Abstract
Background
Current nodal staging for salivary gland cancer (SGC) is extrapolated from mucosal head and neck squamous cell carcinoma. However, given their unique biology and clinical behavior, it is possible that a SGC-specific nodal staging system would be more accurate.
Methods
Patients from the National Cancer Database with non-metastatic SGC of the head and neck diagnosed from 2004 to 2013 and undergoing surgical resection and neck dissection removing at least 10 lymph nodes (LN) were included. Multivariable models were constructed to assess the association between survival and nodal factors, including number of metastatic LN, extranodal extension, LN size, and lower LN involvement.
Results
Overall, 4,520 patients met inclusion criteria. Increasing number of metastatic LN was strongly associated with worse survival without plateau. The risk of death increased more rapidly up to 4 LN (HR=1.34, 95% confidence interval (CI) 1.27-1.41, P<0.001), and was more gradual for additional LN beyond 4 (HR=1.02, 95% CI: 1.01-1.03, P<0.001). LN size, extranodal extension, and lower LN involvement had no impact on survival when accounting for number of metastatic LN. We used recursive partitioning analysis to create a novel SGC nodal staging system: N0 = 0 LN+, N1 = 1-2 LN+, N2 = 3-21 LN+, and N3 = 22 or more LN+. This system exhibited greater concordance than the current American Joint Commission on Cancer (8th edition) system.
Conclusion
Quantitative nodal burden is an important determinant of survival in SGC. Utilization of this variable may improve SGC staging.
Condensed Abstract
Nodal classification for salivary gland cancer historically has been extrapolated from head and neck squamous cell carcinoma, a biologically distinct disease. Herein, using regression and recursive partitioning analysis, we show that number of positive lymph nodes can be used to create a nodal classification for salivary gland cancer that outperforms the current American Joint Committee on Cancer 8th edition system.
Keywords: salivary gland cancer, lymph nodes, staging, neck dissection
INTRODUCTION
Salivary gland cancers (SGC) are a rare, heterogeneous collection of malignancies arising from the major or minor salivary glands in the head and neck that are primarily managed surgically. In addition to grade and tumor stage, one of the primary factors associated with recurrence and survival in SGC is the presence of nodal metastases (1–4), which may show variation according to the site of the primary (1, 2, 5, 6). Current nodal staging systems for SGC are extrapolated from mucosal head and neck squamous cell carcinoma (HNSCC) (7). However, given that SGC has distinct biology, clinical behavior, and treatment paradigms in comparison to HNSCC, it is possible that nodal staging specific for patients with salivary malignancies could outperform current methodology.
The number of cervical lymph nodes containing metastases is emerging as a powerful predictor of outcome in head and neck cancer (8–11). In oral cavity cancers (8), larynx cancers (11), and hypopharynx cancers (11), the number of pathologically positive lymph nodes has been shown to strongly correlate with survival, representing a better metric of prognosis than classic nodal factors included in the American Joint Committee on Cancer (AJCC) 8th edition staging system such as lymph node size, laterality, and extranodal extension. In addition, the AJCC 8th edition nodal staging for p16-positive oropharyngeal cancer is now entirely based on the number of pathologic lymph nodes. However, less is known about the impact of quantitative nodal burden in SGC.
With this background, we sought to define a novel nodal staging system for SGC using data from patients with SGC undergoing surgical resection and neck dissection in the National Cancer Database (NCDB). We hypothesized that similar to other head and neck cancers, quantitative nodal metastatic burden is a central factor for predicting survival outcomes in SGC. We moreover assessed the comparative impact of a variety of other nodal factors including size, extranodal extension, and lower lymph node involvement.
METHODS
Data source
The NCDB is a hospital-based registry representing approximately 70% of all newly diagnosed cancer cases in the United States. It comprises data from more than 1,500 commission-accredited cancer programs (12). The NCDB is a registry maintained by the Commission on Cancer of the American College of Surgeons and the American Cancer Society. There are established criteria to certify the quality of the submitted data, as well as an application process to obtain the data. However, upon distribution of the data, the Commission on Cancer of the American College of Surgeons and the American Cancer Society are not responsible for the analysis and conclusions presented in this manuscript. All data in this study were abstracted from the NCDB, de-identified and investigated. This study was deemed exempt from review by the Cedars-Sinai Medical Center institutional review board.
Histologies Included
The histologies included in this study were based on the two most recent WHO SGC classification systems (13, 14). Included International Classification of Diseases O-3 histology codes were 8012, 8022, 8041, 8047, 8200, 8201, 8255, 8260, 8290, 8310, 8410, 8430, 8440, 8480, 8481, 8500, 8525, 8550, 8562, 8571, 8574, 8940, 8941, and 8980. We excluded all squamous cell carcinomas even if involving the major salivary glands, given these commonly represent nodal metastases from cutaneous head and neck sites.
Patients
All adult patients ≥18 years old diagnosed from 2004 to 2013 from the NCDB with invasive cancers of salivary histology arising from either the major salivary glands or other head and neck subsites who underwent surgical resection and neck dissection as their primary treatment modality were included (n = 34,959). The Consolidated Standards of Reporting Trials (CONSORT) describes the patients included in this analysis (Supplementary Figure 1). Exclusion criteria included patients with non-invasive histology (n = 63), patients with distant metastases at presentation or unknown data of distant metastases (n = 1,850), patients with unknown follow-up details (n = 3,536), patients with no surgery at the primary site (n = 186), missing pathological T-stage (n = 397) or pathological N stage (n = 12,364) information, any oncological therapy prior to surgery (n = 841), and unclear sequence of treatment (n = 265). Patients with adenosquamous histology (n = 163) were excluded as this represents a malignancy of the surface epithelium and not of the salivary glands (14).
In order to exclude biopsies or incidentally removed lymph nodes in the primary specimen, neck dissections yielding less than 10 LNs were excluded (n = 10,354). We also excluded patients without data on LN count (n = 420). This left 4,520 patients, who formed the study cohort. The top quintile of patients in terms of the number of cases treated at their treating facility were defined as receiving treatment at high-volume facilities, and all other patients were considered to have received treatment at lower-volume facilities.
