Abstract
Microcystin toxin-producing cyanobacteria are known to have harmful effects on humans and animals. We have developed a loop-mediated isothermal amplification (LAMP)-based detection method by targeting the microcystin synthetase B gene (mcyB), the gene responsible for the production of microcystin. The sensitivity of the method was found to be 1 fg per reaction, and it was 1000-fold higher than the conventional PCR. The LAMP method was able to amplify the target gene with a minimum amount of dNTP (0.4 mM), which further reduces the cost of reaction. The improved LAMP assay could detect the presence of the toxin-producing cyanobacteria in water samples within 2 h of time, which demonstrates the rapidness of the method. Freshwater samples were screened using the developed LAMP, and seven water samples collected from lakes and a bird sanctuary tested positive for mcyB gene harboring cyanobacteria, and negative in all other drinking waters. Hence, the developed LAMP could be a possible alternative to the existing molecular methods for screening for microcystin in environmental samples with greater sensitivity.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1402-0) contains supplementary material, which is available to authorized users.
Keywords: Microcystin synthetase, LAMP, Microcystin, Cyanobacteria, McyB
Introduction
Microcystins (MCs) are hepatotoxic metabolites of certain cyanobacterial species that are found in freshwater, brackish, and irrigation water supplies. Cyanobacterial species such as Microcystis, Planktothrix, Nostoc, and Anabaena are known to produce more than 100 different types of microcystins (Rinehart et al. 1994; Zastepa et al. 2017). Accumulation of microcystin in the aquatic environment is a threat to both aquatic species and humans. Microcystins have potential toxic effects such as growth retardation, reproductive system problems, and changes in the neurotransmitter system in fishes (Hou et al. 2017; Yan et al. 2017). Irrigation of microcystin-contaminated water resulted in bioaccumulation of toxin in plants (Bittencourt-Oliveira et al. 2016). Consumption of the toxin-accumulated plants and water adversely affects mankind. Microcystin interferes with the DNA damage repair pathway, which triggers the expression of proto-oncogenes in humans (Lone et al. 2015). International Agency for Research on Cancer has classified the microcystins as a possible carcinogen (Fan et al. 2014). Microcystin toxicity alters the mobility and morphology of sperm and reduces the level of testosterone that decreases the male reproduction capacity (Zhou et al. 2012).
Microcystin-producing cyanobacteria have been detected by different techniques such as LCMS (Vudathala et al. 2017), aptamer-based immunoassay (Xiang et al. 2014), ELISA (Preece et al. 2015), and cellular biosensors (Mankiewicz-Boczek et al. 2015). Even though ELISA is one of the essential diagnostic methods, it is unsuitable for screening environmental water samples and it is more expensive. Many of the above-reported methods are not suitable for direct detection of toxin-producing cyanobacteria. Molecular diagnostic methods are a potential alternative for early detection of toxin-producing cyanobacteria. Molecular methods based on multiplex PCR (Barón-Sola et al. 2012), qPCR (Sipari et al. 2010) and HRM qPCR (Manali et al. 2017) have been used to detect the microcystin-producing cyanobacterial species. However, these methods require specialized equipment, which limits their on-field application for screening microcystins.
The loop-mediated isothermal amplification (LAMP) method amplifies the target gene under isothermal conditions with an optimum temperature between 60 and 65 °C. The strand displacement activity and auto-cycling activity of Bst DNA polymerase with high processivity resulted in an enhanced amplification rate, and helped the LAMP detect the target in less time. LAMP reactions utilize four designed primers including two outer (F3 and B3) and two inner primers (FIP and BIP) that recognize six distinct regions of the target gene. The initial LAMP reaction produces a stem-loop structure of DNA, and subsequent cyclic reactions will result in 109 copies of target in less than an hour. Hence, the LAMP method will amplify even a small quantity of the target DNA, which helps in the early detection of the pathogen. The amplified products consist of a stem-loop with several inverted repeats of the target gene, which result in the ladder-like bands in agarose gel electrophoresis (Notomi et al. 2000). LAMP assay is highly specific to the target due to its primer specificity. A water bath is adequate to amplify the target gene in an isothermal condition which enables its applicability for on-field screening of environmental samples.
