Abstract
Approximately 20–25% of traumatic brain injury (TBI) subjects develop acute lung injury (ALI), but the pathomechanisms of TBI-induced ALI remain poorly defined. Our previous work has shown that the inflammasome plays a critical role in TBI-induced secondary pathophysiology and that inflammasome proteins are released in extracellular vesicles (EV) after TBI. Here we investigated whether EV-mediated inflammasome signaling contributed to the etiology of TBI-induced ALI. C57/BL6 male mice were subjected to controlled cortical impact (CCI), and the brains and lungs were examined for inflammasome activation and ALI at 4 and 24 h after TBI. We show that TBI releases EV containing inflammasome proteins into serum that target the lung to cause ALI, supporting activation of a neural-respiratory-inflammasome axis. Administration of a low-molecular-weight heparin (enoxaparin, a blocker of EV uptake) or treatment with a monoclonal antibody against apoptosis speck-like staining protein containing a caspase recruitment domain (anti-ASC) after adoptive transfer of EV isolated from TBI-injured mice significantly inhibited inflammasome activation in the lungs of recipient mice resulting in improved ALI scores.This axis constitutes an important arm of the innate inflammatory response in lung pathology after TBI and targeting this axis represents a novel therapeutic treatment for TBI-induced ALI.
Keywords: : acute lung injury, extracellular vesicles, inflammasome, innate immune response, traumatic brain injury
Introduction
Severe traumatic brain injury (TBI) is a major public health concern and is a leading cause of mortality and morbidity throughout the world.1 The pathophysiology of TBI is complex with injury-induced inflammation being an important secondary injury mechanism and target for novel treatment strategies.2 Inflammation is a critical response of the immune system to infection and disruption of tissue homeostasis.3 Persistent inflammation may lead to tissue damage and chronic inflammatory disease.3 The innate immune response is activated by danger-associated molecular patterns (DAMPs) or by pathogen-associated molecular patterns (PAMPs) that stimulate PRRs (pattern recognition receptors).4 Emerging studies have revealed that inflammasome complexes comprising large molecular platforms for caspase-1 activation and downstream inflammatory cytokine production play a critical role in innate immune inflammatory responses and contribute to various pathologies and metabolic dysfunctions.5 Inflammasome activation has also been associated with formation of the pyroptosome, a large supramolecular assembly of apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), resulting in a caspase-1 dependent form of cell death termed pyroptosis.6
In addition to direct injury to the brain, TBI may lead to complications in other organs, such as the lungs. TBI patients, in particular, develop ALI (acute lung injury) and ARDS (acute respiratory distress syndrome) with some studies reporting an incidence as high as 30%.7 ALI/ARDS is a major public health problem, and in spite of significant advances in understanding of the pathophysiology of the disease, mortality and morbidity remain high.8
Systemic inflammatory factors may lead to pulmonary dysfunction and lung injury after TBI.9 A flood of secreted inflammatory mediators, including cytokines, chemokines, and DAMPs are released by injured cells and contribute to inflammation in the brain and also affect distal organs such as the lungs.7
One of the most widely studied DAMPs is HMGB1, a protein that serves as an early mediator of inflammation in various pathogenic states, including TBI.10 HMGB1 is a highly conserved 215 amino-acid non-histone nucleosomal regulatory protein that can be quickly translocated to the cytoplasm after injury.11 The mechanism restricting release of HMGB1 during apoptosis has been described ensuring the differential release only during necrosis and not apoptosis. Some evidence suggests that inflammasomes are responsible for HMGB1 release in the extracellular compartment.12 These are important attributes as apoptosis takes place under normal cell turnover and does not require the induction of a sterile inflammatory response. A recent study shows that HMGB1, through binding with receptor for advanced glycation end products (RAGE) may play an important role in TBI-induced pulmonary dysfunction.12 It appears that cytosolic HMGB1 associates with AIM2 and is required for caspase-1 activation,13 and the processing of interleukin (IL)-1β and IL-18 after TBI.14
Various pathomechanisms may contribute to pulmonary complications after TBI, including increased vascular permeability leading to capillary leakage and infiltration of proteinaceous debris.15 EV are membrane-contained vesicles that play a role in cell-to-cell communication16 and play a role in the development of ALI in a LPS (lipopolysaccharide)-induced murine model.17 EV carry bioactive cytokines such as IL-1β and inflammasome proteins.18,19 EV may trigger an immune response and amplify inflammation via its cargo to neighboring and surrounding cells. Currently, there is a scarcity of experimental evidence for the role of the inflammasome and pyroptotic cell death in TBI-induced ALI.
Here, we tested if EV-mediated inflammasome signaling contributes to the pathomechanism of TBI-induced ALI. We show that EV released into the peripheral circulation following TBI mediate inflammasome signaling in the lungs and therefore play a central role in the innate immune response after TBI. Previous studies have shown that EV uptake is inhibited in vitro using heparin and forms of low-molecular-weight heparin, such as enoxaparin.20 We show that treatment with enoxaparin and an ASC monoclonal antibody reduce ALI after adoptive transfer of serum-derived EV from TBI-injured mice. Our findings provide evidence for a neural-respiratory-inflammasome axis and identify potential pharmacological agents that may reduce TBI-induced lung damage.
Methods
Animals and TBI
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Miami Miller School of Medicine (Animal Welfare Assurance A3224-01) and were done according to the NIH Guide for the Care and Use of Laboratory Animals. The ARRIVE guidelines, introduced by a team led by the UK National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs), were followed when conducting this study. C57/BL6 male mice, 8–12 weeks old and weighing 24–32 g, were prospectively randomized to experimental groups (sham, 4 h, 24 h) for TBI, and experimental groups (naïve, sham-saline, untreated, enoxaparin, anti-ASC) for adoptive transfer and treatment. For TBI experiment groups, sham animals underwent surgical procedure but were not injured. For adoptive transfer and treatment studies, the sham-saline group underwent surgical procedures and received saline as vehicle treatment. Naïve animals underwent no surgical procedures. A sample size of 5–6 was used for each group based on power analysis (using G* power analysis, with an effect size F = 0.85, α set at 0.05) and historical data.21,22 All mice were housed in the viral antigen free (VAF) animal facility at the Lois Pope Life Center at the University of Miami on 12-h light/dark cycles; food and water were supplied ad libitum.
Mice were anesthetized with ketamine and xylazine (intraperitoneal, i.p.) and TBI was performed using a controlled cortical impact (CCI) model, as previously described23–25 Injury was induced using the ECCI-6.3 device (Custom Design & Fabrication, Richmond, VA) at 6 m/sec velocity, 0.8 mm depth, and 150 msec impact duration.26 Anesthetized animals were not physiologically monitored during and after TBI or sham surgery. However, animals were observed post-op; they were kept on a heating pad and body temperature was controlled with a rectal probe, and maintained at 37°C, in our operation room and then they were transferred to the animal quarters. Animals were sacrificed at 4 and 24 h after TBI. Sham animals were anesthetized and subjected to the same pre-surgical incision as injured animals.
