Abstract
The Toxicology Investigators Consortium (ToxIC) Case Registry was established by the American College of Medical Toxicology in 2010. The Registry collects data from participating sites with the agreement that all bedside medical toxicology consultations will be entered. The objective of this eighth annual report is to summarize the Registry’s 2017 data and activity with its additional 7577 cases. Cases were identified for inclusion in this report by a query of the ToxIC database for any case entered from 1 January to 31 December 2017. Detailed data was collected from these cases and aggregated to provide information which includes demographics (e.g., age, gender, race, ethnicity), reason for medical toxicology evaluation (e.g., intentional pharmaceutical exposure, envenomation, withdrawal from a substance), agent and agent class, clinical signs and symptoms (e.g., vital sign abnormalities, organ system dysfunction), treatments and antidotes administered, fatality, and life support withdrawal data. Females were involved in 50.4% of cases. Transgender demographic information collection was initiated in 2017 to better represent the population and there were 36 cases involving transgender patients. Adults aged 19–65 were the most commonly reported age group. Non-opioid analgesics were the most commonly reported agent class, with acetaminophen again the most common agent reported. There were 93 fatalities reported in 2017. Treatment interventions were frequently reported with 30.6% receiving specific antidotal therapy. Major trends in demographics and exposure characteristics remained similar to past years’ reports. While treatment interventions were commonly required, fatalities were rare.
Electronic supplementary material
The online version of this article (10.1007/s13181-018-0679-z) contains supplementary material, which is available to authorized users.
Keywords: Poisonings, Overdose, Surveillance, Epidemiology, Medical toxicology
Introduction
The year 2017, the eighth full year of operation of the Toxicology Investigators Consortium (ToxIC), was marked by a number of achievements and continued robust data collection. A summary of the data collected is provided in the body of this report. Major changes and achievements are described below.
On January 1, 2017, ToxIC moved to the REDCap data collection platform. This provided an even greater level of data security than we had with our previous interface and allowed for the incorporation of two factor authentication of identity before the database was accessed for data entry or searching.
Given the growth of ToxIC and large amount of associated information, policies, and background information, a unique ToxIC website was developed throughout 2016 and went live in 2017 at https://www.toxicregistry.org. The reader of this Annual Report is referred to this website for additional information about ToxIC.
In light of the evolving complexity of ToxIC, it was felt that it was necessary to embark on a long-term strategic plan. That plan was completed in 2017 and its implementation was initiated. Continued implementation is expected throughout 2018. It is hoped that the implementation of the strategic plan will lead to further sophistication of our data collection and analysis, research, post-marketing surveillance, and toxicovigilance activities. It is also hoped that the strategic plan implementation will serve as a direction to ensure the continued long-term sustainability of the ToxIC program.
In 2017, there were 7577 novel cases entered from 40 separate sites comprising 63 separate facilities. The year 2017 saw the closure of three projects, the evolution and growth of existing efforts, and the launch of three of new focused sub-Registries.
A study, in the form of a sub-Registry, on diagnostic modalities in caustic ingestions, which collected data for nearly the entire 8 years of ToxIC’s existence, was completed and the sub-Registry was closed by virtue of not being migrated to the REDCap platform. Data analysis collected in this project is now underway.
The focused data collection, also in the form of a sub-Registry, on electrocardiographic QRS widening and the antidotal use of sodium bicarbonate was also closed in 2017. The analysis of data from this project is also underway. Hypotheses emerging from this analysis will be potentially tested once this data analysis is completed.
Our data collection of prescription opioid misuse was closed by virtue of it not being migrated to REDCap. With the continued and growing concern about opioid toxicity, it was felt that a new and more robust approach to this critical problem was necessary. The epidemic of opioid abuse and misuse is a major public health concern and new efforts related to this problem by ToxIC are in development. As described below, one such project relating to pediatric opioid exposures has already been instituted.
Our study on the use of lipid resuscitation therapy continues and currently represents the largest known prospective collection of patients treated with this modality. Data analysis for this project is also underway; however, patient accrual is still ongoing. Following our preliminary analysis, the need for objective definable end-points became apparent and were instituted in our mid-2017 database modifications.
The North American Snakebite sub-Registry continues to thrive and currently represents the largest prospective collection of data on these snakebites in existence. The publications and published abstracts from presentations at national professional meetings deriving from this sub-Registry are listed on https://www.toxicregistry.org.
Our extracorporeal substance removal sub-Registry continues to collect unique data on drug clearances by these techniques. Also continuing is the data collection of prognostic factors following drug overdose, now in its fourth year.
Two of our new sub-Registries deal with critical issues related to pediatric exposures: opioids and marijuana. Both of these projects went live with the launch of REDCap on January 1, 2017. Also at that time a sub-Registry on plant and mushroom exposures was initiated. These uncommon but extremely important exposures are very hard to study given the few cases encountered. By aggregating the prospective experience of the network of ToxIC investigators, it is anticipated that a robust series of cases on individual plant and mushroom exposures will be characterized.
There were eight peer-reviewed publications derived from the ToxIC Registry and published by ToxIC investigators in 2017. In addition, there were 17 published abstracts from professional meetings in 2017.
In 2017, ToxIC continued to be supported by a grant on cardiovascular toxicity from the National Institutes of Health, the continuation of our contract with the US Food and Drug Administration, and further unrestricted grant support from BTG International. The latter was used to support the North American Snake Bite Registry.
Ongoing investigator-initiated research projects can be found on the ToxIC website.
This eighth ToxIC Annual Report summarizes the main points of the data collected in our main Registry in 2017. Data from our sub-Registries are published separately.
Methods
A detailed description of the creation and design of the ToxIC Registry has been previously reported [1]. To be part of the consortium, all medical toxicologists at participating institutions agree to enter data into the ToxIC Registry on all medical toxicology consultations performed. Cases are entered on a password-protected encrypted online data collection form. The site uses the REDCap (Research Electronic Data Capture) interface and is hosted by Vanderbilt University. The content of the database is maintained with oversight by the ToxIC Leadership Group. The Registry is compliant with the Health Insurance Portability and Accountability Act and does not collect any protected health information or otherwise identifying fields. Registry participation is pursuant to the participating institutions’ Institutional Review Board approval and compliant with their policies and procedures. The Registry has also been independently reviewed by the Western IRB and determined not to meet the threshold of human subject research under federal regulation 45 CFR 46 and associated guidance.
Data collected on each case includes presenting signs and symptoms, clinical course, treatments, limited patient demographics, outcome, laboratory values, and circumstances of, and reasons for, the toxicological exposure. As in prior reports, the term consultation is used in this report to describe any in-person encounter with a medical toxicologist in which a formal evaluation was conducted and placed in the medical record. Such encounters may include admission to a medical toxicology inpatient service, or evaluation by a medical toxicologist as a consulting physician in an emergency department, inpatient unit, or outpatient clinic. The online data collection interface is formatted to ensure data entry remains organized and searchable. Free text entry fields allow providers to provide further detail or supplementary information. As part of the Registry’s toxicosurveillance mission, one component of the standard data form is a continuously monitored sentinel detection field that signals novel or unusual cases.
For this report a search of the database was performed to identify cases recorded from January 1, 2017, through December 31, 2017. This descriptive report summarizes case demographics, source and location of consultation, and reasons for encounter and provides case frequencies by individual agent class and treatment provided. We also have included several more focused analyses of particular interest. These dealt with pediatric exposures, marijuana edibles, the use of extracorporeal membrane oxygenation (ECMO), coingestants associated with opioid exposures, and gabapentinoid misuse.
In the tables describing individual agents or agent classes, unless otherwise indicated, cells with fewer than five occurrences were not listed as separate items but are further grouped as “miscellaneous.” Percentages noted in tables for individual agents represent their relative proportion within their respective agent class.
For clinical signs or symptoms, the tables provide the percentage of individual signs or symptoms relative to the total number of Registry cases in 2017. Signs and symptoms include the presence or absence of a toxidrome, vital sign abnormalities, and a variety of organ system-based derangements which may arise from a toxic exposure. For each sub-heading in the data collection instrument, investigators are required to either select an abnormality, or “None,” to improve the accuracy of data collection and to avoid missing data fields. In the detailed treatment tables, percentages for each treatment modality represent the relative frequency among all treatments rendered.
Results
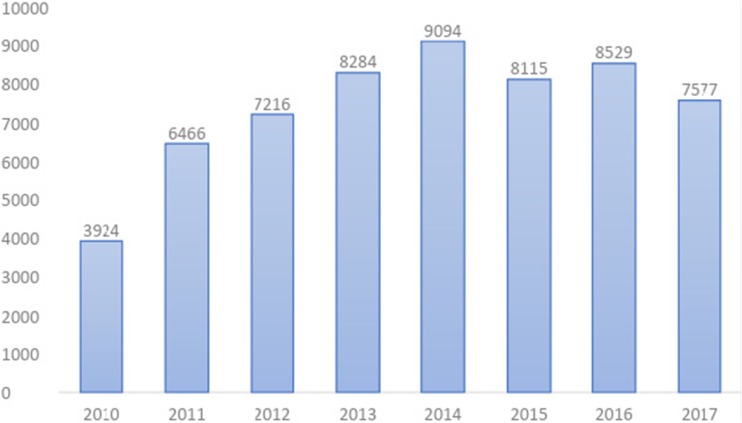
In 2017, there were a total of 7577 cases reporting toxicologic exposures to the ToxIC Registry. This is a decrease from the prior 2 years (Fig. 1). Table 1 lists all individual sites that contributed cases in 2017.
Fig. 1.
ToxIC Registry total case count by year, 2010–2017
Table 1.
Participating institutions providing cases to ToxIC in 2017
| Arizona | |
| Phoenix | |
| Banner - University Medical Center Phoenix | |
| Phoenix Children’s Hospital | |
| California | |
| Loma Linda | |
| Loma Linda University Medical Center | |
| Los Angeles | |
| University of Southern California Verdugo Hills | |
| Keck Medical Center of University of Southern California | |
| San Francisco | |
| San Francisco General Hospital | |
| Colorado | |
| Denver | |
| Children’s Hospital Colorado | |
| Denver Health Medical Center | |
| Porter and Littleton Adventist Hospital | |
| Swedish Medical Center | |
| University of Colorado Medical Center | |
| Connecticut | |
| Hartford | |
| Hartford Hospital | |
| Georgia | |
| Atlanta | |
| Children’s Healthcare of Atlanta Egelston | |
| Children’s Healthcare of Atlanta Hughes Spalding | |
| Elmhurst Medical Center | |
| Emory University Hospital | |
| Grady Health System | |
| Grady Memorial Hospital | |
| Illinois | |
| Evanston | |
| Evanston North-Shore University Health System | |
| Indiana | |
| Indianapolis | |
| IU-Eskenazi Hospital | |
| IU-Indiana University Hospital | |
| IU-Methodist Hospital-Indianapolis | |
| IU-Riley Hospital for Children | |
| Massachusetts | |
| Boston | |
| Beth Israel Deaconess Medical Center Boston | |
| Boston Children’s Hospital | |
| Worcester | |
| University of Massachusetts Memorial Medical Center | |
| Michigan | |
| Grand Rapids | |
| Spectrum Health Hospitals | |
| Missouri | |
| Kansas City | |
| Children’s Mercy Hospitals & Clinics | |
| St. Louis | |
| Washington University School of Medicine | |
| Nebraska | |
| Omaha | |
| University of Nebraska Medical Center | |
| New Mexico | |
| Albuquerque | |
| University of New Mexico | |
| New Jersey | |
| New Brunswick | |
| Robert Wood Johnson University Hospital | |
| Newark | |
| NJMS/Rutgers | |
| New York | |
| Manhasset | |
| North Shore University Hospital | |
| New York | |
| Bellevue Medical Center | |
| Mount Sinai Hospital | |
| NYU Langone Medical Center | |
| Staten Island | |
| Staten Island Hospital | |
| Staten Island University Hospital | |
| Rochester | |
| Highland Hospital | |
| Strong Memorial Hospital | |
| Syracuse | |
| Upstate Medical University-Downtown Campus | |
| North Carolina | |
| Charlotte | |
| Carolinas Medical Center | |
| Greenville | |
| Vidant Medical Center | |
| Oregon | |
| Portland | |
| Oregon Health & Science University Hospital | |
| Pennsylvania | |
| Lehigh Valley | |
| Lehigh Valley Hospital Cedar Crest | |
| Lehigh Valley Hospital Muhlenberg | |
| Philadelphia | |
| Hahnemann University Hospital | |
| Mercy Fitzgerald Hospital | |
| Mercy Hospital of Philadelphia | |
| St. Christopher’s Hospital for Children | |
| Pittsburgh | |
| UPMC Magee Women’s Hospital | |
| UPMC Mercy Hospital | |
| UPMC Presbyterian/Shadyside | |
| Texas | |
| Dallas | |
| Children’s Medical Center Dallas | |
| Parkland Memorial Hospital | |
| University of Texas Southwestern Clinic | |
| William P Clements University Hospital | |
| Houston | |
| Ben Taub General Hospital | |
| Texas Children’s Hospital | |
| Utah | |
| Salt Lake City | |
| Primary Children’s Hospital | |
| University of Utah Hospital | |
| Virginia | |
| Charlottesville | |
| University of Virginia Health Systems | |
| Richmond | |
| Virginia Commonwealth University Medical Center | |
| Wisconsin | |
| Milwaukee | |
| Children’s Hospital of Wisconsin | |
| Froedtert Memorial Lutheran Hospital | |
| Isreal | |
| Haifa | |
| Rambam Health Care Campus | |
| Canada | |
| Toronto | |
| Hospital for Sick Children | |
| Thailand | |
| Bangkok | |
| Vajira Hospital |
Demographics
Tables 2 and 3 summarize selective demographics including gender, age, race, and ethnicity. In 2017, 50.4% of cases involved female patients. Sixty patients (0.8%) were pregnant (Table 2). New fields were added in 2017 to better represent the population and include transgender patient data. There were 14 male-to-female transgender patients and 22 female-to-male, making up 0.5% of patients in the database.
Table 2.
ToxIC case demographics—age and gender
| N (%) | |
|---|---|
| Gender | |
| Male | 3718 (49.1) |
| Female | 3820 (50.4) |
| Transgender | |
| Male to female | 14 (0.2) |
| Female to male | 22 (0.3) |
| Not recorded | 3 (0.0) |
| Pregnant | 60 (0.8) |
| Age (years) | |
| < 2 | 268 (3.5) |
| 2–6 | 371 (4.9) |
| 7–12 | 253 (3.3) |
| 13–18 | 1468 (19.4) |
| 19–65 | 4803 (63.4) |
| 66–89 | 376 (5.0) |
| > 89 | 13 (0.2) |
| Unknown | 25 (0.3) |
| Total | 7577 (100) |
Table 3.
ToxIC case demographics—race and Hispanic ethnicity
| N (%) | |
|---|---|
| Race | |
| Caucasian | 4504 (59.4) |
| Unknown/uncertain | 1456 (19.2) |
| Black/African | 999 (13.2) |
| Asian | 350 (4.6) |
| Mixed | 136 (1.8) |
| American Indian/Alaska Native | 111 (1.5) |
| Other | 16 (0.2) |
| Native Hawaiian or Pacific Islander | 5 (0.1) |
| Total | 7577 (100) |
| Hispanic ethnicitya | |
| Hispanic | 854 (11.3) |
| Non-Hispanic | 5283 (69.7) |
| Unknown | 1439 (19.0) |
| Total | 7577 (100) |
One case not recorded as Hispanic or non-Hispanic ethnicity
aHispanic ethnicity as indicated exclusive of race
Age distribution in 2017 was similar to past years [2–8]. The majority of patients were adults age 19–65 (63.4%), followed by adolescents age 13–18 (19.4%). Children age 12 and under made up 11.8% of cases.
The most commonly reported race was Caucasian (59.4%), followed by Black/African (13.2%) and Asian (4.6%) (Table 3). Unknown or uncertain race was reported in 19.2% of cases, similar to 2016 data [8]. Hispanic ethnicity was reported in 11.3% of cases (Table 3). Nineteen percent of cases reported unknown ethnicity. Race and ethnicity are self-reported by patients, or in cases where a patient is unable to report, it may be reported by the examining medical toxicologist to the best of their ability.
Table 4 summarizes the referral sources of inpatient and outpatient medical toxicology encounters. The majority of inpatient cases (56.7%) were generated from the Emergency Department. Only 0.3% of inpatient encounters were referred from poison centers. Outpatient toxicology evaluations were predominantly referred by primary care or other outpatient physicians (43.5%) or were self-referrals (37.1%).
Table 4.
