Abstract
Beginning with the larval stages, marine pufferfish such as Takifugu niphobles contain tetrodotoxin (TTX), an extremely potent neurotoxin. Although highly concentrated TTX has been detected in adults and juveniles of these fish, the source of the toxin has remained unclear. Here we show that TTX in the flatworm Planocera multitentaculata contributes to the toxification of the pufferfish throughout the life cycle of the flatworm. A species-specific PCR method was developed for the flatworm, and the specific DNA fragment was detected in the digesta of wild pufferfish adults. Predation experiments showed that flatworm larvae were eaten by the pufferfish juveniles, and that the two-day postprandial TTX content in these pufferfish was 20–50 μg/g. Predation experiments additionally showed flatworm adults were also eaten by pufferfish young, and after two days of feeding, TTX accumulated in the skin, liver and intestine of the pufferfish.
Introduction
Tetrodotoxin (TTX), named after the Piscean order Tetradontiformes, is also known as pufferfish toxin after one of the main group of fishes in the order. TTX is a non-peptidic potent neurotoxin that specifically binds to voltage-gated sodium channels on excitable membranes of muscle and nerve tissues1,2. Apart from pufferfish, it has been found in various taxonomic groups of animals, including amphibians (e.g., California newt Taricha torosa3; Costa Rica frog Atelopus spp.4), fishes (e.g., goby Yongeichthys criniger5), cephalopods (e.g., blue-ringed octopus Hapalochlaena maculosa6), gastropods (e.g., the Japanese ivory shell Babylonia japonica7; the opisthobranch Pleurobranchaea maculata8,9), crustaceans (e.g., the xanthid crab Atergatis floridus10), starfishes (e.g., Astropecten spp.11,12), flatworms (e.g., Planocera spp.13–16; Stylochoplana sp.17) and ribbonworms (e.g., Cephalothrix sp.14,18). Furthermore, TTX production has been observed in several species of bacteria that are symbiotic with pufferfish and are obtained from their prey19–22.
It has generally been understood that the Takifugu pufferfish species accumulate TTX via the food web, consisting of several steps, starting with TTX producing marine bacteria22,23. This speculation has been supported by several studies that have shown that non-toxic pufferfish are obtained when they are artificially cultured after hatching and fed non-toxic diets, and that these cultured non-toxic pufferfish become toxic when administered TTX orally24–28. However, tracing the toxification via the food chain exclusively to marine bacteria is unlikely to account for the amount of TTX in the pufferfish body, as bacteria produce minute amounts of toxin21,29–32. However, TTX levels produced by bacterial cultures under potentially non-optimum conditions might be significantly less than those synthesized by bacteria colonizing pufferfish organs. Indeed, the issue remains unsettled, and the toxification process in pufferfish still remains unclear. Recently, our lab showed that the pufferfish Takifugu niphobles fed on the naturally TTX-laden eggs of another pufferfish Takifugu pardalis, suggesting that T. niphobles effectively increased its own toxicity by feeding on the toxic eggs from another toxic species33. That report also proposed that TTX is progressively concentrated in a “TTX loop” that includes TTX-bearing organisms at higher trophic levels in the food web33.
Other studies from our lab have shown that although the source of TTX is largely unidentified in wild-caught juveniles of the pufferfish T. niphobles, larval pufferfish do contain minute amounts of TTX provided by their mother34,35. We have also shown that the larvae of the planocerid flatworm Planocera multitentaculata possess highly concentrated TTX in the body, and eggs are laid May through July, coinciding with the spawning season of the pufferfish T. niphobles in rocky inshore waters36. Therefore, in the present study, we investigated the contribution of the planocerid flatworm to the toxification of the pufferfish T. niphobles throughout its life history.
Results
Toxicity of the wild pufferfish juveniles
The concentration and total amount of TTX in the juveniles from Oiso (obtained at three different time periods) and Katase are shown in Table 1. These values correspond to 3.16 ± 1.37 mouse unit (MU)/g (2.65 ± 2.16 MU/individual) in August, 2010 (n = 12); 3.32 ± 1.35 MU/g (4.57 ± 2.14 MU/individual) in August, 2011 (n = 9); and 21.68 ± 21.67 MU/g (2.50 ± 4.92 MU/individual) in July, 2015 (n = 21) for the juveniles obtained from Oiso, while for those from Katase (n = 24) in July, 2016 and 2017, the numbers shown translate to 54.75 ± 50.25 MU/g (11.70 ± 15.61 MU/individual) and 64.85 ± 38.21 MU/g (14.47 ± 12.60 MU/individual), respectively.
Table 1.
Toxicity (concentration) and amount of TTX in wild pufferfish Takifugu niphobles juveniles. Data are represented mean ± standard deviation.
| Date | Locality | No. of individuals | Total length (mm) | Body weight (g) | Toxicity (ng/g) | TTX amount (ng/ind.) |
|---|---|---|---|---|---|---|
| 2010 August | Oiso (35°18′N, 139°19′E) | 12 | 27.6 ± 6.2 | 0.8 ± 0.5 | 694 ± 301 | 583 ± 475 |
| 2011 August | Oiso (35°18′N, 139°19′E) | 9 | 34.1 ± 1.8 | 1.4 ± 0.2 | 731 ± 298 | 1006 ± 471 |
| 2015 July | Oiso (35°18′N, 139°19′E) | 21 | 19.8 ± 6.0 | 0.2 ± 0.2 | 4770 ± 4768 | 550 ± 1082 |
| 2016 July | Katase (35°18′N, 139°28′E) | 24 | 20.0 ± 2.4 | 0.2 ± 0.1 | 12044 ± 11054 | 2573 ± 3435 |
| 2017 July | Katase (35°18′N, 139°28′E) | 9 | 17.9 ± 5.8 | 0.2 ± 0.1 | 14267 ± 8406 | 3183 ± 2773 |
High-throughput sequencing
Next generation sequencing (NGS) analysis against mitochondrial cytochrome c oxidase subunit I (COI) showed that a sequence essentially identical to that of the flatworm P. multitentaculata was detected in the intestinal contents from the wild juvenile pufferfish T. niphobles captured from waters off Katase, Kanagawa, Japan in July 2016 (all of the three individuals analyzed in this study, Tables 2, S1 and S2) and 2017 (three of nine individuals analyzed in this study, Tables 3, S3 and S4). This sequence constituted 0.04–10.2% (all of three individuals), and 0.5–1.8% (three of nine individuals), of all the orthologous sequences from other TTX-bearing and non-toxic organisms found in the gut contents of T. niphobles, in 2016 and 2017, respectively. The nucleotide sequences of the partial COI gene obtained from the intestinal tract of T. niphobles are included in supplementary file “NGS_seq(2016).docx” and “NGS_seq(2017).docx”, and OTU ID are represented in Tables S2 and S4.
Table 2.
