Abstract
In the past decade since kisspeptin/neurokinin B/dynorphin (KNDy) cells were first identified in the mammalian hypothalamus, a plethora of new research has emerged adding insights into the role of this neuronal population in reproductive neuroendocrine function, including the basis for GnRH pulse generation and the mechanisms underlying the steroid feedback control of GnRH secretion. In this mini-review, we provide an update of evidence regarding the roles of KNDy peptides and their postsynaptic receptors in producing episodic GnRH release and assess the relative contribution of KNDy neurons to the “GnRH pulse generator.” In addition, we examine recent work investigating the role of KNDy neurons as mediators of steroid hormone negative feedback and review evidence for their involvement in the preovulatory GnRH/LH surge, taking into account species differences that exist among rodents, ruminants, and primates. Finally, we summarize emerging roles of KNDy neurons in other aspects of reproductive function and in nonreproductive functions and discuss critical unresolved questions in our understanding of KNDy neurobiology.
This review discusses key research from the last decade that has provided new insights into the control of mammalian reproduction by kisspeptin/neurokinin B/dynorphin (KNDy) neurons.
Nearly four decades ago, Ernst Knobil (1) identified the presence of a hypothalamic pulse generator that regulates the episodic release of GnRH from the hypothalamus. Changes in the frequency and amplitude of pulsatile GnRH release, and in turn pulsatile release of the gonadotropins LH and FSH from the anterior pituitary gland, are essential for the appropriate control of fertility through steroid hormone production and gamete development at the level of the gonads (1–4). In a feedback loop, steroid hormones act through an afferent neuronal network to provide critical information to GnRH neurons, regulating their pattern of activity. Identification of the neuronal structures comprising the pulse generator, and the afferent pathways through which gonadal steroids (in addition to other internal and external cues such as stress, nutrition, and day length) regulate pulsatile GnRH release, have been and remain fundamentally unresolved questions in reproductive neuroendocrinology.
In 2003, it was discovered that mutations in the gene encoding the kisspeptin receptor G protein-coupled receptor 54 (GPR54) or otherwise known as Kiss1R, led to hypogonadotropic hypogonadism (5, 6), providing compelling evidence that kisspeptin-positive afferents to GnRH neurons are critical for maintaining GnRH release needed for fertility. Subsequent studies testing the role of kisspeptin in animal models confirmed that kisspeptin activates GnRH neurons via GPR54 to robustly stimulate LH (and therefore GnRH) release (7, 8). The necessity of kisspeptin-GPR54 signaling at GnRH neurons was later highlighted by the specific deletion of GPR54 from GnRH neurons, which resulted in infertility, and impressively by the restoration of fertility in a GPR54-global knockout mouse by GPR54 expression in GnRH neurons alone (9).
Kisspeptin-expressing neurons in the rostral periventricular area of the third ventricle (RP3V) of rodents or the preoptic area (POA) of other mammals and the arcuate nucleus of the hypothalamus (ARC) are sites of the highest density of kisspeptin expression within the mammalian brain (10). The vast majority of these neurons express receptors critical for steroid hormone feedback by estrogen, progesterone, and testosterone: estrogen receptor α (ERα), progesterone receptor, and androgen receptor, respectively (11–16). Estradiol increases Kiss1 mRNA levels in neurons located within the RP3V but reduces Kiss1 in the ARC, introducing the possibility of populations with divergent roles in positive and negative steroid hormone feedback (14) (discussed further below). Specifically, the suppression of ARC Kiss1 expression by estradiol, along with its expression of gonadal steroid receptors, was one of the first clues suggesting that kisspeptin cells in this region may play an important role in negative feedback control of pulsatile GnRH release.
In 2007, multiple-label immunofluorescence studies in the sheep made the critical discovery that almost all kisspeptin neurons in the ARC coexpressed the tachykinin neurokinin B (NKB), as well as the endogenous opioid peptide (EOP) dynorphin (17). This was consistent with previous immunofluorescence studies in the rat (12) and sheep (18) that recorded high coexpression of NKB with dynorphin in neurons of the ARC. The importance of NKB as a regulator of fertility was highlighted by the 2009 study that revealed human mutations in the genes encoding NKB (TAC3) or its receptor (TAC3R) manifest as defects in GnRH release and subsequent hypogonadism (19). These human mutations provided a strikingly similar neuroendocrine profile to human and murine GPR54 mutations, although the genetic knockout of TAC3R in mice elicited a milder subfertile phenotype (20). Given robust functional support for dynorphin as a mediator of progesterone negative feedback in the ewe, this single ARC population appeared to contain three distinct neuropeptides, each of which was strongly implicated in the control of GnRH release. Due to the high degree of colocalization of the three peptides, and for simplicity, this population was abbreviated as “KNDy” (kisspeptin/neurokinin B/dynorphin) neurons (21). The colocalization of KNDy peptides was later demonstrated in the mouse (22), rat (23), cow (24), goat (25), and nonhuman primate (26). Because immunohistochemistry studies have been primarily based on multiple combinations of fluorescent visualization of two of the three neuropeptides, we recently used fluorescent in situ hybridization for simultaneous visualization of mRNA transcripts for all three peptides within individual ARC cell bodies (Fig. 1), confirming that virtually all ARC kisspeptin cells coexpress NKB and dynorphin mRNA (27). Although the KNDy population is conserved across most mammalian species, the degree of coexpression varies between species, sex, and gonadal steroid hormone status. Further, although prodynorphin mRNA has been reported within the infundibular nucleus of pre- and postmenopausal women (28), immunohistochemical labeling detects few dynorphin-positive cell bodies in the infundibular nucleus of young human males (29). Therefore, although questions remain as to their presence in humans, the unique colocalization of three KNDy peptides, each of which were implicated in the control of fertility and GnRH episodic release, provided a strong candidate for the GnRH pulse generator, long suspected to be located within the mediobasal hypothalamic/ARC region (30, 31).
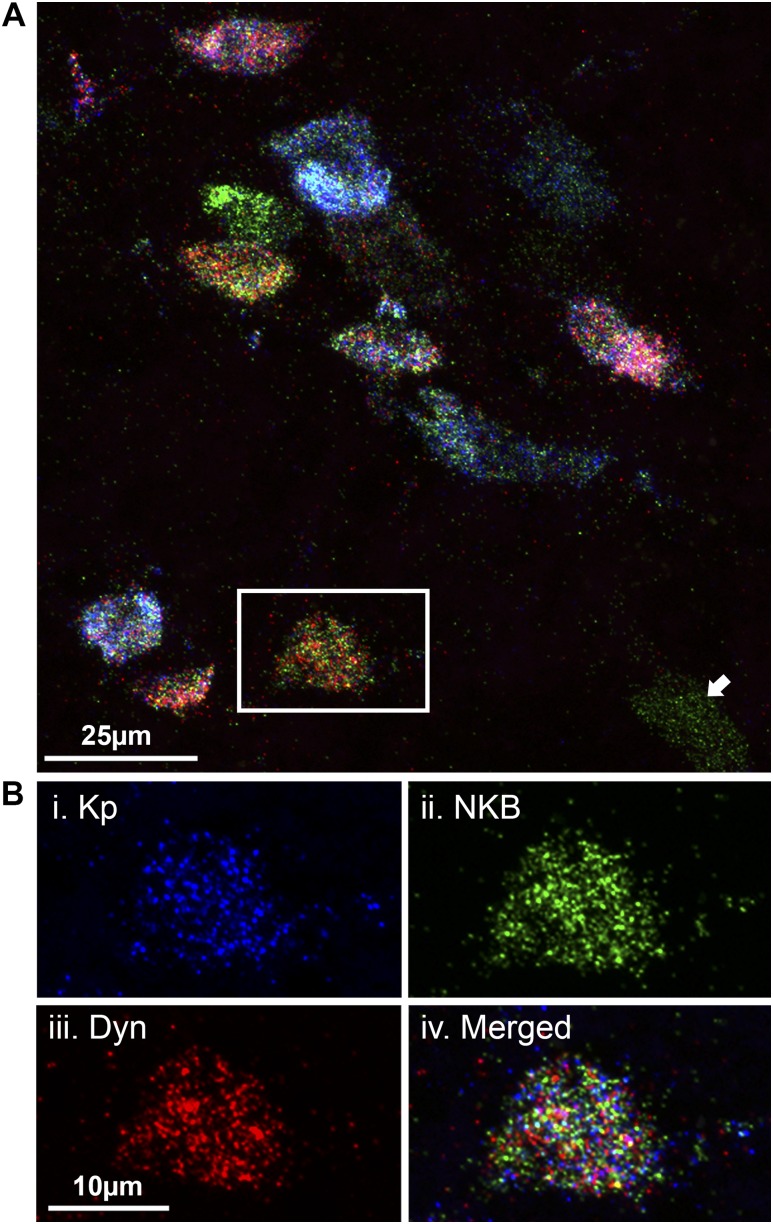
Figure 1.
In situ hybridization using RNAscope technology permits three-plex detection of ARC cells colocalizing kisspeptin (Kp), NKB, and dynorphin (Dyn) mRNA. (A) Confocal image (1-µm optical section, ×60 magnification) of kisspeptin (blue), NKB (green), and Dyn (red) mRNA transcripts within ARC cell bodies in the ovariectomized, estradiol-treated ewe. Kp, NKB, and Dyn mRNA is expressed above background threshold levels within all cell bodies except the cell marked with an arrow, which only expresses NKB. (B) High-magnification example image of (i) Kp, (ii) NKB, and (iii) Dyn transcripts expressed in a single cell body (iv, merged image).
