Abstract
BACKGROUND
Patients who travel a long distance (≥50 miles) for cancer care have improved outcomes. However, to the authors’ knowledge, the prevalence of long travel distances for treatment by patients with head and neck squamous cell carcinoma (HNSCC), and the effect of travel distance on overall survival (OS), remains unknown.
METHODS
The authors used the National Cancer Data base from 2004 through 2013 to identify patients with HNSCC undergoing definitive treatment. Travel distance for treatment was categorized as short (<12.5 miles), intermediate (12.5-49.9 miles), and long (50-249.9 miles). The primary outcome, OS, was evaluated using Cox shared-frailty modeling. A secondary outcome, factors associated with intermediate and long travel distances, was evaluated using multivariable hierarchical logistic regression.
RESULTS
Among 118,000 patients with HNSCC, 62,753 (53.2%), 40,644 (34.4%), and 14,603 (12.4%) patients, respectively, traveled short, intermediate, and long distances for treatment. After adjusting for relevant covariates, long travel distance was associated with treatment at academic and high-volume centers. Patients of black race, of Hispanic ethnicity, with Medicaid insurance, and who were treated with nonsurgical treatment were less likely to travel long distances for treatment (P<.001). Traveling a long distance for treatment was associated with improved OS on multivariable analysis (adjusted hazard ratio, 0.93; 95% confidence interval, 0.89-0.96) compared with a short distance.
CONCLUSIONS
Traveling a long distance for HNSCC treatment is associated with improved survival, especially for patients receiving nonsurgical management. Racial and ethnic disparities in travel for HNSCC treatment exist. As regionalization of care continues, future work should identify and address reasons for racial and ethnic disparities in travel that may prevent access to care at high-volume facilities.
Keywords: head and neck cancer, health services, quality of care, racial disparities, travel distance
INTRODUCTION
Numerous studies have demonstrated a positive relationship between treatment at a high-volume facility and improved outcomes such as survival for a variety of malignancies,1,2 including head and neck squamous cell carcinoma (HNSCC).3–5Although it is responsible for approximately 12,000 deaths annually in the United States,6 HNSCC is relatively uncommon and requires complex, multidisciplinary management for optimal outcomes.7 Therefore, some have advocated for the regionalization of HNSCC care to high-volume centers.8 Receiving care at high-volume centers, which generally are located in large urban areas, may require some patients to travel greater distances.9 Although traveling a greater distance for cancer treatment has been associated with improved outcomes for patients with prostate, colon, esophageal, liver, and pancreatic malignancies,10–12 to our knowledge the effect of travel distance on survival in patients with HNSCC remains unknown.
Prior work has demonstrated the existence of racial disparities in travel for cancer care,10,13 especially for travel to high-volume facilities.12,14 The increasing travel requirements that have occurred contemporaneously with the progressive regionalization of cancer care may be creating another barrier to equitable, quality cancer care and contributing toward worsening racial and ethnic disparities in cancer outcomes.14 However, to our knowledge, the prevalence of long travel distances for treatment by patients with HNSCC has not been described and the question of whether racial disparities in patterns of travel for HNSCC exist has not been investigated to date.
Given the areas of uncertainty in knowledge regarding travel patterns among patients with HNSCC, we sought to answer the following questions: 1) what effect does travel distance have on overall survival (OS)?; 2) how frequently do patients travel a long distance (50-249.9 miles) for the treatment of HNSCC?; and 3) which patients are likely to travel long distances for HNSCC treatment?
MATERIALS AND METHODS
Data Source
The National Cancer Data Base (NCDB) is a hospital-based cancer registry that is a joint program of the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society. The NCDB annually collects high-quality and internally appraised data from >1500 CoC-accredited hospitals in the United States.15
Study Cohort
The Medical University of South Carolina institutional review board deemed this study exempt from review. This article was reviewed and approved by the American College of Surgeons CoC. The NCDB from 2004 through 2013 was used to examine travel distance among patients with HNSCC undergoing curative-intent treatment. HNSCC diagnoses were filtered using International Classification of Disease for Oncology, Third Edition codes (Supporting Table 1) and SCC histology codes. A total of 131,147 patients were identified as undergoing treatment at the reporting facility (to ensure correct correlation of travel distance for treatment). Patients with an American Joint Committee on Cancer (AJCC) clinical M classification of 1 or unknown (4350 patients), those who received palliative-intent treatment (2431 patients), those with a time to treatment initiation ≥180 days (3567 patients), and those with a travel distance ≥250 miles or unknown (2799 patients)10,13 were excluded, producing a cohort of 118,000 patients.
Study Variables
The primary outcome measure was OS. Travel distance to the reporting facility is provided by the NCDB as the greatest circle distance (in miles) between the patient’s and hospital’s ZIP code centroid.16 In this study, travel distance was categorized into short (<12.5 miles), intermediate (12.5-49.9 miles), and long (50-249.9 miles) based on prior work.10,13,17,18
Patient-level covariates included age, sex, race, ethnicity, educational attainment, income, rurality, and insurance. Race and ethnicity were presented according to the Standards for the Classification of Federal Data on Race and Ethnicity as approved by the Office of Management and Budget.19 Clinical factors included severity of comorbidity, oncologic characteristics (tumor site, AJCC clinical stage), treatment year, and treatment modality. Treatment modality was categorized as surgery, surgery plus adjuvant therapy (radiotherapy [RT] or chemoradiation [CRT]), primary RT, and primary CRT. Hospital-level covariates included treatment facility type and annual facility volume,12 which was grouped in quartiles (1-9, >9 to 17, >17 to 43, and >43 cases/year).4 Other categorical variables were grouped as previously described.20
Statistical Analysis
Descriptive statistics were generated to characterize patient-level and hospital-level characteristics and bivariate analyses were conducted to evaluate their relationship with travel distance. Differences between travel distance groups were analyzed using chi-square tests. Multivariable hierarchical, multinomial logistic regression analysis was performed to analyze the relationship between covariates and travel distance for treatment. A 2-level hierarchical regression model (patient-level/hospital-level characteristics and US geographic region) was chosen to assess the relationship between patient-level/hospital-level variables with travel distance because the data likely are correlated.13 That is, cancer care provider density is heterogeneous across the United States as a whole but more homogenous within regions of the United States, and therefore patients within the same region face similar travel distance choices when accessing care.17
The relationship between travel distance and survival was analyzed using a multivariable Cox shared-frailty model for the same reasons that a hierarchical logistic regression was performed. Associations between covariates were investigated using the variable inflation factor before modeling. The overall variable inflation factors for all variables were <5 (except insurance, for which it was 6), and therefore none were deemed collinear. Variables significant at an α level of .05 on univariable analysis were entered into the multivariable Cox shared-frailty model. Possible interaction effects between travel distance, survival and race, subsite, AJCC clinical stage, treatment modality, facility volume, and facility type were examined. Interaction terms for race, subsite, and treatment modality were significant and were included in a separate survival model.
