Abstract
Background
There are no data on the prevalence of cytomegalovirus (CMV) shedding from a representative sample of the US population. This information is critical for understanding and preventing CMV.
Methods
We tested urine specimens from CMV IgG-positive participants aged 6–49 years in three racial/ethnic groups from the National Health and Nutrition Examination Surveys (NHANES) 1999–2004 for the presence of CMV DNA with real-time polymerase chain reaction (PCR) assay. We examined the association of sociodemographic characteristics with shedding prevalence and viral loads.
Results
Among 6,828 CMV IgG-positive subjects tested, 537 had CMV DNA detected in urine—a shedding prevalence of 9.70%. Among persons 6–49 years, shedding prevalence was 3.83%. The prevalence of urinary shedding was inversely associated with increasing age (26.60%, 6.50%, and 3.45% in CMV IgG-positive subjects aged 6–11, 12–19, and 20–49 years, respectively; P < 0.001 for trend test and pairwise comparisons). Urinary viral load also decreased significantly with age (mean: 2.97, 2.69, and 2.43 log10 copies/mL in those age groups, respectively; P < 0.001 for trend test and pairwise comparisons).
Conclusions
Urinary CMV shedding and viral loads decreased dramatically by age, likely reflecting higher rates of primary CMV infection and longer duration of shedding in younger individuals. The findings demonstrate that children 6–11 years of age continue to shed CMV at higher rates and viral loads than adolescents and adults and thus may still be an important source for CMV transmission.
Keywords: Cytomegalovirus, Shedding, Viral Load
INTRODUCTION
Human cytomegalovirus (CMV) is a member of the herpesvirus family. A CMV-seronegative individual becomes IgG-seropositive after initial infection with CMV (primary infection). The virus becomes latent in the host after primary infection, but can reactivate periodically (reactivation).[1] A seropositive person can also be infected with a different strain of CMV (reinfection), which may also become latent and subsequently reactivate. As a result of either primary or non-primary infection (which includes reinfection and reactivation), individuals may shed CMV intermittently in body fluids such as saliva, urine, and genital secretions,[2] and be infectious to others. Though most CMV infections are mild or asymptomatic, CMV infection can have serious clinical consequences in fetuses and immunocompromised individuals. Congenital CMV infection is the leading viral cause of birth defects and developmental disabilities in the United States.[3] An estimated 20,000 infants per year in the United States are born with congenital CMV infection,[3] resulting in around 4,000 children with developmental neurologic disabilities each year.[4] Moreover, CMV is the most common opportunistic viral infection in individuals with immunosuppression and an important cause of morbidity.[5, 6]
CMV shedding has been well studied in immunocompromised populations, such as HIV-infected individuals and organ transplant recipients.[7–11] Among immunocompetent populations, the frequency of CMV shedding has been studied in infants, young children, adolescents, women, men, and even in astronauts during spaceflights.[12–18] However, the sample sizes in these studies were small and the selected populations under study made it difficult to generalize findings to the general population. We evaluated the archived urine specimens from the National Health and Nutrition Examination Survey (NHANES) 1999–2004 to provide population-based estimates of CMV shedding and explore risk factors associated with CMV shedding.
MATERIALS AND METHODS
Design and data
NHANES 1999–2004 were conducted by the National Center for Health Statistics (NCHS). Data were obtained using a complex, multistage probability sampling design to select a sample representative of the civilian noninstitutionalized household population of the United States.[19] CMV IgG status was previously determined for participants of NHANES 1999–2004 aged 6–49 years.[20] For the current study, available stored urine specimens were requested for the IgG-positive participants within three racial/ethnic groups: non-Hispanic White, non-Hispanic Black, and Mexican American as age and sex associated IgG seroprevalence was only available among these three racial groups.[20] As 14.8% CMV IgG-positive persons did not have urine specimens available, the examination weights were adjusted for the incomplete specimens after combination of the data from three cross-sectional waves of continuous NHANES (1999–2000, 2001–2002, and 2003–2004). The new adjusted weights were created by multiplying the weighted proportion of available urine samples for that participant’s sex, age, and race/ethnicity with the weights calculated in the prior IgG seroprevalence study, which had also adjusted for the weighted proportion of available serum samples for each participant’s age, race/ethnicity, and sex group.[20] Publicly accessible data [21] on demographic characteristics associated with CMV IgG seroprevalence were used to examine association with urinary CMV shedding. Sociodemographic characteristics were categorized as in a previous report on CMV IgG seroprevalence.[20] Household income was categorized by family poverty ratio, defined as the ratio of household income to the federal poverty threshold, as low (<1.3), middle (1.3–3.5), or high (>3.5). The household crowding index was defined by the number of persons per room as low (<0.5), average (0.5–1), or high (>1).[20] Individuals were categorized by age as children (6–11years), adolescents (12–19 years), or adults (20–49 years).
