Abstract
Background
Zika, a disease caused by Zika virus infections, has recently emerged and caused outbreaks in many parts of the world. The clinical manifestations of Zika are usually mild, mostly presenting as an exanthematic febrile disease, but on some occasions, it might be associated with microcephaly after intrauterine infection, and Guillain-Barré Syndrome. Zika virus is primarily transmitted by mosquito bites, but other means of transmission have been described, and potential risk for blood transmission has been reported in French Polynesia and Brazil.
Methods
To investigate the risk of Zika virus infection after a blood transfusion in an area of Brazil where a possible transmission by a platelet concentrate has been described. Using a mini-pool format, 1857 blood donations were evaluated by real-time reverse transcriptase polymerase chain reaction designed to detect Zika virus RNA.
Results
After testing samples individually from positive mini-pools, the prevalence of Zika virus RNA was only 0.16%, a result probably associated to the low circulation of this virus in the study area. In addition, it was evident that the implementation of post-surveillance programs is important to detect Zika virus infections in blood donors, as the post-donation surveillance program detected two blood donors with the disease in this study.
Conclusion
This study shows that the risk for Zika virus transmission by blood transfusion is real, even in regions with a low circulation of the disease, but the combination of the detection of Zika virus RNA by polymerase chain reaction and post-donation surveillance might reduce the risk of transmission by blood transfusions.
Keywords: Zika, Blood transfusion, Transfusion risk, ZIKV
Introduction
Zika, a disease caused by the Zika virus (ZIKV), is usually characterized by rash, low-grade fever and mild constitutional symptoms.1, 2 However, according to data released from studies in French Polynesia, most ZIKV infections remain asymptomatic.3 Although the prevalence of asymptomatic ZIKV infections in Brazil is not known, infections by this virus were first reported as a dengue-like disease in 2015 and since then, significant outbreaks have occurred in several regions of the country, especially in the northeastern region.4 Although hard data are still missing, there is evidence that ZIKV infections might also be associated with more severe presentations, such as Guillain-Barré Syndrome and microcephaly in newborn babies whose mothers were infected with ZIKV during pregnancy. This arthropod-borne virus is primarily transmitted by the bites of female Aedes mosquitoes, but new transmission routes have been described in outbreaks occurring in the Pacific region and the Americas.5, 6, 7 Interestingly, the first isolated and fully sequenced autochthonous transmitted ZIKV in the Americas came from a region where the Zika outbreak has not been very significant, the southeastern region of Brazil.8 This virus was isolated in the city of Campinas, São Paulo State, in a patient who underwent a liver transplantation and received a platelet concentrate transfusion with probable transfusion transmission being reported.9 Due to this local transmission and the report from French Polynesia on the risk of ZIKV transmission by blood,5 it is of paramount importance to define the real risk of ZIKV transmission either through blood or blood components, and which preventive measures should be taken to minimize this risk. This study reports the results of an investigation to detect the frequency of ZIKV RNA using minipools comprised of blood donated in the same city where the first Brazilian ZIKV was isolated, and reinforce the importance of post-donation surveillance to detect ZIKV infections in blood donors.
Objectives
To identify the prevalence of ZIKV RNA in blood donations in the blood center of the Universidade Estadual de Campinas (UNICAMP).
Methods
Blood donors
A total of 1857 blood donations from the Campinas metropolitan region were screened for ZIKV RNA by real-time reverse transcription polymerase chain reaction (rRT-PCR). Samples were collected from all donations on Fridays for four consecutive weeks in February and March 2016. According to the Municipal Epidemiological Surveillance Agency10 the highest incidence of arthropod-borne diseases is in the summer and thus, it is believed that this would be the best time to investigate ZIKV transmission by blood transfusion. During the pre-donation evaluation, donors were asked about symptoms of infectious diseases in the weeks leading up to the interview, known risk factors for viral infections and having been in areas of risk for Zika outside of Brazil in the previous 30 days. The blood donations were screened for the usual blood-borne pathogens. The blood components produced from these donations would be released for transfusion independently of any screening test for ZIKV RNA. This study was approved by the Ethics Committee on Human Research of the Faculdade de Medicina de Ribeirão Preto of the Universidade de São Paulo (FMRP USP). The complementary tests were authorized by the donors who signed informed consent forms before the blood donation.
