Abstract
To assess the prognostic impact of pretreatment albumin-to-alkaline phosphatase ratio (AAPR) in patients with upper tract urothelial carcinoma (UTUC), the data of 692 patients, operated between 2003 and 2016 in our center, were retrospectively assessed. The threshold of AAPR was defined as 0.58 by using the receiver-operating curve analysis. Overall survival (OS), cancer-specific survival (CSS), and recurrence-free survival (RFS) were evaluated using the Kaplan-Meier method. And the univariate and multivariate Cox’s regression models were performed to identify independent prognostic predictors. The results showed that AAPR <0.58 was significantly related to higher pT stage and grade, concomitant variant histology, anemia and larger tumor size. Additionally, patients with a lower AAPR had an inferior survival outcomes than those with an AAPR ≥0.58 (all P < 0.001). Multivariate analysis suggested that the lower AAPR was also an independent risk factor for poor OS (HR 1.587, 95%CI: 1.185–2.126; P = 0.002), CSS (HR 1.746, 95%CI: 1.249–2.440; P = 0.001), and RFS (HR 1.337, 95%CI: 1.027–1.739; P = 0.031). Moreover, subgroup analysis demonstrated the lower AAPR was related to worse prognosis in high-grade UTUC patients; but in those with low-grade disease, no relationship between them was observed. In conclusion, our results found that the decreased AAPR was independently related to poor survival outcomes in UTUC patients. Using the AAPR for subclassification of high-grade UTUC seems to further identify a poor prognostic group and contribute to clinical decisions making.
Introduction
Upper tract urothelial carcinomas (UTUCs) accounts approximately 5% of urothelial carcinomas (UCs), including renal pelvicalyceal and ureteric urothelial carcinomas1. Due to the exposure of aristolochic acid in Chinese herbs and arsenic in water, the incidence of UTUC in Asian countries, especially in Taiwan district, was found to be much higher than that in western countries1. The data shows approximately 60% of UTUC are invasive at the time of diagnosis, and their prognosis are relatively poor1. At present, radical nephroureterectomy is still the standard care for treatment of patients with invasive, non-metastatic UTUC1. However, there remains a high risk of local or distant recurrence with nodal disease found in up to 30% of patients after RNU2. Thus recently, increasing number of people raised interests in the pre- and post-operative prognostic predictors. Tumor stage and grade are the best-established prognostic predictors in UTUC, however, most of the clinicopathological features can be accurately determined after surgical excision. Identifying preoperative prognostic markers could help stratify patients with worse outcomes or with a high risk of recurrence who may possibly benefit from neoadjuvant chemotherapy. At the moment, some blood-based markers have been established, including hemoglobin, albumin-globulin ratio, lactate dehydrogenase, neutrophil-to-lymphocyte ratio, white blood cell and albumin3–7. However, the most studies were conducted with small samples and their results were inconsistent. The albumin-to-alkaline phosphatase ratio (AAPR), which has recently been proved to be a significantly prognostic predictor for hepatocellular carcinoma and metastatic nasopharyngeal carcinoma, but it has not yet been studied in UTUC patients8,9.
Therefore, the present study focused on the prognostic role of preoperative AAPR in UTUC patients undergoing RNU in our center.
Material and Methods
Patients
The data of 780 patients diagnosed with UTUC between 2003 and 2016 were retrospectively gathered at our center. Only patients received RNU were included in this study, thus 19 patients who received conservative treatments before RNU were excluded. Thirty-six patients were excluded from the present study as a result of missing data. In addition, patients with the previous cystectomy for invasive bladder cancer (n = 8), patients underwent RNU plus radical cystectomy (n = 6) and patients with concomitant non-urothelial carcinomas (n = 10) and those with liver diseases that could affect ALB or ALP levels (n = 9) were also excluded; none patients received neo-adjuvant chemotherapy before surgery. (Fig. 1) The study was approved by the Ethics Committee of West China Hospital and the methods were carried out in accordance with the approved guidelines. For this type of study, informed consent is not required.
Figure 1.
The patient selection flowchart.
Methods
The methods have been fully described in our previous papers10,11. Simply, all RNU specimens were respectively evaluated by two pathologists according to standard procedures. Baseline clinicopathological features and laboratory assessments within 30 days before RNU including hemoglobin, ALB and ALP, were obtained from the hospital database. The receiver-operating characteristic (ROC) curve and Youden index (Youden index = sensitivity + specificity-1) were applied to select the cut-off value of AAPR12. The AAPR of 0.58 was chosen as the threshold value as it had the maximum Youden index value.