Tissue collection
All animals were anesthetized with ketamine and xylazine, prior to perfusion. Animals then underwent tracheal perfusion. Lungs were infused with 4% paraformaldehyde (PFA) using a tracheal catheter at 20 cm H2O and then fixed in 4% PFA overnight at 4°C. Fixed lung tissues were paraffin embedded and 5-μm sections were processed.27 Animals then underwent decapitation and right cortical tissue was collected for protein isolation and molecular analyses.
Pyroptosome isolation assay
Mice lung tissue lysates were filtered through a 5-μm low-binding polyvinylidene difluoride (PVDF) membrane (Milipore). Supernatant was centrifuged at 2700 × g for 8 min. The pellet was resuspended in 40 μL of 3[(3-cholamidopropyl) dimethylammonio]-propanesulfonic acid (CHAPS) buffer (20 mmol/L HEPES-KOH, pH 7.5, 5 mmol/L MgCl2, 0.5 mmol/L EGTA, 0.1 mmol/L phenylmethylsulfonyl fluoride, protease inhibitor cocktail, and 0.1% CHAPS). The pyroptosome was pelleted by centrifugation at 2700 × g for 8 min. The pellet was then resuspended and incubated in 27.8 μL of CHAPS buffer with 2.2 μL of disuccinimidyl substrate for 30 min at room temperature to cross-link ASC dimers. Lastly, an equal amount of 2 × Laemmli buffer was added and proteins were analyzed by immunoblotting using antibodies to ASC and Gasdermin-D (GSD).
Nuclear and cytoplasmic extraction
Nuclear and cytoplasmic fractions were extracted using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific) according to manufacturer instructions. Briefly, mice lung tissue samples were cut into 20- to 100-mg pieces and centrifuged at 500 × g for 5 min. Tissue pieces were the homogenized with the Cytoplasmic Extraction Reagent and centrifuged at 16,000 × g for 5 min. Then the supernatant (cellular extract) was removed and the pellet was centrifuged with Nuclear Extraction Reagent (Thermo Scientific) at 16,000 × g for 10 min. This supernatant corresponded to the nuclear fraction, which was removed and stored at −80°C.
Immunoblotting
Lung and brain tissue samples were snap-frozen in liquid nitrogen. Two-millimeter sections of right lower lung and right cortical tissue were homogenized in extraction buffer containing protease and phosphatase inhibitor cocktail (Sigma, St Louis, MO) and resolved in 4–20% Tris-TGX Criterion gels (Bio-Rad, Hercules, CA)19 using antibodies to caspase-1 (Novus Biologicals), ASC (Santa Cruz), IL-1β (Cell Signaling), IL-18 (Abcam), AIM2 (Santa Cruz), and HMGB1 (Millipore). Quantification was performed using Image Lab and all data were normalized to β-actin.
Immunohistochemistry
Tissue sections were deparaffinized in xylene and then rehydrated using ethanol and Tris buffer saline. Immunohistochemical procedures were then carried out for double staining.27 Sections were incubated overnight with antibodies against caspase-1 and ASC (Millipore), AIM2 (Santa Cruz), HMGB1 (Millipore), and SPC (Millipore). Immunostained lung sections of sham, 4 h, and 24 h mice were examined with a Zeiss laser scanning confocal microscope (Zeiss, Inc., Thornwood, NY). Lung sections were analyzed by individuals who were blinded to the groups.
EV isolation
EV were isolated from serum from TBI-injured mice and sham mice using the Total Exosome Isolation (TEI) solution (Invitrogen). 100 μL of each sample were centrifuged at 2000 × g for 30 min. The supernatant was then incubated with 20 μL of TEI for 30 min followed by centrifugation at 10,000 × g for 10 min. The pellet was resuspended in 100 μL of PBS. EV were characterized by the expression of CD81 and by Nanosight Tracking analysis (Supplementary Fig. 1; see online supplementary material at http://www.liebertpub.com/neu).
Adoptive transfer of EV
Serum-derived EV from TBI and sham mice were injected into naïve mice through the jugular vein at a dose of 1.0 × 1010 particles/g/body weight.28 Particle count was measured by Nanosight Tracking analysis and samples were diluted accordingly. Prior to surgery animals were anesthetized with ketamine and xylene. Serum-derived EV were transferred and lung and brain tissues were collected 24 h after injection for analysis.
Enoxaparin and anti-ASC treatment
Serum-derived EV from TBI mice were injected into naïve mice through a jugular vein injection. One hour later, enoxaparin (3 mg/kg) and anti-ASC (5mg/kg) were administered to recipient animals. The following groups were used: 1) the naïve group received no treatment, 2) the sham-saline group was used as a negative control and underwent jugular vein injection of only saline, 3) the untreated group received EV from TBI mice without any treatment and was used as a positive control, 4) the ENOX group received EV from TBI mice and enoxaparin, and 5) the anti-ASC group received EV from TBI mice and anti-ASC. To carry out studies in a double-blinded and unbiased manner the treatment bottles were masked with opaque tape. Randomization to the study groups was performed by a research assistant who was not involved in the delivery of treatment and data analyses. In addition, study investigators were unaware of the randomization until study completion. Lung and brain tissues were collected 24 h after injection for analysis.
Histology and lung injury scoring
Lung tissue sections were stained by a standard hematoxylin and eosin (H&E) method for histology, morphometry and ALI scoring. Lung sections were scored by a pathologist (who was blinded to the groups) using the ALI Scoring System from the American Thoracic Society Workshop Report.29
Statistical analysis
Data were analyzed using a Student's t test for two groups and a one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests, (GraphPad Prism version 7.0) for two or more groups. D'Agostino-Pearson test was used to test for normality. Data are expressed as mean ± standard error of the mean (SEM). P values of significance used were *p < 0.05.