Multiple sensitivity analyses were performed to confirm the robustness of the results. To ensure that the relationship between travel distance and survival was not an artifact of the categorization strata, the multivariable Cox shared-frailty model was repeated using travel distance as a continuous variable with adjusted hazard ratios (aHRs) reported for 10-mile travel increments.10,13 Although prior studies analyzing travel distance excluded patients traveling >250 miles,10,13,17,18 we performed an additional sensitivity analysis including those patients who traveled >250 miles. Because of the biological and prognostic differences between carcinogen-mediated and human papillomavirus (HPV)-related HNSCC,21 we performed a sensitivity analysis excluding patients with HPV-related oropharyngeal SCC (using Collaborative Stage Site-Specific Factor 10 codes 020-060). Because the NCDB did not record HPV status until 2010, but many patients from 2004 through 2010 likely had HPV-related cancers, an additional sensitivity analysis was performed excluding all patients with oropharyngeal SCC.
Data analysis was performed using SAS statistical software (version 9.4; SAS Institute Inc, Cary, North Carolina) and R packages (R Foundation, Vienna, Austria). All statistical tests were 2-sided with statistical significance set at a P value of .05. Measures of precision of point estimates are presented as 95% confidence intervals (95% CIs).
RESULTS
Demographic, Clinicopathologic, and Treatment Characteristics
A total of 118,000 patients with HNSCC undergoing treatment from 2004 through 2013 were included in the analysis. The patient demographic, clinicopathologic, and treatment characteristics and their relationship to travel distance for treatment are presented in Table 1. Overall, 53, 34, and 12% of patients traveled short, intermediate, and long distances, respectively, for treatment of HNSCC. The mean travel distance was 5, 115, 94 miles for short, intermediate, and long distances, respectively.
TABLE 1.
Sociodemographic, Oncologic, and Treatment Characteristics Stratified by Travel Distance for Treatment
| Characteristic | Short Distance N = 62,753 |
Intermediate Distance N = 40,644 |
Long Distance N = 14,603 |
P |
|---|---|---|---|---|
| Variable | No. (%) | No. (%) | No. (%) | |
| Age, y | <.001 | |||
| <50 | 10,247 (16.3) | 7474 (18.4) | 2812 (19.3) | |
| 50-59 | 20,262 (32.3) | 13,543 (33.3) | 4837 (33.1) | |
| 60-69 | 17,225 (27.4) | 11,597 (28.5) | 4062 (27.8) | |
| ≥70 | 15,019 (23.9) | 8030 (19.8) | 2892 (19.8) | |
| Sex | .008 | |||
| Male | 47,090 (75.0) | 30,696 (75.5) | 10,843 (74.3) | |
| Female | 15,663 (25.0) | 9948 (24.5) | 3670 (25.7) | |
| Race | <.001 | |||
| White | 51,496 (82.1) | 36,766 (90.5) | 13,295 (91.0) | |
| Black | 8894 (14.2) | 2622 (6.5) | 841 (5.8) | |
| Other | 2363 (3.7) | 1256 (3.0) | 467 (3.2) | |
| Ethnicity | <.001 | |||
| Non-Hispanic | 55,710 (88.8) | 36,973 (91.0) | 13,406 (91.8) | |
| Hispanic | 3195 (5.1) | 954 (2.3) | 306 (2.1) | |
| Other/unknown | 2363 (3.7) | 1256 (3.0) | 467 (3.2) | |
| Charlson/Deyo Comorbidity Score | <.001 | |||
| 0 | 49,087 (78.2) | 32,087 (78.9) | 11,506 (78.8) | |
| 1 | 10,407 (16.6) | 6699 (16.5) | 2457 (16.8) | |
| ≥2 | 3259 (5.2) | 1858 (4.6) | 640 (4.4) | |
| Insurance | <.001 | |||
| Private | 26,154 (41.7) | 18,358 (45.2) | 6002 (41.1) | |
| Medicare | 23,775 (37.9) | 14,677 (36.1) | 5327 (36.5) | |
| Medicaid | 6801 (10.8) | 3511 (8.6) | 1491 (10.2) | |
| Uninsured | 3841 (6.1) | 2467 (6.1) | 997 (6.8) | |
| Other | 2182 (3.5) | 1631 (4.0) | 786 (5.4) | |
| Education, quartiles | <.001 | |||
| Highest | 14,671 (23.5) | 8193 (20.2) | 1835 (12.6) | |
| Second highest | 19,699 (31.4) | 13,258 (32.6) | 4781 (32.7) | |
| Second lowest | 16,767 (26.7) | 11,757 (28.9) | 4944 (33.9) | |
| Lowest | 11,502 (18.3) | 7401 (18.2) | 3024 (20.7) | |
| Unknown | 24 (0.0) | 25 (0.1) | 19 (0.1) | |
