Introduction
Breast cancer is the second most common malignancy after melanoma to metastasize to skin.1 Approximately 30% of individuals with metastatic breast cancer (MBC) go on to have cutaneous metastases, frequently presenting as firm nodules, diffuse infiltrative or ulcerative lesions, often in proximity to an old mastectomy scar.2, 3 Cutaneous metastases of breast cancer (CMOBC) is a therapeutic challenge and is associated with increased morbidity. Progression of disease often results in severe pain, chest wall ulceration, bleeding, and superinfection.2 Optimal management of these highly morbid lesions is an active area of investigation. Apart from improving quality of life, recent evidence suggests that skin-directed treatment of CMOBC with topical imiquimod can provide durable systemic responses that prolong survival.3, 4
We present 3 cases of CMOBC treated with cryotherapy in combination with either topical fluorouracil 5% (5FU) or topical imiquimod. All patients had striking responses both locally and systemically. We hypothesize that there may be a role for topical therapy in palliation of CMOBC. Current evidence suggests there may be synergy between systemic therapies and the antitumor response induced by skin-directed therapies: cryotherapy generating an in situ vaccination of tumor and topical 5FU or imiquimod generating and sustaining a favorable inflammatory milieu.3, 5, 6, 7, 8 Although large-scale controlled studies are needed to determine if skin-directed therapy plays a role in the treatment of MBC, these results suggest combined therapy may be effective for CMOBC.
Case presentations
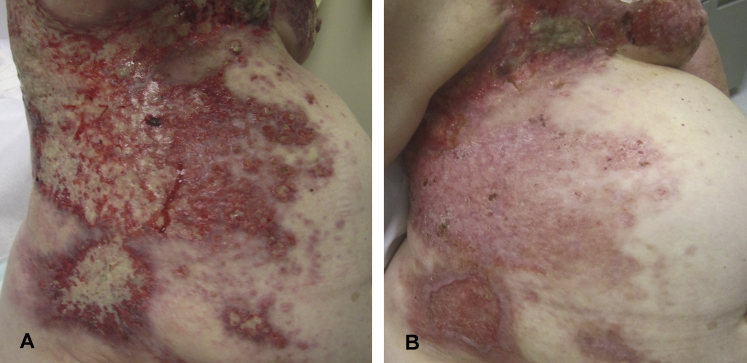
Patient 1 is a woman in her 60s with ER+, HER2+ MBC referred for treatment evaluation of CMOBC. Prior treatments included mastectomy, radiation, multiple lines of chemotherapy, and targeted and endocrine therapy. On presentation, her treatment regimen included letrozole and radiation to left axillary lymph nodes. Despite these treatments, she required frequent transfusions and multiple daily dressing changes because of bleeding from her CMOBC. Physical examination found extensive friable metastases on her trunk (Fig 1, A). Treatment with 5FU cream twice daily to her right lower abdomen was initiated with reduction in pain, bleeding, and drainage within the first week. Treatment with cryotherapy every 2 weeks to 4 to 6 areas was subsequently started. After just 2 cryotherapy treatments, her lesions healed appreciably, only requiring dressing changes every 2 days. She completed a 4-month course of topical 5FU and cryotherapy with dramatic cutaneous response, most prominent in the area treated with 5FU (right lower abdomen; Fig, 1, B). She was continued on letrozole and palliative radiation with sustained improvement at 6 months.
Fig 1.
Clinical response of patient 1 to treatment with 5FU and cryotherapy. A, Clinical image, initial presentation. Numerous eroded, exophytic, bleeding, and friable plaques affecting the back, chest, and abdomen of patient 1 before treatment with 5FU and cryotherapy. B, Clinical image, 4-month follow-up. Significant clinical response, most prominent over the right side of the abdomen of patient 1 after 4 months of skin-directed treatment combined with systemic letrozole and radiation.
