Introduction
Malignant peripheral nerve sheath tumor (MPNST) is an uncommon, aggressive sarcoma that arises from peripheral nerves and metastasizes hematogenously, most often to lung and bone.1 Approximately half of MPNSTs arise from plexiform neurofibromas in patients with neurofibromatosis type I (NF1).1 The remaining half occur sporadically, 10% of which arise from previously irradiated tissue.1 The epithelioid variant of MPNST is uncommon, accounting for less than 5% of cases.1 The most important aspect of treatment for MPNST is achieving clear surgical margins, as survival decreases dramatically with positive margins.1 Similar to other soft tissue sarcomas (STS), adjunctive chemoradiotherapy is used if wide margins cannot be achieved, even though MPNST is notoriously resistant to these therapies; the average 5-year survival rate for patients with MPNST is 50% to 60%, with even lower rates for metastatic disease.1 There are only 2 reports of MPNST metastasizing to the skin,2, 3 and there are no reports of using intralesional methotrexate to slow progression of cutaneous metastases.
Case report
A 62-year-old man with epithelioid MPNST metastatic to the lungs, ribs, and lymph nodes was referred to the dermatology department for evaluation of painful, growing scalp nodules. Three years prior, he had MPNST of the neck diagnosed after presenting with facial weakness and earaches. He had no history of NF1. The patient underwent extensive surgical resection of the initial cervical tumor followed by adjuvant radiation caused by positive surgical margins. After metastases developed, he was enrolled in multiple clinical trials and treated with doxorubicin, trabectedin, and everolimus, in combination with bevacizumab, and temozolomide. All of these treatments were eventually discontinued because of disease progression. On presentation to our clinic, he was receiving radiation for bony metastases and taking pazopanib, a tyrosine kinase inhibitor (TKI) indicated for metastatic STS unresponsive to first-line agents.
On initial examination, there were 3 firm, pink nodules on the occipital scalp and temporal scalp bilaterally ranging in size from 1.2 to 1.6 cm (Fig 1, A). Biopsy found small nests and strands of S100 positive and MITF negative deeply infiltrative tumor cells with marked nuclear pleomorphism and high mitotic activity (Fig 2). The decision was made to administer intralesional methotrexate in an effort to relieve the patient's pain and prevent further disease progression. Two weeks after the injection of 50 mg (25 mg/mL) of methotrexate divided between the scalp nodules, all lesions had grown significantly, now ranging in size from 1.5 to 3.7 cm. There was also interval development of 2 nodules in the right axilla and 4 nodules on the right side of the neck (Fig 3). Two weeks after repeat administration of methotrexate, his disease continued to progress, with scalp nodules ranging in size from 2.7 to 6.8 cm (Fig 1, B). Additionally, there were now 7 nodules on the right side of the neck. At this appointment, the patient was short of breath and fatigued. He was admitted to the hospital where anemia, a large pleural effusion, and new internal metastases were diagnosed. Pazopanib was discontinued, and comfort measures were pursued. The patient passed away 2 weeks later.
Fig 1.
A, Cutaneous metastasis of malignant peripheral nerve sheath tumor. Firm, pink nodule measuring 1.6 × 0.8 cm on right temporal scalp at the time of initial consultation with the dermatology department. B, Right temporal scalp metastasis measuring 6.8 × 5.7 cm 2 weeks after the second injection of intralesional methotrexate. This photograph was taken approximately 5 weeks after the photograph in Fig 1, A.
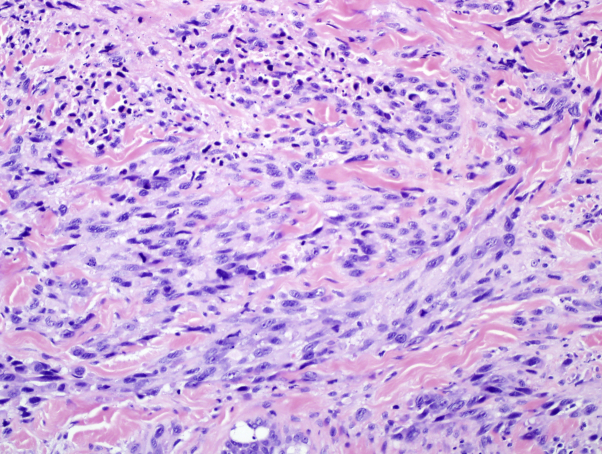
Fig 2.
Cutaneous metastasis of malignant peripheral nerve sheath tumor. Biopsy of the right temporal scalp nodule shows small nests and strands of deeply infiltrative tumor cells with marked nuclear pleomorphism and high mitotic activity. The specimen stained positively for S100, but negatively for MITF. (Hematoxylin-eosin stain; original magnification: ×400.)
Fig 3.
Cutaneous metastasis of malignant peripheral nerve sheath tumor. Rapid growth of firm neck nodules that developed within 3 weeks of the patient's initial presentation to the dermatology department.