Results
Severe TBI increases AIM2 inflammasome proteins and HMGB1 expression in the brain of mice
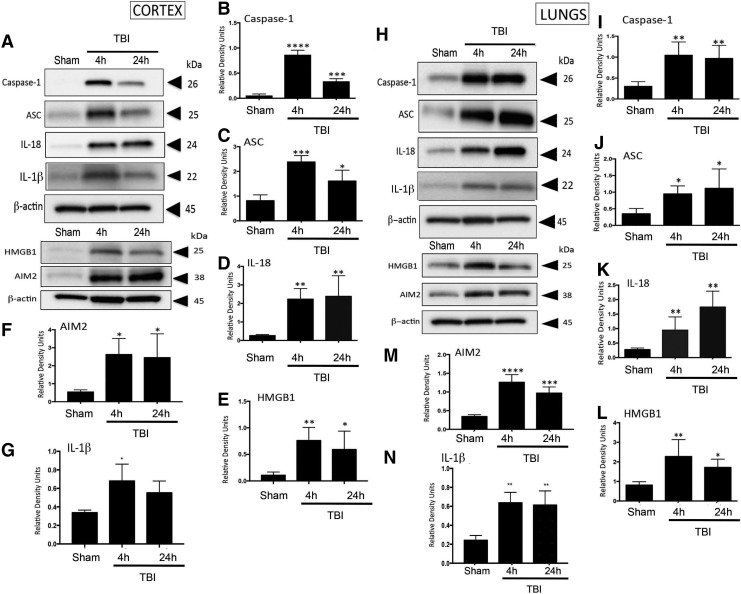
Excessive levels of the pro-inflammatory cytokine IL-1β and IL-18, and inflammasome proteins are associated with secondary damage after fluid-percussion brain injury.30 To determine whether severe CCI induced processing of pro-inflammatory cytokines and alterations in levels of inflammasome proteins, we analyzed cortical brain lysates for the levels of the caspase-1 (Fig. 1A,B; p < 0.001), ASC (Fig. 1A,C; p = 0.003), IL-18 (Fig 1A,D; p = 0.0042), AIM2 (Fig. 1A,F; p = 0.0197), and IL-1β (Fig. 1A,G; p = 0.0141) at 4 and 24 h after injury. Levels of caspase-1, ASC, AIM2, and IL-Iβ peaked at 4 h after CCI and decreased by 24 h. The time-course for maturation of inflammatory cytokines differed slightly, but the levels peaked by 24 h after TBI. In addition there was a significant increase in HMGB1 (Fig. 1A,E; p = 0.0121) at 4 and 24 h after injury. These data indicate that following severe CCI in mice, the levels of the AIM2 inflammasome are significantly elevated in the cortex following injury.
FIG. 1.
Inflammasome activation in C57/BL6 mouse cortical and lung tissue post-TBI. (A) Representative immunoblot of active caspase-1, ASC, IL-18, IL-β, HMGB1, and AIM2 after TBI. (B–G) Active caspase-1, ASC, IL-18, HMGB1, AIM 2, and IL-β, are significantly elevated in cortical tissue at 4 and 24 h post-TBI. Data presented as mean ± SEM; ****p < 0.001, ***p < 0.01, p < 0.05 compared with sham. N = 5–6 per group. (H) Representative immunoblot of active caspase-1, ASC, IL-18, IL-β, HMGB1, and AIM2 in lung tissue. (I–N) Active caspase-1, ASC, IL-18, HMGB1, AIM2, and IL-1β are significantly elevated in lung tissue 4 and 24 h after TBI. Data presented as mean ± SEM. N = 5–6 per group, **p < 0.01., *p < 0.05 compared with sham. ASC, apoptosis speck-like staining protein containing a caspase recruitment domain; IL, interleukin; SEM, standard error of the mean; TBI, traumatic brain injury.
Severe TBI increases AIM2 inflammasome protein and HMGB1 expression on the lungs of mice
To determine whether CCI induced inflammasome activation in the lungs, we performed immunoblot analysis of lung lysates for caspase-1 (Fig. 1H,I; p = 0.0026), ASC (Fig. 1H,J; p = 0.0427), IL-18 (Fig. 1H,K; p = 0.0025), IL-1β (Fig. 1H,N; p = 0.0012) AIM2 (Fig. 1H,M; p < 0.001), and NLRP3 (p = 0.0047) (Supplementary Fig. 1). Increased levels of caspase-1, ASC, IL-18, and AIM2 were significantly increased at 4 h and 24 h after injury. However, the time-course of the increase in protein expression differed slightly from that observed in brain in that they peaked at 24 h after CCI. Because the HMGB1-RAGE axis plays a role in the mechanism by which TBI induces lung dysfunction,12 we analyzed lung lysates for levels of HMGB1 protein expression. Figure 1H,L (p = 0.0158) shows that HMGB1 expression increased at 4 and 24 h after TBI, indicating that the AIM2 inflammasome and HMGB1 play a role in the inflammatory response in the lungs post-TBI.
TBI induces changes in lung morphology and induces ALI
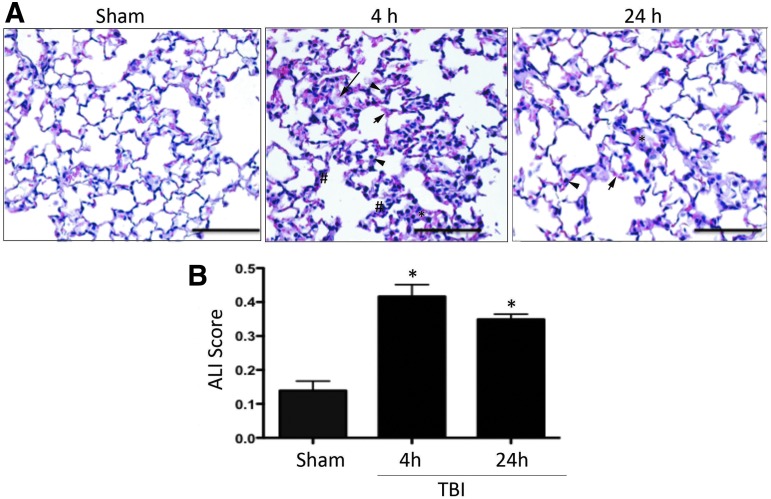
ALI is characterized by inflammatory processes, which lead to alveolar and interstitial edema as well as infiltration of inflammatory cells into the alveolar airspace.31 Histopathological analysis of lung tissue (Fig. 2A) indicated that severe TBI causes substantial changes in the lung architecture and morphology at 4 and 24 h after injury. Sham animals showed a normal alveolar morphology, whereas injured animals showed acute changes in alveolar edema (long arrows). In addition, there is evidence of neutrophil infiltration (arrow heads) and changes in morphology of alveolar capillary membranes (*) at both time-points. Injured animals showed signs of interstitial edema, which was more pronounced at 4 h post-injury (short arrows). Injured animals also showed evidence of thickening of the interstitial area and the alveolar septum (#).
FIG. 2.
TBI induces alveolar morphological changes and acute lung injury in mice. (A) H&E staining of lung sections from sham and injured animals at 4 and 24 h. Sections show evidence of neutrophil infiltration (arrow heads), changes in morphology of alveolar capillary membranes (asterisk, *), interstitial edema (short arrows), and evidence of thickening of the interstitium and the alveolar septum (pound, #). (B) Acute lung injury scoring is significantly increased in injured animals when compared with sham at 4 and 24 h. Data presented as mean ± SEM. N = 5–6 per group, **p < 0.01, *p < 0.05 compared with sham. H&E, hematoxylin and eosin; SEM, standard error of the mean; TBI, traumatic brain injury. Color image is available online at www.liebertpub.com/neu
To confirm that severe injury induces ALI, histological sections were analyzed using the ALI scoring system defined by the American Thoracic Society.29 ALI scores were significantly elevated in TBI-injured animals compared with sham (Fig. 2B; p = 0.0017).