In NHANES 1999–2004, data on sexual behaviors were only available for adults (≥20 years of age). These data included age at first intercourse, number of lifetime sexual partners, number of sexual partners in the past year, number of sexual partners in past month, number of instances of intercourse without a condom in past month, and self-reported ever having been diagnosed with any of four sexually transmitted diseases (STDs): chlamydia, gonorrhea, genital herpes, or genital warts.
Urinary CMV testing
DNA was extracted from urine specimens by treatment with the QIAamp MinElute Media kit (Qiagen, Valencia, CA), then processed with the Qiacube automated extractor (Qiagen, Valencia, CA), following instructions that were customized by the manufacturer for processing urine. Detection of CMV DNA was performed with polymerase chain reaction (PCR) targeting the viral immediate early 2 (IE-2) region [22] using the MX 3005P Real-time PCR System (Agilent Technologies, New Castle, DE). PCR testing was performed in duplicate for all specimens, and two positive results were required for specimens to be reported as positive. The commercially standardized human CMV DNA quantitated with digital PCR by Advanced Biotechnologies, Inc. (Eldersburg, MD, USA) was included on every PCR plate to quantify the viral loads. The urinary CMV shedding and viral load data are publicly accessible.[23]
Statistical analyses
The nationally representative prevalence estimates on urinary shedding were age standardized using the direct method to the 2000 projected U.S. census population using the following age groups: 6–11, 12–19, and 20–49 years.[24] The age-adjusted estimates of urinary CMV shedding prevalence among all subjects were examined to understand the force of infection in the population, assuming the untested CMV IgG-negative subjects were free of urinary shedding. The estimates of shedding prevalence were also calculated among IgG-positive individuals to assess the prevalence of sources for transmission. All prevalence estimates were calculated using the aforementioned adjusted weights with SAS Survey Procedures (SAS Inc., Cary, NC). Risk factors associated with CMV IgG seroprevalence, such as socioeconomic status and household crowding, were examined for their association with CMV urinary shedding after adjustment for age in a logistic regression model. The association of sexual behaviors with urinary CMV shedding was only examined in CMV IgG-positive adult subjects. The urinary viral loads were log10 transformed, and unweighted statistical methods were used to assess the distribution of viral loads across different risk factors examined in urinary shedding after adjustment for age.
The NCHS Research Ethics Review Board approved this study. Informed consent was obtained from all participants.
RESULTS
Prevalence of urinary shedding
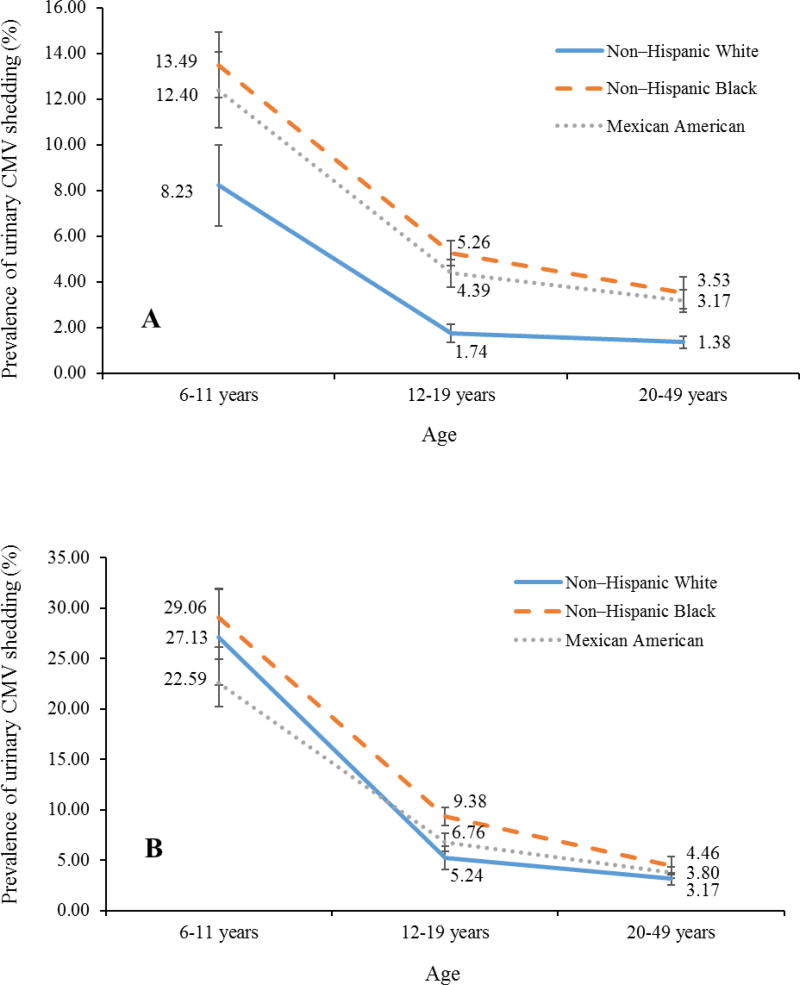