This mini-review will serve as an update of our original review of KNDy neurons (32) and highlight evidence generated within the last decade on their role in the central control of reproductive neuroendocrine function. We will review anatomical, physiological, and pharmacological studies that implicate KNDy neurons as critical components of the GnRH pulse generator, as well as evidence for the role of KNDy neurons in steroid hormone feedback regulation, taking into consideration species differences that appear to exist in the precise roles of each KNDy peptide. Finally, we will summarize other roles, including nonreproductive ones, which have emerged for KNDy neurons and present some critical unanswered questions about this population as a basis for future research.
The “KNDy Hypothesis” and GnRH Pulse Generation
Where do kisspeptin, NKB, and dynorphin act?
The hypothesis that KNDy neurons form a critical component of the GnRH pulse generator was initially based on the colocalization of three neuropeptides with opposing effects on GnRH/LH release, kisspeptin and NKB being stimulatory and dynorphin inhibitory. In brief, the so-called “KNDy hypothesis” stated that NKB was the signal responsible for pulse onset by triggering activation among KNDy neurons, kisspeptin served as the output signal from KNDy neurons driving GnRH secretion, and dynorphin served as the signal terminating each pulse. This hypothesis was supported by evidence that (1) axons containing KNDy peptides were in direct synaptic contact with the majority of KNDy cell bodies, forming an interconnected population perhaps capable of producing bursts of synchronized firing to coordinate pulsatile release of GnRH, (2) receptors for kisspeptin and NKB were anatomically segregated in GnRH and KNDy neurons, respectively, and (3) pharmacological manipulation of the postsynaptic receptors for kisspeptin, NKB, and dynorphin, GPR54, NK3R, and κ-opioid receptor (KOR), respectively, altered pulsatile GnRH release in manners consistent with their predicted roles (25, 33–37). However, many aspects of the roles and sites of action of each peptide for initiating, driving, and terminating GnRH release to create an individual pulse remained unclear. The pharmacological, genetic, electrophysiological, and anatomical evidence described below outlines the latest evidence on the neuronal circuitry and cellular location of postsynaptic receptors that generate pulsatile GnRH secretion, and a summary of the updated KNDy hypothesis can be found in Fig. 2.
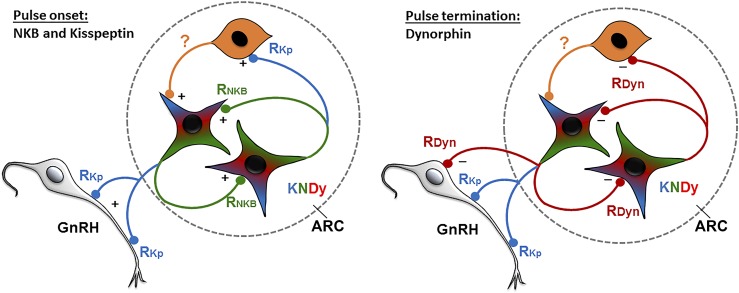
Figure 2.
Proposed model for the control of KNDy neuron activity to drive episodic GnRH/LH secretion. Each GnRH pulse is initiated by NKB (green) acting upon reciprocally-connected KNDy neurons to stimulate kisspeptin (blue) release. Kisspeptin drives GnRH (gray) secretion and activates unidentified GPR54/Kiss1R containing ARC neurons (orange) that reinforces the stimulatory actions of NKB on KNDy neurons. GnRH release is then terminated by the release of dynorphin (red) from KNDy neurons acting directly on KNDy neurons, GnRH neurons, and/or unidentified KOR-containing neurons. The color in each terminal indicates the biologically active transmitter (potentially due to selective expression of postsynaptic receptor) and does not reflect selective transport of that peptide to the terminal. Dashed oval represents the ARC. RDyn, KOR; RKp, GPR54/Kiss1R; RNKB, NK3R.
Within KNDy circuitry
The presence of reciprocal connections between KNDy neurons suggests the population is capable of producing synchronized firing to generate and coordinate the pulsatile release of GnRH. To support this hypothesis, KNDy neurons must express the appropriate postsynaptic receptors for KNDy peptides. As predicted, multilabel immunofluorescence confirmed expression of NK3R in the majority of KNDy neurons of the mouse, rat, and sheep (12, 22). In the last decade, the generation of transgenic mice that conditionally express green fluorescent protein (GFP) under the control of Kiss1 or Tac2 has allowed for in vitro electrophysiological characterization of ARC KNDy neurons in acute brain slices (38–41), which confirmed KNDy neuron action potential firing activity was increased by both NKB and the NK3R agonist senktide (41, 42). However, it should be noted that there is considerable redundancy in the control of episodic GnRH secretion by different tachykinin receptors (discussed in the “Redundancy in tachykinin signaling” section below).
KOR, the EOP receptor with highest affinity for dynorphin, was detected by in situ hybridization within only 20% and 6% of KNDy neurons in the female and male mouse, respectively (22, 43). As Navarro et al. (22) suggested, this unexpectedly low colocalization may be due to insufficient sensitivity of in situ hybridization for low levels of mRNA. This was supported by single-cell RT-PCR analysis that detected KOR in 41% of KNDy neurons in male mice (41). In contrast, a recent immunofluorescence study in the sheep reported KOR in over 90% of KNDy neurons (44). The species difference in KOR colocalization is consistent with pharmacological data that suggests dynorphin may play different functional roles in pulse generation in the rodent than in the goat and sheep (discussed further in “The role of dynorphin in pulse termination” below). Interestingly, the majority of KNDy neurons recorded in mouse brain slices have reduced firing activity following bath application of dynorphin, an effect that is mimicked by KOR agonists (41, 42). Although this may indicate a higher percentage of KNDy neurons express KOR than previously reported in rodents, an indirect action of dynorphin on KNDy neuron activity is also possible. Therefore, the role of dynorphin action directly on KNDy neurons to alter GnRH pulse release is not yet clear in the rodent brain. It should also be noted that a number of non-KNDy neurons in the sheep ARC express KOR, and therefore a role for these neurons in the actions of dynorphin on episodic GnRH release cannot be ruled out (44).
The kisspeptin receptor GPR54 has been detected in the ARC of the primate brain using in situ hybridization, with expression levels increasing between the juvenile period and midpuberty in correspondence with rising LH levels (45). Although a transgenic LacZ knockin mouse model did not find evidence for GPR54 in the ARC (46), mRNA for this receptor has been detected by in situ hybridization within the ARC of sheep and mice (47–49). However, dual in situ hybridization and immunohistochemistry experiments in sheep (49) and rat (50) indicate that GPR54 is not localized within KNDy neurons and instead suggest these receptors are expressed within ARC pro-opiomelanocortin (POMC) and tuberoinfundibular dopamine neurons (50). Further, kisspeptin does not have an effect on ARC kisspeptin neuron electrical activity in mouse brain slices (42), indicating that, unlike NKB and dynorphin, kisspeptin likely does not act directly upon KNDy neurons to modulate pulsatile secretion. This lack of evidence for direct actions of kisspeptin upon KNDy neurons aligns with data that GPR54 expression in GnRH neurons is sufficient for fertility in mice (9). However, kisspeptin activation of arcuate neurons has been recorded in mouse brain slices collected from GPR54 knockout mice (51), indicating kisspeptin may act through other postsynaptic receptors in the ARC. The role of these receptors in pulse generation is less clear and may instead reflect subpopulations of neurons involved in nonreproductive functions.
Projections to GnRH neurons: kisspeptin and dynorphin
A lack of robust evidence for GPR54 expression within KNDy neurons suggests kisspeptin does not act through reciprocal connections to control pulse generation. Far more likely, kisspeptin acts as a stimulatory output to directly drive GnRH neuron activity. In the ewe, 78% to 86% of preoptic GnRH neurons contain GPR54 (49), and 45% to 60% of these neurons in both the POA and mediobasal hypothalamus (MBH) receive inputs from KNDy neurons that colocalize kisspeptin and the vesicular glutamate transporter vGlut2 (52). In the mouse, approximately 80% of GnRH neurons express GPR54 (7, 46) and respond to kisspeptin in mouse brain slices (9), an effect that is eliminated in global- and GnRH neuron-targeted GPR54 knockout mice (9). Although this might reflect kisspeptin input from non-ARC populations, the knockdown of ARC kisspeptin expression using virally delivered kisspeptin antisense or lesion of these neurons with a saporin conjugate in female rats disrupts estrous cyclicity and reduces the frequency of pulsatile LH release (53–55). In a recent study, the temporary inhibition of ARC kisspeptin neuron activity using optogenetic techniques reduced LH pulse frequency and amplitude during illumination (56). NK3R is not expressed by GnRH cell bodies in the sheep (57) and rodent (58, 59) brain, although NK3R protein has been reported to be localized in GnRH neuron axon terminals in the rat (59). In support of the latter finding, activation of NK3R at GnRH nerve terminals in the median eminence induces GnRH release in an effect that is independent of kisspeptin (60). However, the GnRH neuron soma does not respond to NKB in mouse brain slices (43, 60), supporting the notion that kisspeptin, but not NKB, acts as a stimulatory drive of LH release at GnRH neurons through GPR54 signaling.
