Abstract
Current treatment strategies provide minimal results for patients with castration-resistant prostate cancer (CRPC). Attempts to target the androgen receptor have shown promise, but resistance ultimately develops, often due to androgen receptor reactivation. Understanding mechanisms of resistance, including androgen receptor reactivation, is crucial for development of more efficacious CRPC therapies. Here, we report that the RON receptor tyrosine kinase is highly expressed in the majority of human hormone-refractory prostate cancers. Further, we show that exogenous expression of RON in human and murine prostate cancer cells circumvents sensitivity to androgen deprivation and promotes prostate cancer cell growth in both in vivo and in vitro settings. Conversely, RON loss induces sensitivity of CRPC cells to androgen deprivation. Mechanistically, we demonstrate that RON overexpression leads to activation of multiple oncogenic transcription factors (namely, β-catenin and NF-κB), which are sufficient to drive androgen receptor nuclear localization and activation of AR responsive genes under conditions of androgen deprivation and support castration-resistant growth. In total, this study demonstrates the functional significance of RON during prostate cancer progression and provides a strong rationale for targeting RON signaling in prostate cancer as a means to limit resistance to androgen deprivation therapy.
Abbreviations: CRPC, castration-resistant prostate cancer; AR, androgen receptor; ADT, androgen deprivation therapy; RTK, receptor tyrosine kinase; COPA, cancer outlier profile analysis; OE, overexpressing; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling; CSS, charcoal-stripped serum
Introduction
In spite of recent advances in the knowledge and understanding of prostate cancer, effective therapeutic strategies for men with aggressive castration-resistant prostate cancer (CRPC) remain elusive, resulting in an estimated 26,730 deaths in the United States in 2017 [1]. Men diagnosed early with localized prostate cancer have positive clinical responses when treated with surgery and radiation; however, a large population of men either experience recurrence following surgery or are diagnosed with metastatic disease. A primary driver of prostate cancer is the androgen receptor (AR), and for men with recurrent and/or metastatic disease, the treatment is often androgen deprivation therapy (ADT). ADT serves as a form of chemical castration to limit the supply of androgens available for activation of AR signaling. The timeframe in which ADT is effective varies between patients; however, once resistance develops (termed CRPC), the outlook is poor and median survival is only ~19 months [2]. The advent of new compounds targeting AR signaling in CRPC, such as abiraterone or enzalutamide, has provided a slight increase in overall survival; however, resistance inevitably develops and leads to death of the patient [3], [4]. New approaches are needed for treating men with prostate cancer at this stage of disease, where the majority of deaths from prostate cancer occur and currently all available therapeutic options have been ineffective at providing long-term benefits to patients.
Numerous reports have cited a crucial role for receptor tyrosine kinases (RTKs) in promoting cancer, and subsequently the use of small molecule RTK inhibitors has been proposed as a novel treatment for men with CRPC [5]. An example of this is cabozantinib, which was shown to reduce the progression of some pathological variables in select CRPC patients but was unable to significantly improve overall survival [5]. An explanation for this lack of efficacy is that many RTK inhibitors (like cabozantinib) have several targets, and this minimal specificity prevents effective doses to be reached due to unacceptable adverse events in the patient [5]. By understanding the importance of the various targets of such inhibitors, we have the capability to formulate compounds with enhanced specificity for the critical players that promote CRPC, which can in return provide more promising clinical outcomes.
An emerging target of several RTK inhibitors is the RON receptor tyrosine kinase/macrophage stimulating 1 receptor (MST1R). RON is a cell surface RTK that is primarily expressed on epithelial cells and select macrophage populations, where activation of RON will function to reduce inflammation and promote wound healing [6], [7], [8], [9], [10], [11]. Overexpression of RON has been observed in a number of solid cancers, and within cancer, RON promotes phenotypes such as survival, proliferation, migration/invasion, angiogenesis, and stemness [12], [13], [14], [15]. Specifically, in prostate cancer, previous studies have established the RON receptor as critical for cancer development and progression [6], [12], [16], [17]. Selective overexpression of RON in the prostate epithelium of mice induces prostate intraepithelial neoplasia with invasion or adenocarcinoma in the majority of animals, indicating RON as sufficient to drive prostate cancer [16]. The necessity of RON signaling for prostate cancer growth was determined through genetic loss of RON signaling in the probasin driven T-antigen (PB-TAg/TRAMP) murine model of prostate cancer, where mice deficient in RON signaling developed significantly smaller prostate tumors compared to control mice [13], [17]. Furthermore, plasma levels of the ligand for RON (hepatocyte growth factor-like protein/macrophage stimulating protein) positively correlate with prostate cancer progression in patients and were observed to be significantly elevated in CRPC patients [18]. Mechanistically, our laboratory has connected RON to activation of several oncogenic signaling pathways in prostate cancer, such as NF-κB/RELA, STAT3, and BCL-2 signaling [12], [13], [14], [19]. Despite the strides made in understanding the function of RON in prostate cancer, research regarding its role in promoting CRPC has been minimal but is of the utmost importance due to the high number of deaths each year resulting from this form of disease.
In this report, we demonstrate that the RON receptor is highly expressed in CRPC. We have investigated the consequences of RON expression for prostate cancer growth in vivo in response to castration using murine transplantation models and in vitro in response to androgen deprivation using sphere forming assays. Strikingly, we show that RON expression is sufficient for prostate tumor growth following androgen withdrawal. Notably, we provide direct evidence of AR activation mediated through the autonomous stimulation of two RON-dependent transcriptional effectors, β-catenin and NF-κB, highlighting the impact of RON signaling as a mechanism of resistance to ADT. The conclusions of this study establish the importance of RON signaling in CRPC and provide strong rationale for the development of specific inhibitors targeting the RON receptor signaling pathway as treatment for this devastating disease.
Results
RON Expression Is Elevated in CRPC Patient Samples and Is Critical for Tumors to Develop Castration Resistance
RON receptor signaling has been established as a central factor in promoting prostate cancer in several murine models [6], [12], [13], [16], [17]. To assess the significance of RON expression in human prostate cancer, multiple human data sets were analyzed using the Cancer Outlier Profile Analysis (COPA) method [20], [21], [22], [23], [24], [25], [26], [27]. COPA identifies novel oncogenic drivers by taking into account the heterogeneity of tumors through assessing overexpression in subsets of cancers, as opposed to performing a simple t test over an entire class of cancer [28]. Analysis indicates that RON has comparable COPA scores to the prominent oncogene ERG in prostate cancer, highlighting the potential importance of RON in this disease (Table 1).
Table 1.
COPA Analysis for RON and ERG in Prostate Cancer Datasets
| RON | ERG | |||||
|---|---|---|---|---|---|---|
| COPA score | Rank | % | COPA score | Rank | % | Data set |
| 3.801 | 5% | 95 | 3.781 | 5% | 95 | Barwick |
| 13.789 | 6% | 75 | 4.3 | 8% | 75 | Welsh |
| 8.972 | 4% | 95 | 6.894 | 9% | 95 | Varambally |
| 1.94 | 6% | 75 | 1.727 | 9% | 75 | Chandran |
| 1.282 | 10% | 75 | 3.343 | 1% | 75 | LaPointe |
| 7.73 | 10% | 95 | NA | NA | NA | Demichelis |
| NA | NA | NA | 76.976 | 3% | 95 | Magee |
| 3.322 | 10% | 90 | 5.472 | 1% | 90 | Grasso |
COPA identified RON and ERG as significantly overexpressed in a subset of tumors from the Oncomine 4.4 database (threshold by: top 10%, fold change >2 and P < 1E-4). RON was shown to have a higher COPA score than ERG in four of the six databases in which RON and ERG were both identified. NA, not applicable. Rank is relative to other outlier genes identified in the data set. % is the percentile for gene expression intensity within the dataset.