Among 6,828 urine specimens tested from CMV IgG-positive subjects (of 13,001 NHANES subjects with CMV IgG data within the three racial/ethnic groups studied), CMV DNA was detected in 537 specimens, for an overall age-adjusted prevalence of 3.83% (95% confidence interval [CI]: 3.22–4.44). In contrast to the increased prevalence of IgG seropositivity with age (40.16%, 48.78%, and 60.21% in those aged 6–11, 12–19, and 20–49 years, respectively; P < 0.001 for trend), the prevalence of shedding significantly decreased with age (9.75%, 2.67%, and 1.87% in those aged 6–11, 12–19, and 20–49 years, respectively; P < 0.05 for all pairwise comparisons). The trend of decreasing shedding with age was consistent among all three racial/ethnic groups (Figure 1, Panel A). Similar to the association with lower CMV IgG seroprevalence, lower prevalence of shedding was observed among non-Hispanic White, US-born persons, those with higher household income levels, higher household education levels, and low crowding indexes. No difference in urinary shedding was observed by sex or health insurance status (Table 1).
Figure 1.
Changes in Prevalence of Urinary Cytomegalovirus Shedding with Age by Race in All Subjects (Panel A) and in CMV IgG-Positive Subjects Only (Panel B) in NHANES 1999–2004
Table 1.
Prevalence of Urinary CMV Shedding among All Persons and CMV IgG-Positive Persons Aged 6–49 Years in NHANES 1999–2004
| Number with shedding detected |
All subjects (N = 13,001) | IgG-positive subjects (N = 6,828) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Characteristics | Sample size |
Age-adjusted prevalence (95% CI) a |
Age-adjusted P value |
Sample size | Age-adjusted prevalence (95% CI) a |
Age-adjusted P value |
|
| Overall | 537 | 13,001 | 3.83 (3.22–4.44) | 6,828 | 9.70 (8.43–10.98) | ||
| Race/ethnicity | |||||||
| Non-Hispanic White | 109 | 5,098 | 2.86 (2.01–3.71) | Reference | 1,852 | 8.89 (6.72–11.06) | Reference |
| Non-Hispanic Black | 221 | 3,881 | 6.50 (5.40–7.60) | <0.001 | 2,266 | 12.24 (10.39–14.08) | 0.06 |
| Mexican American | 207 | 4,022 | 5.67 (4.68–6.65) | <0.001 | 2,710 | 9.20 (7.83–10.56) | 0.82 |
| Sex | |||||||
| Male | 223 | 6,216 | 3.40 (2.61–4.20) | Reference | 2,902 | 9.12 (7.42–10.82) | Reference |
| Female | 314 | 6,785 | 4.29 (3.58–5.00) | 0.49 | 3,926 | 10.26 (8.66–11.85) | 0.03 |
| Age | |||||||
| 6–11 years | 214 | 2,037 | 9.75 (7.31–12.18) | Reference | 818 | 26.60 (21.15–32.06) | Reference |
| 12–19 years | 192 | 5,178 | 2.67 (2.09–3.25) | <0.001 | 2,526 | 6.50 (5.15–7.85) | < 0.001 |
| 20–49 years | 131 | 5,786 | 1.87 (1.40–2.33) | <0.001 | 3,484 | 3.45 (2.69–4.39) | < 0.001 |
| Country of birth | |||||||
| Born in the 50 US states or DC | 422 | 10,928 | 3.64 (2.97–4.32) | Reference | 5,078 | 9.77 (8.29–11.25) | Reference |
| Other | 115 | 2,073 | 6.40 (4.70–8.11) | < 0.001 | 1,749 | 8.87 (6.69–11.05) | 0.52 |
