Abstract
The medial septum (MS) impacts hippocampal activity and the hippocampus, in turn, regulates midbrain dopamine (DA) neuron activity. However, it remains to be determined how MS activation impacts midbrain DA activity. This question was addressed by infusing NMDA (0.75 µg/0.2 µL) into the medial septum of anesthetized male Sprague-Dawley rats and recording dopamine neuron activity in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc). MS activation increased (71%) the number of spontaneously active DA neurons in the VTA, and decreased (40%) the number of active DA neurons in the SNc. Effects in both the VTA and SNc required the ventral subiculum, but were differentially dependent on cholinergic and GABAergic mechanisms within the vSub and rostral and caudal subregions of the ventral pallidum, respectively. MS activation also decreased amphetamine-induced locomotor behavior, which was dependent on GABAergic inputs to the hippocampus. These findings demonstrate that the MS differentially regulates meso-striatal DA transmission via distinct pathways.
Introduction
Dopamine (DA) transmission is involved in reward prediction and association, salience signaling, and fine motor control, among other constructs [1, 2]. Disruptions in DA signaling are central to symptom presentation of several devastating neuropsychological disorders, including schizophrenia, depression, Parkinson’s, and addiction [2–4]. As such, characterizing DA transmission, and its afferent regulatory circuits, is critical to understanding both DA’s role in normal behavior and its disruption in diseases.
One pathway that potently regulates DA neuron activity arises from the ventral subiculum (vSub) of the hippocampus and affects DA neuron activity in the ventral tegmental area (VTA; [5]). This pathway, which connects to the VTA via the nucleus accumbens (NAc; [5]) and ventral pallidum (VP; [6]), regulates the proportion of DA neurons that are spontaneously active in VTA (i.e., population activity). This function is critical because only spontaneously active DA neurons are able to burst fire [7], the behaviorally salient output of the DA system [8, 9]. Thus, by regulating DA population activity, the vSub-NAc-VP pathway controls the magnitude of behaviorally relevant, phasic DA release, providing a “context” for incoming stimuli.
Due to this critical role, identifying the structures that regulate the vSub-NAc-VP pathway has clear implications for both understanding DA transmission in an intact system and treatment discovery. Interestingly, the medial septum (MS), a sub region of the cholinergic basal forebrain, sends dense cholinergic, GABAergic, and glutamatergic [10, 11] projections that make widespread connections in the hippocampus [12], including in the vSub [11, 13]. This septohippocampal pathway is a potent regulator of hippocampal function, with previous studies demonstrating that the MS drives theta rhythmicity in the hippocampus [14, 15] and is necessary for spatial learning and memory [16–18]. Due to the established role of the MS in hippocampal function and the importance of the vSub in midbrain DA regulation, it is important to determine the impact of the MS on DA transmission. Indeed combined lesion of the cholinergic and GABAergic septohippocampal projections increased amphetamine-induced hyperlocomotion [19], a behavioral paradigm strongly correlated with spontaneous DA neuron activity in the midbrain [20–22].
Another knowledge gap related to the vSub-NAc-VP pathway is that no studies have examined its role in the regulation of substantia nigra pars compacta (SNc) DA transmission, a region that is affected by NAc stimulation [23] and has a clear role in psychopathology [24]. The comparison between regulation of VTA and SNc by this pathway is imperative because it was recently demonstrated that DREADD inactivation of rostral ventral pallidum (rVP) terminals in VTA prevented cue-induced reinstatement of cocaine-seeking behavior, whereas inactivation of caudal ventral pallidum (cVP) terminals in VTA or rVP terminals in SNc had no effect on cocaine-seeking reinstatement [25]. This presents the possibility that the VP could differentially regulate VTA and SNc DA activity along its rostral-caudal axis, suggesting an important additional layer of complexity within the vSub-NAc-VP pathway that has yet to be examined experimentally.
In vivo electrophysiological recordings were used to evaluate how the MS impacts DA activity in the VTA compared to the SNc and the necessity of the previously described vSub-NAc-VP pathway in this effect. Additionally, the behavioral consequence of MS activation was determined using the amphetamine-induced hyperlocomotion paradigm.
Materials and Methods
Animals
Experiments were performed using adult male Sprague-Dawley rats (325–425 g, Envigo, Frederick, MD). Rats were housed in pairs with ad libitum access to food and water in a temperature and humidity controlled room until used. Experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh according to National Institute of Health Guide for the Care and Use of Laboratory Animals.
Electrophysiological recordings
Rats were given an initial dose of chloral hydrate (400 mg/kg i.p.) and secured to a stereotaxic frame (Kopf). A 26 gauge needle was implanted in the tail vein for periodic supplementation of chloral hydrate. Body temperature was maintained using a Fintronics temperature system. A burr hole was drilled in the skull and a guide cannula lowered into the medial septum (MS; AP: + 0.5, ML: 0, DV: −5.8 mm from bregma). NMDA (0.75 µg in 0.2 µL; Sigma-Aldrich) mixed in Dulbecco’s phosphate buffered saline (dPBS; Sigma-Aldrich) or dPBS was then infused ( 0.2 µL/min) and the internal cannula was left in place for 3 min following the infusion. After 10 min, electrophysiological recording of the VTA or SNc began and typically lasted 1.0–2.5 h. Chemical stimulation was deliberately used for these experiments to produce stable, long-duration excitation, as might be expected for extended task performance; and the dose of NMDA corresponds with previous studies demonstrating DA population activity changes that lasted for 1–3 h [5–7, 22]. Because this chemical stimulation protocol could be affected by diffusion of NMDA to adjacent brain regions, an analysis of cannula placements adjacent to the MS was included (see Figure S3). For experiments outlined in Figs. 2–5, a second infusion was made just prior to NMDA activation of the MS in the same animal, using the same procedure (see Fig. 1d). These were tetrodotoxin (TTX, 1 µM, 0.5 µL), bicuculline (bicuc; 12.5 ng in 0.5 µL), or scopolamine (scop; 8 µg in 1.0 µL) into the vSub (AP: −6.0, ML: 4.5 in mm from bregma, DV: −8.5 in mm from skull) or Bicuculline (1 ng in 0.2 µL) into the rostral (rVP) or caudal (cVP) ventral pallidum (rVP = AP: 0.45, ML: 1.5, DV: −7.35 in mm from bregma; cVP = AP: 0.7, ML: 2.9, in mm from bregma, DV: −8.0 in mm from skull).
