Abstract
Cannabinoids combined with opioids produce synergistic antinociceptive effects, decreasing the lowest effective antinociceptive opioid dose (i.e., opioid-sparing effects) in laboratory animals. Although pain patients report greater analgesia when cannabis is used with opioids, no placebo-controlled studies have assessed the direct effects of opioids combined with cannabis in humans or the impact of the combination on abuse liability. This double-blind, placebo-controlled, within-subject study determined if cannabis enhances the analgesic effects of low dose oxycodone using a validated experimental model of pain and its effects on abuse liability. Healthy cannabis smokers (N = 18) were administered oxycodone (0, 2.5, and 5.0 mg, PO) with smoked cannabis (0.0, 5.6% Δ9 tetrahydrocannabinol [THC]) and analgesia was assessed using the Cold-Pressor Test (CPT). Participants immersed their hand in cold water (4 °C); times to report pain (pain threshold) and withdraw the hand from the water (pain tolerance) were recorded. Abuse-related effects were measured and effects of oxycodone on cannabis self-administration were determined. Alone, 5.0 mg oxycodone increased pain threshold and tolerance (p ≤ 0.05). Although active cannabis and 2.5 mg oxycodone alone failed to elicit analgesia, combined they increased pain threshold and tolerance (p ≤ 0.05). Oxycodone did not increase subjective ratings associated with cannabis abuse, nor did it increase cannabis self-administration. However, the combination of 2.5 mg oxycodone and active cannabis produced small, yet significant, increases in oxycodone abuse liability (p ≤ 0.05). Cannabis enhances the analgesic effects of sub-threshold oxycodone, suggesting synergy, without increases in cannabis’s abuse liability. These findings support future research into the therapeutic use of opioid-cannabinoid combinations for pain.
Introduction
In the United States, an estimated 11.2% of the adult population suffers from chronic pain [1] and nearly 20% of patients presenting with acute and chronic non-cancer pain are prescribed opioids [2]. Between 1999 and 2015, opioid prescribing tripled [3] along with the number of deaths attributed to opioid analgesics, with an estimated 17,500 fatalities in 2015 relative to 6160 reported in 1999 [4]. With recent recognition of the significant health risks associated with high doses of opioids including opioid use disorder [5] and overdose [6, 7] physicians are asked to limit the number of prescriptions written, shorten the duration of opioid therapy, and decrease the total daily doses prescribed [8]. As awareness of the risks of prescription opioid use grows, medical cannabis use is also garnering widespread acceptance, with over half of the United States passing medical cannabis laws [9]. Pain is the primary indication for use by patients who are prescribed cannabis [10], and chronic pain is one indication for which strong evidence exists supporting the use of cannabinoids (National Academies of Sciences, Engineering, and Medicine [62, 11]). Yet recent systematic reviews concluded that there is limited or inconclusive evidence supporting the use specifically of cannabis-based products for neuropathic pain and insufficient evidence supporting its use for other types of chronic pain [12, 13]. These findings exemplify the need for more rigorously controlled clinical trials in this area.
With increased access to cannabis and more conservative opioid prescribing, evidence suggests that patients are substituting cannabis for opioids. For example, opioid analgesic prescriptions filled by Medicare Part D enrollees fell significantly in states with medical cannabis laws [14], and patients with chronic pain report over 60% reduction in their opioid use in these states [15]. In lieu of full substitution, some pain patients report that cannabis increases the analgesic effects of their opioids [16] or decreases the opioid dose needed for therapeutic effect [17]. Moreover, some randomized controlled studies have demonstrated analgesic effects of cannabinoids in patients taking opioids for chronic and cancer pain [18–20]. These data suggest that cannabis may (1)increase the pain-relieving properties of opioids and consequently decrease the total dose used, or (2) provide adequate analgesia on its own thus acting as a substitute. However, there are no data from placebo-controlled studies directly addressing whether cannabis can decrease the effective analgesic doses of opioids. Furthermore, to date, no studies have investigated the impact of opioid-cannabinoid drug combinations on abuse liability, a critical aspect when considering the therapeutic utility of two drugs that have significant abuse liability when administered alone.
Based on animal studies, combining opioids and cannabinoids for pain relief is hypothesized to provide superior clinical therapeutic effectiveness than opioid administration alone by increasing the analgesic potency of the opioid and therefore decreasing its effective analgesic dose (termed opioid-sparing effects). Although this effect has yet to be confirmed in humans, preclinical evidence regarding the pro-analgesic effects of co-administration of mu-opioid agonists and cannabinoids abounds, predominantly with Δ9 tetrahydrocannabinol (THC), a partial CB1 and CB2 receptor agonist [21] and the main psychoactive component of cannabis [22]. Combining THC and mu-opioid agonists has been reported to have additive or synergistic effects across a range of routes of administration in rodents (i.e., [23–28]) and non-human primates, depending on the efficacy of the opioid agonist (i.e., [29–31]). Achieving analgesia with lower opioid doses may also decrease adverse effects related to opioid use that diminish their therapeutic utility, including constipation, respiratory depression, and the development of opioid tolerance and dependence [32]. For instance, although chronic administration of a CB1 or mu-opioid receptor agonist alone produces antinociceptive tolerance and physiological dependence, co-administration of the drugs prevents these effects in rodents [33, 34]. In addition to reducing the development of tolerance and dependence, CB1 receptor agonists also reduce the discriminative stimulus and reinforcing effects of opioid agonists in non-human primates [30, 35]. This potential for cannabinoids to decrease the abuse liability of opioids has profound implications for the most significant adverse effects of opioids; that is, the risk of opioid use disorders and associated fatalities [4, 36]. Based on the preclinical literature, co-administration of cannabinoids, specifically CB1 receptor agonists like THC, would potentially decrease the risk of developing an opioid use disorder.
Few controlled clinical studies have sought to identify the opioid-sparing effects of cannabinoids; one assessed the impact of vaporized cannabis on opioid analgesia and pharmacokinetics, however this was under non-placebo controlled conditions. In addition, that study was not designed to assess if cannabis could decrease the effective opioid analgesic dose [37]. Other studies have used various cannabinoid preparations and routes of administration (i.e., oral THC or THC:CBD oromucosal spray) and have either lacked an opioid control (i.e., opioid placebo) or failed to include more than one opioid dose, again making it difficult to conclude whether cannabinoids can decrease the effective opioid dose for analgesia [18–20, 38, 39]. Furthermore, while one study assessed the effects of cannabis and opioid co-administration on subjective intoxication [37], no studies to date have assessed the abuse liability of the drug combination, a critical endpoint when determining if the combination can mitigate risks of abuse associated with opioid administration.
This within-subject, randomized, placebo-controlled, double-blind study sought to determine the opioid-sparing effects of cannabis by assessing analgesia and abuse liability of sub-threshold and lowest-effective doses of oxycodone (2.5 and 5.0 mg, respectively) when administered alone or in combination with smoked cannabis over six experimental sessions in a healthy, cannabis-smoking population. The sub-threshold dose was chosen specifically to assess potential synergistic effects of the drug combination that may not have been apparent with higher doses. Analgesia was assessed using the Cold Pressor Test (CPT) an experimental test of pain that has predictive validity for medications used for chronic pain (opioids [40–42], gabapentin [43], and lamotrigine [44]). Assessing analgesic effects using this elicited pain test in a non-pain population afforded robust experimental control by excluding significant confounding variables that occur when studying a pain population including (1) current use of analgesics and (2) fluctuations in baseline pain across session days.
