Abstract
Objectives
Previous studies have shown marital status differences in incidence and prevalence of cardiovascular disease and cardiovascular mortality. This study examines the consequences of partnership on biomarkers related to cardiovascular health of older men and women in Germany and England (C-reactive protein, HbA1c, systolic and diastolic blood pressure; and total cholesterol).
Methods
Data used is from older adults (60 +) from the German Survey of Health and Retirement Europe SHARE (n=955) and the English Longitudinal Study of Ageing ELSA (n=9707). Life course partnership is measured using the timing (age at first partnership), quantum (number of partnerships) and partnership trajectory. OLS for C-reactive protein, logistic regressions for systolic and diastolic blood pressure, and multinomial logistic regressions for cholesterol are used to investigate the associations between life course partnership characteristics and biomarkers, accounting for early age socioeconomic and health conditions.
Results
Timing of first partnership is associated with poor cardiovascular health in England, number of partnership transitions with poor health in Germany, and partnership trajectories are associated with cardiovascular health both in Germany and England. Men in trajectories with multiple marriages have higher CRP, and are more likely to have elevated systolic and diastolic BP. Trajectories containing single marital disruption for men and women are no longer associated with poor health after accounting for selection effects of childhood conditions. Respondents in widowed partnership trajectories have poorer cardiovascular health compared to those in intact committed relationships, whereas cohabitation trajectories do not differ in the associations with biomarkers from those in intact marriage.
Conclusion
The results offer better understanding of the pathways through which family events and processes are linked to health and support the hypothesis that adversity related to partnerships over the life course accumulates and contributes to worse cardiovascular health in later life measured by objective health measures.
Keywords: Biomarkers, Cardiovascular health, Marital status, Aging, Life course
Highlights
-
•
This study investigates the longitudinal accumulated effects of partnership on cardiovascular health using haemostatic and inflammatory biomarkers in later-life, C-reactive protein, HbA1c, systolic and diastolic blood pressure; and total cholesterol.
-
•
Based on life course theory of cumulative disadvantage, the study finds support that the benefits and risks of marital status accumulate over the life-course. The effects are visible on biomarkers of older adults in both Germany and England in models that account for conditions in early life, health behaviors in adult life and sociodemographic factors.
-
•
Data used is from older adults (60 +) form the German Survey of Health and Retirement Europe SHARE (n=955) and the English Longitudinal Study of Ageing ELSA (n=9707). Life course partnership is measured using the timing (age at first partnership), quantum (number of partnerships) and partnership sequence type.
1. Introduction
Studies investigating self-rated health, chronic conditions, functional limitations, and mortality have found protective effects of marriage across countries, income groups, age groups, and even gender (Bardage et al., 2005, Hemström, 1996, Johnson et al., 2000, Schoenborn, 2004). There is a consistent evidence that married individuals report better overall health (Lindström, 2009, Zheng and Thomas, 2013), and better cardiovascular health (Holt-Lunstad et al., 2008, Kriegbaum et al., 2013, Loucks et al., 2005, Schiller et al., 2012). The rates of mortality for married persons are lower compared to the non-married (Malyutina et al., 2004, Molloy et al., 2009, Zhu and Gu, 2010) and research has repeatedly shown the married have fewer chronic conditions, fewer limitations with mobility, and they tend to be less depressed (Hughes & Waite, 2009).
From a life course perspective, health outcomes are the result of the cumulative influence of multiple risks and protective factors experienced during the life course, including early-life health and socioeconomic position, and health-related behaviours like smoking, drinking, and diet (Halfon and Hochstein, 2002, Ben-Shlomo and Kuh, 2002, Harris, 2010). The life course paradigm is useful for understanding how health differentials among older adults arise. However, the importance of social relationships over the life course, especially those pertaining to marriage, but also other partnership forms like cohabitation, in shaping differences in later life health are often overlooked. Nonetheless, some recent studies linking marital status and health have gone beyond focusing on one-time transitions to marriage, divorce or widowhood, and examined the relationship between marital trajectories and health in a life course perspective. The findings have consistently shown that those in intact first marriages have been found to have better health compared to the rest on a range of health measures, including incidence of chronic diseases, cancer and self-rated health (Dupre et al., 2009, Dupre and Meadows, 2007, Green et al., 2012).
These studies represent a significant step forward in documenting the importance of marital trajectories for health outcomes. However, data limitations have precluded the use of more refined health measures that provide information for the biological risks related to marital trajectories, and in addition, to life course partnership characteristics, e.g. timing and partnership transitions. Biological risk measures such as biomarkers capture “under-the-skin processes” involved in the development of several major diseases prevalent among older adults, in particular cardiovascular disease and type 2 diabetes (McFarland, Hayward, & Brown, 2013).
This study investigates how life course partnership is associated with cardiovascular health in later life using biomarkers and physiological measures of cardiovascular health (C-reactive protein (CRP), HbA1c, systolic blood pressure, diastolic blood pressure, total cholesterol) in Germany and England. The study builds on previous literature in that it investigates the extent to which partnership timing, transitions, and trajectories are associated with worse CVH in later life. Understanding these relationships is important for several reasons. First, although biological risk is frequently used to explain the marriage – health association, only a handful of studies have explicitly tested how marital biographies are related to biological risk (McFarland et al., 2013, Ploubidis et al., 2015). Those that have, have not been able to go beyond using marriage as the only committed partnership form under investigation and excluded cohabitation entirely. In addition, these studies refrained from exploring the mechanisms that reveal how partnership trajectories relate to specific biomarkers, or were unable to account for selection processes that predispose individuals to poor health and also affect partnering chances and success over the life course. Additional evidence for this relationship will contribute to the literature on the direct effects of marriage on morbidity and mortality. Second, self-reported health measures in the marriage and health literature may have reflected reporting biases. By using biomarkers this can be avoided, but more importantly, we are able to investigate unreported and underdiagnosed health risks related to the development of major heart diseases.
An overwhelmingly large literature already focuses on the relationship between martial life courses and mortality, however these studies tend to exclude biological pathways (Kravdal and Syse, 2011, Grundy and Tomassini, 2010). Third, this study provides clues as to how partnership over the life course influences the etiology of prevalent health conditions such as cardiovascular disease and type 2 diabetes. For instance, certain individuals may be more affected than others by marital trajectory, or other partnership characteristics may differ in their impact on biological risk. Previous studies have focused on the US (McFarland et al., 2013) and UK (Ploubidis et al., 2015), whereas this study uses harmonized data form Germany and England to provide a comparative perspective on the life course partnership – CVH relationship for ageing populations that have different base-level characteristics, both with regard to partnership formation trajectories, as well as health status. Finally, the analyses contribute to an ongoing discussion regarding the differential effects of life course partnership characteristics on health for men and women.
2. Background
2.1. Marriage and biomarkers
Most of the research linking partnership and cardiovascular health using biomarkers has focused only on transitions to marriage and marital quality. The findings of these studies support a dyadic biopsychosocial model of marriage and health, which indicates that stress and relationship quality directly affect the cardiovascular system, and relationship quality moderates the effect of stress. Such effects on negative marital quality have been evident in small sample studies on C-reactive protein (CRP) (Shen, Farrell, Penedo, Schneiderman, & Orth-Gomer, 2010), diastolic ambulatory blood pressure (Cundiff, Birmingham, Uchino, & Smith, 2015), changes in blood pressure over time (Birditt, Newton, Cranford, & Ryan, 2015), as well as onset and management of diabetes in later life (Liu, Waite, & Shen, 2016).
Other studies investigating the relationship between marital interaction, quality during daily life and cardiovascular disease (CVD) have used biomarkers as mediators in the marital status–cardiovascular health relationship. Kozo Tanno et al., 2013 used systolic blood pressure, total cholesterol, high density lipoprotein-cholesterol, albumin, and C-reactive protein (CRP), whereas another study used systolic and diastolic BP (Joseph, Kamarck, Muldoon, & Manuck, 2014). However, biomarkers were used as controls without offering a substantive explanation how they moderate and mediate the association between marital quality and CVH. A notable exception is the study of Shen and colleagues that used C-reactive protein to investigate the prospective influence of marital status, social support, and depression on the mortality of patients with chronic heart failure, but found no mediating effect of CRP (Shen, Xu, & Eisenberg, 2017).