Fig. 1.
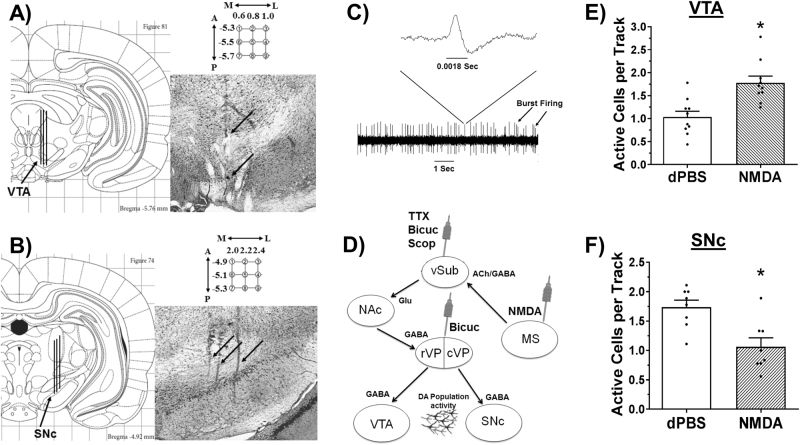
MS activation increases VTA and decreases SNc DA neuron activity: (a) Population activity in the VTA was measured in 9 sequential electrode tracks in the anatomical range depicted. Following the conclusion of the 9th track (top arrow), Chicago Sky Blue is ejected from the electrode leaving a blue dot for histological verification (bottom arrow). (b) Population activity is measured similarly in the SNc. Arrows indicate 3 tracks across the medial-lateral span of the SNc. (c) Representative DA recording and individual spike. The time bar equals 1 s, demonstrating a frequency of approximately five spikes per second. Arrows indicate two sets of spikes that are occurring in bursts. (d) Sites of pharmacological manipulation along the proposed pathway from MS to midbrain. MS-medial septum, vSub-ventral subiculum, NAc-nucleus accumbens, rVP/cVP-rostral/caudal ventral pallidum, VTA-ventral tegmental area, SNc-substantia nigra pars compacta, Bicuc-bicuculline, Scop-scopolamine. MS activation (NMDA, 0.75 µg in 0.2 µL) (e) increased the number of active DA neurons in the VTA and (f) decreased the number of active DA neurons in the SNc compared to vehicle (dPBS; *P < 0.05). Black dots indicate data from individual rats (N = 8–10 rats per group).
Fig. 3.
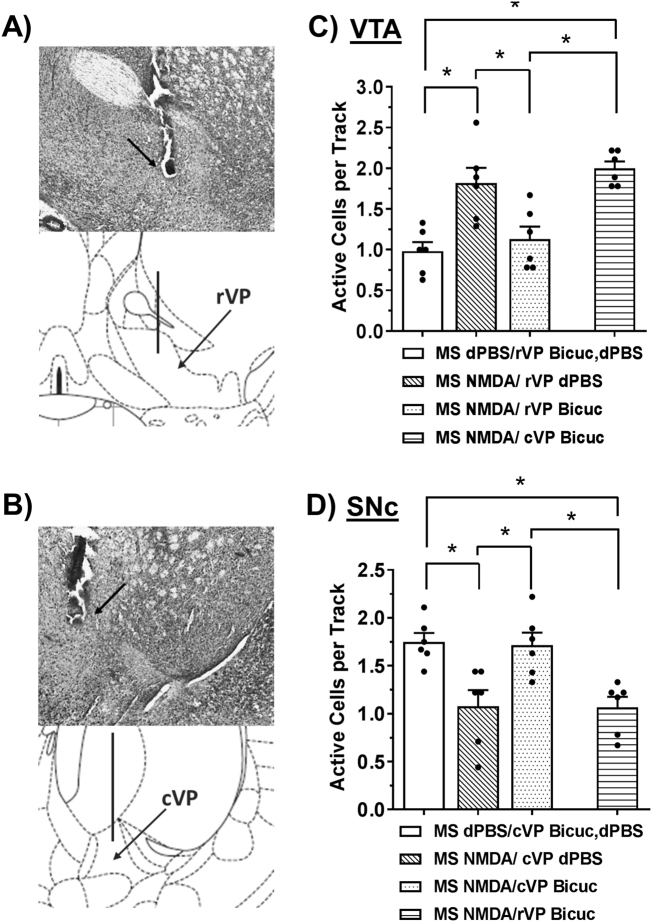
DA neuron activation in VTA selectively requires the rostral VP, whereas DA neuron inhibition in SNc selectively requires the caudal VP. Representative histology and illustration for the target region of the (a) rostral ventral pallidum (rVP) and (b) caudal ventral pallidum (rVP). The line in the illustration denotes the cannula placement and the arrow in the histology indicates the ventral termination of the cannula track. (c) Bicuculline (bicuc; 1 ng in 0.2 µL, N = 6 rats per group) infusion into the rVP selectively prevented MS activation-induced increases in the number of spontaneously active DA neurons in VTA (*Tukey’s P < 0.05). (d) Bicuculline (1 ng in 0.2 µL, N = 6 rats per group) infusion into the cVP selectively prevented MS activation-induced decreases in the number of spontaneously active DA neurons in SNc (*Tukey’s P < 0.05).