| Income, quartiles | <.001 | |||
| Highest | 18,638 (29.7) | 12,199 (30.0) | 1556 (10.7) | |
| Second highest | 16,228 (25.9) | 11,235 (27.6) | 3253 (22.3) | |
| Second lowest | 14,393 (22.9) | 10,195 (25.1) | 5383 (36.9) | |
| Lowest | 13,441 (21.4) | 6952 (17.1) | 4373 (29.9) | |
| Unknown | 53 (0.1) | 63 (0.2) | 38 (0.3) | |
| County type | <.001 | |||
| Metropolitan | 58,821 (93.7) | 28,752 (70.8) | 6334 (43.4) | |
| Urban | 2632 (4.2) | 9832 (24.2) | 6807 (46.6) | |
| Rural | 47 (0.1) | 1265 (3.1) | 1157 (7.9) | |
| Unknown | 1253 (2.0) | 794 (2.0) | 305 (2.1) | |
| Site | <.001 | |||
| Oral cavity | 14,072 (22.4) | 11,516 (28.3) | 5980 (41.0) | |
| Oropharynx | 23,563 (37.5) | 15,178 (37.3) | 4603 (31.5) | |
| Hypopharynx | 3374 (5.4) | 1744 (4.3) | 504 (3.5) | |
| Larynx | 21,744 (34.7) | 12,206 (30.0) | 3516 (24.1) | |
| AJCC clinical stage grouping | <.001 | |||
| I | 15,639 (24.9) | 10,229 (25.2) | 3289 (22.5) | |
| II | 9630 (15.3) | 6274 (15.4) | 2363 (16.2) | |
| III | 11,704 (18.7) | 7299 (18.0) | 2462 (16.9) | |
| IV | 25,780 (41.1) | 16,842 (41.4) | 6489 (44.4) | |
| Treatment modality | <.001 | |||
| Surgery | 12,147 (19.4) | 10,703 (26.3) | 6044 (41.4) | |
| Surgery plus adjuvant | 12,490 (19.9) | 8707 (21.4) | 3864 (26.5) | |
| Radiotherapy | 20,998 (33.5) | 10,575 (26.0) | 1781 (12.2) | |
| Chemoradiation | 17,118 (27.3) | 10,659 (26.2) | 2914 (20.0 | |
| Facility type | <.001 | |||
| Community | 6190 (9.9) | 3408 (8.4) | 325 (2.2) | |
| Comprehensive community | 25,535 (40.7) | 14,776 (36.4) | 2661 (18.2) | |
| Academic | 22,918 (36.5) | 17,869 (44.0) | 10,179 (69.7) | |
| Integrated network | 6793 (10.8) | 3547 (8.7) | 972 (6.7) | |
| Unknown | 1317 (2.1) | 1044 (2.6) | 466 (3.2) | |
| Facility annual volume, quartiles | <.001 | |||
| 1-9 | 18,505 (29.5) | 7920 (19.5) | 1022 (7.0) | |
| >9 to 17 | 17,808 (28.4) | 10,839 (26.7) | 1927 (13.2) | |
| >17 to 43 | 15,817 (25.2) | 10,164 (25.0) | 3069 (21.0) | |
| >43 | 10,623 (16.9) | 11,721 (28.8) | 8585 (58.8) | |
| Region of United States | <.001 | |||
| New England | 3915 (6.4) | 2118 (5.3) | 210 (1.5) | |
| Middle Atlantic | 9980 (16.2) | 5035 (12.7) | 1164 (8.2) | |
| South Atlantic | 13,883 (22.6) | 9826 (24.8) | 3097 (21.9) | |
| East North Central | 13,785 (22.4) | 7882 (19.9) | 2341 (16.6) | |
| East South Central | 3904 (6.4) | 4031 (10.2) | 1911 (13.5) | |
| West North Central | 3854 (6.3) | 3171 (8.0) | 2400 (17.0) | |
| West South Central | 3694 (6.0) | 2962 (7.5) | 1207 (8.5) | |
| Mountain | 2291 (3.7) | 1394 (3.5) | 780 (5.5) | |
| Pacific | 6130 (3.7) | 3181 (8.0) | 1027 (7.3) | |
| Year of diagnosis | <.001 | |||
| 2004-2005 | 10,120 (16.1) | 5902 (14.5) | 2065 (14.1) | |
| 2006-2007 | 10,838 (17.3) | 6603 (16.2) | 2367 (16.2) | |
| 2008-2009 | 13,032 (20.8) | 8183 (20.1) | 2943 (20.2) | |
| 2010-2011 | 13,809 (22.0) | 9311 (22.9) | 3380 (23.1) | |
| 2012-2013 | 14,953 (23.8) | 10,645 (26.2) | 3848 (26.4) |
Abbreviations: AJCC, American Joint Committee on Cancer.
Factors Associated With Increasing Travel Distance
A hierarchical multinomial logistic regression analysis was performed to identify factors associated with intermediate and long travel distances for treatment (Table 2). African American patients had 69% lower odds of traveling a long distance (adjusted odds ratio [aOR], 0.31; 95% CI, 0.28-0.34) compared with white patients. Hispanic individuals traveled a long distance less frequently than non-Hispanic individuals (aOR, 0.54; 95% CI, 0.46-0.62). Those patients with Medicaid (aOR, 0.75, 95% CI, 0.69-0.82) or no insurance (aOR, 0.82; 95% CI, 0.74-0.91) had a lower odds of traveling long distances compared with those with private insurance. Compared with patients undergoing surgical management, patients treated with RT (aOR, 0.21; 95% CI, 0.20-0.23) or CRT (aOR, 0.29; 95% CI, 0.27-0.32) were less likely to travel long distances for treatment.