RNA extraction and real-time reverse transcription polymerase chain reaction
The nucleic acids were extracted on the same day as the donation from minipools containing samples from six blood donors using a silica membrane protocol developed by BioManguinhos (Rio de Janeiro, Brazil) and an automated, robotic system [BioRobot MDx Universal System (Qiagen, Switzerland)]. ZIKV RNA detection as well as NAT screening for human immunodeficiency virus-1 (HIV-1), Hepatitis C virus (HCV), and hepatitis B virus (HBV) were performed in minipools of donations using the NAT HIV/HCV/HBV Kit (Bio-Manguinhos, Rio de Janeiro, Brazil). Extracted RNAs were frozen at −80 °C and ZIKV RNA testing was performed after 24–36 h. In order to obtain the most reliable rRT-PCR results, Lanciotti's rRT-PCR protocol was initially used and confirmed by the rRT-PCR protocol described by Pyke et al.2, 11 Samples were considered positive if they amplified ZIKV RNA with a crossing threshold (CT) of less than 40 and samples with a CT between 35 and 40 were submitted to confirmation tests by an additional rRT-PCR. Donor samples in rRT-PCR positive pools were tested individually to identify the positive donor. All rRT-PCR included internal control samples that tested positive for the HIV-1, HCV and HBV genomes, and positive ZIKV controls consisting of dilutions of ZIKV prepared in Vero cells. In short, rRT-PCR for ZIKV used 4 μL aliquot from each minipool and 6 μL of amplification reaction containing 2.5 μL of reaction mix (TaqMan Fast Virus 1-Step Master Mix), and 125 nM of each primer and probe diluted in nuclease free water.
Sequencing and phylogenetic analysis of the Zika virus detected in positive samples
Positive samples were sequenced by Sanger technology, using the BigDye Direct Cycle Sequencing kit protocol and primers that amplify a 500-base pair region inside the NS1 gene of the ZIKV. Sequencing was performed in an ABI 3500 sequencer (Applied Biosystems) and data was analyzed using MEGA 7 software. The analysis involved 13 NS1 gene sequences from distinct ZIKV sequences available in the NCBI website. A total of 474 positions were analyzed in the final dataset because all positions containing gaps and missing data were eliminated.
Results
Characteristics of the blood donors
The donations studied here corresponded to 42% of all blood donations performed in the blood center during the study period. Blood donors consisted of 1092 male and 765 female donors with a median age of 35.9 ± 11.3 years old (range: 17–69 years). None of the donors reported symptoms compatible with ZIKV infection or other infections at the time of donation, but post-donation surveillance based on positive rRT-PCR results identified two donors reporting Zika-like symptoms a few days after donation. One of them reported a pruritic rash and arthralgia in the interphalangeal joints beginning two days after donation that lasted for five days and the other one complained of low-grade fever, rash, body aches and generalized arthralgia beginning the day following donation that lasted for four days. Both donors had uneventful courses of the disease and recovered without sequelae; due to the low impact on their daily activities, neither of them reported their disease to the blood center. A third donor who was positive for ZIKV remained asymptomatic. An important contribution of this study is that the result of the rRT-PCR was obtained within 48–72 h after donation preventing all blood components produced from these three donations from being transfused.
Real-time reverse transcription polymerase chain reaction results
Of the 344 minipools tested, two yielded positive ZIKV rRT-PCR results and when the individual samples of these minipools were tested, three samples were positive for ZIKV RNA, with CT values of 25.02, 27.19 and 38.04. For each reaction, a sample prepared from a viral culture with a concentration of 1 × 102 copies/μL and a positive sample from a patient diagnosed with ZIKA were used as positive controls.
Some samples were assigned to more than one pool, with the purpose of completing the rRT-PCR test routine. Three additional minipools were initially positive, but when the individual samples were tested, none turned out to be positive, indicating that, probably, these minipool test results were false positive. However, another possible explanation for these cases would be the occurrence of two samples with low viral load in the same pool, which together would reach the detection threshold of the method. None of these blood donors developed Zika after donating.
