Abstract
Background
In the last few years, accumulating evidence has indicated that numerous long noncoding RNAs (lncRNAs) are abnormally expressed in gastric cancer (GC) and are associated with the survival of GC patients. This study aimed to conduct a meta-analysis on 19 lncRNAs (AFAP1 antisense RNA 1 [AFAP1-AS1], CDKN2B antisense RNA 1 [ANRIL], cancer susceptibility 15 [CASC15], colon cancer associated transcript 2 [CCAT2], gastric adenocarcinoma associated, positive CD44 regulator, long intergenic noncoding RNA [GAPLINC], H19, imprinted maternally expressed transcript [H19], HOX transcript antisense RNA [HOTAIR], HOXA distal transcript antisense RNA [HOTTIP], long intergenic non-protein coding RNA 673 [LINC00673], metastasis-associated lung adenocarcinoma transcript 1 [MALAT1], maternally expressed 3 [MEG3], promoter of CDKN1A antisense DNA damage activated RNA [PANDAR], Pvt1 oncogene [PVT1], SOX2 overlapping transcript [Sox2ot], SPRY4 intronic transcript 1 [SPRY4-IT1], urothelial cancer associated 1 [UCA1], X inactive specific transcript [XIST], ZEB1 antisense RNA 1 [ZEB1-AS1] and ZNFX1 antisense RNA 1 [ZFAS1]) to systematically estimate their prognostic value in GC.
Methods
The qualified literature was systematically searched in PubMed, Web of Science, Embase and Cochrane Database of Systematic Reviews (up to March 16, 2018), and one meta-analysis relating to the relationship between lncRNA expression and overall survival (OS) of GC patients was performed. The only evaluation criterion of survival results was OS.
Results
A total of 6,095 GC patients and 19 lncRNAs from 51 articles were included in the present study. Among the listed 19 lncRNAs, 18 lncRNAs (other than SPRY4-IT1) showed a significantly prognostic value (P<0.05).
Conclusion
This meta-analysis suggested that the abnormally expressed lncRNAs (AFAP1-AS1, ANRIL, CASC15, CCAT2, GAPLINC, H19, HOTAIR, HOTTIP, LINC00673, MALAT1, MEG3, PANDAR, PVT1, Sox2ot, UCA1, XIST, ZEB1-AS1 and ZFAS1) were significantly associated with the survival of GC patients, among which AFAP1-AS1, CCAT2, LINC00673, PANDAR, PVT1, Sox2ot, ZEB1-AS1 and ZFAS1 were strong candidates in predicting the prognosis of GC patients.
Keywords: long noncoding RNA, gastric cancer, prognosis, meta-analysis
Introduction
In the last few years, accumulating evidence has indicated that numerous long noncoding RNAs (lncRNAs) are abnormally expressed in gastric cancer (GC) and are associated with the survival of GC patients.1–113 GC is the fourth most diagnosed tumor type and the third most common origin of tumor-related death all over the world.114,115 Although the incidence and mortality of GC are declining, >24,590 individuals are diagnosed with GC per year, of which 10,720 die from GC in the USA.116 Although diagnosis and treatment strategies have been improved, the number of surviving cases remains low, since diagnosis often occurs in the late stages.116,117 Thus, the molecular characteristics about the carcinogenesis of GC and the recognition of new biomarkers for GC are urgently needed.
lncRNA is a new type of noncoding RNA that has a length of >200 nucleotides (nt) and lacks important open reading frameworks and can be divided into five main categories (sense, antisense, bidirectional, intronic and intergenic).118 Abundant evidence has demonstrated that lncRNAs play significant regulatory roles in tumor biology via various mechanisms affecting transcriptional and posttranscriptional levels.118–120 Currently, for both cell behavior and clinicopathological factors, significant advances with respect to lncRNA effects on GC have been discovered.121
On account of the obvious expression differences between normal and malignant tissues as well as causal roles of lncRNAs in cancer development, lncRNAs are now attracting increasing attention, which has led to numerous investigations of the correlation between lncRNA states and clinical results in GC. Nevertheless, most of these studies were performed with small samples, and there were inconsistently observed connections. Consequently, we conducted a meta-analysis to determine the accurate role of lncRNAs in the prognosis of GC patients, which possibly supplied us with new insights into the clinical value of combined detection in forecasting prognostic results and determining promising biomarkers in GC treatment strategies.