DNA sequences from the intestinal contents of the pufferfish Takifugu niphobles juveniles collected at Katase in July 2016.
| Organism | Acc. No. | Sequence identity (%) | Number of sequences from: | ||
|---|---|---|---|---|---|
| Pufferfish S1 | Pufferfish S2 | Pufferfish S3 | |||
| TTX-bearing organisms | |||||
| Flatworm Planocera multitentaculata | LC190986 | 99 | 9873 | 70 | 35 |
| Newt Cynops pyrrhogaster | EU880313 | 90 | 54 | 0 | 0 |
| Goby Yongeichthys criniger | KT894736 | 99 | 0 | 3 | 6 |
| Pufferfish Chelonodon patoca | KU692427 | 100 | 3 | 0 | 2 |
| Non-toxic organismsa | |||||
| Annelida, Oligochaeta | 408 | 79 | 65 | ||
| Annelida, Polychaeta | 171 | 392 | 1553 | ||
| Arthropoda, Arachnida | 2875 | 0 | 565 | ||
| Arthropoda, Crustacea | 81406 | 11104 | 21260 | ||
| Arthropoda, Crustacea, Amphipoda | 6 | 19416 | 68362 | ||
| Arthropoda, Crustacea, Copepoda | 853 | 70016 | 280 | ||
| Arthropoda, Diplopoda | 0 | 0 | 1 | ||
| Arthropoda, Insecta | 359 | 347 | 681 | ||
| Bacillariophyta, Thalassiosirales | 251 | 0 | 0 | ||
| Mollusca, Bivalvia | 0 | 5 | 0 | ||
| Mollusca, Gastropoda | 918 | 240 | 6 | ||
| Platyhelminthes, Polycladida | 0 | 0 | 47 | ||
| Unidentified sequence | 161 | 0 | 1 | ||
| Total number of sequences | 97338 | 101672 | 92864 | ||
| TTX amount (μg/individual) | 4.6 | 7.5 | 15.5 | ||
Table 3.
DNA sequences from the intestinal contents of the pufferfish Takifugu niphobles juveniles collected at Katase in July 2017.
| Organism | Acc. No. | Sequence identity (%) | Number of sequence from pufferfish individual: | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | |||
| TTX-bearing organisms | |||||||||||
| Flatworm Planocera multitentaculata | LC190986 | 100 | 0 | 0 | 0 | 936 | 0 | 272 | 833 | 0 | 0 |
| Ribbonworm Cephalothrix simula | GU726607 | 86 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Sea snail Rapana venosa | KP976378 | 100 | 0 | 0 | 0 | 101 | 0 | 0 | 2 | 0 | 0 |
| Non-toxic organismsa | |||||||||||
| Annelida, Oligochaeta | 2965 | 0 | 3503 | 5330 | 6434 | 16106 | 27615 | 14824 | 7891 | ||
| Annelida, Polychaeta | 0 | 2922 | 908 | 605 | 447 | 1992 | 2422 | 916 | 1408 | ||
| Arthropoda, Chelicerata | 0 | 0 | 33 | 0 | 0 | 86 | 0 | 0 | 0 | ||
| Arthropoda, Crustacea | 18026 | 17521 | 34623 | 47809 | 51895 | 35203 | 12205 | 31368 | 19178 | ||
| Arthropoda, Crustacea, Copepoda | 15506 | 791 | 5828 | 50 | 3 | 710 | 134 | 1445 | 21038 | ||
| Arthropoda, Insecta | 2070 | 0 | 835 | 373 | 132 | 0 | 263 | 178 | 644 | ||
| Mollusca, Bivalvia | 0 | 0 | 783 | 1536 | 2115 | 1418 | 126 | 39 | 268 | ||
| Mollusca, Gastropoda | 3204 | 0 | 0 | 317 | 69 | 367 | 3150 | 23 | 0 | ||
| Nematoda, Ascaridida | 0 | 4359 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Platyhelminthes, Polycladida | 0 | 0 | 38 | 0 | 0 | 2 | 0 | 0 | 0 | ||
| Platyhelminthes, Strigeidida | 0 | 164 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Platyhelminthes, Tricladida | 0 | 0 | 0 | 202 | 0 | 9 | 0 | 0 | 0 | ||
| Vertebrata, Teleostei | 0 | 105 | 0 | 67 | 18 | 1027 | 22 | 0 | 0 | ||
| Chlorophyta, Microthamniales | 0 | 0 | 0 | 22 | 0 | 0 | 0 | 0 | 0 | ||
| Fungi, Ascomycota | 0 | 0 | 0 | 34 | 0 | 0 | 0 | 0 | 0 | ||
| Fungi, Microbotryomycetes | 0 | 479 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Unidentified sequence | 594 | 1834 | 251 | 252 | 112 | 2 | 11 | 5 | 648 | ||
| Total number of sequences | 42365 | 28175 | 46802 | 57634 | 61225 | 57196 | 46783 | 48798 | 51075 | ||
| TTX amount (μg/individual) | 2.8 | 2.9 | 10 | 2.7 | 3.7 | 4.3 | 5.8 | 2.8 | 1.6 | ||
Planocerid-specific PCR and detection of planocerids from pufferfish gut contents
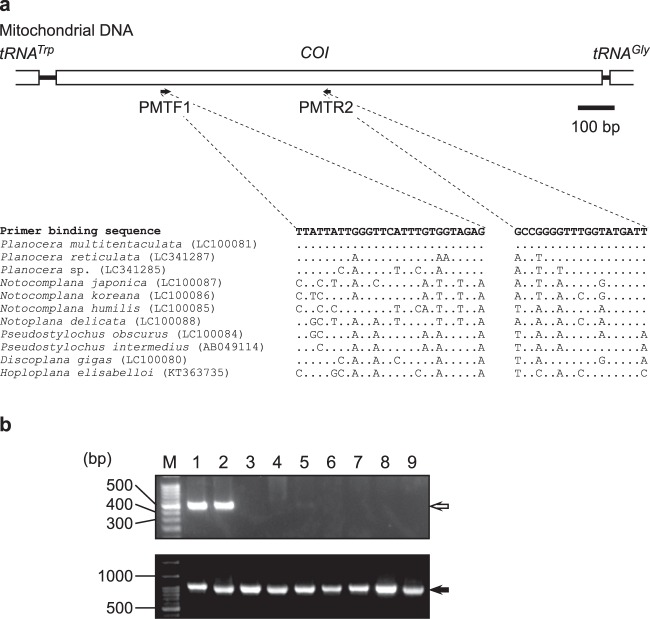
A PCR-based method with P. multitentaculata-specific primers was developed for detection of the flatworm from the intestinal contents of pufferfish: 28S ribosomal RNA (rRNA) gene amplified by PCR with universal primers from all flatworm samples, whereas DNA fragments encoding the mitochondrial COI gene were detected only in P. multitentaculata, with length 429 bp (Fig. 1). No fragment was observed in the related species, P. reticulata.
Figure 1.