Statistical analysis
Missing data patterns among grade, ENE, LN size, margins and LN involvement were assessed by the method proposed by Little, and were deemed not missing completely at random (15). Missing rates among the variables were 26.8% for grade, 26.5% for ENE, 13.7% for LN size, 5.7% for margins, and 5.1% for LN involvement. Missing data were imputed using multiple imputation using Fully Conditional Specifications implemented by the multiple imputation by chained equations (MICE) algorithm as described by Van Buuren and Groothuis-Oudshoorn and the predictive mean matching method as described by Rubin (16, 17).
The primary outcome was overall survival, as assessed from time of diagnosis to date of death or last follow-up. Baseline characteristics were compared with the Wilcoxon-Mann-Whitney test and 2-sample t tests for continuous variables and with the chi-square tests for categorical covariates. The median follow-up time was calculated with the reverse Kaplan-Meier method. Estimated survival functions were generated via the Kaplan-Meier method and compared with a log-rank test (18). Univariate and multivariable survival analyses were performed with Cox proportional hazards model (19). Variable selection was performed with backwards selection, optimizing for Akaike information criterion. The proportional hazards assumption was assessed using Schoenfeld residuals (20). The number of positive LN and number of LN examined were analyzed as continuous variables and modelled non-linearly with respect to overall survival using restricted cubic splines. The optimal number of knots was selected based on the Akaike information criterion. Knot locations were placed in default quantiles as described by Harrell. (21). For number of positive LN, 4 knots were placed at the 5th, 35th, 65th, and 95th percentiles corresponding to 1, 2, 6, and 37 positive nodes. For number of LN examined, 3 knots were placed at the 10th, 50th, and 90th percentiles, corresponding to 11, 26, and 52 LN. Change points were identified by fitting a piecewise linear regression model on the log relative hazard of number of positive LN and number of LN examined (22, 23).
Recursive partitioning analysis (RPA) with a conditional inference tree was used to develop a novel nodal staging system. The conditional inference tree was created using independent nodal predictors of mortality, and estimated by binary recursive partitioning in a conditional inference framework developed by Strasser and Weber (24–26). Performance of the RPA-derived nodal staging system was compared to the American Joint Commission on Cancer (AJCC) 8th edition staging system using c-indices in patients with determinable AJCC stage. Internal validation was performed using bootstrapping with 1000 replicates to correct for possible optimism in c-indices.
All statistical analyses were performed with R (version 3.4.0) with 2-sided tests and significance level of .05.
RESULTS
Patient cohort
Overall, 4,520 patients met inclusion criteria, including 2196 node-positive patients and 2324 node-negative patients (Supplementary Table 1). Median follow-up was 54.3 months. The mean number of LN examined was 28.1 (± SD 16.3), and the mean number of identified positive metastatic nodes was 4.2 (± SD 10.2). In total, 78.9% (N=1733) and 57.5% (N=1337) of node-positive and node-negative patients underwent adjuvant radiotherapy, respectively (P<0.001). Similarly, 30.4% (N=667) of node-positive and 5.3% (N=123) of node-negative patients received adjuvant chemotherapy (P<0.001). The proportion of patients with positive lymph nodes varied substantially by histology, with positive lymph nodes found in 75% of salivary duct carcinoma, 65% of adenocarcinoma, 55% of other carcinomas (including carcinoma ex-pleomorphic adenoma), 41% of mucoepidemoid carcinoma, 34% of adenoid cystic carcinoma, and 26% of acinic cell carcinoma neck dissection specimens, respectively.
Number of positive metastatic lymph nodes
In univariate analysis, increasing number of metastatic LN strongly predicted for worse overall survival (OS) (p<0.001) (Table 1). The estimated 5-year OS rates were 81.7%, 60.6%, 36.9%, 30.1%, and 13.9%, for those with 0, 1-2, 3-9, 10-19, and 20 or more metastatic LNs, respectively (Supplementary Figure 2A). A similar impact of number of metastatic LN was seen in N2b (Supplementary Figure 2B) subgroup. After adjusting for other clinical and demographic factors using multivariable Cox regression (Table 1), the number of positive metastatic LN remained strongly associated with overall survival (p<0.001). Using a 4-knot restricted cubic spline function, mortality risk escalated continuously with increasing number of metastatic nodes without plateau (Figure 1). Given the nonlinear relationship between mortality and the number of metastatic LN, a change point at 4 metastatic LN was identified. The hazard ratio per metastatic LN increased steeply up to 4 metastatic LN (HR 1.34; 95% CI 1.27-1.41; p<0.001). Beyond this, each additional metastatic LN increased the risk of death, though more slowly (HR 1.02; 95% CI 1.01-1.03; p<.001) (Table 2).
Table 1.
Univariate and multivariable predictors of overall survival for patients with salivary gland malignancies undergoing surgery and neck dissection using Cox regression. Final multivariable models were determined after stepwise backwards selection.
