Abstract
Dopamine neurons in the ventral tegmental area (VTA) influence learned behaviors and neuropsychiatric diseases including addiction. The stress peptide corticotrophin-releasing factor (CRF) contributes to relapse to drug and alcohol seeking following withdrawal, although the cellular actions are poorly understood. In this study, we show that presynaptic CRF type 1 receptors (CRF-R1) potentiate GABA release onto mouse VTA dopamine neurons via a PKC-Ca2+ signaling mechanism. In naive animals, activation of CRF-R1 by bath application of CRF or ethanol enhanced GABAA inhibitory postsynaptic currents (IPSCs). Following 3 days of withdrawal from four weekly cycles of chronic intermittent ethanol (CIE) vapor exposure, spontaneous IPSC frequency was enhanced while CRF and ethanol potentiation of IPSCs was intact. However, withdrawal for 3 weeks or more was associated with reduced spontaneous IPSC frequency and diminished CRF and ethanol responses. Long-term withdrawal was also accompanied by decreased sensitivity to the CB1 receptor agonist WIN55212 as well as greatly enhanced sensitivity to the CB1 antagonist AM251. Inclusion of BAPTA in the internal recording solution restored the responsiveness to CRF or ethanol and reduced the potentiating actions of AM251. Together, these data suggest that GABAA inhibition of VTA dopamine neurons is regulated by presynaptic actions of CRF and endocannabinoids and that long-term withdrawal from CIE treatment enhances endocannabinoid-mediated inhibition, thereby suppressing CRF facilitation of GABA release. Such findings have implications for understanding the impact of chronic alcohol on stress-related, dopamine-mediated alcohol-seeking behaviors.
Introduction
Maladaptive changes in the mesocorticolimbic reward system contribute to neuropsychiatric disorders, including addiction [1, 2]. Both stress and drugs of abuse (including alcohol) target reward pathways, originating from dopamine neurons in the ventral tegmental area (VTA) [3, 4]. The transition from moderate controlled drinking to heavy drinking often involves intermittent bouts of binge consumption of ethanol, culminating in dependence [5]. Acute administration of ethanol increases dopamine release from mesolimbic terminals [6–8]. Excessive alcohol consumption is a stressor that dysregulates brain reward systems, including the VTA [5].
Converging evidence shows that the stress hormone corticotropin-releasing factor (CRF) modulates dependence-induced ethanol (EtOH) intake and that CRF signaling itself becomes dysregulated [9]. CRF activates two G protein-coupled receptors, type 1 (CRF-R1) and 2 (CRF-R2), reported to couple both protein kinase A (PKA) and protein kinase C (PKC) signaling cascades [10, 11]. In the rodent VTA, CRF+ immunoreactive glutamate and GABA axon terminals synapse onto tyrosine hydroxylase-positive dopamine neurons [12]. Also, nhanced CRF-R1-mediated signaling in the VTA is required for excessive ethanol consumption in rodents [13, 14].
Intra-VTA infusions of CRF-R1 antagonists decreased intermittent ethanol intake in stressed and non-stressed mice [13]. Although the VTA CRF-R1 action coincides temporally with repeated withdrawal, CRF-R2 likely also plays a role [15]. Systemic administration of CRF-R1 antagonists that reduce self-administration in rats also reduce the withdrawal-induced enhancement of GABA release when infused locally into the CeA [16, 17]. In the VTA, activation of GABAA receptors are known to limit dopamine cell bursting [18] and suppression of GABAA transmission switches dopaminergic neurons into a burst firing pattern [19–21]. However, the regulation of GABA release in the VTA by CRF-R1 before or during alcohol dependence is, by comparison to the central nucleus of the amygdala (CeA), less well understood.
Understanding the interaction between CRF-R1 and EtOH on GABA VTA terminals is also interesting considering the link between ethanol and the endocannabinoid (type 1 cannabinoid (CB1) receptor) regulation of VTA GABA release. Acute administration of ethanol [6, 8] or a CB1 agonist [22] enhances the activity of VTA dopamine neurons and dopamine levels in the nucleus accumbens [23, 24]. Conversely, CB1 receptor antagonists decrease both the ethanol-related voluntary intake in rodents [25–27] and increases accumbal dopamine levels [28]. Such ethanol actions are absent in CB1 knockout mice [29]. Because the acute withdrawal from ethanol robustly elevates endocannabinoid production [30], the role of CRF-R1 on GABA transmission (and by association VTA output) may be altered during alcohol dependence.
Here we test the hypothesis that in VTA dopamine neurons, CRF regulates GABAA transmission through a presynaptic mechanism and prior ethanol dependence alters this relationship through a functional change involving endocannabinoids. To evaluate these points, we first examined the acute actions of CRF on GABA release in the VTA in brain tissue of naive mice and identify a presynaptic CRF-R1-mediated mechanism involving PKC and intracellular calcium stores that facilitates GABA release onto VTA DA neurons. Second, we treated mice with repeated cycles of chronic intermittent ethanol (CIE) vapor exposure, a well-established rodent model of alcohol dependence known to generate recurring surges of CRF [31]. We then showed that in the weeks following CIE exposure, CRF action is suppressed by a persistent functional enhancement of endocannabinoid-mediated CB1 receptor inhibition at VTA GABA synapses. This resulting shift in the opposing (facilitatory and suppressive) systems that regulate dopamine neuron activity would tend to favor VTA output. Together, these data demonstrate an enduring adaptation that is distinct from the classic somatic withdrawal symptoms that typically recede in 48 h [32] and may play a key role in alcohol addiction.
Materials and methods
Slices and solutions
All experiments were conducted according to the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals. Horizontal brain slices (220 µm) of the ventral midbrain were prepared from adult (P60–P160) male C57BL-6J mice as described previously [33]. Slices were cut using a Vibratome (Leica) in ice-cold artificial cerebrospinal fluid (ACSF) containing 126 mM NaCl, 2.5 mM KCl, 1.2 mM MgCl2, 2.4 mM CaCl2, 1.4 mM NaH2PO4, 25 mM NaHCO3, 11 mM d-glucose and 0.4 mM ascorbate, and MK-801 (0.01 mM). Slices were stored in oxygenated (95% O2–5% CO2; 33 °C) ACSF containing MK-801 (0.01 mM). Electrophysiological recordings were performed at 33 °C. The perfusion rate of oxygenated ACSF (95% O2–5% CO2) was 2 ml/min. Neurons were visualized with an Olympus BX51WI (Olympus) microscope.
Whole-cell recordings
The identification of VTA dopamine cells was based on prior experiences using tyrosine hydroxylase (TH)-GFP mice. In horizontal mouse sections (Supplemental Fig. 1), the VTA was identified as the region medial to the medial terminal nucleus of the accessory optic tract (MT) and substantia nigra compacta. Otherwise recordings were performed on putative dopamine neurons in brain slices as described previously [34] using Axopatch 700B amplifier (Axon Instruments). Recordings were collected using AxoGraph X (AxoGraph), filtered (1–2 kHz) and digitized (2–5 kHz). Neurons were voltage clamped at −60 mV using 1.5–2.0 MΩ pipettes. Unless otherwise noted, pipette internal solution contained in mM: 57 KCl, 57 K-methylsulfate, 20 NaCl, 1.5 MgCl2, 5 HEPES, 0.1 EGTA, 2 mM ATP, 0.3 mM GTP, and 10 phosphocreatine (pH 7.3; 265–270 mOsm). Series resistance (3–10 MΩ) was compensated at 80%.
Putative dopamine neurons were identified by: large cell bodies (20 µm), pacemaker-like firing (1–5 Hz), an Ih current (>300 pA), and a mGluR/sK channel-mediated current observed only in dopamine neurons in midbrain slices [35]. An immunocytochemical study in C57BL-6J mice using tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis, indicated the Ih is predictive of dopamine content [36]. We acknowledge topographical differences exist between VTA neurons, but these were not examined in these studies.
Cells were visualized using a 60× water-immersion objective on an upright microscope (Olympus America). Inhibitory postsynaptic currents (IPSCs) were recorded at ~30% of maximal amplitude and isolated pharmacologically with NBQX (3 µM), SCH-23390 (1 µM), CGP-55845 (10 µM), and sulpiride (200 nM). Miniature IPSCs (mIPSCs) were recorded in the presence of tetrodotoxin (TTX; 200 nM). The spontaneous IPSC (sIPSC) and mIPSC amplitudes and inter-event interval times were measured with Axograph X. CRF-R1/R2 antagonists were incubated (10–15 min) prior to application of CRF. The paired pulse protocol was applied with a 50 ms interval (0.2 ms duration, 0.06 Hz).