The cluster of ten microcystin synthetase genes (mcyA-J) produces different types of microcystin. The toxin producers can be identified by detecting the microcystin synthetase genes (Kaebernick et al. 2002). In the previous studies, various genes such as mcyA (Dong et al. 2016; Oh et al. 2013), mcyE (Rantala et al. 2006), and mcyB (Misson et al. 2012) were used as molecular markers for detecting the microcystin-producing cyanobacteria. The mcyB gene is known to be involved in the addition of l-Leu and d-MeAsp to the growing polypeptide chain of microcystin biosynthesis. Since mcyB gene plays an important role in the formation of microcystin, detecting the microcystin-producing species using the gene as a marker could be more sensitive and reliable.
In the current study, we developed mcyB gene targeting LAMP with greater sensitivity and rapidness in detecting the microcystin-producing cyanobacteria. The developed method was also tested with fresh water samples collected in and around Chennai, Tamil Nadu, India.
Materials and methods
Collection and growth of the bacterial strain
Microcystis aeruginosa (NIES-843) strain was procured from the National Institute of Environmental Studies (NIES), Japan. The culture was grown in a BG11 medium under the following conditions: 25 °C ± 1 °C, 60 µmol photons m−2 s−1 and 14:10 h of light/dark cycle (Manali et al. 2017). All the medium components used in this study were purchased from Himedia Pvt Ltd., India.
Cloning of the mcyB gene
The genomic DNA from M. aeruginosa (NIES-843) was isolated using the CTAB method (Murray and Thompson 1980). The mcyB gene was amplified using PCR with the gene-specific primers (FP: 5′ CCCCAGGCAAGCAGAAATTCAGGA 3′ and RP: 5′ CCCCATAGCAACCACC GTCAA AGG 3′). The PCR was performed with 15 mM MgCl2, 40 mM KCl, 10 mM Tris–HCl (pH 8.0), 10 pmol forward and reverse primers, 10 mM dNTP, 1U Taq DNA polymerase and 100 ng of template DNA. PCR was performed for 35 cycles with the following conditions: 95 °C for 60 s, 55 °C for 60 s and 72 °C for 60 s. The amplified mcyB gene was cloned into a pGEM-T Easy Vector (Promega, USA), and transformed into Escherichia coli Top10 strain (Invitrogen). The positive clones were selected and the plasmids harboring the mcyB gene (pMCYB) were isolated and subsequently used for both PCR and LAMP assays.
Design of LAMP primers
Different mcyB genes sequences were retrieved from a gene sequence database repository (http://www.ncbi.nlm.nih.gov), and multiple sequence alignment was performed (http://www.genome.jp/tools-bin/clustalw). The unique and conserved regions of the mcyB gene were identified. The conserved portion of the gene was used as a target region for LAMP primer designing using Primer Explorer V4 (http://www.primerexplorer.jp). LAMP primers contain a pair of inner (F3 and B3) and outer primers (FIP and BIP). FIP has the sense sequence of F2 and the complementary sequence of F1, while BIP contains sense sequence of B1 and the complementary sequence of B2 (Fig. 1). The LAMP primers recognize six distinct regions in the mcyB gene.
Fig. 1.
LAMP primer design. a Location and sequence of LAMP targets and priming sites for the mcyB gene. b Sequences of LAMP primers. Primer FIP consisted of the F1 complementary sequence and F2 direct sequence. Primer BIP consisted of B1 direct sequence and B2 complementary sequence
Optimization of LAMP
The LAMP reaction was performed with a final volume of 25 µL containing 10 mM dNTP, 20 mM Tris–HCl, 10 mM KCl, 10 mM (NH4)2SO4, 8 mM MgSO4, 0.1% Tween 20, 5 pmol F3, 5 pmol B3 primers, 40 pmol FIP, 40 pmol BIP primers, 8U Bst DNA polymerase (New England Biolabs, USA) and 150 ng of pMCYB. The isothermal LAMP reactions were performed at 65 °C using a water bath (Rathinasabapathi et al. 2015). The amplified products were visualized on 2% agarose gel and stained with ethidium bromide. LAMP reaction conditions were optimized to improve the amplification efficiency and to reduce the cost and time of the reaction. Different concentrations of MgCl2 (2, 4, 6 and 8 mM) and dNTP (0.2, 0.4, 0.6 and 1.2 mM) were tested to find the optimum LAMP conditions. The reaction time of LAMP was varied from 30 to 60 min to evaluate the rapidness of the LAMP in detecting mcyB gene.