Several studies conducting meta-analysis have confirmed that greater marital quality is related to better health across various health outcomes, among which biological markers like bloodpressure were included as well (Manfredini et al., 2017, Robles et al., 2014). Meta-analysis studies have noted that most of the empirical studies investigating marital status/marital quality and health showed better outcomes for married persons, whereas men who were single generally had the poorest health. In addition, gender differences were found only when biomarker outcomes like blood pressure were used, as women’s health was more strongly associated with marital quality (Robles et al., 2014). The studies that examined the relationship between marital quality and glucose control in diabetes showed no significant relationship between marital quality and glucose control (Trief et al., 2001, Trief et al., 2006).
2.2. Life course partnership and health
Research has also consistently showed that cardiovascular mortality is lower for married individuals compared to their non-married counterparts (Ebrahim et al., 1995, Eaker et al., 2007, Malyutina et al., 2004, Molloy et al., 2009, Johnson et al., 2000, Ikeda et al., 2007). Older married men in particular enjoy better cardiovascular health (Blumenthal et al., 1995, De Leon et al., 1992, Holt-Lunstad et al., 2008, Kriegbaum et al., 2013, Loucks et al., 2005, Schiller et al., 2012, Venters et al., 1986). Although research has shown marital status differences in mortality and cardiovascular disease, most of the studies did not manage to go beyond investigating marriage and divorce (Eaker et al., 2007, Malyutina et al., 2004, Ikeda et al., 2007).
Life course scholars commonly stress the principle of timing – the meaning and significance of a role, a transition, or an event depends on when it occurs in an individual’s developmental trajectory (Elder & Giele, 2009). The most common sense of timing is transition timing. Depending on when it occurs in the life course, the meaning of a transition differs and affects an individual differently (Wheaton, 1990). Both formal norms prescribed in legislation and institutional arrangements and informal norms are part of the societal scripts that guide individuals with regard to the optimal occurrence and timing of major life-events in the family domain (Neugarten, 1979, Settersten and Hägestad, 1996). Scripts most often refer to timing (when events occur), ordering (in which order events occur) and quantum (how many events occur) of the expected events (Liefbroer and Billari, 2010, Settersten and Hägestad, 1996). Individuals are aware of their alignment with the proscribed norms regarding the timing, ordering and the quantum of desired events (e.g. marriage) (Neugarten, 1979), which enables them to define whether they are “on-time” or “off-time” regarding certain events (Neugarten, Moore, & Lowe, 1965).
Ill-timed and unexpected events (e.g. bereavement due to spousal death), disruptive transitions (e.g. marital disruption or divorce), and disorderly status sequences may have profound and lasting consequences for individual’s health in later life. Lifetime cumulative adversity (LCA), as well as cumulative advantage/disadvantage theory (CAD) describe a “process where initial relative advantage (or disadvantage) associated with structural location and resources results in systematic divergence in life course processes across individuals or groups over time” (see Corna, 2013). Life course theories of cumulative disadvantage state that benefits or risks of marital status may accumulate over the life course (Ben-Shlomo and Kuh, 2002, Kuh and Shlomo, 2004, Kuh et al., 2003). This study investigates whether there is evidence for cumulative disadvantage processes affecting health in later life by using objective measures of health that are not prone to reporting bias compared to self-reported health measures.
To date, research linking marital histories to health has studied cumulative time spent in different states (e.g., married, divorced, widowed), age at first marriage, and the number of marital disruptions (divorce, separation, widowhood). There is consensus about time spent married, as scholars argue that the cumulative effects of social, emotional, and financial support from one’s partner and social control of harmful health-related behaviors are positively related to health. Empirically, this has been supported for outcomes such as self-rated health and disability (Grundy & Holt, 2000), incident chronic disease (Dupre & Meadows 2007), and mortality (Brockmann and Klein, 2004, Dupre et al., 2009, Henretta, 2010, Lund et al., 2004). In addition, time spent in a disrupted marital state (divorce/separation/widowhood) has been found to be negatively related to health (Berntsen and Kravdal, 2012, Dupre and Meadows, 2007, Hughes and Waite, 2009).
Less is known about the effects of age at first marriage, as studies argue that an optimal time for marriage is one that does not interfere with educational and labor market participation to maximize the health benefits of marriage. Early age at first marriage has been linked to disadvantaged socioeconomic pathways and increased likelihood of marital disruption (Heaton, 1991), as well as health (Dupre et al., 2009, Grundy and Holt, 2000). However, other studies have found that late marriage is protective or have reported a positive linear relationship between age at marriage and health (Dupre et al., 2009, Hughes and Waite, 2009, McFarland et al., 2013). Therefore, following the lack of consensus regarding the association between age at marriage and health outcomes, we focus on timing of first committed partnership including marriage and cohabitation.
Marital disruptions are of interest to life course scholars who study health as they have been linked to various health outcomes. In addition to reducing marriage duration, marital disruptions present stressful life events that might infer negative health consequences (Booth & Amato, 1991). On one hand, several studies have reported that although marital disruptions impact health in the short term, these effects dissipate over time (Thierry, 2000, Williams and Umberson, 2004). On the other hand, multiple studies have found that the presence and number of marital disruptions is harmful for later health, including unhealthy behaviors such as smoking and drinking (Keenan, Ploubidis, Silverwood, & Grundy, 2017), chronic conditions (Dupre and Meadows, 2007, Hughes and Waite, 2009, Zhang, 2006), mobility limitations (Hughes & Waite, 2009), and biological risk markers (McFarland et al., 2013).
Off time events, such as early or late first transitions to partnership can be regarded as out-of-time transitions, thus I assume that early and late partnership are negatively related to cardio vascular health indicators across countries (H1). In addition, partnership life course trajectories may link to the accumulation of risk processes in several distinct ways. Unexpected states in the partnership trajectory such as divorce and widowhood, as well as their multiplicity, can be regarded as an adversity with the potential to affect cardiovascular health negatively. I distinguish between the overall partnership transitions individuals experience and the multiplicity of partnership disruptions. I expect that greater number of partnership transitions is negatively associated with cardio vascular health indicators across countries (H2). In addition, to account for the types of events, I expect that partnership trajectories that include disruptions (e.g. divorce) are negatively related with CVH (H3a), and that multiple disruption events in partnership trajectories are negatively related with cardio vascular health indicators across countries (H3b).
Studies have also showed that poor start in life is likely to influence the chances of getting married and partnering (Lamb, Lee, & DeMaris, 2003), but also influence late life. CAD has frequently been used to explain how early-life exposure to stressful events (including poor health and SES in childhood) may influence late-life health (Brandt et al., 2012, Mazzonna, 2014). Thus, when investigating how life course partnership affects later life health it is crucial to acknowledge that selection into certain partnership states (e.g. early marriage, non-marriage, or cohabitation) based on pre-existing conditions preceding adulthood may be affecting health in later life as well.
3. Data and methods
The study uses harmonized data from Germany (Survey of Health, Ageing and Retirement in Europe or SHARE) and England (English Longitudinal Study of Ageing or ELSA). Data include information on early-life socioeconomic status and family situation, partnership history over the life course, as well as a variety of health measures including observer-measured indicators of health. SHARE and ELSA are nationally representative longitudinal panel surveys of non-institutionalized respondents aged 50 or older. SHARE has been designed as a European counterpart of ELSA and the questions and survey protocols have been harmonized. SHARE includes 18 European countries; however biomarkers have only been collected in Germany. The information on partnership and selection variables on childhood health and socioeconomic conditions comes from the third waves of SHARE (Release 1.0.0) collected in 2008 (Börsch-Supan et al., 2011, Schröder, 2011) and ELSA (Scholes et al., 2009, Ward et al., 2009). Previous assessment of the reliability of SHARELIFE comparing reported histories with external macro indicators found the retrospective reports reliable (Havari & Mazzonna, 2015). Biomarker data used are from the fourth waves of SHARE (Release 1.1.1) collected in 2011 (Börsch-Supan et al., 2013, Malter and Börsch-Supan, 2013) and ELSA (Cheshire et al., 2012, Steptoe et al., 2012). The sample is restricted for men and women aged 50–80 and the oldest old are excluded as frequently they are not able to provide blood samples and neither sample captures well those resident in institutions. Due to missing cases we use multivariate imputation by chained equations (MICE, 50 imputations) (Royston and White, 2011, White et al., 2011). For characteristics of non-imputed data and imputation procedure see Supplementary file Table A1.
