Abstract
BACKGROUND
Supportive parenting during childhood has been associated with many positive developmental outcomes for offspring in adulthood, including fewer health-risk behaviors. Little is known about the neural mechanisms underlying these associations.
METHODS
The present study followed rural African Americans (n = 91, 52% female) from late childhood (ages 11–13) to emerging adulthood (age 25). Parent-child communication was assessed at ages 11, 12, and 13. Functional magnetic resonance imaging was used at age 25 to measure resting state functional connectivity (rsFC) of the anterior salience network (ASN). Harmful alcohol use and emotional eating were also assessed at age 25. Structural equation modeling was used to test pathways from parent-child communication at ages 11–13 to harmful alcohol use and emotional eating at age 25, via rsFC of the ASN.
RESULTS
Greater parent-child communication across ages 11–13 forecast greater rsFC of the ASN at age 25, which, in turn, was associated with lower harmful alcohol use and emotional eating at age 25. Significant indirect effects through the ASN were present for both outcomes.
CONCLUSIONS
These findings indicate the importance of parenting in late childhood for adaptive behaviors and suggest a pathway via higher ASN coherence. This network was implicated in both harmful alcohol use and emotional eating, corroborating evidence of overlap in brain regions for dysregulated substance use and eating behaviors, and revealing divergent pathways. These findings support the value of prevention and intervention efforts targeting parenting skills in childhood toward fostering long-term, adaptive neurocognitive development.
Keywords: Parenting, Resting State Functional Connectivity, Salience Network, Emotional Eating, Harmful Alcohol Use, African Americans
Harmful alcohol use and emotional eating represent preventable but significant risk behaviors, given their potential to culminate in health problems such as alcohol dependence (1) and obesity (2). Moreover, accumulating evidence has conceptualized alcohol abuse and overeating as related behaviors that share the topographical feature of overconsumption, possess similar underlying neurocognitive reward pathways, and result in multifaceted adverse consequences (3, 4). Elucidating developmental processes that facilitate or deter overconsumption may help to improve understanding of the occurrence of these behaviors and inform prevention and intervention efforts intended to alleviate them. To date, however, limited research has addressed either the neural networks that underlie these adverse health behaviors or the psychosocial antecedents that contribute to their development.
The present study was designed to address these concerns by investigating childhood factors that contribute to neural processes that, in turn, are associated with health-risk behaviors. More specifically, this study examined the influence of parenting in late childhood on resting state functional connectivity (rsFC) of the anterior salience network (ASN) in emerging adulthood and two subsequent behavioral correlates of rsFC of the ASN in emerging adulthood, namely, harmful alcohol use and emotional eating. Study hypotheses were tested using a sample of rural African Americans who were followed from late childhood through emerging adulthood. This developmental stage represents a pivotal time for adverse health behaviors, particularly among African Americans living in the rural South; this population may be particularly vulnerable to these behaviors as they seek ways to cope with limited vocational and educational opportunities due to racial discrimination and community disadvantage (5).
A growing body of literature has demonstrated the effect of parenting on structural and functional features of brain development in children (6–9). For example, negative parent-child interactions during childhood have been found to influence brain connectivity in neural networks relevant to emotion regulation, threat detection, and reward anticipation (10). Similarly, during adolescence, a positive parent-child relationship as defined by parent-child communication behaviors (i.e., support, disclosure, and conflict) predicted ventral striatum activation to rewards over time (11). Conversely, hostile family relationships (i.e., high conflict, low cohesion) forecast increases over time in prefrontal cortex activation during a cognitive control task (12). However, little research has examined the ways in which parenting may influence functional connectivity, another key indicator of overall brain functioning. Although speculation has surrounded the effect of parenting on rsFC in adulthood (13), parental influences on integrative brain networks remain understudied, particularly with African American samples. Accordingly, the first aim of the current study was to investigate the influence of parenting in late childhood on rsFC in emerging adulthood.
