Abstract
Humpback whale (Megaptera novaeangliae) populations typically undertake seasonal migrations, spending winters in low latitude breeding grounds and summers foraging in high latitude feeding grounds. Until recently, a broad scale understanding of whale movement has been derived from whaling records, Discovery marks, photo identification and genetic analyses. However, with advances in satellite tagging technology and concurrent development of analytical methodologies we can now detail finer scale humpback whale movement, infer behavioural context and examine how these animals interact with their physical environment. Here we describe the temporal and spatial characteristics of migration along the east Australian seaboard and into the Southern Ocean by 30 humpback whales satellite tagged over three consecutive austral summers. We characterise the putative Antarctic feeding grounds and identify supplemental foraging within temperate, migratory corridors. We demonstrate that Antarctic foraging habitat is associated with the marginal ice zone, with key predictors of inferred foraging behaviour including distance from the ice edge, ice melt rate and variability in ice concentration two months prior to arrival. We discuss the highly variable ice season within the putative foraging habitat and the implications that this and other environmental factors may have on the continued strong recovery of this humpback whale population.
Introduction
Migration is a large-scale class of animal movement driven by resource quality or availability (e.g., breeding habitat, seasonal food resources)1. Perhaps the most classic concept of migration is the long-distance movements of many birds and mammals characterised by breeding at one end of the migratory pathway and feeding at the other. Most humpback whale (Megaptera novaeangliae) populations undertake seasonal migration, spending the winter in low latitude breeding grounds and the summer foraging in high latitude and productive feeding grounds2. Historically, our understanding of whale migration has been informed via examination of whaling records and Discovery mark recoveries (a metal tube stamped with a unique serial number, that was fired into the whale, remaining embedded until the whale was captured and flensed3), which provide a coarse description of the spatial and temporal characteristics of movement. More recently, non-lethal methods such as photo identification (for example4) and genetic analyses (for example5) have provided similar point location data. These data, however, provide no detail on the movements in between mark and recapture.
Achieving a more detailed understanding of movement has been difficult for marine mammals because they are highly mobile, diving animals that spend relatively little time at the surface6. Whales are no exception with the majority of movement occurring in remote, often inhospitable, areas with no survey effort7. The development of satellite tagging technologies has provided an extremely valuable, non-lethal technique to collect high-resolution movement data over biologically relevant time scales1. For example, satellite tags recently revealed novel migratory pathways of New Caledonia humpback whales that utilise seamount habitats both during the breeding season and while migrating8. Combining historical whaling and sightings data, Branch et al.9 hypothesised that pygmy blue whales (Balaenoptera musculus brevicauda) migrate between Australia and Indonesia along Australia’s western coastline – a migratory path which has since been confirmed using satellite telemetry10. In these and other cases (for example11), satellite telemetry provided new detailed movement information and identified habitat important to the conservation and management of whale species.
Improvements in tagging technology, particularly advances in sensor, storage and transmission capabilities, have brought improvements in the amount and quality of data received12,13. Concurrently, statistical techniques have evolved and analytical methods such as state space modelling (SSM) are increasingly being applied to tag based movement data14–16. SSM methods combine a process model (for animal movement) with an observation model (for the tracking data), to provide an estimation of the unobserved behavioural state of the animal17. This approach enables inference about the nature of that behaviour – for example, two-state models commonly differentiate more localised search (foraging, resting) behaviour from more directed transiting behaviour (migration18). These inferred behaviours can be examined relative to the animal’s biophysical environment, enabling telemetry data to provide an understanding of the ecological factors influencing animal movement and habitat selection (e.g.19). The influence of directly measured and/or remotely sensed factors that may generate or concentrate resources can therefore be explored; for example environmental variables such as sea ice concentration20, bathymetric depth and gradient21, chlorophyll-a concentration22 and sea surface temperature21,23 have all been demonstrated to influence the “search” behaviour of cetaceans.
Obtaining a detailed understanding of the movement of wide ranging species such as humpback whales is important for informing management policy in the face of environmental variability and long term change associated with anthropogenic forcing14,24. The east Australian humpback whale population, designated as breeding stock E1 by the International Whaling Commission (IWC), migrates along the east coast of Australia and was hunted to near extinction in the 1950s and early 1960s25,26. However this population is now considered 58–98% recovered at a population size of 24,545 whales (95% CI 21,631–27,851) with no evidence that the observed exponential rate of growth is slowing down27. Over multiple years (2008–2010) we deployed satellite tags on east Australian humpback whales near both the feeding and breeding grounds to collect the first high resolution movement data for this population. We aimed to describe migratory movement and identify foraging habitat. Using a SSM to infer foraging behaviour we investigate one factor that we hypothesise supports this population’s sustained recovery: access to productive feeding grounds. Here we identify the environmental factors that characterise the key Antarctic foraging habitat, discuss usage of ice associated habitats in the face of change and the role of supplemental feeding in temperate grounds.
Results
Whale movement
Thirty humpback whales were tracked during three austral summers (2008/09, 2009/10 and 2010/11; Fig. 1 and Table 1) over a period of 3 to 155 days with a mean (±SD) track duration of 50 ± 35 days (Table 2). Based on the filtered state-space location estimates, migrating whales travelled 2850 ± 1377 km (range: 103–5272 km, n = 21) from their tagging location, travelling a mean distance of 78 ± 22 km per day before crossing the 60 °S parallel into the Southern Ocean. On the Antarctic feeding grounds south of 60 °S, tracked whales covered a mean distance of 1885 ± 1567 km (range: 248–6315 km, n = 20), travelling 52 ± 18 km per day. In temperate waters and while migrating south, whales travelled at a speed of 3.32 ± 0.85 kmh−1 and when south of 60 °S slowed to 2.19 ± 0.74 kmh−1.
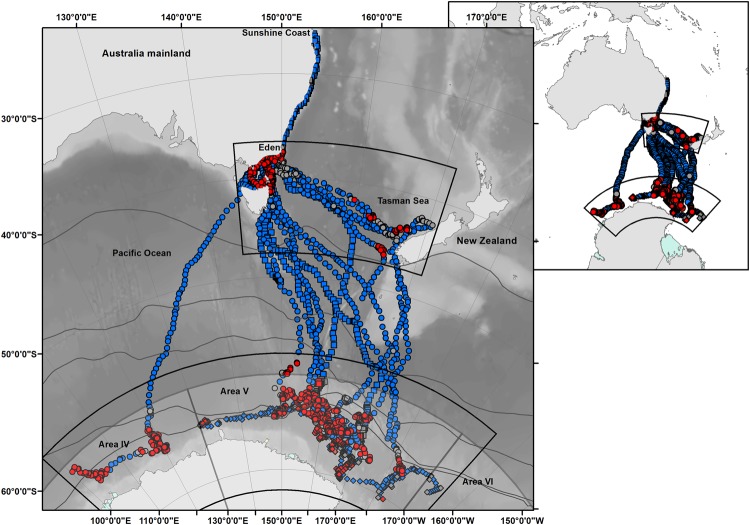
Figure 1.
Migration pathways for 30 humpback whales satellite-tagged off the eastern coast of Australia. (Eden deployment – circles, Sunshine Coast deployment – squares) and in Antarctica (diamonds). (a) Shows tracks across the entire geographic range and depicts IWC Antarctic Management Areas IV, V and VI as white boxes. Location estimates from the state-space model are coloured according to the behavioural state estimate: ‘search’ (red), ‘transit’ (blue) and ‘uncertain’ (grey). Grey lines show climatological oceanic frontal positions102. Background shading indicates bathymetry derived from the GEBCO Digital Atlas103,104.
Table 1.
Summary of satellite tags deployments and the resulting tracking information.
