Abstract
Background
Aspergillus spp. induce elevated levels of several cytokines. It remains unknown whether these cytokines hold value for clinical routine and enhance diagnostic performances of established and novel biomarkers/tests for invasive aspergillosis (IA).
Methods
This cohort study included 106 prospectively enrolled (2014–2017) adult cases with underlying hematological malignancies and suspected pulmonary infection undergoing bronchoscopy. Serum samples were collected within 24 hours of bronchoalveolar lavage fluid (BALF) sampling. Both, serum and BALF samples were used to evaluate diagnostic performances of the Aspergillus-specific lateral-flow device test (LFD), Aspergillus PCR, β-D-glucan, and cytokines that have shown significant associations with IA before .
Results
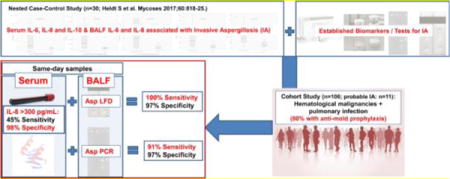
Among 106 cases, 11 had probable IA, and 32 possible IA; 80% received mold-active antifungals at the time of sampling. Diagnostic tests and biomarkers showed better performance in BALF versus blood, with the exception of serum interleukin (IL)-8 which was the most reliable blood biomarker. Combinations of serum IL-8 with either BALF LFD (sensitivity 100%, specificity 94%) or BALF PCR (sensitivity 91%, specificity 97%) showed promise for differentiating probable IA from no IA.
Conclusions
High serum IL-8 levels were highly specific, and when combined with either the BALF Aspergillus-specific LFD, or BALF Aspergillus PCR also highly sensitive for diagnosis of IA.
Keywords: Hematological malignancy, Aspergillus, Mold infection, Serum, BAL, IL-8, mold-active antifungals, galactomannan, prophylaxis
Introduction
Invasive mold infections (IMI) including invasive aspergillosis (IA) are associated with high morbidity and mortality among patients with underlying hematological malignancies [1, 2]. Mycological diagnosis is challenging, with cultures of bronchoalveolar lavage fluid (BALF) having a low sensitivity [3, 4]. Consequently, fungal biomarkers such as galactomannan [GM], beta-D-glucan [BDG] and also molecular diagnostic tests (polymerase chain reaction [PCR]) of blood or BALF, have emerged and are now widely used for diagnosing IA [4, 5]. However, performance of these biomarkers and tests has been shown to be far from perfect, particularly among patients receiving mold-active prophylaxis or treatment, which has been shown to reduce sensitivity of diagnostic tests for IA [5–10]. Despite continuing advances in the diagnostic arsenal, with emerging new diagnostic tests such as the Aspergillus-specific lateral-flow device test [LFD], and new biomarkers [11] on the horizon, IA remains difficult to diagnose.
Performance of currently available biomarkers and tests for early diagnosis of IA and IMI may be enhanced by combination with sensitive and specific immunological markers. In fact, Aspergillus spp. have been shown to induce T-helper cell (Th) 1 and Th17 subsets resulting in elevated levels of several cytokines [12, 13], and recent studies have indicated - after adjusting for multiple covariates also associated with higher cytokine levels - that particularly Interleukin (IL)-8 and IL-6 may show promise as diagnostic markers [14, 15]. It remains however unknown whether these cytokines hold value for clinical routine. Also the added benefit of cytokine testing in addition to, for example, GM testing or PCR has not been evaluated yet.
The objective of this prospective cohort study was to determine the diagnostic potential of IL-8, IL-6 and other cytokines, as well as established and emerging tests for IA and IMI in patients with underlying hematological malignancies in a setting that uses mold-active prophylaxis.
Materials and Methods
This prospective cohort study comprised paired routine serum and BALF samples obtained on the same day from cases with underlying hematological malignancies who underwent routine bronchoscopy due to suspicion of pulmonary infections.
Study cohort
In total 122 cases undergoing bronchoscopy were prospectively enrolled at the Medical University of Graz, Austria, between April 2014 and July 2017. Key inclusion criteria were i.) adult patients with ii.) underlying hematological malignancy who were iii.) at risk for IPA according to the attending clinicians (e.g. febrile neutropenia, induction chemotherapy for acute myeloid leukemia, allogeneic stem cell transplantation) and had iv.) a BALF sample obtained in clinical routine due to suspicion of infection. All patients who met inclusion criteria between April 2014 and July 2017 and signed an informed consent were included in the cohort. After signing the informed consent serum and whole blood samples were collected at the day of bronchoscopy. A total of 16 cases had to be excluded due to the following reasons: 1.) same day blood samples (i.e. collected within 24-hours) were not available (n=14); 2.) BALF volume after routine testings’ were insufficient for further diagnostic work up within the study protocol (n=1); 3.) hematological malignancy was suspected but not confirmed because of mortality within days of admission (n=1). After exclusion of these 16 cases, 106 patients remained in the final analysis.
Biomarker Testing
Conventional culture as well as BALF and serum GM concentrations (Platelia EIA; Bio-Rad Laboratories, Vienna, Austria) were prospectively determined in clinical routine at the Medical University of Graz. Given that the vast majority of patients received mold-active antifungals at the time of bronchoscopy cut-offs of 0.5 GM optical density index (ODI) were used for serum and BALF, following previous evidence that the 0.5 ODI cutoff is preferable in patients on mold-active antifungals [16].
BDG testing was performed in part prospectively and in part retrospectively at the Medical University of Graz, using the commercial available Fungitell® assay (Cape Cod Diagnostics, Falmouth, MA) with an adopted protocol suitable for use on a routine BCS XP® coagulation analyzer, as described previously [17]. BDG testing was only performed in serum samples, as BALF BDG testing yields very low specificity, which is explained by the fact that non-pathogenic Candida colonization in the lung is leading to high BDG values [18–20]. For serum BDG we used the recommended cut-off of ≥80 pg/mL to define positivity.