Postoperative surveillance was performed according to the recommendation of EAU guideline1. In simple term, physical examination, blood laboratory tests and chest radiography were performed at every visiting; cystoscopy and urinary cytology were done at 3 month, and then annually for at least 5 years. Chest/abdomen CT/MRI were performed every 6 month for 2 years, and then annually to detect any postoperative recurrence or metastasis. If necessary, other tests such as bone scan can be applied.
Statistical Analysis
The Pearson’s chi-squared test and Student’s t-test were utilized to assess the categorical variables and continuous variables, respectively. The relationship between AAPR and clinicopathological features were analyzed by logistic regression analyses. The time-to-event analyses were performed using Kaplan–Meier method, and the log-rank test was applied to compare distributions. To identify the independent prognostic factors, univariate and multivariate Cox regression analyses were performed. Hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) and two-side P values were reported. All variables that had a P value < 0.1 in univariate analysis were included in the multivariate model. A P <0.05 was considered to indicate statistical significance. The software SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was applied for all statistical analyses in this study.
Result
A total of 692 participants were finally included in the present study with a mean age of 65.8 ± 11.4 years old. The baseline characteristics of the cohort were shown in Table 1. Among them, 460 patients underwent open RNU and 232 patients had laparoscopic RNU. Only 3.4% (n = 23) of patients had the chronic kidney disease (CKD) stage >3. 22 (3.2%) patients had a history of bladder cancer and 74 (10.7%) patients were found with a concomitant carcinoma in the bladder. The patients were divided into two groups according to their AAPR value < or ≥0.58 (AAPR <0.58, n = 443 and AAPR ≥0.58, n = 249). There was no difference between two groups in age, gender, tumor side and location, surgical approaches, renal function, multifocaity and adjuvant therapy. While, the logistic regression analyses found that lower AAPR was associated with advanced tumor stage (P < 0.001; RR = 1.778 for T3 and RR = 2.941 for T4) and high tumor grade (P = 0.010; RR = 1.574), concomitant variant histology (CVH) (P = 0.013; RR = 1.634) and anemia (P = 0.001; RR = 1.707) as well as larger tumor size (P = 0.020; RR = 1.476).
Table 1.
The relationship between AAPR and clinicalpathological parameters in the present cohort (n = 692).
| Variables | Total (n = 692) | AAPR <0.58 (n = 443, 64.0%) | AAPR ≥0.58 (n = 249, 36.0%) | P |
|---|---|---|---|---|
| Gender (Male vs Female) | 398/294 | 246/197 | 152/97 | 0.173 |
| Age (>67 vs ≤67 years) | 341/351 | 211/130 | 130/119 | 0.268 |
| Smoking (Yes vs No) | 197/495 | 120/323 | 77/172 | 0.293 |
| Tumor side (Right vs Left) | 336/356 | 206/237 | 130/119 | 0.155 |
| Surgical approach | 0.210 | |||
| Open RNU | 460(66.5) | 302(68.2) | 158(63.5) | |