| PTT | Gender | Tagging location | Deployment date (UTC) | First location (UTC) | Last location (UTC) | Track duration (days) | Transmitting days | Track distance (km) | Number of search patches | Total search time (days) | Max. search patch duration (days) | Min. search patch duration (days) | Search locations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 88718 | Male | AU-Eden | 24/10/2008 | 24/10/2008 | 24/01/2009 | 93 | 92 | 5776 | 3 | 50 | 19 | 14 | AU-EC (36), ANT (14) |
| 88723 | Unknown | AU-Eden | 24/10/2008 | 24/10/2008 | 13/01/2009 | 82 | 81 | 5848 | 2 | 26 | 15 | 11 | TS (11), ANT (15) |
| 88732 | Male | AU-Eden | 24/10/2008 | 24/10/2008 | 27/11/2008 | 35 | 35 | 2571 | 1 | 8 | 8 | 8 | TS (8) |
| 88733 | Female* | AU-Eden | 24/10/2008 | 24/10/2008 | 4/12/2008 | 42 | 42 | 4042 | 0 | NA | NA | NA | NA |
| 88735 | Male | AU-Eden | 24/10/2008 | 24/10/2008 | 1/12/2008 | 39 | 38 | 1529 | 3 | 30 | 14 | 5 | AU-EC (30) |
| 88743 | Male | AU-Eden | 24/10/2008 | 24/10/2008 | 5/11/2008 | 13 | 13 | 583 | 0 | NA | NA | NA | NA |
| 88746 | Male | AU-Eden | 24/10/2008 | 24/10/2008 | 12/11/2008 | 20 | 20 | 1720 | 0 | NA | NA | NA | NA |
| 88725 | Male | AU-Eden | 25/10/2008 | 25/10/2008 | 13/01/2009 | 81 | 80 | 5520 | 3 | 61 | 29 | 15 | AU-EC (29), ANT (32) |
| 88744 | Male | AU-Eden | 25/10/2008 | 25/10/2008 | 27/10/2008 | 3 | 3 | 103 | 0 | NA | NA | NA | NA |
| 88745 | Male | AU-Eden | 25/10/2008 | 25/10/2008 | 12/11/2008 | 19 | 14 | 1429 | 0 | NA | NA | NA | NA |
| 88738 | Male | AU-Eden | 27/10/2008 | 27/10/2008 | 23/12/2008 | 58 | 58 | 4639 | 4 | 29 | 17 | 3 | AU-EC (7), ANT (22) |
| 88722 | Female | AU-Eden | 28/10/2008 | 28/10/2008 | 16/11/2008 | 20 | 20 | 1329 | 1 | 13 | 13 | 13 | AU-EC (13) |
| 88729 | Female | AU-Eden | 29/10/2008 | 29/10/2008 | 3/02/2009 | 98 | 97 | 7202 | 3 | 56 | 37 | 3 | AU-EC (3), ANT (53) |
| 88717 | Female* | AU-Eden | 31/10/2008 | 31/10/2008 | 29/11/2008 | 30 | 30 | 2025 | 1 | 8 | 8 | 8 | TS (8) |
| 88728 | Female* | AU-Eden | 31/10/2008 | 31/10/2008 | 1/02/2009 | 94 | 93 | 6312 | 3 | 37 | 16 | 5 | ANT (37) |
| 88741 | Female | AU-Eden | 1/11/2008 | 1/11/2008 | 4/04/2009 | 155 | 153 | 10324 | 6 | 93 | 28 | 4 | AU-EC (4), ANT (89) |
| 53348 | Male | ANT | 21/02/2010 | 21/02/2010 | 24/03/2010 | 32 | 26 | 1566 | 1 | 20 | 20 | 20 | ANT (20) |
| 53736 | Female | ANT | 21/02/2010 | 17/02/2010 | 6/04/2010 | 49 | 39 | 2486 | 1 | 9 | 9 | 9 | ANT (9) |
| 96385 | Female | ANT | 22/02/2010 | 22/02/2010 | 7/03/2010 | 14 | 14 | 487 | 1 | 5 | 5 | 5 | ANT (5) |
| 98138 | Male | ANT | 22/02/2010 | 21/02/2010 | 3/04/2010 | 42 | 42 | 1425 | 0 | NA | NA | NA | NA |
| 96403 | Female | ANT | 25/02/2010 | 26/02/2010 | 18/03/2010 | 21 | 18 | 1821 | 0 | NA | NA | NA | NA |
| 96386 | Female | ANT | 1/03/2010 | 1/03/2010 | 31/05/2010 | 92 | 92 | 4547 | 5 | 30 | 21 | 1 | ANT (30) |
| 96390 | Female | ANT | 8/03/2010 | 8/03/2010 | 28/03/2010 | 21 | 21 | 962 | 1 | 13 | 13 | 13 | ANT (13) |
| 96398 | Male | ANT | 8/03/2010 | 8/03/2010 | 22/04/2010 | 46 | 46 | 2643 | 5 | 25 | 7 | 3 | ANT (25) |
| 96412 | Female | ANT | 8/03/2010 | 8/03/2010 | 22/03/2010 | 15 | 15 | 860 | 2 | 9 | 5 | 4 | ANT (9) |
| 98139 | Male | AU-SC | 13/10/2010 | 13/10/2010 | 22/11/2010 | 41 | 41 | 2697 | 0 | NA | NA | NA | NA |
| 64235 | Male | AU-SC | 14/10/2010 | 14/10/2010 | 29/11/2010 | 47 | 47 | 4335 | 1 | 3 | 3 | 3 | ANT (3) |
| 98100 | Female | AU-SC | 14/10/2010 | 14/10/2010 | 1/12/2010 | 49 | 49 | 3580 | 0 | NA | NA | NA | NA |
| 98114 | Male | AU-SC | 14/10/2010 | 31/10/2010 | 9/12/2010 | 40 | 40 | 3225 | 0 | NA | NA | NA | NA |
| 98129 | Female* | AU-SC | 15/10/2010 | 15/10/2010 | 29/01/2011 | 107 | 104 | 6539 | 1 | 2 | 2 | 2 | ANT (2) |
For tagging location: AU-Eden is Eden, Australia; ANT is Antarctica; AU-SC is Sunshine Coast, Australia. For search location: AU-EC is east coast Australia, ANT is Antarctica, TS is Tasman Sea and number of days searching in each location is given in parentheses. *Indicates where gender was inferred by behaviour (with calf) at time of tagging (determined via biopsy otherwise).
Table 2.
Summary movement statistics for thirty satellite tracked humpback whales.
| Number of whales tagged | Track length (days; mean ± SD) | Track distance (km; mean ± SD) | Initial migratory trajectory | Temperate search patches | Travelled south of 60 °S | Antarctic search patches | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| South | West (crossing the 146 °E meridian) | East (crossing the 160 °E meridian) | East coast Australia: Eden, Bass Strait and Tasmania | Tasman Sea | ||||||
| AU-Eden* | 16 | 55 ± 42 | 3809 ± 2854 | 8 | 1 | 6 | 7 | 3 | 8 | 7 |
| AU-SC | 5 | 57 ± 28 | 4075 ± 1500 | 4 | 0 | 1 | 0 | 0 | 3 | 2 |
| ANT | 9 | 37 ± 25 | 1866 ± 1235 | NA | NA | NA | NA | NA | 9 | 7 |
| Total | 30 | 50 ± 35 | 3271 ± 2414 | 12 (57%) | 1 (5%) | 7 (33%) | 7 (33%) | 3 (14%) | 20 (67%) | 16 (53%) |
Numbers indicate individual animals, with percentages (%) given in parentheses. Trajectory and temperate search patch statistics apply only to whales tagged off Eden and Sunshine Coast, Australia. *One tag failed before transmitting a clear migratory trajectory.
The 21 whales tagged off the eastern Australian coast migrated south along the coastline and across the Bass Strait (separating mainland Australia and Tasmania) during the month of October (Fig. 2a). Throughout November, 12 whales migrated south via the east coast of Tasmania (one tag failed prior). One whale migrated via the west coast of Tasmania and continued in a south westerly direction into the Pacific Ocean then moved onto the Antarctic feeding grounds (Figs 1, 2b and Table 2). Seven whales travelled eastwards into the Tasman Sea crossing the 160 °E meridian whilst still in temperate waters (Fig. 2a). Three of these whales spent time off the south west coast of New Zealand’s South Island (14th to the 29th November 2008) while the other individuals continued transit into the Southern Ocean.
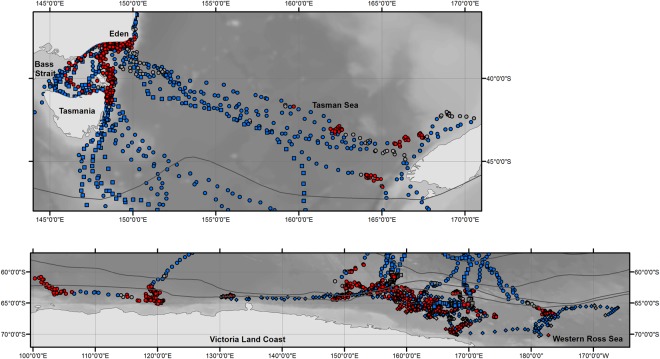
Figure 2.
Detail of inset areas shown in Fig. 1. (a) The temperate, and (b) the Antarctic foraging zones outlined as black boxes in Fig. 1. Location estimates from the state-space model are coloured according to the behavioural state estimate: ‘search’ (red), ‘transit’ (blue) and ‘uncertain’ (grey). Grey lines show climatological oceanic frontal positions102. Background shading indicates bathymetry derived from the GEBCO Digital Atlas103.
In total eleven humpback whales tagged in east Australian waters travelled south of 60 °S (Fig. 2b), with first arrival dates of 29th November 2008 (n = 8) and the 21st November 2010 (n = 3), respectively. The mean date of arrival at 60 °S was the 10th December in 2008 (median: 7th December 2008) and the 4th December in 2010 (median: 23rd November 2010). All humpback whales with transmitting tags were in the Antarctic feeding grounds by January (the latest arrival was an Eden tagged whale 88718 on the 1st January 2009) where location data continued to be transmitted until May (Antarctic tagged whale 96386 – last data transmitted on 31st May 2010).
Whale behaviour
The state-space model clearly distinguished between two behavioural states for the migrating whales (nominally, search and transit; Table 3). The main parameters governing the movement processes (θ – turn angles, and γ – movement persistence; see18) were very well discriminated for the two states. The turn angles clearly showed frequent reversals during search (mean turning angles concentrated around 176° to 192°) as opposed to few when transiting (mean turning angles concentrated near zero, around −1° to 1.5°; Table 3). In general, credible intervals around parameter estimates were tight, with no overlap in parameter estimates between the two states. The estimated movement persistence was notably higher for those animals tagged within east Australian waters (γ1 > 0.8), which undertook the longest migrations, as compared with those animals tagged on the Antarctic foraging grounds (γ1 ~ 0.5). Overall, whales spent on average 29.7 ± 27.8% (0–76.9%) of their time in search behaviour; 14.1 ± 22.7% when north of 60 °S (n = 21) and 29.8 ± 23.6% (n = 20) when south of 60 °S. There was relatively low uncertainty in the behavioural state estimates for the two Australian deployment campaigns with 15.6% (Eden) and 13.7% (Sunshine Coast) of estimates falling between 1.25 and 1.75; this was somewhat higher for the Antarctic deployment at 34.6%.