Drugs
CRF (human/rat), UCN (rat), and UCN-3 (human) were obtained from American Peptide (Sunnyvale, CA). All other compounds were obtained from Tocris Bioscience (Minneapolis, MN) or Abcam (Cambridge, MA). An ethanol concentration (50 mM; Sigma Aldrich) was selected for its relevance to previous work [37].
Ethanol treatment
Experiments using ethanol- and air-treated mice were performed in age-matched animals. Because increased ethanol drinking after CIE is only observed in males [38], females were not investigated in this study. Treated animals were subjected to CIE exposure model as described previously [31, 39–43]. Briefly, ethanol (CIE) mice received pyrazole (1 mmol/kg) plus a loading dose of ethanol (3 g/kg) prior to entering the CIE chamber each day during four exposures (16 h/day, 4 days on, and 72 h off) of ethanol exposure in custom inhalation chambers. Ethanol vapor concentrations were monitored daily to ensure stable blood ethanol concentration levels (BEC: 175–225 mg/dl assessed weekly) [41]. Air-exposed (control) mice received pyrazole before entering the cambers and were exposed to control (air) inhalation conditions. After 4 weeks of treatment, mice were removed from the vapor chambers and returned to their home cage. The repeated exposure to ethanol results in somatic withdrawal symptoms that recede in ~48 h [32]. Mice were killed 3 (short-term withdrawal) or 25–45 days (long-term withdrawal) following the final ethanol or air exposure for tissue collection.
Statistical analysis
Data were analyzed offline on a Macintosh computer (Apple) using AxoGraph X (AxoGraph X) and Chart (AD Instruments). Data are presented as the mean ± SEM and analyzed using t-tests (paired and unpaired) or analysis of variance (ANOVA) (one-way or two-way) followed by Dunnett’s post hoc in Prism (GraphPad). Differences were considered significant if p < 0.05.
Results
CRF potentiates GABAA receptor-mediated IPSCs via presynaptic CRF-R1
We recorded stimulus-evoked GABAA-mediated inhibitory postsynaptic currents (eIPSCs) from VTA dopamine neurons in acute brain slices from C57BL-6J mice. The concentration dependence of two non-selective agonists (CRF and UCN) and one CRF-R2 agonist (UCN-3) were evaluated after 10 min of bath application, over a range of 10–300 nM (Fig. 1a). Both CRF and UCN potentiated the amplitude of eIPSCs with near maximal effects at 200 nM (Fig. 1a; CRF: t(12) = 20.19, p < 0.0001 relative to baseline; UCN: t(10) = 26.01, p < 0.0001 relative to baseline). UCN-3 had no measurable effect on eIPSCs (Fig. 1a, 200 nM UCN-3: t(8) = 1.148, p = 0.1884 relative to baseline). The half-maximal effective concentration (EC50) for CRF and UCN was 34 nM and 41 nM, respectively (Fig. 1a). At 300 nM, CRF also caused a persistent inward current (8.1 ± 3.1 pA), consistent with previous reports [44]. At the end of the experiments, the GABAA receptor antagonist picrotoxin (picro, 100 µM) was applied to confirm the specificity of GABAA receptor-mediated currents (Fig. 1a).
Fig. 1.
Presynaptic CRF-1 receptors potentiate GABAA IPSCs in VTA DA neurons. a Traces of stimulus-evoked, picrotoxin-sensitive IPSCs showing potentiation by CRF (200 nM) and urocortin (UCN; 200 nM), but not the CRF-R2 agonist UCN-3 (300 nM). For the timeline, the amplitude of the evoked current was normalized for each cell using the mean amplitude recorded during the first 5 min and plotted as function of time (% of baseline, mean ± SEM). Although CRF (200 nM) and UCN (200 nM) potentiated IPSCs, UCN-3 did not. The smooth curves in the agonist concentration–response plot are the best fit to the data by the logistic equation. b CRF (300 nM) increased the amplitude of the first eIPSC (S1), with little measurable change in the second eIPSC (S2). Inset shows the first eIPSCs (S1) normalized to the second eIPSC (S2). CRF significantly decreased the paired pulse ratio (PPR; IPSC2/IPSC1, ***p < 0.001. c CRF (300 nM) enhances the frequency of spontaneous IPSCs (sIPSCs). Inset shows magnification of sIPSCs. Both UCN and CRF increased the frequency of sIPCS relative to their baseline (***p < 0.001). d, e The CRF (300 nM) potentiation is blocked by co-application of (e) CRF-R1 antagonists (CP154156 (CP154) or antalarmin (AA), but not (d) CRF-R2 antagonists (K41498 (K414) or astressin-2B (A2B) (*p < 0.05). f The CRF (300 nM) shift in the PPR is reduced by CRF-R1 blockers (CP154 or AA), but not CRF-R2 blockers (K414 or A2B). One-way ANOVA, *p < 0.05 vs CRF alone (Dunnett’s post test). g The CRF (200 nM) enhancement of sIPSC frequency is prevented by co-application of CRF-R1 blockers (CP154 or AA), but not CRF-R2 blockers (K414 or A2B). One-way ANOVA, ***p < 0.001 vs CRF alone (Dunnett’s post test). a–g Number of cells/mice per group: a CRF 13/7; UCN 11/6, UCN-3 9/4 for both timelines and concentration–response curves; b 7/4; c 7/5, d, e K414 9/5, A2B 9/5, CP154 8/4, AA 7/3; f 7/3-4 per group; g CRF+K414 7/4, and the following groups contained 8-9/4-5: UCN+A2B, CRF+A2B, CRF+CP154, and CRF+AA
To assess whether the CRF acted at a pre- or postsynaptic site, we recorded paired pulse ratios (PPRs, IPSC2/IPSC1, Fig. 1b). PPRs correlate inversely with release probability [45]. CRF (300 nM) increased the amplitude of the first eIPSC (Fig. 1b, S1, paired t-test t(6) = 9.625, p < 0.0001), with little measurable change in the second eIPSC (Fig. 1b, S2, paired t-test t(6) = 0.7661, p = 0.2663). This resulted in a significant decrease in the PPR in the presence of CRF (Fig. 1b inset, paired t-test, t(6) = 8.2601, p = 0.0002), thus suggesting a presynaptic locus for CRF actions on GABAA eIPSCs.
As other modifications of short-term plasticity can alter the PPR independent of changes in release probability [46], we confirmed a presynaptic locus of CRF action measuring the frequency and amplitude of sIPSCs and mIPSCs. CRF or UCN increased the sIPSC frequency in all cells tested (Fig. 1c; CRF: t(7) = 4.567, p = 0.0003; UCN: t(7) = 4.324, p = 0.0003). Similar increases were observed with CRF on mIPSC recordings (medium containing 200 nM TTX; CRF: 68.8% ± 4.26% increase relative to baseline; t(7) = 5.885, p = 0.0023). CRF did not alter sIPSC or mIPSC amplitudes (sIPSCs: 5.56% ± 4.6%, t(14) = 0.03597, p = 0.4860; mIPSCs: 2.94% ± 11.13%, t(7) = 0.5172, p = 0.3105). Taken together, these results indicate a presynaptic action of CRF on GABA synapses on to VTA DA neurons.
CRF potentiation of GABAA receptor-mediated IPSCs requires CRF-R1
To assess whether the CRF facilitation of GABAA currents was mediated by CRF-R1 or -R2, CRF was applied in the presence of blockers for CRF-R1 (300 nM CP154156, CP154; 200 nM antalarmin (AA)) or CRF-R2 (300 nM K41498, K414; 200 nM astressin-2B (A2B)). Slices were incubated with antagonists 10 min prior to and during CRF challenges. The resulting changes in evoked currents, the PPR, and the frequency of sIPSCs was examined.
Neither of the CRF-R2 blockers, K414 or A2B, prevented the CRF potentiation of eIPSCs (Fig. 1d; CRF + K414: t(6) = 3.564, p = 0.0121; CRF + A2B: t(7) = 4.234, p = 0.0223). However, co-application of the CRF-R1 antagonists CP154 or AA blocked the CRF increase of eIPSCs (Fig. 1e; CRF + CP154 t(5) = 0.03185, p = 0.5241; CRF+AA: t(7) = 0.02354, p = 0.7123). To verify that these effects were mediated by presynaptic CRF-R1, we evaluated the PPR (Fig. 1f) and sIPSC frequency (Fig. 1g) in the presence of selective CRF receptor antagonists. A one-way between-subject ANOVA evaluating the effects of the four antagonists on the CRF-mediated changes in the PPR (Fig. 1f) or in the frequency of sIPSC (Fig. 1g) indicated significant differences at the p < 0.05 level (PPR F(4, 22) = 3.884, p = 0.0156; frequency F(4, 64) = 13.16, p < 0.0001). Separate post hoc tests (multiple comparisons Dunnett’s) revealed that the CRF changes in the PPR (Fig. 1f) were absent when blocking CRF-R1 with CP154 (p = 0.9997) or AA (p = 0.9745), but were observed during co-application of the CRF-R2 blockers K414 (p = 0.0137) or A2B (p = 0.0413). Post hoc tests revealed that the CRF changes in frequency (Fig. 1g) followed the same pattern: CRF changes were absent when blocking CRF-R1 with CP154 (p = 0.9831) or AA (p = 0.7991), but observed during co-application of the CRF-R2 blockers K414 (p < 0.0001) or A2B (p < 0.001). Thus, presynaptic CRF-R1 receptors regulate GABA release.