Fig. 4.
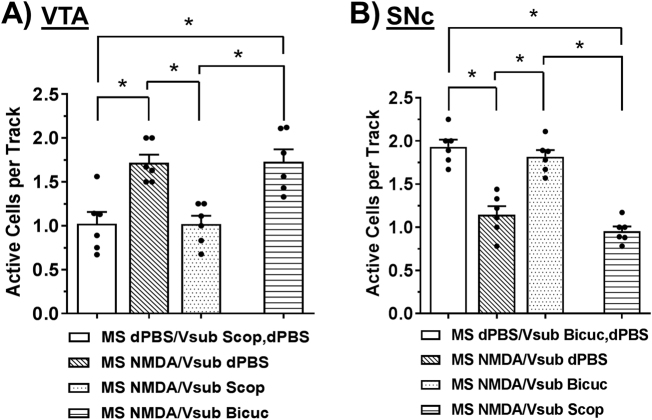
DA neuron activation in VTA selectively requires cholinergic inputs to vSub, whereas DA neuron inhibition in SNc selectively requires GABAergic inputs to vSub. (a) Scopolamine (Scop; 8 µg in 1.0 µL, N = 6 rats per group), but not bicuculline (Bicuc; 12.5 ng in 0.5 µL), infusion into the vSub selectively prevented MS activation-induced increases in the number of active DA neurons in VTA (*Tukey’s P < 0.05). (b) Bicuculline, but not scopolamine, infusion (N = 6 rats per group) into the vSub selectively prevented MS activation-induced decreases in the number of active DA neurons in SNc (*Tukey’s P < 0.05).
Following the experiment specific drug infusions, a glass recording electrode broken back under microscopic control to an impedance of 8–12 MOhms was filled with Chicago Sky Blue (Sigma-Aldrich) in a 2 M NaCl solution and lowered into brain in nine sequential vertical “tracks” (see Fig. 1a,b), moving lateral or posterior 0.2 mm for each new track (VTA = AP: 5.3–5.7 mm posterior from bregma, ML: 0.6–1.0 mm lateral from sinus, and DV: 6.5–9.0 mm from the brain surface; SNc = AP: 4.9–5.3 mm posterior from bregma, ML: 2.0–2.4 mm lateral from sinus, and DV: 6.5–9.0 mm from the brain surface). This recording pattern provided a measure corresponding to the total number of active DA neurons recorded within an animal, or population activity, that was then averaged across the total number of tracks recorded (cells/track). Therefore, DA population activity was normalized within each animal and then analyzed across animals. Coordinates were determined using an atlas [26] and follow a previously described pattern [27]. Electrodes were lowered slowly, until an active DA neuron was encountered. DA neurons were identified using well-established criteria for DA neurons occurring in the areas of the midbrain that were sampled, including (1) slow (2–10 Hz), irregular, or bursting firing pattern; (2) long-duration (>2.2 ms) biphasic action potential with initial segment-somatodendritic positive phase break; and (3) temporary cessation of firing during tail or foot pinch [28–30]. Once identified, neurons were recorded for 3 min and assessed for firing rate and burst firing properties (burst = action potentials occurring with an interspike interval of ≤80 ms and terminating with an interspike interval >160 ms). At the conclusion of the ninth track, electrophoretic ejection of Chicago sky blue dye marked the recording location for histological confirmation of electrode site.
Amphetamine-Induced hyperlocomotion
Prior to experimentation, rats were anesthetized with isoflurane, a burr hole was drilled in the skull, guide cannulae were lowered into the MS or into both the MS and vSub, and the cannulae were secured with bone screws and dental cement. Following a one week recovery period in a reverse light/dark cycle room (lights on 7:00 p.m. to 7:00 a.m.), rats were infused with either NMDA or dPBS in the MS (experiment 1) or NMDA or dPBS in the MS and bicuculline in the vSub (experiment 2) and placed in an open-field arena (Coulborn). Locomotor activity was measured by beam breaks in the x–y plane and analyzed in 5 min epochs. Systemic injections of d-amphetamine (0.75 mg/kg) or saline were given after a 30 min baseline period, and the post-amphetamine/saline sessions lasted for 60 min.
Histology
Following each experiment, rats were given an overdose of i.v. chloral hydrate or placed in a CO2 induction chamber and decapitated. Brains were removed, fixed in 8% paraformaldehyde solution, cryoprotected in a 25% sucrose solution, sectioned (60 µm coronal sections), mounted on gelatin-coated slides, and stained with cresyl violet for histological verification of cannula and electrode placement. All data included in this analysis had placements confirmed by stereotaxic atlas [26].
Statistical analysis
Detection and analysis of DA neuron activity was performed using LabChart and NeuroExplorer, and Locomotor behavior was recorded using TruScan software. Electrophysiological measures included DA population activity (cells/track), neuron firing rate (Hz), and percentage of spikes occurring in bursts (%), which were collapsed within each rat. The total number of DA neurons is reported but all analyses were performed across rats, not individual neurons or tracks to ensure independent samples. Behavioral dependent measures included total distance traveled (centimeter; cm). All measures (reported in mean ± SEM), were analyzed by ANOVA. For electrophysiology experiments a one-way ANOVA was performed using intracranial drug condition (combination of intracranial drug infusions, example MS NMDA/vSub TTX) as a between subjects variable. Post hoc analyses were performed using the Tukey’s test. The behavior was analyzed with a two-way ANOVA with intracranial drug condition (combination of intracranial NMDA/dPBS or Bicuculline/dPBS infusions) as a between subjects and time point (5 min epochs) as a within-subjects variable. Post hoc analyses were performed using the Bonferroni test to correct for the large number of pair-wise comparisons. Significance is defined as P < 0.05 (IBM SPSS Statistics 22).
