Abstract
INTRODUCTION
The ability of Alzheimer disease (AD) cerebrospinal fluid (CSF) biomarkers [amyloid beta peptide 1–42 (Aβ42), total tau (t-tau) and phosphorylated tau (p-tau)] to discriminate AD from related disorders is limited. Biomarkers for other concomitant pathologies [e.g., CSF α-synuclein for Lewy body (LB) pathology] may be needed to further improve the differential diagnosis.
METHODS
CSF total α-synuclein, phosphorylated α-synuclein (pS129), and AD CSF biomarkers were evaluated with Luminex immunoassays in 367 participants, followed by validation in 74 different, neuropathologically-confirmed cases.
RESULTS
CSF total α-synuclein, when combined with Aβ42 and either t-tau or p-tau, improved the differential diagnosis of AD vs frontotemporal dementia, LB disorders, or other neurological disorders. The diagnostic accuracy of the combined models attained clinical relevance (Area Under Curve ~0.9) and was largely validated in neuropathologically-confirmed cases.
CONCLUSIONS
Combining CSF biomarkers representing AD and LB pathologies may have clinical value in the differential diagnosis of AD.
Keywords: Alzheimer disease, Differential diagnosis, Biomarkers, Cerebrospinal fluid, α-Synuclein
1. Introduction
Investigations using biochemical measures in cerebrospinal fluid (CSF) as Alzheimer disease (AD) biomarkers have shown great promise, and such CSF biomarkers have been incorporated into recent guidelines for informed diagnosis of AD [1]. Specifically, CSF markers of core AD pathology [i.e., amyloid beta (Aβ) peptide 1–42 (Aβ42) reflecting Aβ in plaque burden, and total tau (t-tau) and phosphorylated tau (p-tau) for assessing neurofibrillary tangles in the brain] provide both high sensitivity and specificity (80% or above) in differentiating patients with AD or mild cognitive impairment (MCI; prodromal AD) from healthy controls (HC) [2–4]. However, the diagnostic accuracy of these CSF biomarkers in the differential diagnosis of AD and other dementias is limited (40–80% sensitivity and specificity) due to substantial overlap in the CSF levels of these proteins [4–9]. A recent large-scale international multicenter study [5] suggested that the limited utility of these core CSF biomarkers to discriminate AD from a variety of related disorders could be due to overlap in the underlying primary pathologies, and introduction of additional CSF biomarkers reflecting other types of pathologies could be of value to optimize the differential diagnosis [4, 5], though reliance on clinical diagnoses might underestimate the accuracy of CSF biomarkers [10].
Among the concomitant non-AD type pathologies in AD, α-synuclein (α-syn)-positive Lewy bodies (LBs), the pathological hallmark of another family of neurodegenerative diseases including Parkinson disease (PD) and dementia with LBs (DLB), can be observed in up to 50% of familial and sporadic AD patients at autopsy [11–13]. We have reported that CSF total α-syn and phosphorylated α-syn at Ser129 (pS129) help differentiate PD from AD and other related neurodegenerative diseases [14–16]. More recently, we also found that CSF total α-syn improved the diagnostic and prognostic performance of CSF Aβ42 and tau in AD [17, 18]. In this study, to test whether inclusion of CSF α-syn that represents brain LB pathology could improve the differential diagnosis of AD and other dementias, we further evaluated the utility of CSF total α-syn and pS129 in the differential diagnosis in a relatively large clinical cohort, followed by validating our findings in a separate cohort of neuropathologically-confirmed cases.
2. Methods
2.1 Subjects
Two cohorts of research participants were recruited at the AD Core Center (ADCC), the Penn Memory Center (PMC), the Frontotemporal Degeneration (FTD) Center, the Amyotrophic Lateral Sclerosis (ALS) Center, the Parkinson disease and Movement Disorder Clinic (PD&MDC) and the Penn Udall Center for Parkinson’s Research at the University of Pennsylvania (UPenn) [19]. The clinical or discovery cohort (n=540) of clinically diagnosed participants included 165 AD, 105 MCI, 70 FTD [including 60 behavioral variant FTD (bv-FTD) and 10 corticobasal syndrome (CBS)], 79 LB disorders [LBD; including 16 DLB and 63 PD/PD with dementia (PDD)], 41 ALS, 11 progressive supranuclear palsy (PSP), and 69 HC (see Table 1 and Supplementary Table 1). The validation cohort contained 102 neuropathologically-confirmed cases, including 40 AD, 23 frontotemporal lobar degeneration with and without AD (FTLD; 17 FTLD, and 6 FTLD-AD), 30 PD/LB-related pathology with and without AD (LRP) (3 PD, 4 PD-AD, 21 LRP-AD, and 2 LRP-TDP), and 6 ALS (see below, Table 2, and Supplementary Table 2 for more details; note that 3 HC cases with an unremarkable burden of any significant brain pathology were not included in the analyses in the current study due to the small case number). The clinical diagnoses were made applying clinical diagnostic criteria for AD [1], bv-FTD [20], CBS [21], primary progressive aphasia [22], DLB [23], PD/PDD [24, 25], ALS [26], PSP [27], and HC as previously reported [19, 28, 29]. For the purposes of this study, patients diagnosed as CBS, bv-FTD, FTD-motor neuron disease, progressive non-fluent aphasia (PNFA) and semantic dementia (SD) were classified as FTD, while subjects with AD and logopenic progressive aphasia (LPA) were classified as AD. As per current conventions, the term FTD was used for the clinical diagnosis and the term FTLD for the neuropathologically-confirmed diagnoses. Informed consent to be included in research studies and to perform the autopsy was obtained in all cases from the patients or legal representatives in accordance with Pennsylvania state law. The study and all protocols were approved by the Institutional Review Boards of the UPenn and the University of Washington (UW).
Table 1.
Characteristics of the clinical cohort (CSF hemoglobin <500 ng/mL).