| Laparoscopic RNU | 232(33.5) | 141(31.8) | 91(36.5) | |
| CKD stage | 0.748 | |||
| CKD 1 | 109(15.8) | 74(16.7) | 35(14.1) | |
| CKD 2 | 304(43.9) | 191(43.1) | 113(45.4) | |
| CKD 3 | 256(37.0) | 162(36.6) | 94(37.8) | |
| CKD4-5 | 23(3.4) | 16(3.6) | 7(2.8) | |
| Hydronephrosis (Yes vs No) | 427/265 | 276/167 | 151/98 | 0.684 |
| Anemia (Yes vs No) | 278/414 | 198/245 | 80/169 | 0.001 |
| Tumor location | 0.488 | |||
| Pelvicalyceal | 372(53.8) | 241(54.4) | 131(52.6) | |
| Ureteric | 199(28.8) | 121(27.3) | 78(31.3) | |
| Both | 121(17.5) | 81(18.3) | 40(16.1) | |
| Tumor stage, n (%) | <0.001 | |||
| Tis, Ta, T1 | 211(30.5) | 116(26.2) | 95(38.2) | |
| T2 | 139(20.1) | 83(18.7) | 56(22.5) | |
| T3 | 241(34.8) | 165(37.2) | 76(30.5) | |
| T4 | 101(14.6) | 79(17.8) | 22(8.8) | |
| Tumor grade, n (%) | 0.011 | |||
| Low | 180(26.0) | 101(22.8) | 79(31.7) | |
| High | 512(74.0) | 342(77.2) | 170(68.3) | |
| Lymph node status, n (%) | 0.008 | |||
| pN0 | 84(12.1) | 56(12.6) | 28(11.2) | |
| pNx | 541(78.2) | 333(75.2) | 208(83.5) | |
| pN+ | 67(9.7) | 54(12.2) | 13(5.2) | |
| Lymph node resection, n (%) | 151(21.8) | 110(24.8) | 41(16.4) | 0.011 |
| LVI (Positive vs Negative) | 104/588 | 68/375 | 36/213 | 0.825 |
| Tumor size (>3 vs ≤3 cm) | 469/223 | 314/129 | 155/94 | 0.022 |
| Surgical margin status (Positive vs Negative) | 54/638 | 39/404 | 15/234 | 0.237 |
| Multifocality (Present vs Absent) | 113/579 | 65/378 | 48/201 | 0.133 |
| CVH (With vs Without) | 159/533 | 115/328 | 44/205 | 0.014 |
| Bladder cancer status, n (%) | 0.126 | |||
| No | 596(86.1) | 385(86.9) | 211(84.7) | |
| Previous | 22(3.2) | 17(3.8) | 5(2.0) | |
| Concomitant | 74(10.7) | 41(9.3) | 33(13.3) | |
| Adjuvant therapy (Yes vs No) | 285/407 | 171/272 | 114/135 | 0.077 |
| Albumin (Mean ± SD) | 39.82 ± 4.82 | 39.14 ± 4.76 | 41.06 ± 4.68 | <0.001 |
| ALP (Mean ± SD) | 81.05 ± 27.89 | 92.71 ± 27.5 | 60.30 ± 12.07 | <0.001 |
*Note: AAPR: Albumin-to-Alkaline Phosphatase Ratio; CVH, concomitant variant histology; LVI, lymphovascular invasion; RNU, radical nephroureterectomy.
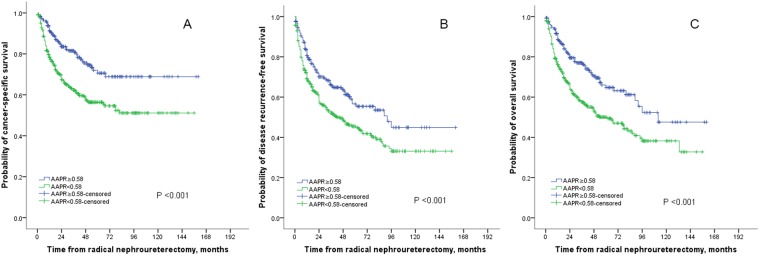
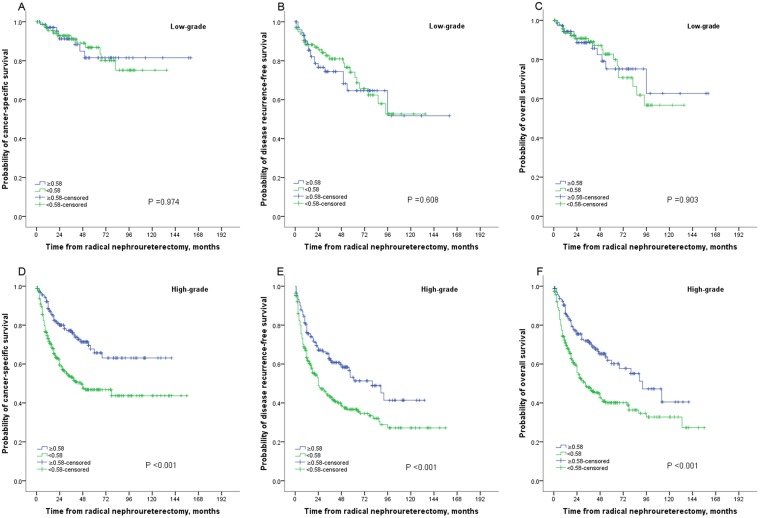
The median follow-up duration was 42 (IQR: 20–75) months. At the last follow-up, 249 patients died of all-causes and 199 patients died of UTUC. The 5-year overall survival (OS), cancer-specific survival (CSS), and disease recurrence-free survival (RFS) were 50.0%, 56.3%, 45.4% in patients with AAPR <0.58, and 64.8%, 70.6%, and 56.6%, respectively, in their counterparts. Kaplan–Meier curves showed that AAPR <0.58 was significantly associated with worse OS, CSS and RFS (all P < 0.001; see Fig. 2A–C). In addition, Kaplan–Meier curves suggested that AAPR <0.58 was also significantly related to higher mortality in subgroup with high-grade UTUC; however, in patients with low-grade disease, there was no difference between two groups (Fig. 3A–F). ROC curves found that AAPR had the higher AUC (area under curve) values for OS (AAPR 0.577, ALB 0.519, ALP 0.548), CSS (AAPR 0.583, ALB 0.520, ALP 0.570), and RFS (AAPR 0.557, ALB 0.502, ALP 0.540).