The possibility that endogenous release of CRF tonically activates CRF receptors was also tested using selective antagonists. Application of either of the CRF-R1 or CRF-R2 antagonists alone, without CRF, produced no measurable change in eIPSCs (one-way ANOVA, F(4, 41) = 0.3584, p = 0.8367), nor in the frequency of sIPSCs (one-way ANOVA F(4, 30) = 0.0194, p = 0.9618), suggesting the absence of a measurable tone on CRF receptors in slices prepared from naive mice. Together, the results from the PPR studies and sIPSC/mIPSC recordings are consistent with a presynaptic action of CRF via CRF-R1 on GABA synapses on to VTA DA neurons.
CRF-R1 acts through PKC and calcium-induced calcium release
CRF receptors couple to the Gs alpha subunit to activate adenylyl cyclase, as well as the Gq alpha subunit to activate PLC–PKC [47]. To better understand the intracellular pathway regulating the CRF potentiation of GABA onto dopamine (DA) cells, we evaluated eIPSCs during co-manipulation of CRF-R1 and the PKC enzyme.
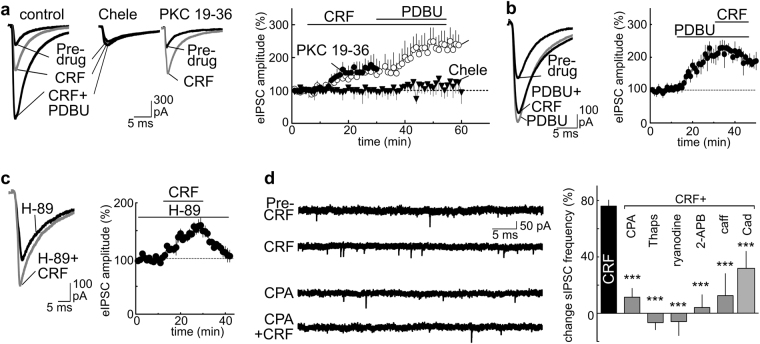
As previously shown, CRF increased the amplitude of eIPSCs (Fig. 2a; t(9) = 4.6327, p = 0.0143) and these responses were further potentiated by phorbol-ester-dybutyrate (PDBU), an activator of PKC (Fig. 2a; t(7) = 4.1032, p = 0.0178). However, if PDBU (1 µM) was applied first, application of CRF (300 nM) produced no additional increase in eIPSC amplitude (Fig. 2b). The lack of a CRF action in the presence of PDBU was not due to a ceiling effect of maximal chloride conductance, because at the end of the experiment a more intense synaptic stimulation (~35%) further increased the inward current (158.4% ± 51.6% relative to baseline, t(2) = 16.99, p = 0.0017). The PDBU potentiation of eIPSC amplitude was not reduced by the CRF-R1 antagonist K41498 (PDBU vs PDBu + K414: 0.56% ± 11.7%, t(5) = 0.3577, p < 0.3676), suggesting an activation of PKC activity downstream of CRF-R1.
Fig. 2.
The CRF potentiation of GABAA receptor IPSCs requires PKC and intracellular calcium stores. a The CRF (200 nM) potentiation of eIPSCs is further increased by the PKC activator Phorbol 12,13-dibutyrate (PDBU; 1 µM). Bath incubation of slices with the PKC inhibitor chelerythrine (Chele, 1 µM), blocked the actions of CRF (200 nM) and PDBU (1 µM), whereas inclusion of the cell impermeable PKC inhibitor PKC 19-36 (300 nM) in the internal recording solution did not. The timeline shows the eIPSC amplitude as a percentage of pre-drug baseline ± SEM, normalized for each cell using the mean amplitude recorded during the first 5 min of pre-drug application and plotted as a function of time. b Application of PDBU (1 µM) prior to CRF (200 nM) reduces the CRF potentiation of eIPSCs. c Incubation of brain slices with the PKA inhibitor (H-89: 10 µM; incubation > 30 min) did not prevent the CRF potentiation of eIPSCs. d Summary bars showing pharmacological manipulation of calcium stores reduces the actions of CRF. Drugs include antagonists/inhibitors of: Ca2+-ATPases [cyclopiazonic acid (CPA 10 µM), thapsigargin (Thaps, 5 µM)], ryanodine receptors (ryanodine, 50 µM), IP3 receptors (2-aminoethoxydiphenyl-borate (2-APB, 10 µM)); voltage-dependent calcium channels (cadmium, Cad 300 µM). Also tested is caffeine (caff, 10 mM), an agonist of ryanodine receptors used to deplete intracellular calcium stores. CRF (300 nM) data in bar graph are duplicate from Fig. 1c, shown again for comparison. One-way ANOVA, ***p < 0.001 vs CRF alone (Dunnett’s post test). a–d Number of cells/mice per group: a control 10/5, Chele 15/7, PKC 19-36 8/4; b 6/3; c 10/5; d CRF 15/7, CRF+CPA 8/4, CRF+Thaps 5/3, CRF+ryanodine 6/4, CRF+2-APB 5/3, CRF+caff, 6/3, Cad 8/4
To corroborate that PKC may be involved in this response, we performed similar experiments in the presence of various PKC or PKA inhibitors. Experiments performed using an internal solution containing the selective, non-membrane permeable pseudo-substrate blocker of PKC, PKC 19-36 (5 µM internal solution; infusion >20 min) [48] showed that CRF still potentiated eIPSCs, suggesting an action independent of postsynaptic PKC (Fig. 2a; t(5) = 4.2340, p = 0.0117). However, treatment of slices with chelerythrine (chele: 1 μM, >30 min), which has been reported to inhibit the catalytic subunit of PKC [49, 50], did block the effects of both CRF and the PKC activator PDBU on eIPSCs (Fig. 2a; CRF+chele t(7) = 0.03016, p = 0.5781) [49]. Although this suggests that CRF may enhance GABAA IPSCs via a presynaptic PKC-dependent mechanism, chelerythrine is not universally accepted to be a PKC inhibitor and has other actions unrelated to PKC [51–54]. So, caution is warranted. However, we noted that incubation with the membrane permeant PKA inhibitor H89 (10 μM; >30 min incubation; present during recording) did not prevent the CRF potentiation of IPSCs (Fig. 2c; t(4) = 4.0124, p = 0.0223). If indeed the CRF-R1 action in mice is PKC dependent, then this would differ from the CRF-R2/PKA-dependent mechanism we previously observed in rats [34].
Neuropeptides can regulate transmitter release by a kinase-dependent mobilization of calcium release from intracellular stores [47]. Accordingly, we observed that depletion of stores by incubating slices (>15 min) with cyclopiazonic acid (CPA; 10 µM) reduced the CRF facilitation of mIPSC frequency (29.2% ± 2.84% decrease relative to pre-CPA; t(9) = 10.89, p < 0.0001), but not the amplitude (mIPSC amplitudes 9.26% ± 13.12% decrease relative to baseline, t(9) = 0.5246, p = 0.3063). As the frequency of the recordable mIPSCs decreased with CPA, we confirmed the results by also measuring sIPSCs. Slices were treated with CPA, thapsigargin (5 µM), ryanodine, caffeine, or 2-APB (Fig. 2d)—drugs that either block IP3/ryanodine receptors or deplete calcium stores [55–57]. Because the frequency of sIPSCs varied considerably between slices/cells even under control conditions without antagonist (range 4–8 Hz, mean 4.39 ± 0.43 Hz), we determined the CRF-related increase in the frequency within cells before CRF (in the presence of the antagonist) and during CRF. A one-way between-subject ANOVA comparing CRF responses in the presence of the antagonists indicated significant differences (Fig. 2d; F(6, 45) 16.21, p < 0.0001). Post hoc tests (multiple comparisons Dunnett’s) indicated the change was significant for all the compounds (Fig. 2d; p < 0.0001 all drugs, except cadmium; cadmium, p < 0.006). A summary of the frequency and amplitude of sIPSCs with different treatments is shown in Table 1. The observation that these different treatments all reduced the CRF action on sIPSCs (Fig. 2d), indicates a need for functional calcium stores. It was notable that treatment with the non-selective calcium channel blocker cadmium reduced, but did not block, the CRF action on sIPSCs (Fig. 2d). Thus, although CRF facilitation does not require extracellular calcium, calcium entering through voltage-gated calcium channels likely potentiates the calcium release from intracellular stores (calcium-induced calcium release) [55].