Table 3.
Posterior sample means and 95% credible intervals (CIs) for movement parameters estimated using a hierarchical state-space behavioural switching model.
| Deployment campaign | N | State 1 ‘transit’ | State 2 ‘search’ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| γ1 | θ1 | γ2 | θ2 | ||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| ANT | 9 | 0.515 | 0.428–0.625 | 0.352 | 0.214–0.470 | −0.018 | −0.098–0.065 | 3.294 | 3.111–3.494 |
| AU-Eden | 16 | 0.821 | 0.785–0.856 | 0.111 | 0.045–0.177 | 0.021 | −0.012–0.053 | 3.074 | 2.692–3.425 |
| AU-SC | 5 | 0.837 | 0.797–0.876 | 0.160 | 0.003–0.664 | 0.027 | −0.017–0.071 | 3.350 | 1.779–4.813 |
Turning angles (θ) are given in radians, the movement persistence parameter (γ) is the correlation in speed and direction. Subscripts indicate behavioural state. The state-space model was run on each campaign separately, with N indicating each sample size (number of whales).
Ten out of 21 animals (47.6%) tagged during migration in east Australian waters had ‘search’ behaviour identified at locations within temperate waters; these were all animals tagged off Eden. Three of these whales undertook short search periods (of three days) near the tagging location from October 24, 2008, whilst four of these whales undertook search extending into the Bass Strait and/or along the east coast of Tasmania ranging in patch duration from four to 35 days (Fig. 2a, Tables 1 and 2). For those whales that travelled eastwards, search patches of between seven and 10 days were located in the Tasman Sea and off the south west coast of New Zealand’s South Island (n = 3; Fig. 2a, Tables 1 and 2).
Within the Antarctic feeding grounds (Fig. 2b) search behaviour was documented for 20 whales over three consecutive austral summers (Tables 1 and 3). Search behaviour was detected soon after animals tagged off eastern Australia (2008/09 and 2010/11) arrived south of 60 °S, with a mean start date for inferred foraging on the 17th December in 2008 (median: 11th December 2008; n = 7) and the 18th of December in 2010 (median: 18th December 2010; n = 2). The majority of search behaviour was concentrated between 145–175 °E (Fig. 2b). Over all seasons, from whales tagged both on migration and on their feeding grounds, there are three small areas containing both transit and search behaviours that overlap or are closely adjacent between years. Two of these areas are located at approximately 60.5 °S to 61.5 °S and 158 °E (Eden and Sunshine Coast deployments – austral summer 2008/09 and 2010/11 respectively), and at 64.7 °S to 66.4 °S and 169.5 °E (Eden, Antarctic and Sunshine Coast deployments – austral summers 2008/09, 2009/10 and 2010/11 respectively). The third area was farther east, at 65.5 °S to 67.2 °S near 178 °W (Eden and Antarctic deployments – austral summers 2008/09 and 2009/10 respectively). This coherence in behaviour between years suggests the potential for persistent space use and well established migratory pathways.
Behaviour-environment associations
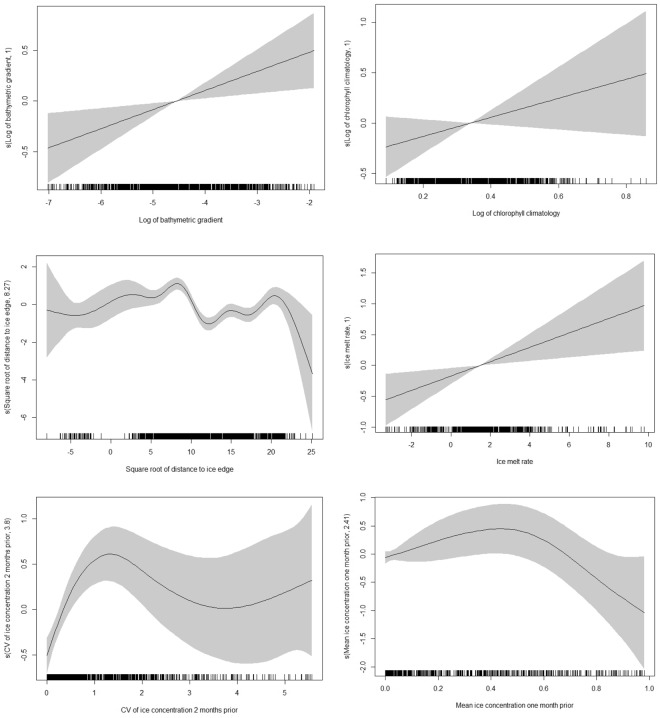
Whale behavioural state (search and transit) varied in relation to a suite of environmental variables (p-values < 0.05, Table 4; full model summary in Supplementary S2, Table S2.3). The GAMM smooths indicate that humpback whales were more likely to adopt search behaviour at higher bathymetric gradients (Fig. 3a) and where ice melt rate was high (Fig. 3d). Non-linear relationships indicated a higher occurrence of search behaviour approximately 65 km from the ice edge (Fig. 3c), where ice concentration variability was moderately high 2 months prior (Fig. 3e) and at mid-range ice concentrations one month prior (Fig. 3f). Search behaviour was not significantly related to the seasonal chlorophyll-a climatologies (Fig. 3b), nor was deployment campaign a significant predictor.
Table 4.
Significance of environmental predictors influencing whale behaviour.
| Environmental variable | p-value | Number of times significant/100 |
|---|---|---|
| log bathyg | 0.007 | 20 |
| log CHLa | 0.114 | 2 |
| sqrt dist ice | <0.001 | 91 |
| melt rate | 0.008 | 73 |
| ice cv lag 2 | <0.001 | 96 |
| ice mn lag 1 | 0.026 | 26 |
P-values indicate approximate significance of GAMM smooth terms based on Chi-sq. statistics. Second column indicates the number of times each environmental variable105–109 was determined to be significant (based on p-value < 0.05) under the resampling procedure (n = 100 iterations, resampling randomly from the state-space model posterior estimates for behavioural state and location).
Figure 3.
Smooths of generalized additive mixed modelling. (GAMM) terms showing the influence of environmental variables on whale ‘search’ behaviour (b). Locations of observations are shown as tick marks on the x-axes. Solid lines are the estimates of the smooths, grey areas indicate standard errors of the estimated smooths. The y-axis indicates the effect of the smooth function of each covariate upon the probability of being in ‘search’ behaviour; with a lower (higher) value indicating reduced (increased) probability. (a) Bathymetric gradient, (b) summer chlorophyll climatology, (c) distance to ice edge, (d) ice melt rate, (e) coefficient of variation (CV) of ice concentration two months prior, and (f) mean ice concentration one month prior.
The resampling procedure examined the retention of model predictors given the uncertainty inherent in the behavioural state and location estimates. This showed that inclusion of the three ice-related variables was the most highly resilient to uncertainty (Table 4). The ice CV and the distance to ice edge were retained as significant in over 95% and 90% of the models fit during the resampling procedure, respectively. Ice melt rate was retained as significant in over 70% of the resampling models whereas the other variables were less resilient (more sensitive) to uncertainty. This result highlights the prominent role that features of the marginal ice zone play in influencing the search behaviour of humpback whales.
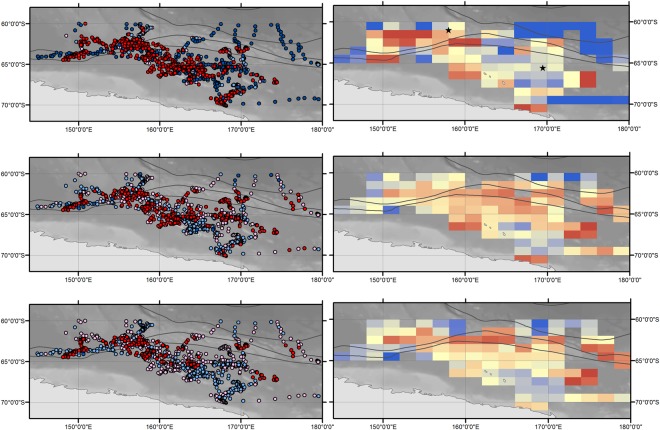
The concentration of search behaviour occurring between 145–175 °E (Fig. 4a) was generally well captured using our environmental model to predict whale movement behaviour (Fig. 4b). The predicted probabilities of search behaviour were typically reduced when uncertainty was incorporated via the resampling procedure, although the spatial patterns remain similar overall (Fig. 4c). When spatially aggregated these predictions may give an indication of habitat areas important for foraging (i.e. high mean probabilities of whales undertaking search behaviour, shown as warmer colours in Fig. 4d–f), opening the possibility for more broad scale habitat predictions on the basis of environmental drivers. For ease of interpretation, the two areas of persistent space use that are located between 145–175 °E and reported within ‘Whale behaviour’ are represented by the star symbol in Fig. 4d.
Figure 4.
Maps showing whale behavioural state estimates. (a) Behavioural state estimates from the hierarchical state-space switching model. Panel focusses on the area of most concentrated search behaviour between 145–175 °E. Predicted probabilities of being in search behaviour obtained from (b) the GAMM using environmental predictors, and (c) averaging the GAMM predictions (n = 100) across the resampling procedure (see Methods). Colours represent the gradient from transit (P < 0.25, dark blue) through to search (P > 0.75, red) at 0.25 intervals. Right hand panels: (d,e and f) spatially aggregate these probabilities into 1° latitude ×2° longitude grid cells by averaging across individual whales. Higher mean probabilities of search behaviour shown as warmer colours. The star symbols in (d) represent areas of persistent use between tracking years. Background shading indicates bathymetry derived from the GEBCO Digital Atlas103.
