Graphical abstract
Keywords: Subaortic stenosis, Subaortic membrane, Discrete
Highlights
-
•
DSS is generally an acquired and progressive condition.
-
•
Subaortic stenosis often takes the form of a discrete crescentic fibromuscular ridge in the LVOT.
-
•
DSS should be considered when an elevated aortic Doppler gradient is seen in the presence of normal aortic leaflet mobility.
-
•
Surgery is considered when the peak instantaneous gradient is >50 to 60 mm Hg.
-
•
DSS has a high rate of recurrence following surgical intervention.
Introduction
Discrete subaortic stenosis (DSS) is a condition generally diagnosed in the pediatric population, but it is becoming increasingly recognized in adult patients. Transthoracic echocardiography is indispensable for the assessment of dyspnea. Unfortunately, attenuation of ultrasound due to increased body mass index, among other factors, may significantly impair the quality of two-dimensional (2D) images, and transesophageal echocardiography may be required to better clarify an uncertain diagnosis.
Case Presentation
A 61-year-old woman with a history of breast cancer, increased body mass index, and prior smoking presented with several months of increasing exertional dyspnea (New York Heart Association functional class II). Cardiovascular examination revealed an ejection systolic murmur but was otherwise unremarkable. Electrocardiography demonstrated sinus bradycardia and nonspecific T-wave flattening in the inferolateral leads. Pulmonary function tests demonstrated relatively preserved lung function. Transthoracic echocardiography was arranged for further assessment.
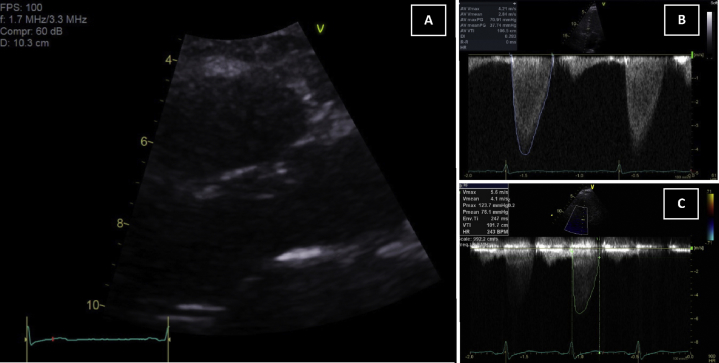
Unfortunately, the 2D transthoracic images were limited by the patient's increased body mass index. The most salient finding was a significantly elevated gradient on CW Doppler interrogation of the aortic valve (mean gradient, 37 mm Hg; Figure 1A). The aortic valve leaflets, however, appeared to have normal leaflet excursion without obvious calcification but was poorly seen on 2D imaging (Figure 1B). Aside from mild concentric hypertrophy of the left ventricle, all other aspects of cardiac size and function were within normal limits, including left and right ventricular size, and the estimated systolic pulmonary pressure. The patient was subsequently referred for transesophageal echocardiography.
Figure 1.
(A) Transthoracic echocardiography from parasternal long-axis view in systole, demonstrating seemingly patent LVOT and absence of significant aortic valve calcification. Continuous-wave Doppler through the aortic valve in the apical five-chamber window, demonstrating an early peaking spectral profile with a mean gradient of 37 mm Hg at rest (B) and 76 mm Hg after exercise (C).
Transesophageal echocardiography revealed a thin ridge of tissue in the anterior left ventricular outflow tract (LVOT) approximately 6 mm from the insertion of the right coronary cusp of the aortic valve (Figure 2A, Video 1). Color Doppler demonstrated significant flow acceleration in the LVOT and below to the aortic valve (Figure 2B). Cross-plane imaging of the LVOT revealed the true extent of the LVOT membrane, which was a crescentic fibromuscular membrane encircling the LVOT, narrowing to 0.8 to 0.9 cm2 during systole (Figures 3A and 3B, Video 2). Three-dimensional assessment confirmed that this was a discrete fibromuscular membrane (Figures 4A and 4B, Video 3). There was mild aortic regurgitation.