Table 1.
Frequency (Hz) and amplitude (pA) of sIPSCs
| Frequency | Amplitude | ||
|---|---|---|---|
| Group | +Drug | (Hz) | (pA) |
| Naive | – | 4.23 ± 0.72 | 47.94 ± 7.52 |
| CPA | 4.62 ± 0.26 | 47.81 ± 10.18 | |
| 2APB | 4.59 ± 0.49 | 49.61 ± 6.09 | |
| thaps | 3.86 ± 0.37 | 47.67 ± 4.93 | |
| RyR | 5.98 ± 0.41 | 43.38 ± 4.56 | |
| Caff | 1.77 ± 0.32 | 53.92 ± 6.90 | |
| Cad | 6.33 ± 1.17 | 46.31 ± 6.13 | |
| ST-air | – | 3.41 ± 0.57 | 42.40 ± 3.49 |
| ST-CIE | – | 6.84 ± 0.40 | 47.86 ± 4.23 |
| LS-air | – | 4.23 ± 0.33 | 41.02 ± 5.21 |
| LT-CIE | – | 3.40 ± 0.39 | 42.08 ± 5.84 |
CPA cyclopiazonic acid (10 µM), 2APB 2-aminoethoxy-diphenyl-borate (10 µM), Thaps thapsigargin (5 µM), RyR ryanodine (50 µM), caff caffeine (10 mM), Cad cadmium (300 µM), ST-air short-term air, LT-air long-term air, ST-CIE short-term CIE, LT-CIE long-term CIE
Mean ± SEM
Reduced CRF and EtOH effects on GABAA currents after long-term withdrawal from CIE exposure
Similar to other brain regions, ethanol can enhance GABA release onto VTA dopamine cells [58, 59]. Using VTA slices from naive mice, we measured the frequency of GABAA IPSCs during ethanol application (50 mM; 10 min) and confirmed that the potentiation also occurs under our recording conditions (Fig. 3a; t(5) = 5.733, p = 0.0023). The ethanol action may require CRF-R1 and the release of calcium from intracellular stores, as observed in other brain regions [37], and so we wondered if this also occurs in the VTA. To evaluate this, we measured the ethanol enhancement of sIPSC frequency in slices from naive mice during bath application of: (1) ethanol, (2) ethanol plus the CRF-R1 antagonist CP154, or (3) ethanol plus CRF. A one-way ANOVA comparing responses under these conditions indicated no significant differences (Fig. 3a; F(2, 32) = 0.1467, p = 0.8644). The absence of an additive effect of CRF on ethanol or any reduction in the ethanol action by the CRF-R1 antagonist suggests that the “stress-like” actions of ethanol may reflect intracellular alterations somewhere downstream of the CRF-R1 receptor.
Fig. 3.
Reduction in responses to CRF or ethanol following long-term withdrawal from chronic intermittent ethanol (CIE) exposure. a In neurons from naive mice, bath application of CRF (300 nM) or ethanol (EtOH; 50 mM) increases the sIPSC frequency relative to their baseline to a similar extent. Ethanol responses are not significantly altered by co-application of the CRF-R1 antagonist CP154156 (CP154; 300 nM; incubation > 12 min) or CRF (CRF+EtOH). The CRF traces are shown for comparison. See Fig. 1c for average CRF data; ***p < 0.001 compared to baseline. b Schematic showing experimental timeline for chronic intermittent ethanol (CIE) exposure. Habituation (5 days) was followed by vapor chamber exposure to ethanol (or air for controls) over 4 cycles (16 h per day; 4 days per week). Blood ethanol concentrations (BEC) were measured after each cycle and averaged ~210 mg/dl—a concentration sufficient to produce tolerance and dependence (see Materials and methods). After the final air/CIE treatment, mice were returned to the home cage. Brain slices were later prepared to compare physiological responses after a period of short-term withdrawal (ST; 3 days after treatment) or long-term withdrawal (LT; pooled responses from 20, 30 and 45 days after treatment and reported as a group). c Sample sIPSC traces from LT-air (top) and LT-CIE (bottom) cells before (baseline) and during bath application of CRF (300 nM) or ethanol (EtOH; 50 mM). d Summary graph showing differences in baseline frequency of sIPSC in ST and LT withdrawal groups without application of CRF or ethanol. One-way ANOVA, ***p < 0.001 ST-CIE vs LT-CIE (Dunnett’s post test). e During ST withdrawal, CRF (200 nM) and ethanol (EtOH; 50 mM) enhance sIPSC frequency similar to control levels. f During LT withdrawal, the CRF (200 nM) and EtOH (50 mM) action is reduced in CIE group relative to controls. Two-way ANOVA, p < 0.0001 for effect of treatment. a–f Number of cells/mice per group: a EtOH 6/4, CP154+EtOH 6/4, CRF+EtOH 7/5; d ST-air 5/4; ST-CIE 6/5, LT-air 5/4, LT-CIE 11/6; e ST-air-CRF 18/9; ST-CIE-CRF 13/6, ST-air-EtOH 10/5, ST-CIE- EtOH 17/8; f LT-air-CRF 18/9; LT-CIE-CRF 11/6, LT-air-EtOH 10/7, LT-CIE-EtOH 9/6
CIE exposure produces dependence and robustly activates CRF stress systems [31, 39, 41]. So, we predicted that CIE treatment (Fig. 3b) would also alter the GABAA responses to CRF and ethanol. To test this, we prepared VTA slices from mice to evaluate the air or CIE treatments under two conditions: short-term (ST) withdrawal of 72 h or a long-term (LT) withdrawal of 20–45 days. We recorded basal frequency of sIPSCs (in Hz) in the ST and LT groups (Fig. 3c, d) and found a significant interaction between treatment and time (two-way ANOVA, F(1, 21) = 4.457, p = 0.0469; post hoc Dunnett’s multiple comparison test: ST- vs LT-CIE p = 0.0007, ST- vs LT-air p = 0.7219). A summary of the frequency and amplitude of sIPSCs with different treatments is shown in Table 1. This suggests that the CIE-induced change in basal release of GABA is temporary: During the ST withdrawal periods, the frequency of sIPSCs increase, whereas during LT withdrawal periods, the spontaneous release of GABA decreases toward control levels.
It was not clear whether the decrease in sIPSCs represented an active change or simply recovery from the acute effects of CIE. On this basis, we examined the actions of CRF and ethanol on sIPSCs starting with the ST withdrawal group. A two-way ANOVA revealed no significant differences in response to CRF or ethanol for sIPSCs (Fig. 3e: treatment (F(1, 20) = 3.435, p = 0.0786), drug (F(1, 20) = 0.0016, p = 0.9682); interaction of treatment × drug (F(1, 20) = 1.14, p = 0.2984)), nor for eIPSCs (treatment (F(1, 20) = 0.3054, p = 0.5866), drug (F(1,20) = 0.1761, p = 0.6792); interaction of treatment × drug (F(1, 20) = 0.0026, p = 0.9625)). This suggests that during ST withdrawal, the mechanisms that facilitate GABA release remains functional. However, a similar comparison after LT withdrawal did reveal differences in the ability of CRF and ethanol to alter the frequency of sIPSCs (Fig. 3c, f). For sIPSCs, a two-way ANOVA showed a significant effect of treatment (F(1, 17) = 142.2, p < 0.0001), but not drug (F(1, 17) = 0.8148, p = 0.3793) or interaction (F(1, 17) = 1.15, p = 0.2986). As a point of control, additional experiments were performed measuring eIPSCs and a two-way ANOVA of eIPSCs also revealed an effect of treatment (F(1, 17) = 10.64, p < 0.0046), but not drug (F(1, 17) = 0.03609, p = 0.8516 or interaction (F(1, 17) = 0.1804, p = 0.6764). Thus, the actions of CRF and alcohol on the release of GABA appear similar, but measurably change following LT withdrawal from CIE treatments.