Discussion
The capability of satellite tags to detail whale movements is markedly building our understanding of how whales move and interact with their environment throughout important migration pathways22,28. This novel information ultimately plays an important role in conservation and management11,29. The tag-derived movements reported here substantially increase our current knowledge of the east Australian humpback whale population by revealing new movement patterns, unidentified temperate feeding locations and providing the first description of the environmental predictors that characterise key foraging habitat in Antarctica.
Satellite tracked humpback whales tagged off eastern Australia travelled south along three different migratory trajectories, detailed for the first time in this study. The majority of animals initially travelled southwards, following the eastern mainland and Tasmanian coastlines. One third of these whales headed in an easterly direction into the Tasman Sea. Of these seven individuals, three tags transmitted long enough to record movement as far as 165 °E, and the New Zealand coastline, before changing direction to transit to Antarctica. The simple information gained from historic Discovery marks hints at these migratory routes, with humpback whales marked off eastern Australia then captured off New Zealand and Antarctica (IWC Antarctic management areas IV and V30). A movement not previously represented within the Discovery mark datasets is that of a lone individual who migrated along a westward trajectory through the Bass Strait and onto Antarctica, venturing as far west as 166 °W (management area VI).
The state-space model indicated areas of search behaviour within temperate habitat for almost half (47.6%) of the individuals tagged off eastern Australia. Temperate search behaviour was demonstrated only amongst those individuals tagged off Eden, Australia where supplemental feeding on small schooling fish and krill has been regularly reported31,32. Whilst apparent search might relate to foraging, resting, breeding or other behaviours33–35, seven humpback whales were directly observed to be feeding off Eden at the time of tagging. Additional areas not previously associated with supplemental feeding were identified through the Bass Strait, along the east coast of Tasmania, and within the eastern Tasman Sea off the southern New Zealand coastline. This temperate search behaviour persisted for longer than 30 days for three individuals within the Bass Strait/Tasmanian region and for 7–10 days in the Tasman Sea off New Zealand.
Traditionally, humpback whales are considered to adopt the “feast or famine” approach to migration that is typical of baleen whales: fasting when not occupying high latitude feeding grounds30. However by temporarily suspending migration to forage, these whales may be able to meet up to 3.4 times their daily energy requirements. This may supplement the energy needed for migration and, in the process, begin to refuel energy reserves prior to reaching their Antarctic feeding grounds31. Riekkola et al.29 inferred supplemental feeding in endangered Oceania humpback whales and hypothesised that supplemental feeding may occur when energetic requirements are not being met on Antarctic feeding grounds. Supplemental feeding has also recently been noted for individuals from other Southern hemisphere humpback whale populations including those off the west coast of Africa36,37 and both the east38 and west39 coasts of South America. Stable isotope analyses of baleen whale plates indicate that supplemental feeding may actually be quite a common strategy for east Australian humpback whales40.
The primary Antarctic foraging grounds of east Australian humpback whales was historically considered to be between 130 °E and 170 °W (IWC Antarctic management area V) as determined through Discovery mark recoveries30. More recently, photo identification data has added further weight to the importance of IWC Antarctic management area V41,42. Our study largely supports this, with the majority of search behaviour of satellite tagged animals occurring between 145°–175 °E. However the tracking data demonstrates that east Australian humpback whales do undertake search behaviour across a wider range between 100 °E and 165 °W; i.e., encompassing IWC Antarctic management areas IV, V and VI. Search patches within Antarctic waters persisted for up to 37 days with individuals transiting amongst up to five patches. This type of movement path, with periods of more tortuous track segments connected by relatively directed track segments, is consistent with those exhibited by other satellite tagged humpback whales foraging on Antarctic feeding grounds off the West Antarctic Peninsula43–45, as well as within the Ross, Amundsen and Bellingshausen Seas29. Marine prey resources are patchily distributed at multiple spatial scales46. So, following the major migration transit south to the polar feeding grounds, animals will typically still need to search between dynamic favourable forage patches within and around the sea ice and Antarctic shelf regions (see e.g.47).
Our study identified important environmental predictors characterising Antarctic foraging habitat of east Australian humpback whales - high variability (in relation to the mean) in ice concentration two months prior to arrival, at a distance of approximately 65 km from the ice edge, and high ice melt rates at the time of ice retreat. These factors are clearly associated with the marginal ice zone. Essentially these humpback whales foraged where the sea ice was present two months prior. This is the first time this area, located off the Victoria Land coast and in the western Ross Sea, has been clearly identified as core foraging habitat for the east Australian humpback whale population, and defined in terms of key biophysical characteristics. The chlorophyll climatology we constructed as a proxy for primary oceanic productivity was only found to be a significant environment predictor in 2% of the statistical models. This is likely due in part to the inability of satellites to measure productivity of closely ice-associated habitats48. Additionally, persistent cloud cover necessitates averaging of remotely sensed chlorophyll measurements (here across a three month period); leading to the loss of temporal information.
The marginal ice zone is defined as the area of transition from dense pack ice (up to 100% cover) through to no ice cover49 and is outer pack ice that persists for ≤100 days in a climatological sense50. It is perhaps not surprising that the marginal ice zone and more specifically, low mean and high variance in ice concentration two months prior, plays an important role in conditioning foraging habitat. Various modelled estimates indicate that it takes approximately 15 to 20 days51, around 30 days52 and less than 90 days53 for the biological cascade that results from the release of new production (by way of ice melt) to trigger the accumulation of zooplankton grazers such as krill on which baleen whales foraging in Antarctica depend54. In fact, productivity peaks within the marginal ice zone one to two months following the point at which maximum open water in the area is achieved, which is a direct reflection of the time it takes for phytoplankton blooms to fully respond to newly created ice-free waters55. Rapid ice retreat has also been shown to enhance production, which concentrates secondary producers and their vertebrate predators54,56. However, changing ice dynamics are not favourable for all Antarctic marine predators (for e.g.57).
Large spatio-temporal variability in sea ice seasonality is a fundamental feature of East Antarctica50 and the area in which the East Australian humpback whales forage. The high variability in the sea ice season between 100 °E and 145 °E occurs primarily in the marginal ice zone region (see Fig. 5c)50. East of 145 °E, high variability in the ice season occurs across the entire ice covered region with the exception of the coastal fast ice zone. Despite such large variability, the foraging habitat of the east Australian humpback whale population has undergone a trend of increasing ice season duration over a 30 year period, through earlier advance and later retreat of the ice edge (see Fig. 6)50. The region east of 145 °E undergoes earlier and rapid ice edge advance with strong ice production occurring in autumn. Due to fewer open water days there has been an overall trend of decreasing sea surface temperature and decreasing net primary productivity, but also a small area of net primary productivity increase located at approximately 64 °S to 66 °S and 160 °E to 165 °E58.
The Antarctic foraging habitat of east Australian humpback whales could also be habitat favourable for krill recruitment and success. Traditionally, increased ice season duration and associated winter ice has been thought to favour krill maturation by providing a winter, ice algae food supply resulting in a high recruitment rate from the spawning season59,60. However, recent evidence suggests that at least in East Antarctica, this relationship may not be so direct and that the winter pack ice may actually be a food-poor habitat for krill61. The marginal ice zone provides a better feeding habitat for krill due to the presence of light, nutrients, grinding ice floes and proximity to open ocean/waves which promote high larval krill growth rates. The complex habitat structure of the marginal ice zone further provides protection important for larval krill survival. Phytoplankton availability in autumn may also govern krill recruitment success with early ice formation in autumn separating adult and larval krill and reducing food competition. As such, time lags in ice-related environmental predictors that date back to the previous autumn may need to be considered in future modelling attempts.
The IWC’s International Decade for Cetacean Research (IDCR) and Southern Ocean Whale Ecosystem Research (SOWER) sightings surveys62,63 and whaling catch records63,64 demonstrate that historically, humpback whales were located (sighted and captured) in relatively high numbers within the same area identified as foraging habitat for the humpback whales tagged during 2008–2010. Our findings showed notably persistent space use, such that different whales moved through the same location or occupied habitat immediately adjacent over three consecutive austral summers. Site fidelity, or persistent space use, has been observed for other humpback whale populations39,45,65,66. In the patchy marine environment, foraging site fidelity may be attributed to the interplay of habitat quality and predictability; ultimately, familiarity with foraging habitat may present significant ecological benefits over the long term67,68. Additionally, for humpback whales, foraging site fidelity is maternally directed with individuals returning to foraging sites that, historically, they first visited with their mother prior to weaning69–71. This behavioural mechanism can act on population structure at an evolutionary time scale72 and may contribute to the lack of recovery for some whale populations that were nearly extirpated due to whaling73.
