Abstract
The incidence of allergic conditions has continued to rise over the past several decades, with a growing body of research dedicated towards the treatment of such conditions. By driving a complex range of changes in the underlying immune response, immunotherapy is the only therapy that modulates the immune system with long-term effects and is presently utilized for the treatment of several atopic condition. Recent efforts have focused on identifying biomarkers associated with these changes that may be of use in predicting patients with the highest likelihood of positive clinical outcomes during allergen immunotherapy (AIT), providing guidance regarding AIT discontinuation, and predicting symptomatic relapse and the need for booster AIT after therapy. The identification of such biomarkers in food allergy has the additional benefit of replacing oral food challenges, which are presently the gold standard for diagnosing food allergies. While several markers have shown early promise, research has yet to identify a marker that can invariably predict clinical response to AIT. Skin prick testing (SPT) and specific IgE have commonly been used as inclusion criteria for the initiation of AIT and prediction of reactions during subsequent allergen challenge; however, existing data suggests that changes in these markers are not always associated with clinical improvement and can be widely variable, reducing their utility in predicting clinical response. Similar findings have been described for the use of allergen-specific functional IgG4 antibodies, basophil activation and histamine release, and type 2 innate lymphoid cells. There appears to be a promising association between changes in the expression of dendritic cell-associated markers, as well as the use of DNA promoter region methylation patterns in the prediction of allergy status following therapy. The cellular and molecular changes brought about by immunotherapy are still under investigation, but major strides in our understanding are being made.
Keywords: Biomarkers, immunotherapy, prognostic, allergen, food allergy
Introduction
As defined by the National Institute of Allergy and Infectious diseases, allergic diseases comprise of symptomatic conditions such as asthma, atopic dermatitis and food allergy (FA). The incidence of these allergic conditions have continued to rise over the past few decades, with an increasing number of individuals having more than one of these allergic conditions. For patients whose symptoms are not abated by conventional pharmacotherapies such as, nasal glucocorticoids and antihistamines, allergen immunotherapy (AIT), in the form of subcutaneous immunotherapy (SCIT) or sublingual immunotherapy (SLIT), is the only safe and effective option [1,2] that reduces symptoms and the need for rescue medications [3–5], improves quality of life, [6] and could provide long-term clinical benefits after cessation of treatment [7–9]. AIT is the only FDA-approved therapy that modifies the underlying immune response in IgE-mediated diseases, such as allergic rhinitis, allergic asthma, stinging insect hypersensitivity, and atopic dermatitis [1,10–13]. Presently, immunotherapy is under research for its application in IgE-mediated FA, and although not FDA-approved, it is gaining popularity in off-label use.
The Immune Response in Allergic Disease
Allergic diseases are due to a dysregulated immune system [14–17] that is associated with an increase in inflammation and the formation of specific IgE antibodies against otherwise harmless environmental and food antigens [18,19]. The inflammatory response is Type 2 T helper (Th2) cell mediated and involves both the innate and the adaptive immune arms [19]. The production of several cytokines is associated with this response, including a cluster of cytokines encoded on chromosome 5q31–33: Interleukin (IL)-3, IL-4, IL-5, IL-9 and IL-13 [20]. IL-17 produced by Type 17 T helper (Th17) cells [21–23]; and IL-25, IL-31, IL-33 and Thymic stromal lymphopoietin (TSLP) produced by tissue cells [20].
There are two phases to the immune response in allergic diseases, including the sensitization phase and the effector phase [24–26]. During the sensitization phase (Figure 1), specific mucosal-resident dendritic cell (DC) subsets capture allergens in the skin, airways, or gut and subsequently internalize and transport the allergens to draining lymph nodes [18]. Within the lymph nodes, the antigens are processed and presented to naïve CD4+ T cells by MHC class II molecules [18] leading to their differentiation into allergen-specific CD4+ Th2 cells which produce high levels of IL-4 and IL-13. Activation of CD4+ Th2 cells occurs through phosphorylation of the trans-acting T cell–specific transcription factor GATA-3 [14,18], driving the production of IgE isotypes by B cells [18]. Natural killer T (NKT) cells and basophils contribute to the process of sensitization by producing IL-4 early in the process [18,27]. As the IgE memory B cells mature, they differentiate into plasma cells and begin to produce large amounts of allergen-specific IgE antibodies (sIgE). These sIgE antibodies bind to the high-affinity FcεRI receptors on the surface of basophils and mast cells. Following the sensitization phase, subsequent exposure to allergen results in crosslinking of the surface bound sIgE to the allergen and activation of mast cells and basophils, leading to clinical symptoms [18].
Fig. 1. Mechanism of sensitization phase in allergic disease.
During the allergic sensitization phase, mucosal dendritic cells (DC) capture allergen, which are transported to neighboring lymph nodes after processing. Within the lymph nodes, allergen-specific Th2 cells proliferate in the presence of IL-4 and endothelial cell-derived Th2 cytokines. Natural killer T (NKT) cells and basophils may provide an early source of IL-4. Activated allergen specific Th2 cells further produce IL-4 and IL-13 which favors B cell isotype class switching to specific IgE cells, which bind to the surface of effector cells such as mast cells and basophils through the high affinity IgE receptor FcεR1 leading to sensitization. During this phase, a memory pool of allergen specific B cells and allergen specific Th2 cells are generated.
Resident tissue cells also contribute to allergen sensitization. While epithelial cells normally form a barrier, providing the first line of defense against environmental allergens, this barrier function is compromised in allergic individuals, allowing allergens to enter the submucosa. This entry results in chronic inflammation and contributes to further sensitization [26,28,29]. Additionally, epithelial cells are able to promote sensitization through the production of various proinflammatory cytokines, including IL-25, IL-31 or TSLP, and IL-33, that act on DCs and type 2 innate lymphoid cells (ILC2s) to further promote a Th2-skewed response [30,31].
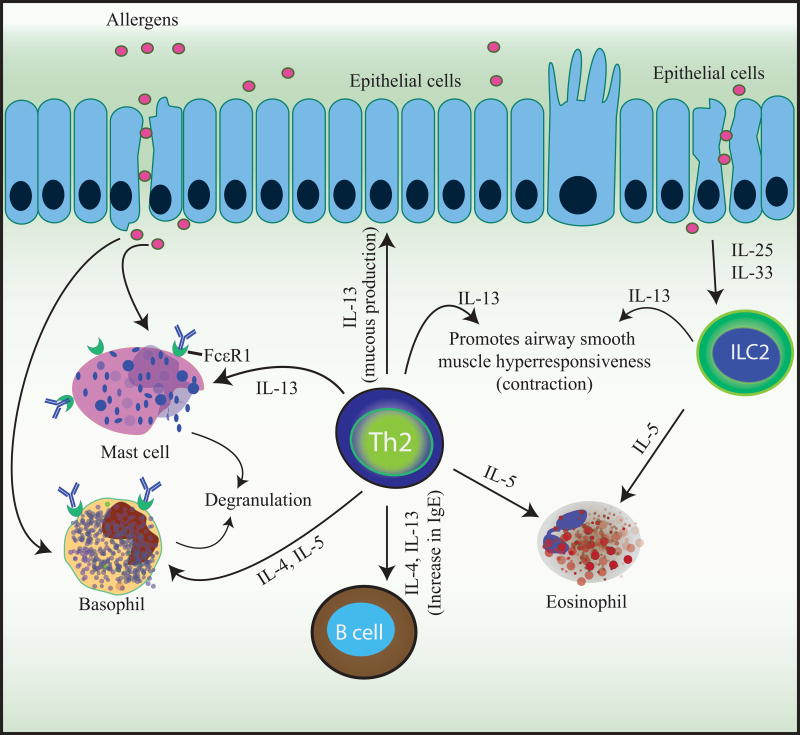
Following the sensitization phase, the effector phase (Figure 2) is initiated when an allergen is presented and causes cross-linking of the sIgE bound to the FcεR1 receptor on sensitized mast cells and basophils [18]. Crosslinking of FcεRI receptors leads to the subsequent release of preformed and de novo synthesized proinflammatory mediators, such as histamine, heparin, and proteases, as well as prostaglandins, leukotrienes, and cytokines which are all responsible for the immediate phase reaction and acute inflammation [18]. The late-phase reaction is facilitated by the accumulation of the inflammatory mediators produced by the effector cells and the activation of memory allergen-specific Th2 cells. These Th2 cells produce IL-4, IL-5, IL-9, and IL-13, which promote eosinophilia, help maintain allergen-specific IgE levels, and recruit additional inflammatory cells to the tissues, worsening the inflammation and tissue damage [18].
Fig. 2. Mechanism of effector phase in allergic disease.
During the effector phase, subsequent encounters to a previously sensitized allergen leads to IgE cross-linking on and activation of basophils and mast cells. Basophil and mast cell degranulation leads to the release of anaphylactogenic mediators such as histamine, proteases, prostaglandins, leukotrienes, and cytokines which are the cause of the immediate symptoms and acute inflammation. As these cytokines accumulate, allergen specific Th2 cells are activated and produce IL-4, IL-5 and IL-13 among other cytokines, which maintain allergen-specific IgE levels, eosinophilia, mucus production, and recruitment of inflammatory cells to inflamed tissues leading to tissue damage.