CB1-mediated inhibition opposes the actions of CRF
To determine the signaling mechanism underlying the blunted CRF and ethanol modulation of GABAA currents after 20–45 days of withdrawal from CIE treatment, we retested the response to CRF and the PKC activator PDBU using eIPSCs, as they are easily discernable due to their large size. While CRF and PDBU responses in LT-air mice were robust (Fig. 4a; CRF: t(6) = 3.3810, p = 0.0221, PDBU: t(4) = 03.6542, p = 0.0323) and resembled those in naive tissue as noted previously, no increase was observed in response to PDBU in slices from LT-CIE mice (Fig. 4a; CRF: t(6) = 0.3415, p = 0.3524, PDBU: t(5) = 0.2153, p = 0.2972). Although acute somatic withdrawal from alcohol is known to increase the synthesis of CRF [60], no evidence of a ceiling effect, nor a CRF tone, was observed, as application of the CRF-R1 antagonist alone produced no significant change in eIPSCs during LT withdrawal (CP154156 (300 nM): 4.95 ± 1.98% increase relative to baseline; t(3) = 0.6470, p = 0.2819). Thus, during LT withdrawal, CRF-R1 did not seem to be tonically activated and some other functional adaptation must emerge in the weeks following chronic ethanol exposure that limits the ability of CRF, ethanol, and PKC-to facilitate GABAergic neurotransmission.
Fig. 4.
The reduction in CRF-R1 activity during LT withdrawal from CIE is associated with enhanced CB1 receptor-mediated inhibition. a Sample eIPSCs traces and summary data showing reduced responding to CRF (300 nM) and PDBU (1 µM) in neurons from CIE treated mice. b,c Sample eIPSCs traces and summary data showing LT-CIE cells are (b) less sensitive to CRF (300 nM) and the CB1 agonist WIN55212 (WIN; 1 µM) but (c) more sensitive to the CB1 antagonist AM251 (1 µM). In (b), note the WIN-related reversal of the CRF potentiation in the air controls. d,e Sample eIPSCs traces and summary data illustrating that enhanced calcium buffering (1 mM BAPTA vs 0.1 mM EGTA in the recording solution) in postsynaptic LT-CIE cells rescues the (d) CRF (300 nM) and PDBU (1 mM) mediated increase in eIPSC amplitude (Two-way ANOVA, ***p < 0.0001 for effect of treatment (internal chelator)),as well as reduces the sensitivity to the (e) CB1 receptor antagonist AM251 (***p < 0.0001). f Schematic of proposed mechanism of CRF regulation of GABA release onto VTA dopamine neurons during LT withdrawal following CIE. Left, in controls, presynaptic CRF-R1 facilitates the release of GABA onto GABAA receptors via PKC mediated recruitment of intracellular calcium. During LT withdrawal from CIE, enhancement of endocannabinoid (eCB) inhibition opposes the CRF action. Chelation of intracellular calcium with BAPTA in the dopamine neuron reduces eCB synthesis, reducing the CB1-mediated suppression of the presynaptic CRF dependent mechanism. (a-d) Number of cells and mice per LT-group: (a) air 8/4, CIE 8/5; (b) air 8/4, CIE 8/5; (c) air 8/4, CIE 8/5;(d) for both EGTA and BAPTA: CRF 7/6, PDBU 6/4; (e) for both EGTA and BAPTA: AM251 6/4. All timelines show eIPSC amplitude % of baseline, mean ± SEM
We and others have shown that activation of CB1 receptors can suppress VTA GABA release [61, 62], and in amygdala neurons this reverses the facilitation produced by acute exposure to ethanol [16]. Consistent with these reports, we noted that application of the CB1 agonist WIN55212 (1 μM) alone in slices from LT-air mice significantly decreased eIPSCs and subsequent co-application of CRF (300 nM) reversed this action of WIN55212 (Supplemental Fig. 2; WIN: 52.8% ± 4.97% decrease relative to baseline; t(4) = 4.085, p = 0.0075; WIN + CRF, 43.6% ± 3.85% increase relative to WIN alone t(4) = 3.918, p = 0.0139). The same concentrations of WIN55212 reversed the CRF (300 nM) potentiation of eIPSCs in LT-air controls (Fig. 4b; pre-CRF vs CRF + WIN: t(4) = 0.25465, p = 0.4701). In contrast, in slices from LT-CIE mice, neither CRF nor co-application of WIN55212 with CRF measurably changed IPSC amplitudes (Fig. 4b; CRF, t(4) = 0.2314, p = 0.3138; WIN, t(5) = 0.2834, p = 0.3807).
To determine if the diminished actions of CRF and WIN55212 in LT-CIE mice reflected an enhancement of endocannabinoid CB1 receptor inhibition, we measured the effects of CB1R antagonist AM251 (1 μM) on eIPSCs. In control LT-air tissue, AM251 produced no change in eIPSC amplitudes when applied after CRF (Fig. 4c; t(6) = 0.2128, p = 0.3781), suggesting the CRF potentiation of eIPSC amplitude was not restricted by inhibitory tone on CB1 receptors under control conditions. Bath application of AM251 (1 μM) alone in the LT-air control group produced no measurable change in eIPSCs (AM251: 8.67% ± 6.13% increase relative to baseline; t(6) = 0.3408, p = 0.7449). This finding with VTA GABAA IPSCs is consistent with our previous published findings by us and others indicating the absence of CB1 receptor tone in drug-naive animals [61, 63]. In tissue from LT-CIE mice, as noted above, CRF applied alone produced no change in IPSC amplitude (Fig. 4c; t(6) = 0.2128, p = 0.3781). However, application of the CB1 antagonist AM251 in the presence of CRF produced a robust enhancement of eIPSC amplitude (Fig. 4c; t(6) = 32.01, p < 0.0001). This suggests that LT withdrawal from CIE treatment is associated with a functional enhancement of CB1 inhibition on GABA synapses of VTA DA neurons. This CB1-mediated tone blocks subsequent modulation of GABA release by CRF or ethanol and may explain the reduction in the basal frequency of sIPSCs observed in Fig. 3d.
As endocannabinoid synthesis requires increases in intracellular calcium in the postsynaptic neuron [64], we tested whether chelating intracellular calcium post-synaptically in the recorded DA neuron would restore CRF and PDBU sensitivity in LT-CIE tissue. Under normal recording conditions (0.1 mM EGTA) in LT-CIE tissue, CRF and PdBU produced minimal changes in eIPSC amplitude (Fig. 4d). In contrast, when recording with an internal solution containing the fast calcium chelator BAPTA (1 mM), both CRF and PDBU enhanced eIPSC amplitude (Fig. 4d). A two-way ANOVA that revealed a significant effect of treatment (internal chelator; F(1, 16) = 51.92, p < 0.0001), but not for drug (F(1, 16) = 3.892, p = 0.0660) or interaction (F(1, 16) = 2.126, p = 0.1641). Thus, chelation of intracellular calcium in the postsynaptic neurons of LT-CIE mice restored the ability of bath applied CRF, which acts on the presynaptic CRF-R1, to potentiate GABAA IPSCs.
As a final test that the LT-CIE treatment functionally enhanced endocannabinoid activity at VTA GABA synapses, we measured changes in the amplitude of eIPSCs in the presence of AM251 under normal recording conditions (0.1 mM EGTA) or altered Ca2+ chelation conditions (BAPTA). In the absence of CRF, AM251 again produced a robust increase in eIPSC amplitude in LT withdrawal (Fig. 4e). We observed that the AM251 potentiation of eIPSCs under normal recording conditions was greatly reduced by inclusion of BAPTA in the recording electrode (Fig. 4e; t(9)=4.975, p = 0.0008). Taken together, these results show in the weeks following cessation of CIE treatment, the presynaptic CRF-R1-mediated plasticity, typically operative at VTA GABA terminals, is suppressed by a functional enhancement of endocannabinoid-activated CB1 receptors (Fig. 4f).
Discussion
We previously reported that in rats activation of presynaptic CRF-R2 facilitated GABA release, and could indirectly reduce glutamate transmission, onto VTA dopamine cells by stimulating presynaptic GABAB receptors [34]. Here we show in mice that CRF increases VTA GABA release via CRF-R1. Acute alcohol produced a similar action [58, 65] and we showed that CRF-R1 antagonism did not prevent alcohol-induced GABA release. Short-term withdrawal from chronic ethanol exposure did not affect the acute actions of CRF or ethanol, but in subsequent weeks the emergence of endocannabinoid inhibition suppressed the CRF potentiation of GABA release. Such a functional rearrangement of GABAergic inhibition would predictably increase the responsiveness of mesolimbic dopamine neurons to excitatory inputs and this hyper-responsiveness could contribute to long-term vulnerability to relapse following chronic ethanol exposure. In addition to new information about CB1 receptor activation following CIE, this work highlights the differential regulation of GABA between two species and shows an effect of CRF-R1 that has been reported in other regions but not the VTA [66–69].