| Household income level | |||||||
| Low | 269 | 4,452 | 5.39 (4.43–6.34) | Reference | 2,765 | 10.51 (8.76–12.25) | Reference |
| Middle | 164 | 4,461 | 3.57 (2.60–5.54) | < 0.001 | 2,324 | 9.91 (7.70–12.11) | 0.29 |
| High | 70 | 3,256 | 2.44 (1.67–3.22) | < 0.001 | 1,268 | 7.89 (5.60–10.18) | 0.03 |
| Household education level | |||||||
| Less than high school | 246 | 4,042 | 5.83 (4.36–7.30) | Reference | 2,723 | 10.75 (8.36–13.14) | Reference |
| High school graduate or GED diploma | 120 | 3,114 | 3.64 (2.36–4.93) | 0.10 | 1,564 | 9.68 (6.97–12.38) | 0.81 |
| More than high school | 160 | 5,466 | 3.12 (2.49–3.75) | < 0.001 | 2,329 | 9.21 (7.40–11.02) | 0.79 |
| Insurance | |||||||
| Insured | 393 | 9,836 | 3.72 (3.02–4.41) | Reference | 4,728 | 9.96 (8.33–11.59) | Reference |
| Not insured | 136 | 3,009 | 4.61 (3.36–5.86) | 0.62 | 2,008 | 8.88 (6.72–11.03) | 0.18 |
| Household crowding index | |||||||
| Low | 52 | 2,567 | 2.29 (1.11–3.46) | Reference | 1,065 | 7.62 (3.64–11.60) | Reference |
| Average | 319 | 8,132 | 3.77 (3.05–4.49) | 0.05 | 4,169 | 9.55 (7.88–11.21) | 0.55 |
| High | 160 | 2,153 | 7.13 (5.65–8.61) | < 0.001 | 1,510 | 12.53 (10.23–14.82) | 0.09 |
weighted prevalence after adjustment for the availability of urine specimens
When we restrict the analysis to CMV IgG-positive subjects only, the age-adjusted prevalence of urinary shedding was 9.70% (95% CI: 8.43–10.98) among 6,828 CMV IgG-positive subjects tested for shedding. The prevalence of shedding significantly decreased with age, from 26.60% to 6.50% to 3.45% in those aged 6–11, 12–19, and 20–49 years, respectively (P < 0.001 for trend test and all pairwise comparisons). The trend of decreasing shedding prevalence with increasing age was consistent across the three racial/ethnic groups (Figure 1, Panel B). Shedding in IgG-positive females was higher than in males (10.26% vs. 9.12%, P = 0.03). There were no differences in urinary shedding prevalence by race/ethnicity, country of birth, household income or education levels, health insurance status, and household crowding among CMV IgG-positive subjects (Table 1).
Sexual behaviors and urinary shedding among CMV IgG-positive subjects
Among the 6,828 CMV IgG-positive participants with urinary shedding data available, 3,484 (56.4%) were 20–49 years old; data on four sexual behavior variables was available for >85% of these participants. Based on weighted analyses, the number of sexual partners within the past year was significantly associated with urinary shedding (P = 0.02), however, there was no association of urinary CMV shedding with ever having had an STD, age at first sexual intercourse, or number of lifetime sexual partners (Table 2). Over 80% of the adults had missing information on the number of sexual partners and frequency of intercourse in the past month, therefore, these two variables could not be analyzed.
Table 2.