Figure 2.
(A) Transesophageal midesophageal long-axis view (140°) demonstrating a thin ridge of tissue in the LVOT (red arrow). (B) Color Doppler demonstrating significant flow acceleration before the aortic valve.
Figure 3.
Transesophageal echocardiography demonstrating the subaortic membrane (red arrow) and relative patency of the LVOT in short axis during end-diastole (A). A cross-plane image (orthogonal views) of the LVOT in systole highlighting the large crescentic fibromuscular membrane (blue arrows) with an estimated orifice area of 0.8–0.9 cm2 (B and C).
Figure 4.
Transesophageal echocardiographic three-dimensional image, LVOT from the left ventricle in diastole (A) and systole (B). The anterior mitral valve leaflet is oriented at 12 o'clock. The red arrows indicate the fibromuscular membrane in systole.
Stress echocardiography was performed to assess for concomitant myocardial ischemia and document functional capacity. Although there was no evidence of ischemia, the maximum instantaneous outflow tract gradient increased to 124 mm Hg (mean gradient, 76 mm Hg) at 100 beats/min, indicating severe obstruction. Exercise capacity was reduced, achieving <5 min on the standard Bruce protocol (7.0 METs).
Invasive coronary angiography demonstrated angiographically normal coronary arteries. The peak-to-peak gradient was 48 mm Hg, and the calculated mean gradient was 42 mm Hg. Left ventriculography was performed in a left anterior oblique cranial view, which best demonstrated the membrane in the LVOT (Figure 5).
Figure 5.
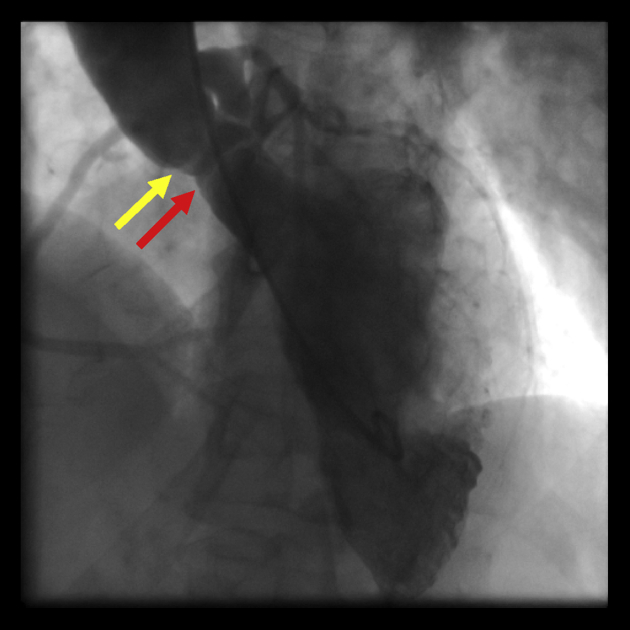
Left ventriculogram (left anterior oblique cranial view) demonstrating the circumferential nature of the thin subaortic membrane (red arrow) and its proximity to the aortic valve (yellow arrow).
The patient was referred for cardiac surgery. At the time of writing, the patient remained stable and free of unplanned hospitalization.
Discussion
DSS can develop at any age, but in the vast majority of cases, it is detected in childhood, although it is rarely seen in infancy. It may be slightly more common in male patients, with cohort studies displaying a 52%–67% male predominance.1, 2, 3 In one large cohort, DSS accounted for 6.5% of adult congenital heart disease,2 and although DSS is frequently categorized as congenital heart disease, it is considered to be an acquired condition.
In this case, the presence of a significant gradient on continuous-wave Doppler interrogation of the aortic valve and flow acceleration on color Doppler below the valve, but the appearance of a valve that opened well on transthoracic echocardiography, provided the clue that the most likely diagnosis was DSS. Transesophageal echocardiography was invaluable in confirming the presence, morphology, and extent of subaortic narrowing.