Location of primers in the 28S rRNA gene from Planocera multitentaculata and various flatworm species. (a) A schematic of the 28S rRNA gene of P. multitentaculata and various flatworm species, and locations of oligonucleotides used as primers for PCR and DNA sequencing. Arrows represent the location and direction of primers. (b) Electrophoretic pattern of PCR products from P. multitentaculata and various flatworm species. White arrow indicates PCR products specific to P. multitentaculata, and black arrow indicates those common to various flatworm species. Lane M, molecular weight marker; lanes 1 and 2, P. multitentaculata; lane 3, Planocera reticulata; lane 4, Planocera sp.; lane 5, Paraplanocera oligoglena; lane 6, Callioplana marginata; lane7, Discoplana gigas; lane 8, Pseudostylochus obscurus; lane 9, Notocomplana humilis. Full-length gels are included in a Supplementary Information file (Fig. S3).
PCR with P. multitentaculata-specific primers amplified the 429 bp long mtDNA fragment from the intestinal content of the pufferfish T. niphobles young, two days after being fed with P. multitentaculata adults in the aquarium. Similarly, a P. multitentaculata-specific band was observed from the intestinal contents from the adult pufferfish captured off Hayama, in July, 2016 (Fig. 2).
Figure 2.
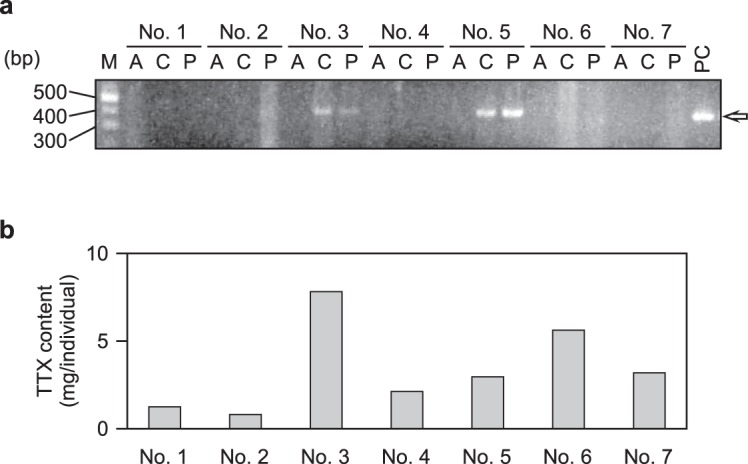
Detection of Planocera multitentaculata in the intestinal contents of young wild Takifugu niphobles individuals (a) and TTX content (mg/individuals) in the pufferfish individuals (b). The arrow indicates PCR products specific to P. multitentaculata. Lane M: molecular weight marker; lane A: anterior part of the intestine; lane C: middle part of the intestine; lane P, posterior part of the intestine; lane PC, positive control (P. multitentaculata). P. multitentaculata-specific band is present in No. 3 and 5 individuals. Full-length gel is included in a Supplementary Information file (Fig. S4).
Toxicity of the planocerid flatworm
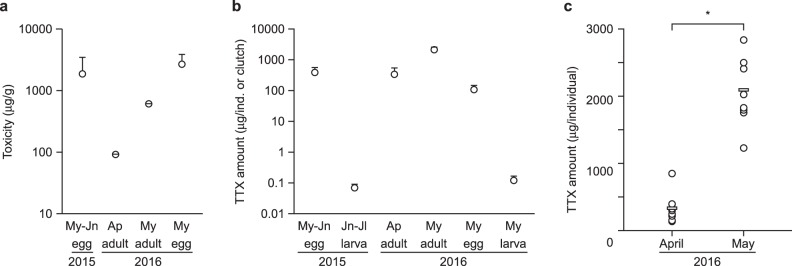
The amount of TTX in the planocerid flatworm P. multitentaculata (n = 9; body weight, 3.49 ± 0.51 g) captured in April 2016 was 91.9 ± 0.9 μg/g (334.0 ± 201.9 μg/individual), corresponding to 418 ± 4 MU/g (1518 ± 918 MU/individual), whereas those captured in May, 2016 (n = 9; body weight, 3.47 ± 0.49 g) were 610.8 ± 2.1 μg/g (2091.4 ± 469.3 μg/individual), corresponding to 2776 ± 10 MU/g (9506 ± 2133 MU/individual). A statistically significant difference was observed between these two sample groups (P < 0.05, Fig. 3, Table S5).
Figure 3.
Toxicity and TTX content of the flatworm Planocera multitentaculata used in this study. Panels (a and b) show the toxicity and TTX content in the flatworm adult, larva and egg, respectively, while panel (c) shows a significant difference in the TTX amount between the flatworm adult collected in April and May 2016. Larvae hatched from the wild eggs were collected in laboratory aquaria. Data of larva/adult and eggs were obtained from individuals and a clutch, respectively. Bars represent means + standard deviation. Student’s t-test was employed for statistical comparison (*P < 0.05 in panel c).
The amount of TTX in the planocerid eggs obtained in 2015 was 1867 ± 1589 µg/g, corresponding to 8486 ± 7223 MU/g, whereas those obtained in 2016 contained 2673 ± 1214 µg/g, corresponding to 12150 ± 5518 MU/g (Fig. 3, Table S5). The amount of TTX in the planocerid larvae in 2015 and 2016 was 69 ± 21 ng/individual, corresponding to 0.314 ± 0.095 MU/individual, and 120 ± 46 ng/individual, corresponding to 0.545 ± 0.209 MU/individual, respectively (Fig. 3, Table S5).
Toxification of pufferfish juveniles fed on flatworm larvae
In the predation experiments, the pufferfish juveniles fed heavily on planocerid larvae and lost equilibrium in approximately 20 min, but then recovered. Multiple reaction monitoring (MRM) patterns showed that the peak in the liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis corresponding to TTX was detected in the planocerid fed-pufferfish juveniles (Fig. S1). The amount of TTX in the whole body of the pufferfish juveniles (18, 39 and 51 dph; days post hatch) was calculated to be 407 ± 102 to 1064 ± 123 ng/individual (19.5 ± 5.4 to 54.8 ± 17.1 μg/g) (Table 4). The peak corresponding to TTX was not detected in the cultured juveniles (18 and 39 dph) of the pufferfish T. niphobles, which fed on non-toxic feeds (Fig. S1).
Table 4.
Toxification of the juvenile pufferfish after feeding on the flatworm larvae.
| Pufferfish (year) | No. of individuals | Total length (mm) | Body weight (g) | TTX content in flatworm larvae (μg)a | TTX concentration in pufferfish (μg/g)b | TTX amount of pufferfish (ng/ind.)b | Ingestion rate (%) |
|---|---|---|---|---|---|---|---|
| Flatworm-fed individual | |||||||
| 18 dph (2016) | 28 (3 replicates) | 6.1 ± 0.2 | 0.006 ± 0.002 | 3.7 | 37.2 ± 21.7 | 116 ± 19 | 2.9 ± 0.5 |
| 39 dph (2015) | 26 (3 replicates) | 7.6 ± 0.8 | 0.008 ± 0.001 | 12.1 | 54.8 ± 17.1 | 407 ± 102 | 3.4 ± 0.8 |
| 51 dph (2015) | 30 (3 replicates) | 11.1 ± 1.6 | 0.022 ± 0.007 | 15.7 | 19.5 ± 5.4 | 474 ± 241 | 3.0 ± 1.5 |
| Non-toxic feed-fed individual | |||||||
| 18 dph (2016) | 9 (1 replicate) | 6.0 ± 1.4 | 0.006 | N/Ac | N/A | N/Dd | N/A |
| 39 dph (2015) | 28 (3 replicates) | 8.0 ± 1.2 | 0.009 ± 0.002 | N/A | N/A | N/D | N/A |
aTTX content in the flatworm larvae fed by a juvenile of the pufferfish was calculated. bPufferfish TTX values represent means of three independent replicate experiments. cN/A: not applicable. dN/D: not detected.