Alterations in an individual’s rsFC can play an integral role in the formation of health risk behaviors and well-being, including substance use (14), maladaptive eating behaviors (15), and negative mood and depression (16). Moreover, given evidence suggesting neurobiological overlaps between addiction to substances and addiction to foods (17–19), similar brain networks may play prominent roles in both harmful alcohol use and emotional eating. One particular network that may contribute to both forms of overconsumption behavior is the anterior salience network (ASN), the central nodes of which are the bilateral dorsal anterior cingulate cortex and bilateral anterior insula (20). Prior work has demonstrated that the ASN functions as a neural mechanism involved in switching activation and deactivation patterns from the central executive network to the default mode network and vice versa (21). Therefore, a more coherent and synchronous ASN (i.e., greater rsFC) may allow more efficient switching between the central executive and default mode networks, thereby facilitating adaptive and regulated behaviors. Given the highly arousing nature of psychoactive substances and food as stimuli, behaviors related to substance use and emotional eating may be particularly relevant to the ASN, with greater connectivity of the ASN potentially associated with lower levels of harmful substance use and unhealthful eating behaviors. For instance, research on smoking has demonstrated reduced rsFC of the ASN in smokers compared with nonsmokers (22, 23). Studies have also found a negative correlation between rsFC of nodes within the ASN and severity of smoking in both smokers with schizophrenia and smokers matched as controls (24, 25). With respect to emotional eating, prior research has implicated disrupted resting-state connectivity in the ASN also as a key network associated with disordered eating (26).
The Present Study
The present longitudinal, multimethod study investigated the influence of parenting during three years in late childhood on rsFC of the ASN in emerging adulthood and, in turn, the contemporaneous association of rsFC of the ASN with harmful alcohol use and emotional eating. We tested the following hypotheses: (a) positive parent-child communication at ages 11–13 would be associated with higher rsFC of the ASN at age 25; (b) higher rsFC of the ASN at age 25 would be associated with less harmful alcohol use and less emotional eating at age 25; and (c) higher ASN connectivity at age 25 would have a significant indirect effect linking parent-child communication at ages 11–13 to less harmful alcohol use and less emotional eating at age 25. Thus, the current study not only addresses a potential etiology of health-risk behaviors but also specifies a functional neural marker that might indicate a pathway to those behaviors. Moreover, the use of a racially homogenous African American sample improves both internal validity and generalizability in this understudied population.
METHODS
Participants and Procedures
A total of 119 right-handed rural African Americans age 25 years were recruited from participants in the Strong African American Families Healthy Adult Project (27) to take part in a neuroimaging session. All participants were right handed and screened to exclude standard MRI research contraindications (e.g., claustrophobia, pregnancy, ferrous metal implants) prior to enrollment. A total of 23 participants were removed for motion (motion correction details available in online supplement) and an additional 5 participants were removed due to other technical problems during preprocessing (i.e., artifacts within the magnetic field), resulting in a final sample of 91 (52% women; mean age 24.92 years of age, SD = 0.58). Comparisons between these 91 participants and the 28 participants who were excluded revealed no significant differences on the parent-child communication, harmful alcohol use, and emotional eating variables (all p > .05). Information on participants’ substance use dependence and psychiatric status is available in supplemental material. The Institutional Review Board of the sponsoring research institution approved all study procedures, and all participants provided written informed consent.
Measures
Parent-Child Communication
Parent-child communication was measured via child report about the primary caregiver using a 9-item scale (28). This scale assessed the frequency of parent-child discussions, the extent of the child’s active involvement in them, and the extent of arguing that occurred. Previous research has supported the validity of this measure with youth at similar ages (29). In the current study, this measure was highly correlated with other parenting measures, including parental emotional support (r = .49), parent-child interactions (r = .49), and nurturant-involved parenting (r = .54). Self-reports were averaged across ages 11, 12, and 13. Example items include, “How often do you and your caregiver talk about your choice of friends?” (response options: 0 = “never” to 3 = “we talk about it a lot”), “When you and your caregiver talk about your choice of friends how does the conversation go?” (response options: 1 = “my caregiver usually does most of the talking and usually just tells me what to do or believe” to 3 = “we usually talk about it openly and we each share our sides of the issue”), and “When you and your caregiver talk about your choice of friends, how often do you end up arguing?” (response options: 0 = “never” to 4 = “always/nearly every time”). Similar questions were asked with respect to “school and school work” and “alcohol”. Scores were coded so that higher scores indicated more positive parent-child communication.
Harmful Alcohol Use
Harmful alcohol use was assessed at age 25 using a four-item subscale of the Alcohol Use Disorders Identification Test (AUDIT; 30). Items in this subscale address consumption that results in harm to the physical or mental health of the individual. Example items included, “How often during the last year have you been unable to remember what happened the night before because you had been drinking?” and “Have you or someone else been injured because of your drinking?” Answers ranged from 0 = “never” to 4 = “daily or almost daily” or 0 = “no” to 4 = “yes, during the last year.” Scores were coded so that higher scores indicated more harmful alcohol use.