Several additional effector cells participate in driving the ongoing allergic reactions. ILCs, especially ILC2, which are present in the sputum, nasal polyps, esophagus and peripheral blood [32–34], control the mucosal environment [31]. Additionally, ILCs interact with surrounding tissue cells, such as ECs, and, through the induction and production of cytokines, promote and contribute to a pro-inflammatory milieu [35]. Type 1 helper (Th1) and NKT cells produce interferon-gamma (IFN-γ), which can either worsen the effector phase by inducing epithelial cell damage, [36,37] or it can counteract the allergic inflammation by opposing the Th2 response [38]. NKT cells, in conjunction with IL-17-producing Th17 cells, also enhance IL-8-mediated neutrophil recruitment [24], thus adding to the inflammation in allergic disease. Additionally, through the production of IL-9 and IL-10, Th9 cells, further promote tissue inflammation [39]. Finally, Th22 cells have been found to contribute to allergic inflammation in atopic dermatitis by promoting epidermal hyperplasia [24,40–42].
Two additional cell types, T regulatory cells (Tregs) and B regulatory cells (Bregs), play an essential role in maintaining tolerance in a healthy individual’s immune system, and they are also key players in the restoration of tolerance after successful AIT [43,44]. Information on Tregs and Bregs has recently been reviewed in [45]. Briefly, tolerance to harmless food and environmental antigens are essential to prevent allergic diseases. In non-allergic individuals, the immune system is a fine balance between the proinflammatory state required to clear pathogens, and the immunosuppressive responses required to prevent chronic inflammation leading to tissue damage. The generation of tolerance to allergens occurs in several tissues including tonsils, GI mucosa, respiratory tract, skin and oral mucosa and leads to the generation of allergen-specific Tregs and Bregs [18] via different immune pathways.
Allergen Immunotherapy
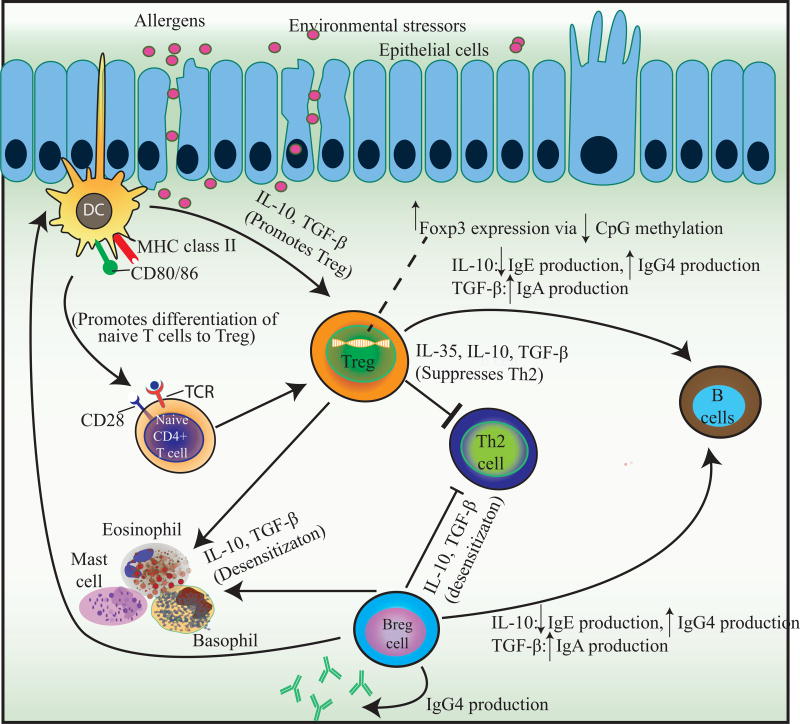
AIT, either environmental or food, increases the threshold of reactivity or desensitizes individuals to allergens. Although maintenance of the state of desensitization appears to require regular allergen exposure in the majority of individuals with food allergy, in some individuals desensitization is maintained ever after a period of cessation of immunotherapy and allergen exposure. However, whether this indicates true tolerance, defined as a permanent immunological changes (a cure) as seen to occur naturally or whether the mechanisms underlying desensitization and natural tolerance differ is still unknown. As biomarkers to determine tolerance are currently unavailable, studies on food allergy are now evaluating the ability to successfully pass a food challenge after a defined period of allergen avoidance after cessation of active therapy (sustained unresponsiveness) [46]. As previously mentioned, environmental AIT is FDA-approved for AR, asthma, and AD. Generally, the subcutaneous route of immunization has been the accepted technique, however, SLIT has also been approved as a non-invasive approach to treat allergic rhinitis [47]. New routes of immunization such as epicutaneous, intradermal, and intralymphatic modalities are currently under study [48,49]. Regardless of the route of immunotherapy, although there are some differences, the same general mechanism of action is elicited [47] (Figure 3).
Fig 3. Mechanism of immune changes after treatment with allergen immunotherapy.
During the course of AIT, several groups of cellular and molecular events take place. Within hours, there is a noted decrease in mast cells and basophil activity and degranulation leading to a decrease in type 1 skin test reactivity. Treg cells and their cytokines suppress Th2-type immune responses and help control the allergic diseases. During AIT, Breg cells are activated and suppress T cells and contribute to IgG4 synthesis, whereas Tregs suppress the allergic process by preventing and inhibiting inflammation in many different cell types.
The mechanisms behind the actions of AIT have mostly been explored in allergic rhinitis and insect hypersensitivity [50], however the same principles most likely apply behind food immunotherapy. For subjects with FA, food immunotherapy is currently under research with studies focusing on the degree of sustained unresponsiveness [51–53], as permanent tolerance has not yet been demonstrated. Over the past decade, research in food immunotherapy has surged [54], and oral immunotherapy (OIT) appears to be efficacious; however, the high rate adverse events during therapy continues to drive research towards adjunct therapies and safer therapeutic alternatives to minimize reactions. SLIT, although well-established in allergic rhinitis, is currently being explored for its efficacy in food immunotherapy, and several clinical trials have shown promising results since the first reported use in 2003 [55]. Epicutaneous immunotherapy (EPIT), a process in which the allergen is contained within a skin patch in soluble form and absorbed into the stratum corneum, is another method that is under exploration for the treatment of FA [51].
In addition to the route of immunotherapy, several potential adjunctive therapies are presently being explored, including the use of omalizumab, an anti-IgE monoclonal antibody. First studied as an adjunct in SCIT, omalizumab demonstrated significant reductions in adverse reactions [56,57]. The potential role of omalizumab with OIT was first evaluated in a pilot study of 11 subjects with cow’s milk allergy [58], and several subsequent studies have been published since [59,60]. The addition of omalizumab accelerates OIT desensitization in high-risk patients and improves the safety profile [61].
Regardless of the indicated allergic disease or method by which AIT is employed, its efficacy is driven by a complex range of changes in the underlying immune response [14–17,27]. Improving our characterization of these changes and the associated biomarkers would be of significant benefit in distinguishing patients who have the highest likelihood of responding to AIT, providing guidance regarding when to discontinue AIT, and predicting symptomatic relapse and administering booster AIT [10].
The identification of biomarkers associated with response to OIT is of particular interest in the setting of food allergies. Presently, the standard of assessing efficacy is through pretreatment and post-treatment double-blind, placebo-controlled food challenges (DBPCFCs); however, this procedure can be resource intensive, time consuming, and carries the risk of moderate-to-severe allergic reactions [62]. Reliable biomarkers could help eliminate the need for food challenges and provide a safer and more convenient alternative to these challenges. In this review, we provide an overview of the potential biomarkers to assess response to both aeroallergen and food immunotherapy.
Biomarkers
Mast cells
As previously mentioned, DBPCFCs and direct allergen exposure represent the current standard for the diagnosis and evaluation of food allergies; however, they can be resource intensive, time consuming, and carry the risk of moderate-to-severe allergic reactions [62]. In effort to avoid these issues, one of the most widely used diagnostic tests to help confirm or evaluate an allergy is the skin prick test (SPT) due to its relative safety, simplicity, and prompt results. By measuring the wheal resulting from exposure to minute amounts of an offending allergen introduced into the superficial layers of the skin, the SPT provides a way to measure and monitor the release of histamine and other inflammatory mediators by mast cells in response to the binding of the allergen to surface-bound IgE [63,64]. While food challenges allow for the direct measurement of an individual’s sensitivity to a given allergen, the SPT may allow for the indirect monitoring of changes in mast cell activity during immunotherapy and prediction of reaction during food challenge. Despite the practical advantages SPT provides over food challenges, there has been debate over its utility as a predictor of response to immunotherapy and the outcomes of such food challenges, as positive SPTs do occur in desensitized individuals [65].
While a wheal diameter of ≥3 mm has generally been regarded as a positive reaction [66], a prospective study of 467 children with suspected cow’s milk, egg, and peanut allergy found this cut-off to be a poor predictor of clinically relevant FA. In order to invariably predict the individuals who reacted at oral food challenge (OFC) before or after immunotherapy, wheal diameter cut-offs of ≥8 mm, ≥7 mm, and ≥8 mm to cow’s, milk, and peanut, respectively, were needed. Interestingly, when the analysis was limited to children aged 2 years or younger, the same ability to correctly predict reaction at food challenge was achieved at lower values, specifically ≥6 mm for cow milk, ≥5 mm for egg, and ≥4 mm for peanut. While patients with SPT values falling below these cut-offs would still need to undergo food challenge to confirm their sensitization status due to the presence of false negatives, these results suggest that the use of higher cut-off values may allow for the accurate indication of FA or failure to desensitize during immunotherapy in patients with values exceeding the cut-offs, thus eliminating the need for further food challenge in those with the highest SPT values [65].