Phylogenetic analysis of positive samples
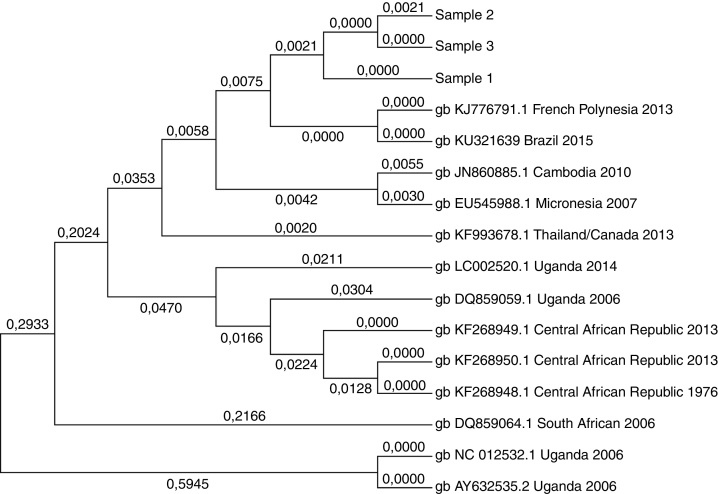
Positive samples were sequenced using Sanger technology and a ZIKV RNA sequence of 474 base pairs within the NS1 gene was compared with ZIKV sequences available in the GenBank (NCBI; Figure 1). The sequences had 99.8% of similarity and 99% to the ZikaSPH2015 virus (KU321639 BRAZIL 2015) originally isolated in the region of this study. Moreover, phylogenetic analysis also confirmed that the viruses detected in this study are closely associated to the French Polynesian strain.
Figure 1.
Molecular phylogenetic analysis of positive samples (samples 1–3). ZIKV RNA sequence of 474 bp within the NS1 gene was compared with ZIKV sequences available in the GenBank. The sequences had a 99.8% of similarity among them and 99% to the ZikaSPH2015 virus (KU321639 BRAZIL 2015).
Discussion
ZIKV is an enveloped, arthropod-borne virus of the Flaviviridae family (genus Flavivirus),12 a genus with several viruses of medical importance, such as yellow fever, West Nile, and dengue fever. As with other viruses in this genus, ZIKV is a single-stranded RNA virus possessing a positive-polarity RNA genome of approximately 11 Kb containing an open reading frame that encodes a polyprotein consisting of three structural proteins [capsid (C), pre-membrane (prM), and envelope (E)], and seven non-structural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5).12 Except for West Nile virus, due to the high rate of asymptomatic infections and the mild disease that usually follows these viral infections, few transmissions in arbovirus-infected transfusions have been proved.13 Furthermore, the duration of viremia is usually short and declines rapidly after the onset of symptoms making it difficult to evaluate the real transmission risk of these viruses by blood transfusion.
Although Musso et al.5 pointed out the potential for ZIKV transmission by blood transfusion during the French Polynesian outbreak, no blood transfusion-transmitted cases of ZIKV infection had been confirmed in the world until a recent report of a well-defined ZIKV transmission that occurred in Brazil.9 Due to the possibility of ZIKV transmission by blood transfusions, a study that aimed to detect ZIKV RNA in donated blood was implemented in the months of February and March of 2016 at the Blood Center of Campinas. Out of almost two thousand blood donor samples examined by two sensitive rRT-PCR procedures, only three samples were found positive for the presence of ZIKV RNA.