Emotional Eating
Emotional eating was assessed at age 25 using an 11-item Emotional Eating Scale (31). The scale measures the strength of the desire to eat that anger, frustration, anxiety, and depression occasion. Answers ranged from 0 = “I have no desire to eat” to 4 = “I have a very strong desire to eat.” The items were coded so that higher scores indicated a stronger desire to eat. Psychometric analyses of this scale have indicated that higher scores are strongly correlated with higher frequency of binge eating (31). Notably, however, preliminary analyses indicated that emotional eating was not significantly correlated with body mass index (M = 29.06, SD = 8.66). This may be due to an artificial limit of body mass index in the current sample, as the practical limitations of MRI methodology disallow participation of individuals higher in body mass index. Therefore, body mass index was not further considered in subsequent analyses.
MRI Acquisition
MRI data were collected using a GE 16-channel Signa HDx 3.0 Tesla scanner at the University of Georgia Bio-Imaging Research Center. Whole-brain high-resolution structural images were acquired for anatomical reference using a high-resolution T1-weighted, fast-spoiled gradient echo scan (TR = 7.8ms; TE = 3.1ms; flip angle, 20°; FOV = 256×256mm; matrix = 256×256; 160 contiguous 1mm axial slices; voxel size = 1mm3). Whole-brain functional images were acquired using T2* echoplanar imaging with a single-shot gradient echo pulse sequence (TR = 2000 ms; TE = 25 ms; flip angle, 90°; FOV = 225×225mm; matrix = 64×64; 38 contiguous 3.5mm axial slices; voxel size = 3.5mm3). The resting state paradigm consisted of two 4-minute imaging runs of 120 brain volumes. Participants were instructed to keep their eyes open, look at a fixation cross, and allow their minds to wander freely.
Image Processing
Data preprocessing for fMRI was conducted using Analysis of Functional Neuroimages software (AFNI; 32). Functional datasets were despiked, slice time shift corrected, and aligned to T1 datasets before warping into Montreal Neurological Institute (MNI) standardized space. The first four volumes of each run were removed to allow the scanner to reach steady state. Volumes with greater than 25% of voxels identified as outliers or intervolume movement greater than 0.2mm along any axis were censored. Data were spatially smoothed using a 6mm full-width half-maximum Gaussian filter and masked to exclude voxels outside the brain. Bandpass filtering was applied to remove low and high frequency noise (.009 to .08 hz), and motion correction was accomplished by including the six standard (de-meaned) motion parameters and their temporal derivatives as regressors of no interest.
Functional Connectivity Analyses and Data Analysis Plan
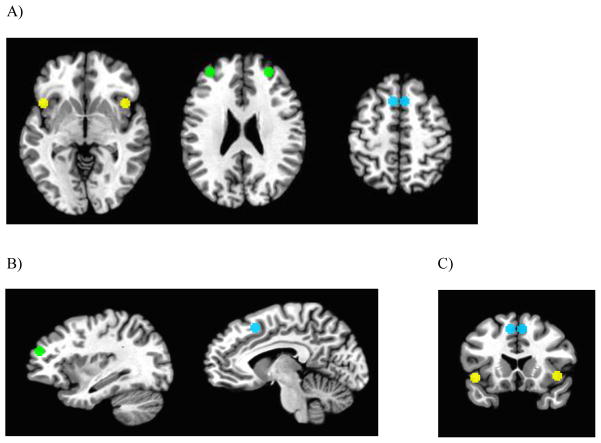
Regions of interest (ROIs) were selected based on the central coordinates of the ASN nodes reported by Shirer et al. (33). Specifically, for each ROI, a 5mm sphere was placed around the reported coordinates (MNI) at the center of mass. As shown in Figure 1, these regions included left dorsal anterior cingulate cortex (x = −6, y = 17, z = 47), right dorsal anterior cingulate cortex (x = 6, y = 17, z = 47), left anterior insula (x = −42, y = 14, z = −3), right anterior insula (x = 43, y = 15, z = −1), left middle frontal gyrus (x = −31, y = 47, z = 22), and right middle frontal gyrus (x = 28, y = 46, z = 26). Raw time series data for each voxel were de-meaned and converted to percent signal change scores to reduce variability between subjects and scanner sessions. ROI seed data were then calculated as the average percent signal change for all voxels contained in a given region. RSFC was quantified using the correlation of the average time series in each ROI with the average time series in all other ROIs in the network using Pearson’s r. Next, r values were converted to Z-scores using Fischer’s r to Z transformation. Finally, the Z-scores of all possible connections were averaged to compute a final value reflecting the connectivity of all possible nodes of the network (referred to as “ASN connectivity” below). The online supplement includes additional details on rationale and methodological details for the current approach.