Toxification of pufferfish young fed on adult flatworms
In these predation experiments, the pufferfish young fed on half of an adult flatworm and appeared to become toxified. MRM patterns showed that the peak in the LC-MS/MS analysis corresponding to TTX was detected in several tissues including intestine, liver and skin of the pufferfish (Fig. S1). The amount of TTX in the body of the toxified pufferfish young (12 months old), fed with a weakly toxic planocerid (152 ± 97 μg) was calculated to be 212 ± 227 (52–819) μg/individual, which corresponds to 964 ± 1032 MU/individual, demonstrating that almost all the TTX (129 ± 60%) in the flatworm was ingested by the pufferfish young (Fig. 4, Table S6). Similarly, the amount of TTX in the whole body of the toxified pufferfish young with a strongly toxic planocerid (1005 ± 305 μg) was calculated to be 181 ± 200 (9–543) μg/individual, which corresponds to 823 ± 909 MU/individual, demonstrating that some of the TTX (19 ± 21%) in the flatworm was ingested by the pufferfish young (Fig. 4, Table S6). TTX in the toxified pufferfish was localized in the liver (59.2 ± 12.7 to 62.0 ± 16.4%), intestine (14.7 ± 7.9 to 16.3 ± 13.9%) and skin (14.0 ± 11.2 to 22.1 ± 15.3%) (Table S7). MRM patterns showed that the peak in the LC-MS/MS analysis corresponding to TTX was not detected in any tissues from the cultured young (12 months old) T. niphobles that were raised on non-toxic feed (Fig. S1).
Figure 4.
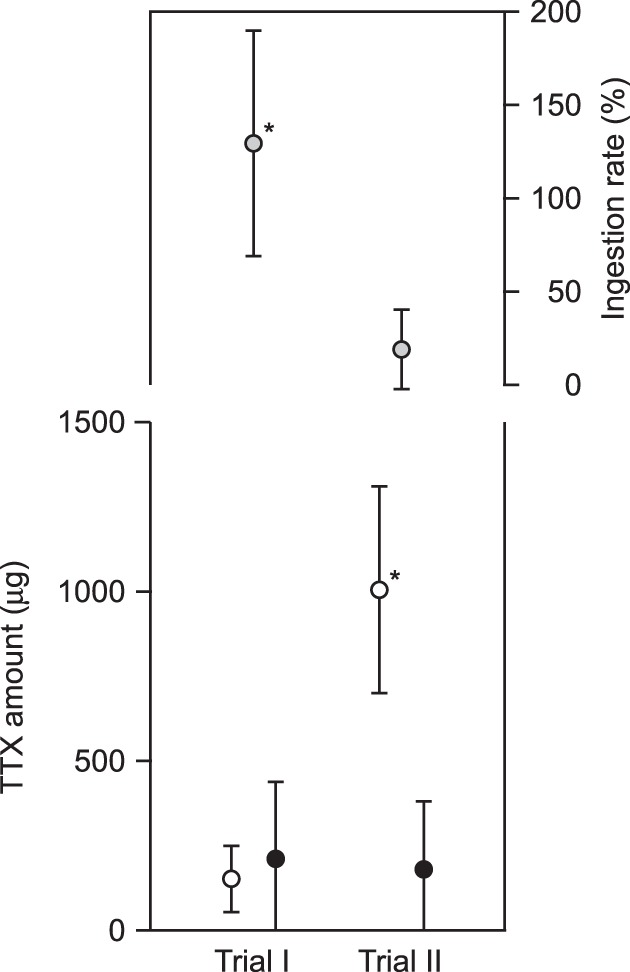
Toxification of the young pufferfish Takifugu niphobles after feeding on the adult flatworm Planocera multitentaculata. Closed circles refer to TTX accumulation in the pufferfish after feeding on half of one flatworm, while the open circles refer to the accumulation in half of one flatworm. Non-toxic pufferfish were used as a predators, and the toxic flatworms were used as prey. The flatworms collected in April and May were subjected to trial I and trial II of the toxification experiment (see text), respectively. Gray circle refers to the rate of accumulation of TTX in the pufferfish after feeding on the flatworm. Values are the mean of nine independent replicate experiments. Bars represent means ± standard deviation. Student’s t-test was employed for statistical comparison (*P < 0.05).
Discussion
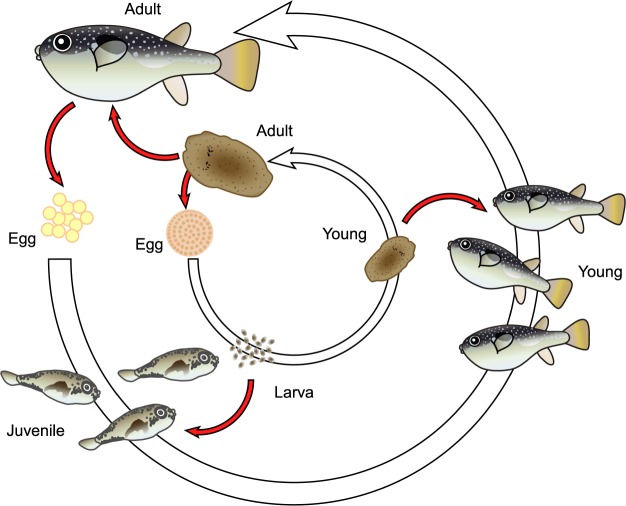
TTX in the offspring of toxic organisms such as pufferfish, octopus, newt and flatworm, appear to be obtained by means of a vertical maternal transfer34–39. This maternally provided TTX provides even just-hatched larvae protection from predators34,35. However, the protection provided by the maternal TTX, at least in pufferfish, decreases with age in the absence of further influx of TTX25,34,40. The larvae and juveniles of pufferfish need to obtain TTX from food in order to continue to protect themselves from predators. Effective toxification of the larval and juvenile pufferfish appears to depend on their feeding on plankton that is laden with highly concentrated TTX. TTX-bearing organisms have been identified from various taxa, including ribbonworms and flatworms, which have microplanktonic stages in their life histories13,14,17,36. Our study shows that the planktonic larvae of the flatworm P. multitentaculata contain highly concentrated TTX (69–120 ng/larva), suggesting that larval stages of the flatworm could serve not merely as suitable food but also a source for the toxification of the pufferfish larvae/juveniles. Considering that toxic flatworm sequences were detected from intestinal contents of wild juveniles and young of the pufferfish T. niphobles, our results suggest that the flatworm contributes to the toxification of the pufferfish T. niphobles throughout its life (Fig. 5).