In addition to the inhibition of KNDy neuron activity, dynorphin may also inhibit GnRH neurons directly. There is significant evidence from studies in sheep that ARC dynorphin neurons mediate progesterone negative feedback (61, 62), and approximately 40% of POA GnRH neurons and 90% of MBH GnRH neurons in the ewe receive synaptic contact from dynorphin fibers (61). Although prior work did not find evidence for KOR mRNA colocalization within GnRH neurons in the rodent brain (63, 64), a recent study detected KOR immunoreactivity in the vast majority of GnRH neurons in both the rat and sheep brain (44). Therefore, as discussed in more detail below, it is possible that dynorphin acts both within the KNDy network and at GnRH neurons to inhibit neurosecretory activity and effectively terminate a GnRH pulse.
In conclusion, the ability of kisspeptin and dynorphin to modulate the activity of GnRH neurons, along with evidence for the expression of the appropriate receptors in these cells, supports functional projections to GnRH neurons. Although KNDy neurons project directly to the GnRH neuron soma in the sheep (32), tract tracing from ARC kisspeptin (presumably KNDy) neurons in mice and rats show minimal evidence for close contact with GnRH cell bodies (23, 65, 66). Although this may indicate the presence of interneurons in the control of GnRH secretion, conditional anterograde transsynaptic tracing studies indicate that KNDy neurons form synaptic connections with GnRH neurons during development in the mouse (67). Therefore, KNDy neurons may target GnRH neuron subcellular compartments not in immediate proximity to the GnRH soma and proximal dendrite in rodents. Filling of GnRH neurons with a low-molecular-weight dye to study the structure of distal GnRH projections revealed a surprising dendritic morphology that transitions into axons near the median eminence (68). These dendritic-axonal projections, termed dendrons, appear to contain sites of synaptic contact as shown by the presence of spines and closely opposed synaptic markers (68). The presence of GnRH dendrons is yet to be reported in other mammalian species but raises the possibility that KNDy neurons form synaptic connections close to the site of GnRH release in the median eminence for fine control of pulsatile secretion. In addition to synaptic regulation, axon terminals from KNDy neurons have been reported in both the internal (23, 66, 69, 70) and external zone (65) of the median eminence and therefore may regulate GnRH pulsatile release through volume transmission to GnRH nerve terminals (71).
Development of KNDy circuitry
Given the proposed role of KNDy neurons in coordinating pulse generation, the functional onset of circuitry between KNDy and GnRH neurons should be timed with the development of pulse generation. Genetic strategies in the mouse revealed that kisspeptin neurons in the ARC (putative KNDy neurons) are first expressed, gain steroid hormone sensitivity, and form connections with GnRH neurons during fetal life (67). Surprisingly, although LH release at this time is low, the measurement of GnRH release in acute brain slices from embryonic mice using fast-scan cyclic voltammetry revealed a strikingly high level of release from embryonic day 18.5 through to postnatal week 1 (72). Although kisspeptin contacts are formed during elevated GnRH secretion, fast-scan cyclic voltammetry–recorded GnRH release during early development remains high in Kiss1-knockout mice (72). Therefore, although the neurocircuitry is present, it remains to be elucidated whether the formation of kisspeptin synapses is necessary to initiate high GnRH release in the neonatal period. Alternatively, high neonatal GnRH release may be an intrinsic feature of GnRH neurons or driven by a nonkisspeptin upstream neuronal population.
Redundancy in tachykinin signaling
Another important addition to our understanding of KNDy neuron function in the last few years is recognition of the considerable redundancy in the control of GnRH secretion in rodents among the three major tachykinin receptor pairs: substance P (SP)–NK1R, neurokinin A (NKA)-NK2R, and NKB-NK3R (73). In retrospect, redundancy in the roles of these tachykinins could have been inferred from the modest reproductive effects of Tacr3 knockouts in mice (20), but it was not directly proposed until a report that antagonists to all three tachykinin receptors were needed to inhibit LH secretion in ovariectomized (OVX) rats (74). Similarly, all three receptors had to be blocked to prevent the stimulatory actions of NKB on KNDy neurons in vitro (42). These data are consistent with the similar affinities of NKB for these receptors (ED50s: NK1R, 70 nM; NK2R, 25 nM; NK3R, 4 nM) (75) and raise the possibility that SP and NKA might also contribute to the control of LH secretion. It should be noted, however, that a specific NK3R antagonist delayed puberty in rats (76), so the degree of redundancy may vary with age and/or endocrine status. There also appear to be some differences between rats and mice in the actions of NK1R and NK2R agonists. Thus, both SP and NKA depolarized murine KNDy neurons in vitro (42), and NK1R and NK2R agonists increased LH secretion in gonadally intact mice (77). However, only the NK2R agonist was stimulatory in intact rats (78). Although these specific agonists all ultimately act by altering kisspeptin release (77), they likely use different signaling pathways because they produce different effects in OVX mice: the NK1R agonist increased LH secretion, whereas the NK2R and NK3R agonists inhibited LH (77). SP most likely acts via NK1R (ED50s: NK1R, 2 nM; NK2R, 2200 nM; NK3R, 18,000 nM), which are found in 49% of KNDy neurons by single-cell quantitative PCR (77). Although the NK2R agonist and NKA produced similar effects to the NK3R agonist in intact and OVX rodents, NKA is unlikely to act via NK3R (ED50s: NK1R, 6 nM; NK2R, 3 nM; NK3R, 1300 nM). However, KNDy neurons do not contain NK2R (77) or respond in vitro to a specific NK2R agonist (79), so the signaling pathway by which NKA acts remains to be determined.
Although these data suggest considerable redundancy in tachykinin signaling, knockout studies (20, 79–81) of Tac2 (NKB), Tacr2 (NK3R), and Tac1 (SP and NKA), have shown that other tachykinins cannot completely compensate for the loss of one signaling pair, particularly in females. Only modest effects were observed in males, with a slight decrease in testicular weight in Tacr3–/– and delayed puberty in Tac1–/– mice, but no effect on fertility. An increase in expression of Tacr2 and a decrease in expression of Pdyn may contribute to the apparent compensation in Tac1 knockout male mice (79). Females of all three genotypes were subfertile, with a decrease in number of pups per litter and a corresponding decrease in number of corpora lutea/ovary. The deletion of Tacr3 had no effect on puberty (20), but disrupted ovarian cycles in adults, whereas the effects of deletion of agonists waned with age: puberty was delayed and estrous cycles were irregular in younger, but not older, mice (80, 81). A similar phenomenon may occur in some humans with disruption of NKB-NK3R signaling who can show spontaneous recovery of fertility as they age (82).
It should be noted that there is little evidence that the redundancy in tachykinin signaling seen in rodents also occurs in sheep, goats, or primates. Thus, although SP-containing fibers make close contacts with KNDy neurons in sheep (73) and goats (83), much higher doses of SP (10- to 20-fold) and NKA (50- to 100-fold) than NKB were needed to stimulate LH secretion in anestrous ewes (84). All three tachykinins are likely acting via NK3R in this species because their relative potency matches the selectivity of this receptor, and few (6%) KNDy and no GnRH neurons contain NK1R in sheep (84). In male monkeys, SP failed to increase LH concentrations at doses that produced flushing of the face (85). In multiunit recording of burst activity in the ARC of goats, 1000 nmol of a specific NK2R agonist was needed to produce the same effect as 10 nmol of an NK3R agonist, whereas the highest dose of a NK1R agonist had no effect (33). Even stronger evidence for a lack of redundancy comes from the ability of specific NK3R antagonists alone to inhibit LH secretion in gonadectomized sheep (86–89), primates (88), and normal men (90) and women (91). This is in marked contrast to the lack of effect of a NK3R antagonist in OVX rats and the modest effect of NK3R mutations in mice (20, 74, 80) and consistent with the much more dramatic effects on puberty and fertility of mutations that disrupt NKB-NK3R signaling in humans (19).
To conclude, neuroanatomical and pharmacological data indicates that tachykinins can act via NK1R, NK2R, or NK3R for the regulation of GnRH/LH release in rodents. In ruminant and primate species, this appears to occur predominantly through NK3R, indicating robust differences in the development of redundant circuits between rodent and nonrodent species. However, the spontaneous restoration of fertility in human patients with TAC3/TAC3R mutations may indicate compensation by other tachykinin circuits that are yet to be fully elucidated in nonrodent species.
The role of dynorphin in pulse termination
There may also be important species differences in the role of dynorphin in the generation of GnRH pulses between ruminants and rodents. In sheep and goats, data with both dynorphin and the KOR antagonist nor-binaltorphimine (nor-BNI) support a critical role for dynorphin in pulse termination. Thus, intracerebroventricular (icv) injection of dynorphin inhibited both LH pulses and the associated bursts of multiunit activity of ARC neurons in OVX goats (25). Likewise, administration of nor-BNI icv to OVX goats (25) or into the ARC of OVX sheep (34) increased LH pulse frequency. The recent identification of KOR in both KNDy and GnRH neurons in sheep (44) raises the possibility that dynorphin could be acting at both sites, and recent preliminary data using internalization of KOR as a marker for dynorphin release support this possibility. Specifically, increased KOR internalization was seen in KNDy neurons shortly after the start of an NKB-induced LH pulse and this internalization increased in tissue collected near the end of a pulse (92). Thus, dynorphin release onto KNDy neurons begins shortly after the start of a pulse and continues for the duration of GnRH secretion, a time course consistent with the stimulatory effects of naloxone on GnRH secretion during a pulse (93). In contrast, KOR internalization into MBH GnRH neurons was observed near the end of the LH pulse, but not at the beginning (94), indicating that dynorphin likely acts at both KNDy and GnRH neurons to terminate each pulse.