Methods and materials
Participants
Volunteers, 21–45 years of age, who met basic inclusion/exclusion criteria after an initial telephone screen came to the laboratory for further screening, received a psychiatric and medical evaluation, and provided a drug use and medical history. Eligible participants currently smoked ≥ three cannabis cigarettes at least three times a week for the four weeks before screening, as determined by urine toxicology and self-report (one ‘blunt’ = two cannabis cigarettes [45]) and were physically healthy, as determined by a physical examination, electrocardiogram, and urine and blood chemistries. Participants also had to have experience with opioids without adverse effects. Volunteers were excluded if they endorsed current pain, used over-the-counter or prescription medications each day, with the exception of oral contraceptives, used illicit drugs other than cannabis, as determined by urine toxicology and self-report, or had problematic alcohol consumption. Those meeting Diagnostic and Statistical Manual (of Mental Disorders), fourth edition revised criteria for Axis I psychopathology were excluded. Pregnant or nursing females were also excluded. Volunteers were told that the study aimed to determine the effects of smoked cannabis and a Food and Drug Administration (FDA)-approved medication on pain and that during each session they would take a capsule and smoke a portion of a cannabis cigarette. Participants were admitted into the study after providing informed consent. Procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute and were in accord with the Declaration of Helsinki.
Design and procedures
The study included six 8-hour outpatient sessions over the course of 4–8 weeks at the New York State Psychiatric Institute. Sessions were separated by >72 h to prevent medication carryover effects and began around 9 AM. Volunteers also participated in two additional sessions assessing the effects of naltrexone (25 mg) on cannabis analgesia; findings will be reported separately. Before study onset, participants were familiarized with computerized tasks, the CPT, and study procedures during a medication-free training. During each session, one capsule containing placebo or oxycodone (2.5 or 5.0 mg) was administered 45 min before cannabis was smoked (0.0 or 5.6% THC). Each combination of cannabis (0.0 and 5.6% THC) and oxycodone strength (0.0, 2.5, and 5.0 mg) was tested. A within-subject design was used in which all participants received all six dose conditions in randomized order.
Experimental session
Participants were instructed not to eat breakfast before sessions as they would be served the same standard breakfast before each session in the laboratory to control for any possible effects of the meal or macronutrients on mood or drug absorption. Participants were also asked not to smoke cannabis or nicotine cigarettes after midnight the night before each session to ensure low carbon monoxide levels in the morning and provide a way to assess any recent cannabis smoking. Upon arrival at the laboratory, carbon monoxide levels were measured to confirm no recent smoking, breath alcohol levels were assessed, urine toxicology screens confirmed no recent use of illicit drugs other than cannabis, and a standardized breakfast was provided.
Before capsule administration, participants completed a baseline pain assessment (CPT and pain ratings). Heart rate and blood pressure were measured using a Sentry II vital signs monitor (Model 6100: NBS Medical Services, Costa Mesa CA). Pupil photographs were taken using a digital pupillometer (VIP-200 Pupillometer, Neuroptics, Inc., San Clemente, CA) under ambient lighting conditions. Participants smoked 70% of an 800 mg cannabis cigarette 45 min after capsule administration, following a cued-smoking procedure [46]. Heart rate, blood pressure, pain assessments (CPT and pain ratings), subjective drug effect ratings, and pupil diameter were assessed at set times throughout the session after capsule administration and cannabis smoking (refer to Table 1). Cannabis’s reinforcing effects were assessed 195 min after smoking (see below for details). During each session, nicotine cigarette smokers were permitted to smoke after carbon monoxide levels were measured (before any assessments) and at predetermined intervals to minimize nicotine withdrawal. At the end of each session participants were given subway fare and left the laboratory after passing field sobriety tasks and verbally agreeing not to drive for the remainder of the day. Table 1 provides details related to timing of assessments relative to capsule administration and cannabis smoking.
Table 1.
Session schedule
| Time (min) | Event |
|---|---|
| −75 | Urine toxicology, breathalyzer, carbon monoxide, breakfast |
| −60 | Pain assessments, BP/HR, pupil measurement, SE-VAS |
| −45 | Capsule administration |
| (0.0, 2.5 or 5.0 mg oxycodone, oral) | |
| −20 | Pain assessments, BP/HR, pupil measurement, capsule-RF, SE-VAS |
| 0 | Cannabis administration |
| (0 or 5.6%, smoked) | |
| 15 | BP/HR, pupil measurement, capsule-RF, cannabis-RF, SE-VAS |
| 30 | Pain assessments, BP/HR, capsule-RF, cannabis-RF, SE-VAS, |
| 60 | Pain assessments, BP/HR, pupil, capsule-RF, cannabis-RF, SE-VAS, |
| 90 | Pain assessments, BP/HR, pupil, capsule-RF, cannabis-RF, SE-VAS, |
| 120 | Pain assessments, BP/HR, capsule-RF, cannabis-RF, SE-VAS |
| 150 | BP/HR, lunch |
| 180 | Pain assessments, BP/HR, pupil, capsule-RF, cannabis-RF, SE-VAS |
| 195 | Choice to purchase 1-3 puffs of marijuana followed by smoking |
| 225 | BP/HR, SE-VAS |
| 255 | BP/HR, SE-VAS |
| 285 | BP/HR, SE-VAS |
| 300 | BP/HR, Field Sobriety Test, participant discharge |
Timing of session events relative to cannabis smoking. Session began at approximately 9 AM
Pain assessments Cold Pressor Test, McGill Pain Questionnaire, and Painful and Bothersome Rating Forms, BP/HR blood pressure and heart rate readings, SE-VAS Subjective Effects -Visual Analog Scale, Capsule-RF Capsule Rating Form, Cannabis-RFC Cannabis Rating Form
Pain assessments
Pain responses were measured before and repeatedly after drug administration (Table 1). Based on earlier reports of smoked cannabis and oral THC’s effects in the CPT [47], these were the primary outcomes for the current study.
Cold pressor test
The cold pressor apparatus consisted of two water coolers, each fitted with a wire cradle and an aquarium pump for water circulation. One cooler was filled with warm water (37 °C) and the other was filled with cold water (4 °C) [47]. Briefly, each CPT began with an immersion of the left hand into the warm-water bath for 3 min. The left hand was then immersed into the cold-water bath, and participants were instructed to report the first painful sensation after immersion and asked to tolerate the stimulus as long as possible before withdrawing their hand (up to 3 min). Pain threshold, defined as latency to first feel pain, and pain tolerance, latency to withdraw the hand from the cold water, were recorded. Staff administering the CPT was the same sex as the volunteer.
Pain Intensity and Bothersomeness Scales (PIB)
Immediately after removing the hand from the cold water, participants rated pain intensity and bothersomeness of the cold water stimulus on a scale from 0 to 10, 0 being “not painful/bothersome at all" and 10 being “most painful/bothersome feeling imaginable.”
