Abstract
We have previously demonstrated that apigenin promotes the expression of antiangiogenic protein thrombospondin-1 (TSP1) via a mechanism driven by mRNA-binding protein HuR. Here, we generated a novel mouse model with whole-body THBS-1 gene knockout on SKH-1 genetic background, which allows studies of UVB-induced acute skin damage and carcinogenesis and tests TSP1 involvement in apigenin's anticancer effects. Apigenin significantly inhibited UVB-induced carcinogenesis in the wild-type (WT) animals but not in TSP1 KO (TKO) mice, suggesting that TSP1 is a critical component of apigenin's chemopreventive function in UVB-induced skin cancer. Importantly, TKO mice presented with the elevated cutaneous inflammation at baseline, which was manifested by increased inflammatory infiltrates (neutrophils and macrophages) and elevated levels of the two key inflammatory cytokines, IL-6 and IL-12. In agreement, maintaining normal TSP1 expression in the UVB-irradiated skin of WT mice using topical apigenin application caused a marked decrease of circulating inflammatory cytokines. Finally, TKO mice showed an altered population dynamics of the bone marrow myeloid progenitor cells (CD11b+), with dramatic expansion of the population of neutrophil progenitors (Ly6ClowLy6Ghigh) compared to the WT control. Our results indicate that the cutaneous tumor suppressor TSP1 is a critical mediator of the in vivo anticancer effect of apigenin in skin, specifically of its anti-inflammatory action.
Abbreviations: BM, bone marrow; COX-2, cyclooxygenase 2; IL, interleukin; MPO, myeloperoxidase; NHEKs, normal human epidermal keratinocytes; NMSC, nonmelanoma skin cancer; TSP1, thrombospondin 1; UVB, ultraviolet B; VEGF, vascular endothelial growth factor
Introduction
There are more cases of skin cancer in the United States population than all other cancers combined. These cancers are on the rise and represent a significant health and economic problem. Nonmelanoma skin cancer (NMSC) occurs in all races worldwide, and more than two million new cases are diagnosed annually in the USA alone. Extensive epidemiologic, clinical, and biological studies have proven that ultraviolet B (UVB) radiation is the major cause of NMSC [1], [2], [3], [4], [5]. Despite significant risk mitigation by the application of sunscreens, a substantial part of the population remains subjected to increasing UVB exposure due to occupational hazards, recreational activities, and climate changes, causing a continued rise in the NMSC incidence [6].
Skin is a critical barrier organ, which maintains organismal defenses against environmental insults. A proper inflammatory milieu in skin relies on reciprocal communications between the epidermal keratinocytes and other cell types [7], [8], [9]. Acute inflammation due to exposure to UVB radiation or xenobiotics involves massive production of cytokines and chemokines, often underpinned by cyclooxygenase (COX)-2 expression [10], [11], [12]. The resultant recruitment of neutrophils [13], monocytes, and macrophages [14] to the site of irradiation and further dynamic interactions between newly recruited inflammatory cells, which continuously produce inflammatory cytokines and matrix-degrading enzymes, further amplify the inflammatory responses, leading to acute responses, such as edema, or chronic changes including inflammation, fibrosis, and cancer [10], [11].
In addition, UVB irradiation can induce potent angiogenic response, vascular leakage, and edema caused by the elevated vascular endothelial growth factor (VEGF) secretion by epidermal keratinocytes and fibroblasts, and exacerbated by attenuated production of an angiogenesis inhibitor thrombospondin-1 (TSP1) [15], [16], [17]. TSP1, a 450-kDa trimeric matricellular protein and the first identified endogenous angiogenesis inhibitor [18], [19] is expressed at high levels in normal skin, especially in epidermal keratinocytes. On the cellular level, TSP1 blocks angiogenesis by causing endothelial cell apoptosis [20]. It impedes angiogenesis by blocking endothelial cell proliferation, chemotaxis [21], and through depletion of circulating endothelial cell progenitors, as was shown using TSP1 peptide mimetics [22]. TSP1 expression is severely downregulated in mouse and human epidermis following UVB irradiation and throughout progressive steps of skin carcinogenesis [15], [23], [24], [25], [26], [27]. In contrast, overexpression of TSP1 in murine epidermis curtails angiogenesis and reduces the incidence of squamous cell carcinoma in a two-stage chemical model of skin carcinogenesis [25].
The antiangiogenic properties of TSP1 are mediated via two cell surface receptors, CD36 and CD47 [28], whereby TSP1 binding to CD36 causes the induction of Fas-Fas ligand [20] or TRAIL–TRAIL-R dependent death [29] predominantly in the endothelial cells. CD47-dependent events entail suppression of endothelial nitric oxide synthase (NOS) activity and NO production [30], [31]. The decreased TSP1 levels have been linked to inflammation in the gut, whereby mice with whole body TSP1 knockout display aggravated symptoms of DSS-induced colitis, an experimental model of inflammatory bowel disease, and this condition can be ameliorated by administration of TSP1 peptide mimetic ABT-510 [32], [33], [34]. However, in patients with atopic dermatitis, circulating TSP1 levels show reverse trend (positive correlation with disease progression) [35], and the exact mechanisms by which TSP1 controls inflammatory responses are not clearly understood. In an earlier study [36], we have confirmed findings by others where UVB irradiation blocks TSP1 expression in skin keratinocytes in vitro and in vivo. Importantly, we demonstrated for the first time that normally high TSP1 expression in UVB-irradiated keratinocytes and skin can be restored by application of a natural bioflavonoid apigenin, which is known for its anti-inflammatory and antiangiogenic properties.
Apigenin (5,7,4′-trihydroxyflavone) is present in a wide variety of food sources including sweet pepper, parsley, thyme, celery, onions, and tea [37]. Topical application of apigenin reduces the incidence and the size of the tumors in both chemical and UVB-induced mouse models of skin carcinogenesis [38], [39]. Apigenin has multiple properties of a successful chemopreventive agent. It inhibits angiogenesis via multiple pathways, including suppression of proangiogenic transcription factor HIF-1α and downstream major angiogenic growth factor VEGF [40], [41], [42] as well as COX-2/prostaglandin E2 related pathways [36], [43], [44]. Several in vitro studies also indicate the inhibition of NOS and IL6/STAT pathways by apigenin [45], [46]. Apigenin also can stabilize and enhance the expression of tumor suppressive p53 [47], [48] and therefore cause cell cycle arrest [49], [50] and apoptosis [51], [52], [53], [54]. In addition, apigenin displays potent anti-inflammatory activity in skin and other tissues [55]. Notably, apigenin inhibits UVB-induced expression of proinflammatory COX-2 in skin through multiple mechanisms [56], [57]. Importantly, we have demonstrated that apigenin, via a mechanism driven by mRNA-binding protein HuR, restores the expression of antiangiogenic TSP1 and that proliferative effects of apigenin as well as its ability to block HIF-1α and COX-2 are, at least in part, mediated through TSP1. Here, we generated a novel mouse model with whole-body THBS-1 gene knockdown on SKH-1 genetic background, which allows studies of UVB-induced acute skin damage and carcinogenesis as well as therapeutic interventions, unhampered by pigmented skin and the coat of hair. Hairless mice with genetic ablation of TSP1 mounted an exacerbated cutaneous response to UVB, both in a short-term model and in a long-term (carcinogenesis) study. Topical application of chemopreventive agent apigenin significantly ameliorated UVB-induced carcinogenesis in the wild-type (WT) animals but not in TSP1 KO (TKO) mice, suggesting that TSP1 is a critical component of apigenin's anticancer and/or chemopreventive action. Importantly, TKO mice presented with the elevated cutaneous inflammation at baseline, which was manifested by increased inflammatory infiltrates (neutrophils and macrophages) and elevated levels of the two key inflammatory cytokines, IL-6 and IL-12. In addition, TKO mice showed a different population dynamics of the bone marrow (BM) myeloid progenitor cells (CD11b+), with dramatic expansion of the population of neutrophil progenitors (Ly6ClowLy6Ghigh) compared to WT control. Our data suggest that, in skin, TSP1 is a critical molecule that suppresses cutaneous carcinogenesis through its antiangiogenic and especially anti-inflammatory action.
Materials and Methods
All chemicals were from Sigma-Aldrich (St Louis, MO) unless otherwise specified. Apigenin was prepared as 50 mM stock in DMSO and kept at −20°C in aliquots.
Generation of TSP1-Null Mice on SKH-1 Background
All housing and experimental procedures were approved by the Animal Care and Use Committee at Northwestern University and performed in a strict adherence with guidelines provided by the National Institutes of Health. Homozygous THBS1 −/− mice on C56BL/6 background (Jackson Labs, Bar Harbor, MN) were back-crossed onto hairless SKH-1 mice (Charles River, Houston, TX), and F5 hairless heterozygotes were selected and crossed onto each other to generate homozygous offspring, which was identified by genotyping (tail-snips). Tail DNA was prepared by proteinase K digestion at 55°C followed by isopropanol precipitation and used for PCR with reverse TSP1 primer (GAGTTTGCTTGTGGTGAACGCTCAG) and a forward TSP1 primer (AGGGCTATGTGGAATTAATATCGG). The expected size of PCR product is 700 bp for the wild type and 400 bp for the TSP null, respectively [58]. The pups homozygous for THBS1 loss were subjected to further back-crossing (six generations) to ensure the gain of the new hairless trait together with the loss of THBS1 expression. Genotyping was performed routinely to confirm knockout status (TransnetYX, Cordova, TN).