3.1. Indicators of life course partnership
Three indicators of partnership are used. An indicator of timing is the age at the first reported transition, regardless of it being marriage or cohabitation. Timing of partnership is grouped in early transition (before 20), on-time transition (between 20–25 and 25–30) and late transition (31 and above). Because the data contains range of ages, age- or cohort-specific average times of marriage are not used to determine the extent to which each respondent’s transition deviates from their counterparts. However, because partnering and marriage are related with fertility and parity, the twenties are taken as a normatively sanctioned baseline for being the appropriate time to partner, distinguishing between early and late twenties.
An indicator of quantum is the total number of partnership transitions from one to another state. This indicator does not discriminate based on the qualities of the sequences however it accounts for the number of times a state changes, as such changes are assumed to serve as stressors to individuals.
Trajectory group is the third indicator that contains a typology that deals with sequencing. Respondents reported the starting and (if relevant) ending dates of all cohabiting and martial unions, as well as the occurrence and timing of all possible marital disruptions such as divorce or death of a spouse. Every trajectory was made up of a number of values that corresponds to the number of years each individual is observed. The state space can take five possible values: S (single), C (cohabiting), M (married), D (divorced), and W (widowed). Individual trajectories were grouped together giving precedence to the order and sequencing. That means that after removing the information about the duration spent in different states, a comprehensive list of all possible sequences emerged (shown in Supplementary file Tables A2 and A3). Based on the hypotheses, the sequences were grouped into: married, widowed, divorced, ever cohabited, multiple marriages and never married (due to low number of never partnered in Germany they are collapsed with widowed). The respondents who experienced any cohabitation were grouped separately as previous research provides mixed evidence about the cohabitation effects on various measures of health (Ploubidis et al., 2015, Kim and McKenry, 2002, Soons and Kalmijn, 2009, Wu et al., 2003), as well as because cohabitation is less common partnership form for older cohorts in Germany and England and may have different associations with health due to stigma, or to selection processes that influence whether individuals cohabit. For details see Supplementary file Technical notes on sequence analyses and Tables A 2, A 3 and A 4.
3.2. Indicators of cardiovascular health
Five indicators of CVH are used in the study: inflammation biomarker C-reactive protein (CRP), HbA1c (glycated hemoglobin), systolic (SP) and diastolic (DP) blood pressure, as well as total cholesterol (TC; a measure of high-density lipoprotein HDL + low-density lipoprotein LDL+ 20% triglyceride level or fats carried in the blood). CRP, HbA1c and TC were measured from dried blood spots taken from respondents who gave written consent (NatCen Social Research, 2014, Schaan, 2013). Blood pressure was measured three times per respondent while they were seated. Systolic BP and diastolic BP were calculated as the average from three measures, respectively. Biomarkers are coded using standard clinical protocol cut-off values for older populations. We use the log of CRP mg/l (Zhou et al., 2014); HbA1c is dichotomized using a cut-off of 6.4% (Bennett, Guo, & Dharmage, 2007); systolic BP is dichotomized using a cut-off of 140 mm/Hg (Port, Demer, Jennrich, Walter, & Garfinkel, 2000); diastolic BP is dichotomized using a cut-off of 90 mm/Hg (Fardella et al., 2000) and total cholesterol is categorized as low (<200 mg/dl), normal (200–239 mg/dl) or high (>240 mg/dl) in Germany (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2002); and low (< 5.2 mmol/l), normal (5.2–6.2 mmol/l) and high (>6.2 mmol/l) in England (Roth et al., 2011). Blood samples were not taken from respondents on anti-coagulants or respondents who refused blood collection.
Some of the respondents use drugs for high blood pressure, diabetes or cholesterol. This may conceal some of the associations between biomarkers and partnership characteristics. Therefore, we coded respondents who are taking medicine for the appropriate illness as having high HbA1c (diabetes medicine); high systolic and diastolic pressure (medicine for high blood pressure) and high cholesterol (lowering cholesterol medicine). The distributions of biomarkers collected via blood spots and the use of drugs for each biomarker in the original data is presented in Supplementary file Table A 5.
3.3. Additional covariates
We included in the analysis information on childhood health and socioeconomic conditions that might influence both life course partnership characteristics, as well as later life health. To control for possible selection effects we use four different indicators for childhood socioeconomic conditions collected in the third waves: housing conditions (people per room; range 0.47–7); cultural capital (number of bookshelves; range 0–5) (Brandt et al., 2012, Deindl, 2013); parental socioeconomic background (main breadwinner’s occupation recoded from ISCO categories: low skilled (farmer (in Germany only), low-skilled blue collar, high-skilled blue collar, and no main breadwinner or other), and high skilled (low-skilled white collar and high-skilled white collar); family situation (if respondent was not living with both biological parents in childhood) and a measure of self-assessed childhood health (1 = very bad to 5 = excellent). We also included covariates measured in the fourth wave, such as a continuous measure of age, but we excluded possible mediators such as parity, education, and health behaviours as they likely both moderate and mediate the relationships between partnership characteristics and health. The characteristics of all covariates included in the analyses are showed in Table 1.
Table 1.
Descriptive sample statistics.
| Germany |
England |
|||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| C-Reactive protein | 0.95 (1.21) | 1.10 (1.38) | 0.62 (1.11) | 0.69 (1.12) |
| High HbA1c | 68.55 | 66.67 | 19.09 | 16.12 |
| High systolic blood pressure | 85.29 | 80.90 | 58.01 | 51.82 |
| High diastolic blood pressure | 73.08 | 67.45 | 44.51 | 40.01 |
| Total cholesterol | ||||
| Low | 19.63 | 21.19 | 19.84 | 20.88 |
| Normal | 20.83 | 24.27 | 20.89 | 12.51 |
| High | 59.54 | 54.55 | 59.26 | 66.61 |
| Age at first union | ||||
| Below 20 | 5.66 | 32.36 | 15.37 | 30.37 |
| 21–25 | 48.64 | 48.73 | 53.48 | 51.30 |
| 26–30 | 30.77 | 13.84 | 20.70 | 12.03 |
| 31 and above | 14.93 | 5.07 | 10.44 | 6.30 |
| Number of transitions | 1.37 (0.82) | 2.35 (1.17) | 2.21 (1.97) | 2.30 (1.89) |
| Partnership type | ||||
| Married | 75.79 | 70.76 | 46.78 | 41.85 |
| Widowed (or never married)a | 6.33 | 10.33 | 7.79 | 13.63 |
| Divorced | 4.52 | 7.41 | 9.48 | 12.21 |
| Ever cohabited | 3.85 | 1.95 | 6.52 | 5.83 |
| Multiple marriages | 9.50 | 9.55 | 19.41 | 18.76 |
| Never partnered | 10.03 | 7.72 2 | ||
| Age | 68.27 (6.52) | 66.59 (6.76) | 64.36 (7.68) | 63.80 (7.96) |
| Absent biological parent at age 10 | 22.17 | 18.00 | 13.18 | 12.53 |
| Breadwinner’s occupation at age 10 | ||||
| Low-skilled | 71.72 | 71.15 | 65.34 | 63.52 |
| High-skilled | 28.28 | 28.85 | 34.66 | 36.47 |
| People per room at age 10 | 1.54 (0.75) | 1.60 (0.75) | 1.74 (0.76) | 1.77 (0.79) |
| Number of bookshelves at age 10 | 2.47 (1.21) | 2.35 (1.17) | 2.47 (1.21) | 2.56 (1.23) |
| Childhood health at age 10 | 2.46 (1.04) | 2.52 (0.97) | 2.11 (1.09) | 2.17 (1.11) |
| n | 442 | 513 | 4446 | 5261 |
Notes: Own calculations. Continues variables are presented as means and standard deviations, categorical variables are presented as percentages.