All blood and BALF samples were initially stored at 4°C. Aliquots of 2 mL were shipped overnight to the Department of Hematology and Oncology, University Hospital of Mannheim, Heidelberg University where a nested Aspergillus PCR assay was performed prospectively in all study samples according to the protocol of Skladny et al. [21, 22]. PCR assays were performed according to the protocol for blood and BALF samples [21]. Extraction of DNA was processed according to the protocol of Sambrook et al. [22]. As an internal control, a 138-bp PCR fragment encoded by the human glucose-6-phosphate dehydrogenase gene was amplified in each clinical sample.
Remaining blood and BALF isolates were stored at −70°C. Cytokine concentrations were determined in serum and BALF samples at the Center for Medical Research of the Medical University of Graz, Austria, between 09/2016 and 10/2017 with a personalized ProcartaPlex® 11plex immunoassay (eBioscience, Vienna, Austria) as described before [14]. Investigators measuring cytokine levels were blinded towards classification of cases into IA and IMI categories and all other clinical and demographic information. The 11 cytokines in the immunoassay were selected based on published literature in human and animal models showing an increase or decrease of these cytokines in blood and/or BALF of cases with IPA [14, 15, 23–28]. The cytokines measured were: IL-4, IL-6, IL-8, IL-10, IL-15, IL-17A, IL-22, soluble IL-2 receptor (sIL-2r), tumors necrosis factor (TNF) α, interferon (IFN) γ, and RANTES (“regulated on activation, normal T cell expressed and secreted”, synonym: chemokine ligand 5). For inclusion into the main analyses we selected only those cytokines that have shown significant associations with IA in the previously conducted nested case-control analysis matched for multiple covariates, including neutrophil status, immunosuppressant and concomitant viral and bacterial infections [14].
Testing with the new Asp LFD (OLM Diagnostics, Newcastle-upon-Tyne, United Kingdom) was performed in the Microbiology Laboratory at the Medical University of Graz in 12/2017. Stored non-hemorrhagic BALF samples where thawed, vortexed, centrifuged at 14,000G and immediately tested by applying 70μL of BALF supernatant to the test, as described previously [19, 29], with results read 10 and 15 minutes later. Following the manufacturer’s instructions, hemorrhagic BALF (after being vortexed and centrifuged) and serum samples were mixed 1:2 with the accompanying buffer, vortexed, heated and centrifuged at 14,000G. 70μL supernatant was applied to the LFD and results were read after 15 minutes and also after 45 minutes.
IA classification and Statistical analysis
IA was graded in accordance with the revised criteria by the European Organization for Research and Treatment of Cancer Invasive Fungal Infections Cooperative Group (EORTC) and the Mycoses Study Group of the National Institute of Allergy and Infectious Disease (MSG) [30], with the exclusion of BDG as a mycological criterion. IMI was graded according to the same criteria but with the inclusion of serum BDG as mycological criterion (i.e., included only in the absence of Pneumocystis or Candida infection).
Our study was conducted in accordance with the Declaration of Helsinki, 2013, Good Clinical Practice. The study protocol was approved by the local ethics committee, Medical University Graz, Austria (EC-numbers 25–221 and 23–343), and registered at ClinicalTrials.Gov (Identifier: NCT02058316 and NCT01576653). Statistical analysis was performed using SPSS, version 24 (SPSS Inc., Chicago, IL, USA). For continuous data, including cytokine levels, receiver operating characteristic (ROC) curves analyses were performed and area under the curve (AUC) values are presented including 95% confidence intervals (95% CI) for the outcomes probable IA diagnosis, possible/probable IA diagnosis, and probable IMI diagnosis (p-values were not corrected for multiple comparisons). Optimal cut-offs for cytokines discriminating patients with probable IA vs. no IA were calculated by using the Youdens index. Utilizing these newly defined cut-offs, performance of cytokines was compared to other tests (Aspergillus PCR and LFD), as well as biomarkers with established cut-offs (BDG and GM), by calculating sensitivity, specificity, positive-predictive value (PPV) and negative-predictive value (NPV), and diagnostic odds ratio (DOR) (a) for probable IA versus no IA (exclusion of possible IA cases; GM as a case defining mycological criterion was not evaluated), and (b) for possible/probable/proven IA versus no IA. Two-sided p-value < 0.05 was taken as cutoff for statistical significance.
Results
Study cohort
A total of 106 cases were included in the final analysis. Eleven cases with probable IA, 32 cases with possible IA (of which 25 had also possible IMI and 7 patients had probable IMI; two had Scedosporium spp. growth in cultures from respiratory samples, and 5 positive serum BDG), and 63 cases classified as not having IA according to EORTC/MSG 2008 criteria. Patients’ characteristics are displayed in Table 1. Overall 85/106 (80%) cases (including 10/11 of those with probable IA and 30/32 of those with possible IA) had received ≥ 2 defined daily doses of mold-active antifungal prophylaxis or treatment at the time concurrent BALF and serum samples were collected (among those with probable IA each three received empirical echinocandin or liposomal amphotericin B treatment, one intravenous voriconazole treatment, two oral voriconazole prophylaxis, and one echinocandin/posaconazole combination treatment). Among the other cases 18 received posaconazole, 17 voriconazole, 17 caspofungin, 14 liposomal amphotericin B, 7 micafungin and 2 anidulafungin at the time of sampling.
Table 1.
Demographic data and underlying diseases of cases with probable, possible invasive aspergillosis (IA), and no evidence for IA as well as probable invasive mold infection (IMI).