ToxIC registry case referral sources by inpatient/outpatient status
| N (%) | |
|---|---|
| Emergency Department (ED) or inpatient (IP)a | |
| ED | 4066 (56.7) |
| Admitting service | 1726 (24.1) |
| Outside hospital transfer | 932 (13.0) |
| Request from another hospital service (not ED) | 239 (3.3) |
| Self-referral | 180 (2.5) |
| Poison Center | 24 (0.3) |
| Primary care provider or other outpatient treating physician | 4 (0.1) |
| Employer/Independent medical evaluation | 2 (0.0) |
| ED/IP total | 7173 (100) |
| Outpatient (OP)/clinic/office consultationb | |
| Primary care provider or other OP physician | 175 (43.5) |
| Self-referral | 149 (37.1) |
| Employer/Independent medical evaluation | 35 (8.7) |
| Poison Center | 24 (6.0) |
| ED | 10 (2.5) |
| Admitting service | 3 (0.7) |
| Outside hospital transfer | 3 (0.7) |
| Request from another hospital service (not ED) | 3 (0.7) |
| OP total | 402 (100) |
Two cases not recorded inpatient or outpatient location
aPercentage based on the total number of cases (N = 7173) seen by a medical toxicologist as consultant (ED or IP) or as attending (IP)
bPercentage based on the total number of cases (N = 402) seen by a medical toxicologist as outpatient, clinic visit, or office consultation
Table 5 reports the reasons for medical toxicology encounters. Similar to prior years, intentional pharmaceutical exposures were the most common reason for encounter (54.7%).
Table 5.
Reason for medical toxicology encounter
| N (%) | |
|---|---|
| Intentional exposure—pharmaceutical | 4144 (54.7) |
| Intentional exposure—non-pharmaceutical | 921 (12.2) |
| Unintentional exposure—pharmaceutical | 610 (8.1) |
| Unintentional exposure—non-pharmaceutical | 322 (4.2) |
| Envenomation—snake | 297 (3.9) |
| Organ system dysfunction | 256 (3.4) |
| Withdrawal—opioid | 217 (2.9) |
| Withdrawal—ethanol | 149 (2.0) |
| Ethanol abuse | 132 (1.7) |
| Interpretation of toxicology data | 123 (1.6) |
| Environmental evaluation | 122 (1.6) |
| Occupational evaluation | 80 (1.1) |
| Envenomation—spider | 55 (0.7) |
| Malicious/criminal | 41 (0.5) |
| Envenomation—other | 38 (0.5) |
| Withdrawal—sedative-hypnotic | 35 (0.5) |
| Withdrawal—other | 16 (0.2) |
| Withdrawal—cocaine/amphetamine | 6 (0.1) |
| Envenomation—scorpion | 5 (0.1) |
| Marine/fish poisoning | 5 (0.1) |
| Not recorded | 3 (0.0) |
| Total | 7577 (100) |
Table 6 presents information on reasons for intentional pharmaceutical exposures. The majority of cases (67.6%) were an attempt at self-harm. Of these cases with an attempt at self-harm, 88.2% represented a suicide attempt.
Table 6.
Detailed reason for encounter—intentional pharmaceutical exposure
| N (%) | |
|---|---|
| Reason for intentional pharmaceutical exposure subgroupa | |
| Attempt at self-harm | 2803 (67.6) |
| Misuse/abuse | 718 (17.3) |
| Therapeutic use | 328 (7.9) |
| Unknown | 294 (7.1) |
| Not recorded | 1 (0.0) |
| Total | 4144 (100) |
| Attempt at self-harm- suicidal intent subclassificationb | |
| Suicidal intent | 2473 (88.2) |
| Suicidal intent unknown | 248 (8.8) |
| No suicidal intent | 79 (2.8) |
| Not recorded | 3 (0.1) |
| Total | 2803 (100) |
aPercentage of total number of cases (N = 4144) indicating primary reason for encounter due to intentional pharmaceutical exposure
bPercentage of number of cases indicating attempt at self-harm (N = 2803)
Agent Classes
Of the 7577 cases entered into the ToxIC Registry in 2017, 2340 cases involved multiple agents for a total of 10,606 individual agent entries. The non-opioid analgesic class was the most common (14.9%), followed by antidepressants (11.4%), opioids (10.4%), and sedative-hypnotic/muscle relaxants (9.2%). Table 7 presents the totals for each of the 40 agent classes in the Registry.
Table 7.
Agent classes involved in medical toxicology consultation
| N(%)a | |
|---|---|
| Analgesic | 1582 (14.9) |
| Antidepressant | 1207 (11.4) |
| Opioid | 1101 (10.4) |
| Sedative-hypnotic/muscle relaxant | 972 (9.2) |
| Ethanol | 723 (6.8) |
| Anticholinergic/antihistamine | 669 (6.3) |
| Sympathomimetic | 658 (6.2) |
| Cardiovascular | 597 (5.6) |
| Antipsychotic | 556 (5.2) |
| Anticonvulsant | 399 (3.8) |
| Envenomation and marine | 375 (3.5) |
| Psychoactive | 223 (2.1) |
| Diabetic medication | 172 (1.6) |
| Lithium | 121 (1.1) |
| Toxic alcohol | 118 (1.1) |
| Metals | 115 (1.1) |
| Cough and cold products | 112 (1.1) |
| Herbal products/dietary supplements | 111 (1.0) |
| Gases/irritants/vapors/dusts | 94 (0.9) |
| Caustic | 89 (0.8) |
| Hydrocarbon | 75 (0.7) |
| Household products | 68 (0.6) |
| Plants and fungi | 65 (0.6) |
| Antimicrobial | 60 (0.6) |
| Endocrine | 38 (0.4) |
| Chemotherapeutic/immunological | 35 (0.3) |
| Other non-pharmaceutical product | 33 (0.3) |
| Gastrointestinal agents | 30 (0.3) |
| Anesthetic | 27 (0.3) |
| Insecticide | 27 (0.3) |
| Anticoagulant | 24 (0.2) |
| Other pharmaceutical product | 18 (0.2) |
| Amphetamine-like hallucinogen | 12 (0.1) |
| Herbicide | 10 (0.1) |
| WMD/riot agent/radiological | 7 (0.1) |
| Anti-parkinsonism drugs | 6 (0.1) |
| Rodenticide | 6 (0.1) |
| Ingested foreign body | 5 (0.0) |
| Pulmonary | 4 (0.0) |
| Fungicide | NR (0.0) |
| Unknown agent | 62 (0.6) |
| Total | 10,606 (100) |
NR no cases reported
aPercentages are out of total number of reported agent entries in 2017; 2340 cases (30.9%) reported multiple agents
Pediatric Agent Classes
Table 8 presents the agent classes reported by age group. There were 3366 individual agent entries in 2017 for 2360 reported pediatric cases with 610 (25.8%) cases involving multiple agents. The top agent classes for all pediatric cases were analgesics (19.5%), followed by antidepressants (13.0%) and anticholinergic/antihistamine (9.2%). By age category, the most common exposures by agent class were opioids for children < 2 years old, cardiovascular drugs for ages 2–6, envenomations for ages 7–12, and analgesics for ages 13–18. Opioids were ranked sixth in agent class frequency for pediatrics with the majority of exposures involving 13–18 year olds, though they were the most common agent class in those under age 2. Envenomations were most commonly reported in the 7–12 year old age category, followed by 13–18 year olds. Caustic exposures were most often seen in 2–6 year olds.
Table 8.
ToxIC 2017—agent classes for pediatric cases by age group
| Exposure rank | Totals | %a | Age < 2 | Age 2–6 | Age 7–12 | Age 13–18 | |
|---|---|---|---|---|---|---|---|
| Analgesic | 1 | 658 | 19.5% | 23 | 20 | 30 | 585 |
| Antidepressant | 2 | 436 | 13.0% | 13 | 25 | 20 | 378 |
| Anticholinergic/antihistamine | 3 | 309 | 9.2% | 10 | 24 | 30 | 245 |
| Unknown/blank | 4 | 234 | 7.0% | 36 | 62 | 36 | 100 |
| Cardiovascular | 5 | 224 | 6.7% | 36 | 82 | 25 | 81 |
| Opioid | 6 | 172 | 5.1% | 37 | 20 | 5 | 110 |
| Envenomation | 7 | 171 | 5.1% | 6 | 37 | 71 | 57 |
| Antipsychotic | 8 | 158 | 4.7% | 5 | 10 | 8 | 135 |
| Sympathomimetic | 9 | 155 | 4.6% | 31 | 24 | 12 | 88 |
| Sedative-hypnotic/muscle relaxant | 10 | 148 | 4.4% | 9 | 16 | 14 | 109 |
| Anticonvulsant | 11 | 110 | 3.3% | 8 | 16 | 14 | 72 |
| Psychoactive | 12 | 67 | 2.0% | 8 | 14 | 3 | 42 |
| Herbal/dietary supp/vitamins | 13 | 61 | 1.8% | 9 | 17 | 4 | 31 |
| Diabetic med | 14 | 59 | 1.8% | 14 | 23 | 1 | 21 |
| Ethanol | 14 | 59 | 1.8% | 5 | 2 | 2 | 50 |
| Cough/cold | 15 | 50 | 1.5% | 1 | 7 | 2 | 40 |
| Metals | 16 | 48 | 1.4% | 8 | 19 | 5 | 16 |
| Household product | 17 | 26 | 0.8% | 11 | 6 | 1 | 8 |
| Hydrocarbons | 18 | 24 | 0.7% | 13 | 9 | 0 | 2 |
| Lithium | 19 | 23 | 0.7% | 1 | 1 | 2 | 19 |
| Antimicrobial | 20 | 22 | 0.7% | 4 | 3 | 2 | 13 |
| Caustic | 21 | 19 | 0.6% | 5 | 10 | 1 | 3 |
| Toxic alcohols | 22 | 18 | 0.5% | 5 | 3 | 2 | 8 |
| Endocrine | 23 | 16 | 0.5% | 1 | 7 | 1 | 7 |
| GI Agent | 24 | 15 | 0.4% | 3 | 2 | 1 | 9 |
| Chemotherapeutic/immune | 25 | 14 | 0.4% | 3 | 2 | 3 | 6 |
| Gases/vapors/irritants/dusts | 25 | 14 | 0.4% | 5 | 2 | 3 | 4 |
| Plants/fungi | 26 | 12 | 0.4% | 3 | 4 | 3 | 2 |
| Anesthetic | 27 | 10 | 0.3% | 3 | 1 | 2 | 4 |
| Other non-pharmaceutical | 28 | 9 | 0.3% | 2 | 2 | 1 | 4 |
| Other pharmaceutical | 29 | 7 | 0.2% | 1 | 1 | 0 | 5 |
| Amphetamine-like hallucinogen | 30 | 4 | 0.1% | 0 | 1 | 1 | 2 |
| Anticoagulant | 31 | 3 | 0.1% | 2 | 1 | 0 | 0 |
| Ingested foreign body | 31 | 3 | 0.1% | 1 | 1 | 0 | 1 |
| Insecticide | 32 | 2 | 0.1% | 2 | 0 | 0 | 0 |
| Pulmonary | 32 | 2 | 0.1% | 0 | 1 | 0 | 1 |
| Rodenticide | 32 | 2 | 0.1% | 1 | 1 | 0 | 0 |
| Parkinson’s med | 33 | 1 | 0.0% | 0 | 1 | 0 | 0 |
| WMD | 33 | 1 | 0.0% | 1 | 0 | 0 | 0 |
| Totals | 3366 | 100.0% |
aPercentages are out of total number of reported agent entries per year; 610 cases (25.8%) reported multiple agents
Table 9 lists the most commonly reported agent classes involved in exposures in children aged 5 or younger. The cardiovascular agent class was the most commonly reported (15.6%), followed by opioids (8.4%). Diabetic medications (5.0%), herbal products/dietary supplements (3.8%), and metals (3.8%) were also reported more frequently in children aged 5 or younger compared to their representation in the Registry as a whole.
Table 9.
Most frequent exposures by agent class- age ≤ 5 years
| N (%)a | |
|---|---|
| Cardiovascular | 106 (15.6) |
| Opioid | 57 (8.4) |
| Sympathomimetic | 52 (7.7) |
| Analgesic | 43 (6.3) |
| Envenomation | 38 (5.6) |
| Antidepressant | 34 (5.0) |
| Diabetic medication | 34 (5.0) |
| Anticholinergic/antihistamine | 33 (4.9) |
| Herbal products/dietary supplements | 26 (3.8) |
| Metals | 26 (3.8) |
| Sedative-hypnotics/muscle relaxant | 24 (3.5) |
| Anticonvulsant | 24 (3.5) |
| Psychoactive | 22 (3.2) |
| Hydrocarbon | 22 (3.2) |
| Household | 17 (2.5) |
| Antipsychotic | 15 (2.2) |
| Caustic | 15 (2.2) |
| Class total | 678 (100) |
aPercentages are out of total number of agent exposures reported in children aged 5 or younger in 2017 (N = 678)
Individual Agents by Class
Tables 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 23, 24, 25, 26, 27, and 28 present the frequencies of individuals agents, organized by agent class as reported to the Registry in 2017. The organization follows past years for consistency with three agents—ethanol, lithium, and amphetamine-like hallucinogens—defined as their own agent class, but reported in conjunction with other agent classes (toxic alcohols, anticonvulsants and mood stabilizers, and psychoactives, respectively) for brevity. For agent classes with few overall entries (less than 100), or for which a single agent made up more than 75% of the cases, or for which the majority of cases were infrequent miscellaneous agents, the results are reported in Tables S1–S16 in the Supplemental Materials.
Table 10.
Analgesics
| N (%) | |
|---|---|
| Acetaminophen | 901 (57.0) |
| Aspirin | 202 (12.8) |
| Ibuprofen | 183 (11.6) |
| Gabapentin | 176 (11.1) |
| Naproxen | 57 (3.6) |
| Pregabalin | 25 (1.6) |
| Salicylic acid | 10 (0.6) |
| Analgesic unspecified | 6 (0.4) |
| Meloxicam | 5 (0.3) |
| Miscellaneousa | 17 (1.1) |
| Class total | 1582 (100) |
aIncludes aminophenazone, diclofenac, etoricoxib, ketorolac, mefenamic acid, unspecified NSAID, other analgesic, phenylbutazone, salicylamide, and salsalate
Table 11.
Antidepressants
| N (%) | |||
|---|---|---|---|
| Other antidepressants | 476 (39.4) | ||
| Bupropion | 252 (21.0) | ||
| Trazodone | 161 (13.3) | ||
| Mirtazapine | 43 (3.6) | ||
| Vilazodone | 9 (0.7) | ||
| Antidepressant unspecified | 8 (0.7) | ||
| Miscellaneousa | < 5 (< 0.4) | ||
| Selective serotonin reuptake inhibitors (SSRIs) | 445 (36.9) | ||
| Sertraline | 128 (10.6) | ||
| Fluoxetine | 116 (9.6) | ||
| Citalopram | 96 (8.0) | ||
| Escitalopram | 80 (6.6) | ||
| Paroxetine | 25 (2.1) | ||
| Tricyclic antidepressants (TCAs) | 166 (13.8) | ||
| Amitriptyline | 105 (8.7) | ||
| Nortriptyline | 28 (2.3) | ||
| Doxepin | 25 (2.1) | ||
| Miscellaneousb | 8 (0.7) | ||
| Serotonin-norepinephrine reuptake inhibitors (SNRIs) | 120 (9.9) | ||
| Venlafaxine | 80 (6.6) | ||
| Duloxetine | 30 (2.5) | ||
| Desvenlafaxine | 6 (0.5) | ||
| Miscellaneousc | < 5 (< 0.4) | ||
| Class Total | 1207 (100) | ||
aIncludes phenelzine and vortioxetine
bIncludes imipramine, clomipramine, amoxapine, and dosulepin
cIncludes fluvoxamine
Table 12.
Opioids
| N (%) | |
|---|---|
| Heroin | 318 (28.9) |
| Oxycodone | 160 (14.5) |
| Tramadol | 106 (9.6) |
| Fentanyl | 87 (7.9) |
| Opioid unspecified | 82 (7.4) |
| Methadone | 79 (7.2) |
| Hydrocodone | 78 (7.1) |
| Buprenorphine | 67 (6.1) |
| Morphine | 38 (3.5) |
| Hydromorphone | 17 (1.5) |
| Codeine | 15 (1.4) |
| Loperamide | 12 (1.1) |
| Naltrexone | 9 (0.8) |
| Oxymorphone | 6 (0.5) |
| Miscellaneousa | 27 (2.5) |
| Class total | 1101 (100) |
aIncludes U47700 (designer opioid), butanoyl-4-fluorofentanyl, methylfentanyl (3- or alpha), diphenoxylate, fluorofentanyl, fluoroisobutyryl fentanyl (4- or para-), methyl norfentanyl, N-allyl norfentanyl, butyrylfentanyl (butyr-), carfentanil, naloxone, normethadone, opium (raw, latex), Papaver somniferum (plant parts), and tapentadol
Table 13.