Never married applies to the German sample only.
3.4. Analytical strategy
Each indicator of cardiovascular health was used as an outcome in a separate regression model. OLS regression was used for the CRP(log), logistic regression for HbA1c, systolic and diastolic BP, and multinomial logistic regression was used for cholesterol. The first set of models shows the association between each measure of life course partnership (timing, quantum, and type), and age. In the second set of models childhood health and SES conditions are added. Due to known gender differences in life course partnership and CVH the analyses were stratified by gender. Additionally, due to country differences in the baseline sample and possible unobserved social factors we stratified the analyses by country.
4. Results
Summary statistics for all variables are provided in Table 1, stratified by gender and country. The German sample consists of 442 men and 513 women; the English sample from 4446 men and 5261 women. Notably, the German respondents have a lower average log of CRP, higher high HbA1c level, high systolic and high diastolic blood pressure than the English, whereas the English respondents on average have higher high total cholesterol.
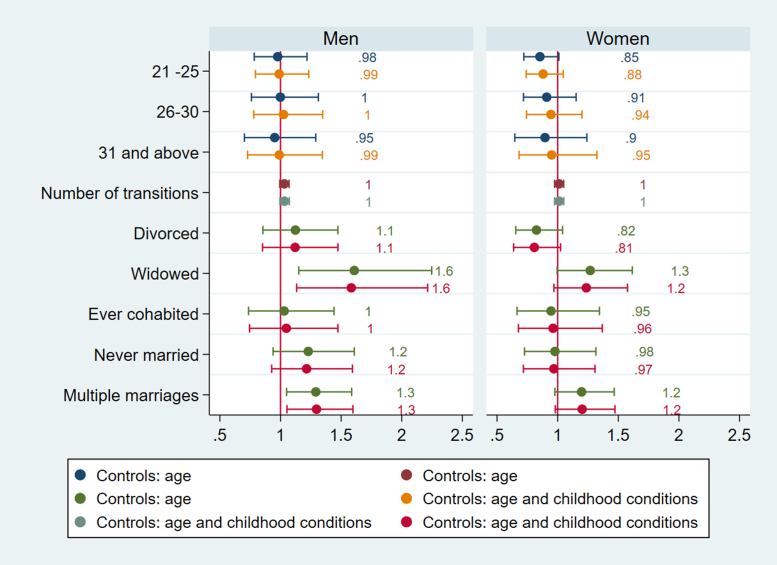
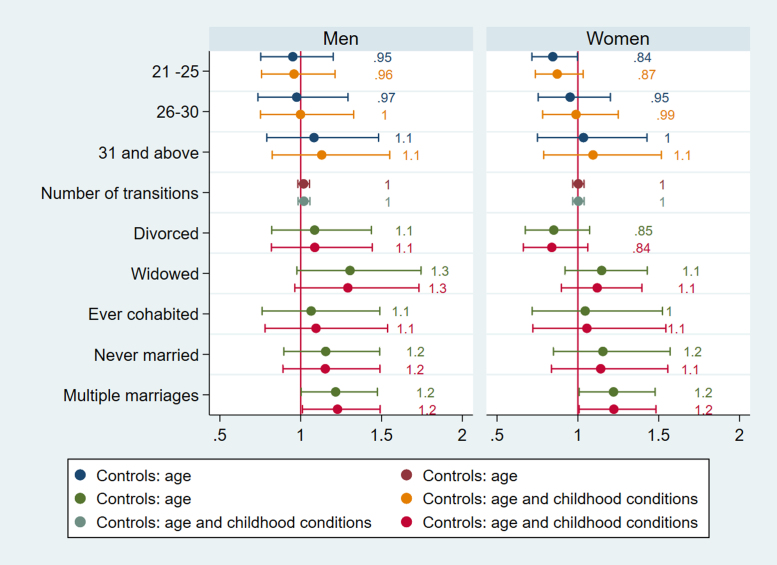
In Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6 and in Supplementary file Tables 7a-b and 8a-b we present the estimated parameters and 95% confidence intervals (or standard errors of OLS estimates) that capture the association between life course partnership and CVH in later life. Figures show the results from two sets of models, the first adjusted for age, and second set is adjusted for selection variables related to childhood conditions at age 10 (people per room, number of bookcases, parental background, absent biological parent). Linear regression coefficients and standard errors are presented for CPR, whereas odds ratios are presented for HbA1c, systolic and diastolic BP, and TC.
Fig. 1.
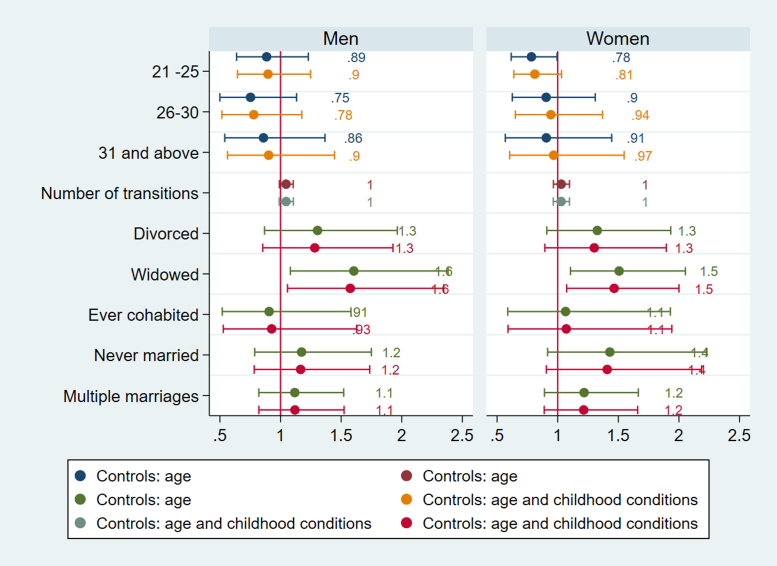
Logistic regressions for HbA1c England.
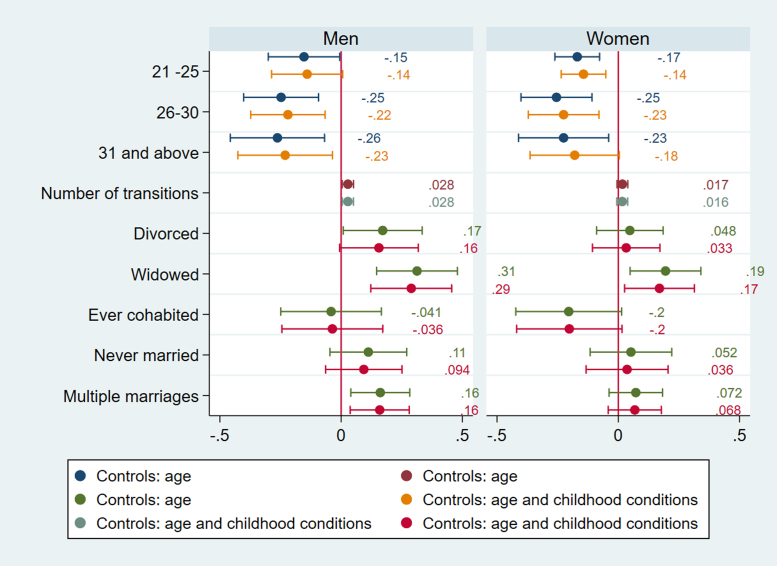
Fig. 2.
OLS regressions for C-reactive protein log England.
Fig. 3.