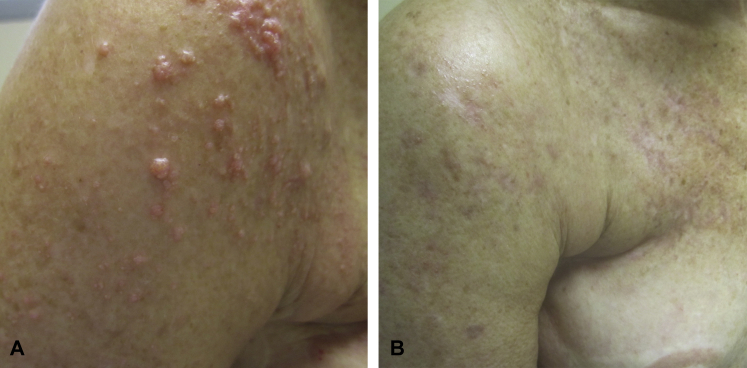
Patient 2 is a woman in her 60s with metastatic triple negative breast cancer. Despite treatment with multiple chemotherapeutics and radiation, her disease progressed prompting initiation of irinotecan salvage chemotherapy and referral to the dermatology department for additional treatment recommendations. On examination, she had cutaneous metastases limited to the right side of her chest and upper arm (Fig 2, A). Treatment with topical imiquimod daily and cryotherapy to 2 to 12 lesions every 3 weeks was started. Despite initial resolution of several lesions, within 1 month she had progressive chest wall involvement, prompting addition of ixabepilone, another chemotherapeutic agent. Regression was noted after the first cycle of ixabepilone, with near complete resolution after the third cycle (Fig 2, B), at which point she had received 3 months of skin-directed treatment.
Fig 2.
Clinical response of patient 2 to treatment with imiquimod and cryotherapy. A, Clinical image, initial presentation. Innumerable papules coalescing into plaques on the right upper arm. B, Clinical image, 3-month follow up. Near-complete resolution of cutaneous metastases on right chest and right upper arm of patient 2 after 3 months of treatment with topical imiquimod and cryotherapy in combination with systemic irinotecan and 2 months of ixabepilone.
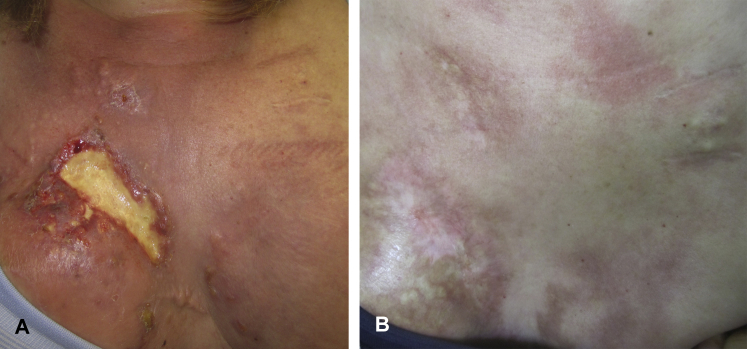
Patient 3 is a woman in her 30s with recurrent metastatic HER2+ MBC who presented for treatment of CMOBC. Neoadjuvant chemotherapy with trastuzumab and lapatinib and a right-sided, skin-sparing mastectomy followed by 1 year of trastuzumab sent her into remission. Two years later, local recurrence developed. Examination found an ulcerating plaque at her mastectomy site. Treatment with 5FU twice daily was initiated with immediate reduction in drainage and bleeding. Cryotherapy every 2 weeks to localized areas of the tumor was subsequently started. Despite initial improvement, her localized tumor completely ulcerated and rapidly progressive cutaneous involvement developed (Fig 3, A) along with nodal and brain metastases. Brain radiation and treatment with docetaxel, pertuzumab, and trastuzumab was initiated. With continued topical therapy, her lesions noticeably improved after 1 cycle of chemotherapy and responded remarkably after 4 cycles. Within 4 months of combined systemic and skin-directed therapy, she achieved a full clinical response with resolution of systemic and cutaneous metastases (Fig 3, B). She continued on pertuzumab and trastuzumab maintenance therapy without evidence of recurrence at 19 months.
Fig 3.