| Demographic data, underlying diseases and other characteristics at the time of sampling (n=106) | Probable IA (n=11) | Possible IA (n=32) | No evidence for IA (n=63) | p-value* | probable IMI (n=18; including probable IA) | |
|---|---|---|---|---|---|---|
| Sex |
Female Male |
6 (55%) 5 (45%) |
13 (41%) 19 (59% |
34 (54%) 29 (46%) |
>0.2 | 7 (39%) 11 (61%) |
| Age [years] | Median (Range) | 55 (49–74) | 60 (26–82) | 57 (27–85) | >0.2 | 59 (49–79) |
| Underlying diseases |
AML / MDS NHL MM ALL Others# |
6 (50%) 2 (18%) 1 (9%) 2 (18%) 0 |
22 (60%) 4 (13%) 0 4 (13%) 2 (6%) |
23 (37%) 18 (29%) 8 (13%) 5 (8%) 9 (14%) |
0.06 | 11 (61%) 3 (17%) 1 (6%) 3 (17%) 0 |
| Other characteristics | Autologous SCT | 2 (18%) | 2 (6%) | 7 (11%) | >0.2 | 3 (17%) |
| Allogeneic SCT | 3 (27%) | 11 (34%) | 13 (21%) | >0.2 | 6 (33%) | |
| GvHD | 2 (18%) | 7 (22%) | 7 (11%) | >0.2 | 5 (28%) | |
| Systemic corticosteroid treatment within 14 days of sampling | 6 (55%) | 6 (19%) | 18 (29%) | 0.08 | 8 (44%) | |
| Positive diagnostic test for viral infections within 14 days of sampling | 5 (45%) | 15 (47%) | 18 (29%) | 0.18 | 11 (61%) | |
| Positive diagnostic test for relevant bacterial pathogens/pneumocystis or toxoplasma in BALF§ | 1 (9%) | 8 (25%) | 12 (19%) | >0.2 | 1 (9%) | |
| Neutropenia (<500/μL) ≤10 days | 3 (27%) | 6 (19%) | 11 (17%) | >0.2 | 4 (22%) | |
| Neutropenia (<500/μL) >10 days | 5 (45%) | 15 (47%) | 8 (13%) | <0.001 | 7 (39%) | |
| Mold-active antifungal prophylaxis/treatment (within 24h before sampling and ≥2 days) | 10 (91%) | 30 (94%) | 45 (71%) | 0.02 | 16 (89%) | |
Bold indicates p-values that met statistical significance.
included in cases with possible IA 1 chronic lymphoid leukemia (CLL) and 1 primary myelofibrosis; and in cases without evidence for IMI 4 CLL, 2 primary myelofibrosis, 2 Hodgkin’s lymphoma, and 1 anaplastic anemia.
Bacterial pathogens: 1 in probable IA, 7 in possible IA, 8 in no IA; Pneumocystis: 1 in possible IA, 3 in no IA; Toxoplasma: 1 in no IA.
Abbreviations: ALL = acute lymphocytic leukemia; AML= acute myelogenous leukemia; GvHD = Graft versus Host Disease; IA = invasive aspergillosis; IMI = invasive mold infection; MDS = myelodysplastic syndrome; MM = multiple myeloma; NHL = Non-Hodgkin lymphoma; SCT = stem cell transplantation.
ROC Curve Analysis for IA
In ROC curve analysis of cytokines serum IL-8 (AUC 0.775, 95%CI 0.618–0.932; p=0.004) and serum IL-6 (AUC 0.751, 95%CI 0.604–0.898; p=0.008) were significantly associated with probable IA, while other cytokines (Table 2; supplemental Tables 1a–d and 2) and also serum BDG (AUC 0.605, 95%CI 0.425–0.784; p=0.271) were not. Among BALF cytokines only IL-8 (AUC 0.732, 95%CI 0.584–0.879; p=0.015) was significantly associated with probable IA. Optimal cut-offs calculated by using Youdens index for serum IL-8 were ≥300 pg/mL, ≥60 pg/mL and ≥14 pg/mL, for serum IL-6 ≥40 pg/mL, and for BALF IL-8 ≥60 pg/mL.
Table 2.
Performance of cytokine levels,* Galactomannan (GM) and beta-D-glucan (BDG) in serum and bronchoalveolar fluid (BALF) for differentiating cases with probable IA (n=11) from those without IA (n=63); those with possible/probable IA (n=43) and those without IA (n=63), and those with probable IMI (n=18) versus without IMI (n=63). Significant differences (p<0.05) are bold (p-values not corrected for multiple comparisions).
| Test performance for differentiating probable IA versus no IA | Test performance for differentiating possible/probable IA versus no IA | Test performance for differentiating probable IMI versus no IMI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Biomarker or Cytokine | AUC | 95% CI | p-value | AUC | 95% CI | p-value | AUC | 95% CI | p-value | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | Lower Bound | Upper Bound | ||||||||
| BALF | BAL GM | – | – | – | – | 0.503 | 0.383 | 0.623 | >0.2 | 0.761 | 0.606 | 0.916 | 0.001 |
| IL-4 | 0.457 | 0.305 | 0.610 | >0.2 | 0.564 | 0.455 | 0.673 | >0.2 | 0.489 | 0.347 | 0.630 | >0.2 | |
| IL-6 | 0.680 | 0.542 | 0.817 | 0.059 | 0.602 | 0.494 | 0.710 | 0.076 | 0.650 | 0.516 | 0.784 | 0.068 | |
| IL-8 | 0.732 | 0.584 | 0.879 | 0.015 | 0.582 | 0.471 | 0.692 | 0.154 | 0.692 | 0.563 | 0.821 | 0.013 | |
| Serum | Serum GM | – | – | – | – >0.2 |
0.607 0.514 |
0.496 0.401 |
0.718 0.626 |
0.061 >0.2 |
0.699 | 0.562 | 0.836 | 0.010 |
| Serum BDG | 0.605 | 0.425 | 0.784 | 0.675 | 0.531 | 0.819 | 0.024 | ||||||
| IL-6 | 0.751 | 0.604 | 0.898 | 0.008 | 0.562 | 0.452 | 0.673 | >0.2 | 0.653 | 0.513 | 0.793 | 0.049 | |
| IL-8 | 0.775 | 0.618 | 0.932 | 0.004 | 0.641 | 0.533 | 0.748 | 0.014 | 0.727 | 0.589 | 0.864 | 0.004 | |
| IL-10 | 0.659 | 0.465 | 0.853 | 0.093 | 0.615 | 0.505 | 0.725 | 0.045 | 0.619 | 0.465 | 0.7774 | 0.124 | |
| IL-17A | 0.683 | 0.495 | 0.870 | 0.055 | 0.603 | 0.493 | 0.714 | 0.072 | 0.632 | 0.483 | 0.781 | 0.088 | |
Only cytokines that have shown significant associations with IA in the previously conducted nested case-control analysis matched for multiple covariates, including neutrophil status, immunosuppressant and concomitant viral and bacterial infections [14], were included in the primary analyses of this study.