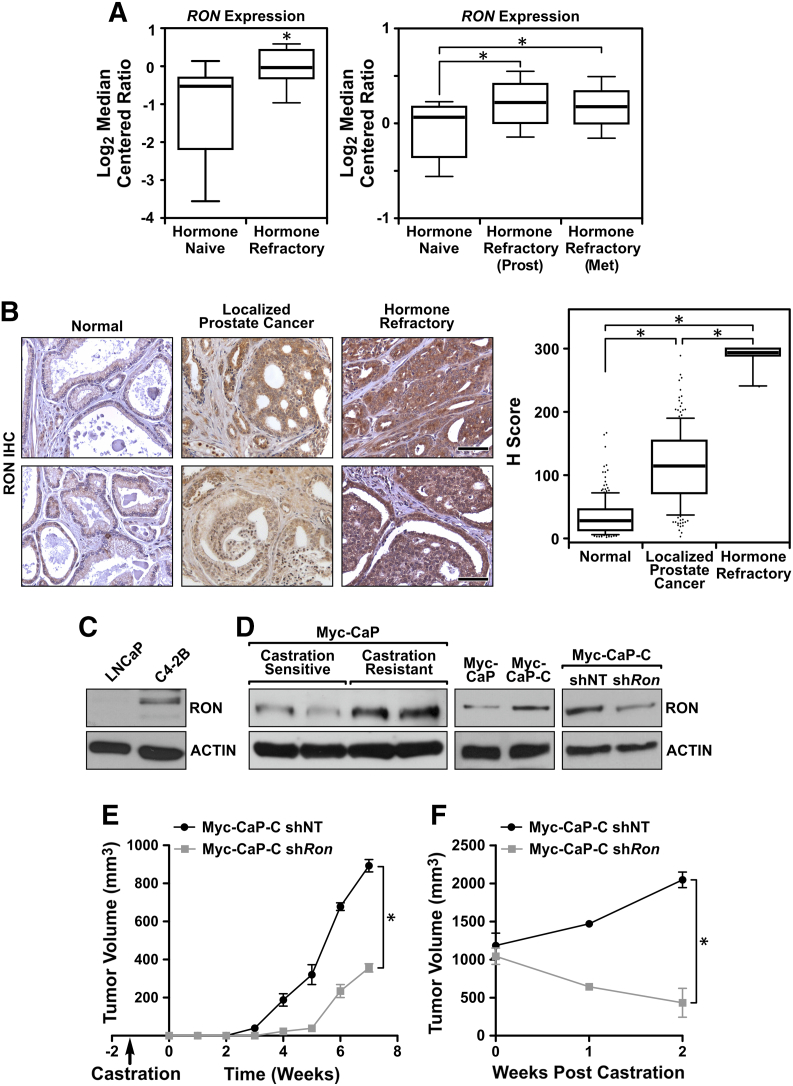
When assessing the expression of RON in human CRPC, data sets comparing hormone-naive versus hormone-refractory prostate cancers were analyzed. RON expression was found to be significantly higher in hormone-refractory samples relative to hormone-naive samples, and this was true whether the hormone-refractory tissue was from the prostate or isolated from a metastatic site (Figure 1A) [29], [30]. To examine whether RON mRNA expression correlated with increased protein expression, RON immunohistochemistry was performed on human prostate tissue microarrays, and expression levels were compared across normal, localized, and hormone-refractory samples. Previous reports have shown RON protein expression is elevated during prostate tumorigenesis, although it has not been reported whether or not RON protein expression changes in response to hormone therapy [12]. In support of previous studies, RON expression was elevated in localized prostate cancer tissue relative to normal tissue (Figure 1B). Strikingly, however, RON expression was drastically higher in hormone-refractory patient samples than both normal and localized samples, suggesting that RON may play a causal role in hormone-refractory disease (Figure 1B).
Figure 1.
RON expression is elevated in CRPC patient samples and critical for tumors to develop castration resistance. (A) Boxplot depicting relative RON expression from the Best (left) and Tamura (right) prostate cancer datasets. Boxplot lists 25th and 75th percentiles along with the group median. Whiskers display 10th and 90th percentiles. (B) Representative images of normal matched (n = 206), hormone-naive (n = 186), and hormone-resistant (n = 20) patient prostate tissues stained for RON, along with boxplot showing H scores of RON staining intensity. Boxplot lists 25th and 75th percentiles along with the group median. Whiskers display 10th and 90th percentiles. Scale bar = 50 μm. (C) Western blot of human LNCaP and C4-2B cell lysates depicting RON expression levels. (D) Western blot of lysates from Myc-CaP tumors following implantation into male FVB mice before (castration sensitive) and after the development of castration resistance, and Myc-CaP and Myc-CaP-C cells depicting RON expression levels following RON knockdown (shRon). (E) Average tumor volume formed from control Myc-CaP-C shNT (black line, n = 4) and Myc-CaP-C shRon (gray line, n = 4) cells grown in precastrated FVB mice. (F) Average tumor volume formed from Myc-CaP-C shNT (black line, n = 4) or Myc-CaP-C shRon (gray line, n = 4) cells following implantation into intact FVB mice and castration at 1000mm3. Data represent mean values ± SEM. *P < .05.
Several reports have established a role for RON in the development and growth of prostate cancer [6], [12], [13], [16], [17]. However, mechanisms linking RON to CRPC, which leads to the death of thousands of men annually, have not been investigated. To experimentally evaluate the requirement of RON in CRPC, multiple in vitro and in vivo CRPC models were assessed. Western blot analysis demonstrated that RON expression was elevated in the castration-resistant C4-2B cell line relative to the parental castration-sensitive human LNCaP cell line (Figure 1C) [31], [32]. To directly determine the requirement of RON in CRPC, intact FVB male mice were injected with Myc-CaP cells to establish androgen-sensitive Myc-CaP prostate tumors [33]. Mice bearing tumors of 1000 mm3 were castrated, and outgrowth of castration-resistant Myc-CaP tumors was monitored temporally. Figure 1D demonstrates that Myc-CaP tumors upregulate RON expression following the development of castration resistance in this model. Furthermore, Western blot analysis of a cell line derived from a castration-resistant tumor, termed Myc-CaP-C, showed increased RON expression relative to parental Myc-CaP cells, similar to what was observed with human cell lines (Figure 1C). Knockdown of RON in Myc-CaP-C cells (Figure 1D, Myc-CaP-C shRon) led to a delay in the growth of castration-resistant tumors following subcutaneous implantation into precastrated FVB mice (Figure 1E). Further, while the growth of Myc-CaP-C tumors was not sensitive to castration following implantation into intact FVB male mice, RON knockdown led to Myc-CaP-C tumor regression following castration (Figure 1F). Overall, these data demonstrate a causal function for RON in driving prostate tumor growth under conditions of androgen deprivation and suggest that RON may be a biomarker for aggressive prostate cancers.
RON Overexpression Mediates Castration-Resistant Growth In Vivo
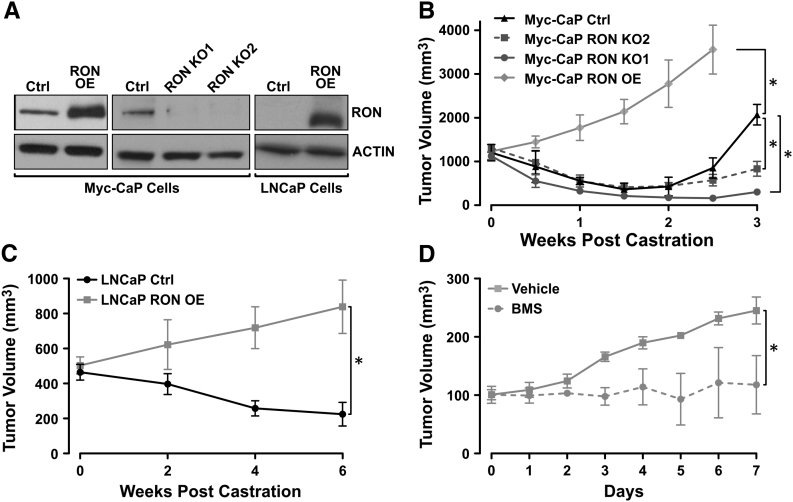
To investigate whether RON overexpression is sufficient to confer castration-resistant growth to Myc-CaP tumors in vivo, RON expression was modulated in Myc-CaP and LNCaP cells. Either RON was exogenously expressed (RON OE, Figure 2A), or CRISPR/Cas9 technology was used to delete RON in Myc-CaP cells (RON KO1 and RON KO2, Figure 2A). RON modulated and control cells were implanted subcutaneously into intact male FVB (for Myc-CaP cells) or immunodeficent (for LNCaP cells) mice, and once tumors were established, mice were surgically castrated and tumor growth was monitored. Strikingly, we observed that exogenous RON expression, in either murine or human prostate cancer cell lines, conferred castration-resistant growth to established tumors compared to control tumors which were sensitive to castration (Figure 2, B and C). Moreover, we found that genetic RON loss resulted in sustained sensitivity of established Myc-CaP prostate tumors to androgen deprivation for at least 3 weeks following castration compared to control cells which allowed androgen-independent growth of tumors during this same time frame (Figure 2B). Considering the importance of RON in CRPC, we next evaluated the effects of targeting RON under castration conditions utilizing Myc-CaP RON OE tumors implanted into precastrated FVB mice. Once tumors reached 100 mm3, mice were treated daily by oral gavage with either vehicle or BMS-777607, a small molecule inhibitor of RON/c-MET family receptor tyrosine kinases [34]. As shown in Figure 2D, pharmacologically inhibiting RON in Myc-CaP RON OE tumors with BMS-777607 blocked castration-resistant tumor growth. These data provide the first direct evidence that therapeutic targeting of RON restores sensitivity to castration in vivo.
Figure 2.
RON overexpression mediates castration-resistant growth in vivo. (A) Western blot of murine Myc-CaP cells depicting expression levels of RON in control (Ctrl), RON-overexpressing (RON OE) ,or CRISPR/Cas9 deleted RON (RON KO1 and RON KO2) and human LNCaP cell lysates depicting expression levels of RON in control (Ctrl) or RON-overexpressing (RON OE) cells. (B) Average subcutaneous tumor volume of Myc-CaP Ctrl (black line, n = 4), Myc-CaP RON OE (gray line, n = 8), Myc-CaP RON KO1 (dark gray line, n = 3), and Myc-CaP RON KO2 (dark gray dashes, n = 3) cells following castration at 1000 mm3 in FVB mice. (C) Average tumor volume of LNCaP Ctrl (black line, n = 3) and LNCaP RON OE (gray line, n = 4) cells following castration at 500 mm3 in athymic nude mice. (D) Average tumor volume of Myc-CaP RON OE cells grown in precastrated FVB mice and treated with DMSO (vehicle, gray line, n = 4) or 50 mg/kg/day BMS-777607 (BMS, gray dashes, n = 4) once castration-resistant tumors reached 100 mm3. Data represent mean values ± SEM. *P < .05.