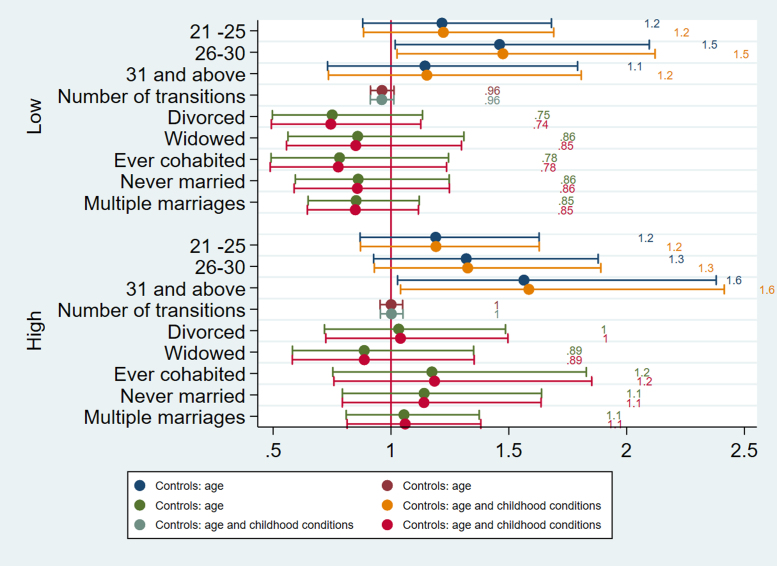
Multinomial logistic regressions for cholesterol England (Men).
Fig. 4.
Multinomial logistic regressions for cholesterol Germany (Men).
Fig. 5.
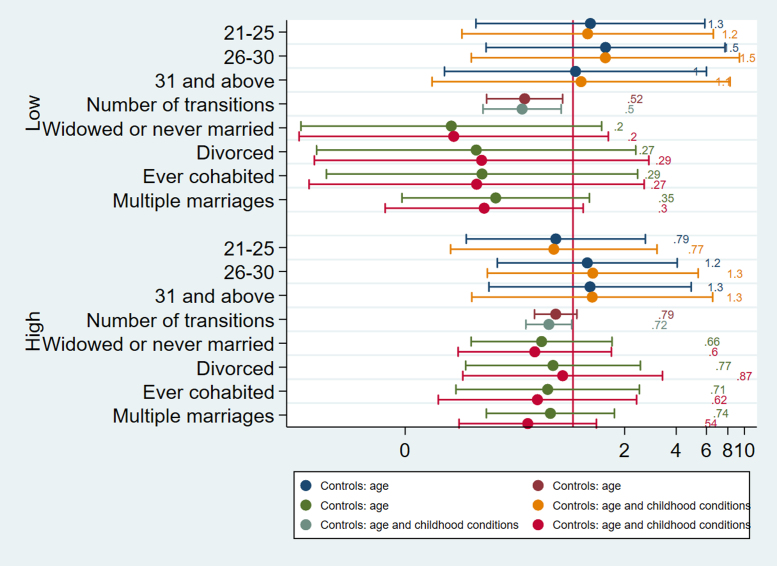
Logistic regressions for systolic BP England.
Fig. 6.
Logistic regressions for diastolic BP England.
4.1. Timing of partnership
The timing of first partnership is associated with poor cardiovascular health in England. Selection accounts for the effects of age at partnership on HbA1c for women in England as women partnered between ages 21–25 are more likely to have high HbA1c (b=0.78) compared to those with first partnership before their twenties (Fig. 1). For both men and women age at first partnership ‘on time’ or later (thus after 20) is associated with lower CRP (for men b= −0.219, CI =−0.373, −0.066; p=.005 for ages 25–30; b= −0.230, CI −0.426, −0.035; p=.02 for ages 31and above; for women b= −0.143, CI =−.236, −0.051; p=.002 for ages 21–25; b=-0.225, CI=-0.371,−0.079; p=.003 for ages 25–30)), but for women late partnership (31 and above) is no longer associated with inflammation after accounting for selection (Fig. 2). For men in England, age at first partnership on time (26–30) is associated with low cholesterol (b=0.388, CI=0.025, 0.752, p=.03), whereas late age at first partnership (31 and above) is associated with high cholesterol (b=0.461, CI=0.039, 0.882, p=.03) (Fig. 3). The results support the expectation that both early and late partnership are negatively associated with cardiovascular health.
4.2. Partnership transitions
The number of partnership transitions is associated with poor cardiovascular health in Germany. For men in Germany the number of partnership transitions is negatively associated with low TC (b=−.683; 95% CI = −1.208, −0.158; p=.01), as those with more transitions are less likely to have low cholesterol, as well as high cholesterol (b=−.324; 95% CI =-0.631, −0.016; p=.01) after accounting for selection processes related to childhood conditions (Fig. 4), thus there is also support for the hypothesis that the number of partnership transitions is negatively associated with cardiovascular health, though only in Germany.
4.3. Partnership trajectories
Partnership trajectories are associated with poor cardiovascular health both in Germany and England. Women in divorced trajectories in Germany are more likely to have elevated HbA1c in the model without selection variables (b= 0.505; 95% CI = 0.262, 0.976; p=.04), but the association does not longer hold in the full model adjusted for childhood conditions. This implies that selection into divorce exist for women in Germany, which also contributes to poor CVH in later life.
For England, men in widowed trajectories are more likely to have high systolic BP (OR= 1.583, CI= 1.133, 2.213, p=.007) whereas this is not the case for women (Fig. 5). Both men and women in widowed trajectories in England have elevated HbA1c compared to stably married persons (OR= 1.575, CI= 1.057, 2.346, p=.026 for men, OR=1.465, CI= 1.074, 2.000, p=.016), accounting for selection based on childhood conditions (Fig. 1). They are also more likely to have high CRP (b= 0.289, CI= 0.122, 0.456, p=.001 for men, b=0.170, CI= 1.026, 0.313, p=.021), but gender difference is apparent for divorce. Only men in divorced categories have higher CRP (b=0.17), but the results show that the association is due to selection (Fig. 2). This implies that selection into divorce exist for men in England, which also contributes to poor CVH in later life.
More notably, only men in trajectories with multiple marriages have higher CRP, and are more likely to have elevated systolic and diastolic BP (Fig. 6) (CRP b= 0.159, CI= 0.037, 0.281, p=.011; systolic BP OR= 1.296, CI=1.053, 1.597, p=.015, diastolic BP OR= 1.227, CI= 1.011,1.491, p=.039). Because the number of partnership transitions is not associated with CRP, systolic or diastolic BP for men, the results imply that it is not the length on the partnership sequence but the accumulation of marriages and disruptive events in between that affect health negatively for men.
In summary, this study finds that number of partnership transitions is associated with poorer cardiovascular health in Germany, whereas age at partnership and trajectories were found to be important for poor health in England.
5. Conclusion and discussion
This study adds to our understanding of the links between partnership over the life course and health in several ways. It is among the first studies to address longitudinal partnership trajectories that include timing, ordering and quantum of partnership events, including states as cohabitation, and how these characteristics relate to objective measures of cardiovascular health using haemostatic and inflammatory biomarkers.
The results suggest that partnership life course trajectories have lasting consequences for men’s and women’s physical health, dependent on the occurrence and timing of major life course events. In particular, groups characterized by early partnership formation, marital disruption, and multiplicity of marital disruption emerged as detrimental for cardiovascular health, with the observed differences only minimally affected by controls for early-life health and socioeconomic conditions. This pattern of results is consistent with previous research findings pertaining to the US, UK and Australia that indicate that marital disruptions (Dupre and Meadows, 2007, Hughes and Waite, 2009), and “on-time” family formation (Dupre et al., 2009, O’Flaherty et al., 2016, Grundy and Holt, 2000; Grundy & Tomassini 2005) are linked to health and mortality.
Although the analyses do not provide direct evidence about the mechanisms that link partnership life course trajectories to cardiovascular health, it is evident that both for men and women, the primary health benefits of the family life course are realized through committed partnership. However, for men only those in intact partnerships get to enjoy the “marriage benefits” on health, as partnership sequences containing multiple partnerships for men are associated with worse biomarker scores for almost all biomarkers used in the study. This association is entirely absent for women (despite women outnumbering men in both samples). It is likely that complex partnership sequences for men interact with other family and work domains (e.g. disrupted work careers, engaging in unhealthy behaviors such as smoking and drinking) to produce long-lasting effects on cardiovascular health.