Figure 1.
Regions of interest forming the salience network for resting state functional connectivity analyses. Panel A, axial views of anterior insula (z = −2), middle frontal gyrus (z = 23), and dorsal anterior cingulate cortex (z = 48), respectively. Panel B, sagittal views of middle frontal gyrus (x = 32) and dorsal anterior cingulate cortex (x = 7), respectively. Panel C, coronal view of dorsal anterior cingulate cortex and anterior insula (y = 15). Left = right in all images.
Preliminary analyses were conducted, including descriptive analyses to determine normality of distributions. Harmful alcohol use was skewed (3.15) and kurtotic (12.18); thus, it was log transformed. After this transformation, all variables fell within acceptable ranges. Next, hypotheses were tested using structural equation modeling (SEM) with Mplus version 7.4 software (34). Participant sex, child maltreatment as measured using the 28-item short form of the Childhood Trauma Questionnaire (35, 36), parental educational attainment, and parental substance use were included as control variables.1 Overall model fit indices were determined by χ2 value, degrees of freedom, corresponding p-value, Root Mean Square Error of Approximation (RMSEA), and Comparative Fit Index (CFI) (37, 38). For all tests of direct and indirect effects, bias-corrected bootstrapping was used (37) with 1,000 draws and corresponding 95% confidence intervals.
RESULTS
For all study variables, descriptive statistics and bivariate correlations are presented in Table 1. We began with a fully saturated model in which all possible pathways were included. Higher parent-child communication at ages 11–13 was significantly associated with higher ASN connectivity at age 25 (b = 0.013, SE = 0.006, p = .019). Furthermore, higher ASN connectivity was associated with less harmful alcohol use (b = −0.209, SE = 0.112, p = .061; 95% CI = [−0.440, −0.004]) and with emotional eating (b = −11.663, SE = 6.376, p = .067; 95% CI = [−25.311, −0.218]). Additionally, sex significantly predicted emotional eating (b = −6.216, SE = 2.087, p = .003) such that females indicated more frequent emotional eating, but sex did not predict ASN connectivity (b = 0.024, SE = 0.039, p = .542) or harmful alcohol use (b = 0.042, SE = 0.040, p = .293). Parental education, alcohol use, and marijuana use did not predict ASN connectivity, emotional eating, or harmful alcohol use. Childhood maltreatment was associated (at marginal significance level) with more harmful alcohol use, but was with ASN connectivity or emotional eating (tabulated results available from first author).
Table 1.
Descriptive statistics and bivariate correlations of study variables.
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | M (SD) | range |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. PC communication ages 11–13 | - | 11.381 (3.230) | 2.5–17 | |||||||
| 2. ASN connectivity age 25 | .251* | - | 0.478 (0.179) | 0.111–1.131 | ||||||
| 3. Harmful alcohol use age 25† | −.099 | −.220* | - | 0.101 (0.196) | 0–0.845 | |||||
| 4. Emotional eating age 25 | −.077 | v.218* | .051 | - | 13.593 (10.361) | 0–44 | ||||
| 5. Child sex (female, 0; male, 1) | .026 | .059 | .113 | −.314* | - | - | - | |||
| 6. Parental education | −.062 | −.084 | .046 | .033 | −.015 | - | 2.330 (0.746) | 1–4 | ||
| 7. Parent alcohol use (no use, 0; use, 1) | −.204 | −.041 | .062 | .071 | .043 | .011 | - | - | - | |
| 8. Parent marijuana use (no use, 0; use, 1) | −.151 | −.080 | −.002 | .031 | .094 | .136 | .306** | - | - | - |
| 9. Childhood maltreatment | −.053 | −.129 | .241* | −.055 | .108 | .063 | .027 | .128 | 31.725 (7.202) | 25–58 |
Log transformed. Ages shown in years. Parental education: 1, less than high school graduation; 2, high school graduation or GED; 3, some college or associate degree; 4, bachelor’s degree or higher.
p < .05,
p < .01.