RON Expression Mediates Castration-Resistant Growth In Vivo Through Oncogenic Signaling Pathways That Enhance Tumor Cell Proliferation and Reduce Apoptosis
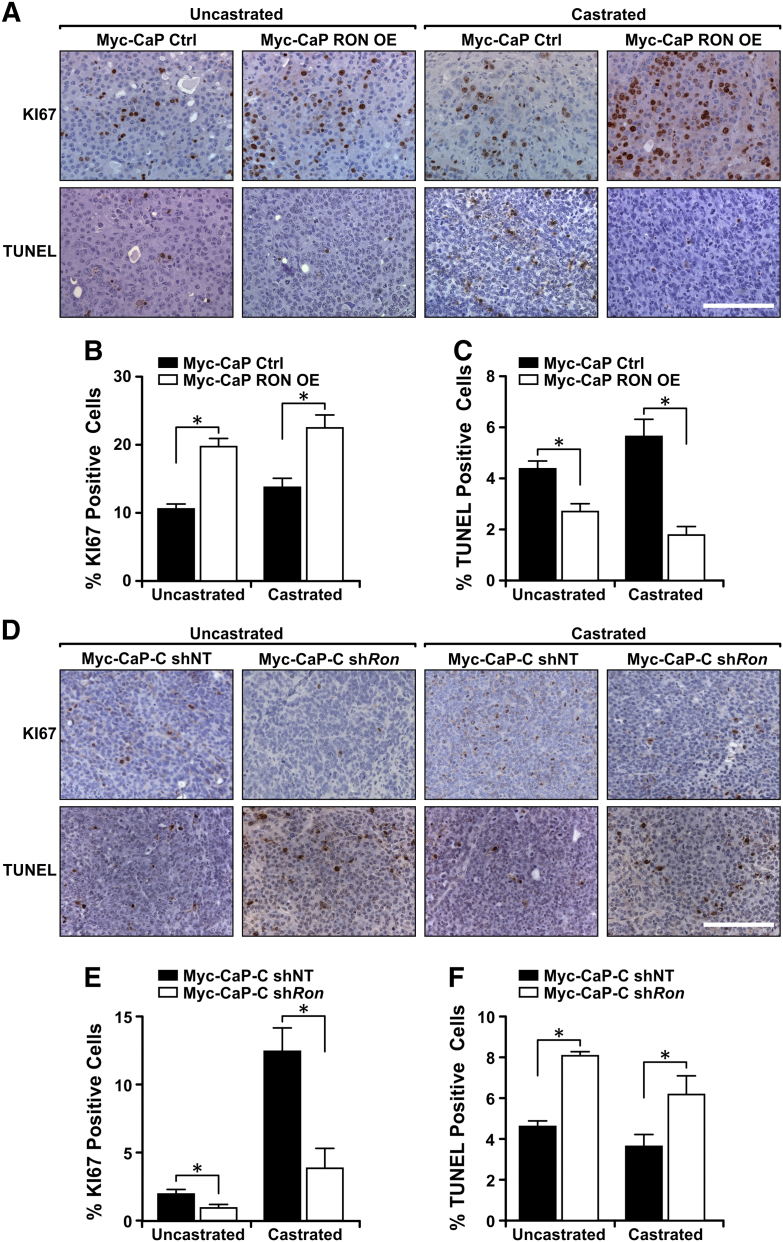
As RON expression levels correlate with the ability of tumors to grow in the context of castration, we next evaluated whether RON modulation would potentiate the effects of castration. As shown in Figure 3, Myc-CaP prostate tumors with RON expression displayed a higher proliferative index as seen by increased numbers of Ki67-positive cells. Further, RON expression was inversely correlated with tumor cell apoptosis as judged by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining. The most dramatic effects of RON modulation were observed under castration conditions, with a 3.2-fold decrease in apoptosis observed in Myc-CaP RON OE tumors and a 1.9-fold increase following RON knockdown in Myc-CaP-C tumors. Correspondingly, under castration conditions, there were a 1.7-fold increase in proliferation in Myc-CaP RON OE tumors and a 3.3-fold decrease in proliferation following RON knockdown in Myc-CaP-C tumors.
Figure 3.
RON expression mediates castration-resistant growth in vivo by enhancing tumor cell proliferation and reducing apoptosis. (A) Representative images of Myc-CaP Ctrl and Myc-CaP RON OE tumors from FVB mice, before and after castration, stained for KI67 and TUNEL. Scale bar = 50 μm. (B & C) Percentage of cells staining positively for KI67 (B) or TUNEL (C) in tumors from Myc-CaP Ctrl and Myc-CaP RON OE cells before and after castration (n = 3-5 independent tumor samples with quantification of at least 3 fields/sample). (D) Representative images of tumors from Myc-CaP-C shNT and Myc-CaP-C shRon cells before and after castration stained for KI67 and TUNEL. Scale bar = 50 μm. (E & F) Percentage of cells staining positively for KI67 (E) or TUNEL (F) in tumors from Myc-CaP-C shNT and Myc-CaP-C shRon cells before and after castration (n = 3-5 independent tumor samples with quantification of at least 3 fields/sample). Data represent mean values ± SEM. *P < .05.
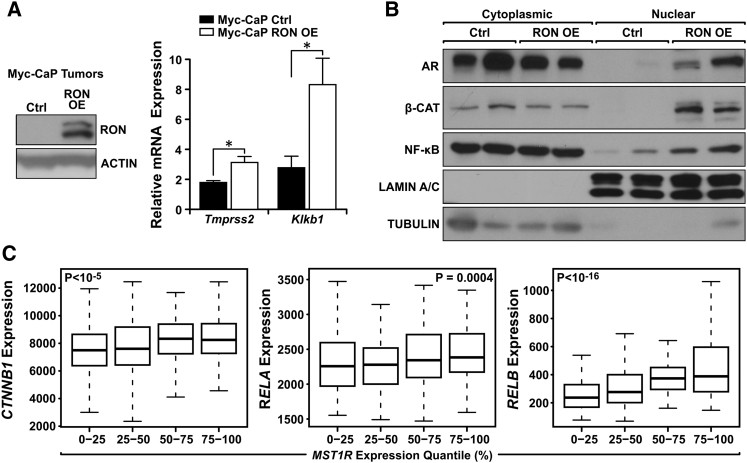
Having established a crucial role for RON expression in prostate cancer growth under castration conditions, we sought to identify the oncogenic signaling pathways mediated by RON (Figure 4). As persistence of AR signaling has been shown to be important in castration resistance, we determined whether RON expression influences AR signaling through the activation of reported AR target genes (Figure 4A) and AR nuclear localization (Figure 4B). The AR target genes Tmprss2 and Klkb1 were both expressed higher in Myc-CaP RON OE castrated tumors compared to Ctrl castrated tumors (Figure 4A). Correspondingly, exogenous RON expression also induced the nuclear localization of AR from Myc-CaP castrated tumors (Figure 4B). We further explored the possibility that RON-dependent signaling pathways may mediate AR activation under conditions of androgen deprivation. As shown in Figure 4B, RON expression facilitated the nuclear localization of two transcription factors, β-CATENIN and NF-κB, that have been associated with aggressive prostate cancers and activation of the AR [35], [36], [37], [38]. To substantiate data from our murine CRPC model, we queried patient samples from The Cancer Genome Atlas database and found a positive correlation between gene expression levels of RON (MST1R) with both β-CATENIN (CTNNB1) and NF-κB (RELA/RELB) (Figure 4C). Collectively, the data suggest that RON-mediated activation of these transcription factors may be responsible for castration-resistant tumor growth.
Figure 4.
RON expression mediates castration-resistant growth in vivo through oncogenic AR, β-CATENIN, and NF-κB. (A) Western blot analysis and qRT-PCR of tumor samples from Myc-CaP Ctrl and Myc-CaP RON OE cells depicting RON protein levels and AR target gene expression of Tmprss2 and Klkb1. Data represent mean values from three independent experiments ± SEM, *P < .05. (B) Western blot analysis of tumors from Myc-CaP Ctrl and Myc-CaP RON OE cells following separation into cytoplasmic and nuclear fractions depicting nuclear localization of the AR, β-CATENIN, and NF-κB. TUBULIN (cytoplasmic) and LAMIN A/C (nuclear) are shown as loading controls. Each lane represents an independent tumor sample. (C) mRNA expression levels of RELA, RELB, and CTNNB1 (β-CATENIN) in different quantiles of MST1R (RON) expression in primary prostate adenocarcinoma samples (from The Cancer Genome Atlas). The P values of correlation (Spearman's rank sum) are indicated in the legend of each plot.