Clinical response of patient 3 to fluorouracil and cryotherapy. A, Clinical image, metastatic progression. Ulcerated plaque at mastectomy site with multiple surrounding metastatic papules coalescing in to plaques on bilateral breasts and upper chest of patient 3 after 4 weeks of skin-directed therapy. B, Clinical image, 5-month follow up. Complete clinical response of cutaneous metastases of patient 3 within 4 months of combined topical and systemic therapy with 5FU, cryotherapy, docetaxel, pertuzumab, trastuzumab, and radiation.
Discussion
Here we describe 3 patients with CMOBC in whom combined skin-directed and systemic therapies produced dramatic clinical responses. In the authors experience, this uncharacteristic response to systemic therapy alone, particularly in heavily pretreated patients, suggests that skin-directed treatments may play a role in the management of CMOBC. We hypothesize that cryotherapy produces an in situ vaccination, whereas topical imiquimod and 5FU create a favorable immune milieu, which together induce specific antitumor immunity that synergizes with systemic therapeutics, resulting in improved clinical outcomes.
Imiquimod, a toll-like receptor 7 (TLR7) agonist used to treat primary skin cancers, is listed by the National Cancer Institute as one of the agents with the highest potential to cure cancer.9 It has been used to treat melanoma in situ, and several reports describe its efficacy treating CMOBC.3, 4, 10, 11 We found that when pretreated with imiquimod and cryotherapy, patient 2 had near-complete resolution of her CMOBC after only 4 cycles of chemotherapy. The unusually rapid response to chemotherapy may be from favorable immunomodulatory antitumor effects induced by imiquimod and cryotherapy. Our hypothesis is supported by findings of Janosky et al6 who showed expansion of antigen-specific T helper 1 cells in 2 patients with CMOBC who had complete and durable response to endocrine therapy after application of topical imiquimod to cutaneous metastases.3, 6 Additionally, studies suggest that imiquimod may promote antitumor immunity via stimulation of proinflammatory cytokines, dendritic cell maturation, and antigen presentation via TLR7 activation as well as direct stimulation of killer plasmacytoid dendritic cells.3, 12 Further studies are needed to elucidate how imiquimod acts on CMOBC.
Encouraged by reports of imiquimod's efficacy treating CMOBC, we tested if 5FU, another topical used to treat primary skin cancers, could induce regression of CMOBC. In patients 1 and 3, we found that when use used alone, 5FU reduced bleeding and drainage of lesions and when combined with cryotherapy and systemic therapy, rapidly decreased tumor burden. Patient 3 had a dramatic response to chemotherapy and radiation with rapid regression of cutaneous and systemic metastases with sustained clearance. We believe that treatment with cryotherapy and topical 5FU is superior to cryotherapy alone given that the abdomen of patient 1 treated with 5FU and cryotherapy showed a better response than areas treated with cryotherapy alone, suggesting that 5FU induces an antitumor activity independent of cryotherapy. Similar to imiquimod, we believe that the dramatic response in both patients is in part owing to a favorable immune milieu induced by 5FU that synergizes with systemic therapies. Further studies are needed, however, to characterize the antitumor responses elicited by 5FU.
Cryotherapy, or tissue destruction by deep freezing and thawing, a common treatment for precancerous skin lesions, has been used to treat solid tumors of skin, breast, liver, and prostate.7, 13 Encouraged by reports highlighting regression of metastases after primary tumor cryoablation, we tested if cryotherapy could enhance regression of CMOBC.13 We found that addition of cryotherapy induced regression of cutaneous metastases in patients 1 and 3, suggesting that cryotherapy induces antitumor activity, which we hypothesize synergizes with systemic treatments given the rapid response elicited in patients 2 and 3 by addition of systemic therapy after pretreatment with skin-directed therapies. The feasibility of our hypothesis is strengthened by evidence that cryotherapy induces release of intact tumor antigens and an immediate local inflammatory response, thereby generating an in situ vaccination against target tumor antigens—a phenomenon described as cryo-immunotherapy.7, 8 Additionally, evidence that antitumor immunity contributes to the response to chemotherapy supports our hypothesis that cryo-immunotherapy may condition the host immune system to achieve an antitumor effect synergistic with some systemic treatments.14
We present 3 cases of MBC with extensive cutaneous metastases, treated with cryotherapy and either imiquimod or fluorouracil in addition to a systemic agent. Each patient showed significant clinical responses including one who continues to have no evidence of systemic or cutaneous disease. Just as cryotherapy followed by imiquimod is an effective way to treat primary skin cancers, we believe cryotherapy combined with imiquimod or 5FU has utility in treatment of CMOBC.15 In light of evidence that imiquimod promotes a proimmunogenic tumor microenvironment in MBC, and antitumor immunity improves response to chemotherapy, we hypothesize that cryotherapy and topical immunomodulators induce tumor-specific immune responses to achieve an antitumor effect synergistic with systemic therapies.14 We acknowledge the limitations of this series, including the small sample size, lack of a control group, and immunohistochemical analysis. Nevertheless, this limited cases series highlights that cryotherapy and topical immune modulators may play a role in management of CMOBC, possibly by generating improved responses to traditional systemic chemotherapeutics and emerging therapies including targeted agents and immune checkpoint blockers. Although results have not been presented, current trials are ongoing to evaluate combination therapy in metastatic melanoma (NCT03276832). Clinical and correlative studies are currently ongoing to develop a therapeutic protocol for CMOBC based on these observations.