In addition to potential variations across age, observed SPT outcomes have also been shown to vary with the extracts and allergens used, the methods and techniques by which the tests are performed and measured, and each individual’s intrinsic skin reactivity [67,68]. Commonly, wheal size is characterized by the mean diameter, defined as the mean value of the longest and the midpoint orthogonal diameter of the wheal; however, several studies have evaluated the use of skin indexes, in which the mean diameter or scanned area of the allergen-induced wheal is divided by the mean diameter or scanned area of the positive histamine-induced control wheal, in an effort to theoretically control for individual skin reactivity and inter-observer variability [69,70]. As hypothesized, significant differences were identified in the mean wheal diameter and skin index (P=0.03 and P<0.001, respectively) of children with suspected FA when comparing patients with a positive food challenge to those with a negative challenge [69]. While this finding would suggest that the skin index may better predict the outcome of food challenge, an additional study in 172 children with cashew nut sensitization found no significant difference in the ability of mean wheal diameter, scanned wheal area, skin index using mean wheal diameters, or skin index using scanned wheal areas to predict the outcome of food challenge to cashew when comparing areas under the curve in an ROC plot [70].
Given the existing data, SPTs may be of potential use in eliminating the need for food challenges in patients with the highest SPT values following immunotherapy; however, further research is needed in the establishment of cut-off values stratified by age and allergen, as well as standardization in the allergen extracts, techniques, and methods of wheal size measurement.
Basophils
Basophils represent less than <1% human leukocytes in peripheral blood [10] and, similar to mast cells, contain proteoglycans and histamine within cytoplasmic secretory granules [71]. In sensitized individuals, incoming allergens can cross-link FcεRI-bound sIgE antibodies on the surface of the basophils, leading to degranulation and the release of histamine, leukotrienes, and other proinflammatory mediators that drive the allergic inflammatory response [72,73]. As a result of AIT, basophil activation is inhibited via allergen-specific IgG antibodies which compete with sIgE for allergen binding and prevent allergen-IgE receptor cross-linking on the basophils. Additionally, the allergen-IgG complexes bind to FcγRIIB, inhibitory IgG receptors which inhibit downstream IgE receptor activation [74–77]. Decreased basophil responsiveness has been demonstrated in both aero-allergen as well as food IT [78–81].
The quantification of basophil responsiveness has been considered as a potential biomarker for the assessment of immunotherapy efficacy. There are two key surface markers on basophils that aid in the monitoring of basophil activation: CD63 and CD203c. CD63 (granule-associated tetraspan) is detected on allergen-stimulated and activated basophils, while CD203c (ectonucleotide pyrophosphatase/phosphodiesterase 3, a type II transmembrane ectoenzyme) is highly selective for basophils in peripheral blood and is induced rapidly on the external surface of the plasma membrane after activation [74,82,83]. A number of other membrane-associated proteins have been identified, including CD13, CD107a, and CD164, each of which is expressed upon activation [10]. CD13 and CD164 follow patterns of expression similar to CD203c, whereas CD107a more closely aligns with that of CD63 [84].
More recently, intracellular expression of fluorochrome-labeled diamine oxidase (DAO) in basophils has been identified as a potential novel biomarker to assess AIT efficacy and the acquisition of tolerance [8]. In a cross-sectional study of AIT [8], grass pollen-induced basophil activation in patients with seasonal allergic rhinitis was found to be significantly diminished in patients who were treated with SCIT or SLIT for grass pollen. The quantification of the reduction in basophil activation and histamine release was performed by measuring DAO using flow cytometry, with reductions being associated with reduced combined symptoms and rescue medication scores [8]; however, studies to date show conflicting results when assessing basophil activation. While a number of studies suggest associations between reductions in basophil activation [85–88], particularly in the reduced expression of CD63, CD203c, and DAO with increases in CD107a [8,89–92], others do not report a suppression of basophil activation in otherwise successful trials of AIT [93–95].
In addition to SCIT and SLIT, changes in basophils have also been noted in the setting of OIT with adjunct omalizumab use [96]. The IgE-mediated response of basophils is facilitated through the expression of tyrosine kinase syk [97], with increased syk expression increasing basophil sensitivity sIgE cross-linking. Basophil activity during treatment with omalizumab is currently thought to be due to two opposing effects, the net effect determining overall response to therapy. In a study involving participants with peanut allergy, the effect of syk on basophil function affected the subsequent efficacy of omalizumab to suppress reactions during the oral challenge following OIT [96,98]. Research has suggested that omalizumab may paradoxically increase the sensitivity of basophils via an increase syk expression [97]; however, omalizumab has also been shown to lower the density of FcεRI and sIgE densities on the basophil surface, leading to a desensitized basophil requiring higher concentrations of allergen for activation[97,99]. This condition has been found to be similar in both food and aeroallergen conditions [96]. Given the current data, the measurement of individual baseline syk expression or IgE-mediated histamine response may have value in predicting the subset of patients with the highest potential for successful treatment with omalizumab in the setting of OIT for FA [98].
Innate Lymphoid Cells
Innate lymphoid cells (ILCs), including ILC1, ILC2 and ILC3 subsets, are similar to lymphocytes, but lack the rearranging antigen receptors [100,101]. Similar to patterns of cytokine production characteristic of polarized CD4+ T cells, ILCs produce large amounts of proinflammatory cytokines, such as IFN-γ, IL-5, IL-13, IL-17, and IL-22 [47], and play a role in the initiation and maintenance of allergic inflammation [47]. Of the three previously mentioned ILC subsets, ILC2s are most closely tied to type 2 allergic inflammation and tissue repair [100]. Their effector function is driven by IL-33 [102], IL-25 [103], TSLP [104], and leukotriene D4, with interactions leading to the production of Th2 cytokines including IL-4, IL-5, IL-9 and IL-13 [100]. The importance of ILC2s in allergic rhinitis was first explored by Doherty et al. in 2014, wherein the team demonstrated an increased presence of peripheral blood ILC2s in subjects exposed to intranasal cat allergen provocation within 4 hours of the exposure, thus demonstrating how acutely ILC2s can be induced in allergic rhinitis [105].
Similar to the study by Doherty et al., a second study involving grass pollen allergic individuals demonstrated increases peripheral blood CD117+ILC2s and IL-13+ILC2s after natural grass pollen exposure during pollen season [106]. Grass pollen SCIT blunted the seasonal increases of CD117+ILC2s, as well as IL-13+ILC2s [106]. Outside of the season, the frequency of the ILC2s were not different among grass pollen allergic and non-allergic controls [106]. In contrast, studies utilizing SLIT have failed to show a reduction in ILC2s; however, this may be due to enumeration of ILC2s in active and placebo groups having been conducted outside of pollen season [107], or that SLIT may not effectively be targeting the ILC2s. Specific studies targeting ILC2 response during immunotherapy for food allergy is presently lacking, with additional studies needed to evaluate its use in predicting clinical outcome in such a setting.
Specific and Total IgE
In addition to clinical symptoms, inclusion criteria for initiating AIT nearly ubiquitously includes elevated serum-specific IgE levels [108–110]. Several studies have shown that, during the first few months of AIT, there is an initial, transient boost in allergen-specific IgE serum levels with no significant clinical change in the individual. Additionally, this increase is only noted peripherally and not in the airways [111]. After 6–12 months, however, a progressive decline in the sIgEs, consistent with a decrease in the allergen-specific plasma cells which reside in the bone marrow, has been well documented in long-term AIT studies [112,113]. This initial increase in allergen-specific IgE levels followed by a gradual decrease has been demonstrated during immunotherapy for food allergy in several studies [55,81,114–116], however, the decrease is not consistently associated with clinical improvement [79,117]. Similar to sIgE, a transient initial increase with subsequent decrease has been reported for total IgE (tIgE); however, there is a wide variation [50,74].
The ratio of sIgE to total IgE (sIgE/tIgE) has also demonstrated conflicting efficacy when used as a marker for the prediction of clinical outcome resulting from AIT. While the ratio has shown promise in the setting of grass pollen and house dust mite SCIT and SLIT [118], a subsequent randomized, controlled, open-label study could not replicate the results. Although others have suggested similar correlations to clinical outcome as those described by in the setting of SCIT and SLIT [5,95,119,118,120], given the conflicting data, further efforts must be made in the establishment and evaluation of quantitative ratio values that can be reliably associated with clinical reactivity. With additional research and refinement, the use of component resolved testing, especially the sIgE/tIgE ratio, may be important as a potential marker for response to AIT for food allergies in the future [121].