Figure 2.
Kaplan–Meier curves for CSS (A), RFS (B) and OS (C) stratified according to APPR value in patients undergoing RNU of UTUC.
Figure 3.
Kaplan–Meier curves for survival outcomes stratified according to APPR value in patients with low- or high-grade disease.
Univariate analysis found the AAPR <0.58 was associated with significantly inferior OS (HR 1.823, 95%CI: 1.374–2.419; P < 0.001), CSS (HR 2.026, 95%CI: 1.464–2.803; P < 0.001) and RFS (HR 1.550, 95%CI: 1.202–1.999; P = 0.001) (Table 2). Also, the pT stage, tumor grade, lymph node metastasis, lymphovascular invasion, CVH, tumor size, positive surgical margin, and anemia were significantly related to survival outcomes. After adjustment for preoperative clinical features, the results of multivariate analysis showed that the pretreatment AAPR was an independent risk factor for OS (HR 1.587, 95%CI: 1.185–2.126; P = 0.002), CSS (HR 1.746, 95%CI: 1.249–2.440; P = 0.001), and RFS (HR 1.337, 95%CI: 1.027–1.739; P = 0.031). Other factors, including pT stage, tumor grade, lymph node metastasis, CVH, tumor size, and anemia were also determined to be independent prognostic predictors in multivariate analysis (Table 3).
Table 2.
Univariable regression analysis of clinicopathological parameters for the prediction of survival outcomes in UTUC patients.
| Variables | Overall survival | Cancer-specific survival | Disease recurrence-free survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Age (>67 years vs ≤67 years) | 1.034 | 0.807–1.325 | 0.789 | 0.944 | 0.716–1.245 | 0.683 | 0.945 | 0.750–1.190 | 0.629 |
| Sex (Male vs Female) | 0.891 | 0.695–1.143 | 0.364 | 0.828 | 0.628–1.093 | 0.183 | 0.869 | 0.689–1.095 | 0.235 |
| Smoking (yes vs no) | 0.904 | 0.681–1.199 | 0.483 | 0.851 | 0.618–1.172 | 0.324 | 0.885 | 0.680–1.152 | 0.364 |
| Tumor site | 0.818 | 0.738 | 0.667 | ||||||
| Pelvicalyceal | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | |||
| Ureteric | 0.941 | 0.702–1.260 | 0.683 | 1.008 | 0.731–1.392 | 0.959 | 0.945 | 0.720–1.241 | 0.686 |
| Both | 1.064 | 0.753–1.503 | 0.724 | 1.157 | 0.793–1.688 | 0.448 | 1.112 | 0.809–1.529 | 0.512 |
| Tumour stage | <0.001 | <0.001 | <0.001 | ||||||
| Tis, Ta, T1 | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | |||
| T2 vs Tis, Ta, T1 | 1.659 | 1.041–2.644 | 0.033 | 1.716 | 0.990–2.974 | 0.054 | 1.518 | 1.008–2.284 | 0.046 |
| T3 vs Tis, Ta, T1 | 3.572 | 2.428–5.255 | <0.001 | 4.054 | 2.579–6.374 | <0.001 | 3.022 | 2.152–4.245 | <0.001 |
| T4 vs Tis, Ta, T1 | 8.725 | 5.788–13.156 | <0.001 | 10.558 | 6.581–16.939 | <0.001 | 7.451 | 5.142–10.798 | <0.001 |
| Tumor grade (high vs low) | 3.104 | 2.141–4.501 | <0.001 | 3.835 | 2.440–6.029 | <0.001 | 2.409 | 1.757–3.303 | <0.001 |
| Lymph node status | <0.001 | <0.001 | <0.001 | ||||||
| pN0 | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | |||
| pNx vs pN0 | 1.484 | 0.960–2.295 | 0.076 | 1.498 | 0.904–2.482 | 0.117 | 1.496 | 1.001–2.237 | 0.050 |
| pN + vs pN0 | 5.318 | 3.222–8.776 | <0.001 | 6.055 | 3.452–10.621 | <0.001 | 5.512 | 3.453–8.797 | <0.001 |
| LVI (positive vs negative) | 2.628 | 1.970–3.507 | <0.001 | 2.843 | 2.074–3.896 | <0.001 | 2.309 | 1.750–3.047 | <0.001 |
| CVH (With vs Without) | 2.176 | 1.671–2.833 | <0.001 | 2.375 | 1.775–3.178 | <0.001 | 1.997 | 1.554–2.565 | <0.001 |
| CKD4–5 vs CKD1–3 | 1.727 | 0.987–3.019 | 0.055 | 1.447 | 0.741–2.824 | 0.279 | 1.417 | 0.812–2.472 | 0.219 |