Results
MS activation produces opposite effects on DA neuron activity in VTA and SNc
To determine whether the MS can affect DA activity, anesthetized rats were infused with NMDA (0.75 µg in 0.2 µL) or dPBS into their MS (N = 10 rats per group; Fig. 2b) and DA neurons were recorded in the VTA in 9 sequential tracks (Fig. 1a). NMDA activation of the MS resulted in a significant (72%) increase in the number of active DA neurons, from 1.0 ± 0.1 (dPBS, 87 total DA neurons) to 1.8 ± 0.1 (NMDA, 137 total DA neurons) average cells per electrode recording track (cells/track; F1,19 = 15.354, P = 0.001; Fig. 1e). A similar pattern (9 sequential tracks) was used to record DA activity in the SNc (N = 8 rats per group, Fig. 1b). In contrast to what was observed in VTA, NMDA activation of the MS produced a significant decrease (by 39%) in the number of active DA neurons in SNc (dPBS = 1.7 ± 0.1 cells/track, 124 DA neurons total, NMDA = 1.1 ± 0.2 cells/track, 74 DA neurons total; F1,15 = 12.033, P = 0.004; Fig. 1f). NMDA activation of the MS did not affect spike frequency (dPBS = 3.6 ± 0.3, NMDA = 4.2 ± 0.2 spikes per second) but did result in a minor increase in the percent of spikes fired in bursts in the VTA (dPBS = 21.9 ± 3.8, NMDA = 34.8 ± 3.3 % spikes in bursts; F1,19 = 6.625, P = 0.019), but not in SNc (spike frequency: dPBS = 3.9 ± 0.3, NMDA = 3.5 ± 0.3 spikes/s; bursting: dPBS = 20.2 ± 2.3, NMDA = 17.6 ± 4.2%; Table S1). NMDA activation of brain regions directly adjacent to the MS resulted in no effect or opposite effects on DA neuron activity, compared to activation of the MS (Figure S3).
Fig. 2.
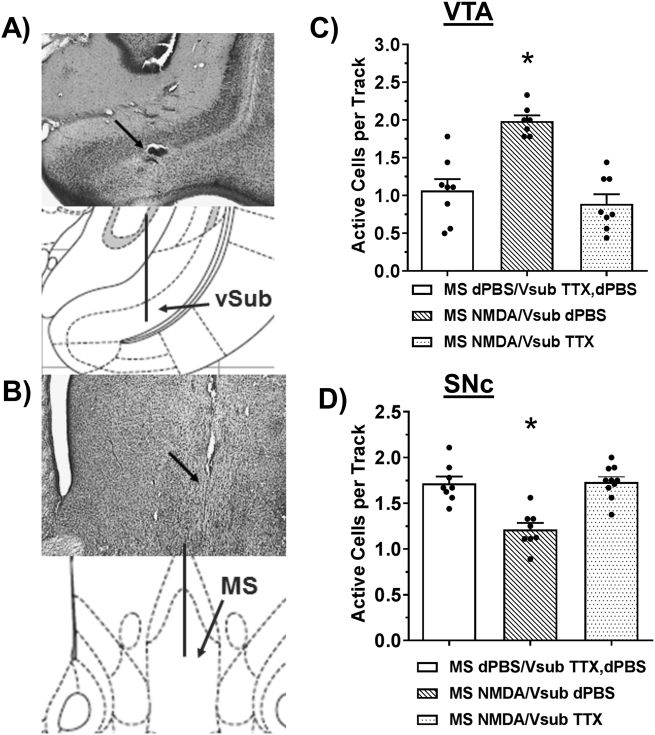
MS regulation of midbrain DA neurons requires the vSub. Representative histology and matching illustration for the target region of the (a) vSub and (b) MS. The line in the illustration denotes the cannula placement and the arrow in the histology indicates the ventral termination of the cannula track. TTX (1 µM, 0.5 µL, N = 7–10 rats per group) inactivation of the vSub prevented changes in the number of spontaneously active DA neurons in the (c) VTA (*MS NMDA/vSub dPBS significantly greater than both other groups, Tukey’s, P < 0.001) and (d) SNc (*MS NMDA/vSub dPBS significant decrease from both groups, Tukey’s, P < 0.001) compared to vehicle infusions (dPBS).
MS regulation of VTA and SNc requires activation of the vSub
To determine if the vSub is necessary for the MS regulation of VTA and SNc DA neuron activity, tetrodotoxin (TTX; 1 µM, 0.5 µL) or dPBS was infused into the vSub (Fig. 2a) just prior to NMDA or dPBS activation of the MS (Fig. 2b). In the VTA (N = 7–8 rats per group; Fig. 2c), NMDA activation of the MS increased the number of spontaneously active DA neurons (overall: F2,22 = 21.136, P < 0.001, MS dPBS/vSub dPBS or TTX = 1.1 ± 0.2 cells/track, 74 DA neurons total, MS NMDA/vSub dPBS = 2.0 ± 0.1 cells/track, 121 DA neurons total; Tukey’s = P < 0.001). This was prevented by TTX infusion into the vSub (0.9 ± 0.1 cells/track, 62 DA neurons total; Tukey’s = P < 0.001). TTX infusion in the absence of NMDA (MS dPBS/vSub TTX) had no effect on DA neuron activity (1.0 ± 0.2 cells/track, 41 DA neurons total), similar to prior studies [20, 22], and was, therefore, combined with the vehicle condition (MS dPBS/vSub dPBS). TTX infusion into the vSub similarly prevented MS activation-induced decreases in DA neuron activity in the SNc (N = 8–10 rats per group, Fig. 2d; F2,25 = 19.409, P < 0.001), restoring DA activity back to vehicle levels (MS dPBS/vSub dPBS or TTX = 1.7 ± 0.1 cells/track, 122 DA neurons total, MS NMDA/vSub dPBS = 1.2 ± 0.1 cells/track, 81 DA neurons total, MS NMDA/vSub TTX = 1.7 ± 0.1 cells/track, 142 DA neurons total). DA neuron spike frequency and bursting were not affected by vSub manipulation in either the VTA or SNc (Table S1).