In rats and mice, there is compelling evidence that dynorphin can inhibit LH pulses via KNDy neurons, but scant data that this normally occurs. Thus, icv administration of dynorphin in castrated rats (95) and intra-ARC (35) and intravenous treatment (22) with a KOR agonist in OVX rats and mice, respectively, inhibited LH secretion. Similar inhibitory effects of dynorphin on the electrical activity of KNDy neurons have been observed in vitro (41, 42, 96). In contrast, nor-BNI has generally been ineffective. It failed to affect LH pulse frequency when given into the third ventricle (97) or ARC (35) of rats. nor-BNI also had no effect on spontaneous electrical activity of murine KNDy neurons in vitro (41, 42), although it blocked the inhibitory actions of dynorphin in this preparation (42). The lack of effect of nor-BNI in vivo was not due to technical problems, because this KOR antagonist was able to block the inhibitory actions of senktide in OVX rats (35, 36) and augment the stimulatory actions of this NK3R agonist in intact male and female rats (78). Thus, most data in rodents indicates that the anatomical substrates (i.e., dynorphin and KOR) are present in KNDy neurons, but that they are only activated in response to an exogenous stimulus (i.e., senktide) and not during normal episodic GnRH secretion. The one exception to this is the recent report that nor-BNI can prolong the slow excitatory postsynaptic potentials induced by optogenetic stimulation of KNDy neurons in vitro that may be important for the synchronization of this neuronal population (37). However, this finding might be considered another example of dynorphin only playing an important role in response to an exogenous stimulus (i.e., optogenetic depolarization). In summary, whereas dynorphin appears to play a key role in the normal termination of GnRH pulses in ruminants and plays a significant role in mediating progesterone negative feedback during the luteal phase of the estrous cycle of both ruminants and primates (discussed further below), it is unlikely to be critical in rodents. Whether this reflects redundancy in EOP signaling similar to that seen with tachykinin signaling in rodents or a completely different mechanism for pulse termination in these species remains to be determined.
What are the minimal components of the pulse generator?
Are KNDy neurons sufficient?
Although it is clear that KNDy neurons play a vital role in generating LH pulsatile release, it is possible that they only form a component of the pulse generator. To address the relative importance of this population in maintaining pulsatile LH release, sophisticated manipulation of genetically identified KNDy neurons in adult mice has recently been achieved. A report using in vivo GCamp fiber photometry recording of the KNDy neuron population in unanesthetized mice revealed synchronized calcium events in near-perfect correlation with the initiation of pulsatile LH secretion (56). This study strongly supports that KNDy neurons compose the GnRH pulse generator identified in earlier multiunit recording studies in the ARC of the primate (98), rat (99, 100), and goat (25). Moreover, conditional activation of KNDy neurons using stimulatory optogenetics was sufficient to elicit pulse-like LH secretion in anesthetized and unanesthetized mice (56, 101). The authors further noted that termination of an inhibitory light stimulus evoked a rebound pulse that was followed by a second pulse after 35 minutes, regardless of endogenous LH pulse frequency, suggesting that silencing of KNDy neurons was sufficient to reset the pulse generator. However, it is not clear how many KNDy neurons were required to drive LH release, as no markers of neuronal activation have been reported in these studies. Surprisingly, use of mice genetically modified to analyze kisspeptin-GnRH neuron connections found that only 36% of RP3V and 22% of ARC kisspeptin neurons were upstream afferents or downstream targets of GnRH neurons (67). These results suggest potential functional heterogeneity among KNDy subpopulations, with only a subset directly regulating GnRH release, although it is possible that a larger percentage of KNDy neurons are recruited via reciprocal connections. In support of the latter, optogenetic stimulation of KNDy neurons in the unilateral ARC produced synchronized activation of contralateral KNDy cells in the in vitro mouse brain slice, suggesting recruitment of bilateral connections to elicit a robust synchronized activation of the KNDy population (37). This suggestion is consistent with tract-tracing studies in rats and goats that examined projections between the ARC on each side of the third ventricle (102, 103) and the synchronized increase in multi-unit activity in the ARC of goats.
Do other neurons contribute?
Although KNDy neurons are clearly critical regulators of episodic GnRH release, a number of other neuropeptides and transmitters have been implicated in the regulation of GnRH pulses from earlier pharmacological studies. Some of these neuropeptide/transmitter systems may act through direct actions on GnRH neurons; however, others may act through KNDy neurons and other circuitry in the ARC. In this regard, it is of interest that the administration of a GPR54 antagonist into the ARC of the ewe and rat increases the LH interpulse interval (34, 104). As mentioned previously, it is likely that KNDy neurons do not express GPR54 (50). Therefore, this observation suggests that kisspeptin may act on non-KNDy ARC neurons to stimulate pulsatile GnRH release. Such non-KNDy cell activation may reinforce the initial stimulation of KNDy neurons by NKB to drive the GnRH pulse. Although further studies are required to identify the neurons involved, GPR54 is expressed within POMC and tuberoinfundibular dopamine neurons (50) and a GPR54 antagonist partially blocks the activation of ARC POMC neurons by kisspeptin (47). Therefore, it is possible that kisspeptin mediates LH release via local inputs to these cells. For example, it is tempting to speculate that kisspeptin-induced release of β-endorphin from POMC neurons might provide redundancy in the EOP-induced termination of GnRH pulses in rodents. However, the role of this circuitry, if any, in pulse generation requires further study.
Role in Steroid Hormone Feedback
Negative feedback
In males, and for the majority of the female cycle, GnRH episodic secretion is reduced by gonadal steroid hormone negative feedback via afferent populations. A key indicator that KNDy neurons mediate steroid hormone feedback comes from widely reported evidence that KNDy peptide expression correlates with changes in circulating steroid hormone levels. In rodents and ewes, the removal of steroid hormones by gonadectomy increases kisspeptin and NKB expression, whereas the reintroduction of estrogen reduces this expression, suggesting a suppression of excitatory peptides that is consistent with negative feedback (14, 105, 106). Similarly, increased kisspeptin and NKB gene expression and hypertrophy of kisspeptin and NKB neurons occur in the infundibular nucleus of postmenopausal women (28, 106, 107), a state associated with reduced estradiol (106, 107).
In contrast, in some species, steroid hormones have an opposing effect on dynorphin in the ARC, potentially providing an increase in inhibitory tone to GnRH neurons. Dynorphin is reduced in postmenopausal women that have increased LH secretion, suggesting a mechanism where decreased inhibitory tone elevates LH despite ovarian failure (28). In the ARC of ewes, estradiol increases dynorphin expression so that there is an increase in the number of cell bodies containing this peptide (108). In addition, progesterone increases dynorphin mRNA expression in the POA, anterior hypothalamic area, and ARC (109), and microimplantation of antagonists to KOR (61) or progesterone receptor (62) in the ARC increases LH pulse frequency. This is consistent with a large body of work from multiple species (110–112), including humans (28, 113, 114), that recognized endogenous opioids as a significant mediator of progesterone negative feedback and identified ARC dynorphin neurons as a key site that mediates progesterone negative feedback. However, these results stand in contrast to findings in mice, in which estradiol treatment dramatically reduces ARC dynorphin expression despite lowering LH release (22, 115). This anomaly likely reflects physiological differences in the estrous cycle between species because rodents lack a true luteal phase.
Despite evidence for correlative changes in KNDy neuropeptide expression related to negative feedback, until recently, studies have failed to detect differences in KNDy neuron activity in castrated vs intact mice, as shown by cFos expression in KNDy neurons (116) and recordings of spontaneous firing activity from Kiss-GFP ARC neurons in mouse brain slices (117, 118). Due to the variation in KNDy cell firing responses, a recent study aimed to detect changes in long-term KNDy neuron firing patterns in male mice brain slices that fluctuate in similar intervals to that of LH pulses. This study identified an increase in episodic activity by castration that was reduced to intact levels by estradiol and DHT (119). In another study, NK3R-mediated activation and KOR-mediated inhibition of KNDy neurons were attenuated and enhanced, respectively, by gonadal steroids in slices from castrated male mice, suggesting direct inhibitory actions of gonadal steroids (41).
ERα is critical for estrogen feedback and subsequent fertility (120–122), and the deletion of ERα specifically from the ARC of adult mice leads to infertility and impaired estradiol negative feedback (123). Although a high percentage of KNDy neurons are coexpressed with ERα (11–16), estradiol is capable of suppressing LH secretion in adult transgenic mice with ERα specifically removed from kisspeptin neurons [kisspeptin estrogen receptor α–knockout (KERKO) mice], despite failing to suppress ARC Kiss1 mRNA, indicating intact negative feedback in spite of high kisspeptin expression (124). In contrast to adult (124) and postpubertal (125) KERKO mice, estradiol failed to suppress ARC Kiss1 mRNA expression and gonadotropin secretion in prepubertal OVX KERKO mice, indicating ERα in kisspeptin cells is required to restrain LH secretion before the onset of puberty (125). In line with these results, KERKO mice exhibit precocious puberty and increased LH secretion until postnatal day 35 (38). This may indicate that kisspeptin neurons containing ERα are highly sensitive to low levels of estradiol prior to puberty, resulting in low ARC kisspeptin expression and GnRH and LH release. However, over puberty, it is possible that these KNDy neurons acquire resistance to estrogen negative feedback so that other ERα-expressing neural circuits are required for full negative feedback control of GnRH release. It should be noted that the deletion of ERα from POMC neurons impairs estradiol negative feedback and fertility in female mice, suggesting that ERα-expressing non-KNDy ARC neurons may be involved (126). Finally, although initial results from adult KERKO mice based on single blood samples did not show any change in estradiol negative feedback, a more recent study using serial blood sampling detected an increase in LH pulse frequency in adult KERKO mice compared with wild-type controls, likely through increased glutamate release by KNDy-KNDy or other glutamatergic upstream inputs (127). Together, these studies support that KNDy neurons are a component of the circuitry mediating estradiol negative feedback but do not exclude the role of other inputs to KNDy or GnRH neurons.