Treatment of Animals
Adult (6-8 weeks old) wild-type (WT) and THBS1−/− (TSP1 KO, TKO) mice were switched to flavonoid-free AIN-93 M diet (Scientific Animal Feeds, Inc., Arlington Heights, IL) 1 week prior to UVB irradiation or apigenin treatment. WT and TKO mice were each assigned to one of three groups. Control (C) group was treated with 200 μl topical vehicle (1:9, v/v DMSO:acetone mix) 1 hour prior to sham radiation; the UVB radiation (R) group was similarly treated with vehicle 1 hour prior to UVB exposure; in the UVB plus Apigenin (AR) group, 5 μmol apigenin in 1:9 v/v DMSO:acetone was topically applied to the dorsal skins 1 hour prior to UVB exposure. To detect early morphologic changes before the appearance of any visible skin lesions after UVB irradiation, Berg et al. have established a robust model in SKH-1 hairless mice exposed to UVB radiation [59]. In short-term studies, we used the same dose of 1300 J/m2 of UVB to irradiate mice (n=5) daily for 5 days. In the long-term tumorigenesis study, mice (n=13 mice in each group except n=11 in WT and TKO C groups) were treated with to 2240 J/m2 of UVB (previously determined to be 1 minimal erythema dose [60]) three times per week for 22 weeks. Animals were sacrificed 24 hours after the final UVB irradiation; dorsal skins were excised, divided into three parts, and processed for various purposes as follows: (1) fixed in 10% neutral-buffered formalin for immunostaining, (2) cryopreserved in OCT compound for immunofluorescence, (3) flash-frozen to generate epidermal lysates for RNA and protein analyses (ELISA and/or Western blotting).
Analysis of Tumor Progression
Tumor onset was monitored three times weekly by exterior examination. Mice were briefly sedated and larger tumors measured with a digital caliper. Dorsal skin areas were also photographed for permanent record. Tumor types at endpoint were identified by histological analyses of hematoxylin and eosin–stained skin sections.
Cell Culture
Primary normal human epidermal keratinocytes (NHEKs) were derived from neonatal foreskin and isolated by Skin Disease Research Center (Northwestern University) as described previously [36]. NHEKs were maintained in M154CF keratinocyte medium supplemented with 10 μg/ml gentamicin and 0.25 μg/ml amphotericin B and human keratinocyte growth supplement (Invitrogen, Carlsbad, CA). The mouse 308 keratinocyte cell line was maintained in suspension in minimum essential media (United States Biological, Swampscott, MA) supplemented with 8% chelexed (Bio-Rad, Hercules, CA) fetal bovine serum, 0.02 mM Ca2+, 2 mM L-glutamine, 0.1 mM nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Cells were grown to 80%-90% confluence and pretreated with apigenin or vehicle control 1 hour prior to UVB irradiation. Apigenin stock solution (50 mM in DMSO) was kept at −20°C and diluted with cell culture medium to required concentration. For UVB exposure, the culture media were removed and saved, cells were rinsed with PBS and irradiated, and the media were replaced.
UVB Irradiation
UVB radiation was provided by FS40T12 lamps (National Biologicals, Twinsburg, OH) with peak emission at 313 nm. To eliminate UVC wavelengths (below 295 nm), Kodacel K6808 filter (Eastman Kodak, Rochester, NY) was used. Cultured cells were irradiated as previously described [57]. For irradiation of mice, same UV lamps and filter were used, and mice were irradiated in a cage, with the lid removed and placed in a chamber with UVB source located at the top of the chamber. The doses of UV irradiation and treatment frequency were described above in the “Treatment of Animals” section.
ELISA
Blood samples were collected by intracardiac puncture in tubes containing 50 mM EDTA. The samples were kept on ice and centrifuged at 2000 rpm for 15 minutes (4°C) to separate cellular elements. Plasma samples were aliquoted and stored at −80°C. Plasma concentrations of IL-6, IL-12, PEDF-BB, and MPB-1 were determined in pooled samples using customized murine cytokine ELISA kit (Signosis, Santa Clara, CA) according to the manufacturer's instructions. Concentrations of VEGF in pooled plasma samples were determined using murine VEGF-specific ELISA kit (R&D Systems, Minneapolis, MN) following the manufacturer's instructions.
Measurement of Myeloperoxidase (MPO) Activity
The UVB-induced neutrophil migration into the skin was evaluated by MPO activity determined by kinetic-colorimetric assay. MPO was extracted by suspending the material in 0.5% hexadecyltrimethylammonium bromide. Skin lysates were diluted 20-fold with 50 mM potassium phosphate buffer (pH 6.0) containing 0.167 mg/mL o-dianisidine dihydrochloride and 0.5% hexadecyltrimethylammonium bromide. The reaction was initiated by adding the substrate, 0.01% peroxide. The MPO activity was determined spectrophotometrically by monitoring the absorbance at 450 nm for a period of 5 minutes.
Immunohistochemistry
Formalin-fixed dorsal skin samples were embedded lengthwise in paraffin and cut into 5-μm–thick sections. Cryopreserved samples were cut into 7-μm sections. Paraffin-embedded sections were used to stain for COX-2, TSP1, and F4/80 (macrophage marker). Staining for CD31 (vascular endothelial marker, PECAM-1) was performed on frozen sections. COX-2 immunostaining of interfollicular epidermal areas was scored by semiautomated analysis of images using HistoQuest 6.0 software (TissueGnostic, Tarzan, CA).
RNA Isolation and Quantitative RT-PCR (Q-PCR) Analysis
Total cellular RNA was isolated using Zymo RNA extraction kit (Genesee Scientific, San Diego, CA) following manufacturer's instructions. Reverse transcription of equal amounts of total RNA was performed using the Super Script III first-strand synthesis system with randomized hexamer primers (Invitrogen). Reverse transcription was first carried out to generate cDNA using total RNA with random oligo primers, dNTPs, and SuperScript III reverse transcriptase (Invitrogen). cDNA products were diluted to 2.5 ng/μl, and SYBR Green qPCR was carried out using the following cycling parameters: 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. A final dissociation step was performed at 95°C to obtain melting curves of the final PCR products. The fluorescence threshold cycle value (Ct) was obtained for each curve and normalized to that obtained for the GAPDH housekeeping gene in the same sample to normalize for discrepancies in sample loading. The differences in Ct values between treated and control samples were then computed and exponentially multiplied to the base of 2 to obtain relative differences in expression levels. All experiments were carried out in duplicates and independently performed at least three times.
Flow Cytometry of Bone Marrow Isolates
Mouse bone marrow (BM) cells were isolated from the tibia and femurs of the TKO and WT control mice bred on SKH-1 background. In brief, after UVB radiation and apigenin treatment, mice were sacrificed and the hind limbs were removed leaving the femur and tibia intact. Excess muscle and tissue were removed from the bones and the bones cut just below the joints. The BM was flushed with 500 μl of calcium- and magnesium-free DPBS using a 27-gauge needle. The BM was passed through a 70-μm cell strainer, centrifuged at 300×g, and resuspended in ACK lysis buffer (Roche) for 10 minutes at 4°C. Cells were washed in PBS and then resuspended in FACS buffer (BioLegend, San Diego, CA). Isolated BM was blocked with anti-CD16/CD32 FC block (BD Biosciences, San Jose, CA) for 20 minutes at room temperature and then stained in 100 μl of antibody mixture for 1 hour at room temperature. The antibodies used for analysis were anti-CD11b conjugated Alexa fluor 647, anti-Ly6C conjugated PE, and anti-Ly6G conjugated brilliant violet (BioLegend). Cells were then washed three times in FACS buffer. Samples were analyzed on Fortessa LSR II Flow Cytometer (BD Biosciences), and the results were read using FCS Express 6 software (DeNovo Software, Glendale, CA).
Statistical Analyses
Statistical analyses of the data were performed in pairwise comparisons using two-sided Student's t test using Prism 6 software (GraphPad Software, Inc., San Diego, CA). The normality of the data was verified using Kolmogorov-Smirnoff test. For survival analysis (Kaplan-Meier curves), statistical significance was determined using log-rank sum test. Multiple group comparisons were evaluated for significance using two-way ANOVA. P values below .05 were considered statistically significant. All data were expressed as the mean ± standard error (SEM).