East Australian humpback whales were hunted along both their migratory corridors and upon their Antarctic feeding grounds during the 20th century25, and may have numbered just 104 individuals when commercial whaling ceased in 196326. This population, assigned the nomenclature E1, is considered to be one of three meta-populations that comprise population E74. The other two meta-populations (New Caledonia E2, and Tonga E3) together with individuals from the central South Pacific collectively comprise the Oceania population. The rapid recovery rate of the east Australian population is just shy of the theoretical upper bound of population increase75 at 11.1% (95% CI of 10.6–11.3%27). Whilst the E1 post-exploitation recovery is strong at between 58–98% recovered27, the Oceania group remains listed as endangered by the International Union for Conservation of Nature76 and is estimated to be well below 50% of pre-exploitation population size29. This group, which was decimated by illegal hunting of Soviet whalers77, mainly forage farther east throughout IWC Antarctic Management Areas V, VI and I (approximately 180 °E to 90 °W)29,78. Tracking data demonstrate a small degree of overlap in foraging range at the eastward limit of the satellite tracks presented in this study. Clearly these populations can mix on their foraging grounds78 which highlights the possibility that the rapid recovery of east Australian humpback whales may only be partly due to forage conditions (access to quality foraging grounds and supplemental feeding). Conversely, the poor recovery of the Oceania population may simply just be the result of their near extirpation. However, Clapham and Zerbini79 hypothesise that the high population growth rate of east Australian humpback whales could be due to temporary immigration by Oceania humpbacks as a consequence of a mating system that results in whales migrating from low density to high density breeding grounds. In light of the annual pregnancy rates recently reported for two Southern Hemisphere humpback whale populations29,80, a revisit of appropriate calving intervals along with further genetic analyses are required to determine the validity of this hypothesis.
The east Australian humpback whale population is perhaps the best monitored population of Antarctic krill consumers in the world, with post-whaling surveys initiated in 1978 and repeated every one to three years since27. Easily counted and sampled whilst on their tropical breeding ground, this population could act as a “sentinel species” providing cost-effective monitoring of the Antarctic sea ice ecosystem27,81. Significant changes are already occurring in key Antarctic forage habitat, with evidence of long-term changes in the sea ice environment50 and associated declines in ocean temperature and net primary productivity58. The capacity for these long lived, large animals with late reproductive age to respond to change is not known, but presumably currently being challenged by rapid environmental change. The future brings potential impacts of even larger scale changes: for example, the possibility of a complete collapse in Antarctic krill populations due to ocean acidification by 230082. Highly mobile species which rely on different habitats during different life history stages are subject to multiple and varied threats across their range, which makes it difficult to understand and predict their ability to adapt83,84. Certainly as this population approaches recovery, ongoing monitoring will be required to identify and address the various impacts of human and economic expansion on migratory pathways and breeding habitat85. However, if we consider that migration has evolved as a successful strategy to manage environmental variability, and persisted as a behaviour throughout global change over millions of years84, then for east Australian humpback whales the act of migration may facilitate their ability to respond to change.
Methods
Tag deployments and tracking information
Satellite tags were deployed on adult humpback whales with a modified version of the Air Rocket Transmitter System ARTS, Restech86 and a purpose-designed projectile carrier at a pressure of 7.5–10 bar. A custom-designed, 80 mm anchor section is attached to a stainless steel cylindrical housing containing a location-only transmitter (SPOT-5 by Wildlife Computers, Redmond, Washington, USA and Kiwisat 202 Cricket by Sirtrack, Havelock North, New Zealand). This superseded anchor design resulted in the anchor section disarticulating upon deployment in order to achieve improved tag retention times while minimising impact87. The tags were sterilised with ethylene oxide prior to deployment and implanted up to 290 mm into the skin, blubber, interfacial layers and outer muscle mass of the whale.
Upon deployment, a small amount of skin and blubber was simultaneously collected for genetic analyses. These were collected using a biopsy dart fired from a modified 0.22 Paxarms system88. Biopsy samples were stored in 70% ethanol and DNA subsequently extracted using a Tissue DNA purification kit for the Maxwell 16 DNA extraction robot (Promega Corporation). The sexes of the tagged whales were determined using a 5′ exonuclease assay of the polymorphisms in the sex-linked Zinc Finger genes as described by Morin et al.89.
Tags were deployed during three austral summers: 2008/09, 2009/10 and 2010/11. The two Australian deployment campaigns were on individuals undertaking their southern migration along the east coast near Eden (37.15 °S, 150.07 °E; n = 16) between October and November 2008 and off the Sunshine Coast (26.51 °S, 153.17 °E; n = 5) during October 2010. Satellite tags were also deployed on individuals whilst on their Antarctic feeding grounds during February and March 2010 (west of the Balleny Islands, approximately 67.25 °S, 152.71 °E; n = 9).
Tags were programmed to transmit to the Argos satellite system at various duty cycles and repetition rates for a maximum of 720 transmissions per day (Table S1). These transmissions are relayed to processing centres which calculate the transmitter’s location by measuring the Doppler Effect on transmission frequency. Transmitted data were processed using a least squares analysis and each location was assigned an estimated error and one of seven associated location classes (see90).
Hierarchical switching state-space model
Argos location data (described in91–93) from whales in each deployment campaign (Eden: n = 16; Antarctica: n = 9; Sunshine Coast: n = 5) was entered into a hierarchical switching state-space model which enables joint estimation over multiple individuals (hSSSM94). This model both accounts for spatial uncertainty in Argos location estimates (via an observation model) and estimates two discrete (categorical) behavioural states (via a movement process model). The hierarchical structure assumes that all individuals move according to a correlated random walk but that that the movement of each individual is characterised by a different diffusivity17. Using a Bayesian approach, the movement process parameters governing each state are estimated: the mean turning angles (θ) and a movement persistence parameter (i.e., the autocorrelation in speed and direction – γ). Here, we nominally label the two states as transit (indicating more directed travel) and search (indicating more localised residency) with the latter putatively indicating foraging behaviour although alternate behaviours, e.g., resting, breeding or social activities may be relevant for particular species in certain areas33,34,95.
For each of the deployment campaigns separately, the hSSSM was fit across animals (AU-Eden: n = 16; AU-SC: n = 5; ANT: n = 9) using a 12 h time step. The hSSSM is fit via Markov chain Monte Carlo (MCMC) simulation implemented in JAGS using the R96 package bsam17,18,94. Two MCMC chains were run in parallel for a total of 100,000 samples each with the first 50,000 discarded as burn-in and the remaining samples thinned to every 50th sample to address autocorrelation. This yielded a total of 1000 samples retained from the joint posterior. Each MCMC sample provides a discrete behavioural state estimate (b, where 1 = ‘transit’, 2 = ‘search’) associated with each location state (longitude, latitude) estimate. From the posterior, the most probable (discrete) behavioural state can then be evaluated for each location, as well as a summary made across all samples e.g., as a posterior mean (giving a value continuous between 1 and 2). For mapping purposes the behavioural state shown is the mean posterior estimate and following Jonsen et al.95 we assign estimates <1.25 as ‘transit’ (blue) and >1.75 as ‘search’ (red). All estimates between 1.25 and 1.75 are shown as ‘uncertain’ (grey). To calculate search patch duration we follow Bailey et al.33 where a patch is comprised of successive location estimates with an estimate greater than 1.75, ending when 3 or more consecutive location estimates have an estimate below 1.75.
Statistical model for behaviour-environment associations
To investigate potential environmental influences on whale search behaviour whilst on the Antarctic forage grounds we used generalized additive mixed models (GAMMs97). The most probable discrete behavioural state estimate (b, where 1 = ‘transit’, 2 = ‘search’) was modelled in response to environmental predictors selected based on their potential to influence the search behaviour of humpbacks whales by concentrating prey. The environmental variables examined, with full details of the data sources and resolution are given in Supplementary S2, Table S2.1. These predictors may act as physical habitat boundaries, influence biological productivity and/or characterise recent ice history. Preliminary investigations examined candidate ice variables at both a one month and two month lag (exploratory models presented in Supplementary S2, Table S2.2). Collinearity amongst predictors98 was assessed using variance inflation factors (ensuring VIF <3) and correlation coefficients (ensuring r < 0.8 – Pearson correlation coefficient). The environmental variables included in the final full GAMM (see Supplementary S2, Table S2.3 for full results) were: bathymetric gradient (bathyg), chlorophyll climatology (CHLa), distance to ice edge (dist ice), ice melt rate (melt rate), the coefficient of variation in ice concentration two months prior (ice cv lag 2) and mean ice concentration one month prior (ice mn lag 1). Deployment campaign was also included as a categorical predictor. We examined spatial and temporal autocorrelation in the Supplementary material (see Supplementary S2, S2.4) and included an animation (see Supplementary S3) depicting each monthly coefficient of variation in ice concentration along with the state space modelled location estimations two months prior, one month prior and throughout the same month to aid in interpretation of this ice cv lag 2 variable.
There is uncertainty inherent in the behavioural state estimate (here the response variable in our GAMMs) for each whale location, and also for each location estimate itself. It is particularly relevant to propagate location uncertainty when sampling environmental variables from spatially gridded remote-sensing data, although this is relatively rarely considered (but see99). To account for the uncertainty in both behavioural states and locations we resampled from the state-space model posterior estimates, thereby adopting a type of sensitivity analysis or bootstrap approach to fitting the GAMMs; this approach to accounting for measurement error is also called multiple imputation. We fit the first GAMM using the hSSSM behavioural state and location estimates summarised as the posterior mean for each location (n = 1442). We then randomly sampled the retained MCMC chains to obtain 100 possible realisations (50 per chain) of these 1442 behavioural and location state estimates.
These new samples drawn from the posteriors were then used to refit the above model 100 times. The discrete behavioural state (b) estimate at each location of the new track realisation was modelled against the relevant set of environmental predictor variables (also extracted at the new posterior sample locations). This enabled assessment of the strength of environmental influences accounting for uncertainty in the state-space model posterior estimates for behavioural state and location. We report the number of times each environmental variable was determined to be significant (based on p-value < 0.05) under the resampling procedure (n = 100 iterations). P-values indicate approximate significance of GAMM smooth terms based on Chi-sq. statistics. The GAMM was fit using a binomial family with a logit link, with individual whale included as a random effect. The GAMM allows for the relationships between behaviour and environmental predictors to be flexible and non-linear. All statistical models were fit using the gamm497 library in R96 which makes use of the modular fitting functions provided by lme4100 and is appropriate for binary data. gamm497 follows the approach taken by package mgcv101 and represents the smooths using R penalized regression spline type smoothers, of moderate rank. We focussed on the Antarctic forage grounds, restricting our behavioural analysis to only those locations at or south of 60 °S (n = 20 whales, N = 1442 locations).