Although it is widely accepted that ERα is an essential component in steroid hormone negative feedback, other estrogen-responsive receptors may be involved. Classical genomic signaling through ERα is sufficient to drive estradiol positive feedback (124, 128). In contrast, mice that possess a mutation within the ERα gene that renders the receptor unable to initiate gene transcription still display 50% of estradiol-induced suppression of LH release and inhibition of kisspeptin expression (129). Although ERα is capable of both genomic and rapid membrane-initiated steroid actions (130), the viral-mediated ablation of the ERα gene in the ARC of the mouse impairs the long-term chronic negative feedback control of LH but leaves the acute suppression of LH by estradiol intact (123). Further, studies of estradiol actions using immortalized cell lines from either RP3V or ARC mouse kisspeptin populations (131, 132) concluded that GPR30 combined with either ERα or ERβ was required to mediate changes in Kiss1 gene expression in the ARC (132). Therefore, it is likely that multiple receptors expressed in KNDy neurons are required for full expression of estradiol negative feedback, although this is clearly an area for additional future work.
The role of KNDy neurons in positive feedback
During the midfollicular phase in females, rising estrogen levels drive a switch from negative to positive feedback (133), leading to a surge in GnRH and LH release (134, 135) that is necessary for ovulation to occur (136). There is strong support in rodents for the role of RP3V kisspeptin neurons in mediating estradiol positive feedback, and the surge is unable to be driven by high estradiol in KERKO mice (124). However, there is also growing evidence that KNDy neurons play a role in regulating surge secretion of LH in nonrodent species. The sheep and primate ARC host a much larger kisspeptin neuron population and exhibit far fewer preoptic kisspeptin neurons compared with the rodent (137). In the ewe and primate, ARC kisspeptin expression increases prior to the GnRH surge (138, 139), and ARC kisspeptin neurons express cFos during LH surge secretion in the ewe (140, 141). Although kisspeptin POA neurons are also activated during surge secretion in the ewe (140–142), it is possible their participation in estradiol positive feedback is redundant as surgical isolation of the MBH does not block the LH surge in ewes (143) or primates (144). It has not yet been determined whether the negative and positive feedback effects of estradiol are mediated through the same KNDy neurons or distinct subgroups within the KNDy population.
In contrast to the primate and ewe, there is no current evidence for KNDy neuron involvement for generating the LH surge in the rodent brain. However, the ablation of KNDy neurons in the rat increases the magnitude of the LH surge (145, 146). This most likely reflects an inhibitory input from dynorphin into the POA that limits the magnitude of the LH surge in rodents.
Other Roles of KNDy Neurons
In addition to their roles in pulse generation and steroid negative feedback, the past few years have also seen increasing evidence of the involvement of KNDy cells in a number of other functions, including those related to other aspects of reproduction as well as other physiological systems. These include the role of KNDy cells as a key central node for external and internal signals regulating seasonal breeding (108, 147–149), puberty (150–152), the effects of stress on the reproductive axis (153, 154), the interaction between metabolic cues and reproduction (155, 156), the influence of gonadal steroids on prolactin secretion (157) and of prolactin on reproduction (158), and the control of thermoregulation (159–161). It is noteworthy that much of this work, as well as that investigating KNDy cells in pulse generation and steroid feedback, has spawned a new frontier for translational work, applying the basic discoveries to development of new treatments for reproductive as well as nonreproductive disease. For example, discovery of the role of KNDy cells and NKB in thermoregulation has led to recent success in the use of NK3R receptor antagonists in randomized, double-blind trials for the treatment of postmenopausal hot flushes (162, 163). Space limitations preclude a full discussion of the recently extended functions of KNDy cells and their therapeutic implications, but the interested reader is referred to the recent papers and reviews cited above.
Unanswered Questions and Opportunities for Future Research
Although much has been learned since the original identification of KNDy cells in 2007, there are still many unanswered questions about this population and its functions, as well as opportunities to apply new technologies to their study. Although strong evidence has accumulated in recent years to support the role of KNDy cells in generating GnRH pulses, identification of the full complement of neurons, and connections responsible for individual GnRH pulses, their dynamics and modulation by internal and external signals remain a major goal; for example, do non-KNDy or kisspeptin cells in other regions, such as the amygdala (164), participate in the regulation of pulse frequency and/or amplitude? A key unresolved question inherent in the “KNDy hypothesis” of pulse generation is how the time lag between pulse onset and termination occurs if NKB and dynorphin are released at the same time. If dynorphin is not the obligatory “stop” signal for pulses in rodents, and is lacking in the human kisspeptin/NKB cell population, then what are the mechanisms in those species responsible for pulse termination? Similarly, a number of questions remain with respect to the role of KNDy neurons in mediating steroid negative feedback. As discussed above, an important area for future research will be to determine the relative roles of different types of estrogen receptors and signaling pathways (genomic and nongenomic) in the normal physiology of KNDy cells. Recent evidence suggests that neuronal-derived estrogens may play a role in positive feedback regulation of GnRH in the primate (165); whether these actions are in part mediated through KNDy or other kisspeptin cells needs to be investigated.
Coupled to research to address these questions are some technical challenges as well as opportunities. For example, there is a pressing need for the ability to achieve inducible, region- and cell-specific manipulation of KNDy peptides/receptors in adult animals; this would allow for discrete testing of their roles in adult physiology, distinct from their influences during development and puberty. In addition, previous studies of KNDy neuroanatomy using immunohistochemical labeling have required tissue sectioning, limiting analysis to subsets of neurons and losing valuable three-dimensional information about the population. Recent developments in wholemount immunohistochemistry and optical tissue clearing combined with improvements in deep-brain imaging now permit three-dimensional imaging of the complete KNDy population in multiple mammalian species (166, 167). These advances in imaging have the potential to reveal unique features that may be critical for dissecting functionally distinct KNDy populations. Further, recent advances in voltage sensing and calcium imaging technologies have made it possible to assess KNDy activity in freely moving animals (56), and these approaches can be applied to study other functions of KNDy cells as well as their dysfunction in animal models of disease (168, 169). Finally, recent technical innovations now allow single-cell, transcriptional profiling of neurons based on their neuropeptide or connectional phenotype (170, 171) and applying this to KNDy cells under a variety of conditions has the potential to reveal unique components of inter- and intracellular signaling pathways important for their biological function.
Summary
Despite the remaining questions and issues, there has been significant progress in the last decade in our understanding of the neuroendocrine role and functional organization of KNDy cells in the mammalian brain. Compelling evidence has accumulated indicating that KNDy neurons are both necessary and sufficient for driving pulsatile GnRH secretion, supporting the hypothesis that KNDy neurons represent the long-sought “GnRH pulse generator.” Complementing this are findings supporting specific roles for each of the KNDy peptides and receptors in the generation of individual pulses, resulting in modifications of the original KNDy hypothesis (Fig. 2). At the same time, there is recognition that significant species differences exist in these roles, as for example with multiple tachykinins and receptors serving as redundant signals for initiating pulses in rodents as opposed to the predominant role that NKB and NK3R play in initiating pulses in ruminants. And although initial data provided contradictory evidence as to whether KNDy cells were required for steroid negative feedback, more recent findings appear to support this functional role but do not exclude the involvement of additional circuitry either upstream or in parallel to KNDy neurons. Finally, the use of new technologies and approaches that allow cell-specific manipulation experiments to be performed in the same animals in which episodic LH is sequentially monitored (56, 127, 168) has been critical in testing the neuroendocrine functions of KNDy cells in vivo. In the near future, application of these approaches and others will likely significantly extend our knowledge not only of KNDy cell function in the normal brain, but also under conditions that mimic reproductive dysfunctions seen in human disease.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants R01 HD39916 (to M.N.L.) and R01 HD082135 (to R.L.G.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ARC
arcuate nucleus of the hypothalamus
- EOP
endogenous opioid peptide
- ERα
estrogen receptor α
- GFP
green fluorescent protein
- GPR54
G protein-coupled receptor 54
- icv
intracerebroventricular
- KERKO
kisspeptin estrogen receptor α–knockout mice
- KNDy
kisspeptin/neurokinin B/dynorphin
- KOR
κ-opioid receptor
- MBH
mediobasal hypothalamus
- NKA
neurokinin A
- NKB
neurokinin B
- nor-BNI
nor-binaltorphimine
- OVX
ovariectomized
- POA
preoptic area
- POMC
pro-opiomelanocortin
- RP3V
rostral periventricular area of the third ventricle
- SP
substance P
References
- 1. Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. [DOI] [PubMed] [Google Scholar]
- 2. Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202(4368):631–633. [DOI] [PubMed] [Google Scholar]
- 3. Savoy-Moore RT, Swartz KH. Several GnRH stimulation frequencies differentially release FSH and LH from isolated, perfused rat anterior pituitary cells. In: Mahesh VB, Dhindsa DS, Anderson E, Kalra SP, eds.Regulation of Ovarian and Testicular Function. Berlin, Germany: Springer; 1987:641–645. [Google Scholar]
- 4. Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376–385. [DOI] [PubMed] [Google Scholar]
- 5. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 6. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–272. [DOI] [PubMed] [Google Scholar]
- 8. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102(5):1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, Schütz G, Herbison AE. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun. 2013;4(1):2492. [DOI] [PubMed] [Google Scholar]
- 10. Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013;784:27–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976–2984. [DOI] [PubMed] [Google Scholar]
- 12. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–726. [DOI] [PubMed] [Google Scholar]
- 13. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401(3):225–230. [DOI] [PubMed] [Google Scholar]
- 14. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. [DOI] [PubMed] [Google Scholar]
- 15. Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141(11):4218–4225. [DOI] [PubMed] [Google Scholar]
- 16. Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143(11):4366–4374. [DOI] [PubMed] [Google Scholar]
- 17. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. [DOI] [PubMed] [Google Scholar]
- 18. Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18(7):534–541. [DOI] [PubMed] [Google Scholar]
- 19. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang JJ, Caligioni CS, Chan Y-M, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology. 2012;153(3):1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hassaneen A, Naniwa Y, Suetomi Y, Matsuyama S, Kimura K, Ieda N, Inoue N, Uenoyama Y, Tsukamura H, Maeda KI, Matsuda F, Ohkura S. Immunohistochemical characterization of the arcuate kisspeptin/neurokinin B/dynorphin (KNDy) and preoptic kisspeptin neuronal populations in the hypothalamus during the estrous cycle in heifers. J Reprod Dev. 2016;62(5):471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Porter DT, Goodman RL, Coolen LM, Lehman MN. Simultaneous visualization of all three KNDy peptide mRNAs in the same neurons on the ovine arcuate nucleus. In: Neuroscience Meeting Planner Program; 11–15 November 2017; Washington, DC. Abstract 785.26.