Sensitivity of LAMP and PCR
PCR and LAMP were performed with various quantities of pMCYB ranging from 1 ng to 1 ag in each reaction. The sensitivity (detection limit) of both the methods was compared. The amplified product of PCR and LAMP was investigated on 1 and 2% agarose gel, respectively, and visualized using ethidium bromide staining under a gel documentation system (Gelstan, Medicare Pvt Ltd, India).
Screening of water samples using LAMP
A total of 21 water samples (250 ml each) were collected in sterile glass containers from different places: (1) lakes and open ponds that provide drinking water for the Chennai city (2) Vedanthangal Bird Sanctuary near Chengalpattu, Kanchipuram district, Tamil Nadu, and (3) chlorinated drinking water samples. The collected water samples were concentrated using a vacuum concentrator (Eppendorf Vacuum Concentrator Plus, Germany) at 60 °C for 75 min. The concentrated samples were stored at − 20 °C and it was used for the analysis. All the assays were performed in triplicates.
Results
Cloning of mcyB gene
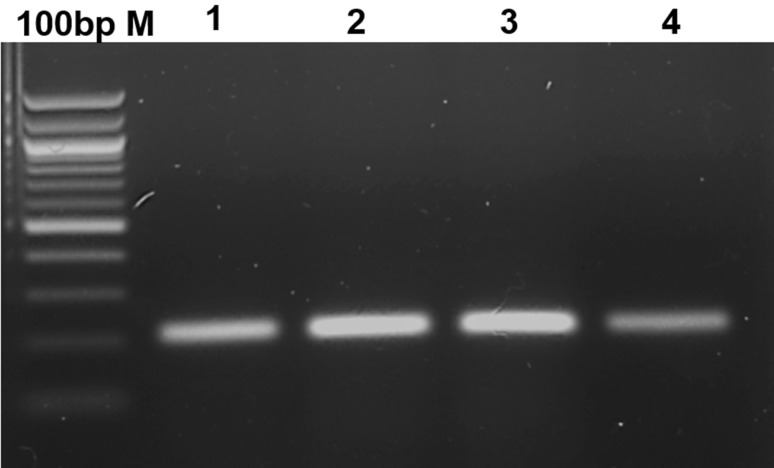
The conserved region of the mcyB gene from M. aeruginosa was cloned into the pGEM-T Easy Vector. The positive recombinant colonies were selected using mcyB gene-specific colony PCR using FP and RP primers (Fig. 2). The plasmid DNA from the positive clones was isolated and used as a template for optimization of PCR and LAMP.
Fig. 2.
Colony PCR amplification of mcyB gene using FP and RP primers. M1-100 bp M, 1–4—transformed colonies from 1 to 4
Optimization of LAMP
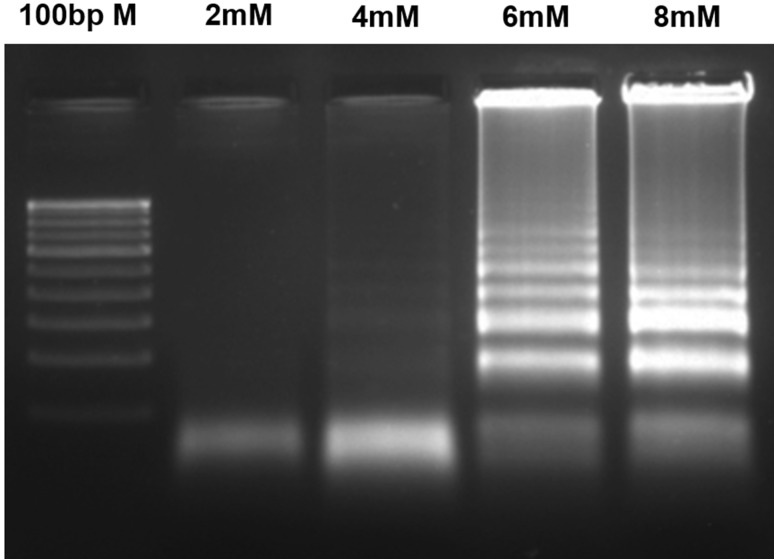
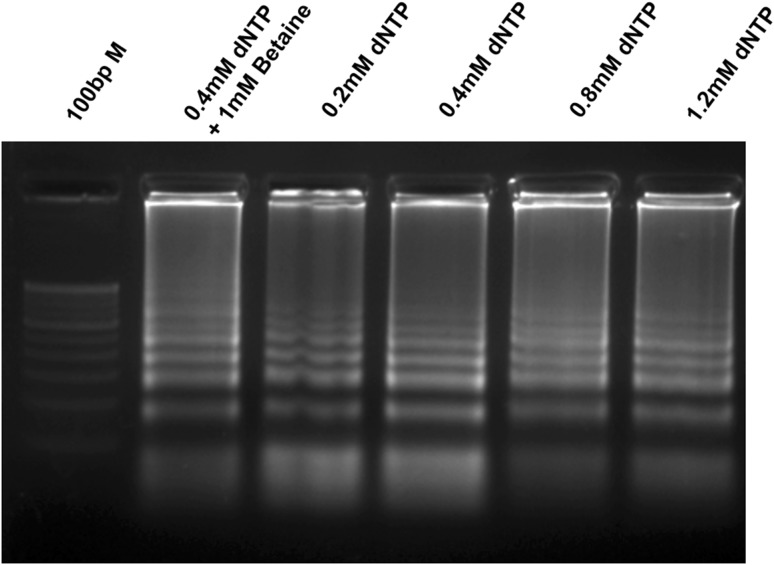
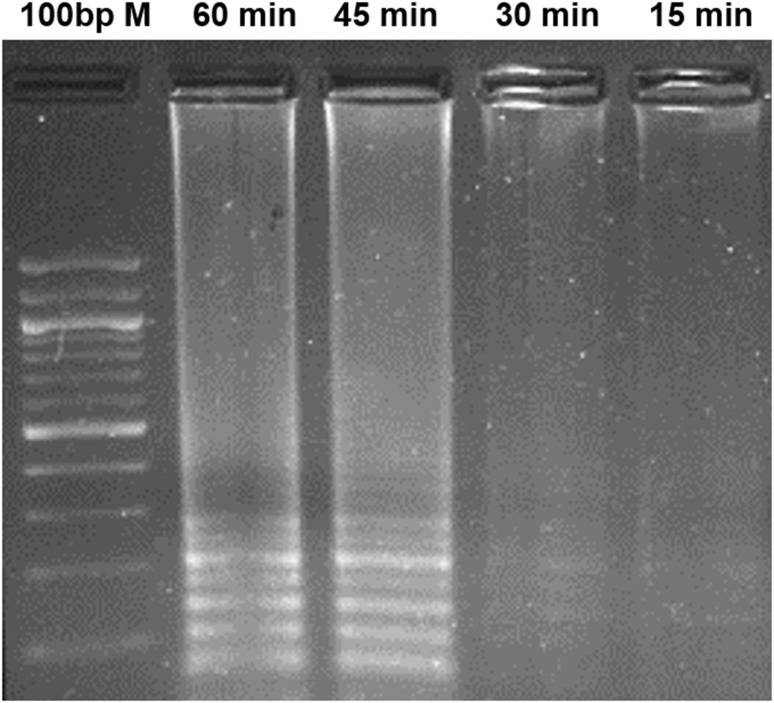