Figure 5.
Role of the toxic flatworm Planocera multitentaculata in the toxification of the pufferfish Takifugu niphobles throughout its life cycle. Red arrows indicate transfer of TTX.
Kono et al.41 reported that dietary administrated TTX in the pufferfish T. niphobles was firstly accumulated into the liver, and then gradually transferred to the skin approximately 200 days after end of TTX administration: TTX content in the skin increased two-fold, while decreasing by 40% in the liver. These results suggest that apart from larval pufferfish, older individuals also use TTX as a chemical defense against predators. This inference is corroborated by the Sakakura et al.42 study, which found the survival rates of the TTX-bearing pufferfish T. rubripes juveniles were higher than those of the non-toxic individuals in a salt-pond mesocosm. In the predation experiments in this study, non-toxic T. niphobles juveniles and young were rapidly and effectively toxified after feeding on larvae and adult specimens of the flatworm P. multitentaculata, respectively, accumulating large quantities of TTX. Thus, the potential for pufferfish to accumulate large quantities of TTX exist; however, this potential does not always appear to be realized, even in laboratory-based predation experiments. Variation in individual toxicity is known to be high in wild Takifugu pufferfish populations23,43. In the present study too, large individual differences in toxicity were observed in T. niphobles young after the toxification experiments, suggesting that variation in individual toxicity might be one of the risk management in the survival strategies in the Takifugu pufferfish because of energy consumption for TTX-bearing in their body. The detection of TTX in young pufferfish at levels higher than the levels contained in the half flatworm they were fed, suggests that the difference might be due to the potential differences in TTX recovery or TTX extraction efficiencies for different tissues.
Flatworms are known to contribute to the toxification of other animals as well; one study reported that dog neurotoxicosis occurred after consuming the side-gilled sea slug Pleurobranchaea maculata in coastal New Zealand8, and a subsequent study revealed that the sea slugs were toxified by feeding on the flatworm Stylochoplana sp.9,17. The extent of pufferfish toxicity varies not only among individuals but also by habitat23, which might be associated with the habitat-specific population size of the planocerid flatworms36. In any cases, although planocerid flatworms contribute to the toxification of organisms at higher trophic levels, further investigation is needed to reveal the source of TTX in the flatworms for a better understanding of the TTX-loop in the marine environments. Our study shows that in the study area, the population size of the flatworm P. multitentaculata is much larger than that of the related species, P. reticulata. Indeed, P. multitentaculata appears to make a greater contribution to the toxification of the larvae and juveniles of the pufferfish, because toxic eggs of P. multitentaculata, and not of P. reticulata, have been observed in the area during the spawning period of the pufferfish36,37. The TTX source of the planktonic flatworms (excluding the maternal TTX) might be key to resolving the missing link to the TTX-loop proposed by our previous study33.
The toxification of the TTX-bearing organisms, including pufferfish, has been thought to be achieved through the classical food webs22,23, although the evidence thus far has largely only been the presence of organisms with indigestible tissues such as the starfish Astropecten polyacanthus, identified from the gut-contents of TTX-bearing organisms, such as a trumpet shell Charonia sauliae44. There has been little evidence of the toxification of other toxic organisms by organisms without indigestible tissues except for the flatworm Stylochoplana sp., which is considered responsible for the toxification of the grey side-gilled sea slug Pleurobranchaea maculata9,17. In our study, in order to determine if wild T. niphobles fed on the flatworm, P. multitentaculata, we developed PCR methods specific to the flatworm based on results from NGS analysis against a generic animal COI gene. Our results suggest that some wild pufferfish feed on the flatworm in the inshore waters around Hayama, Japan, and that future large scale metagenomics analyses of the intestinal contents of TTX-bearing organisms might reveal the mechanism of toxification.
In conclusion, we have shown, by means of TTX quantification, predation experiments, and tools of molecular biology, that the pufferfish T. niphobles can be toxified simply by feeding on the flatworm P. multitentaculata. The TTX content of the flatworm increased in association with increasing body weight36, and flatworms carrying TTX were classified into planocerid and the related species16. These reports and our results would contribute to the elucidation of the pufferfish toxification mechanism.
Materials and Methods
Pufferfish and flatworm
Wild (toxic) pufferfish Takifugu niphobles juveniles (15–34 mm total length, 0.10–0.67 g body weight) were captured in August 2010, August 2011 and July 2015 from coastal waters of Oiso, Japan (35°18′N, 139°19′E) and in July 2016 and July 2017 from coastal waters of Katase, Japan (35°18′N, 139°28′E). Non-toxic juveniles and young were raised from artificially fertilized eggs obtained from wild mature females and males captured in the summer (May–July) of 2015 and 2016 at Enoshima Island, Japan (35°18′N, 139°28′E), and subsequently grown with non-toxic feed (rotifer and artemia) and commercial food pellets in the aquarium (Fig. S1). Wild adult and young specimens were captured during June–July of 2016, and June–July of 2017 off the coast of Hayama, Japan (35°15′N, 139°34′E). Adult specimens of the flatworm Planocera multitentaculata were captured during April–July of 2015 and 2016 also at the coast of Hayama, Japan, while (toxic) flatworm larvae hatched from eggs that were spawned in the laboratory aquaria, by wild parents derived from Hayama.
LC-MS/MS analysis
TTX was extracted from samples with 0.1% acetic acid, the extract was filtered through a membrane of pore size 0.45-μm (SupraPure Syringe Filter, PTEE-Hydrophilic, Recenttec, Taipei, Taiwan) and subjected to analysis using a LC-MS/MS, following Itoi et al.43. Quantification was done using a Quattro Premier XE mass spectrometer (Waters, Milford, MA, USA) equipped with an electrospray ionization (ESI) source coupled to an Acquity UPLC system (Waters), following Itoi et al.33. Chromatographic separation was done using an Atlantis HILIC Silica column (2.1 mm × 150 mm, 5 μm; Waters), coupled to an Atlantis HILIC Silica pre-column (2.1 mm × 10 mm, 5 μm; Waters), with gradient elution of formic acid/acetonitrile. The mass spectrometer was operated in MRM, detecting in positive mode, analyzing two product ions at m/z 162 for quantification of TTX and m/z 302 for confirmation of the compound from the precursor ion at m/z 320. The calibration curve was generated with 1 to 100 ng/ml of TTX standard (Wako Pure Chemicals, Osaka, Japan), which showed good linearity and precision (y = 105.164x + 15.610, r2 = 0.9975). Quantification of TTX was carried out using the data for samples with >1000-fold dilution to remove any matrix effect, since it was recovered from the samples with >1000-fold dilution in accordance with our previous studies33,36. The limit of detection (LOD) of the measurement system was determined based on signal to noise ratio (S/N = 3). The LOD value was calculated at 0.059 ng/ml for TTX. One MU is equivalent to 0.22 μg of TTX, based on the specific toxicity of TTX45.