| Size (>3 cm vs ≤3 cm) | 2.008 | 1.502–2.685 | <0.001 | 2.054 | 1.481–2.850 | <0.001 | 1.871 | 1.435–2.441 | <0.001 |
| Margin status (positive vs negative) | 2.251 | 1.536–3.299 | <0.001 | 2.426 | 1.606–3.665 | <0.001 | 1.979 | 1.362–2.875 | <0.001 |
| Multifocality (present vs absent) | 0.929 | 0.658–1.313 | 0.677 | 1.008 | 0.694–1.464 | 0.967 | 0.957 | 0.696–1.316 | 0.789 |
| Surgical approach (laparoscopic vs open) | 0.726 | 0.540–0.974 | 0.033 | 0.675 | 0.487–0.936 | 0.019 | 0.877 | 0.676–1.138 | 0.323 |
| Tumor side (right vs left) | 1.062 | 0.829–1.360 | 0.635 | 1.098 | 0.833–1.448 | 0.507 | 1.069 | 0.848–1.346 | 0.573 |
| AAPR (<0.58 VS ≥0.58) | 1.823 | 1.374–2.419 | <0.001 | 2.026 | 1.464–2.803 | <0.001 | 1.550 | 1.202–1.999 | 0.001 |
| Anemia (Yes vs No) | 2.012 | 1.570–2.578 | <0.001 | 2.067 | 1.567–2.728 | <0.001 | 1.684 | 1.336–2.122 | <0.001 |
| Adjuvant therapy (yes vs no) | 0.873 | 0.680–1.120 | 0.286 | 0.931 | 0.706–1.230 | 0.616 | 1.111 | 0.882–1.400 | 0.371 |
*Note: AAPR: Albumin-to-Alkaline Phosphatase Ratio; CVH, concomitant variant histology; LVI, lymphovascular invasion; RNU, radical nephroureterectomy.
Table 3.
Multivariable Cox regression analyses of survival outcomes in patients with urinary tract urothelial carcinoma.
| Variables | Overall survival | Cancer-specific survival | Disease recurrence-free survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Tumour stage | <0.001 | <0.001 | <0.001 | ||||||
| Tis, Ta, T1 | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | |||
| T2 vs Tis, Ta, T1 | 1.480 | 0.921–2.378 | 0.105 | 1.504 | 0.857–2.637 | 0.155 | 1.384 | 0.914–2.098 | 0.125 |
| T3 vs Tis, Ta, T1 | 2.197 | 1.446–3.338 | <0.001 | 2.399 | 1.467–3.921 | <0.001 | 2.163 | 1.498–3.122 | <0.001 |
| T4 vs Tis, Ta, T1 | 3.429 | 2.080–5.653 | <0.001 | 3.798 | 2.133–6.763 | <0.001 | 3.560 | 2.251–5.630 | <0.001 |
| Tumor grade (high vs low) | 2.065 | 1.385–3.080 | <0.001 | 2.410 | 1.481–3.923 | <0.001 | 1.682 | 1.198–2.362 | 0.003 |
| Lymph node status | 0.001 | 0.002 | <0.001 | ||||||
| pN0 | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | |||
| pNx vs pN0 | 1.939 | 1.241–3.029 | 0.004 | 1.973 | 1.179–3.301 | 0.010 | 1.907 | 1.267–2.871 | 0.002 |
| pN+ vs pN0 | 2.653 | 1.556–4.524 | <0.001 | 2.869 | 1.582–5.203 | 0.001 | 3.017 | 1.827–4.981 | <0.001 |
| LVI (positive vs negative) | 1.271 | 0.918–1.760 | 0.148 | 1.286 | 0.902–1.833 | 0.165 | 1.072 | 0.780–1.473 | 0.669 |
| CVH (With vs Without) | 1.483 | 1.124–1.958 | 0.005 | 1.561 | 1.150–2.121 | 0.004 | 1.350 | 1.038–1.757 | 0.025 |
| CKD4–5 vs CKD1-3 | 1.138 | 0.639–2.025 | 0.661 | 0.951 | 0.479–1.888 | 0.885 | 0.989 | 0.557–1.756 | 0.970 |
| Size (>3 cm vs ≤3 cm) | 1.578 | 1.159–2.149 | 0.004 | 1.496 | 1.057–2.117 | 0.023 | 1.491 | 1.128–1.972 | 0.005 |
| Margin status (positive vs negative) | 1.200 | 0.805–1.791 | 0.371 | 1.230 | 0.799–1.894 | 0.347 | 1.099 | 0.744–1.624 | 0.635 |
| Surgical approach (laparoscopic vs open) | 0.917 | 0.678–1.240 | 0.574 | 0.884 | 0.631–1.238 | 0.473 | — | ||
| AAPR (<0.58 VS ≥0.58) | 1.587 | 1.185–2.126 | 0.002 | 1.746 | 1.249–2.440 | 0.001 | 1.337 | 1.027–1.739 | 0.031 |
| Anemia (Yes vs No) | 1.588 | 1.222–2.126 | 0.001 | 1.575 | 1.177–2.108 | 0.002 | 1.339 | 1.048–1.712 | 0.020 |
*Note: AAPR: Albumin-to-Alkaline Phosphatase Ratio; CVH, concomitant variant histology; LVI, lymphovascular invasion; RNU, radical nephroureterectomy.