Sedative-hypnotics/muscle relaxants by sub-type
| N (%) | |||
|---|---|---|---|
| Benzodiazepines | 571 (58.8) | ||
| Alprazolam | 220 (22.8) | ||
| Clonazepam | 181 (18.6) | ||
| Lorazepam | 80 (8.2) | ||
| Diazepam | 42 (4.3) | ||
| Benzodiazepine unspecified | 23 (2.4) | ||
| Temazepam | 11 (1.1) | ||
| Chlordiazepoxide | 6 (0.6) | ||
| Miscellaneousa | 8 (0.8) | ||
| Muscle relaxants | 259 (26.7) | ||
| Baclofen | 97 (10.0) | ||
| Cyclobenzaprine | 97 (10.0) | ||
| Carisoprodol | 22 (2.3) | ||
| Tizanidine | 22 (2.3) | ||
| Methocarbamol | 15 (1.5) | ||
| Metaxalone | 5 (0.5) | ||
| Miscellaneousb | < 5 (< 0.5) | ||
| Non-benzodiazepine agonists (“Z” drugs) | 63 (6.5) | ||
| Zolpidem | 58 (6.0) | ||
| Miscellaneousc | 5 (0.5) | ||
| Other Sedatives | 52 (5.3) | ||
| Sedative-hypnotic unspecified | 19 (1.9) | ||
| Buspirone | 17 (1.8) | ||
| Phenibut | 5 (0.5) | ||
| Miscellaneousd | 11 (1.1) | ||
| Barbiturates | 27 (2.8) | ||
| Butalbital | 17 (1.8) | ||
| Phenobarbital | 7 (0.7) | ||
| Miscellaneouse | 3 (0.3) | ||
| Class total | 972 (100) | ||
aIncludes clorazepate, midazolam, flunitrazepam, and oxazepam
bIncludes orphenadrine
cIncludes eszopiclone and zopiclone
dIncludes propofol, chlorzoxazone, etizolam, and clomethiazole
eIncludes butabarbital and barbiturate unspecified
Table 14.
Ethanol and toxic alcohols
| N (%) | |
|---|---|
| Ethanola | 723 (100) |
| Non-ethanol alcohols and glycols | |
| Isopropanol | 44 (37.3) |
| Ethylene glycol | 41 (34.7) |
| Methanol | 18 (15.3) |
| Acetone | 5 (4.2) |
| Miscellaneousb | 10 (8.5) |
| Class total | 118 (100) |
aEthanol is considered a separate agent class
bIncludes benzyl alcohol, butyl ethylene glycol, diethyl ether, diethylene glycol, dipropylene glycol, glycol ethers, propylene glycol, and toxic alcohol unspecified
Table 15.
Anticholinergics and antihistamines
| N (%) | |
|---|---|
| Diphenhydramine | 413 (61.7) |
| Hydroxyzine | 85 (12.7) |
| Doxylamine | 32 (4.8) |
| Chlorpheniramine | 22 (3.3) |
| Benztropine | 21 (3.1) |
| Cetirizine | 18 (2.7) |
| Promethazine | 14 (2.1) |
| Loratadine | 9 (1.3) |
| Trihexyphenidyl | 8 (1.2) |
| Dicyclomine | 7 (1.0) |
| Dimenhydrinate | 7 (1.0) |
| Meclizine | 5 (0.7) |
| Miscellaneousa | 28 (4.2) |
| Class total | 669 (100) |
aIncludes anticholinergic unspecified, antihistamine unspecified, atropine, brompheniramine, buclizine, cyproheptadine, fexofenadine, homatropine, hyoscyamine, mirabegron, oxybutynin, pheniramine, pyrilamine, scopolamine, and triprolidine
Table 16.
Sympathomimetics
| N (%) | |
|---|---|
| Cocaine | 255 (38.8) |
| Methamphetamine | 180 (27.4) |
| Amphetamine | 63 (9.6) |
| Methylphenidate | 36 (5.5) |
| Dextroamphetamine | 30 (4.6) |
| Lisdexamfetamine | 16 (2.4) |
| Sympathomimetic unspecified | 15 (2.3) |
| Methylenedioxy-N-methamphetamine | 10 (1.5) |
| Pseudoephedrine | 9 (1.4) |
| Phentermine | 8 (1.2) |
| Atomoxetine | 7 (1.1) |
| Dexmethylphenidate | 5 (0.8) |
| Phenylephrine | 5 (0.8) |
| Miscellaneousa | 19 (2.9) |
| Class total | 658 (100) |
aIncludes clenbuterol, epinephrine, 2C series drugs, 25I-NBOMe, 3-fluoroethamphetamine, 4-fluoroamphetamine, butylone, cathinone, ephedrine, ethylphenidate, isometheptine, MDPV, norpseudoephedrine, phenylpropanolamine, and tetrahydrozoline
Table 17.
Cardiovascular agents by sub-type
| N (%) | |||
|---|---|---|---|
| Sympatholytics | 150 (25.1) | ||
| Clonidine | 113 (18.9) | ||
| Guanfacine | 34 (5.7) | ||
| Miscellaneousa | < 5 (< 0.8) | ||
| Beta blockers | 139 (23.3) | ||
| Metoprolol | 46 (7.7) | ||
| Propranolol | 39 (6.5) | ||
| Carvedilol | 24 (4.0) | ||
| Atenolol | 19 (3.2) | ||
| Labetalol | 9 (1.5) | ||
| Miscellaneousb | < 5 (< 0.8) | ||
| Calcium channel antagonists | 104 (17.4) | ||
| Amlodipine | 59 (9.9) | ||
| Diltiazem | 24 (4.0) | ||
| Verapamil | 10 (1.7) | ||
| Nifedipine | 9 (1.5) | ||
| Miscellaneousc | < 5 (< 0.8) | ||
| ACE inhibitors | 58 (9.7) | ||
| Lisinopril | 50 (8.4) | ||
| Enalapril | 5 (0.8) | ||
| Miscellaneousd | < 5 (< 0.8) | ||
| Other antihypertensives and vasodilators | 40 (6.7) | ||
| Prazosin | 26 (4.1) | ||
| Hydralazine | 5 (0.8) | ||
| Miscellaneouse | 9 (1.5) | ||
| Antidysrhythmics and other cardiovascular agents | 38 (6.4) | ||
| Atorvastatin | 8 (1.3) | ||
| Amiodarone | 7 (1.2) | ||
| Cardiovascular agent unspecified | 5 (0.8) | ||
| Sotalol | 5 (0.8) | ||
| Miscellaneousf | 13 (2.2) | ||
| Cardiac glycosides | 30 (5.0) | ||
| Digoxin | 28 (4.7) | ||
| Digitoxin | 2 (0.3) | ||
| Diuretics | 29 (4.9) | ||
| Hydrochlorothiazide | 14 (2.3) | ||
| Furosemide | 8 (1.3) | ||
| Spironolactone | 5 (0.8) | ||
| Miscellaneousg | < 5 (< 0.8) | ||
| Angiotensin receptor blockers | 9 (1.5) | ||
| Losartan | 6 (1.0) | ||
| Miscellaneoush | < 5 (< 0.8) | ||
| Class total | 597 (100) | ||
aIncludes dexmetetomidine and methyldopa
bIncludes nadolol
cIncludes felodipine and lercanidipine
dIncludes benazepril and quinipril
eIncludes nitroglycerin, doxazosin, isosorbide, tamsulosin, and terazosin
fIncludes flecainide, gemfibrozil, midodrine, simvastatin, disopyramide, ivabradine, lovastatin, and rosuvastatin
gIncludes chlorthalidone and pamabrom
hIncludes valsartan
Table 18.
Antipsychotics
| N (%) | |
|---|---|
| Quetiapine | 264 (47.5) |
| Olanzapine | 81 (14.6) |
| Risperidone | 40 (7.2) |
| Aripiprazole | 39 (7.0) |
| Haloperidol | 31 (5.6) |
| Chlorpromazine | 19 (3.4) |
| Clozapine | 19 (3.4) |
| Ziprasidone | 17 (3.1) |
| Lurasidone | 13 (2.3) |
| Antipsychotic unspecified | 7 (1.3) |
| Paliperidone | 5 (0.9) |
| Miscellaneousa | 21 (3.8) |
| Class total | 556 (100) |
aIncludes asenapine, brexpiprazole, fluphenazine, loxapine, perphenazine, prochlorperazine, thiothixene, and trifluoperazine
Table 19.
Anticonvulsants and mood stabilizers, and lithium
| N (%) | |
|---|---|
| Lithiuma | 121 (100) |
| Lamotrigine | 108 (27.1) |
| Valproic acid | 91 (22.8) |
| Carbamazepine | 38 (9.5) |
| Topiramate | 38 (9.5) |
| Oxcarbazepine | 31 (7.8) |
| Phenytoin | 30 (7.5) |
| Levetiracetam | 24 (6.0) |
| Divalproex | 10 (2.5) |
| Lacosamide | 9 (2.3) |
| Zonisamide | 5 (1.3) |
| Miscellaneousb | 15 (3.8) |
| Class total | 399 (100) |
aLithium is considered a separate agent class
bIncludes anticonvulsant unspecified, clobazam, felbamate, fosphenytoin, perampanel, primidone, rufinamide, and tiagabine
Table 20.
Envenomations and marine poisonings
| N (%) | |
|---|---|
| Agkistrodon spp. | 108 (28.8) |
| Crotalus spp. | 95 (25.3) |
| Snake unspecified | 38 (10.1) |
| Loxosceles spp. | 32 (8.5) |
| Trimeresurus spp. (Asian pit vipers) | 25 (6.7) |
| Chilopoda spp. (centipedes) | 15 (4.0) |
| Envenomation unspecified | 14 (3.7) |
| Latrodectus spp. | 10 (2.7) |
| Micrurus spp. | 9 (2.4) |
| Hymenoptera | 7 (1.9) |
| Miscellaneousa | 19 (5.1) |
| Class total | 375 (100) |
aIncludes Centruroides spp., Vipera palaestinae, Homalopsis spp. (water snakes), Naja kaouthia, spider unspecified, Acanthanter plasti (Crown of Thorns starfish), animal bite unspecified, ciguetera poisoning, marine toxin unspecified, Micruroides spp., Scolopendra spp. (giant centipedes), scombroid poisoning, scorpion unspecified, and stingrays
Table 21.
Psychoactives
| N (%) | |
|---|---|
| Molly—amphetamine-like hallucinogena | 12 (100) |
| Marijuana | 73 (32.7) |
| Cannabinoid synthetic | 49 (22.0) |
| LSD | 15 (6.7) |
| Cannabinoid non-synthetic | 14 (6.3) |
| Phencyclidine | 11 (4.9) |
| Ketamine | 10 (4.5) |
| Gamma hydroxybutyrate | 9 (4.0) |
| Methylenedioxymethamphetamine | 9 (4.0) |
| Delta-9-tetrahydrocannabinol | 8 (3.6) |
| Miscellaneousb | 25 (11.2) |
| Class total | 223 (100) |
LSD lysergic acid diethylamide
aAmphetamine-like hallucinogens are considered a separate agent class
bIncludes 1,4-Butanediol, 3-methoxyphencyclidine, cannabidiol, disulfiram, donepezil, ethylone, gamma butyrolactone, hallucinogen unspecified, Ipracetin (4-acetoxy-DiPT, 4-acetoxy-N,N-diisopropyltryptamine), nicotine, pharmaceutical tetrahydrocannabinol (THC), psychoactive unspecified, and thujone
Table 23.
Diabetic medications
| N (%) | |
|---|---|
| Metformin | 57 (33.1) |
| Glipizide | 40 (23.3) |
| Insulin | 38 (22.1) |
| Glimepiride | 15 (8.7) |
| Glyburide | 12 (7.0) |
| Miscellaneousa | 10 (5.8) |
| Class total | 172 (100) |
aIncludes diabetic medication unspecified, sitagliptin, alogliptin, exenatide, and liraglutide
Table 24.
Metals
| N (%) | |
|---|---|
| Lead | 32 (27.8) |
| Iron | 26 (22.6) |
| Mercury | 11 (9.6) |
| Cobalt | 10 (8.7) |
| Arsenic | 6 (5.2) |
| Chromium | 6 (5.2) |
| Miscellaneousa | 24 (20.9) |
| Class total | 115 (100) |
aIncludes magnesium, cadmium, copper, gadolinium, manganese, aluminum, antimony, beryllium, cesium, metal unspecified, selenium, titanium, and zinc
Table 25.
Herbal products and dietary supplements
| N (%) | |
|---|---|
| Caffeine | 30 (25.6) |
| Melatonin | 25 (21.4) |
| Herbals/dietary supplements/vitamins unspecified | 17 (14.5) |
| Multiple vitamin | 5 (4.3) |
| Vitamin D | 5 (4.3) |
| Eucalyptus oil | 5 (4.3) |
| Limonene | 3 (2.6) |
| Miscellaneousa | 27 (23.1) |
| Class total | 117 (100) |
aIncludes aloin (aloe vera extract or outer leaves), ashwangandha, black cohosh, Brazil seed (Bertholletia excelsa), citronella oil, dietary supplement unspecified, eugenol (clove oil), herbal (dietary) multibotanical, minerals unspecified, omega-3-acid ethyl esters, orange oil, potassium, senna, sodium chloride, tryptophan, vitamin A, vitamin B1 (thiamine), vitamin B3 (niacin), vitamin B6 (pyridoxine), vitamin C (ascorbic acid), and yohimbine
Table 26.
Gases, irritants, vapors, and dusts
| N (%) | |
|---|---|
| Carbon monoxide | 50 (53.2) |
| Chlorine | 4 (4.3) |
| Natural gas | 4 (4.3) |
| Hydrogen sulfide | 3 (3.2) |
| Miscellaneousa | 33 (35.1) |
| Class total | 94 (100) |
aIncludes acetonitrile, asbestos, bromine, carbon dioxide, chloramine, cyanide, diesel exhaust, dust, duster (canned air), fumes/vapors/gases unspecified, gases/vapors/irritants/dusts unspecified, halon, metal dust unspecified, nitric oxide, nitrogen oxides, ozone, petroleum vapors, polyurethane vapors, radon, smoke, sulfur dioxide, volatile organic compounds (VOC) unspecified, welding fumes, and wood dusts
Table 27.
Household products
| N (%) | |
|---|---|
| Cleaning solutions and disinfectants | 17 (25.0) |
| Sodium hypochlorite ≤ 6% | 13 (19.1) |
| Soaps and detergents | 11 (16.2) |
| Laundry detergent pod | 8 (11.8) |
| Hair product | 4 (5.9) |
| Paint | 4 (5.9) |
| Miscellaneousa | 11 (16.2) |
| Class total | 68 (100) |
aIncludes brake fluid, dishwasher detergent, dishwasher detergent pod, fabric softener, household product unspecified, phenylenediamine (hair dye), rubber cement, and sunscreens
Table 28.
Plants and fungi
| N (%) | |
|---|---|
| Mold unspecified | 29 (44.6) |
| Mushroom, other/unknown | 8 (12.3) |
| Mitragyna speciosa (kratom) | 6 (9.2) |
| Mushroom, psilocibin | 5 (7.7) |
| Miscellaneousa | 17 (26.2) |
| Class total | 65 (100) |
aIncludes Aesculus hippocastanum (horse chestnut), Akuamma (Picralima nitida), Amanita phalloides, Datura stramonium (jimsonweed), Hydrastis canadensis (goldenseal), Lupinus (lupini beans), moonflower, Nerium oleander, strychnine, Toxicoscordion venenosum (death camas), Valerian root, and Vicia faba (fava bean)
Analgesics
Table 10 presents the non-opioid analgesics, the largest class reported in the Registry. Acetaminophen, aspirin, and ibuprofen were the most frequently reported agents in 2017 similar to past years [2–8]. In 2017, gabapentin and pregabalin were moved from the sedative-hypnotics/muscle relaxants class into the analgesics class. In this largest agent class, gabapentin still made up 11.1% of the category.
Antidepressants
Table 11 describes the antidepressant agents. The other antidepressant category was again the most frequent, predominantly due to the frequent reporting of bupropion (21.0%) and trazodone (13.3%). The selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and serotonin-norepinephrine reuptake inhibitors (SNRIs) were reported with similar agent frequencies to past years [7, 8].