High-throughput sequencing
Genomic DNA in the intestinal contents of the juvenile pufferfish T. niphobles was extracted with Fast DNA spin kit for Soil (MO Bio Laboratories, Illkirch, France) based on the manufacturer’s instructions. Fragments of COI gene (approximately 500 bp) were amplified from the extracted genomic DNA by PCR using universal COI primers: 1st-IntF (5′-Seq A-GCTCT TCCCA TCTGT GCCAG CMGCC GCGGT AA-3′) and 1st-HCOmR (5′-Seq B-CTCTT CCGAT CTTAH ACTTC NGGGT GKCCR AARAA TCA-3′), where Seq A (5′-ACACT CTTTC CCTAC ACGAC-3′) and Seq B (5′-GTGAC TGGAG TTCAG ACGTG TG-3′) represent nucleotide sequences targeted by the second PCR primers. A blocking primer (5′-TTACC CACCC CTAGC AGGAA ATCTT GCCCACGCAG G-Spacer C3-3′) was used in the amplicon PCR, to prevent amplification from the pufferfish T. niphobles. A Spacer C3 CpG in the 3′ end of the blocking primer was added to prevent elongation without affecting annealing properties, and minimizing predator DNA amplification. PCR amplification was done under the following conditions: an initial denaturation at 94 °C for 2 min followed by 30 cycles of denaturation at 94 °C for 30 sec, annealing at 67 °C for 15 sec and 52 °C for 30 sec, and extension at 72 °C for 30 sec, with a final extension step at 72 °C for 5 min. PCR products were amplified again using additional forward primer (5′-Adaptor C-Tag sequence-Seq A-3′) and reverse primer (5′-Adaptor D-Seq B-3′), where Adaptors C and D were used for the MiSeq sequencing reaction. The Tag sequence included 8 nucleotides designed for sample identification barcoding. Thermal cycling was done under the following conditions: an initial denaturation at 94 °C for 2 min followed by 12 cycles of denaturation at 94 °C for 30 sec, annealing at 60 °C for 30 sec, and extension at 72 °C for 30 sec, with a final extension step at 72 °C for 5 min. PCR amplicons from each sample were used for high-throughput sequencing on a MiSeq Genome Sequencer (Illumina, CA, USA). The sequences obtained for each sample were grouped based on tag sequences, and average read length of 320 bp was obtained. Negative controls (reactions with no template) were prepared for all steps of the process after DNA extraction to check for contamination. Before analyzing the food organisms, we removed sequences if they met any of the following criteria: <40 bp in length, with a phred-equivalent quality score of <20, containing ambiguous characters, with an uncorrected barcode, or missing the primer sequence. The identities of the phylotypes were analyzed by comparing the sequences against the DDBJ/EMBL/GenBank databases using a BLAST search46.
DNA extraction and PCR amplification
Small tissue samples from adult specimens of the flatworm P. multitentaculata, and intestinal contents from wild specimens of the pufferfish T. niphobles were collected. Total genomic DNA was extracted from the flatworm tissues and the intestinal contents of the pufferfish using the method of Noguchi et al.47 with some modification. Briefly, proteinase K-treated samples were subjected to phenol/chloroform extraction with MaXtract High Density (Qiagen, Germantown, MD, USA). Partial fragments of 28S rRNA gene were amplified by PCR using primers HRNT-F2 (5′-AGTTC AAGAG TACGT GAAAC C-3′) and HRNT-R2 (5′-AACAC CTTTT GTGGT ATCTG ATGA-3′), which were designed with universal primers for the 28S rRNA gene (approx. 1,000 bp) of various polyclads48, whereas those of COI gene were amplified by PCR using P. multitentaculata-specific primers PMTF1 (5′-TTATT ATTGG GTTCA TTTGT GGTAG AG-3′) and PMTR2 (5′-AATCA TACCA AACCC CGGC-3′), which were designed based on the sequences of the COI gene (429 bp) from P. multitentaculata and other polyclads (Fig. 1). PCR amplification was done in a 20 μl reaction mixture containing genomic DNA as a template, 1 unit ExTaq DNA polymerase (Takara Bio, Shiga, Japan), 1.6 μl of 2.5 mM deoxynucleotide triphosphates (dNTP), 5 μl of 5 μM primers, and 2 μl of 10× ExTaq DNA polymerase buffer (Takara Bio). The thermal cycling program for the PCR consisted of an initial denaturation at 95 °C for 1 min followed by 35 cycles of denaturation at 95 °C for 10 s, annealing at 55 °C for 30 s and extension at 72 °C for 45 s.
Direct sequencing
Prior to sequencing the amplified product, the DNA fragment was purified by chloroform extraction, followed by polyethylene glycol (PEG) 8000 precipitation and ethanol precipitation. Both strands were sequenced using a 3130xl genetic analyzer (Applied Biosystems, Foster, CA, USA) and a BigDye Terminator v3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems). The nucleotide sequences of the amplified products were aligned using Clustal Omega49 with those in the DDBJ/EMBL/GenBank databases obtained using a BLAST search46.
Toxification experiment
Pufferfish juveniles vs. planocerid larvae
The toxification experiment was carried out using non-toxic juveniles (within 2 months old) of the pufferfish T. niphobles (8–10 individuals) as the predator and toxic planocerid larvae (2000–3000 larvae) as prey, in a 500-ml beaker. The treatment was repeated three times, except for the control sample in 2016. In the toxification experiment, the non-toxic juveniles of the pufferfish T. niphobles (standard length: 7.2–12.4 mm; body weight: 0.06–0.30 g) fed on the toxic planocerid larvae. After more than two days of feeding, the pufferfish juveniles (8–10 individuals pooled in a sample) and non-toxic control (8–10 individuals pooled in a sample) were subjected to the TTX extraction process followed by LC-MS/MS analysis.
Pufferfish young vs. planocerid adults
The toxification experiment was also carried out in a 50 L glass aquarium using non-toxic young (12 months old) of the pufferfish T. niphobles as the predator and adult flatworm P. multitentaculata as the prey. Since no significant difference in the TTX distribution was observed in both halves of adult flatworm individual (Fig. S2), half the body of a flatworm adult was subjected to LC-MS/MS analysis, and the remaining half fed to a non-toxic pufferfish young, which were kept in a 50 L glass aquaria with a circulating filtration system. The treatment was repeated nine times. After more than two days of feeding on the adult planocerid, the pufferfish young were subjected to TTX extraction followed by LC-MS/MS analysis.
Statistical analysis
The statistical significance of differences in the amount of toxin was analyzed by means of a student’s t-test. Data are given as mean ± standard deviation.
Ethical statement
All animal procedures comply with the Japanese Government Animal Protection and Management Law (No. 105) and Japanese Government Notification on Feeding and Safekeeping of Animals (No. 6).