MS regulation of VTA and SNc is differentially dependent on rostral and caudal VP
To investigate the necessity of the rostral/caudal ventral pallidum (rVP/cVP) in the MS regulation of midbrain DA activity, bicuculline (bicuc; 1 ng in 0.2 µL) or dPBS was infused into the rVP or cVP (N = 6 rats per group; Fig. 3a, b) just prior to NMDA or dPBS activation of the MS. Bicuculline was used to block inputs from the vSub-NAc pathway, as has been shown previously [6]. NMDA activation of the MS increased the number of spontaneously active DA neurons in VTA (Fig. 3c; MS dPBS/rVP dPBS or bicuc = 1.0 ± 0.1 cells/track, 47 DA neurons total, MS NMDA/rVP dPBS = 1.8 ± 0.2 cells/track, 94 DA neurons total; F3,23 = 12.825, P < 0.001; Tukey’s = P = 0.002). This effect was prevented by infusion of bicuculline into the rVP (MS NMDA/rVP bicuc = 1.1 ± 0.2 cells/track, 61 DA neurons total; Tukey’s vs. MS NMDA/rVP dPBS: P = 0.012). In contrast, bicuculline infusion into the cVP did not impact the number of spontaneously active DA neurons in VTA (MS NMDA/cVP bicuc = 2.0 ± 0.1 cells/track, 108 DA neurons total). NMDA activation of the MS decreased the number of spontaneously active DA neurons in the SNc (N = 6 rats per group; Fig. 3d; MS dPBS/cVP dPBS or bicuc = 1.8 ± 0.1 cells/track, 96 DA neurons total, MS NMDA/cVP dPBS = 1.1 ± 0.2 cells/track, 57 Da neurons total; F3,23 = 8.686, P = 0.001; Tukey’s = P = 0.008). In contrast to what was observed in the VTA, this effect was prevented by infusion of bicuculline into the cVP (MS NMDA/cVP bicuc = 1.7 ± 0.1 cells/track, 84 DA neurons total; Tukey’s vs. MS NMDA/cVP dPBS: P = 0.012), but not the rVP (MS NMDA/rVP bicuc = 1.1 ± 0.1 cells/track, 54 DA neurons total). Bicuculline infused into the VP without NMDA activation (MS dPBS/bicuc) had no effect on DA neuron activity, similar to prior studies [6], and was thus combined with the MS dPBS/dPBS condition in both experiments. DA neuron spike frequency and bursting were not affected by VP manipulation in either the VTA or SNc (Table S1).
MS Regulation of midbrain DA activity is differentially dependent on cholinergic and GABAergic mechanisms within the vSub
To determine the necessity of cholinergic and GABAergic inputs in vSub for the effects in VTA and SNc, the muscarinic acetylcholine antagonist scopolamine (8 µg in 1.0 µL; N = 6 rats per group) or dPBS was infused into vSub just prior to NMDA or dPBS activation of the MS. In the VTA, the MS activation-induced increase in the number of active DA neurons (Fig. 4a; MS dPBS/vSub dPBS or scop = 1.0 ± 0.1 cells/track, 50 DA neurons total, MS NMDA/vSub dPBS = 1.7 ± 0.1 cells/track, 88 DA neurons total; F3,23 = 11.959, P < 0.001; Tukey’s, P = 0.002) was completely abolished when scopolamine was infused into the vSub (MS NMDA/vSub scop = 1.0 ± 0.1 cells/track, 50 DA neurons total; Tukey’s vs. MS NMDA/vSub dPBS: P = 0.002). In contrast, scopolamine treatment did not prevent MS activation-induced decreases in DA neuron activity in the SNc (Fig. 4b; MS dPBS/vSub dPBS or bicuc = 1.9 ± 0.1 cells/track, 91 DA neurons total, MS NMDA/vSub scop = 1.0 ± 0.1 cells/track, 47 DA neurons total; F3,23 = 37.274, P < 0.001; Tukey’s, P < 0.001). Alternatively, infusion of GABAA antagonist bicuculline (bicuc; 12.5 ng in 0.5 µL or dPBS; N = 6 rats per group) into the vSub prevented the decrease in active DA neurons in SNc (Fig. 4b; MS NMDA/vSub dPBS = 1.2 ± 0.1 cells/track, 62 DA neurons total, MS NMDA/vSub bicuc = 1.8 ± 0.1 cells/track, 103 DA neurons total; Tukey’s, P < 0.001). Furthermore, bicuculline infusion into the vSub did not prevent increases in the number of active DA neurons in the VTA (Fig. 4a; MS dPBS/vSub dPBS or scop = 1.0 ± 0.1 cells/track, 50 DA neurons total, MS NMDA/vSub bicuc = 1.7 ± 0.1 cells/track, 79 DA neurons total, Tukey’s, P = 0.002). Neither scopolamine nor bicuculline had any effect on DA neuron activity in the VTA or SNc in the absence of NMDA (MS dPBS/vSub bicuc, MS dPBS/vSub scop), thus they were combined with the MS dPBS/vSub dPBS groups. DA neuron spike frequency and bursting in the VTA were not affected by scopolamine or bicuculline infusion, but scopolamine treatment did produce a significant increase in bursting in SNc (F3,23 = 3.38, P = 0.039; Table S1).