Results
TSP1 Plays a Critical Role in Apigenin's Chemopreventive Function to Inhibit UVB-Induced Skin Carcinogenesis
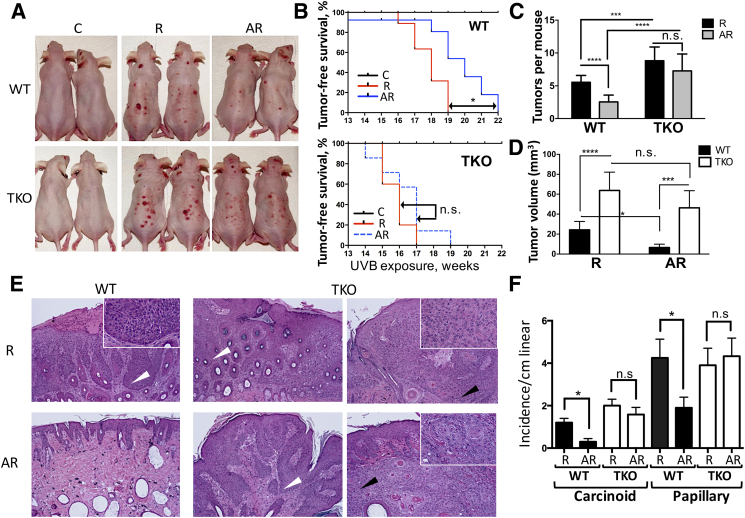
Previous study has shown that topical application of apigenin to mouse skin reduces the number and size of NMSC tumors induced by UVB exposure [39]. More recently, we have demonstrated that the marked UVB-induced suppression of the major endogenous angiogenesis inhibitor TSP1 in the epidermis can be restored by apigenin [36]. To test whether TSP1 may contribute to the anticancer effects of apigenin, we generated a unique model where the hairless mice were bred on THBS1 null genetic background. We then proceeded to assess whether THBS1 knockout enables the UVB-induced carcinogenesis and whether it undermines the protective effects of apigenin in this model. Both WT and TKO mice were divided into three groups: Control (C, n=11); UVB-irradiated (R, n=13); and apigenin-treated, UVB-irradiated group (AR, n=13). The C group was sham-irradiated, while the UVB-irradiated groups were exposed to 2240 J/m2 UVB three times per week for 22 weeks. In the AR group, mice received topical applications of apigenin in DMSO/acetone vehicle (see Methods) 1 hour prior to UVB irradiation, while only vehicle was applied to the C and R groups of animals. Within the timeframe of experiment, none of the mice in the C group developed observable skin lesions regardless of TSP1 expression (Figure 1, A and B). In contrast, both WT and TKO mice developed skin lesions of varying sizes, and TKO mice showed increased susceptibility to the UVB-induced skin carcinogenesis, whereby TKO mice in R group developed first tumors 2 weeks earlier than their wild-type counterparts (Figure 1, A and B). The tumor-free survival was significantly different between WT and TKO groups (P≤.0005 as determined by log-rank test). Moreover, TKO mice presented with significantly more lesions per mouse (Figure 1, A and C). Similarly, the average tumor size in the TKO R group at the end point (22 weeks) was significantly larger than that in the WT R group (Figure 1D).
Figure 1.
UVB-induced skin carcinogenesis and the chemoprevention by apigenin in wild-type (WT) and TSP1 knockout (TKO) SKH-1 hairless mice. Controls (C, n=11) were treated with sham irradiation and control vehicle (acetone:DMSO); R (n=13) were treated with UVB irradiation, 2240 J/m2 3 times per week, 22 weeks; AR (n=13) were treated with 5 μmol of apigenin per mouse 1 hour prior to UVB irradiation. (A) Physical appearance of representative mice from experimental groups 22 weeks after treatment initiation. (B) Analysis of tumor-free survival (Kaplan-Meier curves). The statistical significance is determined by log rank test. (C) Tumor incidence expressed as average tumor number per animal in each group and statistical significance determined by two-way ANOVA. (D) Average tumor volume at 22 weeks after initiation of UVB irradiation. Tumor volume was calculated using the following formula: tumor volume = 4π/3 (L/2)×(W/2)×(H/2), where L is the length, W is the width, and H is the height. Statistical significance is determined by two-sided Student's test. (E) Histopathological analysis of skin cancers in WT and TKO mice after UVB and apigenin treatments. Representative skin sections stained with H&E are shown. White arrowheads point to the papillomas, with no breach in the epithelial basement membrane (inset) and high degree of keratinization. Black arrows point to the more invasive carcinoid tumors with a spindle component (inset). (F) The comparison of different tumor types observed in WT and TKO animals. Statistical significance was calculated by two-sided Student's t test in pairwise comparisons (*P≤.05; ***P≤.001; ****P≤.0001; n.s., not significant).
Strikingly, the chemopreventive effect of apigenin against UVB-induced carcinogenesis was much weaker in TKO mice compared to their WT counterparts. At the end of the 22-week observation period, the number of tumors in the WT AR group was reduced by 55% compared to the WT R group, and this reduction was statistically significant, whereas tumor incidence was decreased by less than 18% between the TKO AR and TKO R groups, and this decrease did not reach statistical significance as was determined by two-way ANOVA (Figure 1, A and C). A subsequent histopathological examination of skin lesions confirmed the tumor-suppressive function of TSP1 in UVB-induced skin carcinogenesis. Histopathological evaluation of H&E skin sections (Figure 1E) showed increased incidence of carcinomas and papillomas in the TKO AR group compared to WT AR group (Figure 1F), suggesting diminished chemopreventive capacity of apigenin against UVB-induced carcinogenesis in the absence of TSP1.
Taken together, our results demonstrate that TSP1 is a critical tumor suppressive protein in the skin that opposes the development of the UVB-induced NMSC and an important constituent of the chemopreventive pathway downstream of apigenin.
Inhibition of UVB-Induced Cutaneous Angiogenesis by Apigenin Is Partially Independent of TSP1
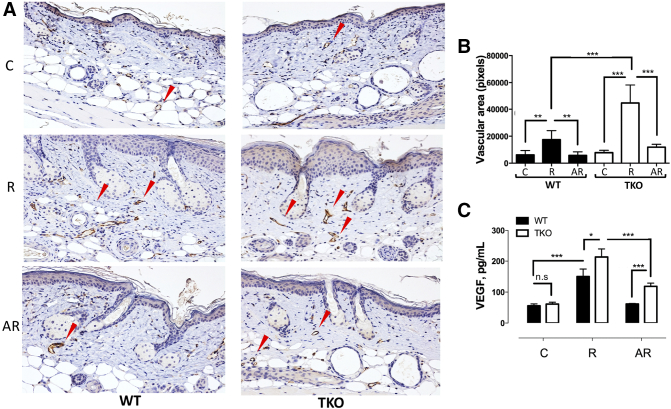
TSP1 is a widely recognized angiogenesis inhibitor, and its loss is among the critical events that allow cancer progression in multiple cancer types including skin cancers. The antiangiogenic effects of apigenin have been reported previously in nonskin tissues. However, the apigenin's effect on cutaneous angiogenesis and the role of TSP1 expression remain elusive. In agreement with previous findings [36], UVB irradiation significantly increased microvascular density (MVD) in the dermis of WT mice, as was measured by IHC for the vascular endothelial marker CD31 (PECAM-1), and this response was severely exacerbated in the skins of TKO mice (Figure 2, A and B). As in short-term experiments, treatment with apigenin dramatically inhibited UVB-induced angiogenesis in the WT mice (Figure 2, A and B). Our previous short-term studies suggested that the inhibition of cutaneous angiogenesis by apigenin was TSP1 dependent. However, we observed significant reduction in the MVD within the AR group of the TKO mice (Figure 2, A and B), suggesting a possibility of TSP1-independent component of the antiangiogenic effect of apigenin. In agreement, we detected a significant increase in plasma VEGF levels after UVB irradiation, which was dramatically suppressed by apigenin treatment both in WT and in TKO mice (Figure 2C), an effect that was likely sufficient to attenuate angiogenesis in the absence of inhibitory TSP1.
Figure 2.
The regulation of angiogenesis by apigenin in WT and TKO mice after short-term UVB exposure. Mice (n=5) were subjected to sham (C) or UVB (R) radiation (1300 J/m2 daily, 5 consecutive days). Topical apigenin (AR) (5 μmol in 0.2 ml DMSO/acetone vehicle mix) was applied daily 1 hour prior to UVB exposure. (A) IHC for the endothelial cell marker CD31. Mice were sacrificed 24 hours after final UVB exposure, and dorsal skins were harvested, formalin-fixed, and stained for CD31 to visualize microvessels. Arrows indicate representative microvessels. (B) Quantitative analysis of the vessel area. Vessel area in digital images of CD31-stained slides was quantitated. At least 20 fields were quantitated for each condition, and mean values and SEMs were calculated. Statistical significance was determined in pairwise comparisons using two-sided Student's t test. (C) The effects of apigenin and TSP1 on systemic VEGF induction by UVB irradiation. VEGF level in mouse plasma was determined using specific ELISA kit and recombinant mouse ELISA as a standard. Statistical significance was determined in pairwise comparisons using two-sided Student's t test (*P<.05; **P<.01; ***P<.001; n.s., not significant).