LAMP primers were designed using the conserved region of mcyB (212 bp) and the primer-binding region, mentioned in Fig. 1. During LAMP, a loop-like structure is created with the help of FIP and BIP primers, which produces the ladder-like DNA fragments in agarose gel electrophoresis. The Bst DNA polymerase displaces the newly synthesized strands using the outer primers (F3 and B3). During the optimization of LAMP conditions, the effects of Mg2+ and dNTP concentrations were evaluated (Figs. 3, 4). In different Mg2+ concentrations between 2 and 8 mM, the amplification of mcyB gene was best observed at 6 mM Mg2+ concentration (Fig. 3). The amplification was observed in all the reactions containing different dNTP concentrations (0.2 mM, 0.4 mM, 0.8 mM and 1.2 mM). However, high-magnitude amplification was observed at 0.4 mM dNTP concentration (Fig. 4). Also, the effect of betaine was tested by the addition of 1M betaine to a reaction mixture containing 0.4 mM dNTP, but it did not enhance the amplification efficiency of the LAMP (Fig. 4). LAMP was performed at different reaction times (15, 30, 45 and 60 min) and a detectable amplification was found in 30 min (Fig. 5). However, the amplification magnitude was in higher at 45 min. Subsequent reactions were performed for 45 min under optimized LAMP conditions of 0.4 mM dNTP and 6 mM Mg2+ concentrations.
Fig. 3.
Effect of different Mg2+ concentrations on LAMP assay using LAMP primers (F3, B3, FIP and BIP). Various final concentrations of MgCl2 were added to the LAMP reactions to evaluate the effect MgCl2. 100 bp M—100 bp Marker; 2 mM—2 mM MgCl2, 4 mM—4 mM MgCl2, 6 mM—6 mM MgCl2, 8 mM—8 mM MgCl2
Fig. 4.