McGill Pain Questionnaire (MPQ)
A 15-item shortened, computerized form of the MPQ was used to assess the sensory and affective dimensions of the pain experience immediately following the CPT. Participants were ask to describe the pain by choosing among a series of possible answers (None [score = 1] to Severe [score = 4]) when prompted by a descriptor (“Throbbing,” “Shooting,” “Stabbing,” etc.). Scores were added across all 15 items to generate a sum score, ranging between 15 and 60. This questionnaire was completed immediately after the PIB.
Subjective drug effects
Ratings of subjective drug effects were measured repeatedly on a scale of 0 mm = no effect to 100 mm = maximum possible effect (Table 1).
Subjective Effect-Visual Analog Scale (SE-VAS)
Participants were asked to rate their mood and physical symptoms on a modified 44-item, computerized VAS that measures affective and physical subjective drug effects (see [47, 48]).
Cannabis Rating Form (Cannabis-RF) and Capsule Rating Form (Capsule-RF)
Subjective cannabis-and capsule-related effects were assessed using two 5-item VASs asking participants to rate the strength of the drug effect, good effect, bad effect, drug liking, and willingness to take the drug again [49].
Cannabis reinforcing effects
Cannabis self-administration was assessed by providing the participants an opportunity to purchase up to 3 puffs ($1/puff, taken from study earnings) of the cannabis that was smoked 3 h earlier. Self-administered cannabis was smoked immediately after the choice according to the puffed-paced procedure.
Drugs
Capsules (size 00 opaque capsules with lactose filler) containing placebo or oxycodone (2.5 or 5.0 mg) were prepared by the New York State Psychiatric Institute Research Pharmacy. Cannabis cigarettes (0.0 or 5.6% THC; ca. 800 mg) were provided by the National Institute on Drug Abuse. Cigarettes were stored frozen in an airtight container and humidified at room temperature for 24 h prior to the session.
Data analysis
Repeated measures analysis of variance (ANOVA) with planned comparisons were used to assess the analgesic and subjective effects of cannabis and oxycodone administered alone and in combination. For each drug condition, pain threshold and tolerance were calculated for each participant as the percent of the baseline pre-drug administration CPT response. Pain ratings were also measured as a function of change from the baseline response. For each dependent measure, seven planned comparisons between dosing conditions were completed. Active cannabis and the two oxycodone strengths were compared to the placebo (three comparisons), and the two drug combinations (active cannabis + 2.5 mg oxycdone and active cannabis + 5.0 mg oxycodone combination) were compared to the placebo (2 comparisons). Lastly, the two drug combinations were compared to active cannabis alone (2 comparisons). Results were considered statistically significant when p values were equal to or less than 0.05 using Huynh-Feldt corrections. One participant held his hand in the cold water for the full 3 min during all CPTs; his data were excluded from analyses.
Results
Demographic characteristics
Table 2 portrays the demographic characteristics of the participants who completed the study. An additional 12 volunteers enrolled, but did not complete the study; nine discontinued for personal reasons, one had a positive toxicology screen for amphetamine, one reported unwanted effects of study medication (nausea), and one provided false information regarding psychiatric and legal history during screening. All participants had experience with prescription opioids with no adverse effects; 14 participants had histories of prescription opioid use for pain only, one participant used prescription opioids for pain and for recreational purposes on one occasion, and three participants had a history of using prescription opioids only for recreational purposes. Recreational use spanned 1–3 occasions. Participants did not have a history of heroin use. Average times since last use prior to study participation was 3.4 ± 4.4 years for therapeutic purposes and 2.6 ± 3.7 years for recreational use.
Table 2.
Demographic characteristics of study participants
| Demographics (N = 18) | |
|---|---|
| Age (years) | 29.9 ± 1.6 |
| Sex (men/women) | 12/6 |
| Race (B/W/M) | 10/5/3 |
| Cannabis use | |
| Years regular use | 12.1 ± 8.5 |
| Days/Wk | 6.6 ± 0.9 |
| $/Wk | 153.8 ± 193.1 |
| Cannabis cigarettes/day | 7.9 ± 5.3 |
| Tobacco use | |
| Daily nicotine smokers | 44% |
| Tobacco cigarettes/day | 4.9 ± 2.9 |
| Alcohol use | |
| Weekly drinkers | 67% |
| Drinks/occasion | 3.3 ± 2.4 |
| Opioid use | |
| Past use for pain only | 78% |
| Recreational use | 22% |
| Occasions of recreational use | 2.0 ± 1.2 |
Note: Data are presented as means (±SD) or as percent
Race is indicated as Black (B), White (W), and Mixed (M)
Analgesic effects
CPT: pain sensitivity and tolerance
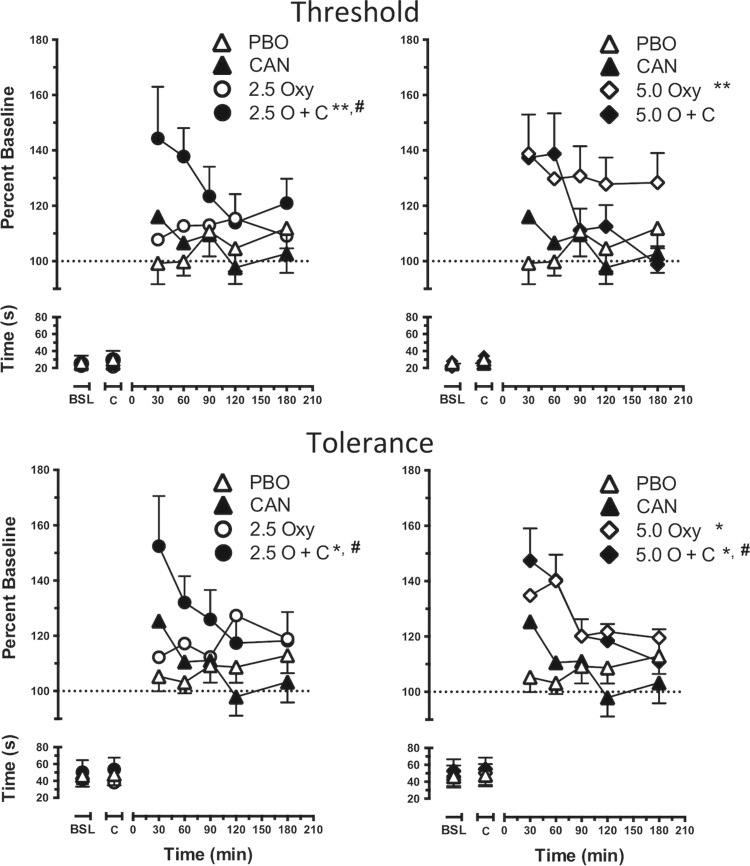
Figure 1 portrays the time course of pain threshold (latency to first report pain, top panels) and pain tolerance (latency to withdraw the hand from cold water, bottom panels) as a function of cannabis strength and oxycodone dose (left column, 2.5 mg oxycodone; right column, 5.0 mg oxycodone). Baseline and post-capsule pain threshold and tolerance did not differ across dosing conditions. Administered alone, only 5.0 mg oxycodone increased pain threshold (F [1, 17] = 7.5, p ≤ 0.01) and tolerance (F [1, 17] = 5.4, p ≤ 0.05) compared to placebo (inactive cannabis and 0.0 mg oxycodone). When administered with active cannabis, 5.0 mg oxycodone also increased pain tolerance compared to the placebo condition and active cannabis alone (F [1, 17] = 5.5, p ≤ 0.05). The combination of active cannabis and 2.5 mg oxycodone increased pain threshold and tolerance relative to the placebo condition (F [1, 17] = 5.9, p ≤ 0.05 and F [1, 17] = 6.5, p ≤ 0.05, respectively) and active cannabis alone (F [1, 17] = 5.2, p ≤ 0.05 and F [1, 17] = 5.5, p ≤ 0.05, respectively).