TABLE 2.
Multivariable Hierarchical Regression Analysis of Factors Associated With Intermediate and Long Travel Distances for Treatment
| Intermediate Versus Short Distance | Long Versus Short Distance | |||
|---|---|---|---|---|
| Variable | Adjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
| Age, y | ||||
| <50 | 1.00 (reference) | 1.00 (reference) | ||
| 50-59 | 0.93 (0.89-0.97) | .001 | 0.94 (0.87-1.01) | .07 |
| 60-69 | 0.93 (0.89-0.98) | .006 | 0.96 (0.88-1.04) | .32 |
| ≥70 | 0.72 (0.68-0.76) | <.001 | 0.73 (0.66-0.81) | <.001 |
| Sex | ||||
| Male | 1.00 (reference) | 1.00 (reference) | ||
| Female | 0.95 (0.92-0.99) | .007 | 0.88 (0.83-0.94) | <.001 |
| Race | ||||
| White | 1.00 (reference) | 1.00 (reference) | ||
| Black | 0.44 (0.41-0.46) | <.001 | 0.31 (0.28-0.34) | <.001 |
| Other | 0.69 (0.63-0.75) | <.001 | 0.72 (0.62-0.84) | <.001 |
| Ethnicity | ||||
| Non-Hispanic | 1.00 (reference) | 1.00 (reference) | ||
| Hispanic | 0.48 (0.45-0.53) | <.001 | 0.54 (0.46-0.62) | <.001 |
| Charlson/Deyo Comorbidity Score | ||||
| 0 | 1.00 (reference) | 1.00 (reference) | ||
| 1 | 0.97 (0.93-1.01) | .17 | 0.87 (0.82-0.93) | <.001 |
| ≥2 | 0.90 (0.83-0.95) | <.001 | 0.73 (0.65-0.82) | <.001 |
| Insurance | ||||
| Private | 1.00 (reference) | 1.00 (reference) | ||
| Medicare | 0.98 (0.94-1.02) | .26 | 0.96 (0.89-1.03) | .22 |
| Medicaid | 0.82 (0.78-0.87) | <.001 | 0.75 (0.69-0.82) | <.001 |
| Uninsured | 0.88 (0.82-0.94) | <.001 | 0.82 (0.74-0.91) | <.001 |
| Other | 1.11 (1.03-1.21) | .009 | 1.50 (1.33-1.69) | <.001 |
| Education, quartiles | ||||
| Highest | 1.00 (reference) | 1 (reference) | ||
| Second highest | 1.46 (1.40-1.53) | <.001 | 1.31 (1.21-1.42) | <.001 |
| Second lowest | 1.90 (1.81-2.01) | <.001 | 1.14 (1.03-1.25) | .008 |
| Lowest | 2.37 (2.22-2.53) | <.001 | 1.02 (0.91-1.14) | .77 |
| Income, quartiles | ||||
| Highest | 1.00 (reference) | 1.00 (reference) | ||
| Second highest | 0.73 (0.70-0.76) | <.001 | 1.81 (1.66-1.97) | <.001 |
| Second lowest | 0.49 (0.46-0.51) | <.001 | 2.17 (1.98-2.39) | <.001 |
| Lowest | 0.27 (0.26-0.29) | <.001 | 1.74 (1.56-1.94) | <.001 |
| County type | ||||
| Metropolitan | 1.00 (reference) | 1.00 (reference) | ||
| Urban | 14.97 (14.15-15.83) | <.001 | 65.59 (60.90-70.65) | <.001 |
| Rural | 118.4 (87.1-161.0) | <.001 | 943.6 (685.8->999) | <.001 |
| Site | ||||
| Oral cavity | 1.00 (reference) | 1.00 (reference) | ||
| Oropharynx | 0.93 (0.89-0.98) | .004 | 0.83 (0.77-0.89) | <.001 |
| Hypopharynx | 0.88 (0.81-0.95) | <.001 | 0.71 (0.62-0.81) | <.001 |
| Larynx | 0.94 (0.90-0.98) | <.008 | 0.80 (0.75-0.86) | <.001 |
| AJCC clinical stage grouping | ||||
| I | 1.00 (reference) | 1.00 (reference) | ||
| II | 1.11 (1.06-1.17) | <.001 | 1.40 (1.29-1.52) | <.001 |
| III | 1.11 (1.05-1.17) | <.001 | 1.52 (1.40-1.66) | <.001 |
| IV | 1.19 (1.13-1.24) | <.001 | 1.83 (1.69-1.98) | <.001 |
| Treatment modality | ||||
| Surgery | 1.00 (reference) | 1 (reference) | ||
| Surgery plus adjuvant | 0.77 (0.74-0.81) | <.001 | 0.53 (0.49-0.57) | <.001 |
| Radiotherapy | 0.61 (0.58-0.64) | <.001 | 0.21 (0.20-0.23) | <.001 |
| Chemoradiation | 0.67 (0.63-0.71) | <.001 | 0.29 (0.27-0.32) | <.001 |
| Facility type | ||||
| Community | 1.00 (reference) | 1.00 (reference) | ||
| Comprehensive community | 1.39 (1.30-1.49) | <.001 | 3.90 (3.32-4.56) | <.001 |
| Academic | 1.71 (1.58-1.85) | <.001 | 12.34 (10.36-14.70) | <.001 |
| Integrated network | 1.00 (0.92-1.10) | .92 | 3.83 (3.32-4.56) | <.001 |
| Facility annual volume, quartiles | ||||
| 1 to 9 | 1.00 (reference) | 1.00 (reference) | ||
| >9 to 17 | 1.72 (1.64-1.81) | <.001 | 1.63 (1.46-1.82) | <.001 |
| >17 to 43 | 2.02 (2.91-2.13) | <.001 | 2.95 (2.62-3.31) | <.001 |
| >43 | 3.68 (3.47-3.91) | <.001 | 13.86 (12.26-15.67) | <.001 |
Abbreviations: CI, confidence interval; AJCC, American Joint Committee on Cancer; OR, odds ratio.