The quick processing of the samples permitted the blood components obtained from these donations to be discarded; in the post-donation follow-up, rRT-PCR-positive donors were interviewed for Zika symptoms aiming at establishing which stage of the disease they were in at the time of donation. Interestingly, only one remained asymptomatic and two of them reported symptoms of Zika after the donation, proving there is a risk of ZIKV transmission in the incubation period and not only from asymptomatic individuals. However, only donors who were positive for ZIKV RNA were interviewed and asked about symptoms. Thus, it is not possible to assess the impact of post-donation surveillance on the prevention of transfusion-transmitted ZIKV infection. The prevalence of ZIKV RNA in this study was 0.16%, a lower rate than the 2.8% reported by Musso et al.5 in their study carried out in French Polynesia. This observation might be explained by the difference in intensity of the ZIKV outbreak in both regions as it has been reported that in the French Polynesian outbreak about 11% of their population was infected by ZIKV.3 On the other hand, the region of Campinas did not have the extensive ZIKV outbreak that was seen in many parts of Brazil; only 252 probable ZIKV infections were reported during this period, with only four being confirmed by rRT-PCR.14 This fact may explain the low prevalence rate of ZIKV RNA in blood donations observed in this study and raise the possibility of higher prevalence rates in other areas of Brazil that experienced more severe outbreaks.
Hence, it is reasonable to believe that a pre-donation question about having come from or having visited areas with increased risk for Zika might be helpful to prevent the transmission of this disease through blood transfusions. However, in 2016, the Brazilian Government considered Zika infection widespread throughout the Brazilian territory and a guidance note was published about clinical screening of blood donors that did not include this recommendation.
In addition, this study confirmed that the ZIKV strain circulating in Brazil is closely related to the French Polynesian strain, ruling out the possibility that the low prevalence rate of ZIKV infections in blood donors of this study is associated to another ZIKV strain circulating in Brazil.
Furthermore, this study sheds more light on the knowledge about risk for ZIKV transmission by blood transfusion showing that this risk is real, even in areas of low prevalence of ZIKV infections. Two of the ZIKV-positive samples had low CTs by rRT-PCR and consequently high viremias that could easily infect recipients of blood or blood products as has happened before in Brazil.9 These results also demonstrated that the frequency of individuals who are infected with ZIKV and do not present any symptoms is high (about 30%). This raises the question as to whether or not blood centers should implement a post-donation surveillance program during outbreaks of ZIKV infections. The importance of this surveillance was demonstrated since it detected Zika in two blood donors who tested positive by rRT-PCR and later developed the disease, but did not report their disease to the blood center due to the low impact of the disease on their daily activities.
Moreover, assessing the risk of transfusion-transmitted ZIKV infection is a challenge to the safety and availability of the blood supply in many areas of the world. The Centers for Disease Control and Prevention (CDC) has issued a document with guidelines to prevent transfusion-transmitted ZIKV infection,15 and the Pan American Health Organization (PAHO) has also issued a document on the recommendations to blood centers in epidemic areas.16 Thus, it is clear from the data presented here that before the risk of transfusion-transmitted ZIKV infections is completely ascertained and before rRT-PCR to investigate ZIKV in blood transfusion services becomes a reality, services located in areas with active ZIKV transmission should very carefully evaluate the suitability of their blood donors. If possible, a robust process of post-donation surveillance should be implemented in these areas to acquire post-donation information before using the donated blood. It is also very important to implement a hemovigilance process to monitor patients after transfusions, where this service is not yet available.
In summary, this is one of the few studies outside of French Polynesia investigating the prevalence of ZIKV RNA in blood donations and shows that the risk is real although it depends on the extension of the outbreak. It is possible that the highest risk occurs in blood donors incubating the disease and not with asymptomatic infections, but this is still subject to confirmation since the number of ZIKV positive donations in this study is very low. Furthermore, viremic donations could have been missed because they were tested by minipools of six samples and not individually, as was performed in the study by Galel et al.,17 where only 50% of the 14 viremic donations were detectable upon 1:6 dilutions simulating minipool testing. The data here show the importance of a post-donation surveillance process to detect the presence of ZIKV contamination of blood components in areas where ZIKV rRT-PCR is not available, and the identification of the real risk of ZIKV transmission by transfusion.