Prevalence of Urinary CMV Shedding with Sexual Behaviors in CMV IgG-Positive Adults (≥20 Years) in NHANES 1999–2004 (N = 3,484)
| Sexual characteristics | Number with shedding detected (sample size) |
Prevalence b (95% confidence interval) |
P value |
|---|---|---|---|
| Ever had sexual transmitted diseases a | 0.63 | ||
| Yes | 9 (296) | 4.22 (1.23–7.21) | |
| No | 106 (2,715) | 3.48 (2.48–4.48) | |
| Number of lifetime sexual partners | 0.49 | ||
| < 10 | 73 (1,982) | 3.27 (2.19–4.35) | |
| ≥ 10 | 40 (1,006) | 3.81 (2.45–5.17) | |
| Age at first intercourse | 0.20 | ||
| < 18 | 80 (1,941) | 3.85 (2.68–5.01) | |
| ≥ 18 | 34 (1,080) | 2.75 (1.46–4.03) | |
| Number of sexual partners in the past year | 0.02 | ||
| ≤ 1 | 84 (2,400) | 3.20 (2.37–4.04) | |
| > 1 | 31 (625) | 5.06 (2.95–7.18) | |
Self-report as ever diagnosed with genital herpes, genital warts, chlamydia, or gonorrhea.
weighted estimates after adjustment for the availability of urine specimens.
Urinary CMV viral loads
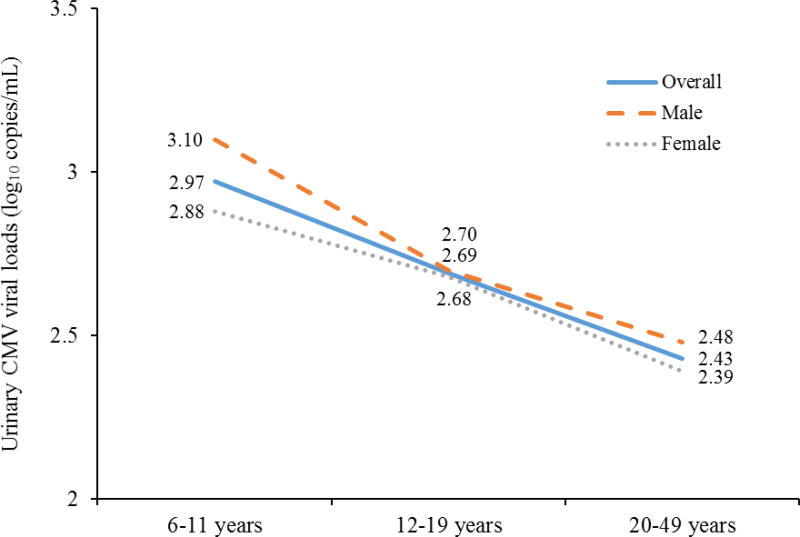
The average CMV viral load in urine was 2.74 log10 copies/mL (95% CI: 2.68–2.80 log10 copies/mL). The viral load decreased significantly with increasing age (mean: 2.87, 2.60, and 2.34 for those aged 6–11, 12–19, and 20–49 years, respectively; P < 0.001 for trend test and pairwise comparisons), and the same trend of decreasing viral loads with increased aged was observed in both males and females. Males had significantly higher viral loads than females (2.81 vs. 2.69 log10 copies/mL, P = 0.04), which was driven by children aged 6–11 years (3.10 vs. 2.88 for males and females, P = 0.03). (Figure 2). Moreover, there was no difference in viral load by sociodemographic factors such as race/ethnicity, country of birth, household income level, household education level, health insurance status, and household crowding index (Table 3).
Figure 2.
Changes in Urinary CMV Viral Loads with Age by Sex among CMV Urinary Shedders in NHANES 1999–2004 (N = 537)
Table 3.