The findings of this study also reveal that not women, but men’s cardiovascular health is more reactive to life course partnership characteristics when biomarkers are used. This is in contrast to previous studies that have investigated marital status and quality using the same biomarkers (CRP, HbA1c, blood pressure) and have found that women’s health was more strongly associated with marital quality (Robles et al., 2014). The gender specific effect of number of transitions on blood pressure and cholesterol level for men points out that cardiovascular reactivity (CVR) to stressful marital interactions might be more pronounced for men. Relationship quality seems to be more important for women, but both absence of partner and frequent partner swaps seem to be detrimental for older men’s cardiovascular health.
The analyses to some extent also contradicts previous studies that have used biomarkers and marital histories, as early men’s transition timing is related to worse biomarker scores (CRP and cholesterol), whereas McFarland et al. (2013) found no relationship between later age of marriage for men and cardiovascular risk. After identifying cohabiting individuals as a distinct group, we were not able to find differences between them and respondents who have stayed in intact marriages. This this contrasts with results from a study that used European data and life course partnership where cohabiting was related to worse health for men (Ploubidis et al., 2015).
Marital disruptions have previously been linked to worse health (Dupre and Meadows, 2007, Hughes and Waite, 2009), but the analysis showed that selection into divorce accounts for the negative relationship between partnership trajectories containing one disruption and bad cardiovascular health. The association between partnership trajectories containing one disruption and health differs for men and women. Men’s divorce in England was found to be related to high CRP (inflammation marker), whereas women’s divorce was associated with HbA1c (diabetes marker) in Germany, and childhood conditions accounted for these associations in both cases. As the results show no association between the number of transitions and biomarkers (except cholesterol) for men, they support the accumulated adversity argument that stressful events have a cumulative negative impact on health.
The observed effects of partnership characteristics differ in size between men and women, as well as between countries. One reason might be due to the sample differences, as studies have found that marital status may be associated with medication use. Additional analysis (not shown, refer to Sensitivity analysis in Supplementary file) where biomarkers exclude medication use show very similar patterns and almost identical association with life course partnership characteristics. By observing sample differences we also note a higher use of drugs in Germany compared to England, although in the UK use of statins is twice as high as in Germany according to OECD Health at glance study (2013). Another implication is that life course partnership status and health are linked by gender-specific, and in addition, country-specific mechanisms. This might be due to English respondents reporting poorer general health, leading to more significant associations between partnership and health in the English sample, but also due to selective mortality, especially pertaining to the German sample.
Several limitations must be noted when interpreting the results of this study. Although we included several biomarkers as CVH outcomes, the outcomes presented here are a narrow range of biomarkers. In addition, we employed retrospective observational data and despite the wealth of the SHARE and the ELSA data, future studies should aim to test the relationship between partnership and CVH using prospective data. Finally, we note that the results are restricted to individuals aged 50–80 at the time of the collection of the biomarkers. The partnership status trajectories, as well as the association between partnership and health, differ for younger cohorts and it is well expected that the relationship between partnership and health will be different in younger and older adults.
Further studies should seek to build on this and devote attention to the mechanisms that link partnership histories and health in later life with social support, health related behaviours and socioeconomic position over the life course. As the overwhelming majority of the literature to date focuses on mortality, it would be beneficial to focus more on health in later life. The implications and the results of such further research can be used to improve the quality of life of individuals, in addition to extending the life course.
Footnotes
The research leading to this publication was supported by funding from the European Research Council Advanced Investigator Grant “Families in Context “ (324211) and the “Healthy ageing, population and society” programme at the University of Groningen, The Netherlands.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ssmph.2018.08.001.
Appendix A. Supplementary material
Supplementary material
.
References
- Bardage C., Pluijm S.M., Pedersen N.L., Deeg D.J., Jylhä M., Noale M., Otero A. Self-rated health among older adults: A cross-national comparison. European Journal of Ageing. 2005;2(2):149–158. doi: 10.1007/s10433-005-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C.M., Guo M., Dharmage S.C. HbA1c as a screening tool for detection of type 2 diabetes: A systematic review. Diabetic Medicine. 2007;24(4):333–343. doi: 10.1111/j.1464-5491.2007.02106.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y., Kuh D. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology. 2002;31(2):285–293. [PubMed] [Google Scholar]
- Berntsen K.N., Kravdal Ø. The relationship between mortality and time since divorce, widowhood or remarriage in Norway. Social Science and Medicine. 2012;75:2267–2274. doi: 10.1016/j.socscimed.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Birditt K.S., Newton N.J., Cranford J.A., Ryan L.H. Stress and negative relationship quality among older couples: Implications for blood pressure. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2015;71(5):775–785. doi: 10.1093/geronb/gbv023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal J.A., Thyrum E.T., Siegel W.C. Contribution of job strain, job status and marital status to laboratory and ambulatory blood pressure in patients with mild hypertension. Journal of Psychosomatic Research. 1995;39(2):133–144. doi: 10.1016/0022-3999(94)00087-l. [DOI] [PubMed] [Google Scholar]
- Booth A., Amato P. Divorce and psychological stress. Journal of Health and Social Behavior. 1991:396–407. [PubMed] [Google Scholar]
- Börsch-Supan A., Brandt M., Hank K., Schröder M., editors. The individual and the welfare state. Life histories in Europe. Springer; Heidelberg: 2011. [Google Scholar]
- Börsch-Supan A., Brandt M., Hunkler C., Kneip T., Korbmacher J., Malter F., Schaan B., Stuck S., Zuber S. Data Resource Profile: The Survey of Health, Ageing and Retirement in Europe (SHARE) International Journal of Epidemiology. 2013 doi: 10.1093/ije/dyt088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt M., Deindl C., Hank K. Tracing the origins of successful aging: The role of childhood conditions and social inequality in explaining later life health. Social Science Medicine. 2012;74(9):1418–1425. doi: 10.1016/j.socscimed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Brockmann H., Klein T. Love and death in Germany: The marital biography and its effect on mortality. Journal of Marriage and Family. 2004;66(3):567–581. 〈http://www.jstor.org/stable/3600213〉 [Google Scholar]
- Cheshire H., Hussey D., Medina J., Pickering K., Wood N., Ward K., Lessof C. National Centre for Social Research; London: 2012. Financial circumstances, health and well-being of the older population in England: The 2008 English Longitudinal Study of Ageing. [Google Scholar]
- Corna L.M. A life course perspective on socioeconomic inequalities in health: A critical review of conceptual frameworks. Advances in Life Course Research. 2013;18(2):150–159. doi: 10.1016/j.alcr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Cundiff J.M., Birmingham W.C., Uchino B.N., Smith T.W. Marital quality buffers the association between socioeconomic status and ambulatory blood pressure. Annals of Behavioral Medicine. 2015;50(2):330–335. doi: 10.1007/s12160-015-9742-z. [DOI] [PubMed] [Google Scholar]
- De Leon C.F.M., Appels A.A., Otten F.W., Schouten E.G. Risk of mortality and coronary heart disease by marital status in middle-aged men in The Netherlands. International Journal of Epidemiology. 1992;21(3):460–466. doi: 10.1093/ije/21.3.460. [DOI] [PubMed] [Google Scholar]
- Deindl C. The influence of living conditions in early life on life satisfaction in old age. Advances in Life Course research. 2013;18:107–114. doi: 10.1016/j.alcr.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Dupre M.E., Beck A.N., Meadows S.O. Marital trajectories and mortality among US adults. American Journal of Epidemiology. 2009;170(5):546–555. doi: 10.1093/aje/kwp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre M.E., Meadows S.O. Disaggregating the effects of marital trajectories on health. Journal of Family Issues. 2007;28(5):623–652. [Google Scholar]
- Eaker E.D., Sullivan L.M., Kelly-Hayes M., D’Agostino Sr R.B., Benjamin E.J. Marital status, marital strain, and risk of coronary heart disease or total mortality: The Framingham Offspring Study. Psychosomatic Medicine. 2007;69(6):509–513. doi: 10.1097/PSY.0b013e3180f62357. [DOI] [PubMed] [Google Scholar]
- Ebrahim S., Wannamethee G., McCallum A., Walker M., Shaper A.G. Marital status, change in marital status, and mortality in middle-aged British men. American Journal of Epidemiology. 1995;142(8):834–842. doi: 10.1093/oxfordjournals.aje.a117723. [DOI] [PubMed] [Google Scholar]
- Elder G.H., Giele J.Z., editors. The craft of life course research. Guilford Press; 2009. [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2002). (September. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), NIH Publication No. 02-5215. Downloaded from 〈http://circ.ahajournals.org/content/106/25/3143.full.pdf〉 on August 10, 2015.