| Characteristics | Univariate Survival Analysis | Multivariable Survival Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR | 95%CI | p | HR | 95%CI | p | |||
| Number of Positive Metastatic Lymph Nodes* | – | – | – | <0.001 | – | – | – | <0.001 |
| Number of Lymph Nodes Examined* | – | – | – | 0.978 | – | – | – | 0.007 |
| Age | 1.039 | 1.036 | 1.043 | <0.001 | 1.026 | 1.021 | 1.032 | <0.001 |
| Sex | ||||||||
| Male | 1.000 | – | – | – | ‡ | |||
| Female | 0.612 | 0.551 | 0.680 | <0.001 | ||||
| Race | ||||||||
| White | 1.000 | – | – | – | 1.000 | – | – | – |
| Black | 0.843 | 0.707 | 1.006 | 0.058 | 1.091 | 0.911 | 1.308 | 0.344 |
| Other | 0.597 | 0.460 | 0.774 | <0.001 | 0.733 | 0.563 | 0.954 | 0.021 |
| Charlson-Deyo comorbidity index | ||||||||
| 0 | 1.000 | – | – | – | 1.000 | – | – | – |
| 1 | 1.408 | 1.232 | 1.608 | <0.001 | 1.131 | 0.986 | 1.296 | 0.078 |
| 2 | 2.077 | 1.667 | 2.589 | <0.001 | 1.711 | 1.363 | 2.146 | <0.001 |
| Facility Type | ||||||||
| Non Academic Center | 1.000 | – | – | – | ||||
| Academic Center | 0.822 | 0.744 | 0.908 | <0.001 | 0.864 | 0.778 | 0.959 | 0.006 |
| Facility Volume | ||||||||
| Low Volume | 1.000 | – | – | – | ‡ | |||
| High Volume | 0.772 | 0.698 | 0.853 | <0.001 | ||||
| Insurance | ||||||||
| Uninsured | 1.000 | – | – | – | 1.000 | – | – | – |
| Private | 0.779 | 0.570 | 1.067 | 0.119 | 0.675 | 0.491 | 0.929 | 0.016 |
| Medicaid | 1.012 | 0.696 | 1.473 | 0.948 | 0.952 | 0.652 | 1.389 | 0.797 |
| Medicare | 1.904 | 1.396 | 2.596 | <0.001 | 0.827 | 0.594 | 1.152 | 0.261 |
| Other/Unknown | 0.977 | 0.657 | 1.454 | 0.911 | 0.656 | 0.436 | 0.987 | 0.043 |
| Year of Diagnosis | 0.997 | 0.977 | 1.017 | 0.772 | ‡ | |||
| Anatomic Site | ||||||||
| Parotid Gland | 1.000 | – | – | – | ‡ | |||
| Submandibular or Sublingual Gland | 0.985 | 0.851 | 1.141 | 0.845 | ||||
| Oral Cavity | 0.660 | 0.538 | 0.810 | <0.001 | ||||
| Other Head and Neck Sites | 0.807 | 0.658 | 0.989 | 0.039 | ||||
| Histology | ||||||||
| Mucoepidermoid | ||||||||
| Carcinoma | 1.000 | – | – | – | 1.000 | – | – | – |
| Adenocarcinoma | 1.511 | 1.332 | 1.714 | <0.001 | 0.863 | 0.758 | 0.984 | 0.028 |
| Adenoid Cystic Carcinoma | 0.909 | 0.773 | 1.069 | 0.250 | 1.277 | 1.078 | 1.514 | 0.005 |
| Salivary Duct Carcinoma | 1.666 | 1.315 | 2.112 | <0.001 | 0.727 | 0.571 | 0.927 | 0.010 |
| Acinic Cell Carcinoma | 0.749 | 0.598 | 0.938 | 0.012 | 1.344 | 1.068 | 1.690 | 0.012 |
| Other Carcinoma, including | ||||||||
| Ex-Pleomorphic Adenoma | 1.529 | 1.273 | 1.837 | <0.001 | 0.901 | 0.746 | 1.087 | 0.274 |
| Grade† | ||||||||
| Low Grade | 1.000 | – | – | – | § | |||
| Intermediate Grade | 2.177 | 1.695 | 2.797 | <0.001 | ||||
| High Grade | 6.165 | 4.929 | 7.710 | <0.001 | ||||
| T-stage | ||||||||
| T1 | 1.000 | – | – | – | 1.000 | – | – | – |
| T2 | 2.193 | 1.814 | 2.651 | <0.001 | 1.615 | 1.331 | 1.960 | <0.001 |
| T3 | 3.524 | 2.936 | 4.229 | <0.001 | 2.039 | 1.687 | 2.465 | <0.001 |
| T4 | 4.626 | 3.877 | 5.519 | <0.001 | 2.647 | 2.199 | 3.187 | <0.001 |
| Metastatic Lymph Node Size† | ||||||||
| 0-3 cm | 1.000 | – | – | – | ‡ | |||
| 3.1-6 cm | 1.998 | 1.740 | 2.295 | <0.001 | ||||
| > 6 cm | 1.883 | 1.364 | 2.601 | <0.001 | ||||
| Lower Lymph Node Involvement† | ||||||||
| No | 1.000 | – | – | – | 1.000 | – | – | – |
| Yes | 3.434 | 3.066 | 3.846 | <0.001 | 1.134 | 0.978 | 1.316 | 0.096 |
| Contralateral (N2c) Lymph Node Involvement | ||||||||
| No | 1.000 | – | – | – | ‡ | |||
| Yes | 2.241 | 1.671 | 3.005 | <0.001 | ||||
| Extranodal Extension† | ‡ | |||||||
| pN0 or no ENE | 1.000 | – | – | – | ||||
| Positive ENE | 3.049 | 2.745 | 3.388 | <0.001 | ||||
| Margin† | ||||||||
| Negative Margin | 1.000 | – | – | – | 1.000 | – | – | – |
| Positive Margin | 1.827 | 1.651 | 2.022 | <0.001 | 1.261 | 1.134 | 1.402 | <0.001 |
| Postoperative Radiation | ||||||||
| No | 1.000 | – | – | – | § | |||
| Yes | 1.228 | 1.098 | 1.374 | <0.001 | ||||
| Postoperative Chemotherapy | ||||||||
| No | 1.000 | – | – | – | 1.000 | – | – | – |
| Yes | 1.950 | 1.734 | 2.193 | <0.001 | 1.161 | 1.017 | 1.324 | 0.027 |
No. of positive metastatic LNs was modeled with 4 knots at the 63rd, 71st, 83rd and 98th quantile (1, 2, 6, and 37 nodes) and No. of LNs examined was modeled with three knots at the 10th, 50th and 90th quantile (11, 26, and 52 nodes).
Missing data were imputed by multiple imputation.
Variables dropped out of the model.
Multivariable model adjusted for post-operative radiation and grade by stratification due to non-proportional hazards.
Figure 1.