Presynaptic CRF-R1 facilitates GABA release
We found that CRF altered the PPR and the frequency of sIPSCs/mIPSCs via GABAA receptors. CRF activated PKC and presynaptic intracellular calcium stores, which serve as reservoirs for release machinery [70]. Calcium entry via voltage-gated calcium channels (VGCC) further augments the release of neurotransmitter [71]. Blockade of VGCCs reduces the frequency of IPSCs [71] and the CRF facilitation in the VTA. However, only manipulation of ryanodine and IP3 receptors abolished the CRF action, indicating that VGCCs amplify CRF-mediated calcium release from stores [55]. Because calcium stores integrate electrical and chemical signals, these CRF pathways are likely necessary for correctly encoding synaptic plasticity in dopamine neurons [55, 72].
CRF G protein-coupled receptors (GPCRs) can activate adenylyl cyclase (AC) as well as PKA and PKC, and the intracellular second messenger cyclic adenosine monophosphate, and thus increase levels of free intracellular calcium (for review see refs. [73–75]). A CRF-R1/PKC action on GABA release also exists in the prefrontal cortex [67] and the amygdala [76]. In contrast, in rats, we previously observed that CRF-R2 facilitated GABA release onto VTA dopamine cells, indicating a potentially important species difference [34]. Our findings here add the VTA to the growing list of CRF neuromodulations, via both inhibitory (D2 and GABA-B, mGluRs, M1-mAChRs) [34, 44, 75, 77] and excitatory (N-methyl-d-aspartate (NMDA); hyperpolarization-activated cyclic nucleotide-gated cation (HCN)) [36, 78] mechanisms.
CRF and EtOH regulation of GABA release
Alcohol-induced inhibition has previously been reported in the VTA, substantia nigra, basolateral amygdala, brainstem, cerebellum, and hippocampus of naive rodents [37]. Although we did not determine the direct site of action for alcohol, the PKC/PKA/Ca2+ signaling pathway is likely involved, as other VTA reports have already shown that acute alcohol increases GABA frequency via 5-HT(2C) receptors, independent of GABA-B and D1-dopamine receptors [58]. Alcohol also increased GABA release in cerebellar interneurons through PKA, PKC, and intracellular calcium pathways, and in the amygdala via PKC [37]. In our study, CRF-R1 antagonism did not prevent alcohol-induced GABA release in the VTA of naive mice (as previously described in the CeA [79]), nor did pretreatment with acute CRF yield additive increases with ethanol in naive mice . This suggests a convergence of the alcohol and CRF signaling system downstream of the CRF-R1 receptor. Future work must determine if alcohol and CRF actions in the VTA shared a common PKC mechanism [76].
Changes following CIE
Following a history of alcohol dependence and withdrawal, CRF expression is upregulated in the long-term in multiple brain areas for a period that outlast the brief heightened hypothalamic–pituitary–adrenal axis activation [5]. We found that basal GABA release was enhanced after brief withdrawal from chronic ethanol exposure, compared to age-matched air control mice, suggesting greater local inhibition of VTA neurons in alcohol-dependent mice. This may be an action of ethanol common to both the VTA and CeA, as CeA showed similar responses in intoxicated rodents immediately following CIE [17, 80]. However, regional specificity of ethanol’s long-term effects on GABA release exist, since 24 h after CIE, GABA release in the dorsal raphe was reduced, but more sensitized to alcohol [81]. Ethanol-induced GABA release in air control mice was consistent with published VTA and amygdala studies [58, 65, 82] and similar to CRF-induced GABA release. Our observation that acute CRF and alcohol stimulated GABA release in alcohol-dependent mice to a similar magnitude as in naive rats indicates a lack of functional tolerance to acute alcohol or CRF actions at VTA-GABAergic synapses. A similar lack of tolerance to alcohol was reported in the CeA of ethanol-intoxicated animals immediately after exposure to CIE treatment (5–7 weeks) [80]. This also means that the previously reported changes in CRF-R1 synthesis, expression, and internalization in other regions were presumably either minimal in the VTA or were resolved after 3 days of withdrawal [83, 84].
Following protracted withdrawal, this scenario reversed: basal GABA release normalized in the VTA of alcohol-dependent mice. Moreover, acute CRF or alcohol-induced GABA release was suppressed in the VTA of alcohol-dependent mice relative to air controls, indicating an emergent adaptation in VTA-GABAergic synapses to protracted withdrawal. Besides CRF-R1, other GPCRs have been implicated in alcohol-induced CeA GABA release, including the CB1 receptor [37]. We found that co-application of a CB1 agonist reversed the acute CRF-induced GABA release in air control mice, suggesting opposing actions of these two neuromodulators at VTA GABA terminals under baseline conditions. In contrast, CRF no longer induced GABA release, nor did ethanol or the PKC activator PDBU, after long-term withdrawal from CIE. The CB1 receptor agonist also did not alter GABA release after long-term withdrawal from CIE. Application of an antagonist for CB1 receptors produced robust increases in GABA responses after long-term withdrawal, which was not seen in matched air controls. These findings suggest the emergence of an endocannabinoid tone during protracted withdrawal from CIE, given that: (1) chelation of calcium with BAPTA in the postsynaptic membrane reduced the actions of the CB1 antagonist on GABA IPSCs and restored the CRF-induced GABA release in a manner that is conventional for endocannabinoids [64], (2) the CB1 agonist was ineffective in altering GABA release, and (3) a CB1 antagonist robustly potentiated GABA release in the presence of CRF.
These results coincide with known actions of repeated exposure to ethanol on CB1 receptor inhibition, evoking a transient downregulation of CB1 followed by a long-term upregulation including increased levels of endogenous cannabinoids [85–88] and studies showing CB1 knockout animals are less sensitive to acute actions of ethanol-associated adaptations [89, 90]. A more complex role of CB1 receptor inhibition in alcohol-associated changes in the CeA has also been reported [91]. Future studies should identify which endocannabinoid mediates this action, whether changes in presynaptic GABA responses induced by acute alcohol are also restored after calcium chelation, and to what extent the function of other GPCRs are impacted.
Functional implications and caveats
Stress plays a pivotal role in alcohol misuse and the magnitude of CRF release during this period determines subsequent stress responsiveness to incentivize excessive drinking [5]. During the weeks or months following the binge-like exposure to ethanol associated with the CIE model [92] the repeated transient homeostatic alterations in CRF signaling eventually stabilize at a new allostatic state [5, 37]. The neuroregulation of GABAergic plasticity in the VTA is crucial for burst firing in dopaminergic neurons [21, 93] and shifts in an enduring manner in the weeks following CIE. The ability of CRF-R1 to potentiate GABA release is suppressed and superseded by a dominating CB1 receptor inhibition of GABA release, which would predictably favor VTA output [94]. In non-dependent animals, the ethanol enhancement of VTA GABA release onto inhibitory GABAA receptors is typically sufficient to overcome the direct stimulatory effect of ethanol on DA neuron activity [58, 65]. After repeated alcohol misuse and dependence, a problematic imbalance arises, potentially contributing to sensitizing the VTA to excitatory stimuli thus contributing to the relapse of alcohol seeking. In the short term, this may contribute to the initial withdrawal associated with CNS hyperexcitability that peaks around 24 h. However, recent evidence shows that protracted abstinence is also associated with elevated anxiety, dysphoria, negative affect, craving, and drug relapse [95]. In mice these withdrawal-related changes in emotional states can last 3 weeks [96] and may promote the high levels of alcohol intake associated with escalated drinking in mice [97, 98].
Although in the current study, we observed long-lasting functional changes in GABA plasticity following CIE exposure in male mice, it remains to be determined whether similar changes extend to females that do not show increased drinking after CIE treatments [38]. Previous studies suggest gender differences would also be anticipated for stress–alcohol interactions. In the CeA, neurons from female rats show reduced sensitivity to acute bath applied alcohol relative to cells from male rats [99]. Likewise, in female mice, stress decreases alcohol intake [100] and human females experience less craving and behavioral arousal in response to alcohol cues than human males [101]. Although we did not examine the contribution of the CRF-binding protein, CIE exposure is known to decrease the expression of CRF-binding protein mRNA to promote increased CRF signaling and alcohol seeking [102, 103]. Future studies are needed to address the role of the CRF binding protein in the dysregulation of GABA inhibition of VTA dopamine neurons following long-term withdrawal from ethanol.