The hypothesized pathogenesis of DSS centers on abnormal LVOT geometry that predisposes to turbulent flow within the LVOT. Proposed anatomic aberrancies that may contribute to turbulent flow include a long, narrow LVOT; a steep aortoventricular septal angle; and aortic valve override of the ventricular septum. It is this turbulent flow that is thought to increase local sheer stress and consequently lead to local reactive cellular proliferation and progressive fibromuscular changes (including the differentiation of fibroblasts into contractile myofibroblasts).4 Although there are rare reports of familial clustering, there is no firm evidence to support a primary genetic etiology.4
In the majority of cases (90%), the subvalvular membrane takes the form of a fibromuscular ridge that encircles the LVOT, but it can also be composed of a diffuse tunnel-like narrowing. Occasionally the defect can involve the base of the aortic cusp or the anterior leaflet of the mitral valve. It frequently occurs in conjunction with other congenital heart disease, 44% of the time in one large study.2 The most common associated condition is a ventricular septal defect, occurring in up to 65% of patients in one cohort.2, 5 Other associated conditions include coarctation of the aorta and a bicuspid aortic valve.6
Progression of DSS is unpredictable. One large retrospective study has reported that the rate of progression is slower in adults, compared with children, although this needs to be interpreted with caution, as the adults may be selected out as having less severe disease.2 Risk factors for progression of LVOT gradient include initial mean gradient > 30 mm Hg, initial aortic valve thickening, and attachment of the subaortic membrane to the mitral valve. The reported rates of progression (peak instantaneous gradient) vary from center to center but are in the range of 1–3 mm Hg/year.2, 3
There is a high prevalence of aortic regurgitation among patients with DSS (up to 80%). When detected, the severity of aortic regurgitation was ≤2+ in approximately 78% of patients, and it progresses slowly.2 Risk factors for the progression of aortic regurgitation include higher peak gradient (>50 mm Hg) and longer distance between the DSS and the aortic valve. The longer distance between the DSS and the aortic valve allows a greater distortion of flow in the LVOT, creating high-velocity turbulent jets that strike and damage the underside of the aortic leaflets, leading to aortic regurgitation.7
Guidelines for intervention vary from center to center, but surgical intervention is generally recommended if the peak instantaneous gradient is >50–60 mm Hg, with a lower threshold in the setting of aortic regurgitation or left ventricular hypertrophy. The timing of surgery is an important consideration, especially in children, because of the risk for recurrence. Reported rates regarding need for reoperation are based on small retrospective studies and range between 15% and 26% during median follow-up periods of 10–15 years.3, 8 Risk factors for reoperation include preoperative peak gradient ≥ 60 mm Hg, close proximity of the subaortic membrane to the aortic valve (<7 mm), peeling of the membrane from the aortic valve, younger age at operation, and female sex.3, 9, 10, 11 Additional routine septal myectomy at the time of initial operation has not been proved to provide additional benefit.3, 8
Although some studies have demonstrated an increased incidence of endocarditis, the consensus guidelines from the American Heart Association no longer recommend routine antibiotic prophylaxis in patients with DSS.12
Conclusions
DSS is generally considered an acquired and progressive condition that is not uncommon in the adult population. Although there are data to guide the threshold for intervention, controversy exists with regard to optimal timing of surgery because of the high risk for recurrence. This case study highlights the importance of transesophageal echocardiography in the assessment of LVOT pathology, especially when there is an unexplained elevated continuous-wave Doppler gradient on aortic valve interrogation, but with a normal appearance of the aortic leaflets on 2D imaging.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2018.01.005.
Supplementary Data
This video demonstrates a simultaneous standard two-dimensional and color Doppler clip of the aortic valve in long axis, acquired by transesophageal echocardiography. The flow acceleration can be clearly seen before the aortic valve.
This video demonstrates a biplane clip of the LVOT. Although there is some movement of the two-dimensional image plane during the cardiac cycle, the extent of the fibromuscular membrane is well demonstrated during systole.
This video demonstrates a three-dimensional image of the LVOT as viewed from the left ventricular aspect of the aortic valve. The dynamic nature of the subvalvular membrane throughout the cardiac cycle is appreciated.