MS activation decreases locomotor response to amphetamine dependent on GABAergic inputs to vSub
To determine the impact of MS activation on locomotor behavior, rats were infused with NMDA (0.75 µg in 0.2 µL) or dPBS into the MS and their total distance traveled (cm) in an open-field arena was measured after systemic amphetamine (0.75 mg/kg, N = 10 rats per group) or saline (N = 4 rats per group) injection (systemic injections given at minute 30, animals receiving amphetamine vs saline injections were analyzed separately). NMDA infusion had no effect on locomotor behavior prior to amphetamine or following saline injection (Figure S1), but significantly decreased locomotion at several of the 5-min time points following the amphetamine injection (Fig. 5; Overall 2-way ANOVA, time point x intracranial drug condition interaction: F51,646 = 2.366, P < 0.001; post hoc Bonferroni test, NMDA vs. dPBS at 40 mins: P = 0.0007, 55 mins: P = 0.0159; see Table S2 for mean ± SEM at each time point). This effect contrasted with prior data indicating that vSub-mediated increases in VTA DA neuron activity led to increases in amphetamine-induced locomotion [20–22]. To examine whether the GABAergic inputs to vSub, which selectively regulated the decrease in SNc DA activity in the previous experiment, were also responsible for the reduction in amphetamine-induced locomotion, bicuculline (N = 11 rats per group, Bicuc; 12.5 ng in 0.5 µL) was infused into the vSub just prior to NMDA or dPBS activation of the MS (i.e., to selectively prevent the SNc population activity decrease, see Fig. 4b). Bicuculline infusion into vSub prevented the MS activation-induced decrease in locomotor behavior at several of the 5-min time points following amphetamine (Fig. 5; Bonferroni test NMDA vs. NMDA/Bicuc at 35 min: P = 0.0002, 40 mins: P < 0.0001, 50 mins: P = 0.0237, 55 mins: P = 0.0065, 65 mins: P = 0.0469; see Table S2 for mean ± SEM at each time point).
Fig. 5.
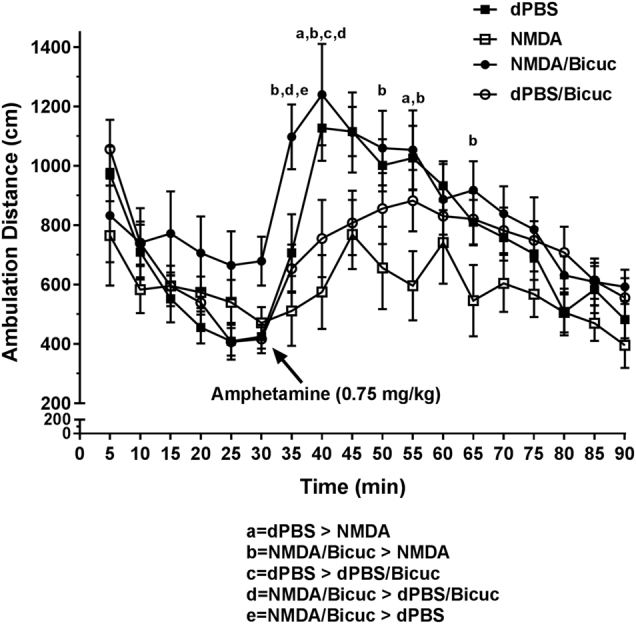
MS activation decreases amphetamine-induced hyperlocomotion via GABAergic inputs to vSub. NMDA (0.75 µg in 0.2 µL) activation of the MS had no effect on basal locomotor behavior, but reduced locomotion following amphetamine injection (0.75 mg/kg; a = Bonferroni test P < 0.05). Bicuculline (Bicuc; 12.5 ng in 0.5 µL) infusion into the vSub just prior to MS activation prevented the decrease in amphetamine-induced hyperlocomotion, restoring locomotor behavior back up to and beyond vehicle (dPBS) levels (b–e = Bonferroni test P < 0.05).
Discussion
These experiments led to several novel findings. First, MS activation increased DA population activity in the VTA and decreased it in the SNc. Second, effects in both regions were dependent on vSub activation; however, vSub cholinergic mechanisms were selectively required for DA population activity increases in the VTA and vSub GABAergic mechanisms were selectively required for DA population activity decreases in the SNc. Third, effects in both the VTA and SNc required the VP, but the rVP was selectively necessary for MS activation-induced effects in the VTA and the cVP in the SNc. Finally, MS activation decreased the hyperlocomotor response to amphetamine, and this effect was mediated by GABAergic inputs to vSub, similar to the effects on DA neuron activity in SNc.
Proposed pathway and mechanism of opposite effects on VTA and SNc DA neurons
Because the pharmacological experiments performed in this manuscript are not pathway specific, there are several potential pathways that interconnect with the MS-vSub-NAc-VP circuit, such as the prefrontal cortex or amygdala [31–33], that could play a role in the effects reported. However, we hypothesize, based on our current data and prior publications, that the pathway we describe here is the most parsimonious explanation for our results (see Figure S2 for a diagram of our proposed pathway). Our principal finding is that MS activation increases DA population activity in the VTA and decreases it in the SNc. Our data show that cholinergic and GABAergic mechanisms within the vSub play a role in this opposite effect, and we propose that this is driven by the cholinergic and GABAergic septohippocampal projections that are known to contact the vSub [13]. However, since GABAergic projections from MS largely target GABAergic interneurons [34], whereas cholinergic projections target both pyramidal neurons and interneurons [35], it is likely that both projections would activate the vSub. This is consistent with our current finding that TTX inactivation of the vSub prevents changes in both VTA and SNc. The vSub likely then activates NAc [5], and while pathways to VTA and SNc from NAc are likely segregated via the shell and core, respectively [33], both NAc efferents to the VP are GABAergic in nature [32, 36]. Therefore, it is likely that the opposite effects on VTA and SNc reside in the presence of the substantia nigra pars reticulata (SNr). Inhibitory interneurons from the SNr densely innervate the SNc [37–39], and the pathway from the VP to the SNr is stronger than to SNc due to the greater sensitivity of GABAA receptors on reticulata GABAergic interneurons compared to compacta DA neurons [37, 38, 40]. Thus, our proposed pathway of MS-vSub-NAcSh activation would inhibit the rVP, disinhibiting the VTA (Figure S2), which is consistent with what we see. In contrast, cVP inhibition would disinhibit the SNr, inhibiting the SNc, leading to the opposite effect of MS activation on VTA and SNc DA activity shown here. This model is consistent with previous reports showing that inactivation/lesion of the globus pallidus [39, 40] and high-frequency stimulation of the NAc [23] also lead to decreases in SNc DA neuron activity.