Abbreviations: AUC: Area under the curve; BALF=bronchoalveolar lavage fluid; BDG=beta-D-glucan; CI: Confidence interval; GM=Galactomannan; IA = invasive aspergillosis; IMI = invasive mold infection; IFN=interferon; IL=interleukin; sIL-2R=soluble IL-2 receptor; RANTES=regulated on activation, normal T cell expressed and secreted (=CCL5); TNF=tumor necrosis factor.
Impact of Bacterial and Viral Infections on Cytokine Levels
In a total of 20 cases with possible (n=8) or no IA (n=12) either relevant bacterial pathogens (n=15), Pneumocystis jirovecii (n=4) or Toxoplasma gondii (n=1) were detected in BALF samples (Table 1). Serum IL-8 (median 61 pg/mL, IQR 17–785 pg/mL vs. median 9 pg/mL, IQR 3–21 pg/mL; p=0.018), serum IL-6 (median 123 pg/mL, IQR 14–808 pg/mL vs. median 24 pg/mL, IQR 9–93 pg/mL; p=0.049) and also BALF IL-8 (median 1756 pg/mL, IQR 713–3665 pg/mL vs. median 485 pg/mL, IQR 197–1109 pg/mL; p=0.012) were all significantly higher in those with probable IA when compared to those with other non-viral infections.
Performance of Biomarkers for probable IA
Table 3 shows performance of tests/biomarkers/cytokines in BALF and in same-day serum samples for differentiating probable IA versus no IA. GM results were used towards classification of IA, and true GM performance was therefore non-evaluable (performance in this study: BALF GM >0.5 ODI sensitivity 91%, specificity 92%; serum GM >0.5 ODI sensitivity 18%, specificity 97%). Overall, tests and biomarkers showed significantly better performance in BALF compared to blood, with the exception of serum IL-8 (cut-off >300 pg/mL: 45% sensitivity and 98% specificity) and IL-6 (cut-off >40; 73% sensitivity and 70% specificity) which proved to be the most reliable blood biomarkers. Performance could not be enhanced by combining serum IL-8 and serum IL-6 (Table 3), but combinations of serum IL-8 (cutoff ≥300 pg/mL) and BALF LFD (i.e., either one or both positive) showed 100% sensitivity (specificity 94% when LFD was read after 10 minutes and 86% when read after 15 minutes), while 91% sensitivity and 97% specificity were achieved when combining serum IL-8 (cutoff ≥300 pg/mL) and BALF Aspergillus PCR.
Table 3.
Performance of diagnostic tests in serum samples (IL-8, IL-6, LFD, Aspergillus PCR, and BDG) and in same-day BALF samples (IL-8, LFD, Aspergillus PCR), as well as combinations, for differentiating probable invasive aspergillosis (IA; n=11) versus no evidence for IA (n=63) ordered by Diagnostic Odds Ratios (DOR). In the right columns: sensitivities for possible IA/possible IMI and possible IA/probable IMI.
| Test/test combination | Sensitivity | Specificity | PPV | NPV | DOR | Positivity in Possible IA / possible IMI cases | Positivity in possible IA / probable IMI cases |
|---|---|---|---|---|---|---|---|
| Serum IL-8 (>300 pg/mL) | 45% (5/11) | 98% (62/63) | 83% (5/6) | 91% (62/68) | 51.7 (5.1–518) | 8% (2/25) | 0% (0/7) |
| Serum IL-8 (>60 pg/mL) | 55% (6/11) | 92% (58/63) | 55% (6/11) | 92% (58/63) | 13.9 (3.1–62.2) | 16% (4/25) | 43% (3/7) |
| Serum IL-8 (>14 pg/mL) | 82% (9/11) | 63% (40/63) | 28% (9/32) | 95% (40/42) | 7.8 (1.6–39.4) | 48% (12/25) | 57% (4/7) |
| Serum IL-6 (>40 pg/mL) | 73% (8/11) | 70% (44/63) | 30% (8/27) | 94% (44/47) | 6.2 (1.5–25.9) | 20% (5/25) | 29% (2/7) |
| Serum LFD (45 min) | 9% (1/11) | 97% (61/63) | 33% (1/3) | 86% (61/71) | 3.0 (0.3–36.9) | 16% (4/25) | 0 |
| Serum BDG (>80 pg/mL) | 45% (5/11) | 75% (47/63) | 24% (5/21) | 89% (47/53) | 2.4 (0.7–9.1) | 4% (1/25)# | 86% (6/7) |
| Blood Aspergillus PCR | 0% (0/10) | 100% (55/55) | – | 85% (55/65) | – | 0 | 0 |
| Serum LFD (15 min) | 0% (0/10) | 98% (54/55) | – | 84% (54/64) | – | 4% (1/25) | 0 |
| BALF LFD (10 min) | 73% (8/11) | 95% (60/63) | 73% (8/11) | 95% (60/63) | 53.3 (9.2–310) | 0 | 0 |
| BALF Aspergillus PCR | 27% (3/11) | 98% (58/59) | 75% (3/4) | 91% (58/64) | 21.8 (2.0–235) | 4% (1/24) | 0 |
| BALF LFD (15 min) | 73% (8/11) | 87% (55/63) | 50% (8/16) | 95% (55/58) | 18.3 (4.0–83.8) | 12% (3/25) | 43% (3/7) |
| BALF IL-8 (>556 pg/mL) | 91% (10/11) | 48% (30/63) | 23% (10/43) | 97% (30/31) | 9.1 (1.1–75.3) | 52% (13/25) | 71% (5/7) |
| BALF IL-8 (>1000 pg/mL) | 73% (8/11) | 67% (42/63) | 28% (8/29) | 93% (42/45) | 5.3 (1.3–22.2) | 36% (9/25) | 43% (3/7) |
| Serum IL-8 (>300 pg/mL) AND/OR Serum IL-6 (>40 pg/mL) | 73% (8/11) | 70% (44/63) | 30% (8/27) | 94% (44/47) | 6.2 (1.5–25.9) | 20% (5/25) | 29% (2/7) |
| Serum IL-8 (>300 pg/mL) AND/OR BALF LFD (10 min) | 100% (11/11) | 94% (59/63) | 73% (11/15) | 100% (59/59) | 304 (15.3–6042) | 8% (2/25) | 0% (0/7) |
| Serum IL-8 (>300 pg/mL) AND/OR BALF LFD (15 min) | 100% (11/11) | 86% (54/63) | 55% (11/20) | 100% (54/54) | 132 (7.2–2432) | 20% (5/25) | 43% (3/7) |
| Serum IL-8 (>300 pg/mL) AND/OR BALF Aspergillus PCR | 91% (10/11) | 97% (57/59) | 83% (10/12) | 98% (57/58) | 285 (23.6–3447) | 12% (3/25) | 0 |
BDG positive in a case with probable IA and Pneumocystis infection and therefore excluded as mycological criterion for probable IMI in that case
Abbreviations: BALF= bronchoalveolar lavage fluid; BDG=beta-D-glucan; IL=Interleukin; IMI=Invasive Mold Infection; LFD=Aspergillus specific Lateral-flow-device Test; NPV=negative predictive value; PPV=positive predictive value;
Performances of tests/biomarkers/cytokines in cases with possible IA/possible IMI as well as cases with possible IA but probable IMI are depicted in Table 3. All cytokines but also the BALF LFD resulted positive in a proportion of cases with possible IA but probable IMI (BDG could not be evaluated as it was used as criterion for defining probable IMI).