Opioids
Table 12 presents the opioid agent class. As in recent years, heroin was the most commonly reported opioid in the Registry in 2017 (28.9%). Oxycodone was again the second most frequently reported agent (14.5%), though its percent contribution to the class was slightly decreased from last year (17.8%) [8]. Overall, oxycodone has declined in its percent contribution to the opioid agent class each year [2–8]. Tramadol increased slightly from 2016, making up 9.6% of reported opioids [8]. Reported fentanyl exposures increased by more than 90% in 2017 making up 7.9% of the opioid class after being steady at 4.1% in both 2015 and 2016 [7, 8]. In the same years, unspecified opioids had increased from 6.5% in 2015 to 8.1% in 2016, but remained fairly stable in 2017 at 7.4%. This increase in fentanyl cases may be a reflection of increased awareness of and testing for fentanyl adulteration of heroin. Additionally, the opioid unspecified class which has had a trending increase since the Registry began in 2010, may be capturing additional cases of adulterated heroin when testing is not available [2–8]. In 2017, the miscellaneous opioids included more specific designer opioids not previously reported to the Registry including butanoyl-4-fluorofentanyl, fluorofentanyl, fluoroisobutyryl fentanyl (4- or para-), methyl norfentanyl, N-allyl norfentanyl, butyrylfentanyl (butyr-), and carfentanil. The designer opioid U47700 and methylfentanyl were also reported after being reported to the Registry for the first time in 2016.
Opioid Coingestants
In 2017, there were 988 total opioid agents reported in 718 unique case entries. The frequency of coingestion was high with 403 (56.1%) out of 718 unique cases having more than one primary agent reported. Coingestion rates varied widely; however, based on specific opioid ingested, ranging from a high of 84.4% of cases for which hydrocodone was reported to a low of 21% when buprenorphine was reported. There were 270 cases (37.6%) with more than one opioid reported as a primary agent. Heroin was the most common opioid reported in 2017 with 318 case entries. The most common coingestions included stimulants occurring in 72 (22.6%) of heroin cases. Cocaine was the most commonly reported stimulant reported with heroin occurring in 49 (15.4%) followed by methamphetamine in 23 (7.2%). Alprazolam was the most commonly reported benzodiazepine reported with heroin occurring in 14 (4.4%). Other opioids were reported in 26 (8.2%) of cases involving heroin. Tramadol was the second most commonly reported opioid in 2017 occurring in 104 case entries. Coingestion was reported in 34 (32.7%) of tramadol cases, and while there were some drugs reported more than once, coingestion involved a variety of other agents. There were 18 unique agents reported along with tramadol; THC, kratom, gabapentin, cyclobenzaprine, and alcohol were all reported more than twice. Oxycodone was reported in 100 case entries with coingestion occurring in 57 (57.0%). Acetaminophen was the most commonly reported drug ingested with oxycodone occurring in 13 cases (13.0%). Benzodiazepines were reported as coingestants in 22 (22.0%) of oxycodone cases and alprazolam was the most commonly reported benzodiazepine in 11 (11.0%) of cases. Fentanyl was reported in 87 case entries with 53 cases (60.9%) involving coingestion and cocaine was the single most common drug, occurring in 13 cases (14.9%). Opioids were the most frequent class of drug reported as coingestion for fentanyl reported for nearly a third of all fentanyl cases with co ingestion. Methadone was reported in 79 case entries with coingestion in 30 (37.9%) and other opioids reported most commonly occurring in 19 cases (24.1%) with heroin being the most common opioid reported with methadone. Other drugs reported with methadone included cocaine in 8 (10.1%) and alprazolam was the most commonly reported benzodiazepine reported in 5 cases (6.3%). Hydrocodone was reported in 77 cases with 39 (50.6%) involving coingestion with acetaminophen as ingestion of the coformulation products for hydrocodone and acetaminophen were common. Sedative ingestion was common with hydrocodone though a variety of sedatives were reported including gabapentin, clonazepam, lorazepam, baclofen, and alcohol and although alprazolam was the most common. Buprenorphine was reported in 66 cases with 16 (24.2%) having coingestion reported. Other opiates, sedatives, cocaine, and benzodiazepines were the most commonly reported substances with buprenorphine. Morphine was reported in 37 cases and 26 (70.3%) had coingestion with opioid coingestion occurring in 10 and oxycodone being the most commonly reported other opioid along with morphine. Non-specific opioids (opioids NOS) were reported in 82 unique entries and 13 cases included co ingestion with sedatives being reported in almost half of these cases. Several different benzodiazepines, alcohol, and gabapentin were reported when non-specified opioid ingestion occurred in case entries.
Overall, alprazolam, followed by clonazepam, were the most common benzodiazepines reported with an opioid reported as primary agent. Illicit opioids had other illicit drugs including cocaine, methamphetamine, fentanyl, or heroin reported as coingestion though commonly abused pharmaceuticals were also not uncommon for illicit opioid cases (e.g., sedatives including alprazolam, gabapentin, and baclofen). Coingestion with other opioids and sedatives was also commonly reported for the pharmaceutical opioids as were illicit drugs, though this was at lower rates than their illicit opioid counterparts.
Sedative-Hypnotics/Muscle Relaxants
Table 13 presents the sedative-hypnotics/muscle relaxants class. Benzodiazepines remained the most commonly reported subclass of sedative-hypnotics/muscle relaxants in 2017. Alprazolam (22.8%) and clonazepam (18.6%) were again the most frequent agents both for the benzodiazepine subclass, as well as for the agent class as a whole. Of note, in 2016, gabapentin was reclassified from the sedative-hypnotics/muscle relaxants class to the non-opioid analgesic class, so is summarized in Table 10. The number of gabapentin cases was approximately stable from 2016. The muscle relaxants baclofen and cyclobenzaprine were reported with equal frequency (10.0%) in 2017. Barbiturates were again infrequently reported with butalbital making up the majority of these (1.8%).
Toxic Alcohols and Ethanol
Table 14 presents data on ethanol and toxic alcohols. As in prior years, ethanol is considered its own agent class. There were 723 ethanol exposures in 2017. Among the non-ethanol alcohols and glycols, isopropanol (37.3%) and ethylene glycol (34.7%) were similarly reported.
Anticholinergics
Table 15 shows the anticholinergics and antihistamines agent class. As in past years, diphenhydramine (59.1%) made up the majority of the agent class, with hydroxyzine (14.9%) following.
Sympathomimetics
Table 16 summarizes the sympathomimetics agent class. Cocaine (38.8%) was the leading agent reported. Methamphetamine (27.4%) and amphetamine (9.6%) were the next most commonly reported, consistent with prior years.
Cardiovascular Agents
Table 17 presents the cardiovascular agent class. In 2017, the sympatholytic subclass was the most commonly reported (25.1%), outnumbering beta blockers (23.3%) for the first time in the Registry. Among all of the cardiovascular agents, clonidine was the most commonly reported agent (18.9%), followed by amlodipine (9.9%), lisinopril (8.4%), and metoprolol (7.7%). Other cardiovascular agent subclasses—other antihypertensives and vasodilators, antidysrhythmics and other cardiovascular agents, cardiac glycosides, diuretics, and angiotensin receptor blockers—each made a smaller contribution to the class, altogether accounting for about one-quarter of the agents.
Antipsychotics
Table 18 shows the antipsychotic agent class. Distribution of agents was similar to prior years. Quetiapine made up early half of the agent class (47.5%). Olanzapine was the next most common (14.6%), followed by risperidone (7.2%) and aripiprazole (7.0%).
Anticonvulsants, Mood Stabilizers, and Lithium
Table 19 presents the anticonvulsants and mood stabilizers, along with lithium. As in past years, lithium is considered its own agent class, but for brevity, is presented along with the anticonvulsants and mood stabilizers. There were 121 cases reporting lithium exposure in 2017. The distribution of anticonvulsants and mood stabilizers followed a similar trend to past years. Lamotrigine was the most common agent in the class (27.1%), followed by valproic acid (22.8%). Carbamazepine and topiramate were equally reported (9.5%).
Envenomations and Marine Poisonings
Table 20 summarizes the envenomations and marine poisonings. Agkistrodon species exposures slightly outnumbered Crotalus species exposures in 2017 (28.8% vs 25.3%). Unspecified snake exposures were the next most common (10.1%). Loxosceles exposures increased from 2016 when they made up 4.2% of the agent class, making up 8.5% of the class in 2017 [8]. Chilopoda species exposures also were reported more frequently than in past years with 15 cases, making up 4% of the agent class.
Psychoactives
Table 21 presents the psychoactive agents and the amphetamine-like hallucinogen methylenedioxymethamphetamine (molly). Molly was reported in 12 cases in 2017, an increase from 2016 when 6 cases were reported [7]. In 2017, the number of marijuana cases surpassed the number of synthetic cannabinoids after a 2-year trend of synthetic cannabinoids being reported more frequently [7, 8].
Marijuana Edibles
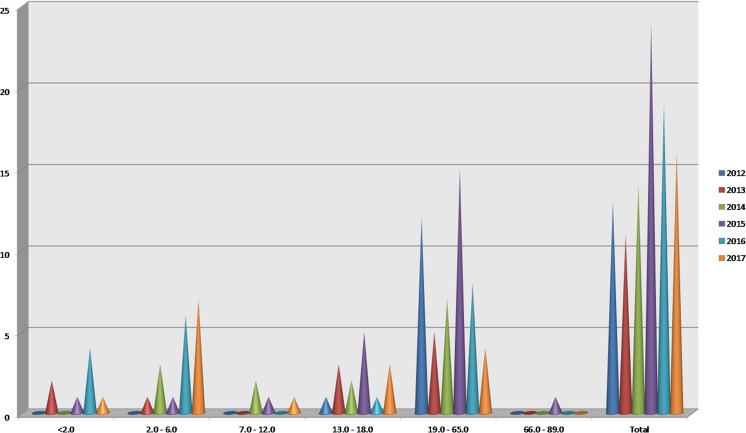
Figure 2 presents annual data on exposures to marijuana edible agents from 2012 through 2017, reported by age range. In 2012, there were no cases of oral marijuana ingestion in children aged 0–6. There were 10 cases reported in this age group in 2017 and 8 in 2017. Use among those aged 66–89 was low with only 1 case reported over the timeframe.
Fig. 2.
Marijuana edibles from 2012 to 2017 by age group
Pediatric Exposures to Drugs of Abuse
Tables 22 A–J present more detailed information about cases involving pediatric exposures to agents that are commonly used as drugs of abuse. If a case was identified reporting a drug of abuse, then additional agents in a polypharmacy exposure were also included in the analysis. Overall, 123 cases involving pediatrics exposed to drugs of abuse were identified. Tables 22 A, B, C, and D present the distribution of these patients by overall race and ethnicity, as well as race and ethnicity broken down by gender. Overall, 59.3% of patients were Caucasian and 21.1% were African American. Tables 22 E and F compare the numbers of single agent exposures and multiple agent exposures overall and by gender. Single agent exposures were more common (61.8%). Table 22 G and H show the most common agent classes involved in these exposures overall and by gender. The top agent classes were psychoactives, sympathomimetics, opioids, and antidepressants. These remained similarly reported among both male and female genders. Table 22 I and J show the reasons for medical toxicology encounters overall and broken down by gender. Intentional non-pharmaceutical exposures were the most commonly reported (38.2%), followed by intentional pharmaceutical exposures (27.6%).
Table 22.
Pediatric exposures to drugs of abuse
| A. Ethnicity | ||||
| Ethnicity | Total number of pediatric patients | % of total | ||
| Hispanic | 20 | 16.2 | ||
| Non-Hispanic | 50 | 40.6 | ||
| Unknown | 53 | 43.1 | ||
| B. Ethnicity by gender | ||||
| Ethnicity by gender | Male | % of males | Female | % of females |
| Hispanic | 15 | 22.4 | 5 | 8.9 |
| Non-Hispanic | 45 | 67.2 | 5 | 8.9 |
| Unknown | 7 | 10.4 | 46 | 82.1 |
| C. Race | ||||
| Race | Total # of pediatric patients | % of total | ||
| Caucasian | 73 | 59.3 | ||
| African American | 26 | 21.1 | ||
| Asian | 2 | 1.6 | ||
| American Indian/Alaska Native | 1 | 0.8 | ||
| Mixed | 2 | 1.6 | ||
| Unknown | 19 | 15 | ||
| D. Race by gender | ||||
| Race by Gender | Male | % of males | Female | % of female |
| Caucasian | 37 | 55.2 | 36 | 64.3 |
| African American | 13 | 19.4 | 13 | 23.2 |
| Asian | 2 | 3.0 | 0 | 0 |
| American Indian/Alaska Native | 0 | 0 | 1 | 1.8 |
| Mixed | 2 | 3.0 | 0 | 0 |
| Unknown | 13 | 19.4 | 6 | 10.7 |
| E. Single and multiple ingestions | ||||
| Single vs multiple ingestion | Total number of pediatric patients | % of total | ||
| Single agent | 76 | 61.8 | ||
| Multiple agents | 47 | 38.2 | ||
| F. Single and multiple ingestions by gender | ||||
| Single vs multiple agents | Males | % of males | Females | % of females |
| Single | 44 | 65.7 | 32 | 57.1 |
| Multiple | 23 | 34.3 | 24 | 42.9 |
| G. Agent classes involved in pediatric exposures to drugs of abuse | ||||
| Agent class | Total number of pediatric patients | % of total | ||
| Psychoactive | 57 | 46.3 | ||
| Sympathomimetic | 54 | 43.9 | ||
| Opioid | 29 | 23.6 | ||
| Antidepressant | 10 | 8.1 | ||
| Sedative-hypnotic/muscle relaxant | 7 | 5.7 | ||
| Anticholinergic/antihistamine | 4 | 3.3 | ||
| Amphetamine-like hallucinogen | 4 | 3.3 | ||
| Antipsychotic | 4 | 3.3 | ||
| Herbals/dietary supplements | 3 | 2.4 | ||
| Analgesics | 3 | 2.4 | ||
| Cardiovascular | 2 | 2.4 | ||
| Alcohol-ethanol | 2 | 2.4 | ||
| H. Agent classes involved in pediatric exposures to drugs of abuse by gender | ||||
| Agent class | Males | % of males | Females | % of females |
| Psychoactive | 32 | 47.7 | 25 | 44.6 |
| Sympathomimetic | 30 | 44.8 | 24 | 42.8 |
| Opioid | 16 | 23.9 | 13 | 23.2 |
| Antidepressant | 3 | 4.5 | 1 | 1.8 |
| Sedative-hypnotic/muscle relaxant | 3 | 4.5 | 4 | 7.1 |
| Anticholinergic/antihistamine | 3 | 4.5 | 1 | 1.8 |
| Antipsychotic | 2 | 2.9 | 2 | 3.6 |
| Herbals/dietary supplements | 2 | 2.9 | 1 | 1.8 |
| Analgesics | 0 | 0 | 3 | 5.4 |
| Cardiovascular | 1 | 1.5 | 4 | 7.1 |
| Alcohol-ethanol | 1 | 1.5 | 1 | 1.8 |
| I. Reason for medical toxicology encounter | ||||
| Reason for encounter | Total number of pediatric patients | % of total | ||
| Intentional pharmaceutical | 34 | 27.6 | ||
| Attempt at self-harm | 16 | 13.0 | ||
| Misuse/abuse | 12 | 9.8 | ||
| Therapeutic use | 2 | 1.6 | ||
| Unknown | 4 | 3.3 | ||
| Intentional non-pharmaceutical | 47 | 38.2 | ||
| Attempt at self-harm | 1 | 0.8 | ||
| Misuse/abuse | 26 | 21.1 | ||
| Use for therapeutic intent | 1 | 0.8 | ||
| Drug concealment | 1 | 0.8 | ||
| Unknown | 18 | 14.6 | ||
| Unintentional pharmaceutical | 13 | 10.6 | ||
| Unintentional non-pharmaceutical | 27 | 21.9 | ||
| Malicious/criminal | 1 | 0.8 | ||
| Interpretation of tox data | 1 | 0.8 | ||
| J. Reason for medical toxicology encounter by gender | ||||
| Reason for encounter by gender | Male | % of males | Female | % of female |
| Intentional pharmaceutical | 20 | 29.8 | 14 | 25.0 |
| Attempt at self-harm | 8 | 11.9 | 8 | 14.3 |
| Misuse/abuse | 9 | 13.4 | 3 | 5.4 |
| Therapeutic use | 1 | 1.5 | 1 | 1.8 |
| Unknown | 2 | 2.9 | 2 | 3.6 |
| Intentional non-pharmaceutical | 28 | 41.8 | 19 | 33.9 |
| Attempt at self-harm | 0 | 0 | 1 | 1.8 |
| Misuse/Abuse | 24 | 35.8 | 2 | 3.6 |
| Use for therapeutic intent | 1 | 1.5 | 0 | 0 |
| Drug concealment | 1 | 1.5 | 0 | 0 |
| Unknown | 2 | 2.98 | 16 | 28.6 |
| Unintentional Pharmaceutical | 8 | 11.9 | 5 | 8.9 |
| Unintentional non-pharmaceutical | 10 | 14.9 | 17 | 30.3 |
| Malicious/criminal | 1 | 1.5 | 0 | 0 |
| Interpretation of tox data | 0 | 0 | 1 | 1.8 |
Diabetic Agents
Table 23 shows diabetic medications. There were 172 diabetic medications reported to the Registry. Metformin was the most commonly reported agent (33.1%). In 2017, glipizide (23.3%) was reported more frequently than insulin (22.1%), a change from prior years.