Third, although the neurons we sampled presumably contribute to drug use/seeking and some portion of this population innervates the accumbens [104, 105], the architecture of the VTA is complex. The topography of the sampled dopamine neurons was not determined [106] and CRF and cannabinoids reportedly exert region-specific actions [107–109]. Lastly, the CB1 antagonist AM251 possesses inverse agonists properties [110] and we cannot rule out the possibility that allosteric modulation of CB1 receptors contributes to our measured responses or the ability of these compounds to decrease the reinforcing properties of alcohol consumption in rodents [25, 111–113]. Collectively, these studies are expected to enhance our understanding of CRF-R1 and CB1 plasticity in the VTA and contribute to the development of better treatments for relapse to alcohol seeking.
Electronic supplementary material
Acknowledgements
NIDA grant R01-DA033342 (AR), NIAAA grants R37AA009986 (JW), U01 AA014095 (HCB), and a pilot grant from the Alcohol Research Center (ARC; P50-AA10761), and VA grant (BX000813 (HCB). We thank Mr. Buchta and Mrs. Pavlinchak for their helpful comments.
Author contributions
BAH and ACR conducted the electrophysiology experiments, performed analysis, and wrote the manuscript. HCB and JJW generated CIE mice and analyzed CIE blood levels.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-018-0106-9).
References
- 1.Cui C, Noronha A, Morikawa H, Alvarez VA, Stuber GD, Szumlinski KK, et al. New insights on neurobiological mechanisms underlying alcohol addiction. Neuropharmacology. 2013;67:223–32. doi: 10.1016/j.neuropharm.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morikawa H, Morrisett RA. Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol. 2010;91:235–88. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–9. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 4.Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–40. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- 5.Becker HC. Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology. 2017;122:115–26. doi: 10.1016/j.neuropharm.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zapata A, Shippenberg TS. Endogenous kappa opioid receptor systems modulate the responsiveness of mesoaccumbal dopamine neurons to ethanol. Alcohol Clin Exp Res. 2006;30:592–7. doi: 10.1111/j.1530-0277.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 8.Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res. 1998;22:367–74. [PubMed] [Google Scholar]
- 9.Pomrenze MB, Fetterly TL, Winder DG, Messing RO. The corticotropin releasing factor receptor 1 in alcohol use disorder: still a valid drug target? Alcohol Clin Exp Res. 2017;39:1609–999. doi: 10.1111/acer.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology. 2014;231:1557–80. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 12.Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol. 2008;506:616–26. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwa LS, Debold JF, Miczek KA. Alcohol in excess: CRF1 receptors in the rat and mouse VTA and DRN. Psychopharmacology. 2013;225:313–27. doi: 10.1007/s00213-012-2820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sparta DR, Hopf FW, Gibb SL, Cho SL, Stuber GD, Messing RO, et al. Binge ethanol-drinking potentiates corticotropin releasing factor R1 receptor activity in the ventral tegmental area. Alcohol Clin Exp Res. 2013;37:1680–7. doi: 10.1111/acer.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, et al. Extended amygdala to ventral tegmental area corticotropin-releasing factor circuit controls binge ethanol intake. Biol Psychiatry. 2017;81:930–40. doi: 10.1016/j.biopsych.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology. 2010;35:1962–72. doi: 10.1038/npp.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–9. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Rev. 1997;25:312–34. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 19.Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995;15:3092–103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paladini CA, Tepper JM. GABA(A) and GABA(B) antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse. 1999;32:165–76. doi: 10.1002/(SICI)1098-2396(19990601)32:3<165::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Paladini CA, Iribe Y, Tepper JM. GABAA receptor stimulation blocks NMDA-induced bursting of dopaminergic neurons in vitro by decreasing input resistance. Brain Res. 1999;832:145–51. doi: 10.1016/s0006-8993(99)01484-5. [DOI] [PubMed] [Google Scholar]
- 22.Cheer JF, Cheer JF, Wassum KM, Heien MLAV, Phillips PEM, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–50. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 24.Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- 25.Arnone M, Maruani J, Chaperon F, Thiébot MH, Poncelet M, Soubrié P, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–6. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 26.Colombo G, Agabio R, Fà M, Guano L, Lobina C, Loche A, et al. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126–30. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- 27.Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25:277–82. [PubMed] [Google Scholar]
- 28.Cheer JF, Cheer JF, Wassum KM, Sombers LA, Heien MLAV, Ariansen JL, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–5. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 30.Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur J Pharmacol. 2003;466:73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- 31.Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–38. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- 32.Diana M, Pistis M, Muntoni A, Gessa G. Mesolimbic dopaminergic reduction outlasts ethanol withdrawal syndrome: evidence of protracted abstinence. Neuroscience. 1996;71:411–5. doi: 10.1016/0306-4522(95)00482-3. [DOI] [PubMed] [Google Scholar]
- 33.Williams JT, North RA, Shefner SA, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984;13:137–56. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- 34.Williams CL, Buchta WC, Riegel AC. CRF-R2 and the heterosynaptic regulation of VTA glutamate during reinstatement of cocaine seeking. J Neurosci. 2014;34:10402–14. doi: 10.1523/JNEUROSCI.0911-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marino MJ, Wittmann M, Bradley SR, Hubert GW, Smith Y, Conn PJ. Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata. J Neurosci. 2001;21:7001–12. doi: 10.1523/JNEUROSCI.21-18-07001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wanat MJ, Hopf FW, Stuber GD, Phillips PEM, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol (Lond) 2008;586:2157–70. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberto M, Varodayan FP. Synaptic targets: chronic alcohol actions. Neuropharmacology. 2017;122:85–99. doi: 10.1016/j.neuropharm.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jury NJ, DiBerto JF, Kash TL, Holmes A. Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol. 2017;58:53–60. doi: 10.1016/j.alcohol.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–96. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- 40.Dhaher R, Finn D, Snelling C, Hitzemann R. Lesions of the extended amygdala in C57BL/6J mice do not block the intermittent ethanol vapor-induced increase in ethanol consumption. Alcohol Clin Exp Res. 2008;32:197–208. doi: 10.1111/j.1530-0277.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 41.Griffin WC, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffin WC, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology. 2009;201:569–80. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez MF, Griffin WC, Melendez RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure leads to the development of tolerance to aversive effects of ethanol in C57BL/6J mice. Alcohol Clin Exp Res. 2012;36:1180–7. doi: 10.1111/j.1530-0277.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PE, Mark GP, et al. CRF enhancement of GIRK channel-mediated transmission in dopamine neurons. Neuropsychopharmacology. 2009;34:1926–35. doi: 10.1038/npp.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol (Lond) 1996;491(Pt 1):163–76. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–96. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- 47.Arzt E, Holsboer F. CRF signaling: molecular specificity for drug targeting in the CNS. Trends Pharmacol Sci. 2006;27:531–8. doi: 10.1016/j.tips.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Yasunari K, Kohno M, Kano H, Yokokawa K, Horio T, Yoshikawa J. Possible involvement of phospholipase D and protein kinase C in vascular growth induced by elevated glucose concentration. Hypertension. 1996;28:159–68. doi: 10.1161/01.hyp.28.2.159. [DOI] [PubMed] [Google Scholar]
- 49.Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–9. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- 50.Ko FN, Chen IS, Wu SJ, Lee LG, Haung TF, Teng CM. Antiplatelet effects of chelerythrine chloride isolated from Zanthoxylum simulans. Biochim Biophys Acta. 1990;1052:360–5. doi: 10.1016/0167-4889(90)90144-3. [DOI] [PubMed] [Google Scholar]
- 51.Yu R, Mandlekar S, Tan TH, Kong AN. Activation of p38 and c-Jun N-terminal kinase pathways and induction of apoptosis by chelerythrine do not require inhibition of protein kinase C. J Biol Chem. 2000;275:9612–9. doi: 10.1074/jbc.275.13.9612. [DOI] [PubMed] [Google Scholar]
- 52.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson LJ, Fields AP. betaII protein kinase C is required for the G2/M phase transition of cell cycle. J Biol Chem. 1996;271:15045–53. doi: 10.1074/jbc.271.25.15045. [DOI] [PubMed] [Google Scholar]
- 54.Gould CM, Antal CE, Reyes G, Kunkel MT, Adams RA, Ziyar A, et al. Active site inhibitors protect protein kinase C from dephosphorylation and stabilize its mature form. J Biol Chem. 2011;286:28922–30. doi: 10.1074/jbc.M111.272526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–79. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 56.Morikawa H, Imani F, Khodakhah K, Williams JT. Inositol 1,4,5-triphosphate-evoked responses in midbrain dopamine neurons. J Neurosci. 2000;20:RC103. doi: 10.1523/JNEUROSCI.20-20-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 58.Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcohol Clin Exp Res. 2008;32:1040–8. doi: 10.1111/j.1530-0277.2008.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–41. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]