IgG4
In contrast to AIT-induced changes in IgE, both allergen and food immunotherapy have been associated with rises in serum allergen-specific functional IgG subtype 4 antibodies (sIgG4) in the first few months of treatment [78–81,122]. In addition to inhibiting the binding of IgE-allergen complexes to B cells and decreasing the activation of B cells, mast cells, and basophils by engaging FcyRIIB (CD32) inhibitor receptors [123,124], sIgG4 antibodies act as a blocking antibody by competing with allergen binding of sIgE, preventing the subsequent activation and degranulation of effector cells, as well as allergen presentation to T cells [75,123,120]. Increases in this antagonistic activity during AIT can be quantified using an immune-solid-phase allergy chip (ISAC) [125,126]. Additionally, a validated flow cytometry-based assay (IgE-FAB) is available for the assessment of IgE-facilitated antigen presentation and activation of T cells during AIT [127].
Several studies have reported a 10–100 fold increase in the concentrations of allergen specific IgG1 and IgG4 during AIT [128,129], with others exploring whether a reliable correlation exists between the levels of allergen-specific IgG4 and clinical outcome [93,130–132]. One of the early immunologic changes noted in OIT includes an increase in food-specific IgG4 levels, supporting its potential use as a predictor of clinical outcome during therapy for food allergy [78–81]; however, in a time and dose-dependent analysis of allergen-IgE binding to B cells and sIgG4 levels [133] during AIT, it was found that the serum inhibitory activity for IgE-FAB, not serum IgG4, best correlated with clinical outcome [132,133], with increases in activity corresponding with positive clinical outcomes. Additional studies have shown that, although sIgG4 remains increased in active treatment groups, levels revert back to near-pretreatment levels after treatment cessation [74]. Conversely, the serum inhibitory activity for IgE-FAB has persistently been shown to remain elevated post-treatment [133,134]. While sIgG4 responses appear to be relevant at the cohort level, these findings suggest that sIgG4 alone cannot reliably be used as a biomarker to predict clinical outcomes or reactivity after both aero-allergen and food AIT at the individual patient level [48].
IgE-blocking activity
Following AIT, there is a serum IgE inhibitory effect that is largely driven by IgA and IgG antibodies [74,135,136]. As previously described, this inhibitory effect prevents allergen binding to IgE, IgE-allergen complex binding to B cells, and the inhibition of basophils [74], thus preventing many aspects of a pro-allergic responsive [120,137,138]. Although several studies have suggested increases in IgE blocking factor (IgE-BF) following AIT are positively correlated with clinical outcome, the instrument used for the IgE-BF assay is no longer being produced. As such, the validation and use of IgE-BF as a predictive biomarker is limited [74].
On the other hand, as mentioned previously, the use of IgE-FAB to detect the binding of allergen-IgE complexes to B cells expressing FcεRII (CD23) is a highly reproducible flow cytometry-based bioassay. Recent studies have suggested an inverse correlation between symptom scores, rescue medication scores, and IgE-FAB [87,133]. Additionally, decreases in IgE-FAB after AIT do appear to correlate with clinical response to grass and birch AIT with an inverse correlation between IgE-FAB and symptom scores and rescue medications [75,126], with increased serum inhibitor activity for IgE-FAB persisting for 2 years [137]; however, despite these findings, no relationship has been discovered between the serum inhibitor activity for IgE-FAB in responders to AIT compared to non-responders [74].
Mucosal IgA has also been implicated in IgE-blocking activity. In mouse models of asthma, the efficacy of SLIT was strongly correlated with the induction of IgA in the respiratory mucosa [47,139]. During AIT, allergen-specific IgAs are produced and are present in the mucosal surfaces as a blocking antibody [113]. Serum IgA has also been found to correlate with response to OIT in the setting of food allergy. In a study of egg OIT, children with egg allergy had lower serum egg white specific IgA compared to healthy controls. In most participants who became tolerant to egg, there was a significant increase of over 28% of egg white specific IgA over time, suggesting a role of allergen specific IgA in food tolerance [140]. Due to the presence of multiple IgE-blocking mechanisms, allergen-specific IgE reactivity has the potential to be used as a proxy for AIT efficacy [141] through the quantification of IgE binding in the presence of blocking IgGs and IgAs [141].
Dendritic cells
There has been interest in monitoring changes in innate immune cells, including peripheral blood monocytes (PBMCs) and DCs, as early predictors of response to immunotherapy due to their role in driving the down-stream orientation of adaptive T cell responses. As DCs mature under the influence of multiple extracellular signals, they may polarize as proallergic DCs (DC2s) producing inflammatory cytokines and promoting the differentiation of naïve CD41 T-cells into IL-5 and 13 secreting Th2 cells. Conversely, others may polarize as tolerogenic DCs (DCregs) driving the differentiation of CD41 T-cells into Tregs [142,143]. These DC-driven changes in the adaptive T cell response, including the promotion of Tregs, Th1 cells, and IgG4 production over allergen-specific Th2 cells, basophils, and specific IgEs, have been documented in those undergoing aero-allergen IT; however, the data linking changes in these markers to clinical response has been limited [144].
In an effort to identify stronger predictors of clinical efficacy, recent efforts have evaluated changes in the expression of markers associated with DCs themselves to highlight potential correlations between changes in peripheral blood DC-associated markers and clinical response to immunotherapy. In a study of PBMCs from patients undergoing SLIT for grass pollen allergy, the expression of DCreg-associated complement component 1 (C1Q) and stabilin-1 (STAB1) were found to be increased in clinical responders compared to non-responders or those in the placebo group[143]. Follow-up study of PBMC-derived DC2 and DCreg-associated markers for additional associations indicated that four-months of SLIT promoted a clear down-regulation of the DC2-associated markers CD141, GATA2, OX40 ligand, and receptor-interacting serine/threonineprotein kinase 4 (RIPK4) in the PBMCs of responders, defined as those with percentage of symptom score improvement above the median at month 4, compared to non-responders. This downregulation was accompanied by a significant increase in the expression of the DCreg-associated complement C1Q subcomponent subunit A (C1QA), FcgRIIIA, ferritin light chain (FTL), and solute carrier organic anion transporter family member 2B1 (SLCO2B1) in responders. While changes in these DC2 and DCreg-associated markers were associated with clinical efficacy after four months of AIT, changes in FcgRIIIA were also found to correlate with the onset of clinical efficacy as early as 2 months of AIT. Further ROC analysis yielded an algorithm using an optimal combination of five of these markers, CD141, GATA3, RIPK4, C1QA, and FcgIIIA, that was able to identify responders from non-responders with a sensitivity and specificity of 90.48% and 61.9%, respectively, when using threshold values that varied at the two and four-month time points [144].
To note, these observed changes in DC-associated markers were associated with clinical improvement regardless of whether or not alterations in known markers of adaptive, allergen–specific responses, including Th2 lymphocytes, type 2 innate lymphoid cells, basophils, IgA, IgE, IgG, and IgG4, were identified in the sera, suggesting changes DC2 and DCreg-associated markers may better correlate with clinical improvement induced by SLIT when compared to previously mentioned markers. While these markers of efficacy still need to be validated in larger cohorts of patients undergoing AIT for other allergens and routes of immunotherapy, they show promise in the early prediction of adaptive immune responses and immunotherapy efficacy on an individual basis [144]. Our understanding of the role of DCs in food allergy in human subjects is limited and further characterization of differences in DC responses in food-allergic individuals undergoing IT is necessary to expand our knowledge of human DC function in food allergy [145].
DNA methylation
Desensitization during immunotherapy is associated with increases in IgG and reductions in specific IgE antibodies, shifts from Th2 responses toward Th1 with increased IFN-γ production, reduced recruitment or deletion of T effector cells (Teffs), and induction of Tregs. While the previously described diagnostic tests have potential as excellent markers of sensitization, they are generally still poor predictors of clinical reactivity and outcomes of AIT, thus making OFCs and direct allergen exposure a necessity in the differentiation of sensitization and clinical allergy. Recently, additional associations between alterations in the methylation patterns of specific DNA promotor regions and response to immunotherapy have come into focus. Studies have suggested that DNA methylation patterns of the fifth carbon in CpG dinucleotides of specific gene promotor regions, especially those associated with Forkhead box protein 3 (FoxP3), a protein associated with the tolerogenic and suppressive function of Tregs, are altered during immunotherapy and may be associated with clinical reactivity, thus making these patterns of methylation promising candidates for the monitoring of response to immunotherapy and prediction of food challenge outcomes. Methylation of these promoter sites influences gene expression, with increased methylation being associated with gene silencing [146]. Alterations in methylation have previously been associated with the shift from Th1 to Th2 responses in those with FA, with methylation studies suggesting that specific immunotherapy may promote the methylation of Th2-related gene promoter regions while decreasing rates of methylation in Th1-associated regions [146,147]. As such, efforts have been made to link these methylation patterns with response to immunotherapy and clinical reactivity.
In a study by Canani et al., peripheral blood mononuclear cell-derived CpG methylation patterns in the promoting regions of specific genes associated with Th2 (IL-4 and IL-5) and Th1 (IL-10 and TNF-γ) cytokines within PBMCs were able to clearly distinguish those with active IgE-mediated cow’s milk allergy from those who outgrew the allergy. IL-4 and 5 are critical to the development of IgE-mediated allergic inflammation in the event that they are not adequately counter-regulated by Th1 cytokines, such as IL-10 and IFN-γ. Those with active allergy exhibited significantly lower methylation of IL-4 and 5-associated regions and significantly higher rates in those associated with IL-10 and IFN-γ when compared to healthy controls, the greatest predictor of active allergy being the methylation rates of the IL-5 associated region. Additionally, methylation patterns in the regions of those with recent acquisition of tolerance to cow’s milk were similar, but not identical, to those of healthy controls [148].