Metals
Table 24 presents the metal agent class. There were 115 agents reported in 2017, with the top agents and frequencies similar to past years. Lead was the most commonly reported (27.8%), followed by iron (22.6%). Cobalt (8.7%) and chromium (5.2%) entries have continued to decrease since they peaked in 2011, coincident with the discontinued use of certain metal-on-metal hip joint prostheses [2–8].
Herbal Products and Dietary Supplements
Table 25 shows the herbal products and dietary supplements agent class. Similar to past years, this category included a diverse group of products, many of which were infrequently reported with less than 5 cases. Caffeine was again the most common agent (25.6%), followed by melatonin (21.4%). Unspecified herbals/dietary supplements/vitamins were the next most common (9.4%).
Gases, Irritants, Vapors, and Dusts
Table 26 shows the gases, irritants, vapors, and dusts category. Carbon monoxide was the most commonly reported agent (53.2%), consistent with prior years [5–8]. Chlorine and natural gas were reported with equal frequency (4.3%). In 2017, a number of infrequently reported miscellaneous compounds made up a large portion of the agent class (35.1%).
Household Agents
Table 27 presents the household product agent class. Cleaning solutions and disinfectants were most commonly reported (25.0%), followed by sodium hypochlorite at a concentration less than or equal to 6% (19.1%). Laundry detergent pod exposures decreased slightly, making up 11.8% of the class in 2017 as compared to 13.3% in 2016.
Plants and Fungi
Table 28 summarizes the plants and fungi agent class. There were 65 agents entered with unspecified mold species being the predominant agent in the category (44.6%). Other or unknown mushroom species was the next most common entry (12.3%). Kratom (Mitragyna speciosa) entries remained stable from 2016 (9.2%) [8]. Infrequent miscellaneous agents made up 26.2% of the class.
Supplemental Tables
Tables S1–S16 can be found in the Supplemental Materials. They present the less frequently reported agent classes, or those agent classes with little diversity, such as fewer than five agent types, or where one agent made up a vast majority of the class. They are briefly described below.
Cough and Cold Preparations
Table S1 presents the 112 cough and cold product agent entries. As in prior years, dextromethorphan made up the majority of the category (84.8%). Unspecified cough and cold products were the next most common (8.0%).
Caustics
Table S2 details the caustics agent class. In 2017, cleaning agents and formaldehyde were the most frequently reported agents (10.1%). Miscellaneous caustics, each reported 4 or fewer times, made up more than one-third of the agent class (36.0%).
Hydrocarbons
Table S3 presents the hydrocarbons agent class. Unspecified hydrocarbons were the most frequently reported (21.3%), similar to prior years. In 2017, lamp oil and petroleum distillates each made up 8% of the agent class, an increase from the past few years [6–8].
Antimicrobials
Table S4 presents the antimicrobial agent class. The class is subdivided into antibiotics, antivirals, and other antimicrobial agents. In 2017, the top antibiotic agents were again amoxicillin (10.0%) and dapsone (8.3%), with isoniazid (5.0%) and levofloxacin (5.0%) following. Among the other antimicrobials, quinine was the most commonly reported (10.0%). The antiviral subclass made up 16.7% of the agent class with amantadine (5.0%) being the most common agent.
Endocrine
Table S5 summarizes the 38 endocrine agents. Levothyroxine was the most commonly reported agent, making up 31.6% of the agent class. Prednisone was the next most commonly reported (15.8%).
Chemotherapeutic and Immunological Agents
Table S6 summarizes the 35 chemotherapeutic and immunological agents. Methotrexate was again the most commonly reported agent (22.9%). The majority of the class (54.3%) was made up of infrequently reported miscellaneous agents.
Other Non-pharmaceuticals
Table S7 presents the 38 other non-pharmaceutical agents. Perfluoro and polyfluoroalkyl substances (PFASs) were the most commonly reported (18.2%). Miscellaneous agents made up 51.5% of the agent class.
Gastrointestinal Agents
Table S8 presents the 30 gastrointestinal agents. Omeprazole (30.0%) and ondansetron (10.0%) were the most commonly reported specific agents. Miscellaneous agents made up 60.0% of the class.
Anesthetics
Table S9 shows the anesthetic agent class. There were 27 anesthetic agents reported with almost half of the class (48.1%) being made up of miscellaneous items. Lidocaine was the most commonly reported agent (29.6%).
Insecticides, Herbicides, Rodenticides, and Fungicides
Table S10 presents the insecticide, rodenticide, and herbicide agent classes. There were no fungicide agents reported in 2017. There were 27 insecticide agent entries. Unspecified pyrethroids were the most commonly reported agents, making up 22.2% of the class. Miscellaneous agents made up 33.3% of the class. Rodenticides and herbicides were infrequently reported.
Anticoagulants
Table S11 summarizes the 24 entries in the anticoagulant class. Warfarin was again the most commonly reported agent and made up the majority of the class (58.3%).
Other Pharmaceuticals
Table S12 presents the other pharmaceutical agent class. There were 18 entries in this category, with no agent contributing more than 3 cases. Hydrogen peroxide less than or equal to 10% and sumatriptan were the most commonly reported, each accounting for 16.7% of the class.
Weapons of Mass Destruction
Table S13 summarizes the potential weapons of mass destruction/riot agents/ radiological agents class. There were 7 entries with 42.9% being botulinum toxin.
Anti-Parkinsonism Agents
Table S14 presents the 6 entries for the anti-parkinsonism agent class. Levodopa/carbidopa and pramipexole were reported in equal numbers each making up 33.3% of the agent class.
Foreign Bodies
Table S15 presents the ingested foreign bodies agent class. These were infrequently reported with 5 entries, 2 of which were batteries (40.0%).
Pulmonary Agents
Table S16 reports the 4 pulmonary agent entries. Albuterol made up 50.0% of the category.
Clinical Signs and Symptoms
The various clinical signs and symptoms categories report information on a diverse range of abnormal clinical findings. In order to be reported as being present, it must meet pre-defined criteria. For example, tachycardia is defined as a heart rate greater than 140 beats per minute. Additionally, each case may report more than one abnormality within a group or across groups. For example, a single case entry may have more than one vital sign abnormality, or may have both a vital sign abnormality and a neurological abnormality. The percentages for these categories are calculated relative to the total number of Registry cases. It is possible for the total to be more than 100%.
Toxidromes
Table 29 summarizes the 2779 toxidrome entries in the Registry in 2017. The frequency of reported toxidromes remained consistent with prior years with the sedative-hypnotic toxidrome being by far the most commonly reported (15.7%). The anticholinergic toxidrome was the next most common (7.3%), followed by sympathomimetic (5.1%), opioid (3.7%), and serotonin syndrome (2.9%).
Table 29.
Toxidromes
| N (%)a | |
|---|---|
| Sedative-hypnotic | 1192 (15.7) |
| Anticholinergic | 550 (7.3) |
| Sympathomimetic | 387 (5.1) |
| Opioid | 280 (3.7) |
| Serotonin syndrome | 217 (2.9) |
| Alcoholic ketoacidosis | 58 (0.8) |
| Sympatholytic | 37 (0.5) |
| NMS | 17 (0.2) |
| Washout syndrome | 16 (0.2) |
| Overlap syndromes (MCS, chronic fatigue, etc.) | 13 (0.2) |
| Anticonvulsant hypersensitivity | 5 (0.1) |
| Miscellaneousb | 7 (0.1) |
| Total | 2779 (36.7) |
NMS neuroleptic malignant syndrome
aPercentage equals number cases reporting specific toxidrome relative to total number of Registry cases in 2017 (N = 7577)
bIncludes cholinergic, fume fever
Major Vital Sign Abnormalities
Table 30 summarizes the 2001 recorded major vital sign abnormalities. This represents 26.4% of the Registry, though cases may be associated with more than one major vital sign abnormality. The vital sign abnormalities remained similar in number to prior years. Tachycardia was the most commonly reported (11.8%). Hypotension was the next most common (6.2%).
Table 30.
Major vital sign abnormalities
| N (%)a | |
|---|---|
| Tachycardia (HR > 140) | 896 (11.8) |
| Hypotension (systolic BP < 80 mmHg) | 471 (6.2) |
| Bradycardia (HR < 50) | 264 (3.5) |
| Bradypnea (RR < 10) | 205 (2.7) |
| Hypertension (systolic BP > 200 mmHg and/or diastolic BP > 120 mmHg) | 123 (1.6) |
| Hyperthermia (temp > 105 °F) | 42 (0.6) |
| Total | 2001 (26.4)b |
HR heart rate, BP blood pressure, RR respiratory rate
aPercentage equals the number of cases relative to the total number of Registry cases in 2017 (N = 7577)
bTotal reflects cases reporting at least one major vital sign abnormality. Cases may be associated with more than one major vital sign abnormality
Clinical Signs and Symptoms—Neurological
Table 31 presents the 6138 entries recording neurological signs and symptoms (81.0%). Coma/central nervous system depression remained the most commonly reported sign (33.3%). Agitation (16.5%) and delirium (11.1%) were the next most common.
Table 31.
Clinical signs and symptoms—neurological
| N (%)a | |
|---|---|
| Coma/CNS depression | 2524 (33.3) |
| Agitation | 1251 (16.5) |
| Delirium | 843 (11.1) |
| Hyperreflexia/myoclonus/tremor | 480 (6.3) |
| Seizures | 427 (5.6) |
| Hallucinations | 312 (4.1) |
| Dystonia/rigidity/extrapyramidal symptoms | 106 (1.4) |
| Numbness/paresthesia | 93 (1.2) |
| Weakness/paralysis | 76 (1.0) |
| Peripheral neuropathy | 26 (0.3) |
| Total | 6138 (81.0)a,b |
CNS central nervous system
aPercentage equals number cases relative to total number of Registry cases in 2017 (N = 7577)
bTotal reflects cases reporting at least one neurological symptom. Cases may be associated with more than one neurological symptom
Clinical Signs—Cardiovascular and Pulmonary
Table 32 presents the cardiovascular and pulmonary clinical signs. The most frequently reported cardiovascular sign was prolonged QTc (6.1%) followed by prolonged QRS (1.8%) and myocardial injury or infarction (1.5%). Among pulmonary signs, respiratory depression was the most commonly reported (10.4%) and made up the majority of this category.
Table 32.
Clinical signs—cardiovascular and pulmonary
| N (%)a | |
|---|---|
| Cardiovascular | |
| Prolonged QTc (≥ 500 ms) | 460 (6.1) |
| Prolonged QRS (≥ 120 ms) | 138 (1.8) |
| Myocardial injury or infarction | 115 (1.5) |
| Ventricular dysrhythmia | 71 (0.9) |
| AV Block (> 1st degree) | 29 (0.4) |
| Total | 813 (10.7)b |
| Pulmonary | |
| Respiratory depression | 785 (10.4) |
| Aspiration pneumonitis | 197 (2.6) |
| Acute lung injury/ARDS | 110 (1.5) |
| Asthma/reactive airway disease | 49 (0.6) |
| Total | 1141 (15.1)b |
ARDS acute respiratory distress syndrome
aPercentage equals number cases reporting signs of symptoms relative to total number of Registry cases in 2017 (N = 7577)
bTotal reflects cases reporting at least one cardiovascular or pulmonary symptom. Cases may be associated with more than one symptom
Clinical Signs—Other Organ Systems
Table 33 presents the other organ system clinical signs. Among these additional categories, metabolic abnormalities were most frequently reported (12.4%). Cases reporting an elevated anion gap (5.2%) and a metabolic acidosis (4.7%) made up the majority of this category. The renal/musculoskeletal category was the next most common and cases were fairly evenly distributed between rhabdomyolysis (5.1%) and acute kidney injury (4.1%). Hematological signs made up 6.7% of the other organ systems. Coagulopathy (2.5%) and leukocytosis (1.8%) were the most commonly reported hematological signs. Among the gastrointestinal/hepatic signs, hepatotoxicity (3.2%) was the most common, followed by gastrointestinal bleeding (0.7%) and pancreatitis (0.6%). Dermatological signs were the least commonly reported category (4.5%). Rash (2.1%) and blister/bullae (1.1%) were the most common dermatological signs reported.
Table 33.
Clinical signs—other organ systems
| N (%)a | |
|---|---|
| Metabolic | |
| Elevated anion gap (> 20) | 394 (5.2) |
| Metabolic acidosis (pH < 7.2) | 357 (4.7) |
| Hypoglycemia (glucose < 50 mg/dL) | 131 (1.7) |
| Elevated osmole gap (> 20) | 60 (0.8) |
| Total | 942 (12.4)b |
| Renal/Musculoskeletal | |
| Rhabdomyolysis (CPK > 1000 IU/L) | 383 (5.1) |
| Acute kidney injury (creatinine > 2.0 mg/dL) | 309 (4.1) |
| Total | 692 (9.1)b |
| Hematological | |
| Coagulopathy (PT > 15 s) | 188 (2.5) |
| Leukocytosis (WBC > 20 K/μL) | 139 (1.8) |
| Thrombocytopenia (platelets < 100 K/μL) | 83 (1.1) |
| Hemolysis (Hgb < 10 g/dL) | 62 (0.8) |
| Methemoglobinemia (MetHgb ≥ 2%) | 21 (0.3) |
| Pancytopenia | 16 (0.2) |
| Total | 509 (6.7)b |
| Gastrointestinal/hepatic | |
| Hepatotoxicity (AST ≥ 1000 IU/L) | 243 (3.2) |
| Gastrointestinal bleeding | 54 (0.7) |
| Pancreatitis | 46 (0.6) |
| Corrosive injury | 34 (0.4) |
| Intestinal ischemia | 3 (0.03) |
| Total | 380 (5.0)b |
| Dermatological | |
| Rash | 162 (2.1) |
| Blister/bullae | 81 (1.1) |
| Angioedema | 54 (0.7) |
| Necrosis | 45 (0.6) |
| Total | 342 (4.5)b |
AST aspartate aminotransferase, PT prothrombin time, WBC white blood cells, Hgb hemoglobin, CPK creatine phosphokinase
aPercentage equals the number of cases reporting specific clinical signs compared to the total number of Registry cases in 2017 (N = 7577)
bTotal reflects cases reporting at least one sign in the category. Cases may be associated with more than one symptom
Fatalities
Tables 34 and 35 summarize cases which reported fatalities. Table 34 includes cases involving single agent exposures, and Table 35 presents those fatalities involving multiple agents. Table S17 in the Supplementary materials presents those fatalities in which it is unknown whether there was a related toxicological exposure.
Table 34.