Subgroup analyses found that the decreased AAPR was also proved to be independently associated with poor OS (HR 1.726, 95%CI: 1.252–2.380; P = 0.001), CSS (HR 1.906, 95%CI: 1.324–2.743; P = 0.001) and RFS (HR 1.509, 95%CI: 1.120–2.033; P = 0.007) in patients with high-grade UTUC; however in patients with low-grade disease, AAPR had a neutral role in OS (HR 0.905, 95%CI: 0.408–2.005, P = 0.805), CSS (HR 0.674, 95%CI: 0.240–1.895, P = 0.455), and RFS (HR 0.727, 95%CI:0.383–1.381, P = 0.273) (Supplementary Tables 1 and 2).
Discussion
The prognosis of patients with UTUC remained unsatisfactory. Previous evidence had found that most of the patients died from UTUC within 1 year of disease recurrence and the probability of surviving 2 years after disease recurrence was only 20%. Thus, to stratify the patients with high risk of poor prognosis was important. However, to accurately stage patients before definitive therapy was unavailable, which limited the management of UTUC. A few retrospective studies had found that patients with UTUC could benefit from the usage of neoadjuvant chemotherapy13,14. Preoperative imaging could not accurately stage UTUC, even preoperatively endoscopic biopsy is rarely sufficient for the determination of the degree of microscopic invasion. Thus, preoperative prognostic markers would be valuable tools to improve the accuracy of risk stratification models.
Anthony et al. reported that AAPR was a powerful prognostic predictor with the highest C-index among liver biochemical parameters8. Then Nie et al. reported that AAPR was independently related to the prognosis of patients with metastatic NPC, and it has better predict ability than ALB or ALP alone9. In our study, we also found the AAPR had the higher AUC compared with ALB or ALP in UTUC patients, indicating that the AAPR better predicted their survival outcomes. Meanwhile, lower AAPR was related to advanced pT stage and high tumor grade, CVH and larger tumor size. Multivariate analysis found lower AAPR was independently associated with poor OS, CSS and RFS in patients with UTUC undergoing RNU.
The mechanism of AAPR in human malignancies including UTUC remains unclear. Increasing evidence shows that the presence of nutritional deficiencies and systematic inflammatory response might play an important role in the development and progress of human cancers and also be associated with inferior prognosis in patients undergoing resection for solid tumors3,15. ALB is a stable and flexible serum protein and modulates the systemic and organ inflammatory reaction, as well as exert antioxidant effects against carcinogens16. Also, low ALB reflected nutrient deficiency exits which could decrease immune function and lead to poor anti-cancer response17. Recently, evidence has found that ALB was a useful prognostic predictor in various malignancies such as hepatocellular carcinoma (HCC), renal carcinoma, prostate cancer, and colorectal cancer as well as UTUC3,4,18–20. ALP is a hydrolase enzyme found primarily in the liver, bile duct, kidney, bone, and placenta. Serum ALP will increase during some pathological conditions, such as HCC, kidney disease and bone metastasis21,22. In addition, ALP has been found to be an independent risk factor for survival outcomes in patients with HCC and nasopharyngeal carcinoma (NPC)23,24. Although Kluth et al. did not find the prognostic value of ALP in patients with UTUC, Sheth et al. reported that ALP ≥116 U/l was related to multiple adverse clinicopathological parameters and poor RFS in high-grade UTUC4,5.