Hospital-level characteristics were found to be associated with the odds of long travel distances for treatment. Patients were 12-fold more likely to travel a long distance for care at an academic center (aOR, 12.3; 95% CI, 10.4-14.7) and 14-fold more likely to travel for care at a high-volume facility (aOR, 13.9; 95% CI, 12.3-15.7).
Subgroup Analysis Examining Reasons for Racial and Ethnic Differences in Travel
Given the observed racial and ethnic disparities in the likelihood of traveling for treatment, subset analyses in African American and Hispanic patients were performed to characterize determinants of travel in these groups. In the subset of African American individuals, any nonprivate form of insurance and residence within a ZIP code with lower income levels were associated with a decreased likelihood of traveling a long distance for HNSCC care (Supporting Table 2). Similar to the entire cohort, African American patients were less likely to travel for RT (aOR, 0.14; 95% CI, 0.10-0.19) and CRT (aOR, 0.19; 95% CI, 0.14-0.26) but more likely to travel a long distance for treatment at an academic (aOR, 10.4; 95% CI, 4.6-23.3) or high-volume (aOR, 10.4; 95% CI, 6.1-17.6) facility on hierarchical logistic regression modeling. In the subset of patients of Hispanic ethnicity (Supporting Table 3), uninsured patients (aOR, 0.47; 95% CI, 0.14-0.81) and those residing within a ZIP code with lower educational levels had lower odds of traveling a long distance. Hispanic individuals were more likely to travel a long distance for treatment at an academic (aOR, 12.5; 95% CI, 3.97-39.44) or high-volume (aOR, 9.59; 95% CI, 4.65-19.80) facility. There was no interaction observed between race, ethnicity, and the odds of traveling a long distance for HNSCC care (P = .07).
Association Between Travel Distance and Survival
In the multivariable Cox shared-frailty model adjusting for relevant covariates (Fig. 1), patients who traveled a long distance for treatment had improved OS compared with patients who traveled a short distance (aHR, 0.93; 95% CI, 0.89-0.96). Other covariates found to be associated with OS included age, race, insurance, comorbidity, subsite, AJCC clinical stage, treatment modality, facility type, and facility volume. In a subset analysis of African American patients, Cox shared-frailty modeling demonstrated that the risk of mortality for a long distance compared with a short distance was unchanged in terms of effect size (aHR, 0.92; 95% CI, 0.82-1.03).
Figure 1.
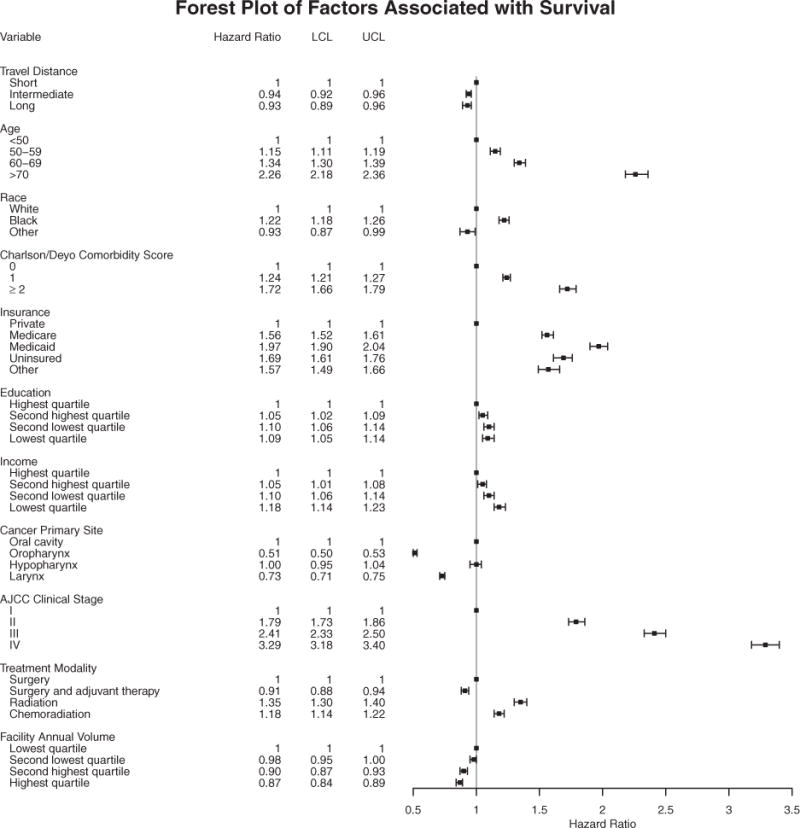
Multivariable Cox shared-frailty model demonstrating the effect of travel distance for treatment and other covariates on survival among 118,000 patients treated for head and neck squamous cell carcinoma. Estimated adjusted hazard ratios are shown as black circles; the 95% confidence intervals are represented by horizontal lines. AJCC indicates American Joint Committee on Cancer; LCL, lower confidence limit; UCL, upper confidence limit.