ASN, anterior salience network; PC, parent-child.
In line with Little’s (39) recommendations, nonsignificant paths that were not central to the current study’s hypotheses—the paths from sex to ASN connectivity and sex to harmful alcohol use, the paths from parental education and substance use to ASN connectivity, harmful alcohol use, and emotional eating, and the paths from childhood maltreatment to ASN connectivity and emotional eating—were trimmed to obtain a final model with fit indices. The resulting model (as presented in Figure 2 with standardized results) showed excellent fit (χ2 = 4.41, df = 6, p = .62; RMSEA < .001; CFI = 1.00), and the pattern of results paralleled the fully saturated model. Higher parent-child communication at ages 11–13 was significantly associated with higher ASN connectivity (b = 0.014, SE = 0.006, p = .012). In turn, higher ASN connectivity was associated with less harmful alcohol use (b = −0.199, SE = 0.104, p = .056; 95% CI = [−0.409, −0.002], standardized p = .037) and less emotional eating at age 25 (b = −11.329, SE = 5.986, p = .058; 95% CI = [−24.171, −0.646], standardized p = .049). In addition, sex significantly predicted emotional eating (b = −6.334, SE = 2.031, p = .002). A significant indirect effect emerged from higher parent-child communication to less harmful alcohol use (b* = −.046, 95% CI [−.117, −.004]) and less emotional eating (b* = −.049, 95% CI [−.139, −.003]) via higher ASN connectivity.
Figure 2.
Structural equation model results of parent-child communication, anterior salience connectivity, harmful alcohol use, and emotional eating with sex statistically controlled. Standardized estimates are presented. PC, parent-child. Ages are shown in years. For clarity of presentation, the following paths are not included in the figure: Parent-child communication ages 11–13 ↔ sex (r = .03, p = .81), sex → emotional eating age 25 (b* = −.31, p < .01), and childhood maltreatment → harmful alcohol use (b* = .22, p < .10). * p < .05, ** p < .01.
Post-hoc Analyses
In order to examine the degree to which current results are specific to the ASN, additional analyses were conducted that investigated the potential intervening roles of other well-established resting state networks. A total of six other networks were examined. These included the default mode network, task active network (both adapted from Anderson et al. (40), central executive network (adapted from Shirer et al. (33), posterior salience network (adapted from Shirer et al. (33), emotion regulation network (adapted from Kohn et al. (41), and a fronto-striatal circuit associated with delay discounting (adapted from van den Bos et al. (42) and Power et al. (43). Results indicated that none of the networks significantly transmitted the effect of parent-child communication onto the adverse health behaviors under consideration (the indirect effects ranged from −.004 to .019 for harmful alcohol use and from −.045 to .008 for emotional eating; all indirect effects were non-significant). Additional analyses tested individual ROI-ROI pairs within the defined salience network; the intervening effect of different individual ROI-ROI pairs involving the insula-dACC connection yielded no significant mediation. Hence, post analyses further supported the unique effect of the ASN in the developmental pathway and the importance of considering the connectivity of the entire network (tabulated results available from the corresponding author).
DISCUSSION
In the present study, we sought to investigate a longitudinal pathway for health-risk behaviors in African American emerging adults. Specifically, analyses explored the effect of parenting at ages 11–13 on rsFC of the ASN at age 25 and its subsequent associations with harmful alcohol use and emotional eating at age 25. These adverse health behaviors confer risk for substantial health problems in adulthood, but may be used as coping strategies during the difficult transitions that accompany emerging adulthood. Thus, the present study helps to address health outcomes among rural African Americans by clarifying the familial antecedents and the neural mechanisms that may underlie these behaviors.
The first hypothesis, that greater parent-child communication at ages 11–13 would be associated with higher rsFC of the ASN at age 25, was supported. This finding is consistent with the broad literature indicating the importance of high-quality parenting for long-term brain development (6, 7), and prior work specifically examining the neural effects of parent-child communication during adolescence (11). The present study demonstrates that these effects apply not only to structural and functional aspects of the brain, but also to rsFC of the ASN. Parent-child communication may facilitate adaptive brain development by reducing levels of catecholamines and cortisol known to alter rsFC (6, 44). By promoting a more synchronous ASN at rest, parent-child communication in late childhood may foster efficient switching between the central executive and default mode network, which is critical for regulated attention to arousing stimuli (21).