Electronic supplementary material
Acknowledgements
This study was supported in part by Research Grant for 2013 from the Nihon University College of Bioresource Sciences (S.I.), the Japan Science Society for 2014 (T.T.), Grant-in-Aid for Challenging Exploratory Research from Japan Society for the Promotion of Science (JSPS) (26660177, S.I.), the Towa Foundation for Food Science & Research (S.I.), and Grant-in-Aid for Scientific Research (B) from JSPS (15H04552, S.I.).
Author Contributions
S.I. and H.S. designed research; S.I., H.U., R.Y., M.T., T. Sato, S.O., Y.W., R.O., H.O., T. Shitto, K.O. and T.T. collected samples, performed research, and analyzed data; E.S. prepared non-toxic pufferfish young; S.I. wrote the main manuscript text, prepared Table 1; H.U. and R.Y. prepared Figures 1 and 2, and the related supporting data; S.I., R.Y., M.T. and T.T. prepared Figures 3 and 4, and the related supporting data; S.I. and R.Y. prepared Figure 5; T. Sato prepared Table 2; R.O. prepared Table 3; S.I., R.Y. and K.O. prepared Table 4, and the related supporting data.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-30696-z.
References
- 1.Colquhon D, Henderson R, Ritchie JM. The binding of labeled tetrodotoxin to non-myelineated nerve fibres. J. Physiol. 1972;227:95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narahashi T. Pharmacology of tetrodotoxin. J. Toxicol.-Toxin Rev. 2001;20:67–84. doi: 10.1081/TXR-100102537. [DOI] [Google Scholar]
- 3.Mosher HS, Fuhrman GJ, Fuhrman FA, Fischer HG. Tarichatoxin-tetrodotoxin, a potent neurotoxin. Science. 1964;144:1100–1110. doi: 10.1126/science.144.3622.1100. [DOI] [PubMed] [Google Scholar]
- 4.Kim YH, Brown GB, Mosher HS, Fuhrman FA. Tetrodotoxin: occurrence in atelopid frogs of Costa Rica. Science. 1975;189:151–152. doi: 10.1126/science.1138374. [DOI] [PubMed] [Google Scholar]
- 5.Noguchi T, Hashimoto K. Isolation of tetrodotoxin from a goby Gobius criniger. Toxicon. 1973;11:305–307. doi: 10.1016/0041-0101(73)90060-3. [DOI] [PubMed] [Google Scholar]
- 6.Sheumack DD, Howden ME, Spence I, Quinn RJ. Maculotoxin: a neurotoxin from the venom glands of the octopus Hapalochlaena maculosa identified as tetrodotoxin. Science. 1978;199:188–189. doi: 10.1126/science.619451. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi T, Maruyama J, Ueda Y, Hashimoto K, Harada T. Occurrence of tetrodotoxin in the Japanese ivory shell Babylonia japonica. Nippon Suisan Gakkaishi. 1981;47:909–914. doi: 10.2331/suisan.47.909. [DOI] [Google Scholar]
- 8.McNabb P, et al. Detection of tetrodotoxin from the grey side-gilled sea slug - Pleurobranchaea maculata, and associated dog neurotoxicosis on beaches adjacent to the Hauraki Gulf, Auckland, New Zealand. Toxicon. 2010;56:466–473. doi: 10.1016/j.toxicon.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Wood SA, et al. Tetrodotoxin concentrations in Pleurobranchaea maculata: temporal, spatial and individual variability from New Zealand populations. Mar. Drugs. 2012;10:163–176. doi: 10.3390/md10010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noguchi T, et al. Occurrence of tetrodotoxin as the major toxin in the xanthid crab Atergatis floridus. Nippon Suisan Gakkaishi. 1983;49:1887–1892. doi: 10.2331/suisan.49.1887. [DOI] [Google Scholar]
- 11.Maruyama J, Noguchi T, Jeon JK, Harada T, Hashimoto K. Occurrence of tetrodotoxin in the starfish Astropecten latespinosus. Experimentia. 1984;40:1395–1396. doi: 10.1007/BF01951908. [DOI] [Google Scholar]
- 12.Maruyama J, et al. Occurrence of tetrodotoxin in a starfish. Astropecten scoparius. Agric. Biol. Chem. 1985;49:3069–3070. [Google Scholar]
- 13.Miyazawa K, et al. Occurrence of tetrodotoxin in the flatworm Planocera multitentaculata (Platyhelminthes) Toxicon. 1986;24:645–650. doi: 10.1016/0041-0101(86)90027-9. [DOI] [PubMed] [Google Scholar]
- 14.Tanu MB, et al. Immunoenzymatic visualization of tetrodotoxin (TTX) in Cephalothrix species (Nemertea: Anopla: Palaeonemertea: Cephalotrichidae) and Planocera reticulata (Platyhelminthes: Turbellaria: Polycladida: Planoceridae) Toxicon. 2004;41:515–520. doi: 10.1016/j.toxicon.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Ritson-Williams R, Yotsu-Yamashita M, Paul VJ. Ecological functions of tetrodotoxin in a deadly polyclad flatworm. Proc. Natl. Acad. Sci. USA. 2006;103:3176–3179. doi: 10.1073/pnas.0506093103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueda H, Itoi S, Sugita H. TTX-bearing planocerid flatworm (Platyhelminthes: Acotylea) in the Ryukyu Islands, Japan. Mar. Drugs. 2018;16:37. doi: 10.3390/md16010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvitti LR, Wood SA, Winsor L, Cary SC. Intracellular immunohistochemical detection of tetrodotoxin in Pleurobranchaea maculata (gastropoda) and Stylochoplana sp. (turbellaria) Mar. Drugs. 2015;13:756–769. doi: 10.3390/md13020756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asakawa M, Ito K, Kajihara H. Highly toxic ribbon worm Cephalothrix simula containing tetrodotoxin in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxins. 2013;5:376–395. doi: 10.3390/toxins5020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simidu U, Noguchi T, Hwang DF, Shida Y, Hashimoto K. Marine bacteria which produce tetrodotoxin. Appl. Environ. Microbiol. 1987;53:1714–1715. doi: 10.1128/aem.53.7.1714-1715.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noguchi T, et al. Vibrio alginolyticus, a tetrodotoxin-producing bacterium, in the intestine of the fish Fugu vermicularis vermicularis. Mar. Biol. 1987;94:625–630. doi: 10.1007/BF00431409. [DOI] [Google Scholar]
- 21.Wu Z, et al. A new tetrodotoxin-producing actinomycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugu rubripes. Toxicon. 2005;45:851–859. doi: 10.1016/j.toxicon.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi T, Arakawa O. Tetrodotoxin – distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs. 2008;6:220–242. doi: 10.3390/md20080011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noguchi T, Arakawa O, Takatani T. TTX accumulation in pufferfish. Comp. Biochem. Physiol. D. 2006;1:145–152. doi: 10.1016/j.cbd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Matsui T, Hamada S, Konosu S. Difference in accumulation of puffer fish toxin and crystalline tetrodotoxin in the puffer fish. Fugu rubripes rubripes. Nippon Suisan Gakkaishi. 1981;47:535–537. doi: 10.2331/suisan.47.535. [DOI] [Google Scholar]
- 25.Matsui T, Sato H, Hamada S, Shimizu C. Comparison of toxicity of the cultured and wild puffer fish Fugu niphobles. Nippon Suisan Gakkaishi. 1982;48:253. doi: 10.2331/suisan.48.253. [DOI] [Google Scholar]
- 26.Saito T, et al. Toxicity of the cultured pufferfish Fugu rubripes rubripes along with their resistibility against tetrodotoxin. Bull. Jpn. Soc. Sci. Fish. 1984;50:1573–1575. doi: 10.2331/suisan.50.1573. [DOI] [Google Scholar]
- 27.Yamamori K, Kono M, Furukawa K, Matsui T. The toxification of juvenile cultured kusafugu Takifugu niphobles by oral administration of crystalline tetrodotoxin. J. Food Hyg. Soc. Jpn. 2004;45:73–75. doi: 10.3358/shokueishi.45.73. [DOI] [PubMed] [Google Scholar]
- 28.Honda S, et al. Toxification of cultured puffer fish Takifugu rubripes by feeding on tetrodotoxin-containing diet. Nippon Suisan Gakkaishi. 2005;71:815–820. doi: 10.2331/suisan.71.815. [DOI] [Google Scholar]
- 29.Miyazawa K, Noguchi T. Distribution and origin of tetrodotoxin. J. Toxicol.-Toxin Rev. 2001;20:11–33. doi: 10.1081/TXR-100103081. [DOI] [Google Scholar]
- 30.Food Safety Commission of Japan. Food Safety Risk Assessment concerning “Liver of Takifugu rubripes Cultivated by the Method Proposed by Saga Prefecture and Ureshinocho in the Prefecture under the Law on Special Zones for Structural Reform (Law No. 189, 2002)”. Risk Assessment Reports – Natural toxins/mycotoxins. (2005).