TBI increases immunoreactivity of inflammasome proteins in type II alveolar epithelial cells
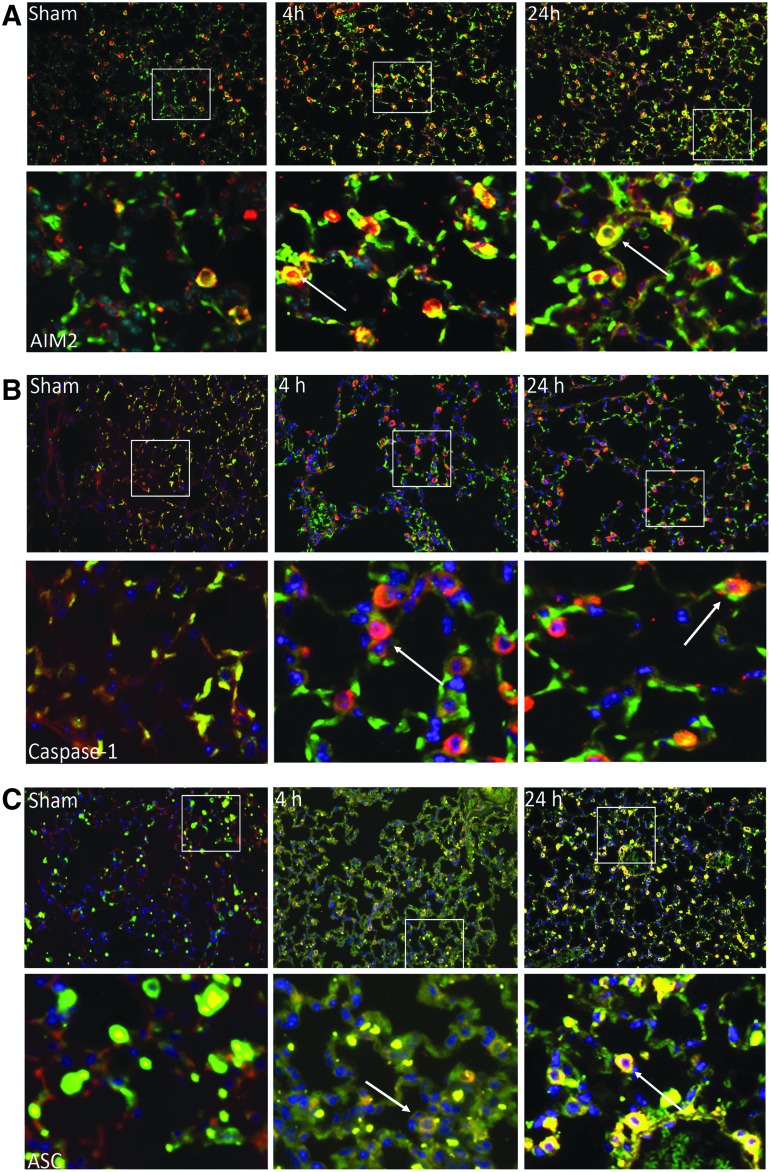
To examine the cellular effects of TBI on inflammasome expression in the lungs after injury, we performed immunohistochemical analysis in lung sections of sham, 4 h, and 24 h injured animals. Lung sections were stained with antibodies against AIM2, caspase-1, and ASC (green), and co-stained with Pro-surfactant protein C (Pro-SPC, red), a marker of type II epithelial cells, and DAPI nuclear staining (blue). As shown in Figure 3, active caspase-1 (Fig. 3A), ASC (Fig. 3B), as well as AIM2 (Fig. 3C) are present in SPC-positive cells (arrow). Immunoreactivity of these inflammasome proteins increased after TBI. Negative control staining for immunohistochemistry is shown in Supplementary Fig. 3 (see online supplementary material at http://www.liebertpub.com/neu). These findings indicate that inflammasome proteins are expressed in type II alveolar epithelial cells and that TBI results in increased immunoreactivity in these cells.
FIG. 3.
Expression of inflammasome proteins in type II alveolar epithelial cells. (A) AIM2, (B) active caspase-1, and (C) ASC immunoreactivity increases in lung tissue after CCI (4 h, 24 h) when compared with sham mice. Confocal images of AIM2, caspase-1, and ASC (green signal) and type II epithelial cells (surfactant protein C, red signal), and DAPI nuclear staining (blue signal). Co-localization of inflammasome protein and type II alveolar epithelial cells demonstrated with arrow. ASC, apoptosis speck-like staining protein containing a caspase recruitment domain; CCI, controlled cortical impact. Color image is available online at www.liebertpub.com/neu
TBI increases nuclear and cytoplasmic HMGB1 expression
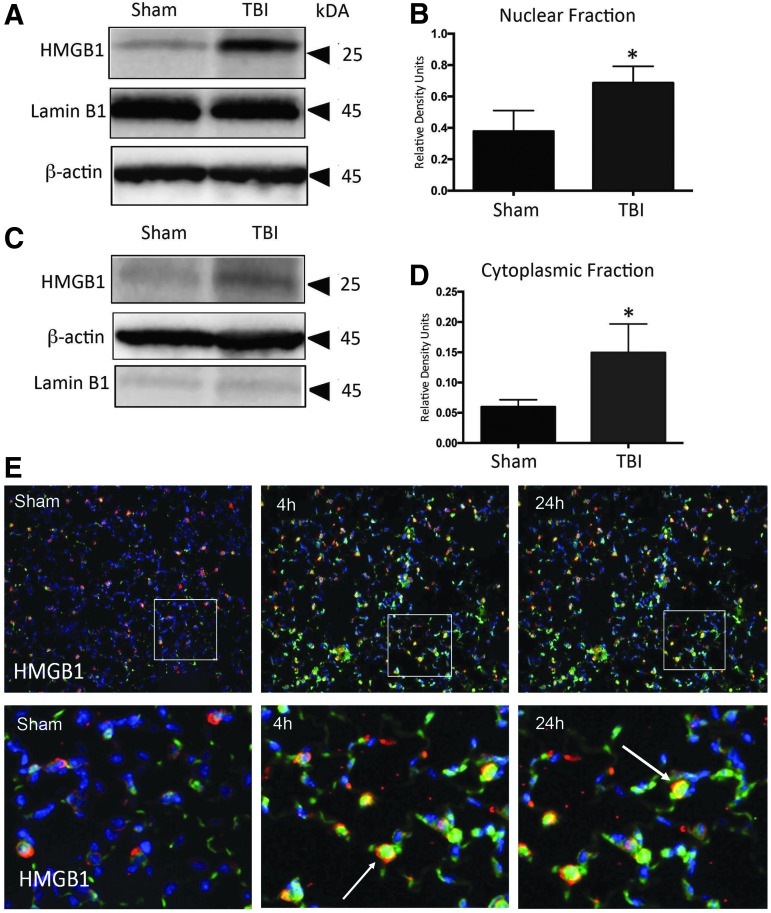
To determine the cellular distribution of HMGB1 in lung cells after TBI, we isolated nuclear and cytoplasmic fractions from lung homogenates (Fig. 4 A,C; p = 0.0337). Immunoblotting indicates that both fractions had significant increases in HMGB1 expression at 4 h post-TBI (Fig. 4B,D; p = 0.0345). We also performed immunohistochemical analysis of HMGB1 to determine the changes in immunoreactivity in lung sections after TBI. Sections were co-stained for HMGB1 (green) and SPC (red), and DAPI nuclear staining (blue). Immunoreactivity of HMGB1 is increased at 4 and 24 h when compared with sham. Weak immunoreactivity of HMGB1 was observed in SPC-positive cells (arrow, Fig. 4E); therefore, suggesting that HMGB1 changes in the injured lung tissue may be cytoplasmic.