Loss of TSP1 Undermines the Apigenin's Ability to Block UVB-Induced Inflammation
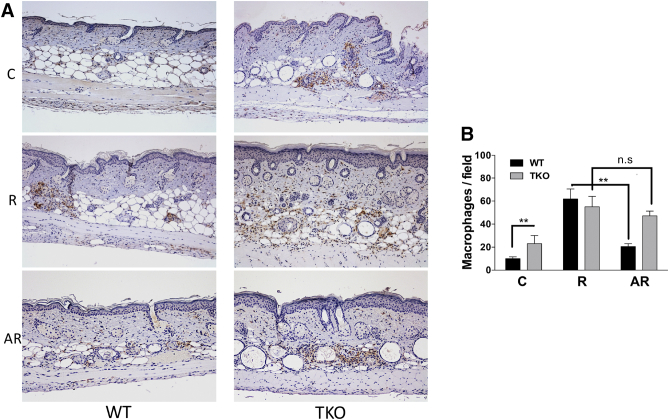
Cutaneous inflammation is one of the major responses to UVB irradiation, and chronic inflammation can lead to carcinogenesis [61], [62], [63], [64]. Compared to its antiangiogenic function, the effect of TSP1 on inflammation is less understood. While some studies demonstrate significant anti-inflammatory activity of TSP1 in the intestine [34], [65], [66], others point to its proinflammatory action in the vasculature and in the kidney, likely indirectly mediated by its fibrogenic action [67], [68], [69]. The anti-inflammatory effects of apigenin are known; however, they have not been linked to TSP1. UVB-induced inflammation was assessed as macrophage infiltration into skin (F4/80 staining, Figure 3A). We found that macrophage infiltration at baseline in sham-irradiated animals was higher in TKO mice compared to the WT controls (Figure 3, A and B). This macrophage infiltration was further increased by the short-term UVB exposure both in WT and in TKO mice (R, Figure 3, A and B) In contrast, apigenin significantly blocked UVB-induced macrophage infiltration only in WT but not in TKO mice (AR, Figure 3, A and B), suggesting that TSP1 was necessary to convey the anti-inflammatory effect of apigenin in skin.
Figure 3.
Loss of TSP1 increases macrophage infiltration and suppresses apigenin's function to inhibit UVB-induced macrophages. (A) Representative immunohistochemical analysis of F4/80 in WT and TKO skin with different treatments. Mice were exposed to short-term UVB irradiation and apigenin treatment as described in Figure 2 (n=5). (B) Quantitative analysis of macrophage presence in skin. Number of macrophages expressing F4/80 was counted and presented as percent of macrophages present in skin of sham-irradiated mice. Statistical significance was determined in pairwise comparisons using two-sided Student's t test (**P<.01; n.s., not significant).
TSP1 Affects the UVB-Induced Inflammatory Cytokine Production in Skin and by Cultured Keratinocytes
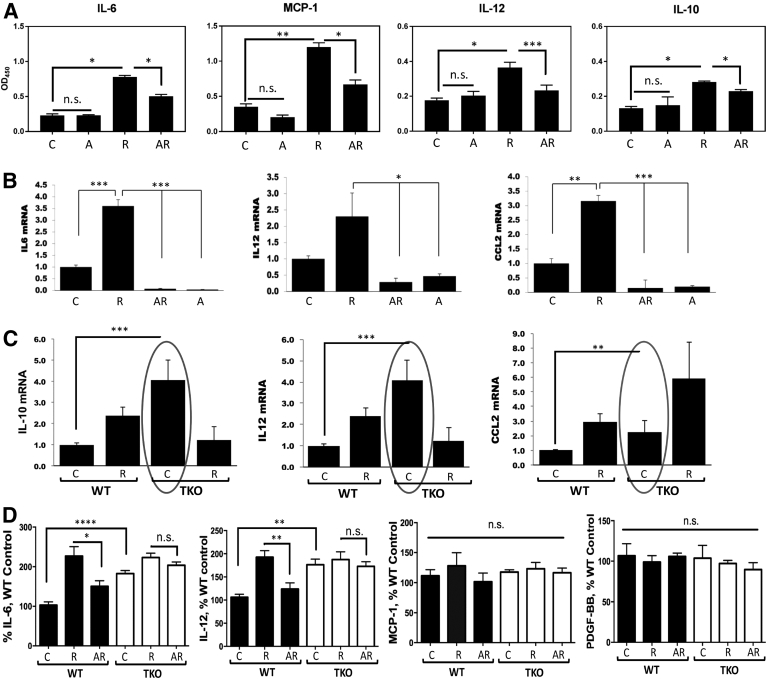
Since TSP1 dramatically altered the ability of apigenin to block macrophage recruitment to the UVB-irradiated skin in vivo, we examined whether the loss of TSP1 alters the expression of inflammatory cytokines, which could control macrophage recruitment to the sites of inflammation. Previous reports have demonstrated that IL-6 promotes malignant growth of skin SCCs by regulating, via STAT3, a network of autocrine and paracrine cytokines including VEGF and MCP-1 [70]. CCL2/MCP-1 can also be expressed in inflammatory environments due to upstream NF-kB activation and is known to play a critical role in UVB-induced skin carcinogenesis [71]. IL-12 promotes of M1 macrophage polarization, which is known to have tumor-suppressive effects [72], and mice deficient in IL-12 are at higher risk of UV-induced carcinogenesis than their wild-type counterparts [73]. At an early stage, however, IL-12 contributes to an overall inflammatory microenvironment, which can have tumor-promoting effects. IL-10, on the contrary, promotes M2 macrophage polarization and immune tolerance, which are associated with tumor promotion [72]. When IL-10 +/+, IL-10 +/−, and IL-10 −/− mice were chronically irradiated with UV, mice carrying at least one IL-10 allele developed skin cancer at an expected rate, whereas IL-10 null mice were protected against carcinogenic effects of UV irradiation [74]. Considering the importance of above-mentioned cytokines in the development of skin inflammation and UVB-induced carcinogenesis, we examined the expression of IL-6, IL-10, IL-12, and CCL2/MCP-1 in our system. First, we investigated the control of cytokine production by apigenin in WT mice (Figure 4A). In WT mice exposed to UVB radiation (1300 J/m2) daily for 5 days, the main mediators of macrophage recruitment, IL-6, CCL2/MCP-1, IL-12, and IL-10, were significantly increased, as was determined by ELISA of skin lysates. Importantly, this induction was strongly inhibited by apigenin, and the changes were especially dramatic in the case of MCP-1/CCL2. Because TSP-1 is known to modulate TGF-β activity [75], we also measured active TGF-β; however, no change was noted (data not shown). The assessment of mRNA levels for these cytokines showed that the regulation occurred at mRNA level (Figure 4B). When cultured human primary keratinocytes were pretreated with vehicle or 50 μM apigenin for 1 hour and subsequently subjected to UVB irradiation (1000 J/m2) for 24 hours, quantitative real-time PCR (Q-PCR) analysis for IL-6, IL-12, and CCL-2/MCP-1 mRNAs showed that UVB irradiation significantly upregulated these proinflammatory cytokines' mRNAs with a 3.7-fold increase for IL-6, 2.3-fold for IL-12, and 3.3-fold for MCP-1/CCL2, respectively. Again, apigenin completely blunted this UVB-induced mRNA upregulation of IL-6, IL-12, and CCL2 (Figure 4B).
Figure 4.
TSP1 regulates cytokine levels in skin. (A) Cytokine protein levels in whole skin lysates of WT mice were measured by ELISA after different treatments. (B) Cytokine mRNA levels in cultured human primary keratinocytes were measured by real-time qPCR. Cells were subjected to 1000 J/m2 UVB irradiation or pretreated with 50 μM apigenin prior to irradiation and harvested at 24 hours after irradiation. (C) Increased cytokine mRNA expression in keratinocytes from TKO mice. Primary keratinocytes were isolated from newborn WT and TKO mice and grew to 80% confluence in dishes, and real-time qPCR was performed to measure the mRNA levels. (D) Cytokine protein levels in plasma of WT and TKO mice were measured by ELISA. All experiments were performed in biological triplicates and technical duplicates. Statistical significance was determined in pairwise comparisons using two-sided Student's t test (*P<.05; **P<.01; ***P<.001; ****, P<.0001; n.s., not significant).
To assess the effect of TSP1 ablation on cytokine production by keratinocytes, we isolated neonatal keratinocytes from WT and TKO mice, subjected them to sham or UVB irradiation (1000 J/m2), and performed Q-PCR analysis of cytokine expression as above. Compared to WT, TKO keratinocytes produced four times higher levels of IL-10 and IL-12 mRNA and more than two-fold higher levels of CCL2 mRNA at baseline (Figure 4C). Intriguingly, only CCL2/MCP-1 was further increased by UVB irradiation in TKO cells, while interleukin levels plummeted, suggesting that they are not essential for macrophage recruitment into irradiated skin of the TKO animals (Figure 4C).