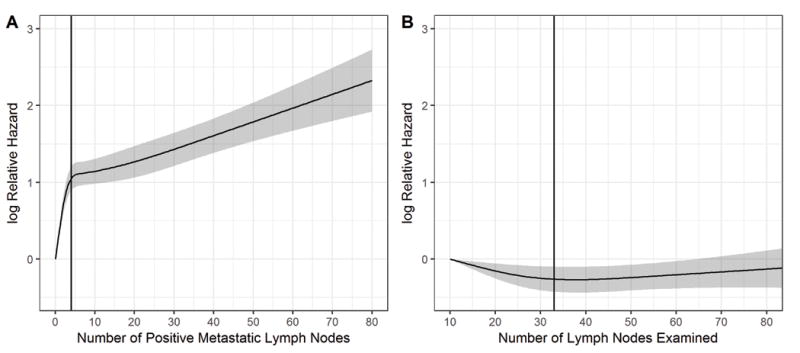
The adjusted hazard ratio of death as a non-linear function of A) number of positive lymph nodes, with 0 positive lymph nodes as a reference, and B) number of lymph nodes examined, with 10 lymph nodes examined as a reference, for patients with salivary gland cancer. The gray area represents the 95% confidence interval of the natural logarithm of the predicted hazard ratios. The black curve represents the smoothed restricted cubic spline plot of the natural logarithm of the predicted adjusted hazard ratio for survival versus number of lymph nodes. The black vertical lines represent the calculated change point of 4 positive lymph nodes and 33 lymph nodes examined, respectively, for the hazard of death as a function of lymph node number.
Table 2.
Summary of hazard ratios for number of positive lymph nodes and number of lymph nodes examined, stratified by changepoint.
| Characteristics | Univariate Survival Analysis | Multivariable Survival Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| HR | 95%CI | p | HR | 95%CI | p | |||
| Number of Positive Metastatic Lymph Nodes† | ||||||||
| ≤4 | 1.483 | 1.417 | 1.552 | <0.001 | 1.337 | 1.267 | 1.410 | <0.001 |
| >4 | 1.018 | 1.013 | 1.022 | <0.001 | 1.020 | 1.014 | 1.025 | <0.001 |
| Number of Lymph Nodes Examined‡ | ||||||||
| ≤33 | 1.012 | 1.003 | 1.021 | 0.009 | 0.988 | 0.979 | 0.998 | 0.017 |
| >33 | 1.014 | 1.008 | 1.019 | <0.001 | 1.003 | 0.996 | 1.010 | 0.354 |
Hazard ratio expressed as 1 unit increment
Multivariable model was stratified on grade and radiotherapy, and adjusted for age, comorbidity, facility type, insurance, histology, t-stage, margins, chemotherapy, and positive regional nodes with 4 knots at the 5th, 35th, 65th, and 95th quantile (1, 2, 6, and 37 nodes)
Multivariable model was stratified on grade and radiotherapy, and adjusted for age, comorbidity, facility type, insurance, histology, t-stage, margins, chemotherapy, and regional nodes examined with three knots at the 10th, 50th and 90th quantile (11, 26, and 52 nodes)
Number of lymph nodes examined
An increasing number of LN examined was associated with improved overall survival in multivariable analyses (p=0.007). As with number of metastatic LN, number of LN examined exhibited a non-linear relationship with mortality. A multivariable model with a 3-knot restricted cubic spline function identified a change point at 33 LN examined. Each additional node harvested (with baseline of 10 LN examined) decreased the risk of death continuously up to this change point (HR 0.988; 95% CI 0.979-0.998; p=0.017) (Figure 1). However, survival did not improve beyond 33 harvested LN (HR 1.003; 95% CI 0.996-1.010; p=0.35) (Table 2).
Metastatic lymph node features
After adjustment for covariates, including positive metastatic LN and number of total nodes examined, extranodal extension, lower neck (Level 4-5), contralateral LN involvement (N2c), and LN size had no significant impact on survival (Table 1).
Proposed nodal staging system
Recursive partitioning analysis (RPA) based on metastatic nodal number generated a novel nodal staging schema (Figure 2: N0 = 0 LN+, N1= 1-2 LN+, N2 = 3-21 LN+, N3 = 22 or more LN+). Kaplan-Meier estimates of the schema and AJCC 8th Edition system of the subset of patients with determinable AJCC 8th edition stage are illustrated in Figures 3A and 3B. The AJCC 8th Edition system N3b nodal category showed a hazard ratio (HR) of 2.732 (95% CI 2.156-3.460, P<0.001) versus N0 patients (Supplementary Table 2), in comparison to a HR of 6.381 (95% CI: 4.724-8.618, P<0.001) for the highest classification (N3 = 22 or more LN) of the proposed system. The optimism-corrected c-index for the proposed system showed improvement in predictive ability (0.797; 95% CI 0.782-0.808) over the AJCC 8th Edition system (0.793; 95% CI 0.777-0.805).
Figure 2.
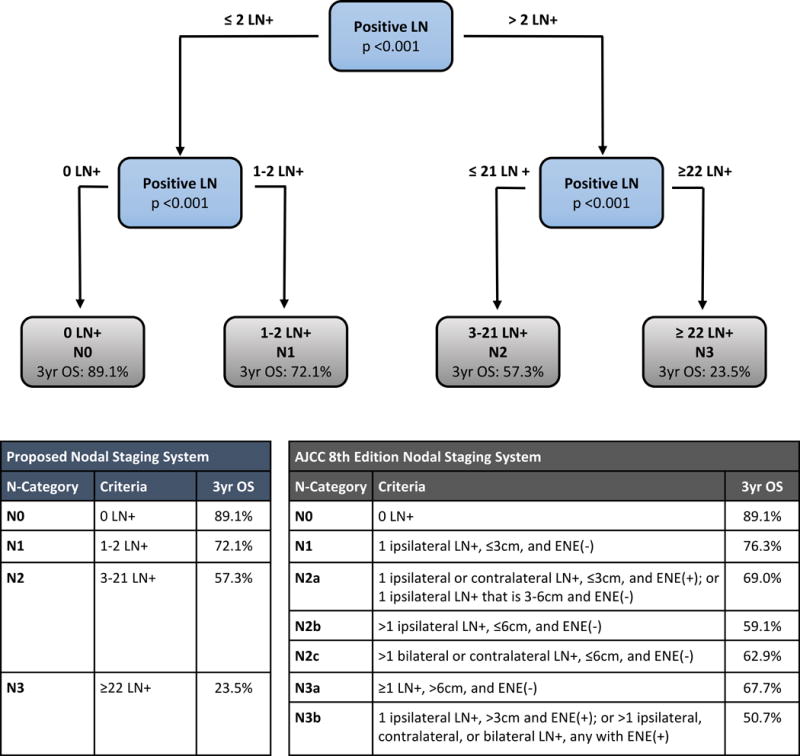
Defining a novel nodal staging system for salivary gland cancer in patients with determinable American Joint Commission on Cancer (AJCC) 8th edition stage, using recursive partitioning analysis based on number of positive lymph nodes.