Conclusion
This study shows that the risk for ZIKV transmission by blood transfusion is real, even in regions with low ZIKV circulation and that the combination of ZIKV RNA detection by rRT-PCR and post-donation surveillance might reduce this risk.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Petersen L.R., Jamieson D.J., Powers A.M., Honein M.A. Zika virus. N Engl J Med. 2016;374(16):1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 2.Lanciotti R.S., Kosoy O.L., Laven J.J., Velez J.O., Lambert A.J., Johnson A.J. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ioos S., Mallet H.P., Leparc Goffart I., Gauthier V., Cardoso T., Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44(7):302–307. doi: 10.1016/j.medmal.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Zika virus outbreaks in the Americas. Wkly Epidemiol Rec. 2015;90(45):609–610. [PubMed] [Google Scholar]
- 5.Musso D., Nhan T., Robin E., Roche C., Bierlaire D., Zisou K. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia November 2013 to February 2014. Euro Surveill. 2014;19(14) doi: 10.2807/1560-7917.es2014.19.14.20761. [DOI] [PubMed] [Google Scholar]
- 6.Besnard M., Lastere S., Teissier A., Cao-Lormeau V., Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia December 2013 and February 2014. Euro Surveill. 2014;19(13) [PubMed] [Google Scholar]
- 7.Foy B.D., Kobylinski K.C., Chilson Foy J.L., Blitvich B.J., Travassos da Rosa A., Haddow A.D. Probable non-vector-borne transmission of Zika virus, Colorado USA. Emerg Infect Dis. 2011;17(5):880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha M.S., Esposito D.L., Rocco I.M., Maeda A.Y., Vasami F.G., Nogueira J.S. First complete genome sequence of Zika virus (Flaviviridae, Flavivirus) from an autochthonous transmission in Brazil. Genome Announc. 2016;4(2) doi: 10.1128/genomeA.00032-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barjas-Castro M.L., Angerami R.N., Cunha M.S., Suzuki A., Nogueira J.S., Rocco I.M. Probable transfusion-transmitted Zika virus in Brazil. Transfusion. 2016;56(7):1684–1688. doi: 10.1111/trf.13681. [DOI] [PubMed] [Google Scholar]
- 10.Secretaria Municipal de Saúde Campinas. DEVISA-Depto de Vigilância em Saúde. Informe Epidemiológico Arboviroses. Available from: http://www.saude.campinas.sp.gov.br/vigilancia/informes/2016/Informe_.Epidemiologico_Arboviroses_3_novembro_2016.pdf [accessed 27.02.18].
- 11.Pyke A.T., Daly M.T., Cameron J.N., Moore P.R., Taylor C.T., Hewitson G.R. Imported Zika virus infection from the cook islands into Australia, 2014. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.4635a54dbffba2156fb2fd76dc49f65e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindenbach B.D., Thiel H.J., Rice C.M. Flaviviruses. In: Knipe D.M., Howley P.M., editors. Fields virology. 6th ed. Wolters Kluwer/Lippencott Williams & Wilkins; Philadelphia, PA, USA: 2013. p. 712. [Google Scholar]
- 13.Petersen L.R., Busch M.P. Transfusion-transmitted arboviruses. Vox Sang. 2010;98(4):495–503. doi: 10.1111/j.1423-0410.2009.01286.x. [DOI] [PubMed] [Google Scholar]
- 14.Secretaria Municipal de Saúde Campinas. DEVISA-Depto de Vigilância em Saúde. Informe Epidemiológico Arboviroses. Available from: http://www.saude.campinas.sp.gov.br/vigilancia/informes/2016/Informe_Epidemiologico_Arboviroses_29_setembro_2016.pdf [accessed 27.02.18].
- 15.US Food and Drug Administration (FDA) 2016. FDA guidance: recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Available from: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm [accessed 16.06.16] [Google Scholar]
- 16.PAHO . 2016. Provisional recommendations for blood services in light of the Zika virus epidemic: potential impact on the spread of the infection and on the availability and safety of blood and blood components. Available from: http://www.paho.org/hq/index.php?option=com_docman&task=doc_details&gid=33664&Itemid=270&lang=en [accessed 27.02.18] [Google Scholar]
- 17.Galel S.A., Williamson P.C., Busch M.P., Stanek D., Bakkour S., Stone M. First Zika-positive donations in the continental United State. Transfusion. 2017;57(3pt2):762–769. doi: 10.1111/trf.14029. [DOI] [PubMed] [Google Scholar]