Methods
Literature search strategy
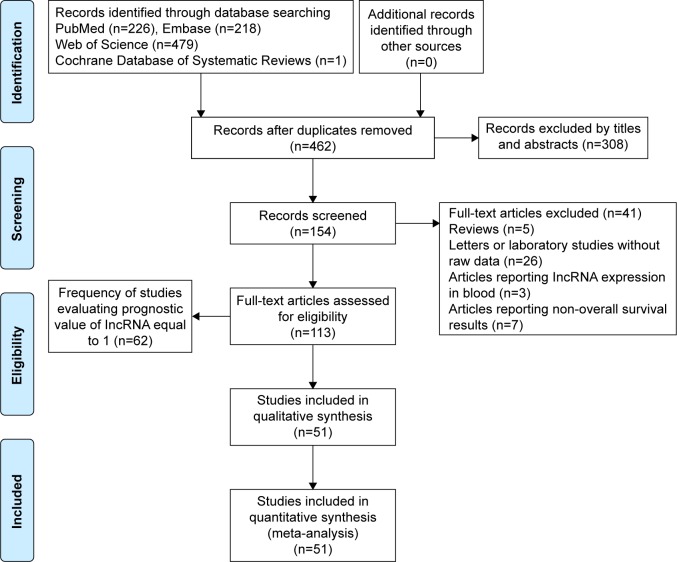
We basically performed a systematic selection of papers published in English from four databases (PubMed, Web of Science, Embase and Cochrane Database of Systematic Reviews). A comprehensive search was conducted using the subject term: lncRNA and gastric cancer. Two authors (Song Gao and Zhi-Ying Zhao) checked the titles and abstracts of the retrieved papers, and Yue Zhang reevaluated uncertain data. Figure 1 shows the flow diagram of the literature search and selection.
Figure 1.
Flow diagram of the literature search and selection.
Abbreviation: lncRNA, long noncoding RNA.
Inclusion criteria
We set up inclusion criteria for qualified papers, which were analyzed using our full-text assessment: 1) articles concerning the pertinence between lncRNA level in cancer tissues and prognosis of GC patients; 2) the survival results were estimated using overall survival (OS) and 3) full-text papers published in English.
Exclusion criteria
Articles that did not meet the abovementioned inclusion criteria, reviews, letters and laboratory studies without raw data were excluded. Articles of non-dichotomous lncRNA expression levels and frequency of studies evaluating prognostic value of lncRNAs equal to 1 were also excluded. If more than one paper had been published on the identical study cohort, only the most well-rounded investigation was selected for this research. In addition, if both of the univariate and multivariate outcomes were covered, only the latter were chosen, since they were adjusted for confounding factors.
Research frequency
Table 1 gives the frequency of investigations reporting prognosis of GC patients, which included the lncRNA name, frequency of researched lncRNA and reference.
Table 1.
Research frequency of lncRNAs in GC
| lncRNA | n | R | lncRNA | n | R | lncRNA | n | R |
|---|---|---|---|---|---|---|---|---|
| AC027119.1 | 1 | 1 | H19 | 4 | 29, 36–38 | NEAT1 | 1 | 83 |
| AC138128.1 | 1 | 2 | HAGLROS | 1 | 39 | NR_003573 | 1 | 7 |
| ADAMTS9-AS2 | 1 | 1 | HIF1A-AS2 | 1 | 40 | OR3A4 | 1 | 84 |
| AFAP1-AS1 | 2 | 3, 4 | HOTAIR | 9 | 41–59 | OTUB1-isoform 2 | 1 | 85 |
| AGAP2-AS1 | 1 | 5 | HOTTIP | 3 | 50–52 | PANDAR | 2 | 86, 87 |
| AK023391 | 1 | 6 | HOXA-AS2 | 1 | 53 | PCAT1 | 1 | 88 |
| AK093735 | 1 | 7 | HXA11-AS | 1 | 54 | PVT1 | 2 | 89, 90 |
| AK123072 | 1 | 8 | KRTI8P55 | 1 | 55 | RP11-119F7.4 | 1 | 91 |
| ANRIL | 2 | 9, 10 | LET | 1 | 56 | RP11-120K18.2 | 1 | 1 |