Figure 3.
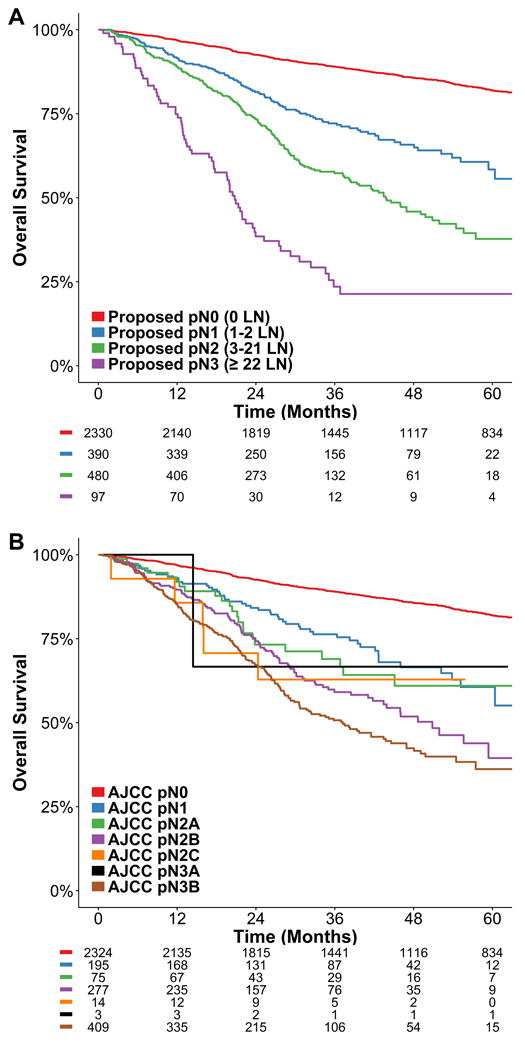
Kaplan-Meier estimates for the A) proposed and B) AJCC 8th edition N classification systems in salivary gland cancers.
Histologic subgroup analysis
Because SGC is a heterogeneous disease comprised of various histologies of different biologic and clinical behavior, we performed an analysis of the risk of mortality as a non-linear function of positive lymph node number, using a 4-knot restricted cubic spline function, for each of the 6 main histologic groups in this study (Supplementary Figure 3, Supplementary Table 3). Although there were some differences in the slope of these functions, including stronger risk of death per lymph node at lower lymph node numbers for less lymphotropic histologies such as adenoid cystic carcinoma and acinic cell carcinoma, increasing number of positive lymph nodes generally was associated with continuously increasing risk of death for all histologic subtypes. We found that our proposed nodal staging system produced excellent separation of survival curves across histologies (Supplementary Figure 4), although there were relatively few acinic cell carcinoma or adenoid cystic carcinoma patients classified as N3 in our system.
Proposed composite stage grouping
Pathologic AJCC T-stage and our proposed nodal classification were both strongly and independently associated with mortality in our study (Supplementary Table 2). Therefore, we designed a novel proposed composite stage grouping, analogous to the AJCC prognostic stage groups, by grouping patients based on the sum of their AJCC T-stage and proposed nodal classification (Stage I: T1N0, Stage II: T2N0/T1N1, Stage IIIA: T3N0/T2N1/T1N2, Stage IIIB: T3N1/T4N0/T2N2/T1N3, Stage IIIC: T4N1/T3N2/T2N3, Stage IVA: T4N2/T3N3, Stage IVB: T4N3). This produced seven relatively similarly sized stage groups with clearly distinct survival curves and incrementally increased mortality (Figure 4). 5-year overall survival was 92.1%, 85.1%, 68.4%, 56.5%, 37.3%, 25.6%, and 5.3% for proposed stages I through IVB, respectively.
Figure 4.
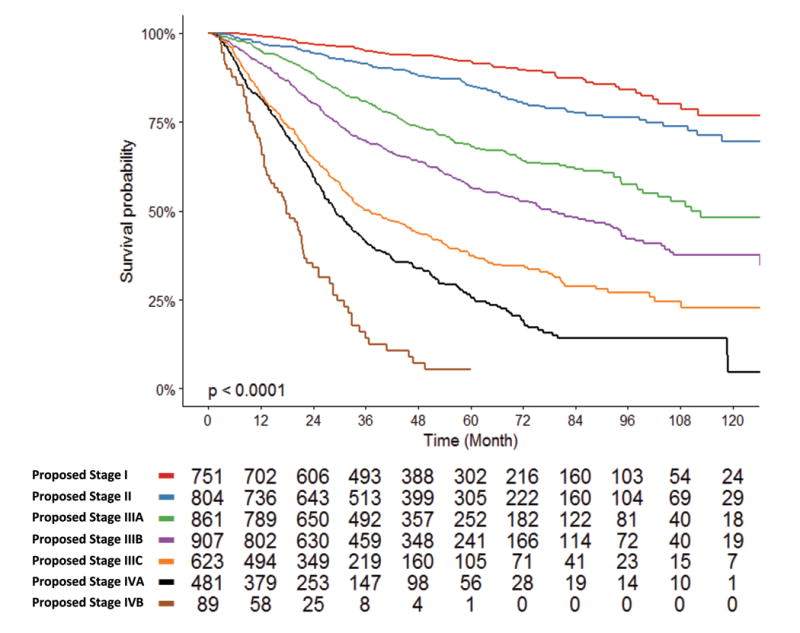
Kaplan-Meier estimates for the proposed composite stage grouping, based on the sum of the American Joint Commission on Cancer (AJCC) 8th edition pathologic tumor classification and the proposed nodal classification. Patients with equal sums of these two classifications were grouped together (Stage I: T1N0, Stage II: T2N0/T1N1, Stage IIIA: T3N0/T2N1/T1N2, Stage IIIB: T3N1/T4N0/T2N2/T1N3, Stage IIIC: T4N1/T3N2/T2N3, Stage IVA: T4N2/T3N3, Stage IVB: T4N3).