Effect of dNTP concentrations and betaine on LAMP assay using LAMP primers (F3, B3, FIP and BIP). The need of betaine for the LAMP was evaluated with the inclusion of 1 M betaine in the reaction. 100 bp M 100 bp Marker
Fig. 5.
Effect of different reaction time on LAMP assay using LAMP primers (F3, B3, FIP and BIP)
Sensitivity of LAMP and PCR
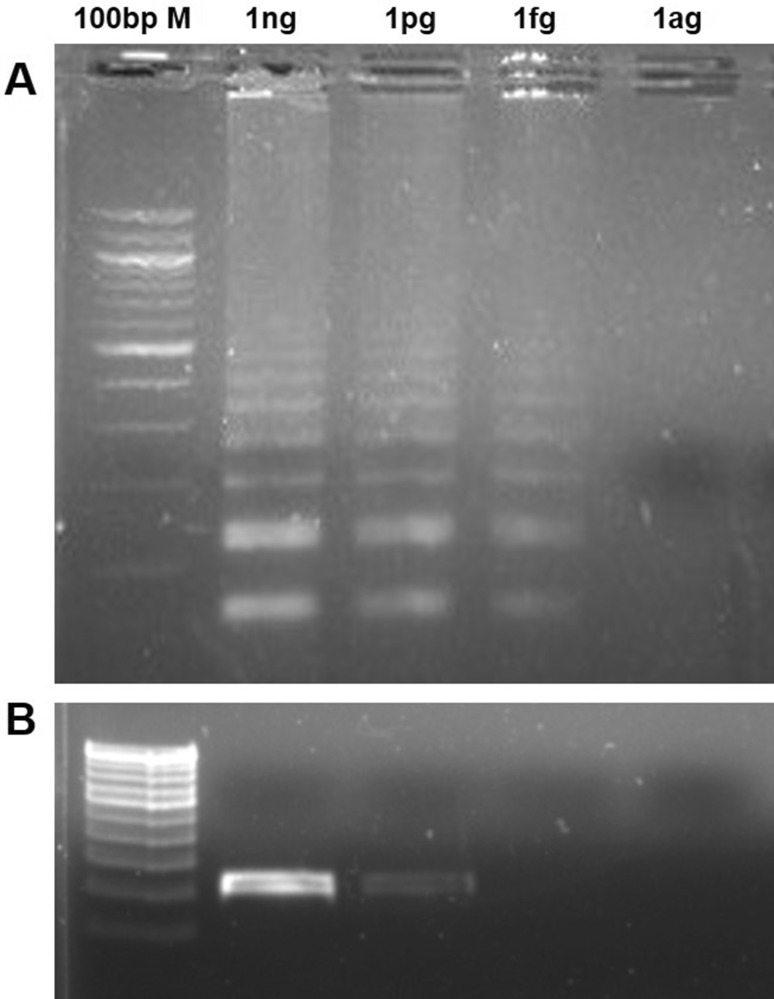
The sensitivity of the LAMP and PCR was evaluated using 1000-fold serial dilutions of pMCYB under optimized conditions. The LAMP outer primers (F3 and B3) were used for PCR amplification of mcyB gene. The LAMP was detected the target gene up to 1 fg per reaction, but the PCR failed to amplify the gene after 1 pg (Fig. 6). The detection limit of the LAMP was, therefore, 1000-fold superior to the conventional PCR reaction.
Fig. 6.
Comparison of the detection limit of the LAMP with PCR. The PCR and LAMP were carried out in different quantities of plasmid harboring mcyB gene ranging between 1 ag and 1 ng per reaction. PCR was performed using F3 and B3 primers and the LAMP assay was carried out with all the four LAMP primers a LAMP assay for the detection of the mcyB gene. b PCR assay for the detection of the mcyB gene. M 100 bp marker, ng nanogram/reaction, pg picogram/reaction, fg femtogram/reaction, ag attogram/reaction
Detection of microcystin-producing cyanobacteria in water samples
The developed LAMP assay was used to screen different fresh water samples around Chennai, Tamil Nadu. We collected 21 freshwater samples from various locations in and around Chennai. The concentrated water samples were directly used for PCR and LAMP analysis. A total of seven samples tested positive for the mcyB gene using the LAMP, where only six samples were identified by PCR (Table 1). LAMP detected the microcystin-producing cyanobacteria in five water samples collected from open lakes and ponds, while PCR identified only four of the samples. Amplification was positive for two water samples collected at the Vedanthangal Bird Sanctuary near Chennai by both the methods. Chlorinated water samples did not show any amplification in PCR and LAMP methods.