Fig. 1.
Cold Pressor Task pain threshold (top panels) and tolerance (bottom panels) as calculated by percent baseline latency (seconds) to report pain and withdraw the hand from cold water. Data are presented as mean values +/- SEM according to cannabis strength, oxycodone dose (2.5 mg, left panels; 5.0 mg, right panels), and time. Placebo oxycodone + inactive cannabis condition = PBO; placebo oxycodone + active cannabis condition = CAN; 2.5 mg oxycodone + inactive cannabis condition = 2.5 Oxy; 2.5 mg oxycodone + active cannabis condition = 2.5 O + C; 5.0 mg oxycodone + inactive cannabis condition = 5.0 Oxy; 5.0 mg oxycodone + active cannabis condition = 5.0 O + C. Baseline response is shown as BSL on the x-axis; response after oxycodone is indicated by C on the x-axis. Significant differences from placebo are indicated by *p ≤ 0.05 and **p ≤ 0.01; significant differences from active cannabis alone are indicated with #p ≤ 0.05
Pain ratings
Pain ratings including MPQ and PIB ratings did not differ between active cannabis or oxycodone, either administered alone or in combination, as compared to placebo (Table 3). Baseline and post-capsule ratings for these measures also did not differ across sessions (average baseline MPQ ratings = 20.0 ± 0.3; average ‘Painfulness’ ratings = 5.9 ± 0.2; average ‘Bothersomeness’ ratings = 5.7 ± 0.2). MPQ ratings increased throughout the sessions for all drug conditions. Lower ratings were observed under active drug conditions compared to placebo, but differences were not statistically significant. Similarly, ‘Painfulness’ and ‘Bothersomeness’ ratings also increased across the session with no significant differences between dose conditions.
Table 3.
Pain ratings
| Drug condition | Oxycodone | 0.0 mg | 0.0 mg | 2.5 mg | 2.5 mg | 5.0 mg | 5.0 mg |
| Cannabis | 0.0 % | 5.6 % | 0.0 % | 5.6 % | 0.0 % | 5.6 % | |
| Subjective effect | MPQ | 2.2 ± 0.5 | 1.5 ± 0.5 | 2.0 ± 0.5 | 0.7 ± 0.6 | 1.7 ± 0.4 | 1.2 ± 0.4 |
| Painfulness | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 | |
| Bothersomeness | 0.6 ± 0.1 | 0.6 ± 0.2 | 0.8 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.0 ± 0.2 |
Mean reductions from baseline in pain ratings ± standard error of the mean (SEM) for the McGill Pain Questionnaire (MPQ), and Painfulness and Bothersomeness scales after administration of placebo (inactive cannabis + 0 mg oxycodone), oxycodone (2.5 and 5.0 mg) and active cannabis (0.0 and 5.6% THC) administered alone or together
Subjective drug effects
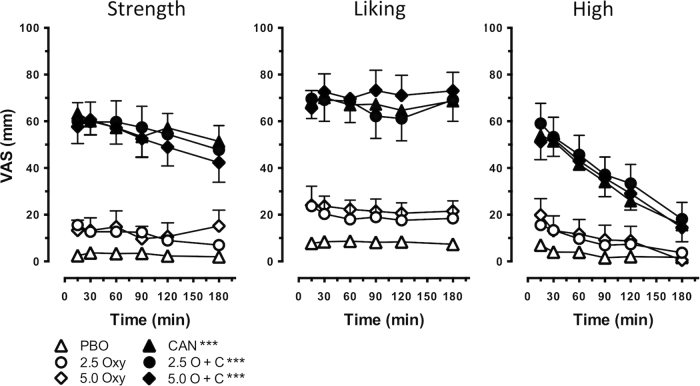
Figure 2 illustrates representative subjective cannabis effects as measured by the Cannabis RF and ratings of ‘High’ measured with the SE-VAS. Ratings of cannabis ‘Strength,’ ‘Liking,’ and ‘High,’ were significantly higher after active cannabis administration relative to placebo (Strength, F [1, 17] = 83.6, p ≤ 0.0001; Liking, F [1, 17] = 53.6, p ≤ 0.0001; High, F [1, 17] = 51.1, p ≤ 0.0001). Oxycodone alone did not increase these ratings. While the combination of 2.5 and 5.0 mg oxycodone and active cannabis increased these ratings relative to placebo (2.5 mg oxycodone in combination with active cannabis, Strength, F [1, 17] = 82.3, p ≤ 0.0001; Liking, F [1, 17] = 51.7, p ≤ 0.0001; High, F [1, 17] = 64.4, p ≤ 0.0001, 5.0 mg oxycodone in combination with active cannabis, Strength, F [1, 17] = 73.7, p ≤ 0.0001; Liking, F [1, 17] = 59.4, p ≤ 0.0001; High, F [1, 17] = 51.9, p ≤ 0.0001), ratings were not higher than those engendered by active cannabis alone. The same effects were observed for other positive subjective effects including ratings of ‘Good’ and ‘Take Again’ (active cannabis alone, Good, F [1, 17] = 57.4, p ≤ 0.0001; Take Again, F [1, 17] = 53.7, p ≤ 0.0001; 2.5 mg oxycodone in combination with active cannabis, Good, F [1, 17] = 52.4 p ≤ 0.0001; Take Again, F [1, 17] = 51.7, p ≤ 0.0001; 5.0 mg oxycodone in combination with active cannabis, Good, F [1, 17] = 67.2, p ≤ 0.0001; Take Again, F [1, 17] = 62.2, p ≤ 0.0001) However, 5.0 mg oxycodone increased ratings of ‘Take Again’ when administered with inactive cannabis relative to placebo (F [1, 17] = 4.8, p ≤ 0.05).
Fig. 2.
Subjective ratings of representative abuse-related subjective effects (‘Strength,’ ‘Liking’) as measured by the Cannabis Rating Form and intoxication (‘High’) as a function of time, cannabis strength, and oxycodone dose. Data are presented as mean ratings +/- SEM. Significant differences from placebo are indicated by ***p ≤ 0.0001
Subjective drug effects related to oxycodone as measure by the Capsule RF are shown in Table 4. Neither active cannabis nor either oxycodone dose alone affected ratings of capsule ‘Strength,’ ‘Good (Drug Quality),’ ‘Liking,’ or ‘Take Again’ compared to placebo. However, the combination of 2.5 mg oxycodone and active cannabis increased these ratings relative to placebo (Strength, F [1, 17] = 4.1, p ≤ 0.05; Good, F [1, 17] = 12.9, p ≤ 0.001; Liking, F [1, 17] = 20.7, p ≤ 0.0001; Take Again F [1, 17] = 20.4, p ≤ 0.001). The combination also increased ratings of ‘Strength,’ ‘Good,’ Liking,’ and ‘Take Again’ relative to cannabis alone (Strength, F [1, 17] = 10.5, p ≤ 0.01; Good, F [1, 17] = 11.8, p ≤ 0.01; Liking, F [1, 17] = 12.2, p ≤ 0.01; Take Again F [1, 17] = 12.2, p ≤ 0.01). The combination of 5.0 mg oxycodone and active cannabis increased ratings of ‘Good’ and ‘Take Again’ relative to placebo (Good, F [1, 17] = 4.4, p ≤ 0.05; Take Again, F [1, 17] = 5.7, p ≤ 0.05).