Interestingly, our results showed that the decreased AAPR contributed to worse survival outcomes in high-grade UTUC; but in terms of low-grade disease, there was no relationship between them. To date, according to the available studies, only few studies had discussed the roles of ALP or ALB in different subgroups in various malignancies, and also their results were inconsistent. Some found they could be used as prognostic markers in patients with non-metastatic diseases, while others recommended they was significantly useful only in those with advanced diseases or with metastatic diseases4,25. Some researchers suggested the dynamic change of ALP or ALB might be related to bone or liver metastasis or metastatic tendency, which may partly explain why low AAPR could contribute to poor prognosis in high-grade UTUC with high metastatic and aggressive ability. Specifically, due to the small sample of low-grade disease and relatively short follow-up duration, our study did not found any significant prognostic predictors in this population. Therefore, the conclusions from patients with low-grade UTUC should be applied with caution and should be validated by future studies with larger samples and longer follow-up time.
Limitations of this study should be described. First, our cohort was retrospectively included which may cause a selection bias. Also, the adjuvant chemotherapies and radiotherapies were variously administered, which may affect the patients‘ outcomes differently. As the additional benefits of the lymphadenectomy pattern for UTUC were not clear to date, thus lymphadenectomy was not routinely performed which may affect the survival outcomes. Moreover, other potential prognostic inflammatory markers such as C-reactive protein, fibrinogen, and cytokines were not available due to limited data which may add additional prognostic value to the model. Despite limitations above, the present study was the first one with a large sample to assess the prognostic impact of AAPR on patients with UTUC after RNU.
Conclusions
The AAPR was a novel derived indicator from routinely available tests. In the present cohort, we demonstrated that lower AAPR was a superior indicator than ALB and ALP, and was independently associated with inferior survival outcomes in UTUC patients. Using the AAPR for subclassification of high-grade UTUC seems to further identify a poor prognostic group and contribute to clinical decisions making. Future prospective studies should be performed to validate its prognostic role.
Electronic supplementary material
Acknowledgements
This study was supported by the National key research and development program of China (Grant No. SQ2017YFSF090096), the Prostate Cancer Foundation Young Investigator Award 2013, the National Natural Science Foundation of China (Grant No. 81300627, 81370855, 81702536, 81770756), Fundings from Science and Technology Department of Sichuan Province (Grant No. 2014JY0219 and 2017HH0063) and Young Investigator Award of Sichuan University 2017.
Author Contributions
Q.W. and L.Y. had the idea for and designed this research. P.T., N.X., J.Z.A. and H.X. collected data, H.X. reviewed all specimens and recorded their pathological features. P.T. and N.X. performed the statistical analyses. L.Y. and L.R.L. contributed to data interpretation. P.T. drafted the paper and N.X. prepared figures. All other authors critically reviewed the article. Q.W. and L.Y. approved the final version.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ping Tan, Nan Xie and Jianzhong Ai contributed equally to this work.
Contributor Information
Lu Yang, Email: wycleflue@163.com.
Qiang Wei, Email: weiqiang933@126.com.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29833-5.