ROC Curve Analysis and Performance of Biomarkers for possible/probable IA and probable IMI
ROC curve analysis for differentiating possible and probable IA from no IA revealed that serum IL-8 (AUC 0.641, 95%CI 0.533–0.748; p=0.014), and – only when extrapolated low levels were included - also serum IL-10 (AUC 0.615, 95%CI 0.505–0.725; p=0.045; after extrapolated levels were excluded AUC dropped to 0.598; p=0.089) were predictive for differentiating between cases with possible/probable IA versus no IA. In contrast, other serum cytokines included into the main analysis, BALF cytokines and also serum GM (AUC 0.607), serum BDG (AUC 0.514), and BALF GM (AUC 0.503) were not discriminative (Table 2). Results for differentiating cases with probable IMI (n=18) versus cases without IMI (n=63) are also depicted in Table 2. Results of other cytokines that were not associated with IA in the previous nested case-control analysis are depicted in supplemental Tables 1a–d and 2).
Performance of all tests/biomarkers/cytokines for differentiating cases with clinical/radiological signs and host factors for IA (i.e., possible or probable cases) versus no evidence for IA is depicted in Table 4 (BALF cultures resulted positive in one case only, showing growth of Scedosporium apiospermum in a case with possible IA and probable IMI). Among all the evaluated tests, serum IL 8 (cutoff ≥300 pg/mL) had the highest DOR in that evaluation.
Table 4.
Performance of diagnostic tests in serum samples (IL-8, IL-6, GM, LFD, Aspergillus PCR, and BDG) and in same-day BALF samples (IL-8, GM, LFD, Aspergillus PCR), for differentiating possible/probable invasive aspergillosis (IA; n=43) versus no evidence of IA (n=63).
| Test/test combination* | Sensitivity | Specificity | PPV | NPV | DOR |
|---|---|---|---|---|---|
| Serum IL-8 (>300 pg/mL) | 16% (7/43) | 98% (62/63) | 88% (7/8) | 63% (62/98) | 12.0 (1.4–102) |
| Serum IL-8 (>60 pg/mL) | 30% (13/43) | 92% (58/63) | 72% (13/18) | 66% (58/88) | 5.0 (1.6–15.4) |
| Serum LFD (45 min) | 12% (5/43) | 97% (61/63) | 71% (5/7) | 62% (61/99) | 4.0 (0.7–21.7) |
| Serum IL-8 (>14 pg/mL) | 58% (25/43) | 63% (40/63) | 52% (25/48) | 83% (40/58) | 2.4 (1.1–5.3) |
| Serum LFD (15 min) | 2% (1/43) | 98% (62/63) | 50% (1/2) | 60% (62/104) | 1.5 (0.1–24.3) |
| Serum GM (>0.5 ODI) | 5% (2/43) | 97% (61/63) | 50% (2/4) | 60% (61/102) | 1.5 (0.2–11.0) |
| Serum IL-6 (>40 pg/mL) | 35% (15/43) | 70% (44/63) | 44% (15/34) | 61% (44/72) | 1.2 (0.5–2.8) |
| Serum BDG (>80 pg/mL) | 28% (12/43) | 75% (47/63) | 43% (12/28) | 60% (47/78) | 1.1 (0.5–2.7) |
| Blood Aspergillus PCR | 0% (0/39) | 100% (55/55) | – | 59% (55/94) | – |
| BALF Aspergillus PCR | 10% (4/42) | 98% (58/59) | 80% (4/5) | 60% (58/96) | 6.1 (0.7–56.7) |
| BALF LFD (10 min) | 19% (8/43) | 95% (60/63) | 73% (8/11) | 63% (60/95) | 4.6 (1.1–18.4) |
| BALF GM (>0.5 ODI) | 23% (10/43) | 92% (58/63) | 67% (10/15) | 64% (58/91) | 3.5 (1.1–11.2) |
| BALF LFD (15 min) | 33% (14/43) | 87% (55/63) | 64% (14/22) | 65% (55/84) | 3.3 (1.2–8.8) |
| BALF IL-8 (>556 pg/mL) | 65% (28/43) | 48% (30/63) | 46% (28/61) | 67% (30/45) | 1.7 (0.8–3.8) |
| BALF IL-8 (>1000 pg/mL) | 47% (20/43) | 67% (42/63) | 49% (20/41) | 65% (42/65) | 1.7 (0.8–3.9) |
Combinations of IL-8 >300pg/mL (serum) and/or positive LFD (BALF read after 10 min and 15 min, respectively), as well as IL-8 >300pg/mL (serum) and/or positive Aspergillus PCR (BALF), were also included.