Similar findings have been demonstrated in the assessment of sustained unresponsiveness following successful desensitization of peanut allergy after 24 months of peanut OIT. Previously desensitized patients were reassessed for tolerance after three and six months of peanut avoidance. Those reacting to food challenge were categorized as non-tolerant, and those without reaction were deemed immune tolerant. Between these groups, there was no significant variation in specific IgE or basophil activation at any time point; however, those who were immune tolerant had significantly increased numbers of antigen-induced Tregs (ai-Tregs) and expression of FoxP3 during OIT and at the three and six-month post-therapy OFCs compared to the non-tolerant and control groups, with significantly lower rates of FoxP3-associated CpG site methylation within ai-Tregs of the immune tolerant group compared to those in the non-tolerant or control groups. Furthermore, the loss of tolerance between three and six months post-therapy was associated with an increase in methylation at the same CpG sites [94]. It should be noted that these associations between immunotherapy and changes in DNA methylation do not appear to be limited to OIT alone. In a study by Swamy et al., subjects successfully treated with dual SLIT to timothy grass and dust mite produced increased levels of allergen-specific suppressive memory Tregs characterized by reduced CpG methylation within their FoxP3 promoter regions [149].
As a whole, these findings suggest that the evaluation of DNA methylation in ai-Tregs could be of value in predicting individual response to immunotherapy and the presence of sustained unresponsiveness without the need for repeat OFCs or direct allergen exposure. In a recent study, Martino et al. analyzed DNA methylation across a wider range of allergy-associated DNA promoter regions. By profiling genome-wide DNA methylation patterns in PBMCs and comparing CpG sites at which methylation levels vary according to FA status, the team determined a DNA methylation signature of 96 CpG sites able to predict clinical outcomes of food challenges with significantly greater accuracy compared to allergen-specific IgE and SPT. These 96 allergy-associated sites, located on genes stimulated by DCs and expressed by plasma and memory B cells, effector cells, Tregs and T cells, had significantly higher levels of methylation in those with positive SPT and OFC compared, classified as food allergic, to those with positive SPT but no reaction at food challenge, classified as food sensitized. Through receiver operating characteristic (ROC) analysis, an optimal methylation cut-off value was determined, accurately predicting OFC outcomes with 96.55% and 89.66% specificity and sensitivity, respectively. Application of this cut-off in validation samples produced posterior probabilities greater than 0.9 in each case with an area under the curve of 0.9774 in the comparison of food allergic patients to food sensitized, with outcome of food challenge correctly predicted in each patient with no misclassification. Predictive performance was strongest when comparing food allergic patients to sensitized; however, methylation scores remained significantly different when comparing both food allergic and sensitized patients to healthy controls [150]. Application of the cut-off value in an additional replication cohort in which methylation scores were derived from total CD4+ T cells, rather than PBMCs, provided correct prediction of food challenge reaction with a reduced accuracy of 79.2%. While differences in food allergic and sensitized methylation scores were preserved despite methylation data being derived from a different cell type, the decrease in specificity and sensitivity strongly suggests that optimal diagnostic cut-off methylation values are cell-specific and would need to be determined for each cell type used as a source of methylation data. Regardless of cell type and cut-off values used, the use of methylation data appears to be most useful in differentiating food allergic patients from those who are sensitized, after patients have been classified as sensitized using SPT and/or specific IgE data, potentially avoiding the need for OFCs in patient who would be difficult to classify based on SPT and IgE data alone [150].
While more studies are needed to develop and refine optimal gene panels, cell sources, and methylation cut-off values, these methylation biomarkers are readily detectable in the blood and appear to be highly promising as predictors of reaction during OFC or direct allergen exposure, especially when used in combination with IgE and SPT data [150].
Summary
We have reviewed some of the major biomarkers found to be important in the process of allergy and immunotherapy. Immunotherapy protocols are being tested for their safety and efficacy in desensitizing individuals to specific allergens; however, recurrence of allergic sensitization is common after discontinuation of therapy. Interestingly, in a subset of individuals, immunotherapy is protective against food allergens even after discontinuation of immunotherapy. Whether this protection is permanent is currently unknown because of inadequate long-term follow-up data. Research on understanding the underlying mechanisms may assist in modifying protocols to improve outcome and enable sustained unresponsiveness, rather than a temporary relief against food allergies. The cellular and molecular changes brought about by immunotherapy are still under investigation, but major strides in our understanding are being made.
Acknowledgments
This work was supported by NIH grant U19AI104209, the Bezos Family Foundation, the FARE Center of Excellence, the Myra Reinhard Foundation, and the Sean N. Parker Center for Allergy and Asthma Research at Stanford University.
Footnotes
Conflict of Interest
The authors of the manuscript (Sayantani B. Sindher, Andrew Long, Swati Acharya, Vanitha Sampath, and Kari C. Nadeau) declare that they have no conflict of interest.
References
- 1.Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127:S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Schunemann HJ, Samolinski B, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130:1049–1062. doi: 10.1016/j.jaci.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 3.Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD001936.pub2. Cd001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bufe A, Eberle P, Franke-Beckmann E, Funck J, Kimmig M, Klimek L, Knecht R, Stephan V, Tholstrup B, Weisshaar C, Kaiser F. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167–173. e167. doi: 10.1016/j.jaci.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 5.Eifan AO, Akkoc T, Yildiz A, Keles S, Ozdemir C, Bahceciler NN, Barlan IB. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy. 2010;40:922–932. doi: 10.1111/j.1365-2222.2009.03448.x. [DOI] [PubMed] [Google Scholar]
- 6.Powell RJ, Frew AJ, Corrigan CJ, Durham SR. Effect of grass pollen immunotherapy with Alutard SQ on quality of life in seasonal allergic rhinoconjunctivitis. Allergy. 2007;62:1335–1338. doi: 10.1111/j.1398-9995.2007.01455.x. [DOI] [PubMed] [Google Scholar]
- 7.Demoly P, Okamoto Y, Yang WH, Devillier P, Bergmann KC. 300 IR HDM tablet: a sublingual immunotherapy tablet for the treatment of house dust mite-associated allergic rhinitis. Expert Rev Clin Immunol. 2016;12:1141–1151. doi: 10.1080/1744666x.2016.1237288. [DOI] [PubMed] [Google Scholar]
- 8.Shamji MH, Layhadi JA, Scadding GW, Cheung DK, Calderon MA, Turka LA, Phippard D, Durham SR. Basophil expression of diamine oxidase: a novel biomarker of allergen immunotherapy response. J Allergy Clin Immunol. 2015;135:913–921. e919. doi: 10.1016/j.jaci.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 9.Durham SR, Walker SM, Varga EM, Jacobson MR, O'Brien F, Noble W, Till SJ, Hamid QA, Nouri-Aria KT. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. doi: 10.1056/nejm199908123410702. [DOI] [PubMed] [Google Scholar]
- 10.Kouser L, Kappen J, Walton RP, Shamji MH. Update on Biomarkers to Monitor Clinical Efficacy Response During and Post Treatment in Allergen Immunotherapy. Curr Treat Options Allergy. 2017;4:43–53. doi: 10.1007/s40521-017-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–562. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 12.Bousquet J, Lockey R, Malling HJ, et al. Allergen immunotherapy: therapeutic vaccines for allergic diseases. World Health Organization. American academy of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1998;81:401–405. doi: 10.1016/s1081-1206(10)63136-5. [DOI] [PubMed] [Google Scholar]
- 13.Bussmann C, Bockenhoff A, Henke H, Werfel T, Novak N. Does allergen-specific immunotherapy represent a therapeutic option for patients with atopic dermatitis? J Allergy Clin Immunol. 2006;118:1292–1298. doi: 10.1016/j.jaci.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 14.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J. 2015;8:17. doi: 10.1186/s40413-015-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol. 2016;137:984–997. doi: 10.1016/j.jaci.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs O, Bahmer T, Rabe KF, von Mutius E. Asthma transition from childhood into adulthood. Lancet Respir Med. 2017;5:224–234. doi: 10.1016/s2213-2600(16)30187-4. [DOI] [PubMed] [Google Scholar]