Table 4.
Abuse-liability ratings of oxycodone
| Drug condition | Oxycodone | 0.0 mg | 0.0 mg | 2.5 mg | 2.5 mg | 5.0 mg | 5.0 mg |
| Cannabis | 0.0% | 5.6% | 0.0% | 5.6% | 0.0% | 5.6% | |
| Subjective effect | Strength | 10.1 ± 1.5 | 16.5 ± 1.9 | 12.6 ± 1.8 | 27.2 ± 2.4**, # | 16.18 ± 1.9 | 20.5 ± 2.1 |
| Good drug | 10.8 ± 1.6 | 11.7 ± 1.7 | 11.1 ± 1.8 | 31.1 ± 2.9***, ## | 11.7 ± 1.7 | 22.6 ± 2.5* | |
| Liking | 11.1 ± 1.7 | 16.7 ± 1.9 | 17.9 ± 2.0 | 39.0 ± 2.7***, # | 18.4 ± 2.1 | 20.6 ± 2.3 | |
| Take again | 6.9 ± 1.3 | 12.1 ± 1.7 | 12.9 ± 2.0 | 39.3 ± 2.8***, ## | 15.9 ± 2.1 | 18.7 ± 2.3* |
Mean subjective ratings ± standard error of the mean (SEM) for the Capsule Rating Form under placebo conditions (inactive cannabis + 0 mg oxycodone), and oxycodone (2.5 and 5.0 mg) and active cannabis administered alone or together. Significant differences from placebo are indicated by **, p ≤ 0.01, and ***, p ≤ 0.001; significant differences from active cannabis alone are indicated with #, p ≤ 0.05, ##, p ≤ 0.01, and ###, p ≤ 0.001.
Reinforcing effects
Figure 3 depicts the number of cannabis puffs self-administered and money spent during each session as a function of cannabis strength and oxycodone dose. Active cannabis was self-administered significantly more than inactive cannabis (F [1, 17] = 7.5, p ≤ 0.01). The combination of active cannabis and 2.5 or 5.0 mg oxycodone was also self-administered more than placebo (2.5 mg oxycodone in combination with active cannabis, F [1, 17] = 20.3, p ≤ 0.0001; 5.0 mg oxycodone in combination with active cannabis, F [1, 17] = 20.3, p ≤ 0.0001); however, no significant differences were observed between the combination of active cannabis and oxycodone compared to active cannabis alone.
Fig. 3.
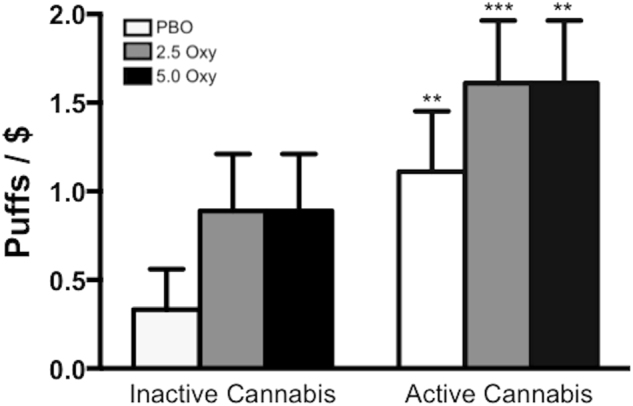
Puffs of cannabis self-administered and money ($) spent as a function of cannabis strength (inactive cannabis, left side; active cannabis, right side) and oxycodone dose (white bars = placebo oxycodone, grey bars = 2.5 mg oxycodone, black bars = 5.0 mg oxycodone). Each puff cost $1. Significant differences from the placebo oxycodone + inactive cannabis condition are indicated by **p ≤ 0.01 and ***p ≤ 0.0001
Physiological effects
Figure 4 portrays the effects of cannabis and oxycodone on miosis and heart rate (Fig. 4). Both doses of oxycodone administered alone decreased pupil diameter relative to the placebo (2.5 mg oxycodone, F [1, 17] = 7.4, p ≤ 0.01; 5.0 mg oxycodone, F [1, 17] = 8.3, p ≤ 0.01) and compared to active cannabis administered alone (2.5 mg oxycodone, F [1, 17] = 7.5, p ≤ 0.01; 5.0 mg oxycodone, F [1, 17] = 17.1, p ≤ 0.0001). Under active cannabis, 5.0 mg oxycodone significantly decreased pupil diameter relative to placebo (F [1, 17] = 9.6, p ≤ 0.01).
Fig. 4.
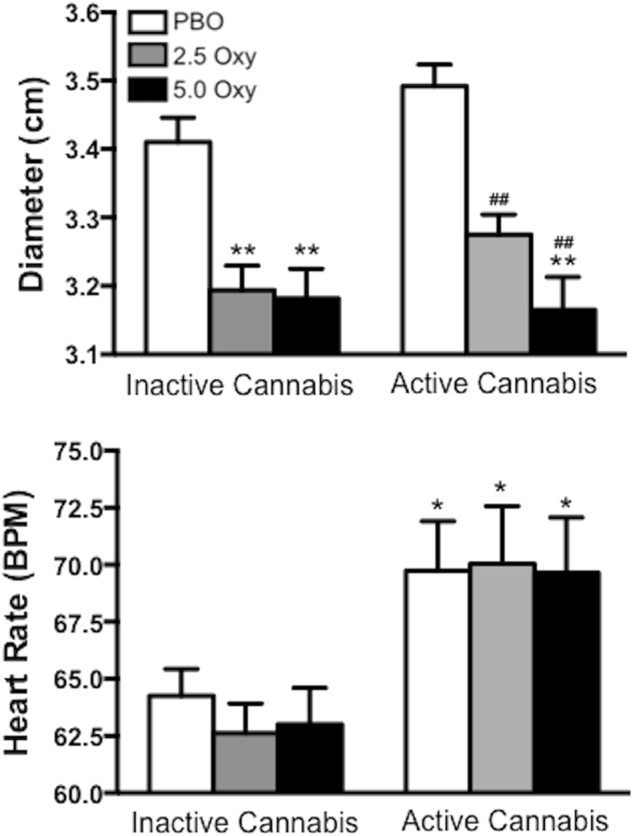
Pupillary (top panel; diameter measured in cm) and cardiovascular (bottom panel; beats per minute) effects as a function of cannabis strength (inactive cannabis, left side; active cannabis, right side) and oxycodone dose (white bars = placebo oxycodone, grey bars = 2.5 mg oxycodone, black bars = 5.0 mg oxycodone). Values represent means across post-smoking time points. Significant differences from the placebo oxycodone + inactive cannabis condition are indicated by *p ≤ 0.05 and **p ≤ 0.01; significant differences from active cannabis alone are indicated with ##p ≤ 0.01
Active cannabis increased heart rate compared to the placebo (F [1, 17] = 7.7, p ≤ 0.05), an effect that was retained when co-administered with 2.5 and 5.0 mg oxycodone (2.5 mg oxycodone in combination with active cannabis, F [1, 17] = 8.6, p ≤ 0.05; 5.0 mg oxycodone in combination with active cannabis, F [1, 17] = 7.4, p ≤ 0.05). Compared to active cannabis alone, the combination of active cannabis and oxycodone did not significantly affect heart rate.