- 28. Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20(12):1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hrabovszky E, Sipos MT, Molnár CS, Ciofi P, Borsay BÁ, Gergely P, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology. 2012;153(10):4978–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karsch FJ. Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinizing hormone. Annu Rev Physiol. 1987;49(1):365–382. [DOI] [PubMed] [Google Scholar]
- 31. Nishihara M, Takeuchi Y, Tanaka T, Mori Y. Electrophysiological correlates of pulsatile and surge gonadotrophin secretion. Rev Reprod. 1999;4(2):110–116. [DOI] [PubMed] [Google Scholar]
- 32. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamamura T, Wakabayashi Y, Ohkura S, Navarro VM, Okamura H. Effects of intravenous administration of neurokinin receptor subtype-selective agonists on gonadotropin-releasing hormone pulse generator activity and luteinizing hormone secretion in goats. J Reprod Dev. 2015;61(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grachev P, Li XF, Kinsey-Jones JS, di Domenico AL, Millar RP, Lightman SL, O’Byrne KT. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153(10):4894–4904. [DOI] [PubMed] [Google Scholar]
- 36. Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O’Byrne KT. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153(1):307–315. [DOI] [PubMed] [Google Scholar]
- 37. Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, Rønnekleiv OK. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5:e16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci USA. 2010;107(52):22693–22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Rønnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner RA. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152(11):4298–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology. 2013;154(8):2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750–2760. [DOI] [PubMed] [Google Scholar]
- 43. Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152(11):4265–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. κ-Opioid receptor is colocalized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology. 2016;157(6):2367–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102(6):2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herbison AE, de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151(1):312–321. [DOI] [PubMed] [Google Scholar]
- 47. Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30(30):10205–10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee DK, Nguyen T, O’Neill GP, Cheng R, Liu Y, Howard AD, Coulombe N, Tan CP, Tang-Nguyen AT, George SR, O’Dowd BF. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446(1):103–107. [DOI] [PubMed] [Google Scholar]
- 49. Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001–1012. [DOI] [PubMed] [Google Scholar]
- 50. Higo S, Iijima N, Ozawa H. Characterisation of Kiss1r (Gpr54)-expressing neurones in the arcuate nucleus of the female rat hypothalamus. J Neuroendocrinol. 2017;29(2). [DOI] [PubMed] [Google Scholar]
- 51. Liu X, Herbison A. Kisspeptin regulation of arcuate neuron excitability in kisspeptin receptor knockout mice. Endocrinology. 2015;156(5):1815–1827. [DOI] [PubMed] [Google Scholar]
- 52. Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto KNDy and GnRH neurones during the preovulatory LH surge in the ewe. J Neuroendocrinol. 2015;27(7):624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beale KE, Kinsey-Jones JS, Gardiner JV, Harrison EK, Thompson EL, Hu MH, Sleeth ML, Sam AH, Greenwood HC, McGavigan AK, Dhillo WS, Mora JM, Li XF, Franks S, Bloom SR, O’Byrne KT, Murphy KG. The physiological role of arcuate kisspeptin neurons in the control of reproductive function in female rats. Endocrinology. 2014;155(3):1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu MH, Li XF, McCausland B, Li SY, Gresham R, Kinsey-Jones JS, Gardiner JV, Sam AH, Bloom SR, Poston L, Lightman SL, Murphy KG, O’Byrne KT. Relative importance of the arcuate and anteroventral periventricular kisspeptin neurons in control of puberty and reproductive function in female rats. Endocrinology. 2015;156(7):2619–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA. 2017;114(47):E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lucas LR, Hurley DL, Krause JE, Harlan RE. Localization of the tachykinin neurokinin B precursor peptide in rat brain by immunocytochemistry and in situ hybridization. Neuroscience. 1992;51(2):317–345. [DOI] [PubMed] [Google Scholar]
- 59. Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–386. [DOI] [PubMed] [Google Scholar]
- 60. Gaskins GT, Glanowska KM, Moenter SM. Activation of neurokinin 3 receptors stimulates GnRH release in a location-dependent but kisspeptin-independent manner in adult mice. Endocrinology. 2013;154(11):3984–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. [DOI] [PubMed] [Google Scholar]
- 62. Goodman RL, Holaskova I, Nestor CC, Connors JM, Billings HJ, Valent M, Lehman MN, Hileman SM. Evidence that the arcuate nucleus is an important site of progesterone negative feedback in the ewe. Endocrinology. 2011;152(9):3451–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sannella MI, Petersen SL. Dual label in situ hybridization studies provide evidence that luteinizing hormone-releasing hormone neurons do not synthesize messenger ribonucleic acid for mu, kappa, or delta opiate receptors. Endocrinology. 1997;138(4):1667–1672. [DOI] [PubMed] [Google Scholar]
- 64. Mitchell V, Prevot V, Jennes L, Aubert JP, Croix D, Beauvillain JC. Presence of mu and kappa opioid receptor mRNAs in galanin but not in GnRH neurons in the female rat. Neuroreport. 1997;8(14):3167–3172. [DOI] [PubMed] [Google Scholar]
- 65. Yip SH, Boehm U, Herbison AE, Campbell RE. Conditional viral tract-tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology. 2015;156(7):2582–2594. [DOI] [PubMed] [Google Scholar]
- 66. Yeo S-H, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152(6):2387–2399. [DOI] [PubMed] [Google Scholar]
- 67. Kumar D, Periasamy V, Freese M, Voigt A, Boehm U. In utero development of kisspeptin/GnRH neural circuitry in male mice. Endocrinology. 2015;156(9):3084–3090. [DOI] [PubMed] [Google Scholar]
- 68. Herde MK, Iremonger KJ, Constantin S, Herbison AE. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J Neurosci. 2013;33(31):12689–12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, Franceschini I. Mapping of kisspeptin fibres in the brain of the pro-oestrous rat. J Neuroendocrinol. 2010;22(10):1101–1112. [DOI] [PubMed] [Google Scholar]
- 70. Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, Ajiki K, Ichikawa M, Okamura H, Maeda KI, Tsukamura H. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol. 2011;23(10):863–870. [DOI] [PubMed] [Google Scholar]
- 71. Lehman MN, Silver R. CSF signaling in physiology and behavior In: Agnati LF, Fuxe K, Nicholson C, Sykova E, eds. Progress in Brain Research: Volume Transmission Revisited. Vol 125 New York, NY: Elsevier; 2000:415–433. [DOI] [PubMed] [Google Scholar]
- 72. Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci. 2014;34(45):15060–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fergani C, Navarro VM. Expanding the role of tachykinins in the neuroendocrine control of reproduction. Reproduction. 2016;153(1):R1–R14. [DOI] [PubMed] [Google Scholar]
- 74. Noritake K, Matsuoka T, Ohsawa T, Shimomura K, Sanbuissho A, Uenoyama Y, Maeda K, Tsukamura H. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev. 2011;57(3):409–415. [DOI] [PubMed] [Google Scholar]