- 60.Merlo-Pich E, Lorang M, Yeganeh M, Rodríguez de Fonseca F, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–47. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–8. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szabo GG, Lenkey N, Holderith N, Andrasi T, Nusser Z, Hajos N. Presynaptic calcium channel inhibition underlies CB1 cannabinoid receptor-mediated suppression of GABA Release. J Neurosci. 2014;34:7958–63. doi: 10.1523/JNEUROSCI.0247-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szabo B, Siemes S, Wallmichrath I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci. 2002;15:2057–61. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- 64.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–82. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 65.Theile JW, Morikawa H, Gonzales RA, Morrisett RA. GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons. Neuroscience. 2011;172:94–103. doi: 10.1016/j.neuroscience.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–4. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- 67.Tan H, Zhong P, Yan Z. Corticotropin-releasing factor and acute stress prolongs serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons. J Neurosci. 2004;24:5000–8. doi: 10.1523/JNEUROSCI.0143-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–65. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, et al. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci. 2008;28:12927–37. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Llano I, DiPolo R, Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron. 1994;12:663–73. doi: 10.1016/0896-6273(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 71.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Caillard O, Ben-Ari Y, Gaïarsa JL. Activation of presynaptic and postsynaptic ryanodine-sensitive calcium stores is required for the induction of long-term depression at GABAergic synapses in the neonatal rat hippocampus. J Neurosci. 2000;20:RC94. doi: 10.1523/JNEUROSCI.20-17-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–43. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23:700–7. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Riegel AC, Williams JT. CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons. Neuron. 2008;57:559–70. doi: 10.1016/j.neuron.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci USA. 2008;105:8410–5. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–7. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- 78.Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–85. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- 79.Herman MA, Kallupi M, Luu G, Oleata CS, Heilig M, Koob GF, et al. Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects. Neuropharmacology. 2013;67:337–48. doi: 10.1016/j.neuropharm.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–66. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lowery-Gionta EG, Marcinkiewcz CA, Kash TL. Functional alterations in the dorsal raphe nucleus following acute and chronic ethanol exposure. Neuropsychopharmacology. 2014;40:590–600. doi: 10.1038/npp.2014.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Varodayan FP, Logrip ML, Roberto M. P/Q-type voltage-gated calcium channels mediate the ethanol and CRF sensitivity of central amygdala GABAergic synapses. Neuropharmacology. 2017;125:197–206. doi: 10.1016/j.neuropharm.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reyes BAS, Fox K, Valentino RJ, van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–8. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- 84.Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–45. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 85.Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- 86.Hungund BL, Basavarajappa BS. Are anandamide and cannabinoid receptors involved in ethanol tolerance? A review of the evidence. Alcohol Alcohol. 2000;35:126–33. doi: 10.1093/alcalc/35.2.126. [DOI] [PubMed] [Google Scholar]
- 87.Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–8. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- 88.Mitrirattanakul S, López-Valdés HE, Liang J, Matsuka Y, Mackie K, Faull KF, et al. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin Exp Res. 2007;31:855–67. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- 89.Lopez-Moreno JA, Echeverry-Alzate V, Buhler KM. The genetic basis of the endocannabinoid system and drug addiction in humans. J Psychopharmacol. 2012;26:133–43. doi: 10.1177/0269881111416689. [DOI] [PubMed] [Google Scholar]
- 90.DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, et al. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci USA. 2013;110:14783–8. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Varodayan FP, Soni N, Bajo M, Luu G, Madamba SG, Schweitze P, et al. Chronic ethanol exposure decreases CB1 receptor function at GABAergic synapses in the rat central amygdala. Addict Biol. 2016;21:788–801. doi: 10.1111/adb.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, et al. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32:3405–13. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lobb CJ, Paladini CA. Application of a NMDA receptor conductance in rat midbrain dopaminergic neurons using the dynamic clamp technique. J Vis Exp. 2010;46:pii: 2275. doi: 10.3791/2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lodge DJ, Grace AA. Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J Pharmacol Exp Ther. 2005;314:201–6. doi: 10.1124/jpet.105.084913. [DOI] [PubMed] [Google Scholar]
- 95.Sinha R, Li CSR. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 96.Lee KM, Coehlo M, McGregor HA, Waltermire RS, Szumlinski KK. Binge alcohol drinking elicits persistent negative affect in mice. Behav Brain Res. 2015;291:385–98. doi: 10.1016/j.bbr.2015.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou Y, Schwartz BI, Giza J, Gross SS, Lee FS, Kreek MJ. Blockade of alcohol escalation and “relapse” drinking by pharmacological FAAH inhibition in male and female C57BL/6J mice. Psychopharmacology. 2017;234:2955–70. doi: 10.1007/s00213-017-4691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Colombo G, Agabio R, Lobina C, Reali R, Zocchi A, Fadda F, et al. Sardinian alcohol-preferring rats: a genetic animal model of anxiety. Physiol Behav. 1995;57:1181–5. doi: 10.1016/0031-9384(94)00382-f. [DOI] [PubMed] [Google Scholar]
- 99.Logrip ML, Oleata C, Roberto M. Sex differences in responses of the basolateral-central amygdala circuit to alcohol, corticosterone and their interaction. Neuropharmacology. 2017;114:123–34. doi: 10.1016/j.neuropharm.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chester JA, de Paula Barrenha G, DeMaria A, Finegan A. Different effects of stress on alcohol drinking behaviour in male and female mice selectively bred for high alcohol preference. Alcohol Alcohol. 2006;41:44–53. doi: 10.1093/alcalc/agh242. [DOI] [PubMed] [Google Scholar]
- 101.Chaplin TM, Hong K, Fox HC, Siedlarz KM, Bergquist K, Sinha R. Behavioral arousal in response to stress and drug cue in alcohol and cocaine addicted individuals versus healthy controls. Hum Psychopharmacol Clin Exp. 2010;25:368–76. doi: 10.1002/hup.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ketchesin KD, Stinnett GS, Seasholtz AF. Binge drinking decreases corticotropin-releasing factor-binding protein expression in the medial prefrontal cortex of mice. Alcohol Clin Exp Res. 2016;40:1641–50. doi: 10.1111/acer.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haass-Koffler CL, Henry AT, Melkus G, Simms JA, Naemmuddin M, Nielsen CK, et al. Defining the role of corticotropin releasing factor binding protein in alcohol consumption. Transl Psychiatry. 2016;6:e953–e953. doi: 10.1038/tp.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ding ZM, Ingraham CM, Rodd ZA, Mcbride WJ. The reinforcing effects of ethanol within the posterior ventral tegmental area depend on dopamine neurotransmission to forebrain cortico-limbic systems. Addict Biol. 2015;20:458–68. doi: 10.1111/adb.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hauser SR, Deehan GA, Toalston JE, Bell RL, Mcbride WJ, Rodd ZA. Enhanced alcohol-seeking behavior by nicotine in the posterior ventral tegmental area of female alcohol-preferring (P) rats: modulation by serotonin-3 and nicotinic cholinergic receptors. Psychopharmacology. 2014;231:3745–55. doi: 10.1007/s00213-014-3508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162:622–34. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holly EN, Boyson CO, Montagud-Romero S, Stein DJ, Gobrogge KL, Debold JF, et al. Episodic social stress-escalated cocaine self-administration: role of phasic and tonic corticotropin releasing factor in the anterior and posterior ventral tegmental area. J Neurosci. 2016;36:4093–105. doi: 10.1523/JNEUROSCI.2232-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26:4901–7. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Linsenbardt DN, Boehm SL. Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: involvement of the posterior ventral tegmental area. Neuroscience. 2009;164:424–34. doi: 10.1016/j.neuroscience.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pertwee RG (editor). Pharmacological actions of cannabinoids. Handbook of experimental pharmacology. Vol 168. Berlin, Heidelberg: Springer; 2005. p. 1–51. [DOI] [PubMed]
- 111.Cippitelli A, Bilbao A, Hansson AC, del Arco I, Sommer W, Heilig M, et al. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur J Neurosci. 2005;21:2243–51. doi: 10.1111/j.1460-9568.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- 112.Gessa GL, Serra S, Vacca G, Carai MAM, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol Alcohol. 2005;40:46–53. doi: 10.1093/alcalc/agh114. [DOI] [PubMed] [Google Scholar]
- 113.Lallemand F, Soubrié P, Witte PD. Effects of CB1 cannabinoid receptor blockade on ethanol preference after chronic alcohol administration combined with repeated re-exposures and withdrawals. Alcohol Alcohol. 2004;39:486–92. doi: 10.1093/alcalc/agh098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.