943–8. doi: 10.1002/jmri.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lingala SG, Zhu Y, Kim YC, Toutios A, Narayanan S, Nayak KS. A fast and flexible MRI system for the study of dynamic vocal tract shaping. Magn Reson Med. 2017;77(1):112–25. doi: 10.1002/mrm.26090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hargreaves BA. Rapid gradient-echo imaging. J Magn Reson Imaging. 2012;36(6):1300–13. doi: 10.1002/jmri.23742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adalsteinsson E, Star-Lack J, Meyer CH, Spielman DM. Reduced spatial side lobes in chemical-shift imaging. Magn Reson Med. 1999;42(2):314–23. doi: 10.1002/(sici)1522-2594(199908)42:2<314::aid-mrm14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CM, Nishimura DG. Reduced aliasing artifacts using variable-density k-space sampling trajectories. Magn Reson Med. 2000;43(3):452–8. doi: 10.1002/(sici)1522-2594(200003)43:3<452::aid-mrm18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Pipe J, Ahunbay E, Menon P. Effects of interleaf order for spiral MRI of dynamic processes. Magn Reson Med. 1999;41(2):417–22. doi: 10.1002/(sici)1522-2594(199902)41:2<417::aid-mrm29>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–62. [PubMed] [Google Scholar]
- 20.Griswold MA, Jackob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–10. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 21.Lustig M, Pauly JM. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn Reson Med. 2010;64(2):457–71. doi: 10.1002/mrm.22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donoho DL. Compressed sensing. IEEE Trans Information Theory. 2006;52(4):1289–306. [Google Scholar]
- 23.Lustig M, Donoho DL, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaing. Magn Reson Med. 2007;58(6):1182–95. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 24.Adluru G, Awate SP, Tasdizen T, Whitaker RT, Dibella EV. Temporally constrained reconstruction of dynamic cardiac perfusion MRI. Magn Reson Med. 2007;57(6):1027–36. doi: 10.1002/mrm.21248. [DOI] [PubMed] [Google Scholar]
- 25.Feng X, Salerno M, Kramer CM, Meyer CH. Kalman filter techniques for accelerated Cartesian dynamic cardiac imaging. Magn Reson Med. 2013;69(5):1346–56. doi: 10.1002/mrm.24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Meyer CH. Semiautomatic off-resonance correction in spiral-imaging. Magn Reson Med. 2008;59(5):1212–9. doi: 10.1002/mrm.21599. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Sica CT, Meyer CH. Fast conjugate phase image reconstruction based on a Chebyshev approximation to correct for B0 field inhomogeneity and concomitant gradients. Magn Reson Med. 2008;60(5):1104–11. doi: 10.1002/mrm.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irarrazabal P, Meyer CH, Nishimura DG, Macovski A. Inhomogeneity correction using an estimated linear field map. Magn Reson Med. 1996;35(2):278–82. doi: 10.1002/mrm.1910350221. [DOI] [PubMed] [Google Scholar]
- 29.Ettema SL, Kuehn DP, Perlman AL, Alperin N. Magnetic resonance imaging of the levator veli palatine muscle during speech. Clef Palate Craniofac J. 2002;39(2):130–44. doi: 10.1597/1545-1569_2002_039_0130_mriotl_2.0.co_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1. Flowchart of the proposed off-resonance correction algorithm to reduce spatial blurring. The off-resonance correction is performed after the model-based reconstruction.
Supporting Video S1. Video of the first slice from the data shown in Fig. 3. This video shows the velum movements when a volunteer pronounces a given sentence, acquired and reconstructed with the proposed method.
Supporting Video S2. Video of the second slice from the data shown in Fig. 3. This video shows the velum movements when a volunteer pronounces a given sentence, acquired and reconstructed with the proposed method.