Urinary CMV Viral Loads among Urinary CMV Shedders (N = 537)
| Characteristics | Mean: log10 copies/mL (95% confidence interval) a |
P value b |
|---|---|---|
| Overall | 2.74 (2.68–2.80) | |
| Race | ||
| non-Hispanic White | 2.78 (2.65–2.91) | Reference |
| non-Hispanic Black | 2.71 (2.62–2.80) | 0.25 |
| Mexican American | 2.75 (2.65–2.84) | 0.75 |
| Sex | ||
| Male | 2.81 (2.71–2.91) | Reference |
| Female | 2.69 (2.62–2.76) | 0.04 |
| Age | ||
| 6–11 years | 2.97 (2.87–3.07) | Reference |
| 12–19 years | 2.69 (2.60–2.77) | <0.001 |
| 20–49 years | 2.43 (2.34–2.53) | <0.001 |
| Country of birth | ||
| Born in the 50 US states or DC | 2.77 (2.70–2.84) | Reference |
| Other | 2.62 (2.51–2.73 | 0.26 |
| Household income level | ||
| Low | 2.80 (2.72–2.88) | Reference |
| Middle | 2.65 (2.55–2.75) | 0.18 |
| High | 2.72 (2.56–2.88) | 0.54 |
| Household education level | ||
| Less than high school | 2.76 (2.68–2.85) | Reference |
| High school graduate or GED diploma | 2.68 (2.55–2.80) | 0.54 |
| More than high school | 2.75 (2.65–2.86) | 0.51 |
| Health insurance status | ||
| Insured | 2.75 (2.68–2.82) | Reference |
| Uninsured | 2.71 (2.59–2.82) | 0.79 |
| Household crowding index | ||
| Low | 2.68 (2.49–2.86) | Reference |
| Average | 2.71 (2.63–2.79) | 0.93 |
| High | 2.82 (2.72–2.93) | 0.56 |
unweighted estimates as analyses were restricted to urinary shedders only.
adjusted with the variables listed in the table.
DISCUSSION
Our study found that the prevalence of urinary CMV shedding was 3.83% among all subjects and 9.70% among CMV IgG-positive persons aged 6–49 years in the US. Both prevalence of CMV urinary shedding and viral loads decreased significantly with increasing age. To our knowledge, this study is the first to present prevalence estimates for CMV shedding based on a nationally representative sample of the US population. Moreover, the sample spans an age range from 6 to 49 years—wider than is typically studied. These findings enrich the literature on CMV shedding and provide reference values for future studies.
While it is well documented that exposure to children 1–4 years of age is an important risk factor for acquiring CMV infection,[25] our findings suggest that older children may also be an important source of CMV transmission. Though both primary and non-primary CMV infection can lead to CMV shedding,[26, 27] shedding is more likely to occur after primary infection and is associated with longer duration of shedding and higher viral loads.[2, 18, 28, 29] Although CMV shedding in the urine typically has higher viral loads than shedding in saliva, [2, 29, 30] urinary shedding is highly correlated with saliva shedding and can be considered a marker, with the caveat that CMV shedding in saliva probably presents more opportunities for exposure and transmission than urine beyond the preschool age. Additionally, the higher CMV prevalence of shedding, higher viral loads, and lower IgG seroprevalence in adolescents compared to adults likely contribute to the higher prevalence and risk of congenital CMV infection seen among infants born to adolescent mothers.[31]
We found that some sociodemographic factors associated with CMV seroprevalence,[20] such as race/ethnicity, household income level, education level, and household crowding index were also statistically significantly associated with urinary shedding in all subjects. However, when the analysis was restricted to IgG-positive subjects, these associations were no longer significant. CMV IgG seroprevalence is determined by risk factors associated with primary CMV infection. In contrast, shedding results from both primary and non-primary infection. The risk factors associated with non-primary infection, particularly reactivation, are likely different from those associated with primary infection.
In addition, the only sexual risk factor we found associated with CMV shedding was a higher number of sexual partners in the past year. We did not detect any association between shedding and some other sexual behaviors that have been associated elsewhere with IgG seroprevalence, such as age of sexual debut, number of lifetime sexual partners, or ever having had STDs.[32] In a previous study, CMV shedding was more prevalent among STD clinic attendees.[2] An explanation for the associations seen between these other sexual risk factors and CMV shedding in previous studies may be that they were conducted in populations in which a greater proportion of participants had active STDs, a proxy for risky sexual behaviors leading to increased risk for primary infection/reinfection with CMV.[2] In contrast, the population sampled by NHANES may be at lower risk for having an active STD at the time of data collection.