| HC | AD | MCI | FTD | LBD | ALS | PSP | |||
|---|---|---|---|---|---|---|---|---|---|
| bvFTD | CBS | DLB | PD | ||||||
| N | 48 | 114 | 63 | 48 | 8 | 10 | 32 | 35 | 9 |
| Age at CSF sampling* | 67.3 (40 – 88) | 69.5 (51 – 91) | 70.5 (50 – 86) | 62.6 (45 – 80) | 66.0 (49 – 87) | 55.3 (28 – 74) | 70.4 (56 – 83) | ||
| Sex (F/M) | 30/18 | 66/48 | 32/31 | 22/34 | 10/32 | 8/27 | 5/4 | ||
| Disease duration at CSF (yr)* | - | 2.9 (−1 – 16) | 2.4 (−1 – 6) | 2.8 (0 – 14) | 6.6 (−1 – 22) | 1.1 (0 – 10) | 3.2 (0 – 7) | ||
| APOE ε4 presence (%) | 29.2 | 50.0 | 38.1 | 28.6 | 23.8 | 20.0 | 11.1 | ||
| CSF Aβ42 (pg/mL)* | 254.7 (94–423) | 144.5 (31–405) | 186.5 (78–355) | 221.5 (82–386) | 227.0 (78–338) | 268.8 (8–464) | 196.4 (110–347) | ||
| CSF t-tau (pg/mL)* | 54.0 (20–161) | 118.0 (21–405) | 86.9 (18–254) | 70.6 (7–203) | 54.4 (20–127) | 60.9 (21–271) | 46.9 (18–80) | ||
| CSF p-tau (pg/mL)* | 21.8 (2–101) | 40.8 (7–135) | 32.5 (5–116) | 21.5 (4–62) | 26.0 (10–69) | 17.6 (6–196) | 15.1 (7–23) | ||
| CSF t-tau/Aβ42* | 0.25 (0.09–1.02) | 1.01 (0.08–4.87) | 0.59 (0.08–2.13) | 0.38 (0.09–1.63) | 0.26 (0.09–0.92) | 0.33 (0.08–3.63) | 0.25 (0.14–0.53) | ||
| CSF p-tau/t-tau* | 0.41 (0.05–1.00) | 0.38 (0.12–1.23) | 0.40 (0.12–1.07) | 0.35 (0.07–1.14) | 0.54 (0.16–1.00) | 0.41 (0.07–6.76) | 0.37 (0.16–0.65) | ||
| CSF α-syn (ng/mL)* | 0.464 (0.179–0.753) | 0.487 (0.219–0.913) | 0.519 (0.180–0.889) | 0.401 (0.082–0.884) | 0.421 (0.142–0.859) | 0.400 (0.119–0.847) | 0.358 (0.138–0.625) | ||
| CSF pS129 (ng/mL)* | 0.085 (0.044–0.159) | 0.084 (0.038–0.165) | 0.082 (0.051–0.157) | 0.087 (0.053–0.199) | 0.084 (0.044–0.134) | 0.088 (0.049–0.156) | 0.091 (0.062–0.157) | ||
| CSF pS129/α-syn* | 0.205 (0.088–0.888) | 0.191 (0.062–0.529) | 0.176 (0.057–0.548) | 0.268 (0.073–1.139) | 0.238 (0.086–0.653) | 0.270 (0.073–1.147) | 0.300 (0.129–0.465) | ||
Data shown is mean (range).
Table 2.
Characteristics of the autopsy cohort (CSF hemoglobin <500 ng/mL).
| AD | FTLD | LRP | ALS | |||||
|---|---|---|---|---|---|---|---|---|
| FTLD | FTLD-AD | PD | PD-AD | LRP-AD | LRP-TDP | |||
| N | 29 | 12 | 4 | 3 | 4 | 12 | 2 | 5 |
| Age at CSF sampling | 70.7 (44–87) | 66.4 (43–83) | 70.0 (52–88) | 57.4 (47–64) | ||||
| Sex (F/M) | 12/17 | 7/9 | 7/14 | 4/1 | ||||
| Disease duration at CSF (yr) | 3.1 (0 – 12) | 3.1 (0 – 9) | 5.3 (1 – 20) | 1.2 (1 – 2) | ||||
| Age at Death | 76.1 (47–92) | 70.7 (44–86) | 74.9 (62–90) | 59.4 (51–67) | ||||
| Survival after CSF (yr) | 5.6 (1.5–13.9) | 4.4 (1.2–10.4) | 4.7 (1.1–10.7) | 1.8 (0.2–4.0) | ||||
| APOE ε4 presence (%) | 55.2 | 12.5 | 52.4 | 20.0 | ||||
| CSF Aβ42 (pg/mL) | 134.3 (39–295) | 195.4 (108–275) | 170.9 (85–274) | 279.2 (203–343) | ||||
| CSF t-tau (pg/mL) | 124.7 (20–304) | 61.3 (22–143) | 82.0 (22–196) | 56.0 (25–102) | ||||
| CSF p-tau (pg/mL) | 34.4 (5–94) | 12.0 (4–45) | 24.1 (4–83) | 8.4 (6–12) | ||||
| CSF t-tau/Aβ42 | 1.08 (0.21–3.40) | 0.35 (0.15–1.15) | 0.57 (0.13–1.72) | 0.19 (0.12–0.30) | ||||
| CSF p-tau/t-tau | 0.45 (0.04–4.70) | 0.21 (0.04–0.55) | 0.32 (0.07–0.83) | 0.19 (0.10–0.40) | ||||
| CSF α-syn (ng/mL) | 0.568 (0.248–1.120) | 0.391 (0.216–0.698) | 0.443 (0.227–0.725) | 0.324 (0.197–0.579) | ||||
| CSF pS129 (ng/mL) | 0.077 (0.044–0.107) | 0.069 (0.051–0.103) | 0.081 (0.046–0.138) | 0.089 (0.071–0.106) | ||||
| CSF pS129/ α-syn | 0.156 (0.051–0.348) | 0.192 (0.103–0.373) | 0.196 (0.075–0.392) | 0.323 (0.138–0,499) | ||||
Data shown is mean (range).
2.2 CSF collection and CSF measurements
All CSF samples were obtained by lumbar puncture as described previously, and samples were immediately stored at −80°C until analysis [30]. CSF total α-syn and pS129 levels were measured at UW by using Luminex immunoassays as previously described [14, 16]. CSF data for Aβ42, t-tau, and p-tau were obtained at UPenn by using the INNO-BIA AlzBio3™ Luminex assay reagents (Innogenetics, Ghent, Belgium) [30–32]. CSF hemoglobin levels were measured as an index of red blood cell contamination, with a human hemoglobin ELISA quantitation kit (Bethyl Lab Inc, Montgomery, TX, USA) as previously described [14].
2.3 Tissue collection and neuropathological assessment
Tissue collection procedures have been previously described [19]. Briefly, a neuropathological diagnosis of AD was assigned if the probability was intermediate or high [33]. The diagnoses of FTLD-TAU, FTLD-TDP and DLB were based on established criteria [23, 34]. FTLD-TAU cases included cases with a diagnosis of argyrophilic grain disease (AGD), progressive supranuclear palsy (PSP), tangle predominant senile dementia (TPSD), and corticobasal degeneration (CBD). See Supplementary Methods for more details.