- 17.Werfel T, Allam JP, Biedermann T, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138:336–349. doi: 10.1016/j.jaci.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Palomares O, Akdis M, Martin-Fontecha M, Akdis CA. Mechanisms of immune regulation in allergic diseases: the role of regulatory T and B cells. Immunol Rev. 2017;278:219–236. doi: 10.1111/imr.12555. [DOI] [PubMed] [Google Scholar]
- 19.Zissler UM, Esser-von Bieren J, Jakwerth CA, Chaker AM, Schmidt-Weber CB. Current and future biomarkers in allergic asthma. Allergy. 2016;71:475–494. doi: 10.1111/all.12828. [DOI] [PubMed] [Google Scholar]
- 20.Agache I, Akdis CA. Endotypes of allergic diseases and asthma: An important step in building blocks for the future of precision medicine. Allergol Int. 2016;65:243–252. doi: 10.1016/j.alit.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Shamji MH, Durham SR. Mechanisms of immunotherapy to aeroallergens. Clin Exp Allergy. 2011;41:1235–1246. doi: 10.1111/j.1365-2222.2011.03804.x. [DOI] [PubMed] [Google Scholar]
- 22.Akdis CA, Akdis M. Advances in allergen immunotherapy: aiming for complete tolerance to allergens. Sci Transl Med. 2015;7:280ps286. doi: 10.1126/scitranslmed.aaa7390. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd CM, Saglani S. T cells in asthma: influences of genetics, environment, and T-cell plasticity. J Allergy Clin Immunol. 2013;131:1267–1274. doi: 10.1016/j.jaci.2013.02.016. quiz 1275. [DOI] [PubMed] [Google Scholar]
- 24.Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012;18:736–749. doi: 10.1038/nm.2754. [DOI] [PubMed] [Google Scholar]
- 25.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao C, Puddicombe SM, Field S, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–556. e541–512. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 27.Palomares O, Martin-Fontecha M, Lauener R, Traidl-Hoffmann C, Cavkaytar O, Akdis M, Akdis CA. Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-beta. Genes Immun. 2014;15:511–520. doi: 10.1038/gene.2014.45. [DOI] [PubMed] [Google Scholar]
- 28.Wawrzyniak P, Akdis CA, Finkelman FD, Rothenberg ME. Advances and highlights in mechanisms of allergic disease in 2015. J Allergy Clin Immunol. 2016;137:1681–1696. doi: 10.1016/j.jaci.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Wawrzyniak P, Wawrzyniak M, Wanke K, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017;139:93–103. doi: 10.1016/j.jaci.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 30.Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Morita H, Moro K, Koyasu S. Innate lymphoid cells in allergic and nonallergic inflammation. J Allergy Clin Immunol. 2016;138:1253–1264. doi: 10.1016/j.jaci.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Doherty TA, Baum R, Newbury RO, Yang T, Dohil R, Aquino M, Doshi A, Walford HH, Kurten RC, Broide DH, Aceves S. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol. 2015;136:792–794. e793. doi: 10.1016/j.jaci.2015.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mjosberg J, Spits H. Human innate lymphoid cells. J Allergy Clin Immunol. 2016;138:1265–1276. doi: 10.1016/j.jaci.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Nagakumar P, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol. 2016;137:624–626. e626. doi: 10.1016/j.jaci.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 35.Morita H, Arae K, Unno H, et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity. 2015;43:175–186. doi: 10.1016/j.immuni.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basinski TM, Holzmann D, Eiwegger T, Zimmermann M, Klunker S, Meyer N, Schmid-Grendelmeier P, Jutel M, Akdis CA. Dual nature of T cell-epithelium interaction in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:74–80. e71–78. doi: 10.1016/j.jaci.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Rebane A, Zimmermann M, Aab A, et al. Mechanisms of IFN-gamma-induced apoptosis of human skin keratinocytes in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;129:1297–1306. doi: 10.1016/j.jaci.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Finotto S, Neurath MF, Glickman JN, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–338. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 39.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 40.Czarnowicki T, Gonzalez J, Shemer A, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol. 2015;136:104–115. e107. doi: 10.1016/j.jaci.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/jci40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wegrzyn AS, Jakiela B, Ruckert B, Jutel M, Akdis M, Sanak M, Akdis CA. T-cell regulation during viral and nonviral asthma exacerbations. J Allergy Clin Immunol. 2015;136:194–197. e199. doi: 10.1016/j.jaci.2014.12.1866. [DOI] [PubMed] [Google Scholar]
- 43.Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol. 2016;138:639–652. doi: 10.1016/j.jaci.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of Treg in immune regulation of allergic diseases. Eur J Immunol. 2010;40:1232–1240. doi: 10.1002/eji.200940045. [DOI] [PubMed] [Google Scholar]
- 45.Yu W, Freeland DM, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751–765. doi: 10.1038/nri.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hussey Freeland DM, Fan-Minogue H, Spergel JM, Chatila TA, Nadeau KC. Advances in food allergy oral immunotherapy: toward tolerance. Curr Opin Immunol. 2016;42:119–123. doi: 10.1016/j.coi.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moingeon P. Biomarkers for Allergen Immunotherapy: A "Panoromic" View. Immunol Allergy Clin North Am. 2016;36:161–179. doi: 10.1016/j.iac.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Moingeon P, Mascarell L. Novel routes for allergen immunotherapy: safety, efficacy and mode of action. Immunotherapy. 2012;4:201–212. doi: 10.2217/imt.11.171. [DOI] [PubMed] [Google Scholar]
- 49.Passalacqua G, Compalati E, Canonica GW. Sublingual immunotherapy: other indications. Immunol Allergy Clin North Am. 2011;31:279–287. ix. doi: 10.1016/j.iac.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 50.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133:621–631. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 51.Kostadinova AI, Willemsen LE, Knippels LM, Garssen J. Immunotherapy - risk/benefit in food allergy. Pediatr Allergy Immunol. 2013;24:633–644. doi: 10.1111/pai.12122. [DOI] [PubMed] [Google Scholar]
- 52.Nowak-Wegrzyn A, Fiocchi A. Is oral immunotherapy the cure for food allergies? Curr Opin Allergy Clin Immunol. 2010;10:214–219. doi: 10.1097/ACI.0b013e3283399404. [DOI] [PubMed] [Google Scholar]
- 53.Rolinck-Werninghaus C, Staden U, Mehl A, Hamelmann E, Beyer K, Niggemann B. Specific oral tolerance induction with food in children: transient or persistent effect on food allergy? Allergy. 2005;60:1320–1322. doi: 10.1111/j.1398-9995.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- 54.McGowan EC, Wood RA. Sublingual (SLIT) versus oral immunotherapy (OIT) for food allergy. Curr Allergy Asthma Rep. 2014;14:486. doi: 10.1007/s11882-014-0486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mempel M, Rakoski J, Ring J, Ollert M. Severe anaphylaxis to kiwi fruit: Immunologic changes related to successful sublingual allergen immunotherapy. J Allergy Clin Immunol. 2003;111:1406–1409. doi: 10.1067/mai.2003.1497. [DOI] [PubMed] [Google Scholar]
- 56.Casale TB, Busse WW, Kline JN, Ballas ZK, Moss MH, Townley RG, Mokhtarani M, Seyfert-Margolis V, Asare A, Bateman K, Deniz Y. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006;117:134–140. doi: 10.1016/j.jaci.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 57.Traister RS, Green TD, Mitchell L, Greenhawt M. Community opinions regarding oral immunotherapy for food allergies. Ann Allergy Asthma Immunol. 2012;109:319–323. doi: 10.1016/j.anai.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 58.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow's milk allergy. J Allergy Clin Immunol. 2011;127:1622–1624. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mansfield L. Successful oral desensitization for systemic peanut allergy. Ann Allergy Asthma Immunol. 2006;97:266–267. doi: 10.1016/s1081-1206(10)60026-9. [DOI] [PubMed] [Google Scholar]
- 60.Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol. 2013;132:1368–1374. doi: 10.1016/j.jaci.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Labrosse R, Graham F, Des Roches A, Begin P. The Use of Omalizumab in Food Oral Immunotherapy. Arch Immunol Ther Exp (Warsz) 2017;65:189–199. doi: 10.1007/s00005-016-0420-z. [DOI] [PubMed] [Google Scholar]
- 62.Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, Bush RK, Metcalfe DD. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol. 1988;82:986–997. doi: 10.1016/0091-6749(88)90135-2. [DOI] [PubMed] [Google Scholar]
- 63.Hill DJ, Heine RG, Hosking CS. The diagnostic value of skin prick testing in children with food allergy. Pediatr Allergy Immunol. 2004;15:435–441. doi: 10.1111/j.1399-3038.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 64.Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344:30–37. doi: 10.1056/nejm200101043440106. [DOI] [PubMed] [Google Scholar]
- 65.Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin Exp Allergy. 2000;30:1540–1546. doi: 10.1046/j.1365-2222.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 66.Eigenmann PA, Sampson HA. Interpreting skin prick tests in the evaluation of food allergy in children. Pediatr Allergy Immunol. 1998;9:186–191. doi: 10.1111/j.1399-3038.1998.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 67.Aas K, Backman A, Belin L, Weeke B. Standardization of allergen extracts with appropriate methods. The combined use of skin prick testing and radio-allergosorbent tests. Allergy. 1978;33:130–137. doi: 10.1111/j.1398-9995.1978.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 68.Dreborg S. Histamine reactivity of the skin. Allergy. 2001;56:359–364. doi: 10.1034/j.1398-9995.2001.056005359.x. [DOI] [PubMed] [Google Scholar]