Activation of Oncogenic β-CATENIN, NF-κB, and AR Is Required for RON to Promote Growth Under Androgen Deprivation
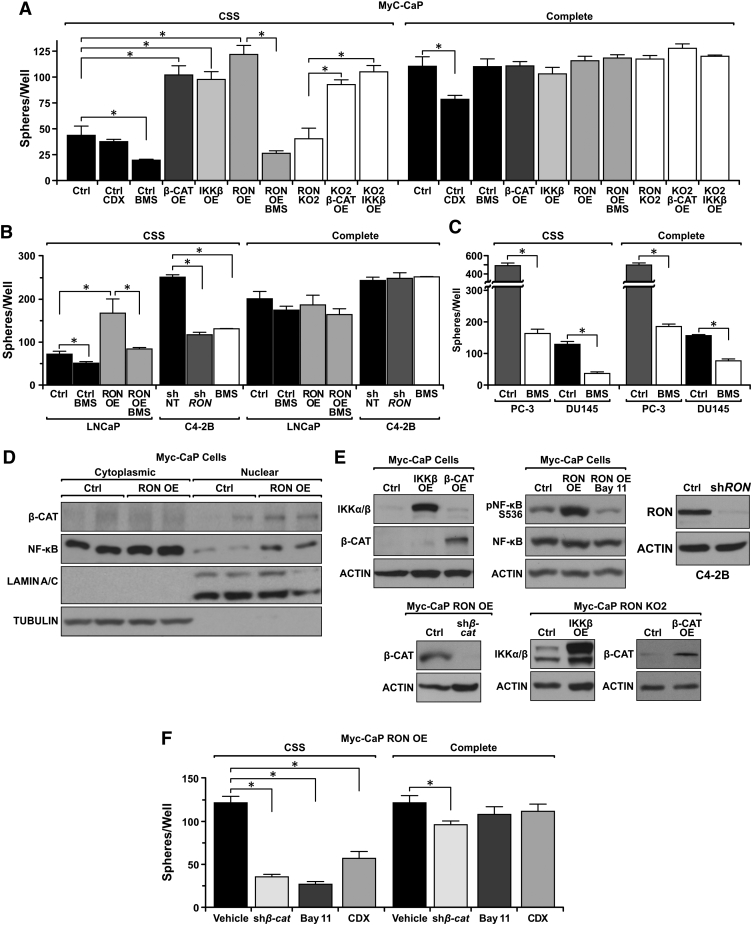
Given the elevated activation of β-CATENIN, NF-κB, and AR in response to RON overexpression, we tested whether RON-mediated activation of these transcription factors is necessary for castration-resistant growth using 3D cell culture assays. Cells were grown in 3D culture conditions under androgen deprived (charcoal-stripped serum, CSS) or in androgen containing (Complete) conditions for 10 days prior to assessing growth. Strikingly, Myc-CaP RON OE cells formed 2.8-fold more spheres compared to Myc-CaP Ctrl cells under conditions of androgen deprivation (Figure 5A). No difference in sphere formation was observed when these same cells were in androgen-containing media (Figure 5A). In addition to Myc-CaP cells, identical results were obtained following ectopic RON expression in the human LNCaP cells wherein RON expression led to significantly greater sphere formation compared to LNCaP Ctrl cells under conditions of androgen deprivation (Figure 5B). Correspondingly, RON knockdown in human C4-2B cells reduced sphere formation relative to control shNT C4-2B cells under androgen deprivation (Figure 5, B and E). These findings suggest that RON expression is essential for sphere formation selectively under conditions of androgen deprivation and provide further rationale for therapeutic targeting of RON in CRPC. To more broadly understand the scope of therapeutic targeting of RON, several androgen-responsive and non–androgen-responsive cell lines were treated with the RON/c-MET family receptor tyrosine kinase inhibitor BMS-777607 under androgen-containing and androgen-depleted conditions. Strikingly, we observed that AR-expressing cell lines (Myc-CaP, Myc-CaP RON OE, LNCaP, LNCaP RON OE, and C4-2B) were only sensitive to the RON inhibitor under androgen-depleted conditions (Figure 5, A and B). Alternatively, cell lines without AR expression (PC-3, DU145) were sensitive to the inhibitor under both androgen-containing and androgen-depleted conditions (Figure 5C). These findings suggest that RON inhibition is a viable therapeutic option for patients with CRPC that is both AR+ and AR−; however, in AR+ patients, RON inhibition should be coupled with androgen deprivation therapy.
Figure 5.
Activation of oncogenic β-CATENIN, NF-κB, and AR is required for RON to promote growth under androgen deprivation. (A) Number of prostate cancer cell spheres formed per well (n = 3 per group) from Myc-CaP Ctrl, RON OE, β-CATENIN OE, IKKβ OE, RON KO2, RON KO2 β-CATENIN OE, and RON KO2 IKKβ OE cells grown in CSS or complete media for 10 days. CDX indicates cells treated Casodex; BMS indicates cells treated with BMS-777607. (B) Number of spheres formed per well (n = 3 per group) of LNCaP Ctrl, LNCaP RON OE, C4-2B shNT, and C4-2B shRON cells following 10 days growth in CSS or complete media. BMS indicates cells treated with BMS-777607. (C) Number of spheres formed per well (n = 3 per group) of PC-3 (treated with vehicle or BMS) and DU145 (treated with vehicle or BMS) cells following 10 days growth in CSS or complete media. (D) Western blot of Myc-CaP Ctrl and Myc-CaP RON OE cells separated into cytoplasmic and nuclear fractions depicting nuclear localization of the β-CATENIN and NF-κB. TUBULIN (cytoplasmic) and LAMIN A/C (nuclear) are shown as loading controls. (E) Western blot of Myc-CaP cells with ectopic IKKβ or β-CATENIN expression. Western blots of Myc-CaP RON KO2 cells depicting ectopic β-CATENIN and IKKβ expression. Western blot for s536 phosphorylated NF-κB and total NF-κB in Myc-CaP Ctrl and Myc-CaP RON OE cells treated with vehicle (DMSO) or Bay-11-7085 (Bay11) for 4 hours. Western blot depicting β-CATENIN knockdown in Myc-CaP RON OE shβ-Catenin cells relative to Myc-CaP RON OE cells. Western blot depicting RON expression levels following knockdown of RON in C4-2B cells. (F) Number of spheres formed per well (n = 3 per group) in Myc-CaP RON OE cells treated with vehicle (DMSO), Bay11-7085 (Bay11), Casodex (CDX), or following knockdown of β-CATENIN (shβ-cat) and Myc-CaP Ctrl cells treated with vehicle (DMSO) or CDX following 10 days growth in CSS or complete media. Data represent mean values from three independent experiments ± SEM, *P < .05.
As our data demonstrate that RON expression promotes AR nuclear localization and the expression of AR target genes under conditions of androgen deprivation (Figure 4), we next sought to examine the requirement of β-CATENIN and NF-κB activation downstream of RON for androgen-independent prostate cancer growth. Similar to tumors, Myc-CaP RON OE cells also exhibited increased nuclear localization of β-CATENIN and NF-κB (Figure 5D). To examine the requirement of β-CATENIN and NF-κB, we ectopically expressed either IKKβ, for activation of NF-κB, or β-CATENIN (Figure 5E) in Myc-CaP cells. Clonal pools of expressing prostate cancer cell lines were then examined for 3D growth under conditions of androgen deprivation. Interestingly, both β-CATENIN and IKKβ expression promoted sphere formation to levels comparable to RON expression in media devoid of androgens (Figure 5A). No changes in sphere formation were observed when the same cells were cultured in complete media (Figure 5A), demonstrating the selectivity of tumor growth for these transcription factors under conditions of androgen deprivation. Having established that RON expression induces nuclear localization of β-CATENIN and NF-κB, we next sought to test whether IKKβ and/or β-CATENIN expression was sufficient to induce prostate cancer cell sphere formation under androgen deprivation. For these studies, Myc-CaP RON KO2 cells were generated which ectopically expressed IKKβ or β-CATENIN (Figure 5E). Overexpression of either protein augmented sphere formation in the RON-deficient cell line compared to control cells under androgen-deprived conditions (Figure 5A). Interestingly, knockout of RON did not reduce sphere formation under androgen-deprived conditions relative to Myc-CaP control cells (Figure 5A).
To further validate β-CATENIN and NF-κB as crucial RON-dependent downstream mediators of AR activation and prostate cancer cell sphere formation under conditions of androgen deprivation, Myc-CaP RON OE cells either had β-CATENIN knocked down or were treated with the NF-κB signaling inhibitor Bay-11-7085 [39]. Remarkably, either β-CATENIN knockdown or NF-κB inhibition significantly reduced sphere formation in Myc-CaP RON OE cells under androgen deprivation conditions (Figure 5F). While no changes were observed in sphere formation in Myc-CaP RON OE cells treated with Bay-11-7085 in complete media (Figure 5F), knockdown of β-CATENIN in Myc-CaP RON OE cells led to a reduction in sphere formation when the cells were cultured in complete media. However, the reduction in sphere formation in complete media was modest (1.3-fold) compared to the large reduction (3.5-fold) observed with androgen depletion (Figure 5F), suggesting the importance of β-CATENIN signaling for prostate cancer growth in both the presence and absence of androgens.