DISCUSSION
In the present study, we demonstrate that the absolute number of positive cervical lymph nodes is a critical predictor of SGC mortality. Each additional metastatic LN increased the risk of death without plateau. The impact was greatest up to 4 positive LN, with each positive LN conferring an added 34% increased risk of death, whereas each positive node beyond this increased relative mortality by 2% (Table 2). Other nodal features including size, contralaterality, extranodal extension, and lower neck involvement, had no impact on survival. The centrality of quantitative metastatic nodal burden in determining survival for SGC is consistent with its importance in other head and neck cancers (8, 11), and suggests that this variable should play a more prominent role in staging and, potentially, adjuvant treatment recommendations.
Using RPA, we designed a novel SGC-specific nodal staging system based on number of positive LN. The RPA-derived staging system exhibited greater concordance than the AJCC 8th edition system, although the magnitude of difference was relatively small. Nevertheless, the proposed nodal classification system has numerous advantages over the AJCC system. It is designed specifically for SGC, rather than extrapolated from head and neck squamous cell carcinoma, a biologically and clinically distinct entity. Thus, the proposed staging system ignores extranodal extension, a strong prognostic factor in head and neck squamous cell carcinoma included in the AJCC 8th edition SGC staging system that has no independent impact on survival in SGC in our study. In addition, our proposed system is relatively simple, given that it is based on a single variable and contains only 3 distinct categories for node positive patients (N1: 1-2 LN+, N2: 3-21 LN+, N3: 22 or more LN+). The proposed staging system also has a relatively even distribution of node-positive patients across stages, whereas certain AJCC 8th edition stages like N2c and N3a patients are very uncommon in SGC. Lastly, the proposed staging system identifies patients with 22 or more LN as an “ultra-high risk” group of patients with more than 6 times the risk of mortality as node-negative patients, which is nearly double the risk of any nodal classification group identified by the AJCC system. Given these advantages, it is possible that the proposed nodal staging system will not only improve prognostication, but more accurately identify patients that would benefit from adjuvant therapy or clinical trial enrollment.
Our results also support the importance of thorough neck dissection in a subset of SGC patients. Metastatic LNs portend a negative prognostic factor in terms of recurrence and long-term survival (27). Thus, therapeutic neck dissection remains an integral part of the management protocol to extirpate possible micrometastatic disease and occult metastases (3, 28, 29). We found that each additional LN harvested above 10 LN improved survival by 1.2% until plateauing beyond 33 LN (Table 2). Although this has a much smaller impact on survival than number of metastatic LN, its importance stems from the fact that it is largely a physician controllable factor. The benefit of increased LN yield is likely the result of multiple factors. This partly may be a function of the therapeutic effect of removing all deposits of microscopic disease. In addition, given that number of metastatic nodes can affect the decision for adjuvant radiation, it is possible that higher LN yields allow more accurate triaging of patients to adjuvant therapy. However, number of LN harvested is also likely a surrogate of quality, both for surgeons and pathologists. It is widely recognized that clinical volume and subspecialty expertise are important drivers of outcome in head and neck cancer (33, 34). However, it is important to note that our results imply only that in SGC patients requiring neck dissection, and more thorough dissection is better than a less thorough dissection, and do not support neck dissection in all unselected SGC patients. All patients in our study had at least 10 LN dissected, and therefore unquestionably represent a relatively high-risk subset of SGC, including a substantial proportion likely harboring clinically positive LNs.
There are multiple limitations of this study. Most significantly, this is a retrospective observational study. Selection bias may influence the administration of adjuvant therapies like radiation, chemotherapy, and hormonal therapy, as well as the type of resection and neck dissection performed. As noted above, we required all patients to have at least 10 LN dissected, and thus our results may not be applicable to low risk SGC where neck dissection in not required. Additionally, SGC is inherently heterogeneous, comprising numerous different histologies. Although we found that our nodal classification system was fairly accurate in each histologic subtype, it is possible that histology-specific SGC staging would outperform our system if sufficient patient numbers were available to develop this. It should also be noted that classifying SGC can be challenging, especially at lower-volume centers without subspecialized pathologists. Thus, it is likely that there is variability in both histological classification and grading across the approximately 1,500 facilities contributing data to the NCDB in comparison to what would be observed with a central pathologic review. NCDB also does not capture certain prognostic factors, like perineural invasion, that could influence patterns of care and survival. Moreover, several important variables were not available for all patients, including grade, extranodal extension, and margins. We used multiple imputation to account for this, but any methodology to account for missing data has limitations and the potential for bias. Lastly, the NCDB has no information on patterns of recurrence, so it is not clear whether the increased mortality risk conveyed by increasing numbers of pathologic lymph nodes is a result of regional recurrence, distant recurrence, or both. This would be an interesting topic for investigation in other large salivary cancer datasets. Despite these limitations, we believe the central findings of this study, that number of positive LN is strongly associated with survival and can improve nodal staging in SGC, are robust.
CONCLUSIONS
Quantitative metastatic LN burden is strongly coupled to mortality in SGC patients, with each additional metastatic LN conferring increased risk of death without plateau. Currently used staging parameters including LN size, contralaterality, and extranodal extension lack independent prognostic value when accounting for number of metastatic nodes. This information will ultimately help triage high-risk patients who may benefit from more aggressive adjuvant therapy.
Supplementary Material
Supplementary Figure 1. Consolidated standards of reporting trials (CONSORT) diagram detailing the study inclusion and exclusion criteria for the cohort analyzed.