2.4 Statistical analysis
All analyses were performed in SPSS 18.0 (IBM, Chicago, IL, USA) or Prism 6.0 (GraphPad Software, La Jolla, CA, USA). Immunoassay data (CSF total α-syn, pS129, Aβ42, t-tau, and p-tau) were Log10 transformed to generate a more normally distributed dataset, and the transformed data was used in all analyses. Correlations between biomarkers are reported as Pearson correlation coefficients. One way analysis of variance (ANOVA) followed by Tukey post-hoc test was used to compare group means. Receiver operating characteristic (ROC) curves for analytes, controlling for age and sex of participants, were generated to evaluate their sensitivities and specificities in distinguishing AD from HC or diseased comparison participants. Area under curve (AUC) was determined as a measure of the overall performance of a diagnostic test (the closer AUC is to 1, the better the overall diagnostic performance), which is also independent of disease prevalence since it is based on sensitivity and specificity [35]. The “optimum” cutoff value for a ROC curve was defined as the value associated with the maximal sum of sensitivity and specificity (i.e., maximizing the Youden index). Stepwise logistic regression was used to determine the best prediction models that included multiple CSF biomarkers as well as age and sex of participants. Values with p<0.05 were regarded as significant.
3. Results
3.1 Correlation among CSF analytes in the whole cohort
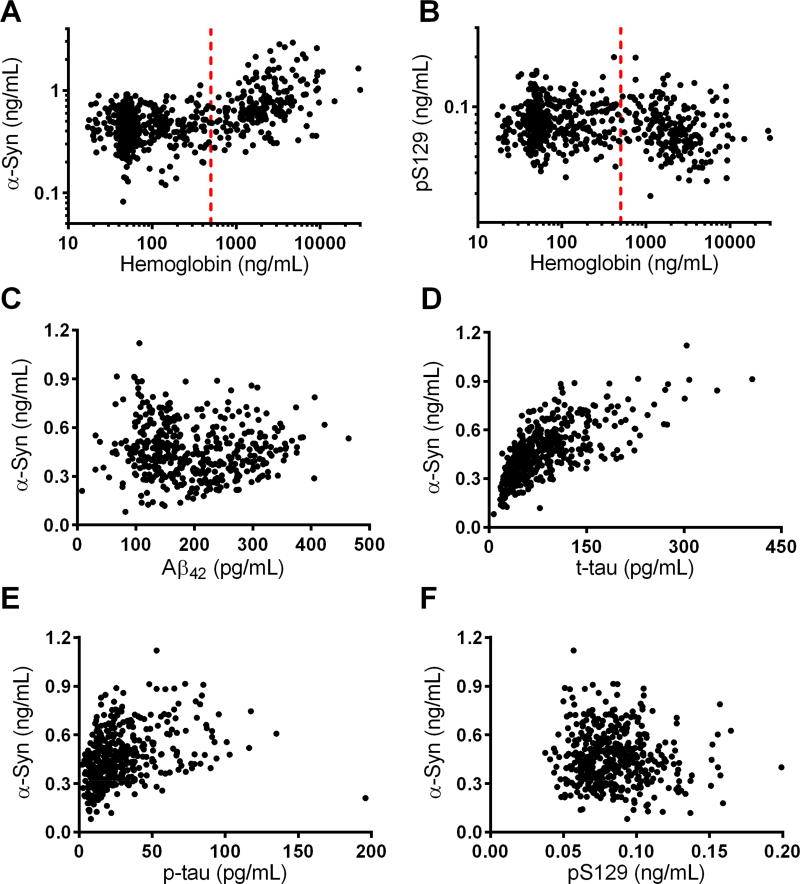
A total of 642 cases were included in the current study. As previously described [14, 15], CSF α-syn showed a strong association with CSF hemoglobin levels (an index of blood contamination in CSF; r=0.523, p<3.8×10−46) (Figure 1A). CSF pS129 showed a significant, although weaker, inverse association with CSF hemoglobin levels (r=−0.182, p<3.6×10−6) (Figure 1B).When using a cutoff of hemoglobin >500 ng/mL in this cohort to exclude blood-contaminated samples, 31.3% of the CSF samples were excluded from all further analyses (n=201), and then both CSF α-syn (p=0.869) and pS129 (p=0.291) showed no significant associations with CSF hemoglobin.
Figure 1. Association of CSF analytes.
(A and B) Associations of CSF α-synuclein (α-syn; A) and α-syn phosphorylated at Ser129 (pS129; B) with CSF hemoglobin values in all subjects (n=642); vertical dashed red line represents the 500 ng/mL cut-off selected to exclude CSF α-syn values due to blood contamination. (C, D, and E) Associations of CSF α-syn with CSF amyloid beta peptide 1–42 (Aβ42; C), total tau (t-tau; D), and phosphorylated p-tau at Thr181 (p-tau; E), after excluding subjects with >500 ng/mL of CSF hemoglobin (n=441 after exclusion). (F) Association of CSF α-syn and pS129 in subjects with ≤500 ng/mL hemoglobin.
After excluding CSF samples with high hemoglobin levels (>500 ng/mL), CSF α-syn showed no association with CSF Aβ42 (r=−0.025, p=0.597) (Figure 1C), but a strong positive correlation with t-tau (r=0.725, p<1.5×10−71) (Figure 1D) as well as a moderate positive correlation with p-tau (r=0.430, p<3.0×10−21) (Figure 1E). In contrast, CSF pS129 showed no association with any of the three classic AD CSF biomarkers (all p>0.07). CSF α-syn and pS129 were not significantly correlated with each other (r=−0.069, p=0.15) in this cohort (Figure 1F).
3.2 Evaluation of diagnostic and differential diagnostic values of CSF α-syn and pS129 in the clinical cohort
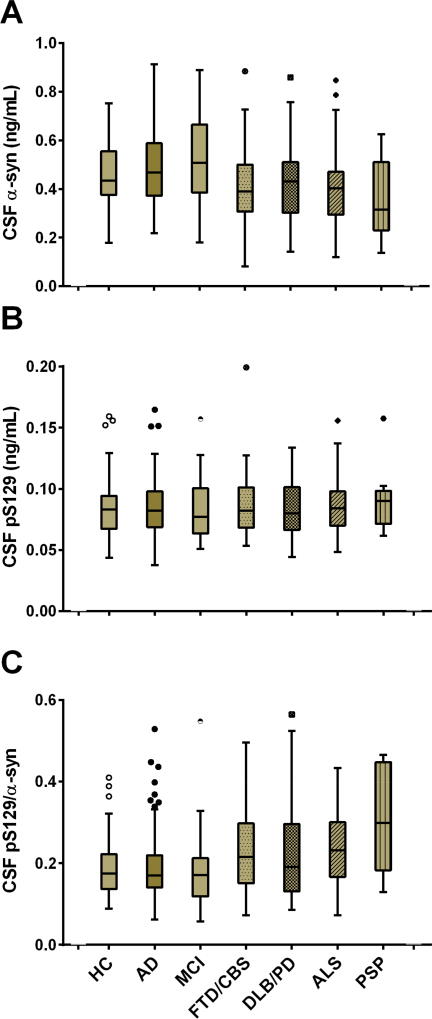
A cohort of 540 cases without neuropathological confirmation was used as the discovery cohort in this study (see Supplementary Table 1 for the whole cohort). As described in the Methods, certain disease groups were combined together based on their similar underlying pathology (e.g., DLB and PD/PDD) to increase the sample size in analyses. In this cohort (n=367 subjects after excluding samples with high hemoglobin levels), CSF α-syn levels were numerically higher in AD compared to HC or LBD (DLB/PD/PDD; p=0.153) (see Table 1 and Figure 2A). However, CSF α-syn was significantly higher in AD compared to FTD (p=0.004) or ALS (p=0.014), and borderline significantly higher in AD compared to PSP (p=0.068). CSF α-syn was also significant higher in MCI compared to FTD (p=0.001), LBD (p=0.034), ALS (p=0.003), or PSP (p=0.023). CSF pS129 showed no differences between AD or MCI and HC, consistent with previous reports [16, 36], or any other diagnostic groups (Figure 2B). Use of the CSF pS129/α-syn ratio did not enhance the performance of CSF total α-syn for AD diagnosis and differential diagnosis (Figure 2C).