Table 1.
Screening of environmental and drinking water samples for the presence of toxic cyanobacteria
| Samples | No of sample screened | PCR+ | LAMP+ |
|---|---|---|---|
| Open lakes and ponds | 9 | 4 | 5 |
| Water samples from bird sanctuary | 2 | 2 | 2 |
| Chlorinated drinking water sample | 10 | – | – |
PCR+ Number of water sample shown positive amplification by PCR, LAMP+ Number of water sample shown positive for mcyb gene by the LAMP
Discussion
Microcystins are the class of hepatotoxin produced by certain cyanobacteria including M. aeruginosa in a freshwater ecosystem. Detection of toxin-producing cyanobacteria through microbiological and biochemical methods are time consuming and laborious. Microcystin-producing cyanobacteria possess gene cluster for microcystin synthetase. The absence of this gene cluster in the non-toxic cyanobacteria provides a way for identifying toxic cyanobacteria. The available PCR- and qPCR-based molecular methods used for the detection of M. aeruginosa have their limitation in screening the water samples. LAMP has the advantages over other methods including (1) higher specificity (2) ability to detect at lower pathogen copies, which was evident in the water samples (3) high level of tolerance to other inhibitory chemicals present in the sample that normally prohibit the PCR reactions (4) rapidness and (5) ability to use a water bath for the amplification reaction. Hence, we have developed a LAMP assay with enhanced sensitivity for detecting microcystin-producing cyanobacteria by targeting the mcyB gene.
The LAMP was optimized to increase the efficiency and reduce the detection time. We have not observed any beneficial effect of betaine on the LAMP assay, which is contrary to many other reports (George et al. 2011; Nagdev et al. 2011; Njiru 2011). However, some previous studies have mentioned an ineffectiveness of betaine in the reaction (Chen et al. 2011; Zhou et al. 2014). Betaine increases the amplification efficiency and sensitivity of GC-rich regions by prohibiting the secondary structure formations. The region in mcyB gene used for the study has 42% GC content, which explains the lack of effect of betaine. Hence, betaine is dispensable for the target sequences with low GC content. The excessive Mg2+ concentration stabilizes the mispriming of primers that decrease the specificity of the assay. Also, it prevents the denaturation of the DNA double strand by stabilizing the duplex structure (Markoulatos et al. 2002). Conversely, low concentrations of Mg2+ fail to amplify the target genes. The significance of optimum Mg2+ concentration in LAMP has been mentioned in many earlier studies (Guan et al. 2010; Nemoto et al. 2009; Zhou et al. 2014). We observed the positive amplification only after 6 mM Mg2+ per reaction was added, which demonstrates the importance of Mg2+ concentration in the LAMP reaction. The mcyB gene was amplified even with 0.2 mM dNTP, and the amplification was consistent from 0.4 mM dNTP, which is less than the other earlier reports (Rathinasabapathi et al. 2015; Zhu et al. 2014). The minimum usage of dNTP reduces the cost of the assay. In this study, the LAMP provides detectable amplification within 30 min and it can detect up to 1 fg of mcyB gene. The sensitivity and detection time are superior to the earlier mcyE gene-based LAMP assay that identified the pathogen only up to 17 pg of microcystin DNA in 60 min (Zhu et al. 2014). The LAMP developed in this study had a 1000-fold higher sensitivity than PCR in detecting the mcyB gene. Hence, the developed method could be used for toxic cyanobacteria detection even before bloom formation.
Screening water samples for the presence of microcystin using a PCR-based method has many limitations such as the need for instruments, the susceptibility of Taq DNA polymerase to inhibitors, and lower sensitivity. No algal blooms were observed in any of the water samples at the time of sample collection. However, LAMP assays could detect the microcystin-producing cyanobacteria in seven of the tested samples within 45 min. None of the chlorinated samples showed a positive result, which could be due to the toxic effect of chlorine components on cyanobacteria. The time taken for analysis is approximately 2 h, as it does not involve culturing and DNA isolation procedures. Greater sensitivity, lesser reaction time, and the suitability for on-field application make the method as a potential alternative to the existing molecular methods for identifying toxic cyanobacteria.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge the SRM Institute of Science and Technology, Tamil Nadu, India, for their financial support and facilities to carry out this project.