Abbreviations: BALF= bronchoalveolar lavage fluid; BDG=beta-D-glucan; IL=Interleukin; LFD=Aspergillus specific Lateral-flow-device Test; NPV=negative predictive value; PPV=positive predictive value;
Discussion
To date, this study represents one of the most complete prospective studies in terms of the number of diagnostic tests for IA evaluated. We found that in a high risk cohort of hematological malignancy patients, where >80% of cases had received mold-active antifungals at the time of sampling, diagnostic tests and biomarkers in BALF showed overall better performance than those in blood. There was one exception, however: high serum IL-8 levels (and to a lesser extend also serum IL-6 levels) were significantly associated with probable IA, and serum IL-8 proved to be the most reliable blood biomarker for probable IA, with better diagnostic performance than serum GM, BDG, LFD and blood Aspergillus PCR. A high serum IL-8 cutoff of >300pg/mL was highly specific, and when combined with either the BALF LFD, or the BALF Aspergillus PCR, also highly sensitive, delivering nearly perfect performance for differentiating probable IA from no IA. Serum IL-8 showed also potential for differentiating patients with possible/probable or proven IA from those without IA, exhibiting the highest cut-off related DOR of all evaluated tests.
Diagnosis of mold infection is challenging, in particular in patients receiving prophylaxis or early empirical treatment with mold-active antifungals [6, 8, 31], and a number of studies have recommended combination of multiple biomarkers to achieve sufficient sensitivity in this setting [4, 7, 8, 32]. Our findings clearly show the overall superior performance of biomarkers in BALF when compared to same day blood samples in those patients. The poor results of all established biomarkers in blood samples might be explained by the high percentage of prior antifungal treatment with consecutively reduced fungal burden in peripheral blood [33], and that this study used one time blood sample testing as opposed to serial screening. As different fungal biomarkers in blood can be found at different stages of fungal diseases, single time point testing may underestimate the diagnostic performance of distinct biomarkers [34].
Significantly better diagnostic performances were observed for serum IL-8 and also serum IL-6 when compared to established blood biomarkers. Studies have shown that cytokines are centrally involved in protective immunity against Aspergillus spp. and other molds [14, 35]. In early stages of IA, conidia are killed by local alveolar macrophages, and IL-8, also known as neutrophil chemotactic factor, is produced by macrophages and epithelial cells as an important chemoattractant for neutrophils [12, 35]. The mechanism of IL-8 increase during IPA has been studied in previous in-vitro studies, reporting an up-regulation of gene transcription by Aspergillus fumigatus proteases as cause of increased release of IL-8 (and also IL-6 which plays an important role in T cell recruitment) by A549 pulmonary epithelial cells and primary epithelial cells [36]. Other studies have shown that in-vitro opsonization of Aspergillus fumigatus conidia with H-ficolin, L-ficolin [23] and M-ficolin, which play essential roles in pathogen recognition and complement activation through the lectin pathway, potentiate IL-8 secretion of A549 lung epithelial cells [15, 37, 38].
We found that in this cohort of high-risk hematological malignancy patients receiving mold-active antifungals at the time of sampling, IL-8 i.) was the most reliable blood biomarker for IA and IMI, ii.) showed excellent specificity when a high cut-off was used and iii.) exhibited close to perfect performance when combined with either the BALF LFD or BALF Aspergillus PCR. The findings of this cohort study therefore confirm those of a smaller previous nested case-control study, which used matched controls based on a number of factors that had been described to be associated with elevated cytokine levels, and found that levels of IL-8 and IL-6 were significantly higher in serum and BALF among cases with IPA versus matched controls [14]. In line, also another very recently published study which evaluated solely BALF cytokines, found that IL-8 was the major discriminator between IPA and no IPA [15]. The cohort study design and the collection of same day serum samples allowed us to get one step further by determining the “real-life” diagnostic potential of these cytokines for IA and IMI without taking into account numerous covariates, and to also evaluate combinations of IL-8 with other IA biomarkers/diagnostic tests, such as the LFD and PCR. Really the most important finding was that all analyses confirmed the potential clinical value of IL-8 in diagnosing IA and to a lesser extend also IMI, as a single stand-alone highly specific test, or even better a highly sensitive and specific test when combined with the BALF LFD or BALF PCR.
Limitations
As an important limitation there were no proven cases and the number of cases with probable IA and also probable IMI was low, despite the study period of more than 3 years, which is the natural result of highly effective anti-mold prophylaxis strategies in place at our center. Overall fungal infections are rare in patients receiving anti-mold prophylaxis (mostly 2%–3% prevalence [39, 40]), and therefore multicenter studies are needed to confirm our findings in larger cohorts. To avoid bias introduced by multiple comparisons and confounding factors, we also had to rely on results of a smaller nested matched case-control analysis for identification of cytokines that were evaluated in the primary analyses of this cohort study. Case-control pairs in this nested analysis were matched for multiple covariates that may affect cytokine levels [17].
Conclusion
In conclusion, high serum IL-8 levels were highly specific, and when combined with either the BALF LFD, or BALF Aspergillus PCR, also highly sensitive, delivering nearly perfect performance for differentiating probable IA from no IA. Our study indicates that serum IL-8 testing may be a valuable addition to clinical routine for diagnosing IA and IMI in high risk patients who receive mold-active antifungals.
Supplementary Material
Highlights.
Blood and BALF biomarkers for diagnosis of invasive aspergillosis were evaluated
Biomarkers showed overall better performance in BALF compared to blood
In blood, IL-8 proved to be the most reliable biomarker showing high specificity
Combination of blood IL-8 with BAL Aspergillus-specific LFD showed high sensitivity
Combination of blood IL-8 with BAL Aspergillus PCR was also highly sensitive for IA
Acknowledgments
The authors acknowledge the support of Jennifer Ober and Sabrina Obersteiner in sample processing and testing, as well as the team of the Clinical Institute of Medical and Chemical Laboratory Diagnostics in providing routinely collected samples and performing routine, GM and BDG testing.