Figure 2. CSF α-syn, pS129, and the pS129/α-syn ratio stratified by clinical diagnosis in the clinical cohort.
CSF total α-syn (A) and pS129 (B) concentrations were measured in the clinical cohort that includes patients with the diagnoses indicated below panel C. The ratio of pS129/α-syn is also shown (C). The boxes extend from the 25th to 75th percentiles. The middle dark lines indicate the medians. The whiskers extend to 1.5 times the height of the box or, if no case has a value in that range, to the minimum or maximum values. Values not included between the whiskers are plotted as outliers with corresponding symbols. No outliers were excluded from the analyses in this study.
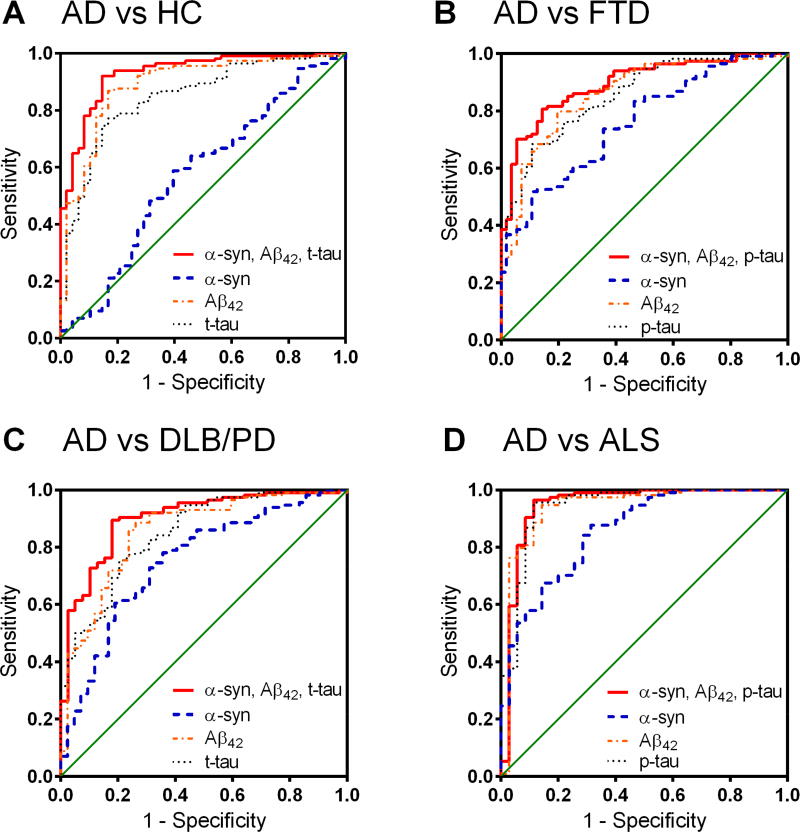
To further evaluate the diagnostic and differential diagnostic values of CSF biomarkers and their combinations, ROC analysis was performed to determine the sensitivities and specificities between AD and HC or patients with other neurodegenerative diseases (see Table 3 and Figure 3). For the comparison between AD and HC, although CSF α-syn alone only provided a poor differentiation and as expected, CSF Aβ42 [AUC=0.890, 95% confidence interval (CI) 0.832–0.948; sensitivity=86.8% (95% CI 79.2–92.4%), specificity=83.3% (95% CI 69.8–92.5%)] or t-tau [AUC=0.848, 95% CI 0.783–0.912; sensitivity=77.2% (68.4–84.5%), specificity=83.3% (69.8–92.5%)] could discriminate the two groups well, the best model was the combination of CSF Aβ42, t-tau, and α-syn, when controlling for age and sex of participants [AUC=0.931, 95% CI 0.890–0.973; sensitivity=92.1% (85.5–96.3%), specificity=85.4% (72.2–93.9%); Figure 3A].
Table 3.
Diagnostic and differential diagnostic values of CSF biomarkers in AD in the clinical cohort.
| α-syn* | pS129/α-syn* | Aβ42* | t-tau* | p-tau* | t-tau/Aβ42* | Combined model** |
|
|---|---|---|---|---|---|---|---|
| AD vs HC | |||||||
| AUC | 0.573 | 0.573 | 0.890 | 0.848 | 0.789 | 0.891 | 0.931† |
| Sens% | 58.8 | 71.9 | 86.8 | 77.2 | 78.9 | 90.4 | 92.1 |
| Spec% | 60.4 | 45.8 | 83.3 | 83.3 | 72.9 | 81.3 | 85.4 |
| p-value | 0.142 | 0.141 | 5.13E-15 | 3.03E-12 | 6.25E-9 | 4.56E-15 | 5.03E-18 |
| AD vs FTD | |||||||
| AUC | 0.760 | 0.767 | 0.860 | 0.831 | 0.857 | 0.873 | 0.893‡ |
| Sens% | 51.8 | 58.8 | 79.8 | 73.7 | 68.4 | 76.3 | 80.7 |
| Spec% | 89.3 | 82.1 | 80.4 | 78.6 | 89.3 | 87.5 | 85.7 |
| p-value | 3.94E-8 | 1.55E-8 | 2.69E-14 | 2.35E-12 | 4.43E-14 | 4.94E-16 | 8.42E-17 |
| AD vs LBD | |||||||
| AUC | 0.751 | 0.746 | 0.854 | 0.849 | 0.768 | 0.886 | 0.900† |
| Sens% | 78.1 | 79.8 | 88.6 | 75.4 | 77.2 | 78.9 | 89.5 |
| Spec% | 64.3 | 64.3 | 73.8 | 79.5 | 70.7 | 89.7 | 82.1 |
| p-value | 1.57E-6 | 2.57E-6 | 1.27E-11 | 8.17E-11 | 3.59E-7 | 1.73E-13 | 9.50E-14 |
| AD vs ALS | |||||||
| AUC | 0.858 | 0.859 | 0.939 | 0.912 | 0.942 | 0.939 | 0.947‡ |
| Sens% | 87.7 | 83.3 | 94.7 | 80.7 | 95.6 | 86.8 | 96.5 |
| Spec% | 68.6 | 71.4 | 85.7 | 88.6 | 88.6 | 88.6 | 88.6 |
| p-value | 1.61E-10 | 1.35E-10 | 4.65E-15 | 1.88E-13 | 2.72E-15 | 1.72E-15 | 1.32E-15 |
| AD vs PSP | |||||||
| AUC | 0.740 | 0.788 | 0.715 | 0.882 | 0.889 | 0.900 | 0.915† |
| Sens% | 70.2 | 78.1 | 76.3 | 67.5 | 81.6 | 90.4 | 80.7 |
| Spec% | 77.8 | 77.8 | 66.7 | 100.0 | 88.9 | 88.9 | 100.0 |
| p-value | 0.017 | 0.004 | 0.032 | 1.41E-4 | 1.04E-4 | 4.34E-5 | 3.51E-5 |
Logistic model contains the CSF marker, age and sex of participants;
Logistic model contains CSF α-syn, Aβ42, t-tau† or p-tau‡, age and sex of participants.