FIG. 4.
TBI increases nuclear and cytoplasmic HMGB1 expression in mice lung tissue. (A) Representative immunoblot of nuclear HMGB1 after TBI. (B) Nuclear HMGB1 is significantly elevated in 4-h injured animals compared with sham. (C) Representative immunoblot of cytoplasmic HMGB1 after TBI. (D) Cytoplasmic HMGB1 is significantly elevated in 4-h injured animals compared with sham. Data presented as mean ± SEM; ****p < 0.001, ***p < 0.01, *p < 0.05 compared with sham. N = 5–6 per group. (E) HMGB1 immunoreactivity increases in lung tissue after CCI when compared with sham mice. Confocal images of HMGB1(green signal) and type II epithelial cells (surfactant protein C, red signal), and DAPI nuclear staining (blue signal). Co-localization of inflammasome protein and type II alveolar epithelial cells demonstrated with arrow. CCI, controlled cortical impact; SEM, standard error of the mean; TBI, traumatic brain injury. Color image is available online at www.liebertpub.com/neu
TBI induces pyroptosis in the lungs of mice
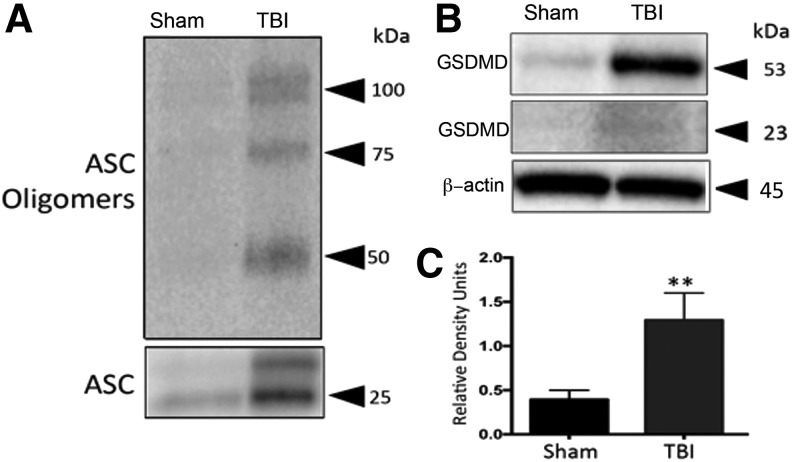
To investigate whether TBI results in pyroptosis in lungs, we isolated the pyroptosome in lung tissue 4 h after TBI. ASC oligomerization was evident by increased levels of ASC dimers (50 kDa) and trimers (75 kDa) as compared with sham animals (Fig. 5A). These results indicate that TBI induces pyroptosome formation in lungs. In addition, there was a significant increase in the levels of the substrate of pyroptosis, GSDMD (Fig. 5B,C; p = 0.0001).
FIG. 5.
Pyroptosome formation in mice lungs 4 h post-TBI. (A) TBI induces laddering of ASC in lung tissue, indicating formation of the pyroptosome, an oligomerization of ASC dimers that leads to activation of caspase-1 and pyroptosis. (B) Representative immunoblot and (C) quantification of gasdermin. Gasdermin-D is significantly elevated in lung tissue post-TBI. Data presented as mean ± SEM. N = 5 per group, **p < 0.01, *p < 0.05 compared with sham. ASC, apoptosis speck-like staining protein containing a caspase recruitment domain; SEM, standard error of the mean; TBI, traumatic brain injury.
Enoxaparin and anti-ASC treatment significantly reduces inflammasome expression and ALI after adoptive transfer of EV from TBI mice
To provide evidence that EV and their cargo are released into the circulation after TBI and that this cargo may induce inflammasome activation in the lungs, we performed an adoptive transfer experiment using serum-derived EV from severe CCI mice. EV preparations were validated using Western blot for EV marker CD81 (Supplementary Fig. 1). Control animals received EV isolated from sham or naïve animals. As shown in Supplementary Fig. 2 (see online supplementary material at http://www.liebertpub.com/neu), active caspase-1 (Supplementary Fig. 2B; p = 0.0048), ASC (Supplementary Fig. 2C; p = 0.0019), IL-18 (Supplementary Fig. 2D; p = 0.0017), AIM2 (Supplementary Fig. 2E; p = 0.0011), and HMGB1 (p = 0.0196; Supplementary Fig. 2F) were significantly elevated at 24 h in the lungs of animals that received the EV from TBI-injured animals when compared with the lungs of animals that receive EV from sham or naïve mice. Further, infiltration of inflammatory cells (arrows) was apparent in lungs treated with EV from TBI mice (Supplementary Fig. 2G). Lastly, the ALI score was also significantly higher in animals that received EV from injured mice (Supplementary Fig. 2H). These studies provide evidence for a neural-respiratory-inflammasome axis in which EV released into the circulation after TBI activate the inflammasome in lung cells contributing to the pathogenesis of ALI.
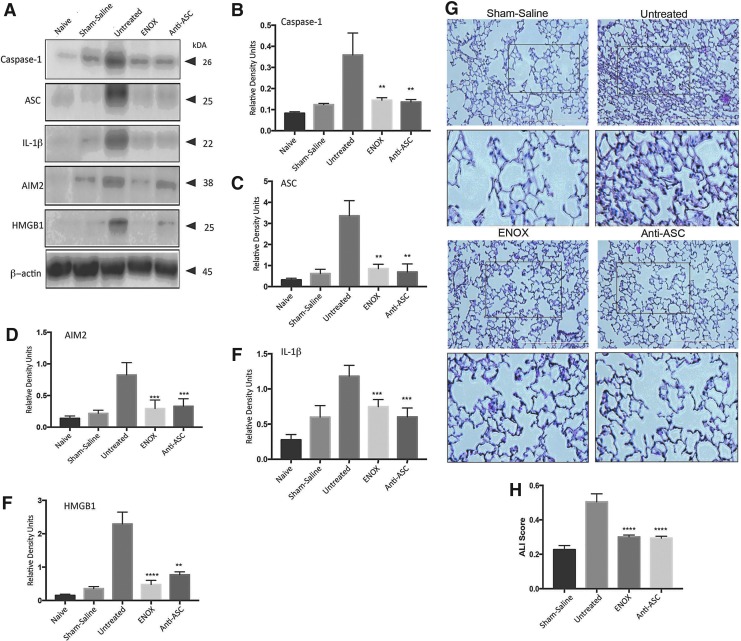
Next we attempted to block exosome uptake by treatment with either enoxaparin and block inflammasome activation with a monoclonal antibody against ASC after the adoptive transfer of EV from injured to naïve mice. Negative control animals received saline, and positive control animals received no treatment. Caspase-1, ASC, IL-1β, AIM2, and HMGB1 were significantly reduced (p ≤ 0.0001; Fig. 6A–F) compared with the untreated (positive control) group after treatment with enoxaparin or anti-ASC. In addition, H&E-stained lung sections showed significantly less neutrophil infiltration into alveolar and interstitial space (Fig. 6G). ALI scores for animals treated with enoxaparin and anti-ASC were significantly lower compared with the untreated group (Fig. 6H; p ≤ 0.0001). Thus, EV released into the circulation after TBI play a role in inflammasome activation in lung cells leading to ALI.