Furthermore, we confirmed that Myc-CaP RON OE cells require AR activation to promote sphere formation under androgen-deprived conditions by treating Myc-CaP RON OE cells with Casodex (CDX) (a well-established AR inhibitor [40]). Treatment of parental Myc-CaP cells with casodex reduced sphere formation under both androgen-deprived and androgen-containing conditions; however, sphere formation for Myc-CaP RON OE cells was only reduced under androgen-deprived conditions (Figure 5, A and F). This information suggests that RON expression is able to bypass the need for ligand-dependent AR activation but that the AR is required downstream of RON for prostate cancer cell growth during androgen deprivation. Moreover, this advocates that early treatment of RON-expressing tumors with combined androgen blockade and RON inhibition may be a successful therapeutic approach.
β-CATENIN and NF-κB Independently Induce AR Activation to Promote Growth Under Androgen Deprivation In Vitro
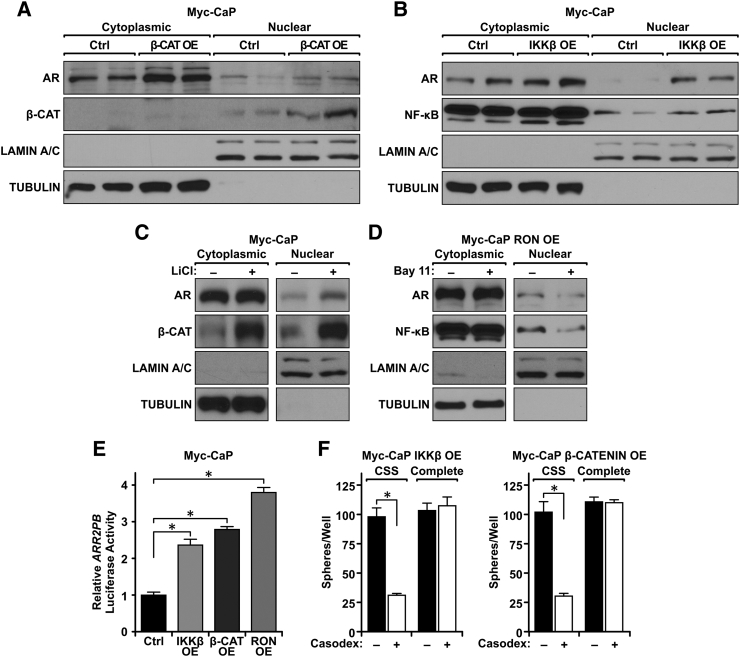
We found activation of β-CATENIN and NF-κB to be crucial for RON to promote sphere formation under androgen deprivation (Figure 5). We next investigated whether these transcription factors are capable of activating AR during androgen withdrawal. To assess AR activation, Myc-CaP β-CATENIN OE and Myc-CaP IKKβ OE cells were grown in CSS and subsequently fractionated into nuclear and cytoplasmic fractions. As depicted in Figure 6A, ectopic β-CATENIN expression led to increased accumulation of nuclear β-CATENIN as well as induced the nuclear localization of AR. Correspondingly, ectopic IKKβ expression induced both NF-κB and AR nuclear localization (Figure 6B). These findings indicate that both β-CATENIN and NF-κB induce AR nuclear localization but may do so independently of one another. Consistent results were obtained when lithium chloride was used to activate β-CATENIN in Myc-CaP Ctrl cells and when Bay-11-7085 was used to inhibit NF-κB pharmacologically in Myc-CaP RON OE cells. Lithium chloride strongly induced both β-CATENIN and AR nuclear localization in Myc-CaP Ctrl cells (Figure 6C), while Bay-11-7085 treatment reduced AR and NF-κB nuclear localization in Myc-CaP RON OE cells (Figure 6D). In addition to nuclear localization, AR reporter activity was elevated upon β-CATENIN OE, IKKβ OE, and RON OE compared to Myc-CaP Ctrl cells as demonstrated by increased expression of the ARR2PB luciferase reporter construct which contains two androgen response elements (Figure 6E). Lastly, to determine if AR activation was required for β-CATENIN or NF-κB to promote growth under androgen deprivation, we treated Myc-CaP β-CATENIN OE and Myc-CaP IKKβ OE cells with casodex and performed an in vitro 3D growth assay under androgen-deprived and androgen-containing conditions. Figure 6F demonstrates that β-CATENIN OE and IKKβ OE prostate cancer cells exhibited a significant reduction in 3D sphere formation under conditions of androgen deprivation when treated with Casodex compared to vehicle-treated controls (Figure 6F). The cell lines exhibited similar sphere formation in complete media regardless of treatment with Casodex. These data illustrate the necessity of β-CATENIN and NF-κB activation to induce AR reactivation during androgen-independent growth. Taken together, these data demonstrate that both β-CATENIN and NF-κB function downstream of RON as a means to promote AR reactivation in times of androgen deprivation for sustained prostate cancer growth.
Figure 6.
β-CATENIN and NF-κB independently induce AR activation to promote growth under androgen deprivation in vitro. (A) Western blot of Myc-CaP Ctrl and Myc-CaP β-CATENIN OE cells from cytoplasmic and nuclear fractions depicting nuclear localization of the AR and β-CATENIN. TUBULIN (cytoplasmic) and LAMIN A/C (nuclear) are shown as loading controls for each fraction. (B) Western blot of Myc-CaP Ctrl and Myc-CaP IKKβ OE cells from cytoplasmic and nuclear fractions depicting nuclear localization of the AR and NF-κB. TUBULIN (cytoplasmic) and LAMIN A/C (nuclear) are shown as loading controls for each fraction. (C) Western blot of Myc-CaP Ctrl cells treated with DMSO (−) or LiCl (+, 4 hours) and fractionated into cytoplasmic and nuclear fractions depicting nuclear localization of the AR and β-CATENIN. TUBULIN (cytoplasmic) and LAMIN A/C (nuclear) are shown as loading controls for each fraction. (D) Western blot of Myc-CaP RON OE cells treated with DMSO (−) or Bay-11-7085 (Bay 11, +, 4 hours) and separated into cytoplasmic and nuclear fractions depicting nuclear localization of the AR and NF-κB. TUBULIN (cytoplasmic) and LAMIN A/C (nuclear) are shown as loading controls for each fraction. (E) Relative luciferase activity normalized to Renilla luciferase for Myc-CaP Ctrl cells, Myc-CaP β-CATENIN OE cells, Myc-CaP IKKβ OE cells, and Myc-CaP RON OE cells following 48 hours growth in CSS (n = 3 per group). (F) Number of spheres formed per well (3 per group) of Myc-CaP β-CATENIN OE and Myc-CaP IKKβ OE cells treated with vehicle (−) or Casodex (+) grown in CSS or complete media. Data represent mean values from three independent experiments ± SEM, performed with three technical replicates, *P < .05.
Discussion
CRPC is a devastating disease with limited treatment options. Our data reveal the RON receptor signaling pathway as a novel target for the treatment of CRPC. We and others have recently reported that RON protein levels are elevated in CRPC cell lines and that RON mRNA expression is elevated in castration-resistant human prostate tumors [12], [41]. Consistent with these reports, we observed that RON protein levels are greatly elevated in hormone-refractory prostate cancer patient samples compared to hormone-naive samples and that this expression is critical for castration-resistant tumors to maintain resistance (Figure 1). Not only is RON expression vital for tumors that are castration resistant, but we show that RON overexpression can drive resistance to castration therapy (Figure 2). These studies are the first to show elevated RON protein levels in hormone-refractory patient samples and to demonstrate the in vivo significance of RON in supporting CRPC. These findings build on prior studies showing that RON is capable of promoting mechanical changes in prostate cancer cells which may be advantageous for survival under androgen-deprived conditions [41]). Based on multiple reports demonstrating the significance of RON in prostate cancer, we postulate that elevated RON levels may serve as a novel biomarker for aggressive prostate cancers, which may require more aggressive treatment strategies.
We further showed that RON-expressing prostate tumors exhibit enhanced tumor cell proliferation and decreased cell death both prior to and following castration conditions in vivo (Figure 3), with the most profound changes occurring postcastration. Mechanistically, we found that, under castration conditions, RON-overexpressing Myc-CaP cells formed tumors with increased activation of AR, β-CATENIN, and NF-κB based on elevated nuclear localization compared to control Myc-CaP cells (Figure 4). Consistent with AR activation, an increase in AR target gene expression was observed (Figure 4). Interestingly, a previous report showed that the relationship between RON and AR may vary between activating or inhibitory, although the exact details for each scenario remained unclear [41]. Our data show that, under castrate conditions, RON has an activating function on AR. Identifying this connection is intriguing because although several therapeutics have been used to limit AR activation in prostate cancer, the ability of providing sustained AR inhibition has remained elusive [42]. Some therapies, such as abiraterone acetate, orteronel, and galeterone, have been used to reduce systemic androgen production to limit AR activation but have yet to provide sustained results [42]. In combination with traditional ADT, these therapies help limit ligand-mediated AR activity but fail at preventing ligand-independent AR activation. Our data suggest that overexpression of the RON receptor may function as a major alternative ligand-independent pathway used by aggressive prostate cancers to activate the AR. This makes targeting the RON receptor signaling pathway a strong candidate for reducing the activation of the AR and eliminating the growth of CRPC.