Figure 3. ROC analysis of CSF biomarkers in the clinical cohort.
(A) Alzheimer disease (AD) vs healthy controls (HC); (B) AD vs frontotemporal degeneration (FTD)/corticobasal syndrome (CBS); (C) AD vs dementia with Lewy bodies (DLB)/Parkinson disease (PD); (D) AD vs amyotrophic lateral sclerosis (ALS). Blue dashed line indicates CSF α-syn alone, orange dot-dashed line indicates CSF Aβ42, black dotted line indicates CSF t-tau or p-tau, and red solid line indicates a combined model of CSF α-syn, Aβ42, and t-tau or p-tau.
For the comparison between AD and FTD groups, CSF α-syn alone (controlling for age and sex of participants) could provide a weak differentiation [AUC=0.760, 95% CI 0.687–0.832; sensitivity=51.8% (42.2–61.2%), specificity=89.3% (78.1–96.0%)], similar to those of CSF Aβ42, t-tau, or p-tau alone (Table 3); a combination of CSF α-syn, Aβ42, and p-tau differentiated AD from FTD well [AUC=0.893, 95% CI 0.845–0.941; sensitivity=80.7% (72.3–87.5%), specificity=85.7% (73.8–93.6%); Figure 3B] and was significantly more informative compared to the best individual CSF biomarker (Aβ42; Z=2.3744, p=0.0176, DeLong’s test[37]). Similarly, CSF α-syn alone could also provide a moderate differentiation for AD vs LBD (DLB/PD/PDD) [AUC=0.751, 95% CI 0.664–0.838; sensitivity=78.1% (69.4–85.3%), specificity=64.3% (48.0–78.4%)], AD vs ALS [AUC=0.858, 95% CI 0.788–0.928; sensitivity=87.7% (80.3–93.1%), specificity=68.6% (50.7–83.1%)], and AD vs PSP [AUC=0.740, 95% CI 0.539–0.940; sensitivity=70.2% (60.9–78.4%), specificity=77.8% (40.0–97.2%)], and adding CSF α-syn to Aβ42, t-tau (or p-tau) enhanced the differential diagnosis [AD vs LBD, AUC=0.900, 95% CI 0.844–0.956, sensitivity=89.5% (82.3–94.4%), specificity=82.1% (62.5–92.5%), Figure 3C; AD vs ALS, AUC=0.947, 95% CI 0.883–1.000, sensitivity=96.5% (91.3–99.0%), specificity=88.6% (73.3–96.8%), Figure 3D; and AD vs PSP, AUC=0.915, 95% CI 0.860–0.970, sensitivity=80.7% (72.3–87.5%), specificity=100.0% (66.4–100%), Table 3].
3.3 Validation of differential diagnostic values of CSF biomarkers in the autopsy cohort
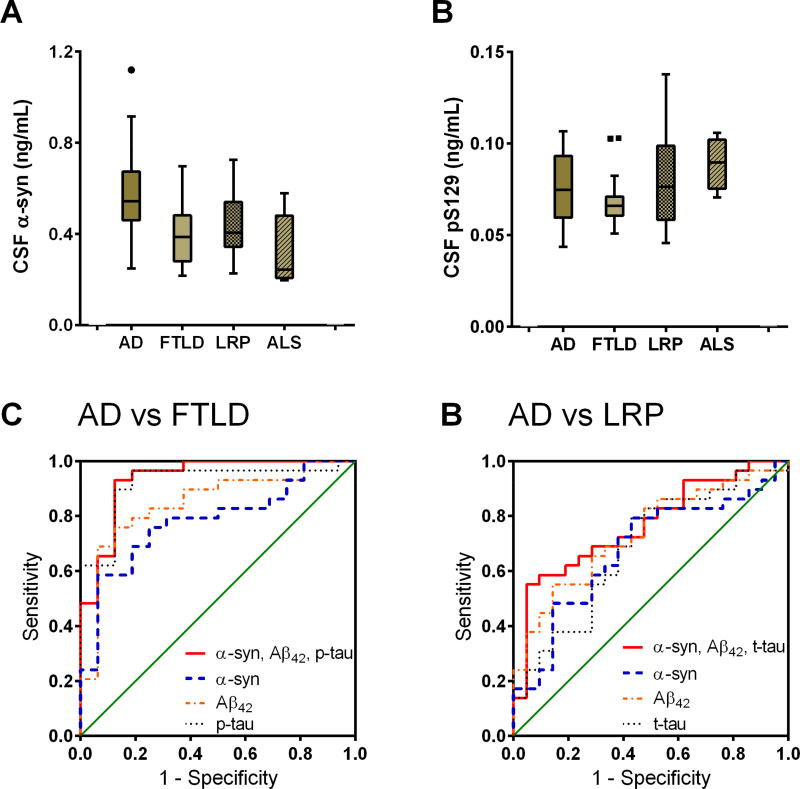
To further validate the differential diagnostic values, we measured the CSF biomarkers in a cohort of neuropathologically-confirmed cases (n=102 in total; 74 after excluding CSF samples with >500 ng/mL hemoglobin levels; see Table 2 and Supplementary Table 2). Due to the limited number of cases, the subjects were categorized into the following pathological groups: AD, FTLD (including FTLD and FTLD-AD), LRP (including PD, PD-AD, LRP-AD, and LRP-TDP), and ALS. As shown in Figure 4, consistent with the results from the clinical cohort, CSF α-syn was substantially higher in AD compared to FTLD, LRP, and ALS, while CSF pS129 didn’t show significant differences among diagnostic groups.
Figure 4. CSF α-syn and pS129 stratified by autopsy diagnosis and the differential diagnosis performance of CSF biomarkers in the validation (autopsy) cohort.