Although rare, it is possible for CMV shedding to occur in IgG-negative individuals shortly after primary infection but before IgG appears. Therefore, our exclusion of IgG-negative subjects might have led to a small underestimation of CMV urinary shedding prevalence, particularly among young subjects more prone to experience primary infection. However, as the time interval between primary infection and IgG appearance is just is a few weeks, and overlaps with the time interval after primary infection but before shedding starts, the effect of underestimate would be small. Another limitation of this study is that the cross-sectional design of NHANES does not allow assessment of the duration or temporal pattern of shedding. Further, the lack of laboratory testing to distinguish primary and non-primary infection makes it impossible to assess the relative contributions of primary vs. non-primary infection to CMV shedding. The findings from current study may not be generalizable to other racial groups, such as Asian and Pacific islanders. Finally, self-reported sexual behaviors may be prone to some biases such as recall and social desirability biases[33] and result in underestimation of the role of sexual behaviors in CMV shedding.
In summary, we found the prevalence of urinary CMV shedding and viral loads decreased dramatically with age, which likely reflects the higher rates of primary CMV infection and longer duration of shedding among those of younger age. In addition to providing nationally representative estimates on CMV shedding prevalence and viral loads in the US, our findings demonstrate that children older than 5 years of age continue to shed CMV at higher rates than adolescents and adults and may still be an important source of CMV transmission.
Main points.
We demonstrated that cytomegalovirus (CMV) shedding prevalence and viral loads decreased with age, but children 6–11 years shed CMV at higher rates with higher viral loads than adolescents and adults, and may still be an important source for CMV transmission.
Acknowledgments
The authors highly appreciated Dr. Kathleen Dooling for her scientific comments and Ms. Mary Ann Hall for her editorial assistance.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONFLICTS OF INTEREST
The authors have no conflicts of interest or funding to disclose.
References
- 1.Revello MG, Gerna G. Pathogenesis and prenatal diagnosis of human cytomegalovirus infection. J Clin Virol. 2004;29(2):71–83. doi: 10.1016/j.jcv.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol. 2011;21(4):240–55. doi: 10.1002/rmv.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–76. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 4.Cannon MJ, Griffiths PD, Aston V, Rawlinson WD. Universal newborn screening for congenital CMV infection: what is the evidence of potential benefit? Rev Med Virol. 2014;24(5):291–307. doi: 10.1002/rmv.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mwintshi K, Brennan DC. Prevention and management of cytomegalovirus infection in solid-organ transplantation. Expert Rev Anti Infect Ther. 2007;5(2):295–304. doi: 10.1586/14787210.5.2.295. [DOI] [PubMed] [Google Scholar]
- 6.Bowen EF, Griffiths PD, Davey CC, Emery VC, Johnson MA. Lessons from the natural history of cytomegalovirus. AIDS. 1996;10(Suppl 1):S37–41. [PubMed] [Google Scholar]
- 7.Lucht E, Brytting M, Bjerregaard L, Julander I, Linde A. Shedding of cytomegalovirus and herpesviruses 6, 7, and 8 in saliva of human immunodeficiency virus type 1-infected patients and healthy controls. Clin Infect Dis. 1998;27(1):137–41. doi: 10.1086/514604. [DOI] [PubMed] [Google Scholar]
- 8.Gautheret-Dejean A, Aubin JT, Poirel L, et al. Detection of human Betaherpesvirinae in saliva and urine from immunocompromised and immunocompetent subjects. J Clin Microbiol. 1997;35(6):1600–3. doi: 10.1128/jcm.35.6.1600-1603.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostad SB, Kreiss JK, Ryncarz AJ, et al. Cervical shedding of cytomegalovirus in human immunodeficiency virus type 1-infected women. J Med Virol. 1999;59(4):469–73. [PubMed] [Google Scholar]
- 10.Betts RF, Freeman RB, Douglas RG, Jr, Talley TE, Rundell B. Transmission of cytomegalovirus infection with renal allograft. Kidney Int. 1975;8(6):385–92. doi: 10.1038/ki.1975.131. [DOI] [PubMed] [Google Scholar]