References
- 1.Roupret M, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur Urol. 2015;68:868–879. doi: 10.1016/j.eururo.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Zigeuner R, Pummer K. Urothelial carcinoma of the upper urinary tract: surgical approach and prognostic factors. Eur Urol. 2008;53:720–731. doi: 10.1016/j.eururo.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Ku JH, Kim M, Choi WS, Kwak C, Kim HH. Preoperative serum albumin as a prognostic factor in patients with upper urinary tract urothelial carcinoma. Int Braz J Urol. 2014;40:753–762. doi: 10.1590/S1677-5538.IBJU.2014.06.06. [DOI] [PubMed] [Google Scholar]
- 4.Sheth KR, et al. Prognostic serum markers in patients with high-grade upper tract urothelial carcinoma. Urol Oncol. 2016;34:418.e419–418.e416. doi: 10.1016/j.urolonc.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Kluth LA, et al. Predictors of survival in patients with disease recurrence after radical nephroureterectomy. BJU Int. 2014;113:911–917. doi: 10.1111/bju.12369. [DOI] [PubMed] [Google Scholar]
- 6.Marchioni M, et al. High Neutrophil-to-lymphocyte Ratio as Prognostic Factor in Patients Affected by Upper Tract Urothelial Cancer: A Systematic Review and Meta-analysis. Clin Genitourin Cancer. 2017;15:343–349.e341. doi: 10.1016/j.clgc.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Cheng YC, et al. The Prognostic Significance of Inflammation-Associated Blood Cell Markers in Patients with Upper Tract Urothelial Carcinoma. Ann Surg Oncol. 2016;23:343–351. doi: 10.1245/s10434-015-4781-z. [DOI] [PubMed] [Google Scholar]
- 8.Chan AW, et al. Albumin-to-alkaline phosphatase ratio: a novel prognostic index for hepatocellular carcinoma. Dis Markers. 2015;2015:564057. doi: 10.1155/2015/564057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nie M, et al. Albumin-to-Alkaline Phosphatase Ratio: A Novel Prognostic Index of Overall Survival in Cisplatin-based Chemotherapy-treated Patients with Metastatic Nasopharyngeal Carcinoma. J Cancer. 2017;8:809–815. doi: 10.7150/jca.17536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibing Y, et al. Effect of concomitant variant histology on the prognosis of patients with upper urinary tract urothelial carcinoma after radical nephroureterectomy. Urol Oncol. 2015;33:204.e209–216. doi: 10.1016/j.urolonc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Tan, P. et al. The prognostic value of preoperative neutrophil-to-lymphocyte ratio in patients with upper tract urothelial carcinoma. Clin Chim Acta, 10.1016/j.cca.2018.06.019 (2018). [DOI] [PubMed]
- 12.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 13.Porten S, et al. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer. 2014;120:1794–1799. doi: 10.1002/cncr.28655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youssef RF, et al. Upper urinary tract urothelial carcinoma with loco-regional nodal metastases: insights from the Upper Tract Urothelial Carcinoma Collaboration. BJU Int. 2011;108:1286–1291. doi: 10.1111/j.1464-410X.2011.10075.x. [DOI] [PubMed] [Google Scholar]
- 15.Lamb GW, Aitchison M, Ramsey S, Housley SL, McMillan DC. Clinical utility of the Glasgow Prognostic Score in patients undergoing curative nephrectomy for renal clear cell cancer: basis of new prognostic scoring systems. Br J Cancer. 2012;106:279–283. doi: 10.1038/bjc.2011.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arroyo V, Garcia-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396–407. doi: 10.1016/j.jhep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Ahluwalia N. Aging, nutrition and immune function. J Nutr Health Aging. 2004;8:2–6. [PubMed] [Google Scholar]
- 18.Chen Z, et al. Prognostic significance of preoperative C-reactive protein: albumin ratio in patients with clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2015;8:14893–14900. [PMC free article] [PubMed] [Google Scholar]
- 19.Chi KN, et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann Oncol. 2016;27:454–460. doi: 10.1093/annonc/mdv594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiraoka A, et al. Proposed New Sub-Grouping for Intermediate-Stage Hepatocellular Carcinoma Using Albumin-Bilirubin Grade. Oncology. 2016;91:153–161. doi: 10.1159/000447061. [DOI] [PubMed] [Google Scholar]
- 21.Damera S, et al. Serum alkaline phosphatase levels associate with elevated serum C-reactive protein in chronic kidney disease. Kidney Int. 2011;79:228–233. doi: 10.1038/ki.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y, Lu Q, Chen HL. Diagnosis of primary liver cancer using lectin affinity chromatography of serum alkaline phosphatase. J Exp Clin Cancer Res. 1997;16:75–80. [PubMed] [Google Scholar]
- 23.Yu MC, et al. Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence? J Gastrointest Surg. 2011;15:1440–1449. doi: 10.1007/s11605-011-1537-3. [DOI] [PubMed] [Google Scholar]
- 24.Jin Y, Yuan MQ, Chen JQ, Zhang YP. Serum alkaline phosphatase predicts survival outcomes in patients with skeletal metastatic nasopharyngeal carcinoma. Clinics (Sao Paulo) 2015;70:264–272. doi: 10.6061/clinics/2015(04)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SE, Byun SS, Han JH, Han BK, Hong SK. Prognostic significance of common preoperative laboratory variables in clear cell renal cell carcinoma. BJU Int. 2006;98:1228–1232. doi: 10.1111/j.1464-410X.2006.06437.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.