| ATB | 1 | 11 | LINC00052 | 1 | 57 | RP11-389G6.3 | 1 | 1 |
| BANCR | 1 | 12 | LINC00152 | 1 | 58 | RP11-499F3.2 | 1 | 1 |
| BC005927 | 1 | 13 | LINC00628 | 1 | 59 | RP11-789C1.1 | 1 | 92 |
| BC032469 | 1 | 14 | LINC00668 | 1 | 60 | RPLPOP2 | 1 | 29 |
| BC041951 | 1 | 15 | LINC00673 | 2 | 61, 62 | SLC26A4 | 1 | 29 |
| BCAR4 | 1 | 16 | LINC00675 | 1 | 63 | SMIM10L2A | 1 | 29 |
| CASC2 | 1 | 17 | LINC00982 | 1 | 64 | SMIM10L2B | 1 | 29 |
| CASC15 | 2 | 18, 19 | LINC01018 | 1 | 65 | SNHG1 | 1 | 93 |
| CCAT1 | 1 | 20 | LINC01234 | 1 | 66 | SNHG6 | 1 | 94 |
| CCAT2 | 2 | 21, 22 | LINC01296 | 1 | 67 | SNHG12 | 1 | 95 |
| CHRDL1 | 1 | 23 | LINC-ROR | 1 | 68 | SNORD116-4 | 1 | 29 |
| CTD-2147F2.1 | 1 | 1 | LINC-UBC1 | 1 | 69 | Sox2ot | 2 | 96, 97 |
| DANCR | 1 | 24 | LOC100130476 | 1 | 70 | SPRY4-IT1 | 2 | 98, 99 |
| DLX6-AS1 | 1 | 1 | LOC553137 | 1 | 65 | TINCR | 1 | 29 |
| E2F1 | 1 | 25 | MACC1 | 1 | 71 | TTTY14 | 1 | 65 |
| EGOT | 1 | 26 | MACC1-AS1 | 1 | 71 | TUG1 | 1 | 100 |
| FENDRR | 1 | 27 | MALAT1 | 4 | 43, 72–74 | UCA1 | 4 | 101–104 |
| FEZF1-AS1 | 1 | 28 | MANCR | 1 | 75 | VPS9D1-AS1 | 1 | 105 |
| FOXD2-AS1 | 1 | 29 | MEG3 | 2 | 76, 77 | XIAP-AS1 | 1 | 106 |
| FRLnc1 | 1 | 30 | MIR31HG | 1 | 78 | XIST | 2 | 107, 108 |
| GACAT3 | 1 | 31 | MIR4435-2HG | 1 | 65 | XLOC_010235 | 1 | 92 |
| GAPLINC | 2 | 32, 33 | MLK7-AS1 | 1 | 79 | ZEB1-AS1 | 2 | 109, 110 |
| GAS5 | 1 | 34 | MLLT4-AS1 | 1 | 80 | ZFAS1 | 2 | 111, 112 |
| GBET1 | 1 | 35 | MRUL | 1 | 81 | ZMAT1 | 1 | 113 |
| GClnc1 | 1 | 15 | MTM | 1 | 82 |
Notes: Highlighted lncRNAs were included in the meta-analysis. n, number of research frequency; R, reference.
Abbreviations: AFAP1-AS1, AFAP1 antisense RNA 1; ANRIL, CDKN2B antisense RNA 1; CASC15, cancer susceptibility 15; CCAT2, colon cancer associated transcript 2; GAPLINC, gastric adenocarcinoma associated, positive CD44 regulator, long intergenic noncoding RNA; GC, gastric cancer; H19, H19, imprinted maternally expressed transcript; HOTAIR, HOX transcript antisense RNA; HOTTIP, HOXA distal transcript antisense RNA; LINC00673, long intergenic non-protein coding RNA 673; lncRNA, long noncoding RNA; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; MEG3, maternally expressed 3; PANDAR, promoter of CDKN1A antisense DNA damage activated RNA; PVT1, Pvt1 oncogene; Sox2ot, SOX2 overlapping transcript; SPRY4-IT1, SPRY4 intronic transcript 1; UCA1, urothelial cancer associated 1; XIST, X inactive specific transcript; ZEB1-AS1, ZEB1 antisense RNA 1; ZFAS1, ZNFX1 antisense RNA 1.