- 69.Ueno H, Yoshioka K, Matsumoto T. Usefulness of the skin index in predicting the outcome of oral challenges in children. J Investig Allergol Clin Immunol. 2007;17:207–210. [PubMed] [Google Scholar]
- 70.van der Valk JP, Gerth van Wijk R, Hoorn E, Groenendijk L, Groenendijk IM, de Jong NW. Measurement and interpretation of skin prick test results. Clin Transl Allergy. 2015;6:8. doi: 10.1186/s13601-016-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishizaka T, De Bernardo R, Tomioka H, Lichtenstein LM, Ishizaka K. Identification of basophil granulocytes as a site of allergic histamine release. J Immunol. 1972;108:1000–1008. [PubMed] [Google Scholar]
- 72.MacGlashan D., Jr Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin Exp Allergy. 2010;40:1365–1377. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schroeder JT, Kagey-Sobotka A, Lichtenstein LM. The role of the basophil in allergic inflammation. Allergy. 1995;50:463–472. doi: 10.1111/j.1398-9995.1995.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 74.Shamji MH, Kappen JH, Akdis M, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy. 2017;72:1156–1173. doi: 10.1111/all.13138. [DOI] [PubMed] [Google Scholar]
- 75.van Neerven RJ, Wikborg T, Lund G, Jacobsen B, Brinch-Nielsen A, Arnved J, Ipsen H. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163:2944–2952. [PubMed] [Google Scholar]
- 76.Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112:915–922. doi: 10.1016/s0091. [DOI] [PubMed] [Google Scholar]
- 77.Kepley CL, Cambier JC, Morel PA, Lujan D, Ortega E, Wilson BS, Oliver JM. Negative regulation of FcepsilonRI signaling by FcgammaRII costimulation in human blood basophils. J Allergy Clin Immunol. 2000;106:337–348. doi: 10.1067/mai.2000.107931. [DOI] [PubMed] [Google Scholar]
- 78.Burks AW, Jones SM, Wood RA, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gorelik M, Narisety SD, Guerrerio AL, Chichester KL, Keet CA, Bieneman AP, Hamilton RG, Wood RA, Schroeder JT, Frischmeyer-Guerrerio PA. Suppression of the immunologic response to peanut during immunotherapy is often transient. J Allergy Clin Immunol. 2015;135:1283–1292. doi: 10.1016/j.jaci.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones SM, Pons L, Roberts JL, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. 300.e291–297. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vickery BP, Scurlock AM, Kulis M, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133:468–475. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buhring HJ, Streble A, Valent P. The basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell activation and allergy diagnosis. Int Arch Allergy Immunol. 2004;133:317–329. doi: 10.1159/000077351. [DOI] [PubMed] [Google Scholar]
- 83.Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991;88:328–338. doi: 10.1016/0091-6749(91)90094-5. [DOI] [PubMed] [Google Scholar]
- 84.Hennersdorf F, Florian S, Jakob A, Baumgartner K, Sonneck K, Nordheim A, Biedermann T, Valent P, Buhring HJ. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res. 2005;15:325–335. doi: 10.1038/sj.cr.7290301. [DOI] [PubMed] [Google Scholar]
- 85.Aasbjerg K, Backer V, Lund G, Holm J, Nielsen NC, Holse M, Wagtmann VR, Wurtzen PA. Immunological comparison of allergen immunotherapy tablet treatment and subcutaneous immunotherapy against grass allergy. Clin Exp Allergy. 2014;44:417–428. doi: 10.1111/cea.12241. [DOI] [PubMed] [Google Scholar]
- 86.Ceuppens JL, Bullens D, Kleinjans H, van der Werf J. Immunotherapy with a modified birch pollen extract in allergic rhinoconjunctivitis: clinical and immunological effects. Clin Exp Allergy. 2009;39:1903–1909. doi: 10.1111/j.1365-2222.2009.03379.x. [DOI] [PubMed] [Google Scholar]
- 87.Schmid JM, Wurtzen PA, Dahl R, Hoffmann HJ. Early improvement in basophil sensitivity predicts symptom relief with grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134:741–744. e745. doi: 10.1016/j.jaci.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 88.Kepil Ozdemir S, Sin BA, Guloglu D, Ikinciogullari A, Gencturk Z, Misirligil Z. Short-term preseasonal immunotherapy: is early clinical efficacy related to the basophil response? Int Arch Allergy Immunol. 2014;164:237–245. doi: 10.1159/000365628. [DOI] [PubMed] [Google Scholar]
- 89.Gokmen NM, Ersoy R, Gulbahar O, Ardeniz O, Sin A, Unsel M, Kokuludag A. Desensitization effect of preseasonal seven-injection allergoid immunotherapy with olive pollen on basophil activation: the efficacy of olive pollen-specific preseasonal allergoid immunotherapy on basophils. Int Arch Allergy Immunol. 2012;159:75–82. doi: 10.1159/000335251. [DOI] [PubMed] [Google Scholar]
- 90.Lalek N, Kosnik M, Silar M, Korosec P. Immunoglobulin G-dependent changes in basophil allergen threshold sensitivity during birch pollen immunotherapy. Clin Exp Allergy. 2010;40:1186–1193. doi: 10.1111/j.1365-2222.2010.03524.x. [DOI] [PubMed] [Google Scholar]
- 91.Nopp A, Cardell LO, Johansson SG, Oman H. CD-sens: a biological measure of immunological changes stimulated by ASIT. Allergy. 2009;64:811–814. doi: 10.1111/j.1398-9995.2008.01900.x. [DOI] [PubMed] [Google Scholar]
- 92.Zidarn M, Kosnik M, Silar M, Bajrovic N, Korosec P. Sustained effect of grass pollen subcutaneous immunotherapy on suppression of allergen-specific basophil response; a real-life, nonrandomized controlled study. Allergy. 2015;70:547–555. doi: 10.1111/all.12581. [DOI] [PubMed] [Google Scholar]
- 93.Gomez E, Fernandez TD, Dona I, et al. Initial immunological changes as predictors for house dust mite immunotherapy response. Clin Exp Allergy. 2015;45:1542–1553. doi: 10.1111/cea.12578. [DOI] [PubMed] [Google Scholar]
- 94.Syed A, Garcia MA, Lyu SC, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Overtvelt L, Baron-Bodo V, Horiot S, et al. Changes in basophil activation during grass-pollen sublingual immunotherapy do not correlate with clinical efficacy. Allergy. 2011;66:1530–1537. doi: 10.1111/j.1398-9995.2011.02696.x. [DOI] [PubMed] [Google Scholar]
- 96.MacGlashan DW, Jr, Savage JH, Wood RA, Saini SS. Suppression of the basophil response to allergen during treatment with omalizumab is dependent on 2 competing factors. J Allergy Clin Immunol. 2012;130:1130–1135. e1135. doi: 10.1016/j.jaci.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.MacGlashan DW., Jr Relationship between spleen tyrosine kinase and phosphatidylinositol 5' phosphatase expression and secretion from human basophils in the general population. J Allergy Clin Immunol. 2007;119:626–633. doi: 10.1016/j.jaci.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 98.MacGlashan DW, Jr, Saini SS. Syk expression and IgE-mediated histamine release in basophils as biomarkers for predicting the clinical efficacy of omalizumab. J Allergy Clin Immunol. 2017;139:1680–1682. e1610. doi: 10.1016/j.jaci.2016.12.965. [DOI] [PubMed] [Google Scholar]
- 99.Ishmael S, MacGlashan D., Jr Early signal protein expression profiles in basophils: a population study. J Leukoc Biol. 2009;86:313–325. doi: 10.1189/jlb.1208724. [DOI] [PubMed] [Google Scholar]
- 100.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 101.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 102.Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, Nakanishi K, Yamanishi K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191:5349–5353. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doherty TA, Scott D, Walford HH, Khorram N, Lund S, Baum R, Chang J, Rosenthal P, Beppu A, Miller M, Broide DH. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol. 2014;133:1203–1205. doi: 10.1016/j.jaci.2013.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134:1193–1195. e1194. doi: 10.1016/j.jaci.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 107.Lombardi V, Beuraud C, Neukirch C, et al. Circulating innate lymphoid cells are differentially regulated in allergic and nonallergic subjects. J Allergy Clin Immunol. 2016;138:305–308. doi: 10.1016/j.jaci.2015.12.1325. [DOI] [PubMed] [Google Scholar]
- 108.Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, Nelson H, Akdis CA. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–1296. e1283. doi: 10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 109.Calderon MA, Casale T, Cox L, Akdis CA, Burks AW, Nelson HS, Jutel M, Demoly P. Allergen immunotherapy: a new semantic framework from the European Academy of Allergy and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL consensus report. Allergy. 2013;68:825–828. doi: 10.1111/all.12180. [DOI] [PubMed] [Google Scholar]
- 110.Cox LS, Casale TB, Nayak AS, Bernstein DI, Creticos PS, Ambroisine L, Melac M, Zeldin RK. Clinical efficacy of 300IR 5-grass pollen sublingual tablet in a US study: the importance of allergen-specific serum IgE. J Allergy Clin Immunol. 2012;130:1327–1334. e1321. doi: 10.1016/j.jaci.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 111.Baron-Bodo V, Horiot S, Lautrette A, et al. Heterogeneity of antibody responses among clinical responders during grass pollen sublingual immunotherapy. Clin Exp Allergy. 2013;43:1362–1373. doi: 10.1111/cea.12187. [DOI] [PubMed] [Google Scholar]