This study was carried out in strict accordance with the approvals and conditions of the Antarctic Animal Ethics Committee for Australian Antarctic Science project 2941. Fieldwork was undertaken with the permission of the Australian Government under EPBC permits 2007–006 and 2007–007.
Electronic supplementary material
Acknowledgements
The data used in this paper was collected from three separate field trips coordinated by many and we are most grateful to all involved. For support in the field we’d particularly like to acknowledge Dave Donnelly, the Sapphire Coast Discovery Centre, the crew of ‘Cat Balou’ and the AWE Science Team: Jean-Benoit Charrassin, Simon Childerhouse, Rochelle Constantine, Paul Ensor, Stephane Gauthier, Jason Gedamke, Curt Jenner, Catriona Johnson, Paul Sagar and vessel and science support crew. For their ongoing persistence and dedication to the difficult task of tag development we’d like to thank Eric King (sadly deceased), Curt Jenner and Micheline Jenner. We would also like to thank James Marthick for assisting with genetic analyses and Brian Miller for comments and suggestions on an earlier version of this manuscript. The Antarctic Whale Expedition was the first voyage of the International Whaling Commission – Southern Ocean Research Partnership (IWC-SORP) which has supported the ongoing development of non-lethal research techniques in order to maximise conservation outcomes for Southern Ocean whales since 2009. We are grateful to two anonymous reviewers for their comments and suggestions that greatly improved the content of this paper. This research was funded by the Australian Government International Whale and Marine Mammal Conservation Initiative. This research was supported under Australian Research Council’s Special Research Initiative for Antarctic Gateway Partnership (Project ID SR140300001) and contributes to the Australian Research Council Discovery Early Career Research Award (DECRA) granted to S.B. under Project DE180100828. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Conceived and designed the experiments: M.C.D., N.J.G. Performed the experiments: M.C.D., N.J.G., S.M.L., A.M.P., N.T.S., D.P. Conducted the genetic analyses: N.T.S., A.M.P. Analysed the data: V.A.G S.B. Wrote the paper: V.A.G., S.B., M.C.D. All authors reviewed the manuscript.
Data availability
See91–93 for permanent links to the satellite tracking data analysed in this study which is held by the Australian Antarctic Data Centre at https://data.aad.gov.au/.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-30748-4.
References
- 1.Dingle, H. Migration: The Biology of Life on the Move. 2nd edn, 352 (Oxford University Press, 2014).
- 2.Dawbin, W. H. The seasonal migratory cycle of humpback whales. Whales, dolphins and porpoises. University of California Press, Berkeley, 145–170 (1966).
- 3.Rayner, G. W. Whale marking progress and results to December 1939. Discovery ReportsXIX (1940).
- 4.Robbins, J. et al. Return movement of a humpback whale between the Antarctic Peninsula and American Samoa: a seasonal migration record (2011).
- 5.Palsbøll PJ, et al. Genetic tagging of humpback whales. Nature. 1997;388:767. doi: 10.1038/42005. [DOI] [PubMed] [Google Scholar]
- 6.Nowacek DP, Christiansen F, Bejder L, Goldbogen JA, Friedlaender AS. Studying cetacean behaviour: new technological approaches and conservation applications. Anim Behav. 2016;120:235–244. doi: 10.1016/j.anbehav.2016.07.019. [DOI] [Google Scholar]
- 7.Mate B, Mesecar R, Lagerquist B. The evolution of satellite-monitored radio tags for large whales: One laboratory’s experience. DSR. 2007;54:224–247. [Google Scholar]
- 8.Garrigue C, Clapham PJ, Geyer Y, Kennedy AS, Zerbini AN. Satellite tracking reveals novel migratory patterns and the importance of seamounts for endangered South Pacific humpback whales. Royal Society open science. 2015;2:150489. doi: 10.1098/rsos.150489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branch TA, et al. Past and present distribution, densities and movements of blue whales Balaenoptera musculus in the Southern Hemisphere and northern Indian Ocean. Mammal Rev. 2007;37:116–175. doi: 10.1111/j.1365-2907.2007.00106.x. [DOI] [Google Scholar]
- 10.Double MC, et al. Migratory movements of pygmy blue whales (Balaenoptera musculus brevicauda) between Australia and Indonesia as revealed by satellite telemetry. Plos One. 2014;9:e93578. doi: 10.1371/journal.pone.0093578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstein BG, Double M, Gales N, Johnston DW, Friedlaender AS. Identifying overlap between humpback whale foraging grounds and the Antarctic krill fishery. Biol Conserv. 2017;210:184–191. doi: 10.1016/j.biocon.2017.04.014. [DOI] [Google Scholar]
- 12.Bograd SJ, Block BA, Costa DP, Godley BJ. Biologging technologies: new tools for conservation. Introduction. Endanger Spec Res. 2010;10:1–7. doi: 10.3354/esr00269. [DOI] [Google Scholar]
- 13.Hussey NE, et al. Aquatic animal telemetry: a panoramic window into the underwater world. Sci. 2015;348:1255642. doi: 10.1126/science.1255642. [DOI] [PubMed] [Google Scholar]
- 14.Evans K, Lea M-A, Patterson T. Recent advances in bio-logging science: Technologies and methods for understanding animal behaviour and physiology and their environments. DSR. 2013;88–89:1–6. [Google Scholar]
- 15.Patterson TA, Thomas L, Wilcox C, Ovaskainen O, Matthiopoulos J. State–space models of individual animal movement. Trends Ecol Evol. 2008;23:87–94. doi: 10.1016/j.tree.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Schick RS, et al. Understanding movement data and movement processes: current and emerging directions. Ecol Lett. 2008;11:1338–1350. doi: 10.1111/j.1461-0248.2008.01249.x. [DOI] [PubMed] [Google Scholar]
- 17.Jonsen I, et al. State-space models for bio-loggers: A methodological road map. DSR. 2013;88:34–46. [Google Scholar]
- 18.Jonsen ID, Flemming JM, Myers RA. Robust state–space modeling of animal movement data. Ecology. 2005;86:2874–2880. doi: 10.1890/04-1852. [DOI] [Google Scholar]
- 19.Bestley, S., Jonsen, I. D., Hindell, M. A., Guinet, C. & Charrassin, J.-B. Integrative modelling of animal movement: incorporating in situ habitat and behavioural information for a migratory marine predator. Proceedings of the Royal Society B: Biological Sciences280 (2013). [DOI] [PMC free article] [PubMed]
- 20.Pomerleau C, et al. Bowhead whale Balaena mysticetus diving and movement patterns in the eastern Canadian Arctic: implications for foraging ecology. Endanger Spec Res. 2011;15:167–177. doi: 10.3354/esr00373. [DOI] [Google Scholar]
- 21.Reisinger RR, Keith M, Andrews RD, de Bruyn PJN. Movement and diving of killer whales (Orcinus orca) at a Southern Ocean archipelago. J Exp Mar Biol Ecol. 2015;473:90–102. doi: 10.1016/j.jembe.2015.08.008. [DOI] [Google Scholar]
- 22.Lee D, et al. Spatial distribution of common Minke whale (Balaenoptera acutorostrata) as an indication of a biological hotspot in the East Sea. DSR. 2017;143:91–99. [Google Scholar]
- 23.Cotté C, Guinet C, Taupier-Letage I, Mate B, Petiau E. Scale-dependent habitat use by a large free-ranging predator, the Mediterranean fin whale. Deep Sea Research Part I: Oceanographic Research Papers. 2009;56:801–811. doi: 10.1016/j.dsr.2008.12.008. [DOI] [Google Scholar]
- 24.Hazen EL, et al. Ontogeny in marine tagging and tracking science: technologies and data gaps. Mar Ecol Prog Ser. 2012;457:221–240. doi: 10.3354/meps09857. [DOI] [Google Scholar]
- 25.Mackintosh, N. A. The stocks of whales., (Fishing News Books, 1965).
- 26.Bannister J, Hedley S. Southern Hemisphere group IV humpback whales: their status from recent aerial survey. Memoirs-Queensl and Museum. 2001;47:587–598. [Google Scholar]
- 27.Noad, M., Dunlop, R., Bennett, L. & Kniest, H. Abundance estimates of the east Australian humpback whale population (BSE1): 2015 survey and update. Paper SC/66b/SH/21 presented to the International Whaling Commission Scientific Committee (2016).