FIG. 6.
Treatment with enoxaparin and anti-ASC antibody reduces inflammasome expression in lungs of animals treated with EV from TBI-injured mice. (A) Representative immunoblot showing that caspase-1, ASC, IL-1β, AIM2, HMGB1 are reduced in the lungs of animals that were treated with enoxaparin (3 mg/kg) and IC 100 (5 mg/kg) when compared with untreated positive control animals; (B–F) data presented as mean ± SEM; p < .0.05 compared with sham. N = 4–5 per group. (G) H&E staining of lung sections from saline, untreated, enoxaparin and IC 100 treated mice lungs delivered EV from injured animals. Sections show evidence of neutrophil infiltration, changes in morphology of alveolar capillary membranes, interstitial edema, and evidence of thickening of the interstitium and the alveolar septum. (H) Acute lung injury scoring is significantly decreased in animals treated with enoxaparin or anti-ASC antibody when compared with untreated animals. Data presented as mean ± SEM. N = 4–5 per group, **p < 0.01, *p < 0.05. ASC, apoptosis speck-like staining protein containing a caspase recruitment domain; EV, extracellular vesicles; H&E, hematoxylin and eosin; IL, interleukin; SEM, standard error of the mean; TBI, traumatic brain injury. Color image is available online at www.liebertpub.com/neu
Discussion
TBI is associated with higher rates of various medical complications, in particular pulmonary and CNS (central nervous system) dysfunction. In this study, we show that (a) severe TBI increases HMGB1 and inflammasome protein expression in cortical and lung tissue and induces changes in lung morphology consistent with ALI; (b) TBI results in pyroptosis in lung tissue and increased expression of inflammasome proteins in type II alveolar epithelial cells; and (c) adoptive transfer of EV from TBI mice activates the inflammasome and induces ALI, indicating that brain injury induces the release of EV containing a cargo of inflammasome proteins that are then carried to the lungs resulting in ALI. These findings support the concept of a neural-respiratory-inflammasome axis that contributes to the pathology in TBI-induced ALI. Our data also suggest that blockage of this axis either by inhibition of EV uptake by enoxaparin or treatment with anti-ASC antibody blocked activation of the inflammasome in lung tissue and prevented the development of ALI, indicating a potential therapeutic approach of TBI-induced lung dysfunction.
Recent clinical studies have shown that patients with an isolated severe TBI and no other polytrauma are at high risk for developing ALI/ARDS32; and development of ALI in severe TBI patients is an independent predictor of unfavorable outcomes.33 Moreover, IL-1β is significantly elevated in BAL (bronchoalveolar lavage) fluid from patients with trauma induced-ALI,34 and cytokine-activated neutrophils, including IL-1β, adhere to the pulmonary endothelial surface of capillaries and migrate into the interstitial and alveolar spaces. These findings suggest a role of the inflammasome in ALI. Our findings of TBI-induced morphological changes in lungs are characteristic of ALI.29 These changes include: infiltration of neutrophils into the alveolar and interstitial space, alveolar septal thickening, as well as alveolar edema and hemorrhage. These are the most common histological diagnostic features in the acute phase of ALI. In our study, the morphological changes were most prominent at 4 h post-injury and correlated with increased inflammasome protein expression in lung tissue (Fig. 1H–N).
Our data are consistent with the time-course of ALI in the human population where physiological evidence of widespread damage to cells and structures of the alveolar capillary membrane occurs within hours to days post-injury.15 Another hallmark of ALI is the destruction of type II alveolar cells, which leads to disruption of normal fluid transport.35 Our results are the first to show inflammasome proteins expression in type II alveolar cells after TBI, indicating a role for the innate immune response in these cells.
The inflammasome in the CNS is a key mediator of the innate immune inflammatory response.5 The CNS innate immune system also plays a role in the pathology of pulmonary dysfunction after TBI.7 Our results support the idea that AIM2 inflammasome activation plays a role in TBI-induced ALI in that AIM2 inflammasome proteins such as caspase-1, ASC, and IL-18 are significantly increased in cortical and lung tissue after TBI. Inflammasome activation and IL-1β release may result in pyroptotic cell death.36 The results of our study are consistent with this concept and show oligomerization of ASC and cleavage of the substrate of pyroptosis GSDMD37 in lungs of TBI-injured animals. Thus, it is possible that development of therapeutics that target pyroptosis may block TBI-induced lung injury.
Recently, it has been shown that a systemic inflammatory response also plays a key role in TBI-induced lung injury.38 Specifically, the HMGB1-RAGE ligand receptor pathway serves as a central transduction mechanism for pulmonary dysfunction after TBI.12 HMGB1 also induces AIM2 inflammasome activation.39 Here we found elevated protein levels of HMGB1 in both nuclear and cytoplasmic fractions of lung cells as early as 4 h after TBI. Previous studies have reported an increase in plasma HMGB1 in trauma patients within the first 6 h after injury, and this increased expression is associated with multiple-organ failure.40 Approximately 50% of TBI patients are admitted to the intensive care unit (ICU) for multiple organ damage, and of these 40% die of multiple organ dysfunction.41 Our findings suggest a critical role of HMGB1 as an inducer of the inflammasome in TBI-induced lung injury. However, recent studies have shown a novel release mechanism for HMGB1 via inflammasome signaling.14 Therefore, further research involving the inflammasome and the HMGB1-RAGE axis may give better insight into EV uptake mechanisms into lung cells after injury.
It has been shown that the blood–brain barrier is permeable after TBI as early as 3 h after injury, resulting in damage to the protective barrier between the brain and the intravascular compartment that leads to leakage of proteins and fluid.42 Disruption of the blood–brain barrier after TBI and by other potential routes, including lymphatic or glymphatic transport systems, results in the secretion of inflammatory mediators, which can exacerbate brain inflammation and damage other distal organs.43,44 Our study shows that TBI induces the secretion of EV containing a cargo of inflammasome protein into the circulation. Adoptive transfer of EV from TBI mice to control mice inducing ALI, supports this concept. It is clear that EV carry a range of nucleic acids and proteins that may have significant impact on the phenotype of the recipient cells.45 Our findings indicate that treatment with low-molecular-weight heparin or an anti-ASC antibody 1 h after EV administration interferes with adoptive transfer and blocks inflammasome activation in lung recipient cells. These effects of a low-molecular-weight heparin are consistent with non-anticoagulant,46 anti-inflammatory effects of heparins.47 Low-molecular-weight heparin has been shown to inhibit systemic inflammation,48 and prevent endotoxin-induced ALI in rats.49 In addition, low-molecular-weight heparin alters the protein composition of EV50 and has been suggested to suppress microvesiculation.51 Unfractionated heparin has also been shown to block the transfer of EV between donor and recipient cells.20 However, the present study does not indicate which EV uptake mechanism is involved in the activation of inflammasomes in recipient lung cells. Thus, further studies are necessary to unravel the mechanistic basis for blockage of inflammasome activation by treatment with enoxaparin or anti-ASC after TBI.