The second and third hypotheses were also supported. Higher rsFC of the ASN at age 25 was associated with less harmful alcohol use and less emotional eating at age 25. Higher ASN connectivity at age 25 had a significant indirect effect linking parent-child communication at ages 11–13 to less emotional eating and less harmful alcohol use at age 25. Indeed, a more synchronous ASN showed concurrent positive consequences for health-risk behaviors, and higher quality parenting in childhood was associated with fewer health-risk behaviors via higher anterior salience rsFC. These findings were consistent with Janes et al. (24) which found weaker ASN connectivity during a smoking cue exposure was associated with a greater probability of lapse in smoking cessation treatment.
In contrast to prior research that focused only on coupling of the insula and dorsal anterior cingulate cortex (45), the present study also included the middle frontal gyrus. The middle frontal gyrus, at the coordinates included in the current study, has demonstrated associations with inhibition across multiple tasks and is associated with cognitive control (46). Thus, this region may act as a conduit between the anterior salience and central executive networks and, as a result, more synchrony among the ROIs in the present study may imply an ASN biased towards switching to the central executive network and away from the default network, which may result in behavior characterized by more cognitive control (i.e., less harmful alcohol use and less emotional eating). Taken together, the data indicate that parenting in childhood forecasts regulation of health behaviors in emerging adulthood via higher rsFC of the ASN.
Another interesting feature of the current findings is the lack of correlation between harmful alcohol use and emotional eating. This indicates multifinality (47). That is, harmful alcohol use and emotional eating develop along divergent trajectories rooted in the same pathways of childhood parenting and ASN rsFC. Although it is not surprising to see overlapping brain regions underlying substance use and disordered eating (18), with alcohol use and dysregulated eating, in particular, following divergent trajectories (48), questions arise concerning why an individual with low ASN rsFC migrates down one path versus the other. One possible explanation may be differences in the level of stimulation an individual wants when self-soothing with consumable products. Although both harmful alcohol use and emotional eating can be considered coping behaviors, long-standing research has demonstrated a positive association between sensation-seeking and drinking (49, 50). Recent research, however, has found a negative association between sensation-seeking and compulsive eating (51). Alternatively, environmental factors may play a role, such as a heavily drinking social niche that provides alcohol and normalizes its use as a self-coping behavior.
LIMITATIONS AND CONCLUSIONS
Limitations of the current study must also be noted. First, the sample intentionally included only African Americans as an understudied population that is disproportionately at risk. The homogenous nature of this sample may limit generalizability beyond rural African Americans; however, this characteristic may also restrict potential confounds. Second, the study design limits conclusions regarding causality. In particular, measurement of the rsFC of the ASN and the outcomes of harmful alcohol use and emotional eating around the same time point prevent strict inferences about directionality. Power considerations also precluded a more exhaustive list of control variables. The current approach, however, is consistent with control variables included in other models testing neural pathways linking childhood experiences and later developmental outcomes (52). Although the current results are promising, future research with larger samples is needed that includes a larger set of predictor variables. Furthermore, a reliance on self-report measures (parent-child communication, harmful alcohol use, and emotional eating) may introduce a degree of method bias. The loss of 28 subjects due to fMRI processing may also be a limitation. However, due to the sensitivity of resting state data, it is not unexpected. Finally, functional connectivity of the ASN was only assessed using resting state data and not also during a task.
Despite these limitations, the current results illuminate pathways for two forms of adverse health-risk behaviors in an at-risk population. Harmful alcohol use and emotional eating may indeed be coping strategies for African Americans facing strenuous circumstances as they navigate emerging adulthood in an environment abounding with risk factors and the potential for adverse outcomes. The present study demonstrates that parenting in childhood may act as a protective factor against these health-risk behaviors by influencing rsFC of the ASN which, in turn, might suppress harmful alcohol use and emotional eating. Furthermore, study findings support the role of prevention efforts design to improve parenting skills, given the centrality of parenting to a host of lifelong outcomes.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institute on Drug Abuse awarded to Gene Brody (P30 DA027827) and by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development awarded to Gene Brody (R01 HD030588). We thank the University of Georgia’s Center for Family Research and Clinical Neuroscience Laboratory for data collection, analyses, and management.
Footnotes
We also examined the bivariate association between other potential confounds (e.g., SES risk, intervention status, current stress) and all dependent measures in the study. No significant effects were detected, and hence these variables were not included for improved model parsimony and statistical power.