Data extraction
The survival data were recovered from qualified articles independently by two authors (Song Gao and Zhi-Ying Zhao). Data extracted from them are as follows: researched lncRNA, first author’s name, paper publication year, reference, patient’s nationality, study design, histological type, patient number, neoplasm staging, cutoff value, detected method, follow-up period, survival analysis type, HRs and 95% CIs. The detailed data are shown in Table 2. If HR and 95% CI were not directly shown in the paper, data from survival curve were extracted. Disagreements were discussed with the third investigator (Yue Zhang).
Table 2.
Basic information of included articles
| lncRNA | Study | Country/source | Study design | Sample | Number | Stage | Cutoff | Method | Follow-up (months) | OS | HR (L/H) | HR (H/L) | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFAP1-AS1 | Feng et al3 | China | R | Frozen | 91 | I–IV | None | qRT-PCR | 66 | Multivariate | 3.32 | 1.55–5.90 | |
| AFAP1-AS1 | Qiao et al4 | China | R | Frozen | 87 | I–IV | Median | qRT-PCR | 60 | Univariate | 1.88 | 1.01–3.52 | |
| ANRIL | Zhang et al9 | China | R | Tissue | 120 | I–IV | 3 | qRT-PCR | 60 | Multivariate | 1.74 | 1.04–2.93 | |
| ANRIL | Deng et al10 | China | R | Tissue | 100 | I–IV | None | qRT-PCR | .60 | Univariate | 1.61 | 0.95–2.74 | |
| CASC15 | Yao et al18 | China | R | Tissue | 60 | I–IV | None | qRT-PCR | 60 | Univariate | 2.33 | 1.15–4.72 | |
| CASC15 | Wu et al19 | China | R | Tissue | 88 | I–IV | Mean | qRT-PCR | 60 | Univariate | 1.70 | 0.84–3.47 | |
| CCAT2 | Wang et al21 | China | R | Frozen | 85 | I–IV | Mean | qRT-PCR | 60 | Multivariate | 2.41 | 1.19–5.42 | |
| CCAT2 | Wang et al22 | China | R | Frozen | 108 | I–IV | Median | qRT-PCR | 66 | Multivariate | 2.11 | 1.44–3.20 | |
| GAPLINC | Hu et al32 | China | R | Tissue | 90 | I–IV | Median | RT-qPCR | .60 | Multivariate | 1.48 | 1.16–1.89 | |
| GAPLINC | Liu et al33 | China | R | Frozen | 33 | None | 2.03 | qRT-PCR | 60 | Univariate | 1.77 | 0.57–5.52 | |
| H19 | Li et al36 | China | R | Frozen | 74 | I–IV | Mean | qRT-PCR | 53 | Univariate | 2.26 | 0.58–8.86 | |
| H19 | Zhang et al37 | China | R | Frozen | 80 | I–IV | Mean | qRT-PCR | 60 | Multivariate | 1.14 | 1.01–1.29 | |
| H19 | Chen et al38 | China | R | Tissue | 128 | I–IV | 4.615 | qRT-PCR | 48 | Multivariate | 1.96 | 0.97–3.97 | |
| H19 | Li et al29 | TCGA | R | Tissue | 361 | I–IV | None | Downloaded | .50 | Univariate | 1.79 | 1.26–2.53 | |
| HOTAIR | Endo et al41 | China I | R | Frozen | 36 | I–IV | 1.0 | qRT-PCR | .60 | Univariate | 0.95 | 0.21–4.31 | |
| China II | 32 | 5.12 | 0.96–27.18 | ||||||||||
| HOTAIR | Xu et al42 | China | R | Frozen | 83 | I–IV | None | RT-qPCR | .72 | Multivariate | 2.13 | 1.00–4.50 | |
| HOTAIR | Okugawa et al43 | Japan | R | Frozen | 150 | III–IV | 0.239 | RT-qPCR | 60 | Multivariate | 1.77 | 1.06–2.95 | |
| HOTAIR | Liu et al44 | China | R | Both | 78 | II–IV | Median | qRT-PCR | >40 | Multivariate | 4.08 | 2.07–8.04 | |
| HOTAIR | Zhang et al45 | China | R | Both | 50 | II–IV | Median | qRT-PCR | >45 | Univariate | 2.86 | 1.16–7.03 | |
| HOTAIR | Zhao et al46 | China | R | Tissue | 168 | III–IV | Median | qRT-PCR | >60 | Multivariate | 1.47 | 1.04–2.06 | |