In summary, our data indicate that AIM2 inflammasome signaling plays a central role in the pathomechanism of lung injury after TBI and demonstrates a novel mechanism of TBI-induced ALI involving EV-mediated inflammasome signaling. These data also provide evidence that EV-mediated inflammasome signaling plays a central role involving a neuronal-respiratory-inflammasome axis. Therefore, targeting this axis with antibodies against inflammasome proteins or drugs that block EV uptake may provide a novel therapeutic approach in neurotrauma-induced ALI in all areas of critical care medicine.
Supplementary Material
Acknowledgments
We thank Dr. Tahir Ahmed for scientific discussions and for critically reading the manuscript and Ofelia E. Furones-Alonso for help with blood gas determinations. This project was supported in part with NIH grant R42NS086274 to RWK, NIH grant HL132425 to NK, and R01 NS042133 and NS089443 to WDD.
Author Disclosure Statement
JPdRV, WDD, and RWK are managing members in InflamaCORE, LLC., a company dedicated to discovering novel diagnostic and therapeutic strategies targeting abnormal inflammasome activation in clinical conditions.
References
- 1.Summers C.R., Ivins B., and Schwab K.A. (2009). Traumatic brain injury in the United States: an epidemiologic overview. Mt. Sinai J. Med. 76, 105–110 [DOI] [PubMed] [Google Scholar]
- 2.Bramlett H.M., and Dietrich W.D. (2015). Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J. Neurotrauma 32, 1834–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chovatiya R., and Medzhitov R. (2014). Stress, inflammation, and defense of homeostasis. Mol. Cell 54, 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newton K., and Dixit V.M. (2012). Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Bio. 4 pii: a006049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Rivero Vaccari J.P., Dietrich W.D., and Keane R.W. (2014). Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J. Cereb. Blood Flow Metab. 34, 369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandes-Alnemri T., and Alnemri E.S. (2008). Assembly, purification, and assay of the activity of the ASC pyroptosome. Methods Enzymol. 442, 251–270 [DOI] [PubMed] [Google Scholar]
- 7.Nicolls M.R., and Laubach V.E. (2014). Traumatic brain injury: lungs in a RAGE. Sci. Transl. Med. 6, 252fs234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav H., Nolan M.E., Bohman J.K., Cartin-Ceba R., Peters S.G., Hogan W.J., Gajic O., and Kor D.J. (2016). Epidemiology of acute respiratory distress syndrome following hematopoietic stem cell transplantation. Crit. Care Med. 44, 1082–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rincon F., Ghosh S., Dey S., Maltenfort M., Vibbert M., Urtecho J., McBride W., Moussouttas M., Bell R., Ratliff J.K., and Jallo J. (2012). Impact of acute lung injury and acute respiratory distress syndrome after traumatic brain injury in the United States. Neurosurgery 71, 795–803 [DOI] [PubMed] [Google Scholar]
- 10.Andersson U., and Rauvala H. (2011). Introduction: HMGB1 in inflammation and innate immunity. J. Intern. Med. 270, 296–300 [DOI] [PubMed] [Google Scholar]
- 11.Feldman P., Due M.R., Ripsch M.S., Khanna R., and White F.A. (2012). The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J. Neuroinflammation 9, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber D.J., Gracon A.S., Ripsch M.S., Fisher A.J., Cheon B.M., Pandya P.H., Vittal R., Capitano M.L., Kim Y., Allette Y.M., Riley A.A., McCarthy B.P., Territo P.R., Hutchins G.D., Broxmeyer H.E., Sandusky G.E., White F.A., and Wilkes D.S. (2014). The HMGB1-RAGE axis mediates traumatic brain injury-induced pulmonary dysfunction in lung transplantation. Sci. Transl. Med. 6, 252ra124. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Sun Q., Loughran P., Shapiro R., Shrivastava I.H., Antoine D.J., Li T., Yan Z., Fan J., Billiar T.R., and Scott M.J. (2017). Redox-dependent regulation of hepatocyte absent in melanoma 2 inflammasome activation in sterile liver injury in mice. Hepatology 65, 253–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu B., Nakamura T., Inouye K., Li J., Tang Y., Lundback P., Valdes-Ferrer S.I., Olofsson P.S., Kalb T., Roth J., Zou Y., Erlandsson-Harris H., Yang H., Ting J.P., Wang H., Andersson U., Antoine D.J., Chavan S.S., Hotamisligil G.S., and Tracey K.J. (2012). Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488, 670–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware L.B., and Matthay M.A. (2000). The acute respiratory distress syndrome. N. Engl. J. Med. 342, 1334–1349 [DOI] [PubMed] [Google Scholar]
- 16.Yanez-Mo M., Siljander P.R., Andreu Z., Zavec A.B., Borras F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., Colas E., Cordeiro-da Silva A., Fais S., Falcon-Perez J.M., Ghobrial I.M., Giebel B., Gimona M., Graner M., Gursel I., Gursel M., Heegaard N.H., Hendrix A., Kierulf P., Kokubun K., Kosanovic M., Kralj-Iglic V., Kramer-Albers E.M., Laitinen S., Lasser C., Lener T., Ligeti E., Line A., Lipps G., Llorente A., Lotvall J., Mancek-Keber M., Marcilla A., Mittelbrunn M., Nazarenko I., Nolte-'t Hoen E.N., Nyman T.A., O'Driscoll L., Olivan M., Oliveira C., Pallinger E., Del Portillo H.A., Reventos J., Rigau M., Rohde E., Sammar M., Sanchez-Madrid F., Santarem N., Schallmoser K., Ostenfeld M.S., Stoorvogel W., Stukelj R., Van der Grein S.G., Vasconcelos M.H., Wauben M.H., and De Wever O. (2015). Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buzas E.I., Gyorgy B., Nagy G., Falus A., and Gay S. (2014). Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 10, 356–364 [DOI] [PubMed] [Google Scholar]
- 18.Qu Y., Franchi L., Nunez G., and Dubyak G.R. (2007). Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 179, 1913–1925 [DOI] [PubMed] [Google Scholar]
- 19.de Rivero Vaccari J.P., Brand F., 3rd, Adamczak S., Lee S.W., Barcena J.P., Wang M.Y., Bullock M.R., Dietrich W.D., and Keane R.W. (2015). Exosome-mediated inflammasome signaling after central nervous system injury. J. Neurochem. 136, Suppl. 1, 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atai N.A., Balaj L., van Veen H., Breakefield X.O., Jarzyna P.A., Van Noorden C.J., Skog J., and Maguire C.A. (2013). Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J. Neurooncol. 115, 343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Rivero Vaccari J.P., Lotocki G., Marcillo A.E., Dietrich W.D., and Keane R.W. (2008). A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. 28, 3404–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assis-Nascimento P., Umland O., Cepero M.L., and Liebl D.J. (2016). A flow cytometric approach to analyzing mature and progenitor endothelial cells following traumatic brain injury. J. Neurosci. Methods 263, 57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong Y., Mahmood A., and Chopp M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campolo M., Esposito E., and Cuzzocrea S. (2018). A controlled cortical impact preclinical model of traumatic brain injury. Methods Mol. Biol. 1727, 385–391 [DOI] [PubMed] [Google Scholar]