2017 fatalities reported in ToxIC Registry with known toxicological exposure: single agent
| Age/gendera | Agents involved | Clinical findings | Life support withdrawn | Brain death confirmed | Treatmentb |
|---|---|---|---|---|---|
| 51F | Acetaminophen | ALI, AGT, CNS, DLM, RFX, MA, AG, HPT, HYS, GIB, PLT, AKI | Yes | Unknown | NAC, vasopressors, continuous renal replacement, intubation, IV fluids |
| 14F | Acetaminophen | None listed | No | No | NAC |
| 34F | Acetaminophen | HTN, TC, RD, CNS, MA, HPT, CPT, AKI | No | No | NAC, vitamin K, vasopressors, continuous renal replacement, intubation |
| 35M | Acetaminophen | HT, TC, CNS, MA, HPT, CPT | No | No | NAC, vitamin K, continuous renal replacement, intubation |
| 53F | Acetaminophen | CNS, MA, HPT, CPT, AKI | Yes | No | NAC, vasopressors, corticosteroids, continuous renal replacement, intubation |
| 37M | Acetaminophen | HT, TC, AP, CNS, DLM, MA, AG, HPT, GIB, CPT, WBC, AKI | Yes | Unknown | NAC, hemodialysis, intubation, IV fluids |
| 38M | Acetaminophen | HPT, OTH2 | Yes | No | NAC, thiamine, vasopressors, benzodiazepines, neuromuscular blockers, opioids, continuous renal replacement, intubation, IV fluids, transfusion |
| 14F | Acetaminophen | HPT | No | No | NAC |
| 55F | Amitriptyline | HT, QRS, QTC, RD, CNS, SZ, AKI | No | No | NaHCO3, benzodiazepines, intubation, IV fluids |
| 52F | Amlodipine | HT, BC, RD, CNS, MA | No | No | Calcium, glucagon, insulin-euglycemic therapy, methylene blue, vasopressors, neuromuscular blockers, opioids, continuous renal replacement, CPR, intubation, IV fluids |
| 60M | Amphetamine | MI, ALI, AGT, DLM, RFX, MA, AG, HPT, PLT, AKI, RBM | Yes | No | Fomepizole, NAC, vasopressors, bronchodilators, antiarrhythmics, benzodiazepines, neuromuscular blockers, opioids, continuous renal replacement, CPR, aortic balloon pump |
| 18M | Bupropion | HT, VD, QRS, QTC, MI, RD, CNS, MA | Yes | Yes | Lipid resuscitation, NaHCO3, vasopressors, CPR, intubation, IV fluids |
| 71M | Carbon monoxide | ALI, CNS, AG | No | No | Hydroxocobalamin |
| 48F | Carbon monoxide | HT, QTC, MI, RD, CNS, MA | No | No | Vasopressors, CPR, intubation |
| 33F | Clonazepam | CNS | No | No | None listed |
| 47M | Cocaine | HT, HYT, VD, CNS, MA, GIB, AKI | Unknown | Unknown | Vasopressors, activated charcoal, hemodialysis, continuous renal replacement, CPR, intubation, IV fluids |
| 43F | Cocaine | CNS, MA, AG, HPT, AKI, RBM | No | No | Calcium, lipid resuscitation, naloxone, NaHCO3, intubation, IV fluids |
| 51M | Cocaine | MI, RD, AGT, DLM, SZ, WBC, RBM | Yes | No | Vasopressors, antihypertensives, CPR, intubation, IV fluids |
| 65F | Colchicine | HT, BC, MI, CNS, MA, PCT, AKI, OTH2 | No | No | None listed |
| 23M | Digitoxin | HT, BC, VD, QRS, MA, AG, AKI | Yes | No | Digoxin Fab |
| 18F | Diphenhydramine | AGT | No | No | Physostigmine, benzodiazepines, IV fluids |
| 19M | Diphenhydramine | AGT, DLM | No | No | Benzodiazepines, IV fluids |
| 47F | Doxepin | HT, TC, BP, HYT, VD, QRS, MI, RD, CNS, MA, HYS, CPT, WBC, AKI, RBM | Yes | Unknown | NaHCO3, vasopressors, intubation, IV fluids |
| 49M | Ethanol | RD, CNS, HGY, MA, AG, OG, PNC, GIB, HYS, CPT, PLT, PCT, WBC | Yes | No | Fomepizole, vasopressors, benzodiazepines, glucose, opioids, continuous renal replacement, intubation, IV fluids, transfusion |
| 32M | Ethanol | CNS | No | No | Folate, thiamine, IV fluids |
| 30M | Ethanol | RD, CNS | Unknown | Unknown | Intubation, IV fluids |
| 31F | Ethanol | CNS | Unknown | Unknown | None listed |
| 60M | Ethanol | HT, AP, CNS, HCN, MA, AG, PNC, CPT | Yes | Unknown | Vasopressors, antiarrhythmics, antihypertensives, antipsychotics, benzodiazepines, opioids, intubation, IV fluids |
| 48F | Heroin | HT, VD, CNS, MA, HPT, AKI | Yes | No | NAC, vasopressors, CPR, intubation, IV fluids |
| 25M | Heroin | HT, AP, RD, CNS | Yes | Yes | Naloxone, vasopressors, benzodiazepines, intubation |
| 32M | Heroin | HT, RD, CNS | Unknown | Unknown | Naloxone, vasopressors, CPR, intubation, IV fluids |
| 35M | Heroin | None listed | No | No | Opioids |
| 66M | Metaxalone | HT, CNS, DLM, RFX, AKI | Yes | Yes | Benzodiazepines, intubation, IV fluids |
| 50M | Metformin | HT, RD, CNS, MA, AKI | Yes | Yes | Vasopressors, continuous renal replacement, intubation, IV fluids |
| 67M | Metformin | HT, QTC, ALI, RD, CNS, MA, AG, GIB, CPT, WBC, AKI | Yes | Unknown | Calcium, methylene blue, NaHCO3, thiamine, vasopressors, benzodiazepines, neuromuscular blockers, opioids, corticosteroids, continuous renal replacement, intubation, IV fluids |
| > 89F | Metformin | HT, QTC, CNS, MA, AG, AKI | Unknown | Unknown | IV fluids |
| 57M | Methamphetamine | HT, TC, MI, RD, CNS, MA, AG | Yes | No | None listed |
| 35M | Methamphetamine | HT, TC, QRS, MI, SZ, MA, HPT, CPT, AKI, RBM | Yes | No | Vasopressors, benzodiazepines, intubation, IV fluids |
| 55M | Methanol | SZ, MA, AG, OG | Yes | Unknown | Fomepizole, antiarrhythmics, benzodiazepines, hemodialysis, intubation, IV fluids |
| 84F | Methylene chloride | None listed | No | No | None listed |
| 29M | Methylfentanyl | HT, TC, HYT, MI, RD, CNS, MA, HPT, CPT, AKI, RBM | Yes | No | Naloxone, vasopressors, benzodiazepines, opioids, intubation, IV fluids |
| 16M | Opioid unspecified | HTN, HT, TC, ALI, CNS, MA, HPT | Yes | No | Atropine, naloxone, NaHCO3, vasopressors, antihypertensives, benzodiazepines, opioids, CPR, intubation |
| 54M | Quetiapine | QTC, RD, CNS | No | No | IV fluids |
| 71M | Rasburicase | HT, ALI, CNS, MA, MET, HYS, AKI | No | No | Methylene blue, vasopressors, glucose, continuous renal replacement, intubation, IV fluids, transfusion |
| 65F | Sodium hydroxide | RD, CNS, CRV, INT, OTH2 | Yes | Yes | Vasopressors, opioids, intubation, IV fluids |
| 58F | Verapamil | HT, BC, AVB, AP, RD, CNS, MA, PNC, AKI | No | No | None listed |
| 17M | Vitamin A | HT, TC, VD, RD, CNS, MA, HYS, PLT, WBC | Yes | Unknown | Calcium, insulin-euglycemic therapy, lipid resuscitation, vasopressors, benzodiazepines, CPR, ECMO, intubation |
Fatalities reported with known toxicological exposure: based on response from medical toxicologist “Did the patient have a toxicological exposure?” equals yes with known agent(s)
wk weeks, m months, AG anion gap, AGT agitation, AKI Acute kidney injury, ALI acute lung injury/ARDS, AP aspiration pneumonia, AVB AV block, BC bradycardia, BP bradypnea, CNS coma/CNS depression, CPT coagulopathy, CRV corrosive injury, DLM delirium, EPS dystonia/rigidity, GIB GI bleeding, HCN hallucinations, HGY hypoglycemia, HPT hepatoxicity, HT hypotension, HTN hypertension, HYS hemolysis, HYT hyperthermia, INT intestinal ischemia, MA metabolic acidosis, MET methemoglobinemia, MI myocardial injury/ischemia, NP neuropathy, OG osmole gap, OTH1 rash, OTH2 skin blisters, necrosis, PCT pancytopenia, PLT thrombocytopenia, PNC pancreatitis, PST paresthesia, QRS QRS prolongation, QTc QTc prolongation, RAD asthma/reactive airway disease, RBM rhabdomyolysis, RD respiratory depression, RFX hyperreflexia/tremor, SZ seizures, TC tachycardia, VD ventricular dysrhythmia, WBC leukocytosis, WKN weakness/paralysis, BAL dimercaprol, CPR cardiopulmonary resuscitation, ECMO extracorporeal membrane oxygenation, NAC n-acetyl cysteine, NaHCO3 sodium bicarbonate
aAge in years unless otherwise stated
bPharmacological and non-pharmacological support as reported by medical toxicologist
Table 35.
2017 fatalities reported in ToxIC Registry with known toxicological exposure: multiple agents
| Age/gendera | Agents involved | Clinical findings | Life support withdrawn | Brain death confirmed | Treatmentb |
|---|---|---|---|---|---|
| 52F | Acetaminophen, hydrocodone | None listed | No | No | None listed |
| 86F | Acetaminophen, ibuprofen | TC | Yes | Unknown | NAC, |
| 60F | Alprazolam, clonazepam, quetiapine | RD, CNS | No | No | Intubation, IV fluids |
| 43F | Alprazolam, gabapentin | QRS, QTC, MI, RD, MA, AG | Yes | Yes | NaHCO3, vasopressors, continuous renal replacement, CPR, intubation, IV fluids |
| 35F | Amitriptyline, buprenorphine, pregabalin, lorazepam | HT, TC, QRS, RD, CNS, MA, AG | No | No | Calcium, dantrolene, lipid resuscitation, naloxone, NaHCO3, vasopressors, opioids, intubation, IV fluids |
| 33M | Amitriptyline, clonazepam | TC, QRS, QTC, ALI, AP, RD, CNS, AG, CPT, RBM | No | No | NaHCO3, intubation, IV fluids |
| 29F | Amlodipine, nifedipine, metoprolol | HT, BC, VD, RD, CNS, SZ, HGY, MA, AG, HPT, HYS | No | No | Calcium, glucagon, insulin-euglycemic therapy, NaHCO3, vasopressors, benzodiazepines, glucose, opioids, hemodialysis, CPR, intubation |
| 75M | Atenolol, diltiazem, amiodarone | HT, BC, VD, QRS, RD, EPS | Yes | Yes | Calcium, glucagon, vasopressors, benzodiazepines, neuromuscular blockers, continuous renal replacement, intubation, IV fluids |
| 52F | Chlorzoxazone, amitriptyline, tramadol | CNS, HPT | Unknown | Unknown | NAC |
| 26F | Clonazepam, doxepin, fluoxetine, quetiapine, trazodone, bupropion | HT, BC, VD, RD, CNS, MA | No | No | Naloxone, vasopressors, antiarrhythmics, CPR, cardioversion, intubation, IV fluids, therapeutic hypothermia |
| 11m M | Cocaine, methamphetamine | HT, TC, RAD, AGT | No | No | Benzodiazepines |
| 12F | Diazepam, gabapentin | HT, TC, BP, HYT, RD, CNS, MA, AG | No | No | Naloxone, neuromuscular blockers, intubation, IV fluids |
| 39F | Digoxin, diltiazem, cardiovascular agent unspecified, warfarin | HT, VD, ALI, RD, CNS, MA, CPT, AKI | Yes | Unknown | Calcium, digoxin Fab, glucagon, insulin-euglycemic therapy, lipid resuscitation, NaHCO3, vasopressors, antiarrhythmics, glucose, CPR, cardioversion, intubation, IV fluids, pacemaker |
| 41F | Ethanol, lorazepam | CNS | Unknown | Unknown | Antipsychotics, benzodiazepines, |
| 30F | Fentanyl, quinine, buclizine, methyl norfentanyl | BP, MI, AP, CNS, MA, AKI, RBM | Yes | No | Naloxone, opioids, intubation, IV fluids |
| 34M | Heroin, cocaine, levamisole | HT, BC, AP, RD, WBC | Yes | Yes | CPR, IVF, therapeutic hypothermia |
| 26F | Heroin, cocaine | AVB, CNS, AG, HPT, RBM | Yes | Yes | NaHCO3, vasopressors, opioids, intubation |
| 25M | Heroin, ethanol, alprazolam, cocaine | HT, MI, RD, CNS, MA, AG, HPT, PNC, AKI | Yes | Yes | Vasopressors, CPR, ECMO, intubation, IV fluids, therapeutic hypothermia |
| 20F | Ibuprofen, citalopram, trazodone, penicillin, ethanol | CNS | No | No | NAC, IVF |
| 56M | Imipramine, cyclobenzaprine, hydrocodone | QTC, RD, CNS | No | No | Benzodiazepines, intubation, IV fluids |
| 52M | Methamphetamine, amphetamine | HT, MI, AP, RD, AGT, CNS, MA, HPT, WBC, AKI, RBM | Yes | Unknown | None listed |
| 31M | MDMA, hydromorphone, methadone, marijuana | None listed | Yes | No | None listed |
| 57M | Nortriptyline, gabapentin | QRS, MI, RD, CNS | No | No | NaHCO3, intubation, IV fluids |
| 52M | Olanzapine, hydrochlorothiazide | TC, DLM | No | No | Benzodiazepines, IV fluids |
| 14F | Propofol, ketamine | HT, BC, MI, RD, SZ, MA, AG | Yes | Unknown | Vasopressors, hemodialysis, ECMO, intubation |
| 63M | Propranolol, citalopram | HT, BC, AVB, RD, CNS | No | No | Glucagon, vasopressors, antiarrhythmics, benzodiazepines, neuromuscular blockers, CPR, intubation, IV fluids |
| 33F | Propranolol, escitalopram, lamotrigine | HT, TC, BC, HYT, VD, QRS, QTC, AVB, MI, RD, AGT, CNS, DLM, EPS, RFX, SZ, HGY, MA, AG, PLT, RBM | Yes | No | Anticoagulation reversal, calcium, facto replacement, glucagon, insulin-euglycemic therapy, NaHCO3, vasopressors, antiarrhythmics, benzodiazepines, glucose, intubation, IV fluids, transfusion |
| 13F | Quetiapine, acetaminophen | HT, ALI, CNS, MA, AG, AKI | Yes | Yes | NAC, hemodialysis, ECMO, intubation, IV fluids |
| 47F | Trazodone, ethanol, heroin | QTC, RFX, GIB | Yes | Unknown | CPR, intubation, IV fluids, transfusion |
| 39F | U47700 (designer opioid), venlafaxine, gabapentin, doxepin | BP, ALI, AP, RD, CNS, MA | Yes | Yes | IV fluids |
Fatalities reported with known toxicological exposure: based on response from medical toxicologist “Did the patient have a toxicological exposure?” equals yes with known agent(s)
wk weeks, m months, AG anion gap, AGT agitation, AKI Acute kidney injury, ALI acute lung injury/ARDS, AP aspiration pneumonia, AVB AV block, BC bradycardia, BP bradypnea, CNS coma/CNS depression, CPT coagulopathy, CRV corrosive injury, DLM delirium, EPS dystonia/rigidity, GIB GI bleeding, HCN hallucinations, HGY hypoglycemia, HPT hepatoxicity, HT hypotension, HTN hypertension, HYS hemolysis, HYT hyperthermia, INT intestinal ischemia, MA metabolic acidosis, MET methemoglobinemia, MI myocardial injury/ischemia, NP neuropathy, OG osmole gap, OTH1 rash, OTH2 skin blisters, necrosis, PCT pancytopenia, PLT thrombocytopenia, PNC pancreatitis, PST paresthesia, QRS QRS prolongation, QTc QTc prolongation, RAD asthma/reactive airway disease, RBM rhabdomyolysis, RD respiratory depression, RFX hyperreflexia/tremor, SZ seizures, TC tachycardia, VD ventricular dysrhythmia, WBC leukocytosis, WKN weakness/paralysis, BAL dimercaprol, CPR cardiopulmonary resuscitation, ECMO extracorporeal membrane oxygenation, NAC n-acetyl cysteine, NaHCO3 sodium bicarbonate
aAge in years unless otherwise stated
bPharmacological and non-pharmacological support as reported by medical toxicologist
There were a total of 93 fatalities in 2017, involving 1.2% of Registry cases. Forty-seven cases involved single agent exposures, 21 involved multiple agents, and 25 cases were unknown. The number of fatality cases decreased from 2016, though was similar to the years prior to that [6–8].
Seventeen fatality cases involved at least one opioid agent. Eight cases involved heroin. Two additional cases involved fentanyl or a fentanyl derivative, and 1 case involved U47700. There were 6 opioid fatalities in which only the single opioid agent was involved, the remainder were polypharmacy exposures, and some involved multiple opioids. Sedative-hypnotics/muscle relaxants, including gabapentinoids, were involved in 14 fatality cases. There were five cases that involved both a sedative-hypnotics/muscle relaxant and an opioid.