Discussion
MPNST skin metastases are rare in the literature, with only 2 prior reports,2, 3 one of which describes metastasis to a myocutaneous flap grafted over the site of primary tumor resection.3 Cutaneous metastasis occurs in less than 1% of patients with STS and most frequently involves the scalp.4 Our patient's rapid decline after cutaneous disease development bears testament to the fact that skin metastasis is often a late event in disease progression and portends a dismal prognosis.3, 4 The rarity of MPNST and STS in general has precluded any definitive efficacy trials for metastatic disease, so treatment depends largely on institutional preference.5 We attempted palliative treatment with intralesional methotrexate because of its effectiveness at providing symptomatic relief in certain metastatic STS, namely aggressive fibromatosis, when used systemically.5 Systemic methotrexate can be used as monotherapy or in combination with a cytotoxic agent such as vinorelbine, vinblastine, or doxorubicin.5, 6 Because our patient was taking pazopanib at presentation and previously had poor response to numerous systemic agents, systemic methotrexate was deemed too aggressive for palliative efforts. Additionally, we did not pursue radiation of cutaneous lesions because the patient was already receiving palliative radiotherapy for spinal metastases.
Recent reports suggest that newer targeted therapies may improve survival in select patients with MPNST. There are isolated cases of MPNST responding to the TKI, sorafenib, and 1 case of a remarkable response to the BRAF inhibitor, vemurafenib, in a patient with MPNST positive for the BRAF V600E mutation.7 Up to 12.5% of MPNST could harbor this mutation, leading some to propose genetic profiling of all newly diagnosed MPNST to allow for potential treatment with vemurafenib.7 Even in patients without a BRAF mutation, inhibition of this enzyme may effectively target MPNST; in both sporadic and NF1-associated MPNST, there is unregulated activation of the MAPK pathway, of which BRAF is a downstream effector.7 In addition to the widely accepted role of aberrant MAPK signaling in MPNST tumorigenesis, recent evidence suggests that immune dysregulation may also contribute to disease pathology. Studies on the immunologic profile of human MPNST have shown increased immunogenicity of tumors over time,8 as well as increased PD-L1 expression and CD8+ lymphocyte infiltration in MPNST compared with benign peripheral nerve sheath tumors.9 Clinical trials are currently underway to evaluate the effectiveness of using immunomodulatory agents such as nivolumab, an anti–PD-1 monoclonal antibody, and ipilimumab, an anti–CTLA-4 monoclonal antibody, for the treatment of advanced STS.10
We report this case to alert clinicians to the aggressive nature of MPNST, which can rarely metastasize to the skin. Because these metastases are so unusual, there is minimal guidance on optimal management. The failure of intralesional methotrexate to effectively palliate this patient's cutaneous MPNST metastases suggests that this drug may not be effective at slowing the progression of skin lesions.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Thway K., Fisher C. Malignant peripheral nerve sheath tumor: pathology and genetics. Ann Diagn Pathol. 2014;18:109–116. doi: 10.1016/j.anndiagpath.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Saeed S., Keehn C.A., Morgan M.B. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31(6):419–430. doi: 10.1111/j.0303-6987.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda A., Kusuzaki K., Hirata H. Metastasis of malignant peripheral nerve sheath tumor to free vascularized myocutaneous flap. Oncol Rep. 2005;13(2):295–297. [PubMed] [Google Scholar]
- 4.Wang W.L., Bones-Valentin R.A., Prieto V.G., Pollock R.E., Lev D.C., Lazar A.J. Sarcoma metastases to the skin: a clinicopathologic study of 65 patients. Cancer. 2012;118(11):2900. doi: 10.1002/cncr.26590. [DOI] [PubMed] [Google Scholar]
- 5.Constantinidou A., Jones R.L., Scurr M., Al-Muderis O., Judson I. Advanced aggressive fibromatosis: effective palliation with chemotherapy. Acta Oncol. 2011;50(3):455. doi: 10.3109/0284186X.2010.509105. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg S.A., Tepper J., Glatstein E. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196(3):305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan H.G. Vemurafenib treatment of BRAF V600E-mutated malignant peripheral nerve sheath tumor. J Natl Compr Canc Ne. 2013;11(12):1466–1470. doi: 10.6004/jnccn.2013.0173. [DOI] [PubMed] [Google Scholar]
- 8.Haworth K.B., Arnold M.A., Pierson C.R. Immune profiling of NF1-associated tumors reveals histologic subtype distinctions and heterogeneity: implications for immunotherapy. Oncotarget. 2017;8(47):82037–82048. doi: 10.18632/oncotarget.18301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shurell E., Singh A.S., Crompton J.G. Characterizing the immune microenvironment of malignant peripheral nerve sheath tumor by PD-L1 expression and presence of CD8+ tumor infiltrating lymphocytes. Oncotarget. 2016;7(39):64300–64308. doi: 10.18632/oncotarget.11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Angelo S.P., Mahoney M.R., Van Tine B.A. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomized, phase 2 trials. Lancet Oncol. 2018 doi: 10.1016/S1470-2045(18)30006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]