Discussion
Preclinical studies and population findings provide a strong signal for the potential opioid-sparing effects of cannabinoids [32]. This study sought to determine how active cannabis affected the analgesic dose of a frequently prescribed opioid analgesic, oxycodone, while also assessing the impact of the opioid-cannabis combination on another clinically relevant endpoint, abuse liability. Both active cannabis and a low dose of oxycodone (2.5 mg) were sub-therapeutic, failing to elicit analgesia on their own; however, when administered together, pain responses as measured by the CPT were significantly reduced, pointing to the opioid-sparing effects of cannabis. Oxycodone did not significantly increase cannabis self-administration. However, the combination of 2.5 mg oxycodone and active cannabis produced modest increases in positive subjective ratings related to oxycodone. These are important data to consider in light of findings from observational studies that prescription opioid use is associated with greater likelihood of CUD [49], and that certain features of problematic prescription opioid use (i.e., high doses, non-adherence with medication dosing regimens) were greatest among chronic pain patients who also used cannabis [16]. No consistent changes in oxycodone-induced or cannabis-induced physiological effects were observed when the two drugs were co-administered. Overall, these findings demonstrate opioid-sparing effects of cannabis for analgesia that is accompanied by increases in some measures of abuse liability.
The current findings correspond to preclinical literature demonstrating the additive and synergistic effects of opioids and cannabinoids on antinociception [32]. These results are also similar to previous human laboratory studies and clinical trials demonstrating that the addition of a THC-based cannabinoid (i.e., dronabinol, THC:CBD oral preparation, or vaporized active cannabis) enhances the analgesic effects of an opioid [18–20, 37–39]. This study extends those findings in a number of important areas in that it (1) assessed the effects of smoked cannabis on opioid analgesia and used a placebo comparison, (2) determined cannabis’s effects on multiple doses of oxycodone, and (3) assessed the impact of the combination on markers of abuse liability. Previous controlled laboratory and clinical studies have not been explicitly designed to measure whether THC-based therapies decrease the effective analgesic dose of an opioid, with the exception of one study that assessed the impact of dronabinol and a single, sub-therapeutic dose of morphine on responses to experimental thermal pain in healthy volunteers [39]. They found that ineffective analgesic doses of dronabinol and morphine produced an affective analgesic response (i.e., reduced the negative valence of the nociceptive stimulus) when administered together. However, the combination did not produce sensory analgesia, a different dimension of pain measured by ratings of sensation intensity. The mixed results may have been due to the timing of the experimental pain test. Analgesia was assessed at a single time-point, 105 min after dronabinol (5 mg) administration and 15 min after intravenous morphine (1.4 mg/70 kg) administration. With peak analgesia of dronabinol previously reported to occur 180 min after administration [47], it is possible that peak effects of the dronabinol/morphine combination weren’t captured in that study. Another controlled laboratory study that found potentiation of opioid analgesia with a cannabinoid for one endpoint, failed to find an effect for others; 20 mg dronabinol and 30 mg morphine had an additive analgesic effect in an electrical stimulation pain test, but did not elicit an additive effect in thermal, pressure, or cold experimental pain tests [38].
In the present study pain threshold and tolerance were affected by the combination of oxycodone and cannabis, while pain ratings (MPQ and PIB) assessed after termination of the painful stimulus were not. These discrepant findings point to potential limitations related to the quality of the pain relief associated with opioid-cannabis combinations. Findings from controlled clinical trials with chronic pain populations administered a THC-based cannabinoid as an adjunct to currently prescribed opioid analgesics have also varied. Dronabinol (10 and 20 mg) provided additional analgesia relative to placebo in patients taking opioids for chronic non-cancer pain [19]. In a clinical population with intractable cancer-related pain, THC:CBD oromucosal spray increased analgesia as measured by mean pain severity rating score (NRS), and THC oromucosal spray significantly decreased pain as measured by the Brief Pain Inventory–Short Form (BPI-SF) compared to placebo [18]. A later study in chronic cancer-related pain patients treated with opioids demonstrated the dose-dependent nature of a THC:CBD oromucosal preparation on pain and clinical outcomes; improvement in pain endpoints were observed in the low and medium dose groups, but not in the high-dose group [20]. The lack of consistent additivity or synergy across and within laboratory and clinical studies highlights the importance of dose (both opioid and cannabinoid), route of administration (oral, oromucosal, intrapulmonary), time-course, endpoint, and modality of experimental pain for laboratory studies.
Abuse-related and reinforcing effects of active cannabis were not significantly altered with the administration of oxycodone. However, subjective ratings related to oxycodone abuse liability showed small, but reliable, increases after active cannabis administration warrants consideration. Future studies should assess the impact of cannabis administration on oxycodone’s reinforcing effects, the primary public health concern related to abuse liability of opioid-cannabinoid combinations. Additionally, employing a more sensitive cannabis self-administration procedure may help to detect potentially subtle changes in reinforcement as a function of opioid co-administration. A pharmacotherapeutic strategy that capitalizes on THC’s opioid sparing effects while also minimizing its positive subjective effects should be prioritized. For example, to decrease the intoxication observed with active cannabis while maintaining opioid-sparing effects, the impact of oral THC on low-dose oxycodone analgesia should be assessed; oral THC produces analgesic effects that are longer lasting than smoked cannabis while eliciting lower ratings of intoxication and positive subjective effects [47]. Although oral THC administered with a single dose of morphine failed to elicit synergistic sensory analgesia in experimental pain [39], this may have been due to the time when the drug combination was tested as discussed above. Another possibility is that opioid-sparing effects in volunteers may be most prominent with higher efficacy opioid agonists, like oxycodone, relative to lower efficacy opioids [31].
The current findings provide evidence of the opioid-sparing effects of smoked cannabis; however, these results should be interpreted within the context of experimental and therapeutic limitations. Analgesia was assessed using an experimental pain model in a group of young, healthy, cannabis-experienced participants. Enrolling participants without pain and assessing analgesia using an experimental test that has predictive validity for analgesics (i.e., [40–42]) affords a degree of control that cannot be achieved with a patient population. Baseline pain sensitivity did not differ across sessions, and the influence of concomitant medications on outcomes was avoided, two outstanding factors that would have impacted experimental control had a pain population been utilized. Further, that the participants were current cannabis smokers assured that cannabis would be well-tolerated. These factors limit the generalizability of the current findings supporting opioid-sparing effects of cannabis and cannabinoids to patient populations, many of which are not current cannabis users. Understanding the safety and tolerability of cannabis or cannabinoids in non-cannabis as well as cannabis-exposed patients is an important consideration given that tolerability of cannabinoid products is contingent upon experience [20, 50, 51]. Another significant consideration is that the current study used smoked cannabis because this is the most common method of medical cannabis use [52]. However, the therapeutic utility of smoked cannabis may be limited by respiratory risks including chronic bronchitis [53], the presence of combustion by-products [54, 55], a lack of regulation regarding medical cannabis strength (i.e., THC concentration) and other cannabinoid content [56], and subjective effects related to abuse liability which are not as apparent with other methods of administration (i.e., [47]). Adverse respiratory effects and combustion by-products would be avoided by vaporizing cannabis [61, 63, 64], while the superior bioavailability of THC afforded by the intrapulmonary route relative to oral administration would be preserved [57]. However, other risks associated with smoked administration including intoxication and lack of dose regulation would still be a concern [57, 58] for intrapulmonary cannabis. Other routes of THC administration should be explored that would retain analgesic and opioid-sparing effects, while reducing unwanted subjective effects and other risks. An additional limitation to the study design was that cannabis effects on opioid respiratory depression, a significant risk associated with their use [59], was not assessed.