Further ROC analysis demonstrated that CSF α-syn could differentiate AD from FTLD [AUC=0.782, p=0.002, sensitivity=58.6% (95% CI 38.9–76.5%), specificity=93.8% (69.8–99.8%); Figure 4C], LRP [AUC=0.678, p=0.033, sensitivity=79.3% (95% CI 60.3–92.0%), specificity=57.1% (34.0–78.2%); Figure 4D], and ALS [AUC=0.966, p=0.001, sensitivity=86.2% (95% CI 68.3–96.1%), specificity=100.0% (47.8–100.0%)] well, when controlling for age and sex of participants. Additionally, the combinations of CSF α-syn, Aβ42, t-tau (or p-tau) further improved the differential diagnosis: AD vs FTLD, AUC=0.935, p=1.67×10−6, sensitivity=93.1% (95% CI 77.2–99.2%), specificity=87.5% (61.6–98.4%) for a model of CSF α-syn, Aβ42, and p-tau (Figure 4E); AD vs LRP, AUC=0.767, p=0.001, sensitivity=55.2% (95% CI 35.7–73.6%), specificity=95.2% (76.2–99.9%) for a model of CSF α-syn, Aβ42, and t-tau (Figure 4F); AD vs ALS, AUC=1.000, p=4.23×10−4, sensitivity=100.0% (95% CI 88.1–100.0%), specificity=100.0% (47.8–100.0%) for a model of CSF α-syn, Aβ42, and p-tau. It should be noted that some small sample sizes (e.g., n=5 for ALS) led to wide 95% CIs.
4. Discussion
For the clinically relevant diagnosis and differential diagnosis of AD, it is essential to have a set of biomarkers that discriminate AD from other clinically relevant dementias or neurodegenerative diseases. Previous studies revealed substantial overlaps in CSF biomarker profiles (Aβ42 and t-tau or p-tau) between AD and related disorders, and this significantly limits the utility of these core CSF biomarkers in differential diagnosis [5, 6]. In the current study, we interrogated CSF samples obtained from a relatively large, longitudinally-followed clinical cohort, and we report that higher CSF total α-syn might be relatively unique to AD, and that by combining data on CSF measures of α-syn, Aβ42, and t-tau or p-tau we might be able to provide better diagnostic and differential diagnostic biomarker values for AD. These findings were largely confirmed in a separate cohort of participants who were longitudinally followed to autopsy for neuropathological confirmation of their diagnoses.
Although it was less apparent in the cohort included in this study, there is overlap of CSF Aβ42 and tau values between AD and related disorders as reported in previous studies [5–9]. As discussed previously [5, 6], these observations were not that surprising, because mixed pathology is a common finding at autopsy [38–41], which may reflect converging pathophysiological mechanisms and pathways at late clinical stages [5]. For example, neuropathological and neuroimaging studies have revealed Aβ and tau pathology in LBD patients [42–44], and regional brain Aβ accumulation appears to correlate with domain-specific cognitive performance in PD patients [45]. These results suggest that CSF Aβ42 and tau may detect the increased levels of Aβ and tau pathology in non-AD diseases, limiting the differential diagnostic value of such biomarkers [5], particularly when used alone. Earlier studies [46, 47] reported that CSF p-tau might improve the differential dementia diagnosis (AUC 0.6–0.8), but other large-scale studies [4, 5], including the current study, found that CSF p-tau and t-tau performed largely equally.
In the current study, CSF α-syn levels tended to be higher in AD or MCI compared to related disorders in both cohorts, though the statistical significance was not achieved for AD vs HC and AD vs LBD (DLB/PD/PDD). This is in agreement with several previous large-scale studies, showing significantly higher levels of α-syn in CSF from patients with AD compared to HC [17, 18, 36] or patients with DLB/PDD [48]. The increase is perhaps due to the release of α-syn from damaged neurons during neurodegeneration, similar to what has been hypothesized for the increased levels of CSF tau in AD. However, this cannot be the entire explanation since CSF α-syn and tau do not appear to be increased in most other neurodegenerative diseases that are also associated with α-syn or tau pathology and extensive neuron loss in the brain. We have reported that CSF α-syn and tau could be transported from the central nervous system (CNS) into peripheral blood, and this potential clearance of CNS α-syn and tau via exosomes appeared to be increased in PD compared to HC [49, 50], but not in AD for tau [50] (α-syn clearance in AD has not been tested yet). Whether this is also true for CNS α-syn clearance and whether it could be a major contributor to the increase of CSF α-syn and tau in AD needs further investigation.
Most importantly, we found that the diagnostic accuracy of the combinations of these CSF biomarkers including measures of α-syn, Aβ, and tau was high enough (AUC, 0.8–0.9) to be useful in clinical settings for differentiating patients with AD from those with other related disorders. The diagnostic accuracy of these CSF proteins for differentiating AD from FTD is at least in the same order of magnitude as those obtained with advanced neuroimaging technologies [51, 52] and at a lower cost. Our findings on AD vs LBD is also in line with a previous study [48] reporting a panel of CSF biomarkers including α-syn, tau and Aβ42 could differentiate AD from DLB and PDD with high sensitivity and specificity. It should be emphasized that these results were acquired from a retrospective study under a research setting, the biomarker accuracy and usefulness of the panel need to be further confirmed in prospective diagnostic studies under clinical settings [4]. Additionally, we used AUC as well as the Youden index-determined sensitivity and specificity in this study to assess the overall performance of candidate biomarkers for the differential diagnosis of AD, which usually has higher prevalence rate among related diseases. In some clinical settings (e.g., to screen for a certain disease of very low prevalence, a high specificity and a low false positive rate is required), the performance may need to be re-evaluated by adjusting the cutoff range or considering only a portion of the ROC curve.
However, CSF pS129 levels did not distinguish AD from any of the other diagnostic groups in this study. Previous studies have demonstrated that pS129 is the predominant post-translationally modified form of α-syn in LBs [53, 54] and have also associated CSF pS129 with PD [16, 55]. However, the role of pS129 in AD remains unclear, despite the frequent observation of LBs in AD brains [11–13]. In our previous study [16], we did not observe significant differences in CSF pS129 levels between AD and HC when a small cohort of AD subjects was examined. This was confirmed in a more recent independent study with a much larger AD cohort [36]. In the current study, CSF pS129 and total α-syn were not significantly correlated with each other, indicating different α-syn forms in CSF might behave differently, possibly due to different transportation or clearance mechanisms. Our results also suggest that the transportation or clearance mechanisms for pS129, if different from other general α-syn species, might be less likely affected under different disease settings.