Supplementary Figure 2. Kaplan-Meier estimates of overall survival in salivary gland cancers, stratified by number of positive cervical lymph nodes in A) all patients and B) patients with American Joint Commission on Cancer stage pN2b disease.
Supplementary Figure 3. The adjusted hazard ratio of death as a non-linear function of number of positive lymph nodes, with 0 positive lymph nodes as a reference, for patients with A) mucoepidermoid carcinoma, B) adenocarcinoma, C) adenoid cystic carcinoma, D) salivary duct carcinoma, E) acinic cell carcinoma, and F) other carcinomas, including carcinoma ex-pleomorphic adenoma. The gray area represents the 95% confidence interval of the natural logarithm of the predicted hazard ratios. The black curve represents the smoothed restricted cubic spline plot of the natural logarithm of the predicted adjusted hazard ratio for survival versus number of lymph nodes.
Supplementary Figure 4. Kaplan-Meier estimates for the proposed N classification system (N0= 0 LN+, N1= 1-2 LN+, N2= 3-21 LN+, N3= 22 or more LN+) in A) mucoepidermoid carcinoma, B) adenocarcinoma, C) adenoid cystic carcinoma, D) salivary duct carcinoma, E) acinic cell carcinoma, and F) other carcinomas, including carcinoma ex-pleomorphic adenoma.
Acknowledgments
FUNDING: This work was supported in part by the National Institutes of Health (grant No. R01 CA188480-01A1), National Center for Advancing Translational Sciences (grants No. UL1TR000124 and UL1TR001881-01).
Dr. Zumsteg is on the external advisory board for the Scripps Proton Therapy Center and has been a paid consultant for EMD Serono. Dr. Aro is supported by the Sigrid Jusélius Foundation, and the Finnish Otorhinolaryngology Research Foundation.
Footnotes
The authors declare no conflict of interest relevant to this study.
AUTHOR CONTRIBUTIONS:
Conceptualization: AH, ZZ
Data Curation: ML, ZZ
Formal Analysis: ML
Methodology: AH, ML, SK, MT, ZZ
Supervision: ZZ
Data Interpretation: All Authors
Writing: Original Draft: KA, AH, ZZ
Writing: Review and Editing: All Authors
References
- 1.Lloyd S, Yu JB, Ross DA, Wilson LD, Decker RH. A prognostic index for predicting lymph node metastasis in minor salivary gland cancer. Int J Radiat Oncol Biol Phys. 2010 Jan 1;76(1):169–75. doi: 10.1016/j.ijrobp.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong JG, Harrison LB, Thaler HT, Friedlander-Klar H, Fass DE, Zelefsky MJ, et al. The indications for elective treatment of the neck in cancer of the major salivary glands. Cancer. 1992 Feb 1;69(3):615–9. doi: 10.1002/1097-0142(19920201)69:3<615::aid-cncr2820690303>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Yoo SH, Roh JL, Kim SO, Cho KJ, Choi SH, Nam SY, et al. Patterns and treatment of neck metastases in patients with salivary gland cancers. J Surg Oncol. 2015 Jun;111(8):1000–6. doi: 10.1002/jso.23914. [DOI] [PubMed] [Google Scholar]
- 4.Ali S, Palmer FL, Yu C, DiLorenzo M, Shah JP, Kattan MW, et al. A predictive nomogram for recurrence of carcinoma of the major salivary glands. JAMA Otolaryngol Head Neck Surg. 2013 Jul;139(7):698–705. doi: 10.1001/jamaoto.2013.3347. [DOI] [PubMed] [Google Scholar]
- 5.Zeidan YH, Pekelis L, An Y, Holsinger FC, Kong CS, Chang DT, et al. Survival benefit for adjuvant radiation therapy in minor salivary gland cancers. Oral Oncol. 2015 May;51(5):438–45. doi: 10.1016/j.oraloncology.2015.02.096. [DOI] [PubMed] [Google Scholar]
- 6.Aro K, Tarkkanen J, Saat R, Saarilahti K, Makitie A, Atula T. Submandibular gland cancer: Specific features and treatment considerations. Head Neck. 2017 Oct 30; doi: 10.1002/hed.24981. [DOI] [PubMed] [Google Scholar]
- 7.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al., editors. AJCC cancer staging manual. 8th. 2017. [DOI] [PubMed] [Google Scholar]
- 8.Ho AS, Kim S, Tighiouart M, Gudino C, Mita A, Scher KS, et al. Metastatic lymph node burden and survival in oral cavity cancer. J Clin Oncol. 2017 Nov 1;35(31):3601–9. doi: 10.1200/JCO.2016.71.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts TJ, Colevas AD, Hara W, Holsinger FC, Oakley-Girvan I, Divi V. Number of positive nodes is superior to the lymph node ratio and american joint committee on cancer N staging for the prognosis of surgically treated head and neck squamous cell carcinomas. Cancer. 2016 May 1;122(9):1388–97. doi: 10.1002/cncr.29932. [DOI] [PubMed] [Google Scholar]
- 10.Divi V, Chen MM, Nussenbaum B, Rhoads KF, Sirjani DB, Holsinger FC, et al. Lymph node count from neck dissection predicts mortality in head and neck cancer. J Clin Oncol. 2016 Aug 1; doi: 10.1200/JCO.2016.67.3863. [DOI] [PubMed] [Google Scholar]
- 11.Ho AS, Kim S, Tighiouart M, Gudino C, Mita A, Scher KS, et al. Association of quantitative metastatic lymph node burden with survival in hypopharyngeal and laryngeal cancer. JAMA Oncol. 2017 Nov 30; doi: 10.1001/jamaoncol.2017.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steele GD, Jr, Osteen RT, Winchester DP, Murphy GP, Menck HR. Clinical highlights from the national cancer data base: 1994. CA Cancer J Clin. 1994 Mar-Apr;44(2):71–80. doi: 10.3322/canjclin.44.2.71. [DOI] [PubMed] [Google Scholar]
- 13.Barnes L, Eveson JW, Reichart P, Sidransky D, editors. tumours of the salivary glands. IARC Press; 2005. World health organization classification of tumours. [Google Scholar]
- 14.El-Naggar AK, Chan JKC, Gramdis JR, Takata T, Slootweg PJ, editors. tumours of the salivary glands. 4th. IARC Press; 2017. World health organization classification of tumours. [Google Scholar]