To assess the effect of TSP1 on systemic cytokine levels, we used plasma of WT and TKO mice from C, R, and AR group in ELISA assay. We were able to demonstrate that TKO mice have elevated circulating levels of IL-6 (P = .0003) and IL-12 (P = .0028) compared to their WT counterparts (Figure 4D). However, circulating levels of MCP-1 and PDGF-BB in the plasma of WT and TKO animals remained similar (Figure 4D). Importantly, in WT mice, UVB irradiation doubled IL-6 and IL-12 levels, and apigenin treatment significantly inhibited this increase. However, in TKO mice, the increase in IL-6 and IL-12 levels induced by UVB was not significant, and the inhibitory activity of apigenin on IL-6 or IL-12 was also abrogated in the absence of TSP1 (Figure 4D).
Apigenin Suppresses UVB-Mediated Increases in Neutrophil Populations through TSP1
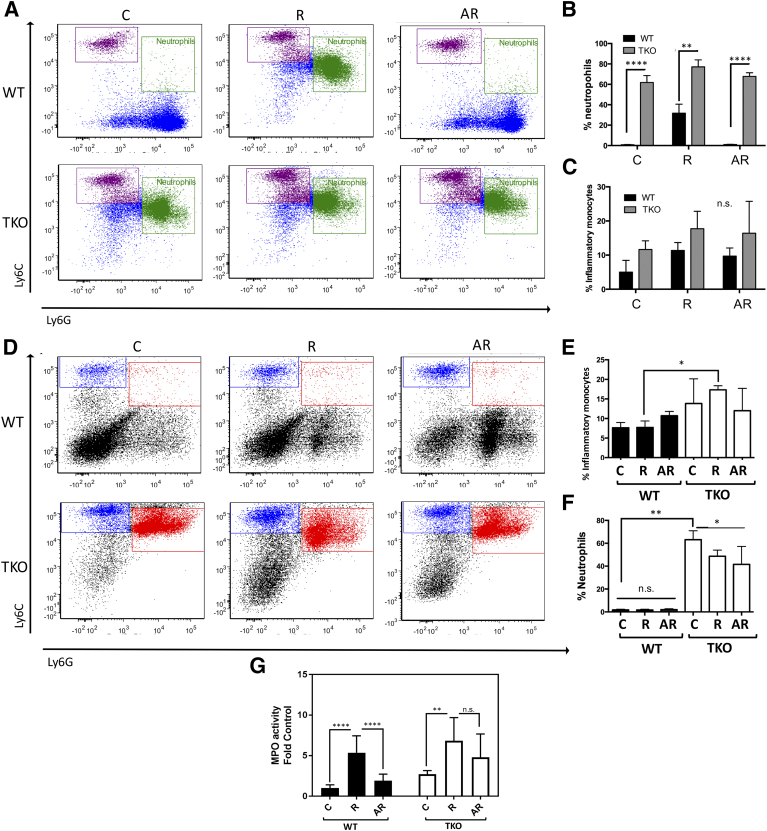
Innate myeloid cells play a critical role in the development of inflammation and subsequent carcinogenesis. With this in mind, we analyzed the effects of apigenin following UVB treatment in both TSP1-expressing and -null mice. UVB radiation led to a substantial increase in Ly6G+ neutrophil precursors in the bone marrow. This effect was attenuated with apigenin treatment prior to UVB radiation (Figure 5, A and B). In contrast, TKO mice had a significantly higher baseline number of neutrophil precursors in comparison to WT mice. In mice lacking TSP1, UVB only caused a slight increase in neutrophil precursors, likely due to the already high numbers presented. Additionally, UVB radiation caused a modest increase in the number of inflammatory monocytes in both WT and TKO mice, but apigenin did not significantly reduce this population, suggesting that inflammatory monocytes are mobilized through alternative mechanisms (Figure 5, A and C). While long-term exposure of WT mice to UVB led to an increase in neutrophil precursors, short-term UVB radiation had no significant effect (Figure 5, D-F). In short, these results demonstrate that apigenin is able to inhibit UVB-induced increases in inflammatory cells (neutrophil precursors).
Figure 5.
TSP1 controls neutrophil population in the bone marrow (BM). (A-C) BM cells were isolated from WT and TKO mice treated to long-term UVB exposure (22 weeks, 2240 J/m2, 3 times weekly) and stained for CD11b, Ly6C, and Ly6G. Flow cytometry results showed populations of monocyte (purple) and neutrophil progenitors (green), and the quantitative analysis of data were also presented. (D-F) BM cells were isolated from WT and TKO mice treated to short-term UVB exposure (5 consecutive days, 1300 J/m2 daily) and stained for CD11b, Ly6C, and Ly6G; the populations of monocyte (blue) and neutrophil progenitors (red) and the quantitative analysis of data are shown. All experiments were performed at least in triplicate. Statistical significance was determined in pairwise comparisons using two-sided Student's t test. (G) MPO activity of skin lysates. Skin lysates were prepared as described in Materials and Methods, and MPO activity of each lysate was measured in triplicates. Data presented as fold of WT control. Statistical significance was determined in pairwise comparisons using two-sided Student's t test (*P<.05; **P<.01; ****P<.0001; n.s., not significant).
Loss of TSP1 Augments MPO Activity in Skin Lysates
MPO is an enzyme produced by neutrophils, and its activity levels reflect the degree of acute inflammation in a tissue. The MPO activity was determined using a kinetic-colorimetric assay as described in Materials and Methods. To determine whether apigenin could reduce UVB-inflammation in murine skin, we compared the average MPO levels, a surrogate for the number of neutrophils that infiltrate into the skin, following UVB exposure (Figure 5G). The MPO levels in skin lysates significantly increased after UVB irradiation, and apigenin-treated WT mice skins showed significantly lower MPO levels (P=.001). Importantly, MPO activity in TKO skin lysates was greatly higher compared to lysates collected from the WT skins (P<.0001).
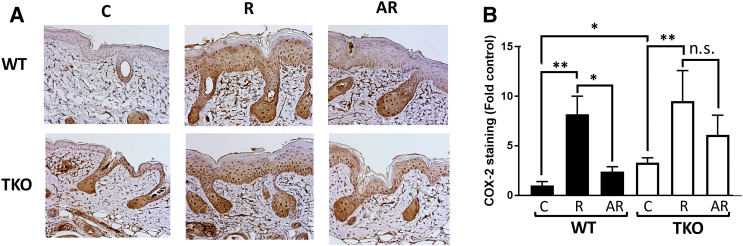
Loss of TSP1 Enhances COX-2 Protein Expression in Mouse Skin and Blunts Apigenin's Effect on COX-2 Expression
COX-2 is an inducible enzyme whose expression is regulated by UVB irradiation. COX-2 has been implicated in the development of skin cancers, and several studies demonstrated a link between TSP1 and COX-2 expression. To determine whether knockout of TSP1 affects COX-2 expression, WT and TKO mice were subjected to short-term UVB irradiation and apigenin treatment, and skin samples were stained with COX-2 antibody. As shown in Figure 6, COX-2 staining was much stronger in the skin of TKO mice compared to the skin of WT animals. Apigenin was less efficient in decreasing UVB-induced COX-2 expression in the epidermis of TKO compared to WT mice.
Figure 6.
Apigenin and TSP1 regulate COX-2 in vivo. (A), Representative IHC of COX-2 in WT and TKO skin after different treatments. Mice were exposed to short-term UVB irradiation and apigenin treatment as described in Figure 2. (B) Epidermal COX-2 immunostaining was scored by semiautomated analysis using HistoQuest 6.0 software. At least 10 interfollicular areas were scored for each sample. Data presented as fold of WT control. Statistical significance was determined in pairwise comparisons using two-sided Student's t test (*P<.05; **P<.01; n.s., not significant).
Discussion
UVB exposure causes acute skin inflammation, which promotes vasodilation and angiogenesis, a condition permissive for cancer progression. In addition, long-term UVB exposure leads to chronic cutaneous inflammation, which plays an important role in initiation, promotion, and progression of skin cancer [76]. Importantly, previous studies point to TSP1 as an important antiangiogenic factor and a tumor suppressor in skin [17], [25], [77]. We and others have previously uncovered anti-inflammatory effects of apigenin owing to its ability to inhibit UVB-mediated COX-2 overexpression and Src kinase activation in epidermal keratinocytes [57], [78]. We have also demonstrated the potential of apigenin to modulate TSP1 expression [36]. Herein, we present two important discoveries. First, using hairless mice on THBS1−/− genetic background, we conclusively demonstrate that TSP1 is a critical component of UVB-induced NMSC. Furthermore, using THBS1−/− (TKO) mice, we show that modulation of TSP1 expression is required for the chemopreventive function of apigenin. We have also found that TKO mice present with increased susceptibility to UVB-induced carcinogenesis with accelerated onset of tumor growth and increased tumor incidence and size. In addition, we demonstrated that TKO mice have diminished susceptibility to the chemopreventive action of apigenin. Importantly, the diminished chemopreventive action of apigenin was predominantly due to the increased cutaneous inflammation, which was manifested by elevated infiltration of neutrophils and macrophages and by higher levels of local and circulating inflammatory cytokines, namely, IL-6 and IL-12. Restoration of normal high levels of TSP1 in UVB-irradiated skin by topical application of apigenin in WT mice was sufficient to inhibit UVB-induced recruitment of macrophages and neutrophils and to attenuate circulating levels of inflammatory cytokines. These anti-inflammatory functions of apigenin were lost in TKO mice.