All the ROC analyses in this study were performed by controlling for age and sex of participants. Several studies have explored if demographic factors, including age, sex, and the APOE ε4 genotype, impact the diagnostic accuracy of CSF AD biomarkers (see review by Mattsson et al [4]). While there is no clear effect of sex on the diagnostic accuracy, age may impact the diagnostic performance of CSF AD biomarkers [4]. The genotype APOE ε4 is strongly associated with reduced CSF Aβ42 in controls, but not with altered CSF t-tau or p-tau levels [4] as well as CSF α-syn levels [17]. However, because APOE ε4 is associated with increased amyloid pathology, rather than artificial reductions of CSF Aβ42, it is not recommended to adjust the CSF Aβ42 cutoff depending on the presence of APOE ε4 [4]. In the current study, adding APOE ε4 status to the models did not change the outcomes (data not shown).
One limitation of this study is that the cohort studied here did not include any subjects with vascular dementia, and thus the performance of CSF α-syn, together with Aβ42 and t-tau or p-tau, on differentiating AD from vascular dementia remains unknown. While this needs to be further investigated in future studies, we previously reported that CSF E-selectin, a biomarker of endothelial function/vascular injury, might be a promising CSF biomarker to pursue as a potential indicator that vascular pathology is contributing to dementia [56]. Thus, CSF E-selectin should be tested in larger cohorts for its ability to differentiate AD from vascular dementia. Another potential limitation is that certain disease groups with similar underlying pathology were combined together (e.g., DLB and PD were combined into overarching LBD or LRP) to increase the sample size in the analyses, and its potential confounding effects need to be further investigated in future larger-scale studies.
In summary, CSF total α-syn, when combined with core AD biomarkers (i.e., Aβ42, t-tau, and p-tau), improved the differential diagnosis of AD vs FTD, LBD (DLB/PD/PDD), and other neurodegenerative disease. The diagnostic accuracy of the combined models described here was high enough to be of clinical value for differentiating AD patients from patients with other related disorders in our cohort. Moreover, the diagnostic performance of these CSF biomarkers was supported by studies of a second cohort of subjects who were longitudinally followed to autopsy for neuropathological confirmation of their diagnoses. Although further validation in independent cohorts is still needed, our results indicate that CSF measures of total α-syn combined with measures of Aβ42, t-tau, and p-tau might have clinical value in the differential diagnosis of AD.
Supplementary Material
Research in Context.
1. Systematic review: The Pubmed database was searched to identify previously published research. Differential diagnosis of AD from other common geriatric dementing disorders remains unresolved. Recent studies found that the utility of the core CSF AD biomarkers (Aβ42, t-tau, and p-tau) to discriminate AD from a variety of related disorders is limited, probably due to overlap in the underlying primary pathologies. It has been suggested that introduction of additional CSF biomarkers reflecting other types of pathologies could optimize differential diagnosis.
2. Interpretation: Our findings indicate that CSF measures of total α-synuclein combined with measures of Aβ42, t-tau, and p-tau, representing LB and AD pathologies, respectively, likely have clinical value in the differential diagnosis of AD.
3. Future directions: Further studies are needed to include additional CSF biomarkers to reflect other comorbid pathologies, such as E-selectin for vascular pathology commonly observed at autopsy of AD brains.
Acknowledgments
We deeply appreciate the participants for their generous donation of samples. This study was supported by grants from the National Institutes of Health (NIH) (U01 NS082137 and U01 NS091272 to J.Z., and P30 AG010124, P50 NS053488 and P01 AG017586-8499 to J.Q.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The sponsors of this study had no role in the study design, data collection, analysis and interpretation, or the writing of the report. The authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: J.Z., J.Q.T., and M.S. supervised the project and designed the studies. L.T., C.G., and H.W. acquired the CSF α-syn, pS129, and hemoglobin data. J.B.T., D.W., A.S.C.-P., D.J.I., M.G., L.M., L.B.E., D.A.W., E.B.L., L.M.S., and J.Q.T. characterized human subjects and provided CSF samples as well as clinical and CSF Aβ42, t-tau and p-tau data. P.H.J. provided antibodies for α-syn immunoassays. M.S., J.B.T., L.T., and J.Z. interpreted the data. M.S. and J.B.T. conducted statistical analysis. C.G. and P.A. provided administrative and technical supports. M.S., J.B.T., J.Z., J.Q.T., and D.W. drafted the manuscript; all authors critically reviewed the paper.
Conflicts of Interest: Nothing to report.
References
- 1.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–44. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 3.Blennow K, Zetterberg H. The past and the future of Alzheimer's disease CSF biomarkers-a journey toward validated biochemical tests covering the whole spectrum of molecular events. Front Neurosci. 2015;9:345. doi: 10.3389/fnins.2015.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattsson N, Lonneborg A, Boccardi M, Blennow K, Hansson O. Clinical validity of cerebrospinal fluid Abeta42, tau, and phospho-tau as biomarkers for Alzheimer's disease in the context of a structured 5-phase development framework. Neurobiol Aging. 2017;52:196–213. doi: 10.1016/j.neurobiolaging.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 5.Ewers M, Mattsson N, Minthon L, Molinuevo JL, Antonell A, Popp J, et al. CSF biomarkers for the differential diagnosis of Alzheimer's disease: A large-scale international multicenter study. Alzheimers Dement. 2015;11:1306–15. doi: 10.1016/j.jalz.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Schoonenboom NS, Reesink FE, Verwey NA, Kester MI, Teunissen CE, van de Ven PM, et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78:47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- 7.Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- 8.Bibl M, Mollenhauer B, Lewczuk P, Esselmann H, Wolf S, Trenkwalder C, et al. Validation of amyloid-beta peptides in CSF diagnosis of neurodegenerative dementias. Mol Psychiatry. 2007;12:671–80. doi: 10.1038/sj.mp.4001967. [DOI] [PubMed] [Google Scholar]
- 9.Struyfs H, Van Broeck B, Timmers M, Fransen E, Sleegers K, Van Broeckhoven C, et al. Diagnostic Accuracy of Cerebrospinal Fluid Amyloid-beta Isoforms for Early and Differential Dementia Diagnosis. J Alzheimers Dis. 2015;45:813–22. doi: 10.3233/JAD-141986. [DOI] [PubMed] [Google Scholar]