Funding
This work was supported by funds of the Gilead Investigator Initiated Study IN-AT-131-1939, and the Oesterreichische Nationalbank (Anniversary Fund, project number 15346).
This work has also partly been carried out with the K1 COMET Competence Center CBmed, which is funded by the Federal Ministry of Transport, Innovation and Technology (BMVIT); the Federal Ministry of Science, Research and Economy (BMWFW); Land Steiermark (Department 12, Business and Innovation); the Styrian Business Promotion Agency (SFG); and the Vienna Business Agency. The COMET program is executed by the FFG (The Austrian Research Promotion Agency, project number 844609), and has been partially supported by grants from the National Institutes of Health (MH 113477, AI106039, AI036214, and MH062512). The funders had no role in study design, data collection, analysis, interpretation, decision to publish, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
D. Buchheidt is consultant to Basilea, Gilead Sciences, Merck Sharp & Dohme/Merck; received research grants from Gilead Sciences and Pfizer; serves on the speakers’ bureau of Astellas, Basilea, Gilead Sciences, Merck Sharp & Dohme/Merck, and Pfizer; received travel grants from Astellas, Gliead Sciences, Merck Sharp & Dohme/Merck, and Pfizer.
M. Hoenigl received research grants from Gilead; served on the speakers’ bureau of Gilead, Basilea and Merck.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Original data of this manuscript have been presented - in part - at ECCMID 2017, Vienna, Austria (poster presentation number 0989), 51st Scientific Meeting of the German speaking Mycological Society (DMykG), Muenster, Germany (oral presentation), and TIMM 2017, Belgrade, Serbia (poster presentation number 098)
Conflicts of interest
J. Prattes received consulting fee from Gilead.
A. Wölfler received speaker honoraria from Merck.
All other authors no conflict.
References
- 1.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective Surveillance for Invasive Fungal Infections in Hematopoietic Stem Cell Transplant Recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 2.Prattes J, Lackner M, Eigl S, et al. Diagnostic accuracy of the Aspergillus-specific bronchoalveolar lavage lateral-flow assay in haematological malignancy patients. Mycoses. 2015;58:461–9. doi: 10.1111/myc.12343. [DOI] [PubMed] [Google Scholar]
- 3.Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–9. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 4.Hoenigl M, Prattes J, Spiess B, et al. Performance of galactomannan, beta-d-glucan, Aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J Clin Microbiol. 2014;52:2039–45. doi: 10.1128/JCM.00467-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchheidt D, Reinwald M, Hoenigl M, Hofmann WK, Spiess B, Boch T. The evolving landscape of new diagnostic tests for invasive aspergillosis in hematology patients: strengths and weaknesses. Curr Opin Infect Dis. 2017;30:539–44. doi: 10.1097/QCO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 6.Hoenigl M, Prattes J, Neumeister P, Wolfler A, Krause R. Real-world challenges and unmet needs in the diagnosis and treatment of suspected invasive pulmonary aspergillosis in patients with haematological diseases: An illustrative case study. Mycoses. 2018;61:201–5. doi: 10.1111/myc.12727. [DOI] [PubMed] [Google Scholar]
- 7.Eigl S, Prattes J, Reinwald M, et al. Influence of mould-active antifungal treatment on the performance of the Aspergillus-specific bronchoalveolar lavage fluid lateral-flow device test. Int J Antimicrob Agents. 2015;46:401–5. doi: 10.1016/j.ijantimicag.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Eigl S, Hoenigl M, Spiess B, et al. Galactomannan testing and Aspergillus PCR in same-day bronchoalveolar lavage and blood samples for diagnosis of invasive aspergillosis. Med Mycol. 2017;55:528–34. doi: 10.1093/mmy/myw102. [DOI] [PubMed] [Google Scholar]
- 9.Springer J, Lackner M, Nachbaur D, et al. Prospective multicentre PCR-based Aspergillus DNA screening in high-risk patients with and without primary antifungal mould prophylaxis. Clin Microbiol Infect. 2016;22:80–6. doi: 10.1016/j.cmi.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Reinwald M, Hummel M, Kovalevskaya E, et al. Therapy with antifungals decreases the diagnostic performance of PCR for diagnosing invasive aspergillosis in bronchoalveolar lavage samples of patients with haematological malignancies. J Antimicrob Chemother. 2012;67:2260–7. doi: 10.1093/jac/dks208. [DOI] [PubMed] [Google Scholar]
- 11.Orasch T, Prattes J, Faserl K, et al. Bronchoalveolar lavage triacetylfusarinine C (TAFC) determination for diagnosis of invasive pulmonary aspergillosis in patients with hematological malignancies. J Infect. 2017;75:370–373. doi: 10.1016/j.jinf.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargo JF, Husain S. Immune correlates of protection in human invasive aspergillosis. Clin Infect Dis. 2014;59:569–77. doi: 10.1093/cid/ciu337. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Vidal C, Viasus D, Carratala J. Pathogenesis of invasive fungal infections. Curr Opin Infect Dis. 2013;26:270–6. doi: 10.1097/QCO.0b013e32835fb920. [DOI] [PubMed] [Google Scholar]
- 14.Heldt S, Eigl S, Prattes J, et al. Levels of interleukin (IL)-6 and IL-8 are elevated in serum and bronchoalveolar lavage fluid of haematological patients with invasive pulmonary aspergillosis. Mycoses. 2017;60:818–25. doi: 10.1111/myc.12679. [DOI] [PubMed] [Google Scholar]
- 15.Goncalves SM, Lagrou K, Rodrigues CS, et al. Evaluation of Bronchoalveolar Lavage Fluid Cytokines as Biomarkers for Invasive Pulmonary Aspergillosis in At-Risk Patients. Front Microbiol. 2017;8:2362. doi: 10.3389/fmicb.2017.02362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinwald M, Buchheidt D, Hummel M, et al. Diagnostic performance of an Aspergillus-specific nested PCR assay in cerebrospinal fluid samples of immunocompromised patients for detection of central nervous system aspergillosis. PLoS One. 2013;8:e56706. doi: 10.1371/journal.pone.0056706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prüller F, Wagner J, Raggam RB, et al. Automation of serum (1–3)-beta-D-glucan testing allows reliable and rapid discrimination of patients with and without candidemia. Med Mycol. 2014;52:455–61. doi: 10.1093/mmy/myu023. [DOI] [PubMed] [Google Scholar]