- 75. Seabrook GR, Bowery BJ, Hill RG. Pharmacology of tachykinin receptors on neurones in the ventral tegmental area of rat brain slices. Eur J Pharmacol. 1995;273(1-2):113–119. [DOI] [PubMed] [Google Scholar]
- 76. Li SY, Li XF, Hu MH, Shao B, Poston L, Lightman SL, O’Byrne KT. Neurokinin B receptor antagonism decreases luteinising hormone pulse frequency and amplitude and delays puberty onset in the female rat. J Neuroendocrinol. 2014;26(8):521–527. [DOI] [PubMed] [Google Scholar]
- 77. Navarro VM, Bosch MA, León S, Simavli S, True C, Pinilla L, Carroll RS, Seminara SB, Tena-Sempere M, Rønnekleiv OK, Kaiser UB. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology. 2015;156(2):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ruiz-Pino F, Garcia-Galiano D, Manfredi-Lozano M, Leon S, Sánchez-Garrido MA, Roa J, Pinilla L, Navarro VM, Tena-Sempere M. Effects and interactions of tachykinins and dynorphin on FSH and LH secretion in developing and adult rats. Endocrinology. 2015;156(2):576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Maguire CA, Song YB, Wu M, León S, Carroll RS, Alreja M, Kaiser UB, Navarro VM. Tac1 signaling is required for sexual maturation and responsiveness of GnRH neurons to kisspeptin in the male mouse. Endocrinology. 2017;158(7):2319–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. True C, Nasrin Alam S, Cox K, Chan YM, Seminara SB. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice. Endocrinology. 2015;156(4):1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Simavli S, Thompson IR, Maguire CA, Gill JC, Carroll RS, Wolfe A, Kaiser UB, Navarro VM. Substance P regulates puberty onset and fertility in the female mouse. Endocrinology. 2015;156(6):2313–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonça BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95(6):2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Okamura H, Yamamura T, Wakabayashi Y. Mapping of KNDy neurons and immunohistochemical analysis of the interaction between KNDy and substance P neural systems in goat. J Reprod Dev. 2017;63(6):571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fergani C, Mazzella L, Coolen LM, McCosh RB, Hardy SL, Newcomb N, Grachev P, Lehman MN, Goodman RL. Do substance P and neurokinin A play important roles in the control of LH secretion in ewes? Endocrinology. 2016;157(12):4829–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kalil B, Ramaswamy S, Plant TM. The distribution of substance P and kisspeptin in the mediobasal hypothalamus of the male rhesus monkey and a comparison of intravenous administration of these peptides to release GnRH as reflected by LH secretion. Neuroendocrinology. 2016;103(6):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Goodman RL, Coolen LM, Lehman MN. A role for neurokinin B in pulsatile GnRH secretion in the ewe. Neuroendocrinology. 2014;99(1):18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li Q, Millar RP, Clarke IJ, Smith JT. Evidence that neurokinin B controls basal gonadotropin-releasing hormone secretion but is not critical for estrogen-positive feedback in sheep. Neuroendocrinology. 2015;101(2):161–174. [DOI] [PubMed] [Google Scholar]
- 88. Fraser GL, Hoveyda HR, Clarke IJ, Ramaswamy S, Plant TM, Rose C, Millar RP. The NK3 receptor antagonist ESN364 interrupts pulsatile LH secretion and moderates levels of ovarian hormones throughout the menstrual cycle. Endocrinology. 2015;156(11):4214–4225. [DOI] [PubMed] [Google Scholar]
- 89. Clarke IJ, Li Q, Henry BA, Millar RP. Continuous kisspeptin restores luteinizing hormone pulsatility following cessation by a neurokinin B antagonist in female sheep. Endocrinology. 2018;159(2):639–646. [DOI] [PubMed] [Google Scholar]
- 90. Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Neurokinin 3 receptor antagonism decreases gonadotropin and testosterone secretion in healthy men. Clin Endocrinol (Oxf). 2017;87(6):748–756. [DOI] [PubMed] [Google Scholar]
- 91. Fraser GL, Ramael S, Hoveyda HR, Gheyle L, Combalbert J. The NK3 receptor antagonist ESN364 suppresses sex hormones in men and women. J Clin Endocrinol Metab. 2016;101(2):417–426. [DOI] [PubMed] [Google Scholar]
- 92. Weems PW, Coolen LM, Hileman SM, Hardy SL, McCosh RB, Goodman RL, Lehman MN. Kappa opioid receptors are internalized in arcuate KNDy cells during GnRH pulse termination in the ewe. In: Proceedings of the Society for Neuroscience; 12–16 November 2016; San Diego, CA. Abstract 60.04.
- 93. Goodman RL, Parfitt DB, Evans NP, Dahl GE, Karsch FJ. Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology. 1995;136(6):2412–2420. [DOI] [PubMed] [Google Scholar]
- 94. Weems PW, Coolen LM, Hileman SM, Hardy SL, McCosh RB, Goodman RL, Lehman MN. Kappa opioid receptors are internalized in Mbh GnRH cells during GnRH pulse termination in the ewe. In: Proceedings of the Endocrine Society; 1–4 April 2016; Orlando, FL. Abstract 472.
- 95. Kinoshita F, Nakai Y, Katakami H, Imura H. Suppressive effect of dynorphin-(1-13) on luteinizing hormone release in conscious castrated rats. Life Sci. 1982;30(22):1915–1919. [DOI] [PubMed] [Google Scholar]
- 96. Ruka KA, Burger LL, Moenter SM. Both estrogen and androgen modify the response to activation of neurokinin-3 and κ-opioid receptors in arcuate kisspeptin neurons from male mice. Endocrinology. 2016;157(2):752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mostari P, Ieda N, Deura C, Minabe S, Yamada S, Uenoyama Y, Maeda K, Tsukamura H. Dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev. 2013;59(3):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Knobil E. Patterns of hypophysiotropic signals and gonadotropin secretion in the rhesus monkey. Biol Reprod. 1981;24(1):44–49. [DOI] [PubMed] [Google Scholar]
- 99. Kimura F, Nishihara M, Hiruma H, Funabashi T. Naloxone increases the frequency of the electrical activity of luteinizing hormone-releasing hormone pulse generator in long-term ovariectomized rats. Neuroendocrinology. 1991;53(1):97–102. [DOI] [PubMed] [Google Scholar]
- 100. Nishihara M, Sano A, Kimura F. Cessation of the electrical activity of gonadotropin-releasing hormone pulse generator during the steroid-induced surge of luteinizing hormone in the rat. Neuroendocrinology. 1994;59(6):513–519. [DOI] [PubMed] [Google Scholar]
- 101. Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci USA. 2015;112(42):13109–13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized GnRH pulse generator activity among kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in goats. J Reprod Dev. 2013;59(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166(2):680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O’Byrne KT. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One. 2009;4(12):e8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148(3):1150–1157. [DOI] [PubMed] [Google Scholar]
- 106. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92(7):2744–2750. [DOI] [PubMed] [Google Scholar]
- 107. Rance NE, Young WS III. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239–2247. [DOI] [PubMed] [Google Scholar]
- 108. Weems P, Smith J, Clarke IJ, Coolen LM, Goodman RL, Lehman MN. Effects of season and estradiol on KNDy neuron peptides, colocalization with D2 dopamine receptors, and dopaminergic inputs in the ewe. Endocrinology. 2017;158(4):831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835–1842. [DOI] [PubMed] [Google Scholar]
- 110. Whisnant CS, Goodman RL. Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biol Reprod. 1988;39(5):1032–1038. [DOI] [PubMed] [Google Scholar]
- 111. Goodman R, Inskeep E. Neuroendocrine control of the ovarian cycle of the sheep. In: Knobil and Neill’s Physiology of Reproduction. New York, NY: Elsevier; 2006:2389–2447.