Treatment implications
The behavioral effects of MS activation were examined by measuring the locomotor response to amphetamine in an open-field arena because this paradigm is correlated with DA neuron population activity [20–22]. Previous studies have shown that increasing activity in the vSub via afferent excitation [22] or a decrease in interneuron function [20] increases both DA population activity in the VTA and amphetamine-induced hyperlocomotion. In this study, however, MS activation increased VTA DA population activity via the vSub, similar to prior studies, but decreased amphetamine-induced hyperlocomotion. Interestingly, the decrease in amphetamine-induced hyperlocomotion following MS activation is aligned with the increase seen after MS lesion [19], and coincides with a decrease in DA population activity in SNc. Furthermore, when bicuculline was infused into the vSub, which selectively prevented the MS activation-induced decrease in SNc DA population activity (see Fig. 4b), the decrease in amphetamine-induced hyperlocomotion was blocked. Thus, the effect of MS activation on this particular behavior seems to be qualitatively different from activating the vSub via other mechanisms and could be driven by the ability of the MS to affect the SNc. This highlights MS regulation of the DA system as a unique afferent pathway and one that has yet to be explored as a treatment target for DA-related disorders. Furthermore, because we demonstrate the ability to change behavioral output via a manipulation that selectively inhibited SNc DA activity decreases (vSub bicuculline infusion prior to activation of MS), these data shed light on the possibility of developing treatments that selectively manipulate one DA system or the other via this pathway. Given that treatments for disorders such as Parkinson’s and schizophrenia come with side effects in part due to their nonselective manipulation of the DA system, the potential selectivity reported here suggests the possibility of providing symptomatic relief with fewer side effects.
The differential impact of the MS on VTA and SNc DA neurons may also have implications for drug abuse. For example, studies on cocaine addiction have shown that initial drug use activates DA transmission in the reward-related ventromedial striatum, but established drug use activates the habit-related dorsolateral striatum [41]. This shift is thought to coincide with the habit formation associated with addiction, and a switch from “liking” to “wanting” [42]. Given the MS maintains a strong but opposite regulatory influence over the VTA and SNc, it is possible that an up- or downregulation of the MS to midbrain pathway could play a role in the switch from meso-accumbal to nigro-striatal DA release in addiction.
Cognitive implications
It is well-established that the MS, via its projection to the hippocampus, plays a key role in learning and memory. For example, lesion of the septohippocampal pathway leads to deficits in spatial memory [16] and spatial working memory [18, 43, 44], whereas optogenetic activation of the MS improves T-maze performance [45] and fear learning [46]. While the mechanism for these effects remain unclear, it is hypothesized that MS regulation of hippocampal theta may play a role [14, 15]. Interestingly, we report that MS activation, via activation of the vSub, increases population activity in VTA DA neurons. These DA neurons are known to project preferentially to the ventromedial striatum; a region associated with goal-directed behavior [33, 47]. In contrast, activation of the MS attenuates DA neurons in the SNc. These are known to project preferentially to the dorsal striatum, which is involved in impulsive, habit-related behavior [33, 47, 48]. Therefore, an alternate possibility is that the MS affects learning and memory by attenuating SNc-driven impulsive responding in favor of VTA-driven goal-directed responding, producing a “stop and consider alternate possibilities” mode of responding. This concept is in accordance with data showing that septohippocampal lesions increase perseverative responding and proactive interference and decrease fear extinction [17, 44].
Summary
This manuscript demonstrated, for the first time, that the MS differentially regulates midbrain DA activity via distinct pathways. One pathway increased VTA DA neuron population activity, selectively requiring cholinergic inputs to vSub and the rostral VP, while the other decreased SNc DA neuron population activity, selectively requiring GABAergic inputs to vSub and the caudal VP. Activation of the MS also decreased amphetamine-induced hyperlocomotion via GABAergic inputs to vSub, similar to DA activity in the SNc. These data effectively describe a novel afferent regulatory system for midbrain DA transmission, and set the stage to determine if the MS’s regulation of midbrain DA activity could be the mechanism by which it exerts its influence on learning and memory and could be a locus of interest for DA-related disorders.
Electronic supplementary material
Acknowledgements
Funding
This work was funded by NIMH grants 1F32MH115550-01 (D.M.B.) and MH057440-11 (A.A.G.).
Competing interests
A.A.G. also received funds from the following organizations: Lundbeck, Pfizer, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, Janssen, Alkermes, Newron. D.M.B. declares no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
The online version of this article (10.1038/s41386-018-0048-2) contains supplementary material, which is available to authorized users.