In 2017, there were 10 pediatric (age 0–18 years) deaths related to toxicologic exposures. The age range for these was 11 months to 18 years. Six of these were single agent exposures and 4 involved multiple agents. Two deaths were single agent exposures to acetaminophen, both in 14 year olds. One death involved an 18 year-old with a bupropion exposure that resulted in hypotension, ventricular dysrhythmia, QRS prolongation, QTc prolongation, myocardial injury, respiratory depression, metabolic acidosis, and central nervous system depression. He was treated with sodium bicarbonate, lipid resuscitation, vasopressors, intubation, and cardiopulmonary resuscitation. There was a death in an 11 month old related to cocaine and methamphetamine who had clinical signs of hypotension, tachycardia, agitation, and reactive airway disease and was treated with benzodiazepines. A 16 year old died related to an unspecified opioid after life support was withdrawn.
There were 38 fatality cases in which life support was withdrawn (0.5% of Registry cases). An additional, 7 cases were unknown whether life support was withdrawn. Brain death was declared in 12 cases, though there were 19 cases in which it was unknown if brain death was declared.
Adverse Drug Reactions
Table 36 presents the drugs most frequently associated with adverse drug reactions (ADRs). Lithium was again the most commonly reported agent in 2017 (4.1%), as it has been in past years. The next most commonly reported agents—valproic acid (3.8%), haloperidol (2.9%), and bupropion (2.7%)—have also been reported in past years. Digoxin was less frequently reported in 2017 and was not one of the top 10 agents as it has been in prior years [5–8]. The sedatives clonazepam (2.4%) and baclofen (2.1%) were among the most commonly reported agents associated with ADRs in 2017. This was a change from prior years, though in 2015, lorazepam was the second most commonly reported ADR [7].
Table 36.
Most common drugs associated with ADRs
| N (%)a | |
|---|---|
| Lithium | 14 (4.1) |
| Valproic acid | 13 (3.8) |
| Haloperidol | 10 (2.9) |
| Bupropion | 9 (2.7) |
| Phenytoin | 9 (2.7) |
| Clonazepam | 8 (2.4) |
| Diphenhydramine | 8 (2.4) |
| Baclofen | 7 (2.1) |
ADRs adverse drug reactions
aPercentages are calculated out of the total number of cases reporting an ADR (N = 339)
Treatment
Antidotal Therapy Administered
Table 37 summarizes antidotal therapies reported to the Registry. Similar to prior years, N-acetylcysteine was the most commonly reported antidote (28.3%), followed by naloxone/nalmefene (21.9%) and sodium bicarbonate (11.8%).
Table 37.
Antidotal therapy
| N (%)a | |
|---|---|
| N-acetylcysteine | 820 (28.3) |
| Naloxone/nalmefene | 633 (21.9) |
| Sodium bicarbonate | 343 (11.8) |
| Thiamine | 267 (9.2) |
| Folate | 157 (5.4) |
| Fomepizole | 102 (3.5) |
| Calcium | 74 (2.6) |
| Physostigmine | 66 (2.3) |
| Glucagon | 63 (2.2) |
| Octreotide | 57 (2.0) |
| Cyproheptadine | 36 (1.2) |
| L-carnitine | 34 (1.2) |
| Vitamin K | 34 (1.2) |
| Atropine | 33 (1.1) |
| Insulin-euglycemic therapy | 32 (1.1) |
| Flumazenil | 31 (1.1) |
| Lipid resuscitation | 27 (0.9) |
| Pyridoxine | 24 (0.8) |
| Fab for digoxin | 16 (0.6) |
| Methylene blue | 11 (0.4) |
| Dantrolene | 8 (0.3) |
| Hydroxocobalamin | 8 (0.3) |
| Bromocriptine | 6 (0.2) |
| 2-PAM | 4 (0.2) |
| Coagulation factor replacement | 3 (0.1) |
| Silimarin | 2 (0.1) |
| Anticoagulation reversal | 2 (0.1) |
| Botulinum antitoxin | 1 (< 0.1) |
| Nitrites | 1 (< 0.1) |
| Total | 2895 (100) |
aPercentages are out of the total number of antidotes administered (2895); 2319 registry cases (30.6%) received at least one antidote. Cases may have involved the use of multiple antidotes
Antivenom Therapy Administered
Table 38 summarizes the antivenom therapies reported in 2017. Similar to prior years, Crotalidae polyvalent immune fab (ovine) was the most commonly reported antivenom (92.0%). Other snake venoms were the next most common and made up 3.7% of the category.
Table 38.
Antivenom therapy
| N (%)a | |
|---|---|
| Crotalidae polyvalent immune fab (ovine) | 173 (92.0) |
| Other snake antivenom | 7 (3.7) |
| Scorpion antivenom | 3 (1.6) |
| Spider antivenom | 2 (1.1) |
| Not recorded | 3 (1.6) |
| Total | 188 (100) |
aPercentages are out of the total number of antivenom treatments administered (N = 188)
Pharmaceutical Supportive Care
Table 39 presents the pharmacological supportive care interventions that were reported in 2017. There were 3574 pharmacological interventions recorded. As in past years, benzodiazepines made up approximately half of the reported pharmacologic interventions (49.1%). Opioids (14.3%) and vasopressors (8.4%) were the next most commonly reported. There were 2501 cases (33.0%) that involved at least one form of pharmacological supportive care.
Table 39.
Supportive care—pharmacological
| N (%)a | |
|---|---|
| Benzodiazepines | 1756 (49.1) |
| Opioids | 511 (14.3) |
| Vasopressors | 302 (8.4) |
| Antipsychotics | 250 (7.0) |
| Neuromuscular blockers | 197 (5.5) |
| Anticonvulsants | 146 (4.1) |
| Glucose (concentration > 5%) | 137 (3.8) |
| Antihypertensives | 83 (2.3) |
| Albuterol (or other bronchodilator) | 62 (1.7) |
| Corticosteroids | 56 (1.6) |
| Antiarrhythmics | 41 (1.1) |
| Beta blockers | 24 (0.7) |
| Vasodilators | 9 (0.3) |
| Total | 3574 (100) |
aPercentages are out of the total number of treatments administered (3574); 2501 registry cases (33.0%) received at least one form of pharmacological treatment. Cases may have involved the use of multiple forms of treatment
Non-pharmaceutical Supportive Care
Table 40 presents the non-pharmacological supportive care interventions. The majority of this category was made up of intravenous fluid resuscitation (72.3%). Intubation/ventilatory management was the next most common intervention (22.9%). Additional interventions were less common with none making up more than 2% of the category. Overall, 3141 Registry cases (41.5%) reported at least one non-pharmacological intervention.
Table 40.
Supportive care—non-pharmacological
| N (%)a | |
|---|---|
| IV fluid resuscitation | 2912 (72.3) |
| Intubation/ventilatory management | 919 (22.9) |
| CPR | 74 (1.8) |
| Transfusion | 36 (0.9) |
| Therapeutic hypothermia | 18 (0.4) |
| Hyperbaric oxygen | 15 (0.4) |
| ECMO | 14 (0.3) |
| Cardioversion | 11 (0.3) |
| Pacemaker | 9 (0.2) |
| Organ transplantation | 3 (0.1) |
| Aortic balloon pump | 1 (0.02) |
| Total | 4012 (100) |
aPercentages are out of the total number of treatments administered (4012); 3141 registry cases (41.5%) received at least one form of non-pharmacological treatment. Cases may have involved the use of multiple forms of treatment. CPR Cardiopulmonary resuscitation, ECMO extracorporeal membrane oxygenation
Chelation Therapy Administered
Table 41 summarizes chelation therapy reported to the Registry in 2017. There were 31 total chelator agents reported in 2017. Twenty-five cases involved the delivery of a single chelating agent, while 5 cases involved two or more agents. The most commonly administered chelator was dimercaptosuccinic acid (DMSA) (38.7%). Deferoxamine was the next most common (25.8%).
Table 41.
Chelation therapy
| N (%)a | |
|---|---|
| DMSA | 12 (38.7) |
| Deferoxamine | 8 (25.8) |
| Dimercaprol | 6 (19.4) |
| EDTA | 5 (16.1) |
| Total | 31 (100) |
aPercentages are out of the total number of chelation treatments administered (31); 25 registry cases (0.3%) received at least one form of chelation treatment. DMSA dimercaptosuccinic acid, EDTA ethylenediamine-tetraacetic acid
Decontamination Interventions Administered
Table 42 presents decontamination interventions. Activated charcoal remained by a large margin the most commonly reported decontamination measure (79.6%). External irrigation (8.1%) and whole bowel irrigation (7.7%) were the next most commonly reported. Gastric lavage was an uncommon intervention (4.6%). Overall, there were 264 cases that reported at least one kind of intervention (3.5%). Some cases reported more than one decontamination method.
Table 42.
Decontamination
| N (%)a | |
|---|---|
| Activated charcoal | 226 (79.6) |
| External irrigation | 23 (8.1) |
| Whole bowel irrigation | 22 (7.7) |
| Gastric lavage | 13 (4.6) |
| Total | 284 (100) |
aPercentages are out of the total number of treatments administered (334); 264 registry cases (3.5%) received at least one form of decontamination
Enhanced Elimination Interventions Administered
Table 43 describes enhanced elimination techniques reported to the Registry. Urinary alkalinization was the most commonly reported intervention (26.4%). Hemodialysis for toxin removal was the next most common (24.8%) and hemodialysis for any other indication made up an additional 18.6% of the treatments in this group. Continuous renal replacement therapy made up 24.8% of the enhanced elimination techniques. There were 226 cases (3.0% of the Registry) reporting at least one enhanced elimination method. Some cases reported more than one.
Table 43.
Enhanced elimination
| N (%)a | |
|---|---|
| Urinary alkalinization | 64 (26.4) |
| Hemodialysis (toxin removal) | 60 (24.8) |
| Continuous renal replacement therapy | 60 (24.8) |
| Hemodialysis (other indication) | 45 (18.6) |
| Multiple-dose activation charcoal | 11 (4.5) |
| Exchange transfusion | 2 (0.8) |
| Total | 242 (100) |
aPercentages are out of the total number of treatments administered (242); 226 registry cases (3.0%) received at least one form of enhanced elimination
Table 44 presents more detailed information about cases in which extracorporeal membrane oxygenation ECMO was delivered from 2013 through 2017. During the 5-year period of data collection, there were 62 cases reporting the use of ECMO. The mean age of patients was 22 years. Most cases involved intentional ingestions.
Table 44.
Cases involving extracorporeal membrane oxygenation (ECMO): a detailed look from 2013 to 2017
| Year | ECMO cases | %a | Age range (mean)b | Deaths | Toxicological etiology? | Type of exposure | Primary agent category | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | U | IN | UN | U | Other | Analgesic | Opioid | CV | Psych | Symp | Antichol | Sed | Otherc | U | |||||
| 2013 | 11 | 0.12% | 2–48 (20.1) | 2 | 8 | 2 | 1 | 5 | 3 | 1 | 2 | 2 | 3 | 2 | 1 | 7 | 1 | |||
| 2014 | 7 | 0.08% | 2–29 (17.4) | 0 | 4 | 1 | 2 | 4 | 1 | 0 | 2 | 1 | 1 | 2 | 1 | 5 | 4 | 2 | ||
| 2015 | 19 | 0.23% | 1–43 (20.1) | 6 | 16 | 3 | 0 | 14 | 1 | 1 | 3 | 5 | 2 | 4 | 4 | 3 | 1 | 1 | 6 | 1 |
| 2016 | 11 | 0.13% | 15–47 (26.6) | 3 | 8 | 0 | 3 | 10 | 0 | 1 | 0 | 2 | 3 | 2 | 3 | 2 | 2 | 2 | ||
| 2017 | 14 | 0.18% | 13–51 (24.4) | 4 | 14 | 0 | 0 | 10 | 2 | 0 | 2 | 3 | 5 | 5 | 3 | 1 | 4 | 6 | 1 | |
| Totals | 60 | 1–51 (22) | 15 | 50 | 6 | 6 | 43 | 7 | 3 | 9 | 10 | 11 | 14 | 15 | 5 | 5 | 11 | 25 | 7 | |
U unknown, IN intentional UN unintentional, CV cardiovascular agents, Psych antidepressant agents and antipsychotic agents, Symp sympathomimetics, Antichol anticholinergics, Sed sedative-hypnotics/muscle relaxants
aPercentages are out of the total number of ToxIC registry cases for that year (N = 7577 for 2017; N = 8529 for 2016; N = 8115 for 2015; N = 9094 for 2014; N = 8284 for 2013)
b2 cases had unknown/unlisted age; these were not included in the means
cOther category included 4 ethanol, 4 gases/irritants/vapors/dusts, 4 diabetic agents, 3 anticonvulsants, 2 psychoactives, 1 chemotherapeutic/immunological agent, 2 antimicrobial, 1 herbicide, 1 insecticide, 1 herbal products/dietary supplements, 1 gastrointestinal agent, and 1 endocrine agent
Discussion
The data collection in this eighth year of the ToxIC case registry has been characterized by continued strong data collection and a number of important observations related to new trends and findings. Although the slight reduction in the number of cases entered is multifactorial, probably a major reason is our continued quality assurance efforts and dropping poorly performing sites from the Consortium.
The Registry serves as a large source of information on poisoning cases as evaluated by bedside medical toxicologists. Although the Toxic Registry is not technically population based, it does have multiple sites broadly around the USA. As such, it may be used in conjunction with other types of registries such as poison centers and health agencies to produce a more detailed understanding of poisoning trends, novel exposures, and their public health implications.
Information on novel exposure surveillance is not included with this annual report, but is being analyzed with results to be reported separately.
Overall, this annual report finds that the most common agent classes, agents, demographics, types of encounters, toxidromes, and treatments remain similar year-to-year. Some notable trends in the Registry as well as trends in a larger national context are discussed.
Use of Extracorporeal Membrane Oxygenation
The ToxIC case registry has been gathering data on extracorporeal membrane oxygenation (ECMO) since 2013. Analysis of these cases is presented in Table 44. Over 5 years, a total of 62 patients were recorded as receiving ECMO, accounting for a very small percentage of patients in the overall registry. The number of cases per year was relatively consistent, other than in 2015 where there were more cases than typically seen. ECMO patients were relatively younger compared to the total registry sample, with an overall mean age of 22 years. Most of the cases were likely to be related to a toxicologic exposure, with intentional exposures predominating. The most frequently encountered agent classes were analgesics, opioids, cardiovascular agents, psychiatric medications, or a diabetic medication, specifically metformin. Though ECMO is generally considered only in the most critically ill patients, the majority of the patients (76%) survived. This may be related to the superior prognosis of critically ill poisoned patients as compared to patients who are ill from other medical causes. Alternatively, there may be an inconsistent reporting of deaths in the Registry which could have increased this figure. Since a patient might die at some time after a toxicology consult and the Registry case entry, the consultant would need to actively go back and update the case after a death occurs.
Exposures in Pediatric Patients
As with the Registry overall, analgesics and antidepressants were the most commonly reported agent classes in pediatric patients (Table 8). The anticholinergic/antihistamine class was the next most common in pediatrics, though it was sixth overall in the Registry. The majority of pediatric patients with anticholinergic/antihistamine exposures were in the 13–18 year old age group. Opioids were ranked sixth in agent class frequency for pediatrics with the majority of exposures involving 13–18 year olds, though they were also the most common agent class in those under age 2.
The most commonly reported pediatric agent classes in ToxIC are notable in that they are dissimilar to those reported by the National Poison Data System (NPDS) [9]. The NPDS describes the most common agent class exposures reported in children less than or equal to 5 years old. When the same subset of patients in the ToxIC database is compared, there are differences in the frequency of agent classes reported. Whereas the NPDS reported the most common pediatric exposures as involving cosmetic and household cleaning products, these were infrequently reported in the ToxIC database. Household products were ranked 15th in frequency in ToxIC, making up just 2.5% of pediatric exposures. The other non-pharmaceutical class made up only 0.3% of pediatric exposures. The leading agent class in children ≤ 5 years old in the ToxIC database was cardiovascular agents (15.6%), though this agent class made up only 2.1% of the exposures in the NPDS [9]. Opioids (8.4%) and sympathomimetics (7.7%) were the next most frequently reported agents classes in the ToxIC database. The NPDS reported foreign bodies/toys/miscellaneous as the fourth most common agent class (6.5%), compared to being one of the least commonly reported agent classes in ToxIC (0.3%).
Of note, the most recent NPDS data available are from 2016, though the agent classes reported are consistent with prior NPDS reports and would not be expected to significantly change in 2017 [10, 11]. Additionally, there are some differences in the categorization of agents into agent classes between the NPDS and ToxIC, though the general trends are still clear. These differences in agent class reporting between the NPDS and ToxIC support the idea that the ToxIC database represents a unique population of patients, with an inclination towards more severe exposures. This is likely due to that fact that the cases entered are ones in which a patient presented for care and were evaluated by a medical toxicologist. Additionally, the differences between agent class frequencies in the two databases may speak to the important role of poison control centers in reducing the overuse of healthcare settings for non-life threatening exposures that can be safely managed at home.