- 25.Kokiko-Cochran O.N., Saber M., Puntambekar S., Bemiller S.M., Katsumoto A., Lee Y.S., Bhaskar K., Ransohoff R.M., and Lamb B.T. (2017). Traumatic Brain Injury in hTau Model Mice: Enhanced Acute Macrophage Response and Altered Long-Term Recovery. J Neurotrauma 35, 73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkins C.M., Cepero M.L., Kang Y., Liebl D.J., and Dietrich W.D. (2013). Effects of early rolipram treatment on histopathological outcome after controlled cortical impact injury in mice. Neurosci. Lett. 532, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S., Platteau A., Chen S., McNamara G., Whitsett J., and Bancalari E. (2010). Conditional overexpression of connective tissue growth factor disrupts postnatal lung development. Am. J. Respir. Cell Mol. Biol. 42, 552–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiklander O.P., Nordin J.Z., O'Loughlin A., Gustafsson Y., Corso G., Mager I., Vader P., Lee Y., Sork H., Seow Y., Heldring N., Alvarez-Erviti L., Smith C.I., Le Blanc K., Macchiarini P., Jungebluth P., Wood M.J., and Andaloussi S.E. (2015). Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 4, 26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matute-Bello G., Downey G., Moore B.B., Groshong S.D., Matthay M.A., Slutsky A.S., Kuebler W.M., and Acute Lung Injury in Animals Study Group. (2011). An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 44, 725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Rivero Vaccari J.P., Lotocki G., Alonso O.F., Bramlett H.M., Dietrich W.D., and Keane R.W. (2009). Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J. Cereb. Blood Flow Metab. 29, 1251–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ragaller M., and Richter T. (2010). Acute lung injury and acute respiratory distress syndrome. J. Emerg. Trauma Shock 3, 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendrickson C.M., Howard B.M., Kornblith L.Z., Conroy A.S., Nelson M.F., Zhuo H., Liu K.D., Manley G., Matthay M.A., Calfee C.S., and Cohen M.J. (2016). The acute respiratory distress syndrome following isolated severe traumatic brain injury. J. Trauma Acute Care Surg. 80, 989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mascia L. (2009). Acute lung injury in patients with severe brain injury: a double hit model. Neurocrit. Care 11, 417–426 [DOI] [PubMed] [Google Scholar]
- 34.Cross L.J., and Matthay M.A. (2011). Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit. Care Clin. 27, 355–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luh S.P., and Chiang C.H. (2007). Acute lung injury/acute respiratory distress syndrome (ALI/ARDS): the mechanism, present strategies and future perspectives of therapies. J. Zhejiang Univ. Sci. B 8, 60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miao E.A., Rajan J.V., and Aderem A. (2011). Caspase-1-induced pyroptotic cell death. Immunol. Rev. 243, 206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V.G., Wu H., and Lieberman J. (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalsotra A., Zhao J., Anakk S., Dash P.K., and Strobel H.W. (2007). Brain trauma leads to enhanced lung inflammation and injury: evidence for role of P4504Fs in resolution. J. Cereb. Blood Flow Metab. 27, 963–974 [DOI] [PubMed] [Google Scholar]
- 39.Liu L., Yang M., Kang R., Dai Y., Yu Y., Gao F., Wang H., Sun X., Li X., Li J., Wang H., Cao L., and Tang D. (2014). HMGB1-DNA complex-induced autophagy limits AIM2 inflammasome activation through RAGE. Biochem. Biophys. Res. Commun. 450, 851–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peltz E.D., Moore M.E., Eckels P.C., Damle S.S., Tsuruta Y., Johnson J.L., Sauaia A., Silliman C.C., Banerjee A., and Abraham E. (209). HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock 32, 17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zygun D.A., Kortbeek J.B., Fick G.H., Laupland K.B., and Doig C.J. (2005). Non-neurologic organ dysfunction in severe traumatic brain injury. Crit. Care Med. 33, 654–660 [DOI] [PubMed] [Google Scholar]
- 42.Hay (2015). Blood-brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. J. Neuropathol. Exp. Neurol. 74, 1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mrozek S., Dumurgier J., Citerio G., Mebazaa A., and Geeraerts T. (2014). Biomarkers and acute brain injuries: interest and limits. Crit. Care 18, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spector R., Robert Snodgrass S., and Johanson C.E. (2015). A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp. Neurol. 273, 57–68 [DOI] [PubMed] [Google Scholar]
- 45.Mulcahy L.A., Pink R.C., and Carter D.R. (2014). Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tyrrell D.J., Horne A.P., Holme K.R., Preuss J.M., and Page C.P. (1999). Heparin in inflammation: potential therapeutic applications beyond anticoagulation. Adv. Pharmacol. 46, 151–208 [DOI] [PubMed] [Google Scholar]
- 47.Campo C., Molinari J.F., Ungo J., and Ahmed T. (1999). Molecular-weight-dependent effects of nonanticoagulant heparins on allergic airway responses. J. Appl. Physiol. (1985)86, 549–557 [DOI] [PubMed] [Google Scholar]
- 48.Luan Z.G., Naranpurev M., and Ma X.C. (2014). Treatment of low molecular weight heparin inhibits systemic inflammation and prevents endotoxin-induced acute lung injury in rats. Inflammation 37, 924–932 [DOI] [PubMed] [Google Scholar]
- 49.Ning F., Wang X., Shang L., Wang T., Lv C., Qi Z., and Wu D. (2015). Low molecular weight heparin may prevent acute lung injury induced by sepsis in rats. Gene 557, 88–91 [DOI] [PubMed] [Google Scholar]
- 50.Shomer E., Katzenell S., Zipori Y., Rebibo-Sabbah A., Brenner B., and Aharon A. (2016). Microvesicles of pregnant women receiving low molecular weight heparin improve trophoblast function. Thromb. Res. 137, 141–147 [DOI] [PubMed] [Google Scholar]
- 51.Sustar V., Jansa R., Frank M., Hagerstrand H., Krzan M., Iglic A., and Kralj-Iglic V. (2009). Suppression of membrane microvesiculation—a possible anticoagulant and anti-tumor progression effect of heparin. Blood Cells Mol. Dis. 42, 223–227 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.