- 15.Little R. A test of missing completely at random for multivariable data with missing values. Journal of the American Statistical Association. 1988;83(404):1198–202. [Google Scholar]
- 16.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Statistical Methods in Medical Research. 2007;16(3):219–42. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 17.Rubin D. Statistical matching using file concatenation with adjusted weights and multiple imputations. Journal of Business & Economic Statistics. 1986;4(1):87–94. [Google Scholar]
- 18.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data wiley series in probability and mathematical statistics. New York: John Wiley & Sons, Inc; 1980. [Google Scholar]
- 19.Cox DR. Regression models and life tables. J Royal Stat Society. 1972;B34:187–220. [Google Scholar]
- 20.Grambsch P, Therneau T. Proportional hazards tests and diagnosgtics based on weighted residuals. Biometrika. 1994;81(3):515–26. [Google Scholar]
- 21.Harrell FE Jr, editor. Regression modeling strategies: With applications to linear models, logistic and ordinal regression, and survival analysis. Springer; 2015. [Google Scholar]
- 22.Muggeo V. Segmented: An R package to fit regression models with broken-line relationships. R News. 2008;8(1):20–5. [Google Scholar]
- 23.Muggeo VM. Estimating regression models with unknown break-points. Stat Med. 2003 Oct 15;22(19):3055–71. doi: 10.1002/sim.1545. [DOI] [PubMed] [Google Scholar]
- 24.Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: A conditional inference framework. Journal of Computational and Graphical Statistics. 2006;15(3):651–74. [Google Scholar]
- 25.Hothorn T, Zeileis A. Partykit: A modular toolkit for recursive partytioning in R. Journal of Machine Learning Research. 2015;16:3905–9. [Google Scholar]
- 26.Strasser H, Weber C. On the asymptotic theory of permutation statistics. Mathematical Methods of Statistics. 1999;8(2):220–50. [Google Scholar]
- 27.Ettl T, Gosau M, Brockhoff G, Schwarz-Furlan S, Agaimy A, Reichert TE, et al. Predictors of cervical lymph node metastasis in salivary gland cancer. Head Neck. 2014 Apr;36(4):517–23. doi: 10.1002/hed.23332. [DOI] [PubMed] [Google Scholar]
- 28.Lim CM, Gilbert M, Johnson JT, Kim S. Is level V neck dissection necessary in primary parotid cancer? Laryngoscope. 2015 Jan;125(1):118–21. doi: 10.1002/lary.24772. [DOI] [PubMed] [Google Scholar]
- 29.Ali S, Palmer FL, DiLorenzo M, Shah JP, Patel SG, Ganly I. Treatment of the neck in carcinoma of the parotid gland. Ann Surg Oncol. 2014 Sep;21(9):3042–8. doi: 10.1245/s10434-014-3681-y. [DOI] [PubMed] [Google Scholar]
- 30.Hong HR, Roh JL, Cho KJ, Choi SH, Nam SY, Kim SY. Prognostic value of lymph node density in high-grade salivary gland cancers. J Surg Oncol. 2015 May;111(6):784–9. doi: 10.1002/jso.23874. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, Hanai N, Hirakawa H, Nishikawa D, Hasegawa Y. Lymph node density is a prognostic factor in patients with major salivary gland carcinoma. Oncol Lett. 2015 Dec;10(6):3523–8. doi: 10.3892/ol.2015.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Fang Z, Dai T, Zhang C, Sun J, He Y. Higher positive lymph node ratio indicates poorer distant metastasis-free survival in adenoid cystic carcinoma patients with nodal involvement. J Craniomaxillofac Surg. 2015 Jul;43(6):751–7. doi: 10.1016/j.jcms.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 33.David JM, Ho AS, Luu M, Yoshida EJ, Kim S, Mita AC, et al. Treatment at high-volume facilities and academic centers is independently associated with improved survival in patients with locally advanced head and neck cancer. Cancer. 2017 Oct 15;123(20):3933–42. doi: 10.1002/cncr.30843. [DOI] [PubMed] [Google Scholar]
- 34.Boero IJ, Paravati AJ, Xu B, Cohen EE, Mell LK, Le QT, et al. Importance of radiation oncologist experience among patients with head-and-neck cancer treated with intensity-modulated radiation therapy. J Clin Oncol. 2016 Mar 1;34(7):684–90. doi: 10.1200/JCO.2015.63.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Consolidated standards of reporting trials (CONSORT) diagram detailing the study inclusion and exclusion criteria for the cohort analyzed.
Supplementary Figure 2. Kaplan-Meier estimates of overall survival in salivary gland cancers, stratified by number of positive cervical lymph nodes in A) all patients and B) patients with American Joint Commission on Cancer stage pN2b disease.
Supplementary Figure 3. The adjusted hazard ratio of death as a non-linear function of number of positive lymph nodes, with 0 positive lymph nodes as a reference, for patients with A) mucoepidermoid carcinoma, B) adenocarcinoma, C) adenoid cystic carcinoma, D) salivary duct carcinoma, E) acinic cell carcinoma, and F) other carcinomas, including carcinoma ex-pleomorphic adenoma. The gray area represents the 95% confidence interval of the natural logarithm of the predicted hazard ratios. The black curve represents the smoothed restricted cubic spline plot of the natural logarithm of the predicted adjusted hazard ratio for survival versus number of lymph nodes.
Supplementary Figure 4. Kaplan-Meier estimates for the proposed N classification system (N0= 0 LN+, N1= 1-2 LN+, N2= 3-21 LN+, N3= 22 or more LN+) in A) mucoepidermoid carcinoma, B) adenocarcinoma, C) adenoid cystic carcinoma, D) salivary duct carcinoma, E) acinic cell carcinoma, and F) other carcinomas, including carcinoma ex-pleomorphic adenoma.