Our results are consistent with previous findings where TSP1 receptors CD36 and CD47 have been shown to profoundly affect inflammatory responses, although these findings are not clearly applicable to the cutaneous responses to UVB irradiation. For example, macrophage-associated CD36 has been shown to stimulate neutrophil killing by macrophages in the context of cardiovascular disease [79]. Similarly, CD36 mediates neutrophil clearance by macrophages during staphylococcal infection of the skin [80] or in the course of inflammatory bowel disease [81]. On the other hand, mice deficient for another TSP1 receptor, CD47, present with defective innate and adaptive immune responses in the presence of infectious agent Candida albicans [82], with increased immune infiltrates to the kidneys and brain, presumably due to increased expression of MIP-2/cxcl3, IL-1/IL-1R1, and IL-17. In other studies, TSP1 has been reported to inhibit inflammatory lymphangiogenesis, presumably via CD36 ligation on monocytes [83], and block the production of inflammatory cytokines by dendritic cells through CD47 [84]. The potential of TSP1 to attenuate cutaneous inflammation has been demonstrated using a mouse model of an acute cutaneous hypersensitivity [85]. However, the effect of TSP1 on UVB-induced inflammation and carcinogenesis has not been demonstrated. Importantly, in the present study, we found TSP1 was critical for chemopreventive action of a natural bioflavonoid apigenin. UVB irradiation caused a similar increase in macrophage infiltration in both WT and TKO mice; however, apigenin attenuated UVB-induced macrophage infiltration only in WT mice and failed to alleviate the effect of UVB irradiation in TKO mice. Importantly, we found that UVB irradiation led to systemic effect on neutrophil progenitors: we observed a substantial increase in Ly6G+ cells in the bone marrow of irradiated mice, and this effect was suppressed by apigenin treatment. In contrast, TKO mice presented with a significantly higher number of neutrophil precursors at baseline. This is in agreement with earlier findings by others demonstrating that TSP1-deficient mice have increased numbers of infiltrating neutrophils and macrophages in the lung tissue [86].
Previous studies using mouse model of acute hypersensitivity reaction noted increased IL-1β, MIP-2, and TNFα production in TSP1-deficient mice [85]. However, TSP1 targets in UVB-induced inflammation were unknown. Our in vivo experiments and in vitro studies revealed an inverse correlation between TSP1 expression and the number of infiltrating neutrophils and macrophages, indicating that TSP1 inhibits recruitment of the cells of myeloid origin to the skin. Analysis of the plasma of mice from C, R, and AR groups revealed dramatic increases in IL-6, IL-10, IL-12, and MCP-1 levels in response to UVB irradiation, which were significantly reduced in response to apigenin treatment in WT but not in TKO mice, suggesting that these cytokines are likely responsible for the differential effects of TSP1 downstream of apigenin on myeloid cell recruitment, inflammation, and carcinogenesis. Further in vitro analysis of neonatal keratinocytes from TKO and WT mice confirmed these findings and pointed to a feed-forward loop in which myeloid-derived innate immune cells (neutrophils) are recruited to the skin in response to a primary burst of cytokine production from UVB-exposed keratinocytes and then themselves become a source of cytokines, especially IL-10 and IL-12. In UVB-irradiated WT mice, however, higher TSP1 production by cutaneous keratinocytes in response to topical apigenin application results in decreased chemokine production and attenuated numbers of neutrophil progenitors in the bone marrow. These events cause a decrease in the overall chemokine levels and macrophage recruitment and lower the permissive capacity of the skin as a “soil” for carcinogenic events caused by UVB irradiation, possibly by reducing immunosuppressive environment (Treg cell recruitment by tumor-associated neutrophils [87]).
COX-2 is a key enzyme in the synthesis of prostaglandins from arachidonic acid, and increased production of COX-2 induced by UVB can cause inflammation, proliferation, angiogenesis, and tumor promotion [88]. Increased COX-2 expression has been observed in multiple cancer types, including skin, colon, and pancreas. [89]. Several studies have shown that COX-2 silencing/inhibition induces an upregulation of TSP1 [90], [91]. In contrast, our previous study showed that TSP1 bioactive peptide mimetic, ABT-898, blocks UVB-induced COX-2 expression in mouse keratinocytes [36]. In agreement, COX-2 was expressed at higher levels in the skins of WT mice compared to the TKO animals, and apigenin lost its previously noted inhibitory capacity against UVB-induced COX-2 induction in TKO mouse skins. These results further implicate TSP1 as an upstream modifier of COX-2 expression. In summary, we have shown that TSP1 plays a pivotal role in control of cutaneous inflammation and carcinogenesis through regulation of myeloid cell recruitment and homeostasis as well as COX-2. On the other hand, we propose a means to manage TSP1 balance in skin by topical application of natural bioflavonoid apigenin, which is more feasible and practical than the use short synthetic peptides for the management of inflammatory disease, such as psoriasis or dermatitis, or as an approach for skin cancer prevention.
Author Contributions
S. M., X. T., and O. V. contributed to the design of research; S. M., X. T., B. B., and M. P. performed the experiments; S. M., X. T., B. B., M. P., and O. V. wrote and corrected the manuscript.
Conflicts of Interest
O. V. has significant financial interest in a biotechnology startup Pamdeca LLC, which develops small bioactive peptides with antiangiogenic and immunomodulatory action.
Funding
The work was funded by the NCI grant R01CA172669 and NEI R24EY022883 (O. V.).
References
- 1.Gruber P., Bhimji S.S. StatPearls Publishing; Treasure Island (FL): 2018 Jan. Cancer, Skin (Integument), StatPearls [Internet] 2017 Oct 6. [Google Scholar]
- 2.Bode AM, Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci STKE. 2003;2003:RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 3.Mancebo SE, Wang SQ. Skin cancer: role of ultraviolet radiation in carcinogenesis. Rev Environ Health. 2014;29:265–273. doi: 10.1515/reveh-2014-0041. [DOI] [PubMed] [Google Scholar]
- 4.Pfeifer GP, Besaratinia A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci. 2012;11:90–97. doi: 10.1039/c1pp05144j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin CL, Ananthaswamy HN. p53 and the pathogenesis of skin cancer. Toxicol Appl Pharmacol. 2007;224:241–248. doi: 10.1016/j.taap.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiom S. Public awareness regarding UV risks and vitamin D—the challenges for UK skin cancer prevention campaigns. Prog Biophys Mol Biol. 2006;92:161–166. doi: 10.1016/j.pbiomolbio.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 8.Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14:289–301. doi: 10.1038/nri3646. [DOI] [PubMed] [Google Scholar]
- 9.Awad F, Assrawi E, Louvrier C, Jumeau C, Giurgea I, Amselem S, Karabina SA. Photoaging and skin cancer: Is the inflammasome the missing link? Mech Ageing Dev. 2018;172:131–137. doi: 10.1016/j.mad.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Maru GB, Gandhi K, Ramchandani A, Kumar G. The role of inflammation in skin cancer. Adv Exp Med Biol. 2014;816:437–469. doi: 10.1007/978-3-0348-0837-8_17. [DOI] [PubMed] [Google Scholar]
- 11.Rundhaug JE, Mikulec C, Pavone A, Fischer SM. A role for cyclooxygenase-2 in ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 2007;46:692–698. doi: 10.1002/mc.20329. [DOI] [PubMed] [Google Scholar]
- 12.Zhan H, Zheng H. The role of topical cyclo-oxygenase-2 inhibitors in skin cancer: treatment and prevention. Am J Clin Dermatol. 2007;8:195–200. doi: 10.2165/00128071-200708040-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 14.Cooper KD, Duraiswamy N, Hammerberg C, Allen E, Kimbrough-Green C, Dillon W, Thomas D. Neutrophils, differentiated macrophages, and monocyte/macrophage antigen presenting cells infiltrate murine epidermis after UV injury. J Invest Dermatol. 1993;101:155–163. doi: 10.1111/1523-1747.ep12363639. [DOI] [PubMed] [Google Scholar]
- 15.Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol. 2005;152:115–121. doi: 10.1111/j.1365-2133.2005.06368.x. [DOI] [PubMed] [Google Scholar]