References
- 1.Choi J.Y., Sullivan I.D. Fixed subaortic stenosis: anatomical spectrum and nature of progression. Br Heart J. 1991;65:280–286. doi: 10.1136/hrt.65.5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver J.M., González A., Gallego P., Sánchez-Recalde A., Benito F., Mesa J.M. Discrete subaortic stenosis in adults: increased prevalence and slow rate of progression of obstruction and aortic regurgitation. J Am Coll Cardiol. 2001;38:835–842. doi: 10.1016/s0735-1097(01)01464-4. [DOI] [PubMed] [Google Scholar]
- 3.van der Linde D., Roos-Hesselink J.W., Rizopoulos D., Heuvelman H.J., Budts W., van Dijk A.P. Surgical outcome of discrete subaortic stenosis in adults: a multicenter study. Circulation. 2013;127:1184–1191. doi: 10.1161/CIRCULATIONAHA.112.000883. [DOI] [PubMed] [Google Scholar]
- 4.Foker J.E. Outcomes and questions about discrete subaortic stenosis. Circulation. 2013;127:1447–1450. doi: 10.1161/CIRCULATIONAHA.113.001619. [DOI] [PubMed] [Google Scholar]
- 5.Kitchiner D., Jackson M., Malaiya N., Walsh K., Peart I., Arnold R. Morphology of left ventricular outflow tract structures in patients with subaortic stenosis and a ventricular septal defect. Br Heart J. 1994;72:251–260. doi: 10.1136/hrt.72.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezon D.S. Fixed subaortic stenosis: a clinical dilemma for clinicians and patients. Congenit Heart Dis. 2013;8:450–456. doi: 10.1111/chd.12127. [DOI] [PubMed] [Google Scholar]
- 7.Motro C., Schneeweiss A., Shem-Tov A., Benjamin P., Kaplinsky E., Hegesh J. Correlation of distance from subaortic membrane to base of the right coronary cusp and development of aortic regurgitation in mild discrete subaortic stenosis. Am J Cardiol. 1989;64:395–396. doi: 10.1016/0002-9149(89)90544-4. [DOI] [PubMed] [Google Scholar]
- 8.Hirata Y., Chen J.M., Quaegebeur J.M., Mosca R.S. The role of enucleation with or without septal myectomy for discrete subaortic stenosis. J Thorac Cardiovasc Surg. 2009;137:1168–1172. doi: 10.1016/j.jtcvs.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Pickard S.S., Geva A., Gauvreau K., del Nido P.J., Geva T. Long-term outcomes and risk factors for aortic regurgitation after discrete subvalvular stenosis resection in children. Heart. 2015;101:1547–1553. doi: 10.1136/heartjnl-2015-307460. [DOI] [PubMed] [Google Scholar]
- 10.Geva A., McMahon C.J., Gauvreau K., Mohammed L., del Nido P.J., Geva T. Risk factors for reoperation after repair of discrete subaortic stenosis in children. J Am Coll Cardiol. 2007;50:1498–1504. doi: 10.1016/j.jacc.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Dodge-Khatami A., Schmid M., Rousson V., Fasnacht M., Doell C., Bauersfeld U. Risk factors for relief after congenital subaortic stenosis. Eur J Cardiothorac Surg. 2008;33:885–889. doi: 10.1016/j.ejcts.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 12.Wilson W., Taubert K.A., Gewitz M., Lockhart P.B., Baddour L.M., Levison M. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video demonstrates a simultaneous standard two-dimensional and color Doppler clip of the aortic valve in long axis, acquired by transesophageal echocardiography. The flow acceleration can be clearly seen before the aortic valve.
This video demonstrates a biplane clip of the LVOT. Although there is some movement of the two-dimensional image plane during the cardiac cycle, the extent of the fibromuscular membrane is well demonstrated during systole.
This video demonstrates a three-dimensional image of the LVOT as viewed from the left ventricular aspect of the aortic valve. The dynamic nature of the subvalvular membrane throughout the cardiac cycle is appreciated.