| HOTAIR | Chen et al47 | China | R | Frozen | 65 | I–IV | 2.35 | qRT-PCR | >60 | Multivariate | 2.00 | 1.06–3.77 | |
| HOTAIR | Feng and Huang48 | China | R | Tissue | 32 | None | None | qRT-PCR | >120 | Univariate | 1.52 | 0.45–5.14 | |
| HOTAIR | Li et al49 | China | R | FFPE | 100 | I–IV | Mean | qRT-PCR | 90 | Univariate | 1.83 | 0.82–4.05 | |
| HOTTIP | Ye et al50 | China | R | Frozen | 98 | I–III | Median | qRT-PCR | 60 | Univariate | 2.06 | 0.97–4.38 | |
| HOTTIP | Yang et al51 | China | R | Frozen | 94 | I–III | Median | RT-qPCR | 54 | Univariate | 1.03 | 0.52–2.05 | |
| HOTTIP | Zhao et al52 | GEO | R | Tissue | 348 | I–IV | None | Downloaded | >150 | Univariate | 1.63 | 1.19–2.23 | |
| LINC00673 | Ba et al61 | China | R | Frozen | 79 | I–IV | Median | qRT-PCR | 66 | Multivariate | 2.56 | 1.01–4.54 | |
| LINC00673 | Huang et al62 | China | R | Tissue | 73 | I–IV | 2 | qRT-PCR | >40 | Multivariate | 2.38 | 1.12–5.06 | |
| MALAT1 | Qi et al72 | TCGA | R | Tissue | 118 | III–IV | None | RT-qPCR | >50 | Univariate | 1.98 | 1.38–2.83 | |
| MALAT1 | Li et al73 | China | R | Tissue | 78 | I–IV | None | qRT-PCR | >60 | Univariate | 2.52 | 1.35–4.68 | |
| MALAT1 | Li et al74 | China | R | FFPE | 150 | I–IV | None | RT-qPCR | >150 | Univariate | 1.38 | 1.03–1.85 | |
| MALAT1 | Okugawa et al43 | Japan | R | Frozen | 150 | III–IV | 0.985 | RT-qPCR | 60 | Univariate | 1.54 | 0.92–2.58 | |
| MEG3 | Sun et al76 | China | R | Frozen | 72 | II–IV | Median | RT-qPCR | 48 | Univariate | 1.93 | 0.99–3.75 | |
| MEG3 | Guo et al77 | China | R | Frozen | 134 | I–IV | None | qRT-PCR | >60 | Univariate | 2.00 | 0.88–4.54 | |
| PANDAR | Ma et al86 | China | R | Tissue | 100 | I–IV | None | qRT-PCR | 36 | Multivariate | 3.68 | 1.13–12.06 | |
| PANDAR | Liu et al87 | China | R | Tissue | 146 | I–IV | Mean | qRT-PCR | 84 | Multivariate | 3.10 | 2.70–3.54 | |
| PVT1 | Kong et al89 | China | R | Tissue | 80 | I–IV | Median | qRT-PCR | 36 | Multivariate | 2.09 | 1.07–4.10 | |
| PVT1 | Yuan et al90 | China | R | Tissue | 111 | I–IV | Median | qRT-PCR | 48 | Multivariate | 2.28 | 1.05–4.93 | |
| Sox2ot | Zhang et al96 | China | R | Frozen | 132 | I–IV | Median | qRT-PCR | >84 | Multivariate | 2.05 | 1.28–3.30 | |
| Sox2ot | Zou et al97 | China | R | Tissue | 155 | None | Median | qRT-PCR | >60 | Univariate | 3.24 | 1.24–6.43 | |
| SPRY4-IT1 | Peng et al98 | China | R | Frozen | 175 | I–IV | Median | qRT-PCR | 60 | Multivariate | 0.82 | 0.31–1.57 | |
| SPRY4-IT1 | Xie et al99 | China | R | Frozen | 61 | I–IV | Median | qRT-PCR | 36 | Univariate | 2.49 | 1.08–5.75 | |
| UCA1 | Zheng et al101 | China | R | Frozen | 112 | I–IV | Median | RT-qPCR | 60 | Multivariate | 2.35 | 1.22–4.52 | |
| UCA1 | Nasrollahzadeh-Khakiani et al102 | TCGA | R | Tissue | 188 | I–IV | Median | Downloaded | >116 | Univariate | 1.11 | 0.72–1.73 | |
| UCA1 | Zuo et al103 | China | R | Frozen | 37 | I–IV | Median | qRT-PCR | 36 | Multivariate | 2.92 | 1.07–7.96 | |
| UCA1 | Gu et al104 | China | R | Frozen | 62 | I–IV | None | qRT-PCR | 60 | Univariate | 1.80 | 0.95–3.38 | |
| XIST | Chen et al107 | China | R | Frozen | 106 | I–IV | Median | qRT-PCR | >90 | Multivariate | 1.72 | 1.32–2.26 | |