- 112.Nouri-Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK, Staple SQ, Aalberse RC, Till SJ, Durham SR. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–3259. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 113.Pilette C, Nouri-Aria KT, Jacobson MR, Wilcock LK, Detry B, Walker SM, Francis JN, Durham SR. Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. J Immunol. 2007;178:4658–4666. doi: 10.4049/jimmunol.178.7.4658. [DOI] [PubMed] [Google Scholar]
- 114.Chin SJ, Vickery BP, Kulis MD, et al. Sublingual versus oral immunotherapy for peanut-allergic children: a retrospective comparison. J Allergy Clin Immunol. 2013;132:476–478. e472. doi: 10.1016/j.jaci.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, Steele P, Driggers S, Burks AW, Wood RA. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–455. 455, e441–445. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, Gorelik M, Schroeder J, Hamilton RG, Wood RA. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol. 2015;135:1275–1282. e1271–1276. doi: 10.1016/j.jaci.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fleischer DM, Burks AW, Vickery BP, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol. 2013;131:119–127. e111–117. doi: 10.1016/j.jaci.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Di Lorenzo G, Mansueto P, Pacor ML, et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol. 2009;123:1103–1110. 1110.e1101–1104. doi: 10.1016/j.jaci.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 119.Li Q, Li M, Yue W, Zhou J, Li R, Lin J, Li Y. Predictive factors for clinical response to allergy immunotherapy in children with asthma and rhinitis. Int Arch Allergy Immunol. 2014;164:210–217. doi: 10.1159/000365630. [DOI] [PubMed] [Google Scholar]
- 120.Wurtzen PA, Lund G, Lund K, Arvidsson M, Rak S, Ipsen H. A double-blind placebo-controlled birch allergy vaccination study II: correlation between inhibition of IgE binding, histamine release and facilitated allergen presentation. Clin Exp Allergy. 2008;38:1290–1301. doi: 10.1111/j.1365-2222.2008.03020.x. [DOI] [PubMed] [Google Scholar]
- 121.Andorf S, Borres MP, Block W, et al. Association of Clinical Reactivity with Sensitization to Allergen Components in Multifood-Allergic Children. J Allergy Clin Immunol Pract. 2017 doi: 10.1016/j.jaip.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wood RA. Food allergen immunotherapy: Current status and prospects for the future. J Allergy Clin Immunol. 2016;137:973–982. doi: 10.1016/j.jaci.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 123.Flicker S, Valenta R. Renaissance of the blocking antibody concept in type I allergy. Int Arch Allergy Immunol. 2003;132:13–24. doi: 10.1159/000073260. doi:73260. [DOI] [PubMed] [Google Scholar]
- 124.Wachholz PA, Durham SR. Mechanisms of immunotherapy: IgG revisited. Curr Opin Allergy Clin Immunol. 2004;4:313–318. doi: 10.1097/01.all.0000136753.35948.c0. [DOI] [PubMed] [Google Scholar]
- 125.Lupinek C, Wollmann E, Baar A, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–119. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lupinek C, Wollmann E, Valenta R. Monitoring Allergen Immunotherapy Effects by Microarray. Curr Treat Options Allergy. 2016;3:189–203. doi: 10.1007/s40521-016-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shamji MH, Wilcock LK, Wachholz PA, Dearman RJ, Kimber I, Wurtzen PA, Larche M, Durham SR, Francis JN. The IgE-facilitated allergen binding (FAB) assay: validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. J Immunol Methods. 2006;317:71–79. doi: 10.1016/j.jim.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–613. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 129.Reisinger J, Horak F, Pauli G, van Hage M, Cromwell O, Konig F, Valenta R, Niederberger V. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J Allergy Clin Immunol. 2005;116:347–354. doi: 10.1016/j.jaci.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 130.Gehlhar K, Schlaak M, Becker W, Bufe A. Monitoring allergen immunotherapy of pollen-allergic patients: the ratio of allergen-specific IgG4 to IgG1 correlates with clinical outcome. Clin Exp Allergy. 1999;29:497–506. doi: 10.1046/j.1365-2222.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- 131.Moverare R, Elfman L, Vesterinen E, Metso T, Haahtela T. Development of new IgE specificities to allergenic components in birch pollen extract during specific immunotherapy studied with immunoblotting and Pharmacia CAP System. Allergy. 2002;57:423–430. doi: 10.1034/j.1398-9995.2002.13248.x. [DOI] [PubMed] [Google Scholar]
- 132.Nelson HS, Nolte H, Creticos P, Maloney J, Wu J, Bernstein DI. Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North American adults. J Allergy Clin Immunol. 2011;127:72–80. 80.e71–72. doi: 10.1016/j.jaci.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 133.Shamji MH, Ljorring C, Francis JN, Calderon MA, Larche M, Kimber I, Frew AJ, Ipsen H, Lund K, Wurtzen PA, Durham SR. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67:217–226. doi: 10.1111/j.1398-9995.2011.02745.x. [DOI] [PubMed] [Google Scholar]
- 134.James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN, Jacobson MR, Kimber I, Till SJ, Durham SR. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509–516. e501–505. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 135.Lichtenstein LM, Norman PS, Winkenwerder WL. Antibody response following immunotherapy in ragweed hay fever: Allpyral vs. whole ragweed extract. J Allergy. 1968;41:49–57. doi: 10.1016/0021-8707(68)90007-5. [DOI] [PubMed] [Google Scholar]
- 136.Platts-Mills TA, von Maur RK, Ishizaka K, Norman PS, Lichtenstein LM. IgA and IgG anti-ragweed antibodies in nasal secretions. Quantitative measurements of antibodies and correlation with inhibition of histamine release. J Clin Invest. 1976;57:1041–1050. doi: 10.1172/jci108346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Durham SR, Emminger W, Kapp A, de Monchy JG, Rak S, Scadding GK, Wurtzen PA, Andersen JS, Tholstrup B, Riis B, Dahl R. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–725. e715. doi: 10.1016/j.jaci.2011.12.973. [DOI] [PubMed] [Google Scholar]
- 138.Petersen AB, Gudmann P, Milvang-Gronager P, Morkeberg R, Bogestrand S, Linneberg A, Johansen N. Performance evaluation of a specific IgE assay developed for the ADVIA centaur immunoassay system. Clin Biochem. 2004;37:882–892. doi: 10.1016/j.clinbiochem.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 139.Razafindratsita A, Saint-Lu N, Mascarell L, Berjont N, Bardon T, Betbeder D, Van Overtvelt L, Moingeon P. Improvement of sublingual immunotherapy efficacy with a mucoadhesive allergen formulation. J Allergy Clin Immunol. 2007;120:278–285. doi: 10.1016/j.jaci.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 140.Konstantinou GN, Nowak-Wegrzyn A, Bencharitiwong R, Bardina L, Sicherer SH, Sampson HA. Egg-white-specific IgA and IgA2 antibodies in egg-allergic children: is there a role in tolerance induction? Pediatr Allergy Immunol. 2014;25:64–70. doi: 10.1111/pai.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wollmann E, Lupinek C, Kundi M, Selb R, Niederberger V, Valenta R. Reduction in allergen-specific IgE binding as measured by microarray: A possible surrogate marker for effects of specific immunotherapy. J Allergy Clin Immunol. 2015;136:806–809. e807. doi: 10.1016/j.jaci.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 143.Zimmer A, Bouley J, Le Mignon M, et al. A regulatory dendritic cell signature correlates with the clinical efficacy of allergen-specific sublingual immunotherapy. J Allergy Clin Immunol. 2012;129:1020–1030. doi: 10.1016/j.jaci.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 144.Gueguen C, Bouley J, Moussu H, et al. Changes in markers associated with dendritic cells driving the differentiation of either TH2 cells or regulatory T cells correlate with clinical benefit during allergen immunotherapy. J Allergy Clin Immunol. 2016;137:545–558. doi: 10.1016/j.jaci.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 145.Ruiter B, Shreffler WG. The role of dendritic cells in food allergy. J Allergy Clin Immunol. 2012;129:921–928. doi: 10.1016/j.jaci.2012.01.080. [DOI] [PubMed] [Google Scholar]
- 146.Bunning BJ, DeKruyff RH, Nadeau KC. Epigenetic Changes During Food-Specific Immunotherapy. Curr Allergy Asthma Rep. 2016;16:87. doi: 10.1007/s11882-016-0665-y. [DOI] [PubMed] [Google Scholar]
- 147.Hong X, Ladd-Acosta C, Hao K, et al. Epigenome-wide association study links site-specific DNA methylation changes with cow's milk allergy. J Allergy Clin Immunol. 2016;138:908–911. e909. doi: 10.1016/j.jaci.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Berni Canani R, Paparo L, Nocerino R, Cosenza L, Pezzella V, Di Costanzo M, Capasso M, Del Monaco V, D'Argenio V, Greco L, Salvatore F. Differences in DNA methylation profile of Th1 and Th2 cytokine genes are associated with tolerance acquisition in children with IgE-mediated cow's milk allergy. Clin Epigenetics. 2015;7:38. doi: 10.1186/s13148-015-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Swamy RS, Reshamwala N, Hunter T, Vissamsetti S, Santos CB, Baroody FM, Hwang PH, Hoyte EG, Garcia MA, Nadeau KC. Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. J Allergy Clin Immunol. 2012;130:215–224. doi: 10.1016/j.jaci.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Martino D, Dang T, Sexton-Oates A, Prescott S, Tang ML, Dharmage S, Gurrin L, Koplin J, Ponsonby AL, Allen KJ, Saffery R. Blood DNA methylation biomarkers predict clinical reactivity in food-sensitized infants. J Allergy Clin Immunol. 2015;135:1319–1328. e1311–1312. doi: 10.1016/j.jaci.2014.12.1933. [DOI] [PubMed] [Google Scholar]