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher J. Holmes, Center for Family Research, University of Georgia.
Allen W. Barton, Center for Family Research, University of Georgia.
James MacKillop, Peter Boris Centre for Addictions Research, McMaster University.
Adriana Galván, Department of Psychology, University of California, Los Angeles.
Max M. Owens, Department of Psychology, University of Georgia.
Michael J. McCormick, Department of Psychology, Auburn University.
Tianyi Yu, Center for Family Research, University of Georgia.
Steven R. H. Beach, Center for Family Research, University of Georgia.
Gene H. Brody, Center for Family Research, University of Georgia.
Lawrence H. Sweet, Department of Psychology, University of Georgia.
References
- 1.Gmel G, Rehm J. Harmful alcohol use. Alcohol Res Health. 2003;27:52–62. [PMC free article] [PubMed] [Google Scholar]
- 2.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND, Wang G-J, Fowler JS, Tomasi D, Baler RD. Food and drug reward: Overlapping circuits in human obesity and addiction. In: Carter CS, Dalley JW, editors. Brain Imaging in Behavioral Neuroscience. Heidelberg, Germany: Springer Science+Business Media; 2012. pp. 1–24. [DOI] [PubMed] [Google Scholar]
- 4.Avena NM, Bocarsly ME, Hoebel BG, Gold MS. Overlaps in the nosology of substance abuse and overeating: The translational implications of “food addiction”. Curr Drug Abuse Rev. 2011;4:133–139. doi: 10.2174/1874473711104030133. [DOI] [PubMed] [Google Scholar]
- 5.Arnett JJ, Brody GH. A fraught passage: The identity challenges of African American emerging adults. Hum Dev. 2008;51:291–293. [Google Scholar]
- 6.Belsky J, de Haan M. Annual research review: Parenting and children’s brain development: The end of the beginning. J Child Psychol Psychiatry. 2011;52:409–428. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 7.Wickrama KAS, O’Neal CW, Holmes C. Towards a heuristic research model linking early socioeconomic adversity and youth cumulative disease risk: An integrative review. Adolesc Res Rev (in press) [Google Scholar]
- 8.Luby JL, Belden A, Botteron KN, Marrus N, Harms MP, Babb C, et al. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167:1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brody GH, Gray JC, Yu T, Barton AW, Beach SRH, Galván A, et al. Protective prevention effects on the association of poverty with brain development. JAMA Pediatr. 2017;171:46–52. doi: 10.1001/jamapediatrics.2016.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17:652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- 11.Qu Y, Fuligni AJ, Galván A, Telzer EH. Buffering effect of positive parent–child relationships on adolescent risk taking: A longitudinal neuroimaging investigation. Dev Cogn Neurosci. 2015;15:26–34. doi: 10.1016/j.dcn.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormick EM, Qu Y, Telzer EH. Adolescent neurodevelopment of cognitive control and risk-taking in negative family contexts. NeuroImage. 2016;124(Part A):989–996. doi: 10.1016/j.neuroimage.2015.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sripada RK, Swain JE, Evans GW, Welsh RC, Liberzon I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacol. 2014;39:2244–2251. doi: 10.1038/npp.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baek K, Morris LS, Kundu P, Voon V. Disrupted resting-state brain network properties in obesity: Decreased global and putaminal cortico-striatal network efficiency. Psychol Med. 2017;47:585–596. doi: 10.1017/S0033291716002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barch DM, Pagliaccio D, Belden AC, Harms MP, Gaffrey MS, Sylvester CM, et al. Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school-age depression. Am J Psychiatry. 2016;6:625–634. doi: 10.1176/appi.ajp.2015.15081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomasi D, Volkow ND. Striatocortical pathway dysfunction in addiction and obesity: Differences and similarities. Crit Rev Biochem Mol Biol. 2013;48:1–19. doi: 10.3109/10409238.2012.735642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: Neurobiological overlaps. Obes Rev. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomasi D, Wang G-J, Wang R, Caparelli EC, Logan J, Volkow ND. Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: Association to striatal D2/D3 receptors. Hum Brain Mapp. 2015;36:120–136. doi: 10.1002/hbm.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neuosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Yuan K, Guan Y, Cheng J, Bi Y, Shi S, et al. The implication of salience network abnormalities in young male adult smokers. Brain Imaging Behav. 2017;11:943–953. doi: 10.1007/s11682-016-9568-8. [DOI] [PubMed] [Google Scholar]