- 16.Yano K, Kajiya K, Ishiwata M, Hong YK, Miyakawa T, Detmar M. Ultraviolet B-induced skin angiogenesis is associated with a switch in the balance of vascular endothelial growth factor and thrombospondin-1 expression. J Invest Dermatol. 2004;122:201–208. doi: 10.1046/j.0022-202X.2003.22101.x. [DOI] [PubMed] [Google Scholar]
- 17.Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, Lawler J, Detmar M. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol. 1999;155:441–452. doi: 10.1016/S0002-9440(10)65140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bornstein P. Thrombospondins: structure and regulation of expression. FASEB J. 1992;6:3290–3299. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- 19.Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 21.Mirochnik Y, Kwiatek A, Volpert OV. Thrombospondin and apoptosis: molecular mechanisms and use for design of complementation treatments. Curr Drug Targets. 2008;9:851–862. doi: 10.2174/138945008785909347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaked Y, Bocci G, Munoz R, Man S, Ebos JM, Hicklin DJ, Bertolini F, D'Amato R, Kerbel RS. Cellular and molecular surrogate markers to monitor targeted and non-targeted antiangiogenic drug activity and determine optimal biologic dose. Curr Cancer Drug Targets. 2005;5:551–559. doi: 10.2174/156800905774574020. [DOI] [PubMed] [Google Scholar]
- 23.Enk CD, Jacob-Hirsch J, Gal H, Verbovetski I, Amariglio N, Mevorach D, Ingber A, Givol D, Rechavi G, Hochberg M. The UVB-induced gene expression profile of human epidermis in vivo is different from that of cultured keratinocytes. Oncogene. 2006;25:2601–2614. doi: 10.1038/sj.onc.1209292. [DOI] [PubMed] [Google Scholar]
- 24.Howell BG, Wang B, Freed I, Mamelak AJ, Watanabe H, Sauder DN. Microarray analysis of UVB-regulated genes in keratinocytes: downregulation of angiogenesis inhibitor thrombospondin-1. J Dermatol Sci. 2004;34:185–194. doi: 10.1016/j.jdermsci.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Hawighorst T, Oura H, Streit M, Janes L, Nguyen L, Brown LF, Oliver G, Jackson DG, Detmar M. Thrombospondin-1 selectively inhibits early-stage carcinogenesis and angiogenesis but not tumor lymphangiogenesis and lymphatic metastasis in transgenic mice. Oncogene. 2002;21:7945–7956. doi: 10.1038/sj.onc.1205956. [DOI] [PubMed] [Google Scholar]
- 26.Bleuel K, Popp S, Fusenig NE, Stanbridge EJ, Boukamp P. Tumor suppression in human skin carcinoma cells by chromosome 15 transfer or thrombospondin-1 overexpression through halted tumor vascularization. Proc Natl Acad Sci U S A. 1999;96:2065–2070. doi: 10.1073/pnas.96.5.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fusenig NE, Boukamp P. Multiple stages and genetic alterations in immortalization, malignant transformation, and tumor progression of human skin keratinocytes. Mol Carcinog. 1998;23:144–158. doi: 10.1002/(sici)1098-2744(199811)23:3<144::aid-mc3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren B, Song K, Parangi S, Jin T, Ye M, Humphreys R, Duquette M, Zhang X, Benhaga N, Lawler J. A double hit to kill tumor and endothelial cells by TRAIL and antiangiogenic 3TSR. Cancer Res. 2009;69:3856–3865. doi: 10.1158/0008-5472.CAN-08-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isenberg JS, Frazier WA, Roberts DD. Thrombospondin-1: a physiological regulator of nitric oxide signaling. Cell Mol Life Sci. 2008;65:728–742. doi: 10.1007/s00018-007-7488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer. 2009;9:182–194. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez LS, Ling J, Nye D, Papathomas K, Dickinson C. Thrombospondin peptide ABT-898 inhibits inflammation and angiogenesis in a colitis model. World J Gastroenterol. 2015;21:6157–6166. doi: 10.3748/wjg.v21.i20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Dee ZP, Chittur SV, Patel B, Stanton R, Wakeley M, Lippert B, Menaker A, Eiche B, Terry R, Gutierrez LS. Thrombospondin-1 type 1 repeats in a model of inflammatory bowel disease: transcript profile and therapeutic effects. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zak S, Treven J, Nash N, Gutierrez LS. Lack of thrombospondin-1 increases angiogenesis in a model of chronic inflammatory bowel disease. Int J Color Dis. 2008;23:297–304. doi: 10.1007/s00384-007-0397-5. [DOI] [PubMed] [Google Scholar]
- 35.Huang SW, Kao KJ. Use of thrombospondin level to predict the clinical course of atopic dermatitis associated with food hypersensitivity or skin infection. J Dermatol Sci. 1996;11:59–63. doi: 10.1016/0923-1811(95)00420-3. [DOI] [PubMed] [Google Scholar]
- 36.Tong X, Mirzoeva S, Veliceasa D, Bridgeman BB, Fitchev P, Cornwell ML, Crawford SE, Pelling JC, Volpert OV. Chemopreventive apigenin controls UVB-induced cutaneous proliferation and angiogenesis through HuR and thrombospondin-1. Oncotarget. 2014;5:11413–11427. doi: 10.18632/oncotarget.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 38.Wei H, Tye L, Bresnick E, Birt DF. Inhibitory effect of apigenin, a plant flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res. 1990;50:499–502. [PubMed] [Google Scholar]
- 39.Birt DF, Mitchell D, Gold B, Pour P, Pinch HC. Inhibition of ultraviolet light induced skin carcinogenesis in SKH-1 mice by apigenin, a plant flavonoid. Anticancer Res. 1997;17:85–91. [PubMed] [Google Scholar]
- 40.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review) Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- 41.Mirzoeva S, Kim ND, Chiu K, Franzen CA, Bergan RC, Pelling JC. Inhibition of HIF-1 alpha and VEGF expression by the chemopreventive bioflavonoid apigenin is accompanied by Akt inhibition in human prostate carcinoma PC3-M cells. Mol Carcinog. 2008;47:686–700. doi: 10.1002/mc.20421. [DOI] [PubMed] [Google Scholar]
- 42.Fang J, Zhou Q, Liu LZ, Xia C, Hu X, Shi X, Jiang BH. Apigenin inhibits tumor angiogenesis through decreasing HIF-1alpha and VEGF expression. Carcinogenesis. 2007;28:858–864. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- 43.Shukla S, Shankar E, Fu P, MacLennan GT, Gupta S. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by apigenin through IkappaBalpha and IKK pathway in TRAMP mice. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silvan S, Manoharan S. Apigenin prevents deregulation in the expression pattern of cell-proliferative, apoptotic, inflammatory and angiogenic markers during 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Arch Oral Biol. 2013;58:94–101. doi: 10.1016/j.archoralbio.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Olszanecki R, Gebska A, Kozlovski VI, Gryglewski RJ. Flavonoids and nitric oxide synthase. J Physiol Pharmacol. 2002;53:571–584. [PubMed] [Google Scholar]
- 46.Yan X, Qi M, Li P, Zhan Y, Shao H. Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci. 2017;7 doi: 10.1186/s13578-017-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McVean M, Xiao H, Isobe K, Pelling JC. Increase in wild-type p53 stability and transactivational activity by the chemopreventive agent apigenin in keratinocytes. Carcinogenesis. 2000;21:633–639. doi: 10.1093/carcin/21.4.633. [DOI] [PubMed] [Google Scholar]
- 48.Tong X, Pelling JC. Enhancement of p53 expression in keratinocytes by the bioflavonoid apigenin is associated with RNA-binding protein HuR. Mol Carcinog. 2009;48:118–129. doi: 10.1002/mc.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000;28:102–110. [PubMed] [Google Scholar]
- 50.McVean M, Weinberg WC, Pelling JC. A p21(waf1)-independent pathway for inhibitory phosphorylation of cyclin-dependent kinase p34(cdc2) and concomitant G(2)/M arrest by the chemopreventive flavonoid apigenin. Mol Carcinog. 2002;33:36–43. doi: 10.1002/mc.10016. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Y, Mao Y, Chen H, Lin Y, Hu Z, Wu J, Xu X, Xu X, Qin J, Xie L. Apigenin promotes apoptosis, inhibits invasion and induces cell cycle arrest of T24 human bladder cancer cells. Cancer Cell Int. 2013;13:54. doi: 10.1186/1475-2867-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim BR, Jeon YK, Nam MJ. A mechanism of apigenin-induced apoptosis is potentially related to anti-angiogenesis and anti-migration in human hepatocellular carcinoma cells. Food Chem Toxicol. 2011;49:1626–1632. doi: 10.1016/j.fct.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 53.Lu HF, Chie YJ, Yang MS, Lee CS, Fu JJ, Yang JS, Tan TW, Wu SH, Ma YS, Ip SW. Apigenin induces caspase-dependent apoptosis in human lung cancer A549 cells through Bax- and Bcl-2-triggered mitochondrial pathway. Int J Oncol. 2010;36:1477–1484. doi: 10.3892/ijo_00000634. [DOI] [PubMed] [Google Scholar]