Previous studies have detailed an association for both β-CATENIN and NF-κB activation with poor clinical prognosis in prostate cancer patients [35], [36]; however, the studies herein discover dual activation of these two transcription factors downstream of RON in the context of CRPC (Figure 5). This information makes targeting RON an appealing approach because targeting this single pathway may have broad negative consequences for tumor progression. Prior work from our group in breast cancer illustrated a similar relationship, with RON activation leading to β-CATENIN and NF-κB activation. However, in breast cancer, a linear relationship was observed, with RON activation of NF-κB being dependent on β-CATENIN [14]. It is interesting to note that, in CRPC, we observed independent activation of β-CATENIN and NF-κB downstream of RON under conditions of androgen deprivation, suggesting that this relationship may be a product of cancer cells being under intense stress brought on by androgen withdrawal. The independent activation of these transcription factors through RON may function as a survival mechanism for cancer, and it would be interesting to test whether or not this mechanism exists in other cancers following different stress conditions, such as after chemotherapy treatment or in conditions of hypoxia. This concept is substantiated by other studies in breast cancer wherein RON expression was shown to be a predictor of recurrent disease [43], [44], [45]. The broad potential of RON as a driver of resistance and recurrence makes targeting RON a viable option for treating aggressive cancers, potentially of many different types, that have risen up following treatment.
Consistent with published studies, we also found that activation of either β-CATENIN or NF-κB leads to enhanced AR activation observed by an increase in AR nuclear localization and ARR2PB promoter luciferase activity (Figure 6) [37], [38]. Further, we observed that RON-dependent activation of either β-CATENIN or NF-κB requires the AR to promote sphere formation under androgen deprived conditions (Figure 6). This is consistent with our experiments showing that RON overexpression is unable to overcome inhibition of the AR under androgen deprivation in vitro, suggesting that RON functions upstream of β-CATENIN, NF-κB, and the AR (Figure 5). Interestingly, others have shown β-CATENIN and AR nuclear localization to be significantly correlated in human CRPC but not in hormone-naive prostate cancer [46]. In another study examining NF-κB and the AR, a strong positive correlation between NF-κB and AR protein expression was observed in human CRPC samples [37]. These prior studies are consistent with our data wherein RON expression is elevated in CRPC samples relative to hormone-naive prostate cancer samples, indicating that an increase in RON expression may be driving the correlations between β-CATENIN, NF-κB, and AR. Identifying an activating role for RON upstream of AR was informative as a previous report showed that RON may inhibit AR signaling in the AR-expressing C4-2B cell line when androgens are present [41]. Our data build upon this report and show that, in AR-expressing cells, under conditions of androgen deprivation, RON functions to drive castration resistance and is associated with AR activation. This information is significant as a large number of patients undergo ADT for the treatment of CRPC. Additionally, we have determined that prostate cancer cells which have adapted to grow in the absence of AR expression still have a requirement for RON overexpression to promote growth. This is illustrated in Figure 5 wherein the growth of AR-expressing cells was reduced by RON inhibition only under androgen-depleted conditions, while in AR-negative cell lines, prostate tumor cell growth was inhibited under both androgen-containing and -depleted conditions. We hypothesize this is due to a reprogramming of cells to more heavily rely on other growth pathways activated by RON, like NF-κB, β-Catenin, STAT3, or AKT, as a response to the loss of AR. Determining which signal transduction pathways are necessary for RON to promote growth when AR expression is lost is an area that requires further study. Together, these reports show that RON plays a critical role in promoting growth of AR expressing and AR-negative cells and further establishes RON as a viable target for men with aggressive prostate cancer.
Further work is needed to establish the mechanism(s) by which RON induces β-CATENIN and NF-κB activation and to uncover how these two proteins facilitate AR activation. Prior work from our group has shown that, in breast cancer, RON induces tyrosine phosphorylation of β-CATENIN at residues 654 and 670, facilitating its activation [19]. Additional studies will be needed to confirm if this relationship exists in the context of CRPC. In regards to NF-κB, RON activation may facilitate activation of upstream members of the NF-κB pathway, such as IKKα/β and/or NIK kinase, as these proteins have been shown by others to be activated by receptor tyrosine kinases [47]. Lastly, others have shown that there are multiple mechanisms possible for β-CATENIN and NF-κB to induce AR activation. For example, one group demonstrated that β-CATENIN can interact with the ligand binding domain of the AR to promote AR transcriptional activity [38], [48]. Regarding NF-κB, others have shown that expression of NF-κB can function to maintain AR expression levels and promote AR transactivation activity [37]. Whether or not these mechanisms are operant downstream of RON activation remain unclear.
In summary, our laboratory and others have demonstrated that RON is a key player in the growth and progression of prostate cancer. Here we show that RON overexpression is sufficient to drive resistance to castration therapy in multiple mouse models and uncovered a crucial role for β-CATENIN, NF-κB, and the AR in this process. This broad activation of several important oncogenic growth pathways makes RON an attractive target for CRPC therapy as opposed to targeting a single pathway downstream; targeting RON will prevent tumors from sustaining growth due to activation of remaining oncogenic pathways. This novel therapeutic approach should be further studied as it may help provide hope to CRPC patients currently without effective therapeutic options.
Materials and Methods
Immunohistochemistry
RON immunohistochemistry was performed on human prostate cancer tissue microarrays from the Prostate Cancer Biorepository Network. Tissue staining and scoring were performed as described [12], [15], [19]. Tumor tissue was fixed and paraffin embedded, and 5-μm sections were stained for Ki67 (ThermoFisher Scientific) and TUNEL (Millipore). Counts were performed using ImageJ software (National Institutes of Health).
Western Blot Analyses
Cells were homogenized in RIPA buffer as described [14]. Nuclear and cytoplasmic extracts were isolated by centrifugation and hypotonic lysis as described [19]. Antibodies for analyses included: RON (SC-322), ANDROGEN RECEPTOR (SC-815), and TUBULIN (SC-5286) from Santa Cruz Biotechnology; β-CATENIN (#9582S), phospho-NF-κB s536 p65 (#3033S), NF-κB (#8242S), IKKα (#2682S), IKKβ (#2678S), and LAMIN A/C (#4777S) from Cell Signaling Technologies; and ACTIN (Cincinnati Children's Hospital Medical Center, Clone C4). Peroxidase-conjugated secondary antibodies (Jackson Laboratories) were applied, and membranes were developed using Pierce ECL2 Western Blotting substrate (ThermoFisher Scientific). Membranes were stripped using Restore Western Blot Stripping Buffer (ThermoFisher Scientific) before reprobing.
Mouse Models
Mice were maintained under specific pathogen-free conditions and experimental protocols approved by the University of Cincinnati IACUC. For murine cell injections, 1.0 × 106 cells were injected subcutaneously into the flanks of 6- to 8-week-old male FVB mice as described [33], [49]. Tumor growth was measured via calipers, and volume was determined by the formula 0.5 × length × width2 [50]. Surgical castration was performed as described when tumors reached 1000 mm3 [33], [49]. Precastrated animals were surgically castrated at 6 weeks of age and allowed to recover for 10 days before injection of cells, as described previously [51]. For in vivo kinase inhibitor studies, precastrated mice were treated with 50 mg/kg/day BMS-777607 (Selleck Chemicals) or methocellulose (vehicle) via oral gavage once tumors reached 100 mm3. For human LNCaP and LNCaP RON OE cells, 5 × 106 cells were injected subcutaneously into athymic nude mice (NCr-Foxn1nu) from Charles Rivers; mice were castrated when tumors reached 500 mm3.
Cell Models
Myc-CaP cells [33] were developed in the laboratory of Charles Sawyers and were obtained from Memorial Sloan Kettering Cancer Center. For tumor formation, 1 × 106 Myc-CaP cells were injected subcutaneously into wild-type FVB male mice; once tumors reached 1000 mm3, the mouse was castrated. Myc-CaP-C cells were generated from a castration-resistant tumor formed following injection of Myc-CaP cells. All Myc-CaP cells were maintained in DMEM with 10% Cosmic Calf Serum and 1% gentamycin [33]. The human prostate cancer cell lines LNCaP and C4-2B were obtained from ATCC and were maintained in RPMI-1640 with 10% fetal bovine serum and 1% gentamycin. The human prostate cancer cell line PC-3 was obtained from ATCC and maintained in F-12 media with 10% fetal bovine serum and 1% gentamycin. The human prostate cancer cell line DU145 was obtained from ATCC and maintained in MEM with 10% fetal bovine serum and 1% gentamycin. All cells were maintained at 37 °C and 5.0% CO2.