- 18.Reischies FM, Prattes J, Pruller F, et al. Prognostic potential of 1,3-beta-d-glucan levels in bronchoalveolar lavage fluid samples. J Infect. 2016;72:29–35. doi: 10.1016/j.jinf.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Prattes J, Flick H, Pruller F, et al. Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am J Respir Crit Care Med. 2014;190:922–9. doi: 10.1164/rccm.201407-1275OC. [DOI] [PubMed] [Google Scholar]
- 20.Krause R, Halwachs B, Thallinger GG, et al. Characterisation of Candida within the Mycobiome/Microbiome of the Lower Respiratory Tract of ICU Patients. PLoS One. 2016;11:e0155033. doi: 10.1371/journal.pone.0155033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skladny H, Buchheidt D, Baust C, et al. Specific detection of Aspergillus species in blood and bronchoalveolar lavage samples of immunocompromised patients by two-step PCR. J Clin Microbiol. 1999;37:3865–71. doi: 10.1128/jcm.37.12.3865-3871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook JE, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. 1989 [Google Scholar]
- 23.Bidula S, Sexton DW, Abdolrasouli A, et al. The serum opsonin L-ficolin is detected in lungs of human transplant recipients following fungal infections and modulates inflammation and killing of Aspergillus fumigatus. J Infect Dis. 2015;212:234–46. doi: 10.1093/infdis/jiv027. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho A, Cunha C, Bistoni F, Romani L. Immunotherapy of aspergillosis. Clin Microbiol Infect. 2012;18:120–5. doi: 10.1111/j.1469-0691.2011.03681.x. [DOI] [PubMed] [Google Scholar]
- 25.Gresnigt MS, Rosler B, Jacobs CW, et al. The IL-36 receptor pathway regulates Aspergillus fumigatus-induced Th1 and Th17 responses. Eur J Immunol. 2013;43:416–26. doi: 10.1002/eji.201242711. [DOI] [PubMed] [Google Scholar]
- 26.Potenza L, Vallerini D, Barozzi P, et al. Characterization of specific immune responses to different Aspergillus antigens during the course of invasive Aspergillosis in hematologic patients. PLoS One. 2013;8:e74326. doi: 10.1371/journal.pone.0074326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceesay MM, Kordasti S, Rufaie E, et al. Baseline cytokine profiling identifies novel risk factors for invasive fungal disease among haematology patients undergoing intensive chemotherapy or haematopoietic stem cell transplantation. J Infect. 2016;73:280–8. doi: 10.1016/j.jinf.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 28.Shen HP, Tang YM, Song H, Xu WQ, Yang SL, Xu XJ. Efficiency of interleukin 6 and interferon gamma in the differentiation of invasive pulmonary aspergillosis and pneumocystis pneumonia in pediatric oncology patients. Int J Infect Dis. 2016;48:73–7. doi: 10.1016/j.ijid.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Hoenigl M, Eigl S, Heldt S, Duettmann W, Thornton C, Prattes J. Clinical evaluation of the newly formatted lateral-flow device for invasive pulmonary aspergillosis. Mycoses. 2018;61:40–3. doi: 10.1111/myc.12704. [DOI] [PubMed] [Google Scholar]
- 30.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornely OA, Lass-Florl C, Lagrou K, Arsic-Arsenijevic V, Hoenigl M. Improving outcome of fungal diseases - Guiding experts and patients towards excellence. Mycoses. 2017;60:420–5. doi: 10.1111/myc.12628. [DOI] [PubMed] [Google Scholar]
- 32.Boch T, Spiess B, Cornely OA, et al. Diagnosis of invasive fungal infections in haematological patients by combined use of galactomannan, 1,3-beta-D-glucan, Aspergillus PCR, multifungal DNA-microarray, and Aspergillus azole resistance PCRs in blood and bronchoalveolar lavage samples: results of a prospective multicentre study. Clin Microbiol Infect. 2016;22:862–8. doi: 10.1016/j.cmi.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis. 2005;40:1762–9. doi: 10.1086/429921. [DOI] [PubMed] [Google Scholar]
- 34.Mery A, Sendid B, Francois N, et al. Application of Mass Spectrometry Technology to Early Diagnosis of Invasive Fungal Infections. J Clin Microbiol. 2016;54:2786–97. doi: 10.1128/JCM.01655-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winn RM, Gil-Lamaignere C, Roilides E, et al. Selective effects of interleukin (IL)-15 on antifungal activity and IL-8 release by polymorphonuclear leukocytes in response to hyphae of Aspergillus species. J Infect Dis. 2003;188:585–90. doi: 10.1086/377099. [DOI] [PubMed] [Google Scholar]
- 36.Borger P, Koeter GH, Timmerman JA, Vellenga E, Tomee JF, Kauffman HF. Proteases from Aspergillus fumigatus induce interleukin (IL)-6 and IL-8 production in airway epithelial cell lines by transcriptional mechanisms. J Infect Dis. 1999;180:1267–74. doi: 10.1086/315027. [DOI] [PubMed] [Google Scholar]
- 37.Ghufran MS, Ghosh K, Kanade SR. A fucose specific lectin from Aspergillus flavus induced interleukin-8 expression is mediated by mitogen activated protein kinase p38. Med Mycol. 2017;55:323–33. doi: 10.1093/mmy/myw066. [DOI] [PubMed] [Google Scholar]
- 38.Houser J, Komarek J, Kostlanova N, et al. A soluble fucose-specific lectin from aspergillus fumigatus conidia–structure, specificity and possible role in fungal pathogenicity. PLoS One. 2013;8:e83077. doi: 10.1371/journal.pone.0083077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duarte RF, Sanchez-Ortega I, Cuesta I, et al. Serum galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective antimold prophylaxis. Clin Infect Dis. 2014;59:1696–702. doi: 10.1093/cid/ciu673. [DOI] [PubMed] [Google Scholar]
- 40.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–59. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