Subgroup Analysis of Interaction Effects of Travel Distance With Other Covariates and Survival
Interaction effects between travel distance, survival and race, subsite, AJCC clinical stage, treatment modality, facility volume, and facility type were examined. Significant interactions were found for race, subsite, and treatment modality (P<.001), but not for disease stage (P = .58), facility volume (P = .95), or facility type (P = .80). A subgroup effects model demonstrating the interaction between travel distance and race, subsite, and treatment modality was developed (Supporting Table 4). Figure 2 shows the interaction between travel distance and race (Fig. 2A), subsite (Fig. 2B), and treatment modality (Fig. 2C). The subgroup analysis shows that for treatment modality, the improved OS observed with increased travel distance was primarily due to the benefit of longer travel for RT and CRT. Interaction testing for subsite demonstrated that the effect of travel distance on OS is mediated through oropharyngeal and oral cavity cancers.
Figure 2.
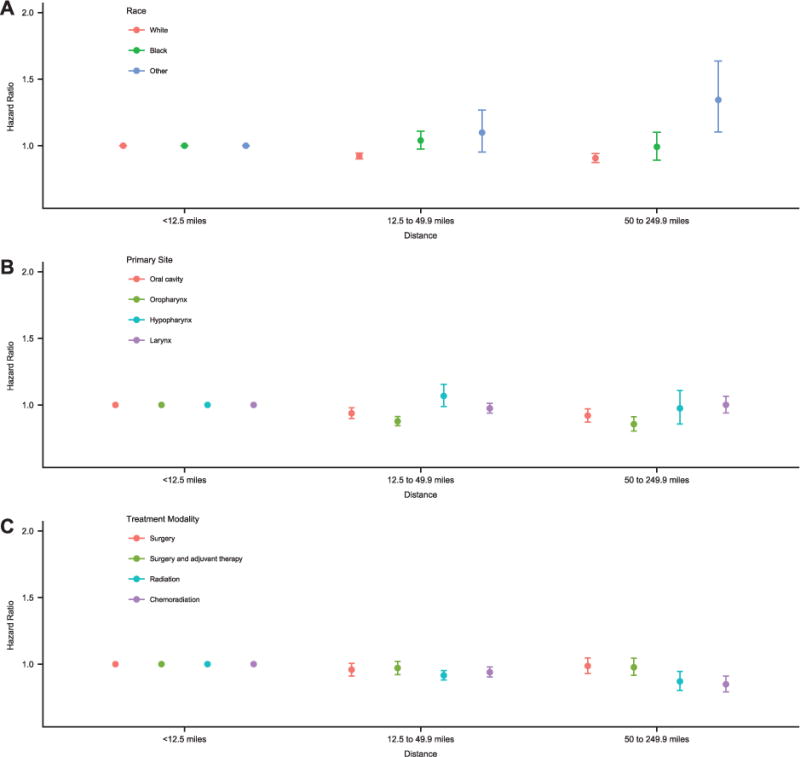
Subgroup analysis demonstrating interactions between covariates and travel distance for treatment for those covariates for which significant interactions existed (race, primary site, and treatment modality). Each hazard ratio is the combined effect of the covariate and travel distance for treatment compared with a reference travel distance of <12.5 miles. Survival according to travel distance varied by (A) race (white, black, or other), (B) primary head and neck subsite (oral cavity, oropharynx, hypopharynx, or larynx); and (C) treatment modality (surgery, surgery and adjuvant therapy, radiotherapy, or chemoradiation).
Sensitivity Analyses
Numerous sensitivity analyses were performed to confirm the robustness of the relationship between longer travel and survival. Increasing travel distance remained associated with improved OS when analyzed as a continuous variable; the risk of death decreased by 1% for every 10-mile increase in travel distance (aHR, 0.99; 95% CI, 0.98-0.99). Including patients who traveled ≥250 miles for treatment did not change the improvement in survival noted with longer travel distance (aHR, 0.92; 95% CI, 0.89-0.95) (Supporting Table 5). Excluding patients with high-risk HPV-positive oropharyngeal SCC (aHR, 0.89; 95% CI, 0.86-0.92) (Supporting Table 6) and all patients with oropharyngeal SCC (aHR, 0.93; 95% CI, 0.90-0.97) (Supporting Table 7) did not alter the relationship between longer travel distance and improved survival. An additional sensitivity analysis excluding patients with oral cavity cancer was performed because these individuals were more likely to travel a long distance for treatment and had improved survival. The association between long travel distance and survival remained unchanged (aHR, 0.89; 95% CI, 0.85-0.93) (Supporting Table 8).
DISCUSSION
In the current study, we demonstrated that patients who traveled a long distance (50-249.9 miles) for HNSCC treatment had a decreased risk of death compared with those who traveled a short distance (<12.5 miles). To our knowledge, the current study is the first to demonstrate a significant association between increasing travel distance and improved OS among patients with HNSCC. The association between travel distance and survival has been examined for other cancer sites and the current study findings are consistent with these prior studies.10–12 The survival benefit observed from increasing travel distance is partially a consequence of the regionalization of care to high-volume centers with resultant improvements in oncologic outcomes,11,12,14 because patients in the current study who traveled a long distance for treatment were found to be significantly more likely to be treated at high-volume and academic centers. In HNSCC,3–5 as in other malignancies,1,2 there is evidence to support a relationship between higher patient volumes and improved outcomes. However, the current study data support the association between long travel distance and survival independent of facility type, facility volume, age, race, insurance status, treatment modality, and a variety of relevant clinical and sociodemographic variables. Similar effect sizes were noted in the African American patient subset as well as in numerous sensitivity analyses. Nevertheless, there is a likely role for unmeasured confounding patient and treatment facility characteristics in this association between longer travel distance and improved survival. Given that long travel distances generally are regarded as a burden to cancer care,17 patients who can overcome the barrier associated with travel distance possibly are more highly motivated, supported socially, health-seeking in their behaviors, and adherent to treatment recommendations.10,11 Future research should identify these additional unmeasured variables that in part mediate the relationship between travel distance and survival and focus interventions on increasing these health-promoting behaviors among all patients with HNSCC.