Compliance with ethical standards
Conflict of interest
The authors declared that there is no conflict of interest regarding the publication of this paper.
References
- Barón-Sola Á, Ouahid Y, del Campo FF. Detection of potentially producing cylindrospermopsin and microcystin strains in mixed populations of cyanobacteria by simultaneous amplification of cylindrospermopsin and microcystin gene regions. Ecotoxicol Environ Saf. 2012;75:102–108. doi: 10.1016/j.ecoenv.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Bittencourt-Oliveira MdC, Cordeiro-Araújo MK, Chia MA, Arruda-Neto JDdT, Oliveira ÊTd S. Lettuce irrigated with contaminated water: photosynthetic effects, antioxidative response and bioaccumulation of microcystin congeners. Ecotoxicol Environ Saf. 2016;128:83–90. doi: 10.1016/j.ecoenv.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang F, Beaulieu JC, Stein RE, Ge B. Rapid detection of viable Salmonellae in produce by coupling propidium monoazide with loop-mediated isothermal amplification. App Environ Microbiol. 2011;77:4008–4016. doi: 10.1128/AEM.00354-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Zeng S, Bai F, Li D, He M. Extracellular microcystin prediction based on toxigenic Microcystis detection in a eutrophic lake. Sci Rep. 2016 doi: 10.1038/srep20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Cai Y, Xie P, Xiao W, Chen J, Ji W, Zhao S. Microcystin-LR stabilizes c-myc protein by inhibiting protein phosphatase 2A in HEK293 cells. Toxicology. 2014;319:69–74. doi: 10.1016/j.tox.2014.02.015. [DOI] [PubMed] [Google Scholar]
- George G, Mony P, Kenneth J. Comparison of the efficacies of loop-mediated isothermal amplification, fluorescence smear microscopy and culture for the diagnosis of tuberculosis. PloS One. 2011 doi: 10.1371/journal.pone.0021007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Guo J, Shen P, Yang L, Zhang D. Visual and rapid detection of two genetically modified soybean events using loop-mediated isothermal amplification method. Food Anal Methods. 2010;3:313–320. doi: 10.1007/s12161-010-9132-x. [DOI] [Google Scholar]
- Hou J, et al. Microcystin-LR retards gonadal maturation through disrupting the growth hormone/insulin-like growth factors system in zebrafish. Ecotoxicol Environ Saf. 2017;139:27–35. doi: 10.1016/j.ecoenv.2017.01.025. [DOI] [PubMed] [Google Scholar]
- Kaebernick M, Dittmann E, Börner T, Neilan BA. Multiple alternate transcripts direct the biosynthesis of microcystin, a cyanobacterial nonribosomal peptide. Appl Environ Microbiol. 2002;68:449–455. doi: 10.1128/AEM.68.2.449-455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lone Y, Koiri RK, Bhide M. An overview of the toxic effect of potential human carcinogen microcystin-LR on testis. Toxicol Rep. 2015;2:289–296. doi: 10.1016/j.toxrep.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manali KM, Arunraj R, Kumar T, Ramya M. Detection of microcystin producing cyanobacteria in Spirulina dietary supplements using multiplex HRM quantitative PCR. J Appl Phycol. 2017;29:1279–1286. doi: 10.1007/s10811-016-1011-4. [DOI] [Google Scholar]
- Mankiewicz-Boczek J, Karwaciak I, Ratajewski M, Gagała I, Jurczak T, Zalewski M, Pułaski Ł. Application of cellular biosensors for detection of atypical toxic bioactivity in microcystin-containing cyanobacterial extracts. Aquat Toxicol. 2015;168:1–10. doi: 10.1016/j.aquatox.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Markoulatos P, Siafakas N, Moncany M. Multiplex polymerase chain reaction: a practical approach. J Clin Lab Anal. 2002;16:47–51. doi: 10.1002/jcla.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misson B, Sabart M, Amblard C, Latour D. Benthic survival of Microcystis: long-term viability and ability to transcribe microcystin genes. Harmful Algae. 2012;13:20–25. doi: 10.1016/j.hal.2011.09.010. [DOI] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4326. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagdev KJ, Kashyap RS, Parida MM, Kapgate RC, Purohit HJ, Taori GM, Daginawala HF. Loop-mediated isothermal amplification for rapid and reliable diagnosis of tuberculous meningitis. J Clin Microbiol. 2011;49:1861–1865. doi: 10.1128/JCM.00824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto J, et al. Rapid and specific detection of the thermostable direct hemolysin gene in Vibrio parahaemolyticus by loop-mediated isothermal amplification. J Food Prot. 2009;72:748–754. doi: 10.4315/0362-028X-72.4.748. [DOI] [PubMed] [Google Scholar]