Cell Transfections
Stable polyclonal cell lines were generated by performing transfection with Lipofectamine 2000 (ThermoFisher Scientific) and selection in puromycin (Invitrogen, 5 μg/ml) or G418 (Invitrogen, 500 μg/ml). LNCaP Ctrl and LNCaP RON OE cells overexpressing human RON were generated as described [12]. The Ron gene was deleted in Myc-CaP cells using CRISPR/Cas9 technology. RON knockout guide RNA was cloned into the PX458 plasmid (Addgene #48138) using the following primers: 5′-caccgCAGAGACTTGATGGCACAGT-3′ and 5′-aaacACTGTGCCATCAAGTCTCTGC-3′. Overexpression of β-CATENIN was performed using wild-type murine β-CATENIN cloned into the p3XFLAG-CMV vector as previously described [19]. Overexpression of IKKβ was performed using the pCDNA-IKK-β construct (Addgene, #23298). The ARR2PB luciferase construct was developed in the laboratory of Robert J. Matusik and was obtained from Vanderbilt University [52]. Luciferase assay was performed as previously described using the pRL-TK renilla plasmid as an internal control [12].
Viral Transduction
Lentivirus short hairpin RNA (Open Biosystems) was used to target murine shRon (RMM3981-9590952), human shRON (RHS3979-9571732), murine sh β-Catenin (RMN1766-96879831), and nonsense shNT (RHS1764). The pCDH backbone and pCDH-CMV-EF1-PURO-RON full-length mouse RON cDNA expression vectors were utilized for control and RON overexpression. Transduction was performed as described [12], [19].
In Vitro Cell Growth Assays and Treatments
Three-dimensional growth assays were performed as described with the substitution of agarose to prevent cell adhesion [13]. Briefly, 2 × 104 cells were plated on top of 1.0% agarose in 6-well plates in media supplemented with cosmic calf serum (Complete, Thermofisher Scientific) or CSS (Midsci). Cells were left untreated or treated with DMSO (vehicle), Bay-11-7085 (Enzo Life Sciences, 1 μM), Casodex (Selleck Chemicals, 10 μM), or BMS-777607 (Selleck Chemicals, 5 μM) daily. After 10 days, images of spheres were taken using a Zeiss Axiovert S100TV inverted microscope (Carl Zeiss Microscopy), and spheres >25 μm in diameter were counted using ImageJ software. The 25-μm threshold was established based on the average sphere size obtained for the control cells. For 2D growth, 2.5 × 104 cells were plated on 12-well plates and counted every 24 hours. Myc-CaP Ctrl or Myc-CaP Ron OE cells were grown in 2D and treated with DMSO (vehicle), LiCl (Sigma, 10 mM, 4 hours), or Bay-11-7085 (Bay 11, 5 μM, 4 hours) when cells were ~70% confluent prior to fractionation.
Quantitative Real-Time (qRT)-PCR
RNA was extracted with TRIzol (Invitrogen), and cDNA was prepared using the High-Capacity cDNA Reverse Transcriptase kit (Applied Biosystems). Quantitative PCR was performed with 2× SYBR Green Master Mix (Roche Diagnostics) on a Mastercycler ep realplex4 (Eppendorf). Data were normalized to an 18S reference gene and analyzed by ΔΔCT. Primer sequences included: Murine Tmprss2 (forward: 5′-AAGTCCTCAGGAGCACTGTGCA-3′; reverse: 5′-CAGAACCTCCAAAGCAAGACAGC-3′), murine Klkb1 (forward: 5′-AAAGTCAGCGGACAACCTGGTG-3′; reverse 5′-AGATGGTGCGACACACAAAGGC-3′), and 18S (forward: 5′-AGTCCCTGCCCTTTGTACACA-3′; reverse: 5′-GATCCGAGGGCCTCACTAAAC-3′).
Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM). Statistical significance was determined by performing Student's t test for pairwise comparisons or ANOVA for comparison of multiple groups using GraphPad Prism software. All in vitro experiments represent the average of at least triplicate experiments. Spearman's rank sum test was utilized to determine the P values of correlation for different quantiles of RON expression for data taken from the TGCA data base. All in vitro experiments represent the average of at least triplicate experiments. Significance was set at *P < .05.
Acknowledgements
We would like to thank Glenn Doermann for assistance with figure preparation.
Footnotes
Disclosure of potential conflicts of interest: The authors declare no conflicts of interest.
Funding: This work was supported by the United States Department of Veterans Affairs research grant 1IOBX000803 (S.E.W.); National Institutes of Health grants T32 CA117846 (S.E.W., N.E.B.), F31-CA200390 (N.E.B.), F31-CA165767 (A.M.P.), and CA125379 (S.E.W.); and Department of Defense Prostate Cancer Research Program Awards (W81XWH-10-2-0056 and W81XWH-10-2-0046) for the Prostate Cancer Biorepository Network.
References
- 1.American Cancer Society . American Cancer Society; Atlanta: 2017. Cancer Facts & Figures 2017. [Google Scholar]
- 2.Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., van der Kwast T., Mason M., Matveev V., Wiegel T., Zattoni F. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Scher H.I., Fizazi K., Saad F., Taplin M.E., Sternberg C.N., Miller K., de Wit R., Mulders P., Chi K.N., Shore N.D. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 4.de Bono J.S., Logothetis C.J., Molina A., Fizazi K., North S., Chu L., Chi K.N., Jones R.J., Goodman O.B., Jr., Saad F. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith M., De Bono J., Sternberg C., Le Moulec S., Oudard S., De Giorgi U., Krainer M., Bergman A., Hoelzer W., De Wit R. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34(25):3005–3013. doi: 10.1200/JCO.2015.65.5597. [DOI] [PubMed] [Google Scholar]
- 6.Gurusamy D, Gray JK, Pathrose P, Kulkarni RM, Finkleman FD, Waltz SE. Myeloid-specific expression of Ron receptor kinase promotes prostate tumor growth. Cancer Res. 2013;73(6):1752–1763. doi: 10.1158/0008-5472.CAN-12-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwama A., Wang M.H., Yamaguchi N., Ohno N., Okano K., Sudo T., Takeya M., Gervais F., Morissette C., Leonard E.J. Terminal differentiation of murine resident peritoneal macrophages is characterized by expression of the STK protein tyrosine kinase, a receptor for macrophage-stimulating protein. Blood. 1995;86(9):3394–3403. [PubMed] [Google Scholar]
- 8.Kulkarni RM, Stuart WD, Waltz SE. Ron receptor-dependent gene regulation of Kupffer cells during endotoxemia. Hepatobiliary Pancreat Dis Int. 2014;13(3):281–292. doi: 10.1016/s1499-3872(14)60254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolaidis N.M., Gray J.K., Gurusamy D., Fox W., Stuart W.D., Huber N., Waltz S.E. Ron receptor tyrosine kinase negatively regulates TNFalpha production in alveolar macrophages by inhibiting NF-kappaB activity and Adam17 production. Shock. 2010;33(2):197–204. doi: 10.1097/SHK.0b013e3181ae8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolaidis NM, Kulkarni RM, Gray JK, Collins MH, Waltz SE. Ron receptor deficient alveolar myeloid cells exacerbate LPS-induced acute lung injury in the murine lung. Innate Immun. 2011;17(6):499–507. doi: 10.1177/1753425910383725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuart WD, Kulkarni RM, Gray JK, Vasiliauskas J, Leonis MA, Waltz SE. Ron receptor regulates Kupffer cell-dependent cytokine production and hepatocyte survival following endotoxin exposure in mice. Hepatology. 2011;53(5):1618–1628. doi: 10.1002/hep.24239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thobe MN, Gurusamy D, Pathrose P, Waltz SE. The Ron receptor tyrosine kinase positively regulates angiogenic chemokine production in prostate cancer cells. Oncogene. 2010;29(2):214–226. doi: 10.1038/onc.2009.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasiliauskas J, Nashu MA, Pathrose P, Starnes SL, Waltz SE. Hepatocyte growth factor-like protein is required for prostate tumor growth in the TRAMP mouse model. Oncotarget. 2014;5(14):5547–5558. doi: 10.18632/oncotarget.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz-Torres SJ, Benight NM, Karns RA, Lower EE, Guan JL, Waltz SE. HGFL-mediated RON signaling supports breast cancer stem cell phenotypes via activation of non-canonical beta-catenin signaling. Oncotarget. 2017;8(35):58918–58933. doi: 10.18632/oncotarget.19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peace BE, Toney-Earley K, Collins MH, Waltz SE. Ron receptor signaling augments mammary tumor formation and metastasis in a murine model of breast cancer. Cancer Res. 2005;65(4):1285–1293. doi: 10.1158/0008-5472.CAN-03-3580. [DOI] [PubMed] [Google Scholar]
- 16.Gray JK, Paluch AM, Stuart WD, Waltz SE. Ron receptor overexpression in the murine prostate induces prostate intraepithelial neoplasia. Cancer Lett. 2012;314(1):92–101. doi: 10.1016/j.canlet.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thobe M.N., Gray J.K., Gurusamy D., Paluch A.M., Wagh P.K., Pathrose P., Lentsch A.B., Waltz S.E. The Ron receptor promotes prostate tumor growth in the TRAMP mouse model. Oncogene. 2011;30(50):4990–4998. doi: 10.1038/onc.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugie S, Mukai S, Yamasaki K, Kamibeppu T, Tsukino H, Kamoto T. Plasma macrophage-stimulating protein and hepatocyte growth factor levels are associated with prostate cancer progression. Hum Cell. 2016;29(1):22–29. doi: 10.1007/s13577-015-0123-5. [DOI] [PubMed] [Google Scholar]