Given the independent association between long travel distances and survival in patients with HNSCC, identifying which patients are least likely to travel long distances for treatment is important to ensure equitable care. African American patients had a >3-fold decrease in the odds of traveling long distances for treatment compared with white patients, and Hispanic patients had a 2-fold decreased odds of traveling a long distance compared with non-Hispanic patients. To the best of our knowledge, the reasons that African American and Hispanic patients are less likely to travel long distances for the treatment of HNSCC compared with white and non-Hispanic patients are not known but are likely multifactorial in nature. Racial and ethnic disparities in travel for cancer treatment have been documented for patients with non-HNSCC12,22; the results of the current study add to the growing literature. Lack of insurance, lack of access to an automobile or someone to drive patients to treatment, and financial toxicity as well as cultural beliefs regarding health care have been shown to contribute toward racial and ethnic differences in travel patterns.22–24 The results of the current study generally are in agreement, because we also observed that certain social determinants of health (insurance, education, and income) contributed toward low rates of traveling long distances for HNSCC care in African American and Hispanic patients. Two exceptions included the finding that higher educational attainment at census tract quartile levels was inversely associated with a higher likelihood of long travel distance in African American patients and that lower census tract income quartiles were not associated with decreased odds of traveling longer for HNSCC care. Whether these racial and ethnic differences in income and education represent true associations or are artifacts of the data collection (ZIP code-level quartiles) is unknown.
Racial and ethnic disparities in HNSCC survival were present in the current study and are well documented in other studies.25,26 These disparities are due in part to inequities in stage of disease at the time of presentation, timely care, guideline-concordant care, and access to care.25,26 The exact roles that racial and ethnic disparities in travel distance play in racial and ethnic disparities in outcomes is unknown. The independent improvement in survival associated with long travel distances for treatment in the African American subset was of the same magnitude as observed in the overall cohort. Although African American patients were more likely to be treated at academic centers and high-volume centers when they traveled a long distance for treatment, the benefit of longer travel persisted independent of these factors for African American individuals as it did for the cohort at large. As cancer care continues to be centralized in high-volume institutions and travel distances for the treatment of HNSCC care increase,14 racial and ethnic differences in travel for HNSCC care may exacerbate existing racial and ethnic disparities in outcomes. Further work is necessary to identify and address barriers related to travel for HNSCC care for African American and Hispanic patients to develop strategies to improve the equity and quality of HNSCC care.
We also found that patients who underwent nonsurgical management were 3-fold less likely to travel long distances for treatment, even though traveling a long distance for nonsurgical management correlated with improved survival. The reasons for discrepancies in willingness to travel for surgical versus nonsurgical care for HNSCC are unknown. However, the fact that patients are more willing to travel for surgery than (chemo)radiation has been documented for other malignancies,10 despite studies demonstrating a volume-outcome relationship for RT.27 Differential travel patterns for the surgical and nonsurgical treatment of HNSCC have implications for multidisciplinary evaluation and management, processes of care that improve survival.7 As cancer care continues to regionalize to high-volume centers, identifying and addressing the barriers to travel for patients receiving nonsurgical modalities will be a critically important part of elevating the quality of care for all patients with HNSCC.
In addition to race, ethnicity, and treatment modality, other factors were found to be associated with the likelihood of traveling a long distance for HNSCC care. As noted in other oncologic sites,10,11,18 patients with more severe comorbidities were less likely to travel, presumably reflecting on the need to be sufficiently healthy to withstand the physical demands of long travel.
Limitations
The current study had important limitations. Because it was a retrospective database study, reasons for the choice of travel distance could not be discerned. These may include factors related to patient motivation, insurance network restrictions, local referral patterns, travel cost, health-seeking behaviors, and social support. The calculation of travel distance to all surrounding hospitals was not possible. Therefore, we were unable to ascertain whether patients voluntarily traveled greater distances to seek care (bypassing a possible treatment facility) when they could have sought care closer to home or know where they stayed during treatment. Provider density, which is known to vary across the United States,17 was not assessed. Hierarchical regression modeling was used to control for this, but whether this technique fully addressed this concern is unknown. Treatment biases inherent in the retrospective observational study design may affect survival. Multilevel Cox models were used to control for this source of bias, but statistical analysis cannot control for relevant variables not captured in the NCDB. In addition, the survival benefit observed with surgery compared with RT, although consistent with other studies using the NCDB,28 was not fully explored and likely is related to the use of RT for oral cavity cancer, differences in the frequency of AJCC stage 1 disease, treatment at academic centers, and treatment at high-volume centers.3 Individual, patient-level socioeconomic information is not available in the NCDB. Adjustments were made for ZIP code-level income and education, but these may be inadequate. Despite these limitations, the current study possesses numerous methodological strengths. It captured patients of all adult ages, with a variety of insurance types, and different treatment modalities; had a national scope, large sample size, and relevant oncologic details; and analyzed treatment at different types of hospitals.
Conclusions
Traveling a long distance for the treatment of HNSCC is associated with improved OS, especially for patients receiving nonsurgical management. Racial and ethnic disparities in travel for HNSCC treatment exist. As regionalization of care continues, future work should identify and address the reasons for racial and ethnic disparities in travel that may prevent access to care at high-volume facilities.
Supplementary Material
Acknowledgments
FUNDING SUPPORT Supported by the Biostatistics Shared Resource of Hollings Cancer Center at the Medical University of South Carolina (P30 CA138313), Comparative Effectiveness Data Analytics Resources (CEDAR) core, Medical University of South Carolina Office of the Provost, and the South Carolina Clinical and Translational Research (SCTR) Institute of the Medical University of South Carolina (NCATS UL1 TR001450).