Conclusion
Cannabinoids may provide a therapeutic strategy to enhance the analgesic effects of opioids while mitigating their serious adverse effects. Smoked cannabis combined with an ineffective analgesic dose of oxycodone produced analgesia comparable to an effective opioid analgesic dose without significantly increasing cannabis’s abuse liability. Yet the combination did increase opioid-related positive subjective ratings. These findings warrant future well-controlled, double-blind, placebo-controlled studies designed to assess the opioid-sparing effects of cannabinoids across therapeutically viable routes of administration, employing multiple nociceptive stimuli, patient populations, and importantly, addressing the impact of the drug combination on other critical endpoints including opioid self-administration, tolerance, and dependence. Such studies will determine the generalizability of these findings and the clinical benefit of combined cannabinoid-opioid therapy to treat chronic pain.
Acknowledgements
The authors acknowledge and appreciate the exceptional assistance of Olivia Derella and Bennett Wechsler in data collection and Dr. Richard Foltin for his assistance with regulatory and computer programming aspects of the study.
Funding
This research was supported by US National Institute on Drug Abuse Grant DA19239, DA009236, and DA027755. ZDC, GB, DR, RB, SDC, and MH have no competing interests in relation to the work described. ZDC and MH have received research funds and partial salary support from Insys Therapuetics. Over the past 3 years, SDC received compensation (in the form of partial salary support) from studies supported by Braeburn Pharmaceuticals, Cerecor, Indivior, MediciNova, and Reckitt-Benckiser Pharmaceuticals. In addition, SDC has served as a consultant to the following companies over the past 3 years: Advances in Pain Management, AstraZeneca, Clinilabs, Collegium Pharmaceutical, Daiichi Sankyo, Depomed, Egalet, Endo, Guidepoint Global, Heptares Therapeutics Limited, Inspirion Delivery Sciences, IntelliPharmaCeutics, Janssen, KemPharm, Mallinckrodt, Neuromed, Opiant, Orexo, Pfizer, and Shire.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16:769–80. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daubresse M, Chang H-Y, Yu Y, Viswanathan S, Shah ND, Stafford RS, et al. Ambulatory diagnosis and treatment of non-malignant pain in the United States, 2000–2010. Med Care. 2013;51:870–8. doi: 10.1097/MLR.0b013e3182a95d86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guy GP, Zhang K, Bohm MK, Losby J, Lewis B, Young R, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66:697–4. doi: 10.15585/mmwr.mm6626a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute on Drug Abuse (2017). Overdose Death Rates. at https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates.
- 5.Edlund MJ, Martin BC, Russo JE, Devries A, Braden JB, Sullivan MD (2013). The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic non-cancer pain: the role of opioid prescription. Clin J Pain. 10.1097/AJP.0000000000000021. [DOI] [PMC free article] [PubMed]
- 6.Dunn KM. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohnert ASB. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 8.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Rep. 2016;65:1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 9.Marijuana Policy Project (2017). State-by-state medical marijuana laws. at https://www.mpp.org/issues/medical-marijuana/state-by-state-medical-marijuana-laws/state-by-state-medical-marijuana-laws-report/.
- 10.Bonn-Miller MO, Boden MT, Bucossi MM, Babson KA. Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abus. 2014;40:23–30. doi: 10.3109/00952990.2013.821477. [DOI] [PubMed] [Google Scholar]
- 11.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 12.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–73. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nugent SM, Morasco BJ, O’Neil ME, Freeman M, Low A, Kondo K, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med. 2017;167:319–31. doi: 10.7326/M17-0155. [DOI] [PubMed] [Google Scholar]
- 14.Bradford AC, Bradford WD. Medical marijuana laws may be associated with a decline in the number of prescriptions for medicaid enrollees. Health Aff. 2017;36:945–51. doi: 10.1377/hlthaff.2016.1135. [DOI] [PubMed] [Google Scholar]
- 15.Boehnke KF, Litinas E, Clauw DJ. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain. 2016;17:739–44. doi: 10.1016/j.jpain.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Degenhardt L, Lintzeris N, Campbell G, Bruno R, Cohen M, Farrell M, et al. Experience of adjunctive cannabis use for chronic non-cancer pain: findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend. 2015;147:144–50. doi: 10.1016/j.drugalcdep.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 17.Lynch ME, Clark AJ. Cannabis reduces opioid dose in the treatment of chronic non-cancer pain. J Pain Symptom Manag. 2003;25:496–98. doi: 10.1016/S0885-3924(03)00142-8. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manag. 2010;39:167–79. doi: 10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, et al. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain. 2008;9:254–64. doi: 10.1016/j.jpain.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13:438–49. doi: 10.1016/j.jpain.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9 -tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–15. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mechoulam R, Shani A, Edery H, Grunfeld Y. Chemical basis of hashish activity. Science. 1970;169:611–12. doi: 10.1126/science.169.3945.611. [DOI] [PubMed] [Google Scholar]
- 23.Cichewicz DL, Welch SP, Smith FL. Enhancement of transdermal fentanyl and buprenorphine antinociception by transdermal delta9-tetrahydrocannabinol. Eur J Pharmacol. 2005;525:74–82. doi: 10.1016/j.ejphar.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Cox ML, Haller VL, Welch SP. Synergy between delta9-tetrahydrocannabinol and morphine in the arthritic rat. Eur J Pharmacol. 2007;567:125–30. doi: 10.1016/j.ejphar.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Kazantzis NP, Casey SL, Seow PW, Mitchell VA, Vaughan CW. Opioid and cannabinoid synergy in a mouse neuropathic pain model: opioid-cannabinoid synergy in neuropathic pain. Br J Pharmacol. 2016;173:2521–31. doi: 10.1111/bph.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh G, Smith PB, Dombrowski DS, Welch SP. The role of endogenous opioids in enhancing the antinociception produced by the combination of delta 9-tetrahydrocannabinol and morphine in the spinal cord. J Pharmacol Exp Ther. 1996;279:608–16. [PubMed] [Google Scholar]
- 27.Welch SP, Stevens DL. Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine, in mice. J Pharmacol Exp Ther. 1992;262:10–18. [PubMed] [Google Scholar]
- 28.Williams J, Haller VL, Stevens DL, Welch SP. Decreased basal endogenous opioid levels in diabetic rodents: effects on morphine and delta-9-tetrahydrocannabinoid-induced antinociception. Eur J Pharmacol. 2008;584:78–86. doi: 10.1016/j.ejphar.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Li JX, McMahon LR, Gerak LR, Becker GL, France CP. Interactions between Δ9-tetrahydrocannabinol and μ opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology. 2008;199:199–8. doi: 10.1007/s00213-008-1157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maguire DR, Yang W, France CP. Interactions between opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. J Pharmacol Exp Ther. 2013;345:354–62. doi: 10.1124/jpet.113.204099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maguire DR, France CP. Impact of efficacy at the -opioid receptor on antinociceptive effects of combinations of -opioid receptor agonists and cannabinoid receptor agonists. J Pharmacol Exp Ther. 2014;351:383–89. doi: 10.1124/jpet.114.216648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen S, Sabioni P, Trigo JM, Ware MA, Betz-Stablein BD, Murnion B, et al. Opioid-sparing effect of cannabinoids: a systematic review and meta-analysis. Neuropsychopharmacology. 2017;42:1752–65. doi: 10.1038/npp.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith PA, Selley DE, Sim-Selley LJ, Welch SP. Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol. 2007;571:129–37. doi: 10.1016/j.ejphar.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cichewicz DL. Modulation of oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2003;305:812–17. doi: 10.1124/jpet.102.046870. [DOI] [PubMed] [Google Scholar]
- 35.Li JX, Koek W, France CP. Interactions between Δ9-tetrahydrocannabinol and heroin: self-administration in rhesus monkeys. Behav Pharmacol. 2012;23:754–61. doi: 10.1097/FBP.0b013e32835a3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CDC (2017). Overview of an epidemic. at https://www.cdc.gov/drugoverdose/data/index.html.