- Fardella C.E., Mosso L., Gómez-Sánchez C., Cortés P., Soto J., Gómez L., Montero J. Primary hyperaldosteronism in essential hypertensives: Prevalence, biochemical profile, and molecular biology 1. The Journal of Clinical Endocrinology Metabolism. 2000;85(5):1863–1867. doi: 10.1210/jcem.85.5.6596. [DOI] [PubMed] [Google Scholar]
- Green K.M., Doherty E.E., Fothergill K.E., Ensminger M.E. Marriage trajectories and health risk behaviors throughout adulthood among urban African Americans. Journal of family issues. 2012;33(12):1595–1618. doi: 10.1177/0192513X11432429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy E., Holt G. Adult life experiences and health in early old age in Great Britain. Social Science and Medicine. 2000;51:1061–1074. doi: 10.1016/s0277-9536(00)00023-x. [DOI] [PubMed] [Google Scholar]
- Grundy E.M., Tomassini C. Marital history, health and mortality among older men and women in England and Wales. BMC public health. 2010;10(1):554. doi: 10.1186/1471-2458-10-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon N., Hochstein M. Life course health development: An integrated framework for developing health, policy, and research. The Milbank Quarterly. 2002;80(3):433–479. doi: 10.1111/1468-0009.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K.M. An integrative approach to health. Demography. 2010;47(1):1–22. doi: 10.1353/dem.0.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havari E., Mazzonna F. Can we trust older people’s statements on their childhood circumstances? Evidence from SHARELIFE. European Journal of Population. 2015;31(3):233–257. [Google Scholar]
- Heaton T. Time-related determinants of marital dissolution. Journal of Marriage and Family. 1991;53:285–295. 〈http://www.jstor.org/stable/352899〉 [Google Scholar]
- Hemström Ö. Is marriage dissolution linked to differences in mortality risks for men and women? Journal of Marriage and the Family. 1996:366–378. [Google Scholar]
- Henretta J.C. Lifetime marital history and mortality after age 50. Journal of Aging and Health. 2010;22:1198–1212. doi: 10.1177/0898264310374354. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J., Birmingham W., Jones B.Q. Is there something unique about marriage? The relative impact of marital status, relationship quality, and network social support on ambulatory blood pressure and mental health. Annals of Behavioral Medicine. 2008;35(2):239–244. doi: 10.1007/s12160-008-9018-y. [DOI] [PubMed] [Google Scholar]
- Hughes M.E., Waite L.J. Marital biography and health at mid-life. Journal of Health and Social Behavior. 2009;50(3):344–358. doi: 10.1177/002214650905000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A., Iso H., Toyoshima H., Fujino Y., Mizoue T., Yoshimura T., Tamakoshi A. Marital status and mortality among Japanese men and women: The Japan Collaborative Cohort Study. BMC Public Health. 2007;7(1):73. doi: 10.1186/1471-2458-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N.J., Backlund E., Sorlie P.D., Loveless C.A. Marital status and mortality: The national longitudinal mortality study. Annals of Epidemiology. 2000;10(4):224–238. doi: 10.1016/s1047-2797(99)00052-6. [DOI] [PubMed] [Google Scholar]
- Joseph N.T., Kamarck T.W., Muldoon M.F., Manuck S.B. Daily marital interaction quality and carotid artery intima medial thickness in healthy middle aged adults. Psychosomatic Medicine. 2014;76(5):347. doi: 10.1097/PSY.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K., Ploubidis G.B., Silverwood R.J., Grundy E. Life-course partnership history and midlife health behaviours in a population-based birth cohort. Journal of Epidemiol Community Health. 2017;71(3):232–238. doi: 10.1136/jech-2015-207051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.K., McKenry P.C. The relationship between marriage and psychological well-being a longitudinal analysis. Journal of Family Issues. 2002;23(8):885–911. [Google Scholar]
- Kravdal H., Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11(1):804. doi: 10.1186/1471-2458-11-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegbaum M., Christensen U., Andersen P.K., Osler M., Lund R. Does the association between broken partnership and first time myocardial infarction vary with time after break-up? International Journal of Epidemiology. 2013;42(6):1811–1819. doi: 10.1093/ije/dyt190. [DOI] [PubMed] [Google Scholar]
- Kuh D., Ben-Shlomo Y., Lynch J., Hallqvist J., Power C. Life course epidemiology. Journal of Epidemiology and Community Health. 2003;57(10):778. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D., Shlomo Y.B. Oxford University Press; 2004. A life course approach to chronic disease epidemiology. [Google Scholar]
- Lamb K.A., Lee G.R., DeMaris A. Union formation and depression: Selection and relationship effects. Journal of Marriage and Family. 2003;65(4):953–962. 〈http://www.jstor.org/stable/3599902〉 [Google Scholar]
- Liefbroer A.C., Billari F.C. Bringing norms back in: A theoretical and empirical discussion of their importance for understanding demographic behaviour. Population, Space and Place. 2010;16(4):287–305. [Google Scholar]
- Lindström M. Marital status, social capital, material conditions and self-rated health: A population-based study. Health Policy. 2009;93(2):172–179. doi: 10.1016/j.healthpol.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Liu H., Waite L., Shen S. Diabetes risk and disease management in later life: A national longitudinal study of the role of marital quality. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2016;71(6):1070–1080. doi: 10.1093/geronb/gbw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks E.B., Berkman L.F., Gruenewald T.L., Seeman T.E. Social integration is associated with fibrinogen concentration in elderly men. Psychosomatic Medicine. 2005;67(3):353–358. doi: 10.1097/01.psy.0000160482.89163.e8. [DOI] [PubMed] [Google Scholar]
- Lund R., Holstein B.E., Osler M. Marital history from age 15 to 40 years and subsequent 10-year mortality: A longitudinal study of Danish males born in 1953. International Journal of Epidemiology. 2004;33:389–397. doi: 10.1093/ije/dyh065. [DOI] [PubMed] [Google Scholar]
- Malter F., Börsch-Supan A., editors. SHARE Wave 4: Innovations & Methodology. MEA, Max Planck Institute for Social Law and Social Policy; Munich: 2013. [Google Scholar]
- Malyutina S., Bobak M., Simonova G., Gafarov V., Nikitin Y., Marmot M. Education, marital status, and total and cardiovascular mortality in Novosibirsk, Russia: A prospective cohort study. Annals of Epidemiology. 2004;14(4):244–249. doi: 10.1016/S1047-2797(03)00133-9. [DOI] [PubMed] [Google Scholar]
- Manfredini R., De Giorgi A., Tiseo R., Boari B., Cappadona R., Salmi R., Gallerani M., Signani F., Manfredini F., Mikhailidis D., P., Fabbian F. Marital status, cardiovascular diseases, and cardiovascular risk factors: A review of the evidence. Journal of Women’ States Health. 2017;26(6):624–632. doi: 10.1089/jwh.2016.6103. [DOI] [PubMed] [Google Scholar]
- Mazzonna F. The long–lasting effects of family background: A European cross–country comparison. Economics of Education Review. 2014;40:25–42. [Google Scholar]
- McFarland M.J., Hayward M.D., Brown D. I’ve got you under my skin: Marital biography and biological risk. Journal of Marriage and Family. 2013;75(2):363–380. doi: 10.1111/jomf.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy G.J., Stamatakis E., Randall G., Hamer M. Marital status, gender and cardiovascular mortality: Behavioural, psychological distress and metabolic explanations. Social Science Medicine. 2009;69(2):223–228. doi: 10.1016/j.socscimed.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NatCen Social Research (2014). (June. English Longitudinal Study of Ageing (ELSA) Wavw 2,4 and 6 User Guide to the nurse datasets.