Acknowledgments
The authors thank Drs Rondi Kauffman, Joanne Mortimer, Arti Hurria, Yuan Yuan, and James Waisman for their contributions to this study.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Lookingbill D.P., Spangler N., Helm K.F. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228–236. doi: 10.1016/0190-9622(93)70173-q. [DOI] [PubMed] [Google Scholar]
- 2.Spratt D.E., Gordon Spratt E.A., Wu S. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol. 2014;32:3144–3155. doi: 10.1200/JCO.2014.55.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams S., Kozhaya L., Martiniuk F. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748–6757. doi: 10.1158/1078-0432.CCR-12-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henriques L., Palumbo M., Guay M.P. Imiquimod in the treatment of breast cancer skin metastasis. J Clin Oncol. 2014;32(8):e22–e25. doi: 10.1200/JCO.2012.46.4883. [DOI] [PubMed] [Google Scholar]
- 5.Knutson K.L., Bishop M.R., Schiffman K., Disis M.L. Immunotherapy for breast cancer. Cancer Chemother Biol Response Modif. 2002;20:351–369. [PubMed] [Google Scholar]
- 6.Janosky M., Sabado R.L., Cruz C. MAGE-specific T cells detected directly ex-vivo correlate with complete remission in metastatic breast cancer patients after sequential immune-endocrine therapy. J Immunother Cancer. 2014;2:32. doi: 10.1186/s40425-014-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sidana A., Chowdhury W.H., Fuchs E.J., Rodriguez R. Cryoimmunotherapy in urologic oncology. Urology. 2010;75:1009–1014. doi: 10.1016/j.urology.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Ablin R.J. Cryoimmunotherapy. Br Med J. 1972;3:476. doi: 10.1136/bmj.3.5824.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy. 2009;1:949–964. doi: 10.2217/imt.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis L.Z., Cohen J.L., High W., Stewart L. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937–946. doi: 10.1111/j.1524-4725.2012.02362.x. [DOI] [PubMed] [Google Scholar]
- 11.Adams S., Novik Y., Oratz R. Clinical trial evidence of the antitumor activity of topical imiquimod for breast cancer skin metastases. J Clin Oncol. 2014;32:3204–3205. doi: 10.1200/JCO.2014.56.1282. [DOI] [PubMed] [Google Scholar]
- 12.Drobits B., Holcmann M., Amberg N. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Invest. 2012;122:575–585. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabel M.S. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1–11. doi: 10.1016/j.cryobiol.2008.10.126. [DOI] [PubMed] [Google Scholar]
- 14.Apetoh L., Ghiringhelli F., Tesniere A. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 15.MacFarlane D.F., El Tal A.K. Cryoimmunotherapy: superficial basal cell cancer and squamous cell carcinoma in situ treated with liquid nitrogen followed by imiquimod. Arch Dermatol. 2011;147:1326–1327. doi: 10.1001/archdermatol.2011.334. [DOI] [PubMed] [Google Scholar]