- 28.Kennedy AS, Zerbini AN, Rone BK, Clapham PJ. Individual variation in movements of satellite-tracked humpback whales Megaptera novaeangliae in the eastern Aleutian Islands and Bering Sea. Endanger Spec Res. 2014;23:187–195. doi: 10.3354/esr00570. [DOI] [Google Scholar]
- 29.Riekkola L, et al. Application of a multi-disciplinary approach to reveal population structure and Southern Ocean feeding grounds of humpback whales. Ecol Indic. 2018;89:455–465. doi: 10.1016/j.ecolind.2018.02.030. [DOI] [Google Scholar]
- 30.Chittleborough R. Dynamics of two populations of the humpback whale, Megaptera novaeangliae (Borowski) Mar Freshw Res. 1965;16:33–128. doi: 10.1071/MF9650033. [DOI] [Google Scholar]
- 31.Owen K, et al. Potential energy gain by whales outside of the Antarctic: prey preferences and consumption rates of migrating humpback whales (Megaptera novaeangliae) Polar Biol. 2017;40:277–289. doi: 10.1007/s00300-016-1951-9. [DOI] [Google Scholar]
- 32.Stamation KA, Croft DB, Shaughnessy PD, Waples KA. Observations of humpback whales (Megaptera novaeangliae) feeding during their southward migration along the coast of southeastern New South Wales, Australia: identification of a possible supplemental feeding ground. Aquat Mamm. 2007;33:165. doi: 10.1578/AM.33.2.2007.165. [DOI] [Google Scholar]
- 33.Bailey H, et al. Behavioural estimation of blue whale movements in the Northeast Pacific from state space model analysis of satellite tracks. Endanger Spec Res. 2009;10:93–106. doi: 10.3354/esr00239. [DOI] [Google Scholar]
- 34.Bestley S, Jonsen I, Harcourt RG, Hindell MA, Gales NJ. Putting the behavior into animal movement modeling: Improved activity budgets from use of ancillary tag information. Ecology and evolution. 2016;6:8243–8255. doi: 10.1002/ece3.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClintock BT, Russell DJ, Matthiopoulos J, King R. Combining individual animal movement and ancillary biotelemetry data to investigate population‐level activity budgets. Ecology. 2013;94:838–849. doi: 10.1890/12-0954.1. [DOI] [Google Scholar]
- 36.Barendse J, et al. Migration redefined? Seasonality, movements and group composition of humpback whales Megaptera novaeangliae off the west coast of South Africa. Afr J Mar Sci. 2010;32:1–22. doi: 10.2989/18142321003714203. [DOI] [Google Scholar]
- 37.Findlay KP, et al. Humpback whale “super-groups” – A novel low-latitude feeding behaviour of Southern Hemisphere humpback whales (Megaptera novaeangliae) in the Benguela Upwelling System. Plos One. 2017;12:e0172002. doi: 10.1371/journal.pone.0172002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alves DS, et al. Record of feeding by humpback whales (Megaptera novaeangliae) in tropical waters off Brazil. Mar Mamm Sci. 2009;25:416–419. doi: 10.1111/j.1748-7692.2008.00249.x. [DOI] [Google Scholar]
- 39.Acevedo J, et al. Evidence of spatial structuring of eastern South Pacific humpback whale feeding grounds. Endanger Spec Res. 2013;22:33–38. doi: 10.3354/esr00536. [DOI] [Google Scholar]
- 40.Eisenmann P, et al. Isotopic evidence of a wide spectrum of feeding strategies in Southern Hemisphere humpback whale baleen records. Plos One. 2016;11:e0156698. doi: 10.1371/journal.pone.0156698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franklin W, et al. Antarctic waters (Area V) near the Balleny Islands are a summer feeding area for some eastern Australian Breeding Stock E (i) Humpback Whales (Megaptera Novaeangliae) J. Cetacean Res. Manage. 2012;12:321–327. [Google Scholar]
- 42.Constantine R, et al. Remote Antarctic feeding ground important for east Australian humpback whales. Mar. Biol. 2014;161:1087–1093. doi: 10.1007/s00227-014-2401-2. [DOI] [Google Scholar]
- 43.Dalla Rosa L, Secchi E, Maia Y, Zerbini A, Heide-Jørgensen M. Movements of satellite-monitored humpback whales on their feeding ground along the Antarctic Peninsula. Polar Biol. 2008;31:771–781. doi: 10.1007/s00300-008-0415-2. [DOI] [Google Scholar]
- 44.Curtice C, et al. Modeling the spatial and temporal dynamics of foraging movements of humpback whales (Megaptera novaeangliae) in the Western Antarctic Peninsula. Movement ecology. 2015;3:13. doi: 10.1186/s40462-015-0041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinstein BG, Friedlaender AS. Dynamic foraging of a top predator in a seasonal polar marine environment. Oecologia. 2017;185:427–435. doi: 10.1007/s00442-017-3949-6. [DOI] [PubMed] [Google Scholar]
- 46.Fauchald P, Tveraa T. Hierarchical patch dynamics and animal movement pattern. Oecologia. 2006;149:383–395. doi: 10.1007/s00442-006-0463-7. [DOI] [PubMed] [Google Scholar]
- 47.Michelot T, et al. Estimation and simulation of foraging trips in land‐based marine predators. Ecology. 2017;98:1932–1944. doi: 10.1002/ecy.1880. [DOI] [PubMed] [Google Scholar]
- 48.Lange BA, et al. Pan‐Arctic sea ice‐algal chl a biomass and suitable habitat are largely underestimated for multiyear ice. Glob Change Biol. 2017;23:4581–4597. doi: 10.1111/gcb.13742. [DOI] [PubMed] [Google Scholar]
- 49.Smith WO., Jr. Phytoplankton dynamics in marginal ice zones. Oceanogr. Mar. Biol. 1987;25:11–38. [Google Scholar]
- 50.Massom R, et al. Change and variability in East Antarctic sea ice seasonality, 1979/80–2009/10. Plos One. 2013;8:e64756. doi: 10.1371/journal.pone.0064756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melbourne-Thomas J, et al. Optimal control and system limitation in a Southern Ocean ecosystem model. DSR. 2015;114:64–73. [Google Scholar]
- 52.Dalpadado P, et al. Productivity in the Barents Sea-response to recent climate variability. Plos One. 2014;9:e95273. doi: 10.1371/journal.pone.0095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lehodey P, et al. Predicting skipjack tuna forage distributions in the equatorial Pacific using a coupled dynamical bio‐geochemical model. Fish Oceanogr. 1998;7:317–325. doi: 10.1046/j.1365-2419.1998.00063.x. [DOI] [Google Scholar]
- 54.Nicol S, Worby A, Leaper R. Changes in the Antarctic sea ice ecosystem: potential effects on krill and baleen whales. Mar Freshw Res. 2008;59:361–382. doi: 10.1071/MF07161. [DOI] [Google Scholar]
- 55.Arrigo, K. R., van Dijken, G. L. & Bushinsky, S. Primary production in the Southern Ocean, 1997–2006. Journal of Geophysical Research: Oceans113 (2008).
- 56.Nicol S, Worby A, Strutton P, Trull T. Oceanographic influences on Antarctic ecosystems: a summary of observations and insights from East Antarctica (0–150 E) The Sea. 2006;14:778. [Google Scholar]
- 57.Hindell, M. A. et al. Decadal changes in habitat characteristics influence population trajectories of southern elephant seals. Glob Change Biol (2017). [DOI] [PubMed]
- 58.Schine C, van Dijken G, Arrigo KR. Spatial analysis of trends in primary production and relationship with large‐scale climate variability in the Ross Sea, Antarctica (1997–2013) Journal of Geophysical Research: Oceans. 2016;121:368–386. [Google Scholar]
- 59.Siegel V, Loeb V. Recruitment of Antarctic krill Euphausia superba and possible causes for its variability. Mar. Ecol. Prog. Ser. 1995;123:45–56. doi: 10.3354/meps123045. [DOI] [Google Scholar]
- 60.Loeb V, et al. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature. 1997;387:897. doi: 10.1038/43174. [DOI] [Google Scholar]
- 61.Meyer B, et al. The winter pack-ice zone provides a sheltered but food-poor habitat for larval Antarctic krill. Nature ecology & evolution. 2017;1:1853. doi: 10.1038/s41559-017-0368-3. [DOI] [PubMed] [Google Scholar]
- 62.Branch TA. Humpback whale abundance south of 60 S from three complete circumpolar sets of surveys. Journal of Cetacean Research and Management (Special Issue) 2011;3:53–69. [Google Scholar]
- 63.Bombosch A, et al. Predictive habitat modelling of humpback (Megaptera novaeangliae) and Antarctic minke (Balaenoptera bonaerensis) whales in the Southern Ocean as a planning tool for seismic surveys. Deep Sea Research Part I: Oceanographic Research Papers. 2014;91:101–114. doi: 10.1016/j.dsr.2014.05.017. [DOI] [Google Scholar]
- 64.Tynan CT. Ecological importance of the Southern Boundary of the Antarctic Circumpolar Current. Nature. 1998;392:708–710. doi: 10.1038/33675. [DOI] [Google Scholar]
- 65.Stevick P, De Godoy LP, McOsker M, Engel M, Allen J. A note on the movement of a humpback whale from Abrolhos Bank, Brazil to South Georgia. J Cetacean Res Manag. 2006;8:297. [Google Scholar]
- 66.Barendse J, Best PB, Carvalho I, Pomilla C. Mother knows best: occurrence and associations of resighted humpback whales suggest maternally derived fidelity to a Southern Hemisphere coastal feeding ground. Plos One. 2013;8:e81238. doi: 10.1371/journal.pone.0081238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bradshaw CJ, Hindell MA, Sumner MD, Michael KJ. Loyalty pays: potential life history consequences of fidelity to marine foraging regions by southern elephant seals. Anim Behav. 2004;68:1349–1360. doi: 10.1016/j.anbehav.2003.12.013. [DOI] [Google Scholar]
- 68.Arthur B, et al. Return customers: Foraging site fidelity and the effect of environmental variability in wide-ranging Antarctic fur seals. Plos One. 2015;10:e0120888. doi: 10.1371/journal.pone.0120888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clapham PJ, et al. Seasonal occurrence and annual return of humpback whales, Megaptera novaeangliae, in the southern Gulf of Maine. Can J Zool. 1993;71:440–443. doi: 10.1139/z93-063. [DOI] [Google Scholar]
- 70.Clapham PJ, Mayo CA. Reproduction and recruitment of individually identified humpback whales, Megaptera novaeangliae, observed in Massachusetts Bay, 1979–1985. Can J Zool. 1987;65:2853–2863. doi: 10.1139/z87-434. [DOI] [Google Scholar]
- 71.Katona, S. K. & Beard, J. A. Population size, migrations and feeding aggregations of the humpback whale (Megaptera novaeangliae) in the western North Atlantic Ocean. Report of the International Whaling Commission (Special Issue 12), 295–306 (1990).