- Neugarten B.L. Time, age, and the life cycle. The American Journal of Psychiatry. 1979 doi: 10.1176/ajp.136.7.887. [DOI] [PubMed] [Google Scholar]
- Neugarten B.L., Moore J.W., Lowe J.C. Age norms, age constraints, and adult socialization. American journal of Sociology. 1965;70(6):710–717. doi: 10.1086/223965. 〈http://www.jstor.org/stable/2774397〉 [DOI] [PubMed] [Google Scholar]
- O’Flaherty M., Baxter J., Haynes M., Turrell G. The family life course and health: Partnership, fertility histories, and later-life physical health trajectories in Australia. Demography. 2016;53(3):777–804. doi: 10.1007/s13524-016-0478-6. [DOI] [PubMed] [Google Scholar]
- Organization for Economic Cooperation and Development (OECD) Staff (2013). Health at a Glance 2013: OECD Indicators. OECD. 〈http://www.oecd.org/els/health-systems/Health-at-a-Glance-2013.pdf〉.
- Ploubidis G., Silverwood R., De Stavola B., Grundy E. Life course partnership status and biomarkers in mid-life: Evidence from the 1958 British birth cohort. American Journal of Public Health. 2015;105(8):1596–1603. doi: 10.2105/AJPH.2015.302644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port S., Demer L., Jennrich R., Walter D., Garfinkel A. Systolic blood pressure and mortality. The Lancet. 2000;355(9199):175–180. doi: 10.1016/S0140-6736(99)07051-8. [DOI] [PubMed] [Google Scholar]
- Robles T.F., Slatcher R.B., Trombello J.M., McGinn M.M. Marital quality and health: Ameta-analytic review. Psychological Bulletin. 2014;140(1):140. doi: 10.1037/a0031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G.A., Fihn S.D., Mokdad A.H., Aekplakorn W., Hasegawa T., Lim S.S. High total serum cholesterol, medication coverage and therapeutic control: An analysis of national health examination survey data from eight countries. Bulletin of the World Health Organization. 2011;89(2):92–101. doi: 10.2471/BLT.10.079947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P., White I.R. Multiple imputation by chained equations (MICE): Implementation in Stata. Journal of Statistical Software. 2011;45(4):1–20. [Google Scholar]
- Schaan B. Collection of biomarkers in the survey of health, ageing and retirement in Europe (SHARE) In: Malter F., Börsch-Supan A., editors. SHARE Wave 4: Innovations & methodology. MEA, Max Planck Institute for Social Law and Social Policy; Munich: 2013. pp. 38–46. [Google Scholar]
- Schiller J.S., Lucas J.W., Ward B.W., Peregoy J.A. Summary health statistics for US Adults: National Health Interview Survey, 2010. Vital and Health Statistics Series 10, Data from The National Health Survey. 2012;252:1–207. [PubMed] [Google Scholar]
- Schoenborn, C.A. (2004). Marital status and health, United States 1999–2002. [PubMed]
- Scholes S., Medina J., Cheshire H., Cox K., Hacker E., Lessof C. National Centre for Social Research; London: 2009. Living in the 21st century: Older people in England. The 2006 English longitudinal study of ageing. (Technical report) [Google Scholar]
- Schröder M. SHARELIFE methodology. Mannheim: Mannheim Research Institute for the Economics of Aging (MEA); 2011. Retrospective data collection in the Survey of Health, Ageing and Retirement in Europe. [Google Scholar]
- Settersten R.A., Jr, Hägestad G.O. What’s the latest? Cultural age deadlines for family transitions. The Gerontologist. 1996;36(2):178–188. doi: 10.1093/geront/36.2.178. [DOI] [PubMed] [Google Scholar]
- Shen B.J., Farrell K.A., Penedo F.J., Schneiderman N., Orth-Gomer K. Waist circumference moderates the association between marital stress and C-reactive protein in middle-aged healthy women. Annals of Behavioral Medicine. 2010;40(3):258–264. doi: 10.1007/s12160-010-9211-7. [DOI] [PubMed] [Google Scholar]
- Shen B.J., Xu Y., Eisenberg S. Psychosocial and physiological predictors of mortality in patients of heart failure: Independent effects of marital status and C-reactive protein. International Journal of Behavioral Medicine. 2017;24(1):83–91. doi: 10.1007/s12529-016-9579-2. [DOI] [PubMed] [Google Scholar]
- Soons J.P., Kalmijn M. Is marriage more than cohabitation? Well‐being differences in 30 european countries. Journal of Marriage and Family. 2009;71(5):1141–1157. 〈http://www.jstor.org/stable/27752530〉 [Google Scholar]
- Steptoe A., Breeze E., Banks J., Nazroo J. Cohort profile: The English longitudinal study of ageing. International Journal of Epidemiology. 2012 doi: 10.1093/ije/dys168. (doi: dys168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno Kozo, Ohsawa Masaki, Itai Kazuyoshi, Kato Karen, Turin Tanvir Chowdhury, Onoda Toshiyuki, Sakata Kiyomi, Okayama Akira, Fujioka Tomoaki. Associations of marital status with mortality from all causes and mortality from cardiovascular disease in Japanese haemodialysis patients. Nephrology Dialysis Transplantation. 2013;28(4):1013–1020. doi: 10.1093/ndt/gfs547. (1 April) [DOI] [PubMed] [Google Scholar]
- Thierry X. Risks of mortality and excess mortality during the first ten years of widowhood. Population: An English Selection. 2000;12:81–110. [Google Scholar]
- Trief P.M., Himes C.L., Orendorff R., Weinstock R.S. The marital relationship and psychosocial adaptation and glycemic control of individuals with diabetes. Diabetes Care. 2001;24(8):1384–1389. doi: 10.2337/diacare.24.8.1384. [DOI] [PubMed] [Google Scholar]
- Trief P.M., Morin P.C., Izquierdo R., Teresi J., Starren J., Shea S., Weinstock R.S. Marital quality and diabetes outcomes: The IDEATel Project. Families, Systems, Health. 2006;24(3):318. [Google Scholar]
- Venters M., Jacobs D.R., Pirie P., Luepker R.V., Folsom A.R., Gillum R.F. Marital status and cardiovascular risk: The Minnesota heart survey and the Minnesota heart health program. Preventive Medicine. 1986;15(6):591–605. doi: 10.1016/0091-7435(86)90064-2. [DOI] [PubMed] [Google Scholar]
- Ward K., Medina J., Mo M., Cox K. National Centre for Social Research; London: 2009. ELSA wave three: Life history interview – A user guide to the data. [Google Scholar]
- Wheaton B. Life transitions, role histories, and mental health. American Sociological Review. 1990:209–223. [Google Scholar]
- White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Williams K., Umberson D. Marital status, marital transitions, and health: A gendered life course perspective. Journal of Health and Social Behavior. 2004;45:81–98. doi: 10.1177/002214650404500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Penning M.J., Pollard M.S., Hart R. “In sickness and in health” does cohabitation count? Journal of Family Issues. 2003;24(6):811–838. [Google Scholar]
- Zhang Z. Marital history and the burden of cardiovascular disease in midlife. Gerontologist. 2006;46:266–270. doi: 10.1093/geront/46.2.266. [DOI] [PubMed] [Google Scholar]
- Zheng H., Thomas P.A. Marital status, self-rated health, and mortality: Overestimation of health or diminishing protection of marriage? Journal of Health and Social Behavior. 2013;54(1):128–143. doi: 10.1177/0022146512470564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Shu B., Yang J., Liu J., Xi T., Xing Y. C-reactive protein, interleukin-6 and the risk of colorectal cancer: A meta-analysis. Cancer Causes Control. 2014:1–9. doi: 10.1007/s10552-014-0445-8. [DOI] [PubMed] [Google Scholar]
- Zhu H., Gu D. The protective effect of marriage on health and survival: Does it persist at oldest-old ages? Journal of Population Ageing. 2010;3(3-4):161–182. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material