- 37.Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL. Cannabinoid–opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844–51. doi: 10.1038/clpt.2011.188. [DOI] [PubMed] [Google Scholar]
- 38.Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain. 2003;105:79–88. doi: 10.1016/S0304-3959(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 39.Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur J Pharmacol. 2006;530:54–58. doi: 10.1016/j.ejphar.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 40.Conley KM, Toledano AY, Apfelbaum JL, Zacny JP. Modulating effects of a cold water stimulus on opioid effects in volunteers. Psychopharmacology. 1997;131:313–20. doi: 10.1007/s002130050298. [DOI] [PubMed] [Google Scholar]
- 41.Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD. Sex differences and hormonal influences on response to cold pressor pain in humans. J Pain. 2006;7:151–60. doi: 10.1016/j.jpain.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Zacny JP, Coalson DW, Young CJ, Klafta JM, Lichtor JL, Rupani G, et al. Propofol at conscious sedation doses produces mild analgesia to cold pressor-induced pain in healthy volunteers. J Clin Anesth. 1996;8:469–74. doi: 10.1016/0952-8180(96)00126-2. [DOI] [PubMed] [Google Scholar]
- 43.Compton P, Kehoe P, Sinha K, Torrington MA, Ling W. Gabapentin improves cold-pressor pain responses in methadone-maintained patients. Drug Alcohol Depend. 2010;109:213–19. doi: 10.1016/j.drugalcdep.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb J, Kamali F. Analgesic effects of lamotrigine and phenytoin on cold-induced pain: a crossover placebo-controlled study in healthy volunteers. Pain. 1998;76:357–63. doi: 10.1016/S0304-3959(98)00068-2. [DOI] [PubMed] [Google Scholar]
- 45.Mariani JJ, Brooks D, Haney M, Levin FR. Quantification and comparison of marijuana smoking practices: blunts, joints, and pipes. Drug Alcohol Depend. 2011;113:249–51. doi: 10.1016/j.drugalcdep.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foltin RW, Brady JV, Fischman MW, Emurian CS, Dominitz J. Effects of smoked marijuana on social interaction in small groups. Drug Alcohol Depend. 1987;20:87–93. doi: 10.1016/0376-8716(87)90079-2. [DOI] [PubMed] [Google Scholar]
- 47.Cooper ZD, Comer SD, Haney M. Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology. 2013;38:1984–92. doi: 10.1038/npp.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haney M, Ward AS, Comer S, Foltin R, Fischman M. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999;14:395–4. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- 49.Hefner K, Sofuoglu M, Rosenheck R. Concomitant cannabis abuse / dependence in patients treated with opioids for non-cancer pain. Am J Addict. 2015;24:538–45. doi: 10.1111/ajad.12260. [DOI] [PubMed] [Google Scholar]
- 50.Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- 51.Tramèr MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323:16–21. doi: 10.1136/bmj.323.7303.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. Toking, vaping, and eating for health or fun. Am J Prev Med. 2016;50:1–8. doi: 10.1016/j.amepre.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 53.Tashkin DP. Smoked marijuana as a cause of lung injury. Monaldi Arch Chest Dis Arch Monaldi Mal Torace. 2005;63:93–100. doi: 10.4081/monaldi.2005.645. [DOI] [PubMed] [Google Scholar]
- 54.Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008;21:494–2. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- 55.Singh R, Sandhu J, Kaur B, Juren T, Steward WP, Segerbäck D, et al. Evaluation of the DNA damaging potential of cannabis cigarette smoke by the determination of acetaldehyde derived N2-ethyl-2’-deoxyguanosine adducts. Chem Res Toxicol. 2009;22:1181–88. doi: 10.1021/tx900106y. [DOI] [PubMed] [Google Scholar]
- 56.Klieger SB, Gutman A, Allen L, Pacula RL, Ibrahim JK, Burris S. Mapping medical marijuana: state laws regulating patients, product safety, supply chains and dispensaries, 2017: State medical marijuana laws. Addiction. 2017. 10.1111/add.13910. [DOI] [PMC free article] [PubMed]
- 57.Newmeyer MN, Swortwood MJ, Abulseoud OA, Huestis MA. Subjective and physiological effects, and expired carbon monoxide concentrations in frequent and occasional cannabis smokers following smoked, vaporized, and oral cannabis administration. Drug Alcohol Depend. 2017;175:67–76. doi: 10.1016/j.drugalcdep.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther. 2007;82:572–78. doi: 10.1038/sj.clpt.6100200. [DOI] [PubMed] [Google Scholar]
- 59.Dahan A, Olofsen E, Niesters M. Pharmacotherapy for pain: efficacy and safety issues examined by subgroup analyses. Pain. 2015;156:S119–26. doi: 10.1016/j.pain.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 60.Comer SD, Metz VE, Cooper ZD, Kowalczyk WJ, Jones JD, Sullivan MA, et al. Comparison of a drug versus money and drug versus drug self-administration choice procedure with oxycodone and morphine in opioid addicts. Behav Pharmacol. 2013;24:504–16. doi: 10.1097/FBP.0b013e328363d1c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooper ZD, Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend. 2009;103:107–13. doi: 10.1016/j.drugalcdep.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda (National Academies Press (US): Washington (DC), 2017). The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. at http://www.ncbi.nlm.nih.gov/books/NBK423845/. [PubMed]
- 63.Pomahacova B, Van der Kooy F, Verpoorte R. Cannabis smoke condensate III: the cannabinoid content of vaporised Cannabis sativa. Inhal Toxicol. 2009;21:1108–12. doi: 10.3109/08958370902748559. [DOI] [PubMed] [Google Scholar]
- 64.Van Dam NT, Earleywine M. Pulmonary function in cannabis users: support for a clinical trial of the vaporizer. Int J Drug Policy. 2010;21:511–13. doi: 10.1016/j.drugpo.2010.04.001. [DOI] [PubMed] [Google Scholar]