| XIST | Ma et al108 | China | R | FFPE | 98 | I–IV | None | qRT-PCR | 54 | Univariate | 2.49 | 1.40–4.42 | |
| ZEB1-AS1 | Li et al109 | China | R | Tissue | 124 | I–IV | Median | qRT-PCR | 72 | Multivariate | 2.36 | 1.41–3.96 | |
| ZEB1-AS1 | Zhang et al110 | China | R | Frozen | 76 | I–IV | Median | qRT-PCR | 90 | Univariate | 2.72 | 1.27–5.84 | |
| KM | R | Tissue | 631 | I–IV | None | Downloaded | >150 | Univariate | 1.95 | 1.52–2.49 | |||
| ZFAS1 | Zhang et al111 | China | R | Frozen | 104 | I–IV | Median | qRT-PCR | 60 | Multivariate | 2.57 | 1.25–6.84 | |
| ZFAS1 | Nie et al112 | China | R | Tissue | 54 | I–IV | Median | qRT-PCR | 36 | Univariate | 2.43 | 0.96–6.17 |
Abbreviations: AFAP1-AS1, AFAP1 antisense RNA 1; ANRIL, CDKN2B antisense RNA 1; Both, frozen and formalin-fixed paraffin-embedded tissues; CASC15, cancer susceptibility 15; CCAT2, colon cancer associated transcript 2; FFPE, formalin-fixed paraffin-embedded; GAPLINC, gastric adenocarcinoma associated, positive CD44 regulator, long intergenic noncoding RNA; GEO, Gene Expression Omnibus; H19, H19, imprinted maternally expressed transcript; HOTAIR, HOX transcript antisense RNA; HOTTIP, HOXA distal transcript antisense RNA; HR (H/L), hazard ratios of high expression versus low expression of lncRNAs; HR (L/H), hazard ratios of low expression versus high expression of lncRNAs; KM, Kaplan–Meier plotter; LINC00673, long intergenic non-protein coding RNA 673; lncRNA, long noncoding RNA; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; MEG3, maternally expressed 3; OS, overall survival; PANDAR, promoter of CDKN1A antisense DNA damage activated RNA; PVT1, Pvt1 oncogene; qRT-PCR, quantitative real-time polymerase chain reaction; R, retrospective; RT-qPCR, reverse transcription quantitative real-time polymerase chain reaction; Sox2ot, SOX2 overlapping transcript; SPRY4-IT1, SPRY4 intronic transcript 1; TCGA, The Cancer Genome Atlas; UCA1, urothelial cancer associated 1; XIST, X inactive specific transcript; ZEB1-AS1, ZEB1 antisense RNA 1; ZFAS1, ZNFX1 antisense RNA 1.
Statistical analysis
Stata version 13.0 (StataCorp LP, College Station, TX, USA) was used for the whole meta-analysis. HR and 95% CI from GC patients were calculated on the basis of survival curve and patient number using Engauge Digitizer version 4.1 and Tierney’s method.122 The random-effect model was used in the whole article because different histological type (frozen, formalin-fixed paraffin-embedded or undefined) from GC patients at different neoplasm staging, cutoff value and lncRNA detected method was used in the single study. The HR was considered significant if its P-value was <0.05 and 95% CI did not contain the value 1. Furthermore, the lncRNA was considered as a strong biomarker of prognosis, if its HR was >2. The Begg’s funnel plot was used to estimate publication bias, and a two-tailed P-value <0.05 was considered as significant. The sensitivity analysis was performed to examine how sensitive the merged HR was if the single study was removed, and if the point of evaluation was outside the 95% CI after it was removed from the whole analysis, a single research was considered as excessive influence.