- 11.Correia-Silva Jde F, Victoria JM, Guimaraes AL, et al. Cytomegalovirus shedding in the oral cavity of allogeneic haematopoietic stem cell transplant patients. Oral Dis. 2007;13(2):163–9. doi: 10.1111/j.1601-0825.2006.01240.x. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal LS, Fowler KB, Boppana SB, et al. Cytomegalovirus shedding and delayed sensorineural hearing loss: results from longitudinal follow-up of children with congenital infection. Pediatr Infect Dis J. 2009;28(6):515–20. doi: 10.1097/INF.0b013e318198c724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noyola DE, Valdez-Lopez BH, Hernandez-Salinas AE, et al. Cytomegalovirus excretion in children attending day-care centers. Arch Med Res. 2005;36(5):590–3. doi: 10.1016/j.arcmed.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Yamada H, Minami M, et al. Screening for vaginal shedding of cytomegalovirus in healthy pregnant women using real-time PCR: correlation of CMV in the vagina and adverse outcome of pregnancy. J Med Virol. 2006;78(6):757–9. doi: 10.1002/jmv.20619. [DOI] [PubMed] [Google Scholar]
- 15.Lange M, Klein EB, Kornfield H, Cooper LZ, Grieco MH. Cytomegalovirus isolation from healthy homosexual men. JAMA. 1984;252(14):1908–10. [PubMed] [Google Scholar]
- 16.Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis. 2000;182(6):1761–4. doi: 10.1086/317624. [DOI] [PubMed] [Google Scholar]
- 17.Shen CY, Chang SF, Lin HJ, et al. Cervical cytomegalovirus infection in prostitutes and in women attending a sexually transmitted disease clinic. J Med Virol. 1994;43(4):362–6. doi: 10.1002/jmv.1890430408. [DOI] [PubMed] [Google Scholar]
- 18.Zanghellini F, Boppana SB, Emery VC, Griffiths PD, Pass RF. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J Infect Dis. 1999;180(3):702–7. doi: 10.1086/314939. [DOI] [PubMed] [Google Scholar]
- 19.Zipf G, Chiappa M, Porter K, et al. National Health and Nutrition Examination Survey: Plan and operations, 1999–2010. National Center for Health Statistics. Vital and Health Statistics Series. 2013;1(56) [PubMed] [Google Scholar]
- 20.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50(11):1439–47. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. [Accessed January 15, 2016];National Health and Nutrition Examination Survey. Available at: http://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics.
- 22.Boppana SB, Ross SA, Novak Z, et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303(14):1375–82. doi: 10.1001/jama.2010.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC. [Accessed May 16, 2016];National Health and Nutrition Examination Survey: Urinary cytomegalovirus shedding. Available at: https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&CycleBeginYear=1999.
- 24.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes. 2001;(20):1–10. [PubMed] [Google Scholar]
- 25.Adler SP, Nigro G. Prevention of maternal-fetal transmission of cytomegalovirus. Clin Infect Dis. 2013;57(Suppl 4):S189–92. doi: 10.1093/cid/cit585. [DOI] [PubMed] [Google Scholar]
- 26.Arora N, Novak Z, Fowler KB, Boppana SB, Ross SA. Cytomegalovirus viruria and DNAemia in healthy seropositive women. J Infect Dis. 2010;202(12):1800–3. doi: 10.1086/657412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy S, Hayward GS, Wheelan S, et al. Detection of a single identical cytomegalovirus (CMV) strain in recently seroconverted young women. PLoS One. 2011;6(1):e15949. doi: 10.1371/journal.pone.0015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Revello MG, Zavattoni M, Sarasini A, Percivalle E, Simoncini L, Gerna G. Human cytomegalovirus in blood of immunocompetent persons during primary infection: prognostic implications for pregnancy. J Infect Dis. 1998;177(5):1170–5. doi: 10.1086/515277. [DOI] [PubMed] [Google Scholar]
- 29.Stowell JD, Mask K, Amin M, et al. Cross-sectional study of cytomegalovirus shedding and immunological markers among seropositive children and their mothers. BMC Infect Dis. 2014;14:568. doi: 10.1186/s12879-014-0568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunkel J, Wolfs TF, Nijman J, et al. Urine is superior to saliva when screening for postnatal CMV infections in preterm infants. J Clin Virol. 2014;61(1):61–4. doi: 10.1016/j.jcv.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Zhang X, Bialek S, Cannon MJ. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis. 2011;52(2):e11–3. doi: 10.1093/cid/ciq085. [DOI] [PubMed] [Google Scholar]
- 32.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–13. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 33.Brener ND, Billy JO, Grady WR. Assessment of factors affecting the validity of self-reported health-risk behavior among adolescents: evidence from the scientific literature. J Adolesc Health. 2003;33(6):436–57. doi: 10.1016/s1054-139x(03)00052-1. [DOI] [PubMed] [Google Scholar]