- Njiru ZK. Rapid and sensitive detection of human African trypanosomiasis by loop-mediated isothermal amplification combined with a lateral-flow dipstick. Diagn Microbiol Infect Dis. 2011;69:205–209. doi: 10.1016/j.diagmicrobio.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KH, Jeong DH, Cho YC. Quantification of toxigenic Microcystis spp. in freshwaters by quantitative real-time PCR based on the microcystin synthetase A gene. J Microbiol. 2013;51:18–24. doi: 10.1007/s12275-013-2354-z. [DOI] [PubMed] [Google Scholar]
- Preece EP, Moore BC, Swanson ME, Hardy FJ. Identifying best methods for routine ELISA detection of microcystin in seafood. Environ Monit Assess. 2015 doi: 10.1007/s10661-014-4255-y. [DOI] [PubMed] [Google Scholar]
- Rantala A, Rajaniemi-wacklin P, Lyra C, Lepisto L, Rintala J, Mankiewicz-boczek J, Sivonen K. Detection of microcystin-producing cyanobacteria in Finnish Lakes with genus-specific microcystin synthetase gene E (mcyE) PCR and associations with environmental factors. App Environ Microbiol. 2006;72:6101–6110. doi: 10.1128/AEM.01058-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinasabapathi P, Hiremath DS, Arunraj R, Parani M. Molecular detection of New Delhi metallo-beta-lactamase-1 (NDM-1) positive bacteria from environmental and drinking water samples by loop mediated isothermal amplification of blaNDM-1. Indian J Microbiol. 2015;55:400–405. doi: 10.1007/s12088-015-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart KL, Namikoshi M, Choi BW. Structure and biosynthesis of toxins from blue–green algae (cyanobacteria) J Appl Phycol. 1994;6:159–176. doi: 10.1007/BF02186070. [DOI] [Google Scholar]
- Sipari H, Rantala-Ylinen A, Jokela J, Oksanen I, Sivonen K. Development of a chip assay and quantitative PCR for detecting microcystin synthetase e gene expressions. App Environ Microbiol. 2010;76:3797–3805. doi: 10.1128/AEM.00452-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vudathala D, Smith S, Khoo L, Kuhn DD, Mainous ME, Steadman J, Murphy L. Analysis of microcystin-LR and nodularin using triple quad liquid chromatography-tandem mass spectrometry and histopathology in experimental fish. Toxicon. 2017;138:82–88. doi: 10.1016/j.toxicon.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Xiang A, et al. An aptamer-based immunoassay in microchannels of a portable analyzer for detection of microcystin-leucine-arginine. Talanta. 2014;130:363–369. doi: 10.1016/j.talanta.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Yan W, Li L, Li G, Zhao S. Microcystin-LR induces changes in the GABA neurotransmitter system of zebrafish. Aquat Toxicol. 2017;188:170–176. doi: 10.1016/j.aquatox.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Zastepa A, Watson SB, Kling H, Kotak B. Spatial and temporal patterns in microcystin toxins in lake of the woods surface waters. Lake Reserv Manag. 2017;33:433–443. doi: 10.1080/10402381.2017.1384415. [DOI] [Google Scholar]
- Zhou Y, Yuan J, Wu J, Han X. The toxic effects of microcystin-LR on rat spermatogonia invitro. Toxicol Lett. 2012;212:48–56. doi: 10.1016/j.toxlet.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Zhou D, et al. Establishment and application of a loop-mediated isothermal amplification (LAMP) system for detection of cry1Ac transgenic sugarcane. Sci Rep. 2014;4:4912. doi: 10.1038/srep04912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Zhang B-F, Wu J-H, Dang C-Y, Lv Y-T, Fan J-Z, Yan X-J. Sensitive and rapid detection of microcystin synthetase E Gene (mcyE) by loop-mediated isothermal amplification: a new assay for detecting the potential microcystin-producing Microcystis in the aquatic ecosystem. Harmful Algae. 2014;37:8–16. doi: 10.1016/j.hal.2014.04.018. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.