- 19.Wagh P.K., Gray J.K., Zinser G.M., Vasiliauskas J., James L., Monga S.P., Waltz S.E. beta-Catenin is required for Ron receptor-induced mammary tumorigenesis. Oncogene. 2011;30(34):3694–3704. doi: 10.1038/onc.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barwick B.G., Abramovitz M., Kodani M., Moreno C.S., Nam R., Tang W., Bouzyk M., Seth A., Leyland-Jones B. Prostate cancer genes associated with TMPRSS2-ERG gene fusion and prognostic of biochemical recurrence in multiple cohorts. Br J Cancer. 2010;102(3):570–576. doi: 10.1038/sj.bjc.6605519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandran U.R., Ma C., Dhir R., Bisceglia M., Lyons-Weiler M., Liang W., Michalopoulos G., Becich M., Monzon F.A. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer. 2007;7:64. doi: 10.1186/1471-2407-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magee J.A., Araki T., Patil S., Ehrig T., True L., Humphrey P.A., Catalona W.J., Watson M.A., Milbrandt J. Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res. 2001;61(15):5692–5696. [PubMed] [Google Scholar]
- 23.Demichelis F., Setlur S.R., Beroukhim R., Perner S., Korbel J.O., Lafargue C.J., Pflueger D., Pina C., Hofer M.D., Sboner A. Distinct genomic aberrations associated with ERG rearranged prostate cancer. Genes Chromosomes Cancer. 2009;48(4):366–380. doi: 10.1002/gcc.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grasso C.S., Wu Y.M., Robinson D.R., Cao X., Dhanasekaran S.M., Khan A.P., Quist M.J., Jing X., Lonigro R.J., Brenner J.C. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lapointe J., Li C., Higgins J.P., Rijn M., Bair E., Montgomery K., Ferrari M., Egevad L., Rayford W., Bergerheim U. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A. 2004;101(3):811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varambally S., Yu J., Laxman B., Rhodes D.R., Mehra R., Tomlins S.A., Shah R.B., Chandran U., Monzon F.A., Becich M.J. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8(5):393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Welsh J.B., Sapinoso L.M., Su A.I., Kern S.G., Wang-Rodriguez J., Moskaluk C.A., Frierson H.F., Jr., Hampton G.M. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61(16):5974–5978. [PubMed] [Google Scholar]
- 28.Tomlins S.A., Rhodes D.R., Perner S., Dhanasekaran S.M., Mehra R., Sun X.W., Varambally S., Cao X., Tchinda J., Kuefer R. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 29.Best C.J., Gillespie J.W., Yi Y., Chandramouli G.V., Perlmutter M.A., Gathright Y., Erickson H.S., Georgevich L., Tangrea M.A., Duray P.H. Molecular alterations in primary prostate cancer after androgen ablation therapy. Clin Cancer Res. 2005;11(19 Pt 1):6823–6834. doi: 10.1158/1078-0432.CCR-05-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K., Furihata M., Tsunoda T., Ashida S., Takata R., Obara W., Yoshioka H., Daigo Y., Nasu Y., Kumon H. Molecular features of hormone-refractory prostate cancer cells by genome-wide gene expression profiles. Cancer Res. 2007;67(11):5117–5125. doi: 10.1158/0008-5472.CAN-06-4040. [DOI] [PubMed] [Google Scholar]
- 31.Thalmann G.N., Anezinis P.E., Chang S.M., Zhau H.E., Kim E.E., Hopwood V.L., Pathak S., Eschenbach A.C., Chung L.W. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54(10):2577–2581. [PubMed] [Google Scholar]
- 32.Horoszewicz J.S., Leong S.S., Chu T.M., Wajsman Z.L., Friedman M., Papsidero L., Kim U., Chai L.S., Kakati S., Arya S.K. The LNCaP cell line—a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- 33.Watson PA, Ellwood-Yen K, King JC, Wongvipat J, Lebeau MM, Sawyers CL. Context-dependent hormone-refractory progression revealed through characterization of a novel murine prostate cancer cell line. Cancer Res. 2005;65(24):11565–11571. doi: 10.1158/0008-5472.CAN-05-3441. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder G.M., An Y., Cai Z.W., Chen X.T., Clark C., Cornelius L.A., Dai J., Gullo-Brown J., Gupta A., Henley B. Discovery of N-(4-(2-amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a selective and orally efficacious inhibitor of the Met kinase superfamily. J Med Chem. 2009;52(5):1251–1254. doi: 10.1021/jm801586s. [DOI] [PubMed] [Google Scholar]
- 35.Jung S.J., Oh S., Lee G.T., Chung J., Min K., Yoon J., Kim W., Ryu D.S., Kim I.Y., Kang D.I. Clinical significance of Wnt/beta-catenin signalling and androgen receptor expression in prostate cancer. World J Mens Health. 2013;31(1):36–46. doi: 10.5534/wjmh.2013.31.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin R., Yi Y., Yull F.E., Blackwell T.S., Clark P.E., Koyama T., Smith J.A., Jr., Matusik R.J. NF-kappaB gene signature predicts prostate cancer progression. Cancer Res. 2014;74(10):2763–2772. doi: 10.1158/0008-5472.CAN-13-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L., Altuwaijri S., Deng F., Chen L., Lal P., Bhanot U.K., Korets R., Wenske S., Lilja H.G., Chang C. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175(2):489–499. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Truica CI, Byers S, Gelmann EP. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000;60(17):4709–4713. [PubMed] [Google Scholar]
- 39.Pierce J.W., Schoenleber R., Jesmok G., Best J., Moore S.A., Collins T., Gerritsen M.E. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272(34):21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 40.Gao W, Kim J, Dalton JT. Pharmacokinetics and pharmacodynamics of nonsteroidal androgen receptor ligands. Pharm Res. 2006;23(8):1641–1658. doi: 10.1007/s11095-006-9024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batth I., Yun H., Hussain S., Meng P., Osmulski P., Huang T.H., Bedolla R., Profit A., Reddick R., Kumar A. Crosstalk between RON and androgen receptor signaling in the development of castration resistant prostate cancer. Oncotarget. 2016;7(12):14048–14063. doi: 10.18632/oncotarget.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferraldeschi R, Welti J, Luo J, Attard G, de Bono JS. Targeting the androgen receptor pathway in castration-resistant prostate cancer: progresses and prospects. Oncogene. 2015;34(14):1745–1757. doi: 10.1038/onc.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee WY, Chen HH, Chow NH, Su WC, Lin PW, Guo HR. Prognostic significance of co-expression of RON and MET receptors in node-negative breast cancer patients. Clin Cancer Res. 2005;11(6):2222–2228. doi: 10.1158/1078-0432.CCR-04-1761. [DOI] [PubMed] [Google Scholar]
- 44.Gyorffy B., Lanczky A., Eklund A.C., Denkert C., Budczies J., Li Q., Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123(3):725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 45.Benight NM, Waltz SE. Ron receptor tyrosine kinase signaling as a therapeutic target. Expert Opin Ther Targets. 2012;16(9):921–931. doi: 10.1517/14728222.2012.710200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajan P., Sudbery I.M., Villasevil M.E., Mui E., Fleming J., Davis M., Ahmad I., Edwards J., Sansom O.J., Sims D. Next-generation sequencing of advanced prostate cancer treated with androgen-deprivation therapy. Eur Urol. 2014;66(1):32–39. doi: 10.1016/j.eururo.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habib AA, Chatterjee S, Park SK, Ratan RR, Lefebvre S, Vartanian T. The epidermal growth factor receptor engages receptor interacting protein and nuclear factor-kappa B (NF-kappa B)-inducing kinase to activate NF-kappa B. Identification of a novel receptor-tyrosine kinase signalosome. J Biol Chem. 2001;276(12):8865–8874. doi: 10.1074/jbc.M008458200. [DOI] [PubMed] [Google Scholar]
- 48.Yang F., Li X., Sharma M., Sasaki C.Y., Longo D.L., Lim B., Sun Z. Linking beta-catenin to androgen-signaling pathway. J Biol Chem. 2002;277(13):11336–11344. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- 49.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464(7286):302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ware JL, DeLong ER. Influence of tumour size on human prostate tumour metastasis in athymic nude mice. Br J Cancer. 1985;51(3):419–423. doi: 10.1038/bjc.1985.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellis L, Lehet K, Ramakrishnan S, Adelaiye R, Pili R. Development of a castrate resistant transplant tumor model of prostate cancer. Prostate. 2012;72(6):587–591. doi: 10.1002/pros.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J., Gao N., DeGraff D.J., Yu X., Sun Q., Case T.C., Kasper S., Matusik R.J. Characterization of cis elements of the probasin promoter necessary for prostate-specific gene expression. Prostate. 2010;70(9):934–951. doi: 10.1002/pros.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]