- 112. Okrasa S, Kalamarz H, Tilton JE, Ziecik AJ. Influence of opioids on LH secretion in gilts during the estrous cycle. J Physiol Pharmacol. 1992;43(4 Suppl 1):105–116. [PubMed] [Google Scholar]
- 113. Reid RL, Quigley ME, Yen SS. The disappearance of opioidergic regulation of gonadotropin secretion in postmenopausal women. J Clin Endocrinol Metab. 1983;57(6):1107–1110. [DOI] [PubMed] [Google Scholar]
- 114. Quigley ME, Yen SS. The role of endogenous opiates in LH secretion during the menstrual cycle. J Clin Endocrinol Metab. 1980;51(1):179–181. [DOI] [PubMed] [Google Scholar]
- 115. Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29(29):9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53(2):367–378. [DOI] [PubMed] [Google Scholar]
- 117. Cholanian M, Krajewski-Hall SJ, Levine RB, McMullen NT, Rance NE. Electrophysiology of arcuate neurokinin B neurons in female Tac2-EGFP transgenic mice. Endocrinology. 2014;155(7):2555–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. de Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology. 2012;153(11):5384–5393. [DOI] [PubMed] [Google Scholar]
- 119. Vanacker C, Moya MR, DeFazio RA, Johnson ML, Moenter SM. Long-term recordings of arcuate nucleus kisspeptin neurons reveal patterned activity that is modulated by gonadal steroids in male mice. Endocrinology. 2017;158(10):3553–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52(2):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors α (ERalpha) and β (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. [DOI] [PubMed] [Google Scholar]
- 122. Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78(4):204–209. [DOI] [PubMed] [Google Scholar]
- 123. Yeo S-H, Herbison AE. Estrogen-negative feedback and estrous cyclicity are critically dependent upon estrogen receptor-α expression in the arcuate nucleus of adult female mice. Endocrinology. 2014;155(8):2986–2995. [DOI] [PubMed] [Google Scholar]
- 124. Dubois SL, Acosta-Martínez M, DeJoseph MR, Wolfe A, Radovick S, Boehm U, Urban JH, Levine JE. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology. 2015;156(3):1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Estradiol restrains prepubertal gonadotropin secretion in female mice via activation of ERα in kisspeptin neurons. Endocrinology. 2016;157(4):1546–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wang L, Burger LL, Greenwald-Yarnell ML, Myers MG Jr, Moenter SM. Glutamatergic transmission to hypothalamic kisspeptin neurons is differentially regulated by estradiol through estrogen receptor α in adult female mice. J Neurosci. 2018;38(5):1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96(1):50–56. [DOI] [PubMed] [Google Scholar]
- 129. McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol. 2008;290(1-2):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, Guihot AL, Boudou F, Sautier L, Vessières E, Kim SH, Lière P, Fontaine C, Krust A, Chambon P, Katzenellenbogen JA, Gourdy P, Shaul PW, Henrion D, Arnal JF, Lenfant F. Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci USA. 2014;111(2):E283–E290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jacobs DC, Veitch RE, Chappell PE. Evaluation of immortalized AVPV- and arcuate-specific neuronal kisspeptin cell lines to elucidate potential mechanisms of estrogen responsiveness and temporal gene expression in females. Endocrinology. 2016;157(9):3410–3419. [DOI] [PubMed] [Google Scholar]
- 132. Treen AK, Luo V, Chalmers JA, Dalvi PS, Tran D, Ye W, Kim GL, Friedman Z, Belsham DD. Divergent regulation of ER and kiss genes by 17β-estradiol in hypothalamic ARC versus AVPV models. Mol Endocrinol. 2016;30(2):217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yamaji T, Dierschke DJ, Hotchkiss J, Bhattacharya AN, Surve AH, Knobil E. Estrogen induction of LH release in rhesus monkey. Endocrinology. 1971;89(4):1034–1041. [DOI] [PubMed] [Google Scholar]
- 134. Clarke IJ, Cummins JT. Increased gonadotropin-releasing hormone pulse frequency associated with estrogen-induced luteinizing hormone surges in ovariectomized ewes. Endocrinology. 1985;116(6):2376–2383. [DOI] [PubMed] [Google Scholar]
- 135. Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology. 1991;129(3):1175–1182. [DOI] [PubMed] [Google Scholar]
- 136. Reame NE, Sauder SE, Case GD, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab. 1985;61(5):851–858. [DOI] [PubMed] [Google Scholar]
- 137. Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010;1364:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol. 2006;18(10):806–809. [DOI] [PubMed] [Google Scholar]
- 139. Vargas Trujillo M, Kalil B, Ramaswamy S, Plant TM. Estradiol upregulates kisspeptin expression in the preoptic area of both the male and female rhesus monkey (Macaca mulatta): implications for the hypothalamic control of ovulation in highly evolved primates. Neuroendocrinology. 2017;105(1):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153(11):5406–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150(12):5530–5538. [DOI] [PubMed] [Google Scholar]
- 142. Hoffman GE, Le WW, Franceschini I, Caraty A, Advis JP. Expression of fos and in vivo median eminence release of LHRH identifies an active role for preoptic area kisspeptin neurons in synchronized surges of LH and LHRH in the ewe. Endocrinology. 2011;152(1):214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pau KF, Kuehl DE, Jackson GL. Effect of frontal hypothalamic deafferentation on luteinizing hormone secretion and seasonal breeding in the ewe. Biol Reprod. 1982;27(4):999–1009. [DOI] [PubMed] [Google Scholar]
- 144. Krey LC, Butler WR, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology. 1975;96(5):1073–1087. [DOI] [PubMed] [Google Scholar]
- 145. Mittelman-Smith MA, Krajewski-Hall SJ, McMullen NT, Rance NE. Ablation of KNDy neurons results in hypogonadotropic hypogonadism and amplifies the steroid-induced LH surge in female rats. Endocrinology. 2016;157(5):2015–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Helena CV, Toporikova N, Kalil B, Stathopoulos AM, Pogrebna VV, Carolino RO, Anselmo-Franci JA, Bertram R. KNDy neurons modulate the magnitude of the steroid-induced luteinizing hormone surges in ovariectomized rats. Endocrinology. 2015;156(11):4200–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bartzen-Sprauer J, Klosen P, Ciofi P, Mikkelsen JD, Simonneaux V. Photoperiodic co-regulation of kisseptin, neurokinin B and dynorphin in the hypothalamus of a seasonal rodent. J Neuroendocrinol. 2014;26(8):510–520. [DOI] [PubMed] [Google Scholar]
- 148. Weems PW, Goodman RL, Lehman MN. Neural mechanisms controlling seasonal reproduction: principles derived from the sheep model and its comparison with hamsters. Front Neuroendocrinol. 2015;37:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Uenoyama Y, Tsukamura H, Maeda K. KNDy neuron as a gatekeeper of puberty onset. J Obstet Gynaecol Res. 2014;40(6):1518–1526. [DOI] [PubMed] [Google Scholar]
- 151. Greenwald-Yarnell ML, Marsh C, Allison MB, Patterson CM, Kasper C, MacKenzie A, Cravo R, Elias CF, Moenter SM, Myers MG Jr. ERα in Tac2 neurons regulates puberty onset in female mice. Endocrinology. 2016;157(4):1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Toro CA, Wright H, Aylwin CF, Ojeda SR, Lomniczi A. Trithorax dependent changes in chromatin landscape at enhancer and promoter regions drive female puberty. Nat Commun. 2018;9(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Grachev P, Li XF, Hu MH, Li SY, Millar RP, Lightman SL, O’Byrne KT. Neurokinin B signaling in the female rat: a novel link between stress and reproduction. Endocrinology. 2014;155(7):2589–2601. [DOI] [PubMed] [Google Scholar]
- 154. Yang JA, Song CI, Hughes JK, Kreisman MJ, Parra RA, Haisenleder DJ, Kauffman AS, Breen KM. Acute psychosocial stress inhibits LH pulsatility and Kiss1 neuronal activation in female mice. Endocrinology. 2017;158(11):3716–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Roa J, Tena-Sempere M. Connecting metabolism and reproduction: roles of central energy sensors and key molecular mediators. Mol Cell Endocrinol. 2014;397(1-2):4–14. [DOI] [PubMed] [Google Scholar]
- 156. Nestor CC, Kelly MJ, Rønnekleiv OK. Cross-talk between reproduction and energy homeostasis: central impact of estrogens, leptin and kisspeptin signaling. Horm Mol Biol Clin Investig. 2014;17(3):109–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Stathopoulos AM, Helena CV, Cristancho-Gordo R, Gonzalez-Iglesias AE, Bertram R. Influence of dynorphin on estradiol- and cervical stimulation-induced prolactin surges in ovariectomized rats. Endocrine. 2016;53(2):585–594. [DOI] [PubMed] [Google Scholar]
- 158. Araujo-Lopes R, Crampton JR, Aquino NS, Miranda RM, Kokay IC, Reis AM, Franci CR, Grattan DR, Szawka RE. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology. 2014;155(3):1010–1020. [DOI] [PubMed] [Google Scholar]
- 159. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Jayasena CN, Comninos AN, Stefanopoulou E, Buckley A, Narayanaswamy S, Izzi-Engbeaya C, Abbara A, Ratnasabapathy R, Mogford J, Ng N, Sarang Z, Ghatei MA, Bloom SR, Hunter MS, Dhillo WS. Neurokinin B administration induces hot flushes in women. Sci Rep. 2015;5(1):8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109(48):19846–19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, Doyle C, Papadopoulou DA, Bloom SR, Mohideen P, Panay N, Hunter MS, Veldhuis JD, Webber LC, Huson L, Dhillo WS. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10081):1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Mohideen P, Lin VH, Stern TP, Panay N, Hunter MS, Webber LC, Dhillo WS. Neurokinin 3 receptor antagonism rapidly improves vasomotor symptoms with sustained duration of action. Menopause. 2018;1. [DOI] [PMC free article] [PubMed]
- 164. Comninos AN, Anastasovska J, Sahuri-Arisoylu M, Li X, Li S, Hu M, Jayasena CN, Ghatei MA, Bloom SR, Matthews PM, O’Byrne KT, Bell JD, Dhillo WS. Kisspeptin signaling in the amygdala modulates reproductive hormone secretion. Brain Struct Funct. 2016;221(4):2035–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kenealy BP, Keen KL, Garcia JP, Kohlenberg LK, Terasawa E. Obligatory role of hypothalamic neuroestradiol during the estrogen-induced LH surge in female ovariectomized rhesus monkeys. Proc Natl Acad Sci USA. 2017;114(52):13804–13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Yeo SH, Kyle V, Morris PG, Jackman S, Sinnett-Smith LC, Schacker M, Chen C, Colledge WH. Visualisation of Kiss1 neurone distribution using a Kiss1-CRE transgenic mouse. J Neuroendocrinol. 2016;28(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Moore AM, Lucas KA, Goodman RL, Coolen LM, Lehman MN. Three-dimensional imaging of KNDy neurons in the mammalian brain using optical tissue clearing and multiple-label immunocytochemistry. Sci Rep. 2018;8(1):2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci USA. 2015;112(2):596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Cernea M, Padmanabhan V, Goodman RL, Coolen LM, Lehman MN. Prenatal testosterone treatment leads to changes in the morphology of KNDy neurons, their inputs, and projections to GnRH cells in female sheep. Endocrinology. 2015;156(9):3277–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, Goldman M, Verstegen AM, Resch JM, McCarroll SA, Rosen ED, Lowell BB, Tsai LT. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20(3):484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Nectow AR, Moya MV, Ekstrand MI, Mousa A, McGuire KL, Sferrazza CE, Field BC, Rabinowitz GS, Sawicka K, Liang Y, Friedman JM, Heintz N, Schmidt EF. Rapid molecular profiling of defined cell types using viral TRAP. Cell Reports. 2017;19(3):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]