- 10.Toledo JB, Brettschneider J, Grossman M, Arnold SE, Hu WT, Xie SX, et al. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol. 2012;124:23–35. doi: 10.1007/s00401-012-0983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippa CF, Fujiwara H, Mann DM, Giasson B, Baba M, Schmidt ML, et al. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer's disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol. 1998;153:1365–70. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ. Antibodies to alpha-synuclein detect Lewy bodies in many Down's syndrome brains with Alzheimer's disease. Ann Neurol. 1999;45:353–7. doi: 10.1002/1531-8249(199903)45:3<353::aid-ana11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton RL. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–84. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010;133:713–26. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, et al. Cerebrospinal Fluid Biomarkers for Parkinson Disease Diagnosis and Progression. Ann Neurol. 2011;69:570–80. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Shi M, Chung KA, Zabetian CP, Leverenz JB, Berg D, et al. Phosphorylated α-synuclein in Parkinsonian disorders. Sci Transl Med. 2012;4:121ra20. doi: 10.1126/scitranslmed.3002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korff A, Liu C, Ginghina C, Shi M, Zhang J. alpha-Synuclein in Cerebrospinal Fluid of Alzheimer's Disease and Mild Cognitive Impairment. J Alzheimers Dis. 2013;36:679–88. doi: 10.3233/JAD-130458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toledo JB, Korff A, Shaw LM, Trojanowski JQ, Zhang J. CSF alpha-synuclein improves diagnostic and prognostic performance of CSF tau and Abeta in Alzheimer's disease. Acta Neuropathol. 2013;126:683–97. doi: 10.1007/s00401-013-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toledo JB, Van Deerlin VM, Lee EB, Suh E, Baek Y, Robinson JL, et al. A platform for discovery: The University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers Dement. 2014;10:477–84. e1. doi: 10.1016/j.jalz.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossman M, Libon DJ, Forman MS, Massimo L, Wood E, Moore P, et al. Distinct Antemortem Profiles in Patients With Pathologically Defined Frontotemporal Dementia. Arch Neurol. 2007;64:1601–9. doi: 10.1001/archneur.64.11.1601. [DOI] [PubMed] [Google Scholar]
- 22.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 24.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–24. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 26.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 27.Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol. 1996;55:97–105. doi: 10.1097/00005072-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Xie SX, Libon DJ, Wang X, Massimo L, Moore P, Vesely L, et al. Longitudinal patterns of semantic and episodic memory in frontotemporal lobar degeneration and Alzheimer's disease. J Int Neuropsychol Soc. 2010;16:278–86. doi: 10.1017/S1355617709991317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie SX, Ewbank DC, Chittams J, Karlawish JH, Arnold SE, Clark CM. Rate of decline in Alzheimer disease measured by a Dementia Severity Rating Scale. Alzheimer Dis Assoc Disord. 2009;23:268–74. doi: 10.1097/WAD.0b013e318194a324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grossman M, Farmer J, Leight S, Work M, Moore P, Van Deerlin V, et al. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer's disease. Ann Neurol. 2005;57:721–9. doi: 10.1002/ana.20477. [DOI] [PubMed] [Google Scholar]
- 31.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark C, Aisen P, Petersen R, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, et al. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–45. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- 33.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–7. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Mackenzie I, Neumann M, Bigio E, Cairns N, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obuchowski NA. Receiver operating characteristic curves and their use in radiology. Radiology. 2003;229:3–8. doi: 10.1148/radiol.2291010898. [DOI] [PubMed] [Google Scholar]
- 36.Majbour NK, Chiasserini D, Vaikath NN, Eusebi P, Tokuda T, van de Berg W, et al. Increased levels of CSF total but not oligomeric or phosphorylated forms of alphasynuclein in patients diagnosed with probable Alzheimer's disease. Sci Rep. 2017;7:40263. doi: 10.1038/srep40263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 38.Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 39.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 40.Sonnen JA, Postupna N, Larson EB, Crane PK, Rose SE, Montine KS, et al. Pathologic correlates of dementia in individuals with Lewy body disease. Brain Pathol. 2010;20:654–9. doi: 10.1111/j.1750-3639.2009.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang BW, Lu E, Mackenzie IR, Assaly M, Jacova C, Lee PE, et al. Multiple pathologies are common in Alzheimer patients in clinical trials. Can J Neurol Sci. 2012;39:592–9. doi: 10.1017/s0317167100015316. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Isla T, Growdon WB, McNamara M, Newell K, Gomez-Tortosa E, Hedley-Whyte ET, et al. Clinicopathologic correlates in temporal cortex in dementia with Lewy bodies. Neurology. 1999;53:2003–9. doi: 10.1212/wnl.53.9.2003. [DOI] [PubMed] [Google Scholar]
- 43.Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–10. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol. 2017;16:55–65. doi: 10.1016/S1474-4422(16)30291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akhtar RS, Xie SX, Chen YJ, Rick J, Gross RG, Nasrallah IM, et al. Regional brain amyloid-beta accumulation associates with domain-specific cognitive performance in Parkinson disease without dementia. PLoS ONE. 2017;12:e0177924. doi: 10.1371/journal.pone.0177924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Struyfs H, Niemantsverdriet E, Goossens J, Fransen E, Martin JJ, De Deyn PP, et al. Cerebrospinal Fluid P-Tau181P: Biomarker for Improved Differential Dementia Diagnosis. Front Neurol. 2015;6:138. doi: 10.3389/fneur.2015.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang W, Huang Q, Yao YY, Wang Y, Wu YL, Wang ZY. Does CSF p-tau181 help to discriminate Alzheimer's disease from other dementias and mild cognitive impairment? A meta-analysis of the literature. J Neural Transm (Vienna) 2014;121:1541–53. doi: 10.1007/s00702-014-1226-y. [DOI] [PubMed] [Google Scholar]
- 48.Hall S, Ohrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. 2012;69:1445–52. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 49.Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, et al. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson's disease. Acta Neuropathol. 2014;128:639–50. doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi M, Kovac A, Korff A, Cook TJ, Ginghina C, Bullock KM, et al. CNS tau efflux via exosomes is likely increased in Parkinson's disease but not in Alzheimer's disease. Alzheimers Dement. 2016;12:1125–31. doi: 10.1016/j.jalz.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moller C, Pijnenburg YA, van der Flier WM, Versteeg A, Tijms B, de Munck JC, et al. Alzheimer Disease and Behavioral Variant Frontotemporal Dementia: Automatic Classification Based on Cortical Atrophy for Single-Subject Diagnosis. Radiology. 2016;279:838–48. doi: 10.1148/radiol.2015150220. [DOI] [PubMed] [Google Scholar]
- 52.Bron EE, Smits M, Papma JM, Steketee RM, Meijboom R, de Groot M, et al. Multiparametric computer-aided differential diagnosis of Alzheimer's disease and frontotemporal dementia using structural and advanced MRI. Eur Radiol. 2016 doi: 10.1007/s00330-016-4691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–52. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 54.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–4. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 55.Majbour NK, Vaikath NN, van Dijk KD, Ardah MT, Varghese S, Vesterager LB, et al. Oligomeric and phosphorylated alpha-synuclein as potential CSF biomarkers for Parkinson's disease. Mol Neurodegener. 2016;11:7. doi: 10.1186/s13024-016-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li G, Xiong K, Korff A, Pan C, Quinn JF, Galasko DR, et al. Increased CSF E-Selectin in Clinical Alzheimer's Disease without Altered CSF Abeta42 and Tau. J Alzheimers Dis. 2015;47:883–7. doi: 10.3233/JAD-150420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.