Pediatric Exposures to Drugs of Abuse
A total of 832 patients from the 2017 ToxIC registry were exposed to drugs of abuse with 123 patients of those being between the ages of 0–18 (14.8%). Pediatric patients exposed to drugs of abuse made up 1.6% of the total Registry. Cases were included after a manual search of pediatric patients for drugs of abuse. Any additional agents in a polypharmacy exposure were also included. The following agents were included: 25I-NBOMe, alprazolam, bupropion, butanoyl-4-fluorofentanyl, caffeine, cannabinoid non-synthetic, cannabinoid synthetic, clonidine, cocaine, delta-9-tetrahydrocannabinol, diphenhydramine, ethanol, fentanyl, fluoxetine, gamma hydroxybutyrate, heroin, hydroxyzine, ketamine, LSD (lysergic acid diethylamide), marijuana, methamphetamine, methylenedioxymethamphetamine, methylnorfentanyl, methylphenidate, molly, N-allyl norfentanyl, nicotine, oxycodone, paliperidone, pharmaceutical THC, phencyclidine, phenylephrine, psychoactive unspecified, quetiapine, sympathomimetic unspecified, tramadol, trazodone, and valproic acid. Those patients presenting for withdrawal were excluded from the data analysis. The majority of the pediatric patients were between the ages of 13–18 (54%) followed by ages 2–6 (22%). Fifty-nine percent identified as Caucasian and non-Hispanic (41%). Pediatric patients were more likely to present due to an intentional exposure of a non-pharmaceutical agent (38.2%). The most commonly reported class of drug was psychoactives (46.3%) followed by sympathomimetics (43.9%). The majority of patients had a single agent exposure (61.8%) as opposed their adult counterparts. There were 2 pediatric deaths related to drugs of abuse. The first was an 11-month-old Caucasian male admitted after an unintentional exposure to cocaine and methamphetamine. The second was a 14-year-old female who received ketamine and propofol. She later developed propofol infusion syndrome and sustained cardiac arrest. The circumstances surrounding ketamine exposure were not described. When analyzed by gender, there were no significant differences in the reason for exposure, agent used or demographics in the pediatric drug of abuse population.
Cannabis—the Trouble with Edibles
Cannabis is the most commonly used illicit drug in the USA, according to the 2016 National Survey on Drug Use and Health [12]. While cannabis is typically consumed through smoke inhalation, the increase in the legalization of cannabis has made “edibles” (products containing cannabis intended for oral consumption) more accessible as the cannabis market continues to evolve [13]. There is often significant variation in the potency of “edibles,” which can contribute to accidental overdose, especially with inexperienced users. Perhaps more troubling is the unintentional ingestion of edibles by young children and adolescents that can result in significant toxicity, including agitation, ataxia, hallucinations, lethargy, myoclonus, respiratory depression, seizures, and tachycardia [14–16].
Since the first edibles cases were reported in the ToxIC Registry in 2012, there has been a substantial increase in the number of cases involving children 0–6 years of age [4]. In 2012, there were no “edibles” cases reported to the ToxIC Registry that involved children 0–6 years of age, compared with 10 cases in 2016 (52.6% of total “edibles” cases) and 8 cases in 2017 (50.0% of total “edibles” cases) [4, 8]. In 2017, among the children 0–6 years of age that had unintentionally consumed a product containing cannabis, CNS depression was reported in five, delirium in three, agitation in one, hallucinations in one, respiratory depression in one, and seizures in one, with no fatalities reported. Certainly, this is a concerning trend, and clinicians should be proactive in educating parents about the dangers that “edibles” pose to young children.
Increase in ToxIC Gabapentinoid (GP) Cases
Gabapentin and pregabalin are substituted derivatives of the neurotransmitter gamma-aminobutyric acid (GABA) labeled in the USA for the treatment of neurologic pain and seizures [17, 18]. Their weak GABA-mimetic features include feelings of relaxation and euphoria reported by some patients and recreational users [17]. As with the benzodiazepines and other addictive GABA-mimetics, withdrawal symptoms can occur and tolerance to the euphoria develops, increasing their addiction potential [17]. This, combined with a global increase in GP prescribing for a variety of on- and off-label uses (e.g., anxiety, insomnia, and recreational drug withdrawal, among others), is thought to help explain the recent spikes in the numbers of GP abuse cases reported to pharmacovigilance databases in Europe [19, 20]. In the USA, although population-level estimates of GP misuse/abuse are not yet available, GP misuse/abuse appears on the rise as well [21]. A recent analysis of data from the Researched Abuse, Diversion and Addiction-Related Surveillance (RADARS®) system, a national prescription drug surveillance system covering all states except Hawaii, reported 407 cases of gabapentin diversion in 41 states from 2002 to 2015 and showed steady annual increases of gabapentin diversion from 2002 to 2015, with a 2015 diversion rate comparable to OxyContin [21]. In 2016, the US National Poison Data System (NPDS) reported 3443 intentional (including misuse/abuse, suicide, and unknown) single agent gabapentin exposures, up from 0 in 2011, the first year it was reported separately [9, 22]. These recent spikes in GP misuse/abuse are mirrored in the numbers of GP cases recorded in ToxIC, up from 54 (1.4% of all ToxIC cases) in 2010 to 201 (2.7% of all ToxIC cases) in 2017. Approximately half the ToxIC 2017 cases were suicide attempts and one-quarter were misuse/abuse cases.
Some speculate that off-label prescriptions are increasing as physicians attempt to provide non-opioid pain relief to their patients, despite the lack of efficacy and long-term safety data for common off-label pain indications [23, 24]. There are also published case reports of GP abuse by patients with substance use disorders (SUDs), especially prescription opioid misusers and heroin users, and of patients attempting to potentiate the effects of methadone or buprenorphine or to get high from the buprenorphine/gabapentin combination [9, 24, 25]. A recent systematic review concluded that those most at risk of GP addiction were patients with current or past SUDs, particularly opioid and multi-drug users, and that the risk of overdose lethality increases with multiple agent exposures, particularly those involving opioids and sedatives [17]. Potential mechanisms include pharmacodynamic (i.e., additive respiratory depression of opioids and gabapentin) and pharmacokinetic (i.e., increased gabapentin absorption in the upper small intestine after slowed motility from opioid ingestion) factors [26]. In the GP misuse/abuse cases in ToxIC, coma/CNS (central nervous system) depression affects approximately half or more of both the single and multiple agent cases, regardless of coingestant category, and respiratory depression is seen in approximately one-third or more of the multiple agent cases. Overall, the ToxIC data illustrate the range of potentially severe medical outcomes associated with GP misuse/abuse and provide complimentary insight on its burden on US healthcare facilities.
Limitations
The ToxIC Registry is a unique database that is able to provide an informed reported relationship between exposures and clinical outcomes due to its involvement of actively practicing medical toxicologists. There are, however, some limitations within the Registry. One of these is a possible bias towards more severe case presentations since cases are only entered if they are evaluated at the bedside by a medical toxicologist. Cases in which the patient did not present for care or in which a medical toxicology consult was not called would not have been reported. Therefore, the Registry likely represents a different population from other agencies such as Poison Control Centers. There may also be a bias in the types of cases reported based on regional variations in drug use and abuse and toxicological exposures. The scope of the ToxIC Registry includes medical toxicologists from multiple, diverse locations, but the entire country is not fully or equally represented. Larger, academic medical centers may be more likely to have practicing medical toxicologists and be more likely to be members of the Consortium. Therefore, some areas of the USA and more rural populations may be underrepresented.
Additionally, there may be a reporting bias towards more complicated or more interesting cases at the level of the individuals reporting cases to the Registry. We attempt to limit this as much as possible by way of a written agreement with all participating sites requiring that all cases that are seen be entered into the Registry. Data regarding substances of exposure, or species of envenomation, relies heavily on patient self-report which is limited by willingness to disclose as well as lack of knowledge about substances involved. This may be particularly true of illicit drugs, making the subset of patients with analytical confirmation of fentanyl analogue exposure particularly valuable. Lastly, improving data quality remains an issue for the registry. While member sites are instructed to complete all applicable data fields in each case, there are still a number of cases with likely incomplete data. This is best demonstrated the fatality cases where no notable signs/symptoms or treatments were reported. Efforts remain underway to improve reporting of data, in particular demographic and fatality data.
Conclusions
The ToxIC Registry continues to be the only database of its kind, poised to collect data on toxicological exposures that have each been evaluated by a bedside medical toxicologist. While limited to the scope of those participating medical toxicologists, this also enhances the ability of the Registry to correlate exposures to clinical findings. Quality improvement efforts have improved the consistent reporting of information, and these efforts continue to be a major focus of the Registry going forward. The most commonly reported exposures, types of encounters, toxidromes, and clinical signs and symptoms overall remain consistent and represent the current practice of participating medical toxicologists. While nearly three-quarters of cases required treatment intervention, fatalities were uncommon.
Electronic Supplementary Material
(DOCX 32 kb)
Acknowledgments
Toxicology Investigators Consortium (ToxIC) Study Group Collaborators:
Algren DA, Alwasiyah D, Beauchamp GA, Bentur Y, Berman AJ, Beuhler MC, Biary R, Bonney C, Boyle KL, Bruccoleri RC, Burns MM, Cahana A, Cannon RD, Caravati EM, Carey J, Chiba T, Christian M, Cook MD, Cumpston K, Davis A, Dribben W, Eisenga BH, Eldos Y, Falkowitz DM, Farkas A, Finkelstein Y, Fisher E, Froberg BA, Ganetsky M, Garlich F, Geib AJ, Gittinger M, Gorodetsky R, Greene SC, Hart K, Hendrickson RG, Hernandez S, Hodgman M, Hoyte C, Judge BS, Kao L, Katz KD, Kessler BD, King JD, Kirschner RI, Kostic M, Leikin JB, Levine M, Lowry JA, Lurie Y, Majlesi N, Malashock H, Manini AF, Marino R, McKay CA Jr., McKeever R, McKeown N, Meadors K, Moore E, Morgan B, Mullins ME, Nacca NE, Niruntarai S, Nogar JN, Olmedo R, Othong R, Riley BD, Rusyniak DE, Schimmel J, Schult R, Schwarz ES, Scoccimarro A, Seifert SA, Shafer S, Smith S, Smolinske SC, Spyres M, Steck A, Stripp M, Sullivan R, Theobald J, Troendle M, Vearrier, Warrick BJ, Watts D, Wills B, Zosel AE.
We also wish to thank study coordinators AB Adefeso, D Hopkins, Julie Licata, Tammy Phan, Andrea Ramirez, Mellissa VandenBerg, and Love Wilson.
Funding Sources
This study received funding from the NIH National Institute on Drug Abuse, 1R56DA38366 and 1R01DA037317-01, a data sharing contract with the US Food and Drug Administration and BTG International Inc. (North America).
Conflicts of Interest
None
Previous Presentation of Data
None
References
- 1.Wax PM, Kleinschmidt KC, Brent J. ACMT ToxIC Case Registry Investigators. The Toxicology Investigators Consortium (ToxIC) Registry. J Med Toxicol. 2011;7(4):259–265. doi: 10.1007/s13181-011-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brent J, Wax PM, Schwartz T, et al. The Toxicology Investigators Consortium Case Registry—the 2010 experience. J Med Toxicol. 2011;7(4):266–276. doi: 10.1007/s13181-011-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiegand TJ, Wax PM, Schwartz T, et al. The Toxicology Investigators Consortium Case Registry—the 2011 experience. J Med Toxicol. 2012;8(4):360–377. doi: 10.1007/s13181-012-0264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiegand T, Wax P, Smith E, Hart K, Brent J. The Toxicology Investigators Consortium Case Registry—the 2012 experience. J Med Toxicol. 2013;9(4):380–404. doi: 10.1007/s13181-013-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhyee SH, Farrugia L, Wiegand T, et al. The Toxicology Investigators Consortium Case Registry—the 2013 experience. J Med Toxicol. 2014;10(4):342–359. doi: 10.1007/s13181-014-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhyee SH, Farrugia L, Campleman SL, et al. The Toxicology Investigators Consortium Case Registry—the 2014 experience. J Med Toxicol. 2015;11(4):388–409. doi: 10.1007/s13181-015-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrugia L, Rhyee SH, Campleman SL, et al. The Toxicology Investigators Consortium Case Registry—the 2015 experience. J Med Toxicol. 2016;12(3):224–247. doi: 10.1007/s13181-016-0580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrugia L, Rhyee SH, Calello DP, et al. The Toxicology Investigators Consortium Case Registry—the 2016 experience. J Med Toxicol. 2017;13(3):203–226. doi: 10.1007/s13181-017-0627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gummin DD, Mowry JB, Spyker DA, Brooks DE, Fraser MO, Banner W. 2016 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th annual report. Clin Toxicol (Phila) 2017;55(10):1072–1252. doi: 10.1080/15563650.2017.1388087. [DOI] [PubMed] [Google Scholar]
- 10.Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. 2015 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 33rd annual report. Clin Toxicol (Phila). 2016;54(10):924–1109. doi: 10.1080/15563650.2016.1245421. [DOI] [PubMed] [Google Scholar]
- 11.Mowry JB, Spyker DA, Brooks DE, McMillan N, Schauben JL. 2014 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd annual report. Clin Toxicol (Phila) 2015;53(10):962–1146. doi: 10.3109/15563650.2015.1102927. [DOI] [PubMed] [Google Scholar]
- 12.Center for Behavioral Health Statistics and Quality. 2016 national survey on drug use and health: detailed tables. Substance Abuse and Mental Health Services Administration, Rockville, MD 2017.
- 13.Ouellette L, Cearley M, Judge B, Riley B, Jones J. Cooking with cannabis: the rapid spread of (mis)information on YouTube. Am J Emerg Med 2017. [DOI] [PubMed]
- 14.Richards JR, Smith NE, Moulin AK. Unintentional cannabis ingestion in children: a systematic review. J Pediatr. 2017;190:142–152. doi: 10.1016/j.jpeds.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Vo KT, Horng H, Li K, Ho RY, Wu AHB, Lynch KL, Smollin CG. Cannabis intoxication case series: the dangers of edibles containing tetrahydrocannabinol. Ann Emerg Med. 2018;71:306–313. doi: 10.1016/j.annemergmed.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Sierzant C, Judge B. Cooking with cannabis: severe toxicity following the use of cannabutter. Clin Toxicol. 2016;54:708. [Google Scholar]
- 17.FDA (U.S. Food and Drug Administration). 2011. Neurontin® (gabapentin) capsules, Neurontin® (gabapentin) tablets, Neurontin® (gabapentin) oral solution. NDA 020235/S-036; NDA 020882/S-022; NDA 021129/S-022 FDA Approved Labeling Text dated 03/01/2011. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020235s036,020882s022,021129s022lbl.pdf; accessed 12 Feb 2018.
- 18.FDA. 2012. Highlights of prescribing information: Lyrica (pregabalin) capsules, CV Lyrica (pregabalin) oral solution, CV. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021446s028lbl.pdf; accessed 12 Feb 2018.
- 19.Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘Suspected Adverse Drug Reactions’ database. CNS Drugs. 2016;30(7):647–654. doi: 10.1007/s40263-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 20.Office for National Statistics [United Kingdom]. 2017. Death related to drug poisoning in England and Wales from 1993 onwards, by cause of death, sex, age and substances involved in the death. Latest release: Deaths related to drug poisoning in England and Wales: 2016 registrations, Released: 2 Aug 2017. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2016registrations; accessed 12 Feb 2018.
- 21.Buttram ME, Kurtz SP, Dart RC, Margolin ZR. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083–1086. doi: 10.1002/pds.4230. [DOI] [PubMed] [Google Scholar]
- 22.Bronstein AC, Spyker DA, Cantilena LR, Jr, Rumack BH, Dart RC. 2011 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol (Phila) 2012;50(10):911–1164. doi: 10.3109/15563650.2012.746424. [DOI] [PubMed] [Google Scholar]
- 23.Goodman CW, Brett AS. Gabapentin and Pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med. 2017;377(5):411–414. doi: 10.1056/NEJMp1704633. [DOI] [PubMed] [Google Scholar]
- 24.Johansen ME. Gabapentinoid use in the United States 2002 through 2015. JAMA Intern Med. 2018;178(2):292–294. doi: 10.1001/jamainternmed.2017.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487–488. doi: 10.1176/appi.ajp.2014.14101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e1002396. doi: 10.1371/journal.pmed.1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 32 kb)