Footnotes
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
AUTHOR CONTRIBUTIONS
Evan M. Graboyes: Conceptualization, formal analysis, methodology, writing-original draft, and writing-review and editing. Mark A. Ellis: Conceptualization, data curation, formal analysis, and writing-original draft. Hong Li: Conceptualization, formal analysis, methodology, and writing-review and editing. John M. Kaczmar, Anand K. Sharma, Eric J. Lentsch, Terry A. Day, and Chanita Hughes Halbert: Conceptualization, methodology, and writing-review and editing.
References
- 1.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 3.David JM, Ho AS, Luu M, et al. Treatment at high-volume facilities and academic centers is independently associated with improved survival in patients with locally advanced head and neck cancer. Cancer. 2017;123:3933–3942. doi: 10.1002/cncr.30843. [DOI] [PubMed] [Google Scholar]
- 4.Gourin CG, Forastiere AA, Sanguineti G, et al. Impact of surgeon and hospital volume on short-term outcomes and cost of laryngeal cancer surgical care. Laryngoscope. 2011;121:85–90. doi: 10.1002/lary.21348. [DOI] [PubMed] [Google Scholar]
- 5.Chen AY, Fedewa S, Pavluck A, Ward EM. Improved survival is associated with treatment at high-volume teaching facilities for patients with advanced stage laryngeal cancer. Cancer. 2010;116:4744–4752. doi: 10.1002/cncr.25364. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer Facts & Figures. 2016 http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed December 28, 2016.
- 7.Lewis CM, Nurgalieva Z, Sturgis EM, Lai SY, Weber RS. Improving patient outcomes through multidisciplinary treatment planning conference. Head Neck. 2016;38(suppl 1):E1820–E1825. doi: 10.1002/hed.24325. [DOI] [PubMed] [Google Scholar]
- 8.Eskander A, Merdad M, Irish JC, et al. Volume-outcome associations in head and neck cancer treatment: a systematic review and meta-analysis. Head Neck. 2014;36:1820–1834. doi: 10.1002/hed.23498. [DOI] [PubMed] [Google Scholar]
- 9.Birkmeyer JD, Siewers AE, Marth NJ, Goodman DC. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003;290:2703–2708. doi: 10.1001/jama.290.20.2703. [DOI] [PubMed] [Google Scholar]
- 10.Vetterlein MW, Loppenberg B, Karabon P, et al. Impact of travel distance to the treatment facility on overall mortality in US patients with prostate cancer. Cancer. 2017;123:3241–3252. doi: 10.1002/cncr.30744. [DOI] [PubMed] [Google Scholar]
- 11.Wasif N, Chang YH, Pockaj BA, Gray RJ, Mathur A, Etzioni D. Association of distance traveled for surgery with short- and long-term cancer outcomes. Ann Surg Oncol. 2016;23:3444–3452. doi: 10.1245/s10434-016-5242-z. [DOI] [PubMed] [Google Scholar]
- 12.Jindal M, Zheng C, Quadri HS, et al. Why do long-distance travelers have improved pancreatectomy outcomes? J Am Coll Surg. 2017;225:216–225. doi: 10.1016/j.jamcollsurg.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massarweh NN, Chiang YJ, Xing Y, et al. Association between travel distance and metastatic disease at diagnosis among patients with colon cancer. J Clin Oncol. 2014;32:942–948. doi: 10.1200/JCO.2013.52.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol. 2009;27:4671–4678. doi: 10.1200/JCO.2008.20.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American College of Surgeons. National Cancer Data Base-data dictionary PUF. 2014 http://ncdbpuf.facs.org/node/259?q=print-pdf-all. Accessed November 21, 2016.
- 17.Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the US. Cancer. 2008;112:909–918. doi: 10.1002/cncr.23229. [DOI] [PubMed] [Google Scholar]
- 18.Lin CC, Bruinooge SS, Kirkwood MK, et al. Association between geographic access to cancer care, insurance, and receipt of chemotherapy: geographic distribution of oncologists and travel distance. J Clin Oncol. 2015;33:3177–3185. doi: 10.1200/JCO.2015.61.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Office of Management and Budget. Standards for the classification of federal data on race and ethnicity. https://obamawhitehouse.archives.gov/omb/fedreg_race-ethnicity/. Accessed April 9, 2018.
- 20.Graboyes EM, Garrett-Mayer E, Sharma AK, Lentsch EJ, Day TA. Adherence to National Comprehensive Cancer Network guidelines for time to initiation of postoperative radiation therapy for patients with head and neck cancer. Cancer. 2017;123:2651–2660. doi: 10.1002/cncr.30651. [DOI] [PubMed] [Google Scholar]
- 21.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 22.Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5:361–366. [PubMed] [Google Scholar]
- 23.Esnaola NF, Ford ME. Racial differences and disparities in cancer care and outcomes: where’s the rub? Surg Oncol Clin N Am. 2012;21:417–437, viii. doi: 10.1016/j.soc.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahal BA, Chen YW, Sethi RV, et al. Travel distance and stereotactic body radiotherapy for localized prostate cancer. Cancer. 2018;124:1141–1149. doi: 10.1002/cncr.31190. [DOI] [PubMed] [Google Scholar]
- 25.Molina MA, Cheung MC, Perez EA, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer. 2008;113:2797–2806. doi: 10.1002/cncr.23889. [DOI] [PubMed] [Google Scholar]
- 26.Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116:1093–1106. doi: 10.1097/01.mlg.0000224939.61503.83. [DOI] [PubMed] [Google Scholar]
- 27.Chen YW, Mahal BA, Muralidhar V, et al. Association between treatment at a high-volume facility and improved survival for radiation-treated men with high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2016;94:683–690. doi: 10.1016/j.ijrobp.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Murphy CT, Galloway TJ, Handorf EA, et al. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J Clin Oncol. 2016;34:169–178. doi: 10.1200/JCO.2015.61.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