References
- 1.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 2.Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann NY Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 3.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524–32. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci. 2015;16:305–12. doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- 5.Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–22. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–73. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 7.Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–61. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- 8.Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–62. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz W. The phasic reward signal of primate dopamine neurons. Adv Pharmacol. 1998;42:686–90. doi: 10.1016/S1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- 10.Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol. 2003;551:927–43. doi: 10.1113/jphysiol.2003.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutar P, Bassant MH, Senut MC, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol Rev. 1995;75:393–27. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- 12.Nyakas C, Luiten PG, Spencer DG, Traber J. Detailed projection patterns of septal and diagonal band efferents to the hippocampus in the rat with emphasis on innervation of CA1 and dentate gyrus. Brain Res Bull. 1987;18:533–45. doi: 10.1016/0361-9230(87)90117-1. [DOI] [PubMed] [Google Scholar]
- 13.Milner TA, Amaral DG. Evidence for a ventral septal projection to the hippocampal formation of the rat. Exp Brain Res. 1984;55:579–85. doi: 10.1007/BF00235290. [DOI] [PubMed] [Google Scholar]
- 14.Huh CY, Goutagny R, Williams S. Glutamatergic neurons of the mouse medial septum and diagonal band of Broca synaptically drive hippocampal pyramidal cells: relevance for hippocampal theta rhythm. J Neurosci. 2010;30:15951–61. doi: 10.1523/JNEUROSCI.3663-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoder RM, Pang KC. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus. 2005;15:381–92. doi: 10.1002/hipo.20062. [DOI] [PubMed] [Google Scholar]
- 16.Lecourtier L, de Vasconcelos AP, Leroux E, Cosquer B, Geiger K, Lithfous S, et al. Septohippocampal pathways contribute to system consolidation of a spatial memory: sequential implication of GABAergic and cholinergic neurons. Hippocampus. 2011;21:1277–89. doi: 10.1002/hipo.20837. [DOI] [PubMed] [Google Scholar]
- 17.Pang KC, Jiao X, Sinha S, Beck KD, Servatius RJ. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: effects on proactive interference. Hippocampus. 2011;21:835–46. doi: 10.1002/hipo.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roland JJ, Stewart AL, Janke KL, Gielow MR, Kostek JA, Savage LM, et al. Medial septum-diagonal band of Broca (MSDB) GABAergic regulation of hippocampal acetylcholine efflux is dependent on cognitive demands. J Neurosci. 2014;34:506–14. doi: 10.1523/JNEUROSCI.2352-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecourtier L, de Vasconcelos AP, Cosquer B, Cassel JC. Combined lesions of GABAergic and cholinergic septal neurons increase locomotor activity and potentiate the locomotor response to amphetamine. Behav Brain Res. 2010;213:175–82. doi: 10.1016/j.bbr.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 20.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–30. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28:7876–82. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmerman EC, Grace AA. The Nucleus Reuniens of the Midline Thalamus Gates Prefrontal-Hippocampal Modulation of Ventral Tegmental Area Dopamine Neuron Activity. J Neurosci. 2016;36:8977–84. doi: 10.1523/JNEUROSCI.1402-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sesia T, Bizup B, Grace AA. Nucleus accumbens high-frequency stimulation selectively impacts nigrostriatal dopaminergic neurons. Int J Neuropsychopharmacol. 2014;17:421–7. doi: 10.1017/S1461145713001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Prim. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 25.Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, et al. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014;17:577–85. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam; Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- 27.Bunney BS, Grace AA. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci. 1978;23:1715–27. doi: 10.1016/0024-3205(78)90471-X. [DOI] [PubMed] [Google Scholar]
- 28.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identif Charact Neurosci. 1983;10:301–15. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 29.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984;4:2866–76. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35:422–30. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Astasheva E, Astashev M, Kitchigina V. Changes in the behavior and oscillatory activity in cortical and subcortical brain structures induced by repeated l-glutamate injections to the medial septal area in guinea pigs. Epilepsy Res. 2015;109:134–45. doi: 10.1016/j.eplepsyres.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Root DH, Melendez RI, Zaborszky L, Napier TC. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol. 2015;130:29–70. doi: 10.1016/j.pneurobio.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–73. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- 35.Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;239:237–46. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- 36.Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann NY Acad Sci. 1999;877:140–56. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- 37.Grace AA, Bunney BS. Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons. Eur J Pharmacol. 1979;59:211–8. doi: 10.1016/0014-2999(79)90283-8. [DOI] [PubMed] [Google Scholar]
- 38.Tepper JM, Lee CR. GABAergic control of substantia nigra dopaminergic neurons. Prog Brain Res. 2007;160:189–208. doi: 10.1016/S0079-6123(06)60011-3. [DOI] [PubMed] [Google Scholar]
- 39.Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995;15:3092–103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Celada P, Paladini CA, Tepper JM. GABAergic control of rat substantia nigra dopaminergic neurons: role of globus pallidus and substantia nigra pars reticulata. Neuroscience. 1999;89:813–25. doi: 10.1016/S0306-4522(98)00356-X. [DOI] [PubMed] [Google Scholar]
- 41.Willuhn I, Burgeno LM, Everitt BJ, Phillips PE. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc Natl Acad Sci USA. 2012;109:20703–08. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 43.Pang KC, Nocera R, Secor AJ, Yoder RM. GABAergic septohippocampal neurons are not necessary for spatial memory. Hippocampus. 2001;11:814–27. doi: 10.1002/hipo.1097. [DOI] [PubMed] [Google Scholar]
- 44.Dwyer TA, Servatius RJ, Pang KC. Noncholinergic lesions of the medial septum impair sequential learning of different spatial locations. J Neurosci. 2007;27:299–303. doi: 10.1523/JNEUROSCI.4189-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blumberg BJ, Flynn SP, Barriere SJ, Mouchati PR, Scott RC, Holmes GL, et al. Efficacy of nonselective optogenetic control of the medial septum over hippocampal oscillations: the influence of speed and implications for cognitive enhancement. Physiol Rep 4. 2016;4:e13048. doi: 10.14814/phy2.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hersman S, Cushman J, Lemelson N, Wassum K, Lotfipour S, Fanselow MS. Optogenetic excitation of cholinergic inputs to hippocampus primes future contextual fear associations. Sci Rep. 2017;7:2333. doi: 10.1038/s41598-017-02542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burton AC, Nakamura K, Roesch MR. From ventral-medial to dorsal-lateral striatum: neural correlates of reward-guided decision-making. Neurobiol Learn Mem. 2015;117:51–59. doi: 10.1016/j.nlm.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25:2771–80. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.