- 72.Palsbøll, P. J. & Arctander, P. Distribution of mtDNA haplotypes in North Atlantic humpback whales: the influence of behaviour on population structure. Marine Ecology-Progress Series, 1–10 (1995).
- 73.Clapham PJ, Aguilar A, Hatch LT. Determining spatial and temporal scales for management: lessons from whaling. Mar Mamm Sci. 2008;24:183–201. doi: 10.1111/j.1748-7692.2007.00175.x. [DOI] [Google Scholar]
- 74.International Whaling Commission Report of the Scientific Committee. Annex H. Report of the Sub-Committee on Other Southern Hemisphere Whale Stocks. Appendix 7. Comprehensive Assessment of Southern Hemisphere humpback whales: proposal for an intersessional workshop. J. Cetacean Res. Manage. (Suppl.) 2006;8:170. [Google Scholar]
- 75.Zerbini AN, Clapham PJ, Wade PR. Assessing plausible rates of population growth in humpback whales from life-history data. Mar. Biol. 2010;157:1225–1236. doi: 10.1007/s00227-010-1403-y. [DOI] [Google Scholar]
- 76.Childerhouse, S. et al. Megaptera novaeangliae (Oceania subpopulation). The IUCN Red List of Threatened Species, doi:10.2305/IUCN.UK.2008.RLTS.T132832A3463914.en (2008).
- 77.Clapham P, Ivashchenko Y. A whale of a deception. Mar. Fish. Rev. 2009;71:44–52. [Google Scholar]
- 78.Schmitt NT, et al. Low levels of genetic differentiation characterize Australian humpback whale (Megaptera novaeangliae) populations. Mar Mamm Sci. 2014;30:221–241. doi: 10.1111/mms.12045. [DOI] [Google Scholar]
- 79.Clapham PJ, Zerbini AN. Are social aggregation and temporary immigration driving high rates of increase in some Southern Hemisphere humpback whale populations? Mar. Biol. 2015;162:625–634. doi: 10.1007/s00227-015-2610-3. [DOI] [Google Scholar]
- 80.Pallin, L. J. et al. High pregnancy rates in humpback whales (Megaptera novaeangliae) around the Western Antarctic Peninsula, evidence of a rapidly growing population. Royal Society Open Science5, 10.1098/rsos.180017 (2018). [DOI] [PMC free article] [PubMed]
- 81.Bengtson Nash SM, et al. Signals from the south; humpback whales carry messages of Antarctic sea‐ice ecosystem variability. Glob Change Biol. 2018;24:1500–1510. doi: 10.1111/gcb.14035. [DOI] [PubMed] [Google Scholar]
- 82.Kawaguchi S, et al. Risk maps for Antarctic krill under projected Southern Ocean acidification. Nature Climate Change. 2013;3:843. doi: 10.1038/nclimate1937. [DOI] [Google Scholar]
- 83.Robinson RA, et al. Travelling through a warming world: climate change and migratory species. Endanger Spec Res. 2009;7:87–99. doi: 10.3354/esr00095. [DOI] [Google Scholar]
- 84.Lennox, R. J. et al. Conservation physiology of animal migration. Conservation Physiology 4, 10.1093/conphys/cov072 (2016). [DOI] [PMC free article] [PubMed]
- 85.Bejder M, Johnston DW, Smith J, Friedlaender A, Bejder L. Embracing conservation success of recovering humpback whale populations: Evaluating the case for downlisting their conservation status in Australia. Mar Policy. 2016;66:137–141. doi: 10.1016/j.marpol.2015.05.007. [DOI] [Google Scholar]
- 86.Heide‐Jørgensen MP, Kleivane L, ØIen N, Laidre KL, Jensen MV. A new technique for deploying satellite transmitters on baleen whales: Tracking a blue whale (Balaenoptera musculus) in the North Atlantic. Mar Mamm Sci. 2001;17:949–954. doi: 10.1111/j.1748-7692.2001.tb01309.x. [DOI] [Google Scholar]
- 87.Gales, N. et al. Satellite tracking of southbound East Australian humpback whales (Megaptera novaeangliae): challenging the feast or famine model for migrating whales. Paper submitted for consideration by the IWC Scientific Committee, IWC Paper SC/61/SH17:12 (2009).
- 88.Krutzen M, et al. A biopsy system for small cetaceans: darting success and wound healing in Tursiops spp. Mar Mamm Sci. 2002;18:863–878. doi: 10.1111/j.1748-7692.2002.tb01078.x. [DOI] [Google Scholar]
- 89.Morin PA, Nestler A, Rubio‐Cisneros NT, Robertson KM, Mesnick SL. Interfamilial characterization of a region of the ZFX and ZFY genes facilitates sex determination in cetaceans and other mammals. Mol Ecol. 2005;14:3275–3286. doi: 10.1111/j.1365-294X.2005.02651.x. [DOI] [PubMed] [Google Scholar]
- 90.CLS. Argos user’s manual. (CLS Argos, 2016).
- 91.Gales, N., Double, M. & Andrews-Goff, V. Tracking summer migration of humpback whales from Sunshine Coast, Queensland to southern waters 2010/11 Australian Antarctic Data Centre - CAASM Metadata, 10.4225/15/5ae67ef1e6ec2 (2018).
- 92.Gales, N., Andrews-Goff, V. & Double, M. Satellite tracks of humpback whales generated from tag deployments during the Antarctic Whale Expedition (AWE) 2009/10. Australian Antarctic Data Centre - CAASM Metadata, 10.4225/15/5ae67dbd96e55 (2018).
- 93.Gales, N., Andrews-Goff, V. & Double, M. Satellite tracking of southbound East Australian humpback whales 2008/09. Australian Antarctic Data Centre - CAASM Metadata, 10.4225/15/5ae679d839e71 (2018).
- 94.Jonsen I. Joint estimation over multiple individuals improves behavioural state inference from animal movement data. Scientific Reports. 2016;6:20625. doi: 10.1038/srep20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jonsen ID, Myers RA, James MC. Identifying leatherback turtle foraging behaviour from satellite telemetry using a switching state space model. Mar Ecol Prog Ser. 2007;337:255–264. doi: 10.3354/meps337255. [DOI] [Google Scholar]
- 96.R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2017).
- 97.Wood, S. & Scheipl, F. gamm4: Generalized additive mixed models using ‘mgcv’ and ‘lme4’. R package version 0.2–5 (2017).
- 98.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution. 2010;1:3–14. doi: 10.1111/j.2041-210X.2009.00001.x. [DOI] [Google Scholar]
- 99.Eckert SA, et al. Modeling loggerhead turtle movement in the Mediterranean: importance of body size and oceanography. Ecol Appl. 2008;18:2008. doi: 10.1890/06-2107.1. [DOI] [PubMed] [Google Scholar]
- 100.Wood, S. N. Generalized additive models: an introduction with R. (Chapman and Hall/CRC, 2017).
- 101.Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 102.Orsi AH, Whitworth T, Nowlin WD. On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep Sea Research Part I: Oceanographic Research Papers. 1995;42:641–673. doi: 10.1016/0967-0637(95)00021-W. [DOI] [Google Scholar]
- 103.IOC, IHO & BODC. Centenary Edition of the GEBCO Digital Atlas, on behalf of the Intergovernmental Oceanographic Commission and the International Hydrographic Organization as part of the General Bathymetric Chart of the Oceans, British OceanographicData Centre (2003).
- 104.Burrough, P. A. & McDonell, R. A. Principles of geographical information systems., (Oxford University Press, 1998).
- 105.Raymond, B. Polar Environmental Data Layers. Australian Antarctic Data Centre - CAASM Metadata, https://data.aad.gov.au/metadata/records/Polar_Environmental_Data (2012 (updated 2014)).
- 106.Johnson R, Strutton PG, Wright SW, McMinn A, Meiners KM. Three improved satellite chlorophyll algorithms for the Southern Ocean. Journal of Geophysical Research: Oceans. 2013;118:3694–3703. [Google Scholar]
- 107.NASA Goddard Space Flight Center, O. E. L., Ocean Biology Processing Group,. Sea-viewing Wide Field-of-view Sensor (SeaWiFS) Ocean Color Data, 10.5067/ORBVIEW-2/SEAWIFS_OC.2014.0 (2014 Reprocessing).
- 108.Cavalieri, D. J., Parkinson, C. L., Gloersen, P. & Zwally, H. J. Sea Ice Concentrations from Nimbus-7 SMMR and DMSP SSM/I-SSMIS Passive Microwave Data, Version 1, 10.5067/8GQ8LZQVL0VL (1996, updated yearly).
- 109.Spreen, G., Kaleschke, L. & Heygster, G. Sea ice remote sensing using AMSR‐E 89‐GHz channels. Journal of Geophysical Research: Oceans113, 10.1029/2005JC003384 (2008).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
See91–93 for permanent links to the satellite tracking data analysed in this study which is held by the Australian Antarctic Data Centre at https://data.aad.gov.au/.