- 23.Bi Y, Yuan K, Guan Y, Cheng J, Zhang Y, Li Y, et al. Altered resting state functional connectivity of anterior insula in young smokers. Brain Imaging Behav. 2017;11:155–165. doi: 10.1007/s11682-016-9511-z. [DOI] [PubMed] [Google Scholar]
- 24.Janes AC, Pizzagalli DA, Richardt S, Frederick BdB, Chuzi S, Pachas G, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran LV, Sampath H, Stein EA, Hong LE. Insular and anterior cingulate circuits in smokers with schizophrenia. Schizophr Res. 2012;142:223–229. doi: 10.1016/j.schres.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steward T, Menchón JM, Jiménez-Murcia S, Soriano-Mas C, Fernández-Aranda F. Neural network alterations across eating disorders: A narrative review of fMRI studies. Curr Neuropharmacol. 2017:15. doi: 10.2174/1570159X15666171017111532. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brody GH, Yu T, Chen Y-f, Kogan SM, Evans GW, Beach SRH, et al. Cumulative socioeconomic status risk, allostatic load, and adjustment: A prospective latent profile analysis with contextual and genetic protective factors. Dev Psychol. 2013;49:913–927. doi: 10.1037/a0028847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brody GH, Flor DL, Hollett-Wright N, McCoy JK. Children’s development of alcohol use norms: Contributions of parent and sibling norms, children’s temperaments, and parent-child discussions. J Fam Psychol. 1998;12:209–219. [Google Scholar]
- 29.Brody GH, Flor DL, Hollett-Wright N, McCoy JK. Children’s development of alcohol use norms: Contributions of parent and sibling norms, children’s temperaments, and parent-child discussions. J Fam Psychol. 1998;12:209–219. [Google Scholar]
- 30.Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 31.Arnow B, Kenardy J, Agras WS. The Emotional Eating Scale: The development of a measure to assess coping with negative affect by eating. Int J Eat Disord. 1995;18:79–90. doi: 10.1002/1098-108x(199507)18:1<79::aid-eat2260180109>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 32.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 33.Shirer WR, Ryali S, Rykhlevskaia EI, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2015. [Google Scholar]
- 35.Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 36.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 37.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 38.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Newbury Park, CA: Sage; 1993. [Google Scholar]
- 39.Little TD. Longitudinal Structural Equation Modeling. New York, NY: Guilford Press; 2013. [Google Scholar]
- 40.Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. Connectivity gradients between the default mode and attention control networks. Brain Connect. 2011;1:147–157. doi: 10.1089/brain.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation — An ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM. Adolescent impatience decreases with increased frontostriatal connectivity. Proc Natl Acad Sci U S A. 2015;112:E3765–E3774. doi: 10.1073/pnas.1423095112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janes AC, Farmer S, Peechatka AL, Frederick BdB, Lukas SE. Insula-dorsal anterior cingulate cortex coupling is associated with enhanced brain reactivity to smoking cues. Neuropsychopharmacology. 2015;40:1561–1568. doi: 10.1038/npp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wager TD, Sylvester C-YC, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. NeuroImage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 47.Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Dev Psychopathol. 1996;8:597–600. [Google Scholar]
- 48.Racine SE, Martin SJ. Exploring divergent trajectories: Disorder-specific moderators of the association between negative urgency and dysregulated eating. Appetite. 2016;103:45–53. doi: 10.1016/j.appet.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 49.Stojek MM, Fischer S, Murphy CM, MacKillop J. The role of impulsivity traits and delayed reward discounting in dysregulated eating and drinking among heavy drinkers. Appetite. 2014;80:81–88. doi: 10.1016/j.appet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norbury A, Husain M. Sensation-seeking: Dopaminergic modulation and risk for psychopathology. Behav Brain Res. 2015;288:79–93. doi: 10.1016/j.bbr.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 51.VanderBroek-Stice L, Stojek MK, Beach SRH, van Dellen MR, MacKillop J. Multidimensional assessment of impulsivity in relation to obesity and food addiction. Appetite. 2017;112:59–68. doi: 10.1016/j.appet.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartholomeusz CF, Whittle SL, Pilioussis E, Allott K, Rice S, Schäfer MR, et al. Relationship between amygdala volume and emotion recognition in adolescents at ultra-high risk for psychosis. Psychiatry Res Neuroimaging. 2014;224:159–167. doi: 10.1016/j.pscychresns.2014.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.