- 31.Wang XJ, Yu RC, Luo X, Zhou MJ, Lin XT. Toxin-screening and identification of bacteria isolated from highly toxic marine gastropod Nassarius semiplicatus. Toxicon. 2008;52:55–61. doi: 10.1016/j.toxicon.2008.04.170. [DOI] [PubMed] [Google Scholar]
- 32.Yang G, et al. A novel TTX-producing Aeromonas isolated from the ovary of Takifugu obscurus. Toxicon. 2010;56:324–329. doi: 10.1016/j.toxicon.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Itoi S, et al. Toxic Takifugu pardalis eggs found in Takifugu niphobles gut: Implications for TTX accumulation in the pufferfish. Toxicon. 2015;108:141–146. doi: 10.1016/j.toxicon.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Itoi S, et al. Larval pufferfish protected by maternal tetrodotoxin. Toxicon. 2014;78:35–40. doi: 10.1016/j.toxicon.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Itoi S, et al. Role of maternal tetrodotoxin in survival of larval pufferfish. Toxicon. 2018;148:95–100. doi: 10.1016/j.toxicon.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Yamada R, et al. Seasonal changes in the tetrodotoxin content of the flatworm Planocera multitentaculata. Mar. Drugs. 2017;15:56. doi: 10.3390/md15030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyazawa K, Jeon JK, Noguchi T, Ito K, Hashimoto K. Distribution of tetrodotoxin in the tissues of the flatworm Planocera multitentaculata (Platyhelminthes) Toxicon. 1987;25:975–980. doi: 10.1016/0041-0101(87)90160-7. [DOI] [PubMed] [Google Scholar]
- 38.Hanifin CT, Brodie ED, III, Brodie ED., Jr. Tetrodotoxin levels in the eggs of the rough-skin newt, Taricha granulosa, are correlated with female toxicity. J. Chem. Ecol. 2003;29:1729–1739. doi: 10.1023/A:1024885824823. [DOI] [PubMed] [Google Scholar]
- 39.Williams BL, Lovenburg V, Huffard CL, Caldwell RL. Chemical defense in pelagic octopus paralarvae: tetrodotoxin alone does not protect individual paralarvae of the greater blue-ringed octopus (Hapalochlaena lunulata) from common reef predators. Chemoecology. 2011;21:131–141. doi: 10.1007/s00049-011-0075-5. [DOI] [Google Scholar]
- 40.Nagashima Y, et al. Change in tetrodotoxin content of the puffer fish Takifugu rubripes during seed production from fertilized eggs to juveniles. Shokuhin Eiseigaku Zasshi. 2010;51:48–51. doi: 10.3358/shokueishi.51.48. [DOI] [PubMed] [Google Scholar]
- 41.Kono M, Matsui T, Furukawa K, Yotsu-Yamashita M, Yamamori K. Accumulation of tetrodotoxin and 4,9-anhydrotetrodotoxin in cultured juvenile kusafugu Fugu niphobles by dietary administration of natural toxic komonfugu Fugu poecilonotus liver. Toxicon. 2008;51:1269–1273. doi: 10.1016/j.toxicon.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Sakakura Y, Takatani T, Nakayasu J, Yamazaki H, Sakiyama K. Administration of tetrodotoxin protects artificially-raised juvenile tiger puffer Takifugu rubripes from predators. Fish. Sci. 2017;83:191–197. doi: 10.1007/s12562-016-1046-0. [DOI] [Google Scholar]
- 43.Itoi S, et al. Seasonal changes in the tetrodotoxin content of the pufferfish Takifugu niphobles. Toxicon. 2016;114:53–58. doi: 10.1016/j.toxicon.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Noguchi T, Narita H, Maruyama J, Hashimoto K. Tetrodotoxin in the starfish Astropecten polyacanthus, in association with toxification of a trumpet shell, “Boshubora” Charonia sauliae. Bull. Jpn. Soc. Sci. Fish. 1982;48:1173–1177. doi: 10.2331/suisan.48.1173. [DOI] [Google Scholar]
- 45.Kawabata, T. Assay Method for Tetrodotoxin. Food Hygiene Examination Manual, Vol. II., pp. 232–240. (Japan Food Hygiene Association, 1978).
- 46.Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res.25, 3389–3402 (1997). [DOI] [PMC free article] [PubMed]
- 47.Noguchi S, et al. Population genetic structure of Scombrops boops (Percoid, Scombropidae) around the Japanese archipelago inferred from the cytochrome b gene sequence in mitochondrial DNA. Mitochondr. DNA. 2012;23:233–239. doi: 10.3109/19401736.2012.668897. [DOI] [PubMed] [Google Scholar]
- 48.Tsunashima T, et al. A molecular framework for the taxonomy and systematics of Japanese marine turbellarian flatworms (Platyhelminthes, Polycladida) Aquat. Biol. 2017;26:159–167. doi: 10.3354/ab00682. [DOI] [Google Scholar]
- 49.Sievers F, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.