- 54.Kaur P, Shukla S, Gupta S. Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: an in vitro and in vivo study. Carcinogenesis. 2008;29:2210–2217. doi: 10.1093/carcin/bgn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Dross RT, Hong X, Essengue S, Fischer SM, Pelling JC. Modulation of UVB-induced and basal cyclooxygenase-2 (COX-2) expression by apigenin in mouse keratinocytes: role of USF transcription factors. Mol Carcinog. 2007;46:303–314. doi: 10.1002/mc.20281. [DOI] [PubMed] [Google Scholar]
- 57.Tong X, Van Dross RT, Abu-Yousif A, Morrison AR, Pelling JC. Apigenin prevents UVB-induced cyclooxygenase 2 expression: coupled mRNA stabilization and translational inhibition. Mol Cell Biol. 2007;27:283–296. doi: 10.1128/MCB.01282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101:982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berg RJ, van Kranen HJ, Rebel HG, de Vries A, van Vloten WA, Van Kreijl CF, van der Leun JC, de Gruijl FR. Early p53 alterations in mouse skin carcinogenesis by UVB radiation: immunohistochemical detection of mutant p53 protein in clusters of preneoplastic epidermal cells. Proc Natl Acad Sci U S A. 1996;93:274–278. doi: 10.1073/pnas.93.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oberyszyn TM, Tober KL, Ross MS, Robertson FM. Inhibitory effects of pentoxifylline on ultraviolet B light-induced cutaneous inflammation. Mol Carcinog. 1998;22:16–25. [PubMed] [Google Scholar]
- 61.Song IY, Balmain A. Cellular reprogramming in skin cancer. Semin Cancer Biol. 2015;32:32–39. doi: 10.1016/j.semcancer.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Cho CH. Effect of NF-kappaB signaling on apoptosis in chronic inflammation-associated carcinogenesis. Curr Cancer Drug Targets. 2010;10:593–599. doi: 10.2174/156800910791859425. [DOI] [PubMed] [Google Scholar]
- 63.Lopes DM, McMahon SB. Ultraviolet radiation on the skin: a painful experience? CNS Neurosci Ther. 2016;22:118–126. doi: 10.1111/cns.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishisgori C. Current concept of photocarcinogenesis. Photochem Photobiol Sci. 2015;14:1713–1721. doi: 10.1039/c5pp00185d. [DOI] [PubMed] [Google Scholar]
- 65.Zhang HP, Wu Y, Liu J, Jiang J, Geng XR, Yang G, Mo L, Liu ZQ, Liu ZG, Yang PC. TSP1-producing B cells show immune regulatory property and suppress allergy-related mucosal inflammation. Sci Rep. 2013;3 doi: 10.1038/srep03345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Punekar S, Zak S, Kalter VG, Dobransky L, Punekar I, Lawler JW, Gutierrez LS. Thrombospondin 1 and its mimetic peptide ABT-510 decrease angiogenesis and inflammation in a murine model of inflammatory bowel disease. Pathobiology. 2008;75:9–21. doi: 10.1159/000113790. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka T, Nangaku M. Angiogenesis and hypoxia in the kidney. Nat Rev Nephrol. 2013;9:211–222. doi: 10.1038/nrneph.2013.35. [DOI] [PubMed] [Google Scholar]
- 68.Bige N, Shweke N, Benhassine S, Jouanneau C, Vandermeersch S, Dussaule JC, Chatziantoniou C, Ronco P, Boffa JJ. Thrombospondin-1 plays a profibrotic and pro-inflammatory role during ureteric obstruction. Kidney Int. 2012;81:1226–1238. doi: 10.1038/ki.2012.21. [DOI] [PubMed] [Google Scholar]
- 69.Kong P, Gonzalez-Quesada C, Li N, Cavalera M, Lee DW, Frangogiannis NG. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am J Physiol Endocrinol Metab. 2013;305:E439–E450. doi: 10.1152/ajpendo.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lederle W, Depner S, Schnur S, Obermueller E, Catone N, Just A, Fusenig NE, Mueller MM. IL-6 promotes malignant growth of skin SCCs by regulating a network of autocrine and paracrine cytokines. Int J Cancer. 2011;128:2803–2814. doi: 10.1002/ijc.25621. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J, Chen L, Xiao M, Wang C, Qin Z. FSP1+ fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am J Pathol. 2011;178:382–390. doi: 10.1016/j.ajpath.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 73.Meeran SM, Katiyar S, Elmets CA, Katiyar SK. Interleukin-12 deficiency is permissive for angiogenesis in UV radiation-induced skin tumors. Cancer Res. 2007;67:3785–3793. doi: 10.1158/0008-5472.CAN-06-3134. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Loser K, Apelt J, Voskort M, Mohaupt M, Balkow S, Schwarz T, Grabbe S, Beissert S. IL-10 controls ultraviolet-induced carcinogenesis in mice. J Immunol. 2007;179:365–371. doi: 10.4049/jimmunol.179.1.365. [DOI] [PubMed] [Google Scholar]
- 75.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 76.Hensler S, Mueller MM. Inflammation and skin cancer: old pals telling new stories. Cancer J. 2013;19:517–524. doi: 10.1097/PPO.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 77.Detmar M. The role of VEGF and thrombospondins in skin angiogenesis. J Dermatol Sci. 2000;24(Suppl. 1):S78–S84. doi: 10.1016/s0923-1811(00)00145-6. [DOI] [PubMed] [Google Scholar]
- 78.Byun S, Park J, Lee E, Lim S, Yu JG, Lee SJ, Chen H, Dong Z, Lee KW, Lee HJ. Src kinase is a direct target of apigenin against UVB-induced skin inflammation. Carcinogenesis. 2013;34:397–405. doi: 10.1093/carcin/bgs358. [DOI] [PubMed] [Google Scholar]
- 79.DeLeon-Pennell KY, Tian Y, Zhang B, Cates CA, Iyer RP, Cannon P, Shah P, Aiyetan P, Halade GV, Ma Y. CD36 is a matrix metalloproteinase-9 substrate that stimulates neutrophil apoptosis and removal during cardiac remodeling. Circ Cardiovasc Genet. 2016;9:14–25. doi: 10.1161/CIRCGENETICS.115.001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castleman MJ, Febbraio M, Hall PR. CD36 is essential for regulation of the host innate response to Staphylococcus aureus alpha-toxin–mediated dermonecrosis. J Immunol. 2015;195:2294–2302. doi: 10.4049/jimmunol.1500500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ortiz-Masia D, Diez I, Calatayud S, Hernandez C, Cosin-Roger J, Hinojosa J, Esplugues JV, Barrachina MD. Induction of CD36 and thrombospondin-1 in macrophages by hypoxia-inducible factor 1 and its relevance in the inflammatory process. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cursiefen C, Maruyama K, Bock F, Saban D, Sadrai Z, Lawler J, Dana R, Masli S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med. 2011;208:1083–1092. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johansson U, Londei M. Ligation of CD47 during monocyte differentiation into dendritic cells results in reduced capacity for interleukin-12 production. Scand J Immunol. 2004;59:50–57. doi: 10.1111/j.0300-9475.2004.01354.x. [DOI] [PubMed] [Google Scholar]
- 85.Velasco P, Huegel R, Brasch J, Schroder JM, Weichenthal M, Stockfleth E, Schwarz T, Lawler J, Detmar M, Lange-Asschenfeldt B. The angiogenesis inhibitor thrombospondin-1 inhibits acute cutaneous hypersensitivity reactions. J Invest Dermatol. 2009;129:2022–2030. doi: 10.1038/jid.2008.447. [DOI] [PubMed] [Google Scholar]
- 86.Zhao Y, Xiong Z, Lechner EJ, Klenotic PA, Hamburg BJ, Hulver M, Khare A, Oriss T, Mangalmurti N, Chan Y. Thrombospondin-1 triggers macrophage IL-10 production and promotes resolution of experimental lung injury. Mucosal Immunol. 2014;7:440–448. doi: 10.1038/mi.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X, Zhang W, Yuan X, Fu M, Qian H, Xu W. Neutrophils in cancer development and progression: Roles, mechanisms, and implications (Review) Int J Oncol. 2016;49:857–867. doi: 10.3892/ijo.2016.3616. [DOI] [PubMed] [Google Scholar]
- 88.Brecher AR. The role of cyclooxygenase-2 in the pathogenesis of skin cancer. J Drugs Dermatol. 2002;1:44–47. [PubMed] [Google Scholar]
- 89.Muller-Decker K, Furstenberger G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol Carcinog. 2007;46:705–710. doi: 10.1002/mc.20326. [DOI] [PubMed] [Google Scholar]
- 90.Sennlaub F, Valamanesh F, Vazquez-Tello A, El-Asrar AM, Checchin D, Brault S, Gobeil F, Beauchamp MH, Mwaikambo B, Courtois Y. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation. 2003;108:198–204. doi: 10.1161/01.CIR.0000080735.93327.00. [DOI] [PubMed] [Google Scholar]
- 91.Stasinopoulos I, O'Brien DR, Wildes F, Glunde K, Bhujwalla ZM. Silencing of cyclooxygenase-2 inhibits metastasis and delays tumor onset of poorly differentiated metastatic breast cancer cells. Mol Cancer Res. 2007;5:435–442. doi: 10.1158/1541-7786.MCR-07-0010. [DOI] [PubMed] [Google Scholar]