Results
Meta-analysis
Table 3 gives the basic information of the merged meta-analysis for researched lncRNAs.
Table 3.
HR with 95% CI of lncRNA expression in GC
| lncRNA | Number of articles | Included articles | HR | 95% CI | Figure | P-value | Heterogeneity (Higgins I2 statistic) | Total patients |
|---|---|---|---|---|---|---|---|---|
| High AFAP1-AS1 | 2 | 3, 4 | 2.47 | 1.41–4.30 | 2 | <0.01 | I2=32.7%, P=0.22 | 178 |
| High ANRIL | 2 | 9, 10 | 1.68 | 1.16–2.43 | 2 | <0.01 | I2=0.0%, P=0.84 | 220 |
| High CASC15 | 2 | 18, 19 | 1.99 | 1.21–3.28 | 2 | <0.01 | I2=0.0%, P=0.54 | 148 |
| High CCAT2 | 2 | 21, 22 | 2.17 | 1.53–3.09 | 2 | <0.01 | I2=0.0%, P=0.76 | 193 |
| High GAPLINC | 2 | 32, 33 | 1.49 | 1.18–1.89 | 2 | <0.01 | I2=0.0%, P=0.76 | 123 |
| High H19 | 4 | 29, 36–38 | 1.51 | 1.05–2.17 | 2 | 0.03 | I2=64.1%, P=0.04 | 643 |
| High HOTAIR | 9 | 41–49 | 1.93 | 1.53–2.43 | 3 | <0.01 | I2=14.0%, P=0.31 | 794 |
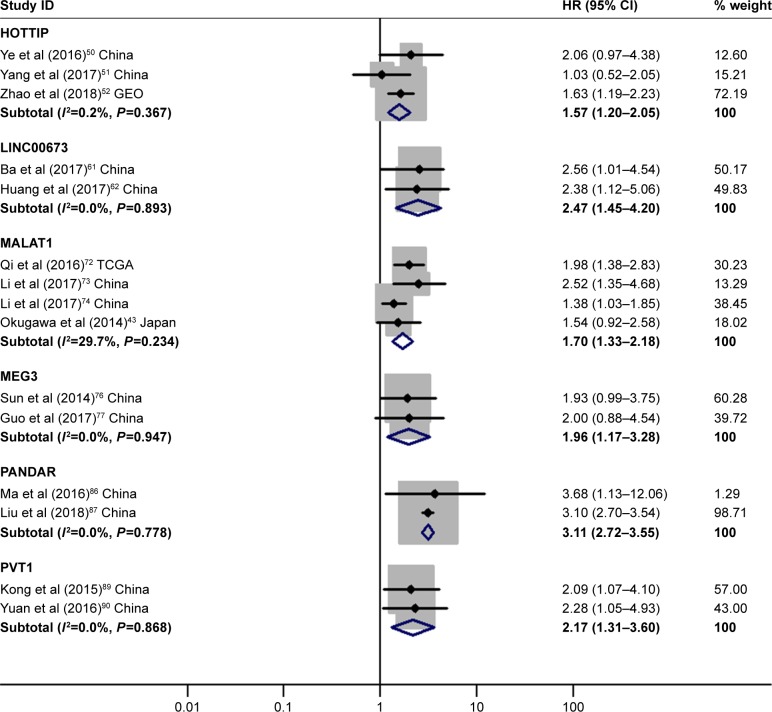
| High HOTTIP | 3 | 50–52 | 1.57 | 1.20–2.05 | 6 | <0.01 | I2=0.2%, P=0.37 | 540 |
| High LINC00673 | 2 | 61, 62 | 2.47 | 1.45–4.20 | 6 | <0.01 | I2=0.0%, P=0.89 | 152 |
| High MALAT1 | 4 | 43, 72–74 | 1.70 | 1.33–2.18 | 6 | <0.01 | I2=29.7%, P=0.23 | 496 |
| Low MEG3 | 2 | 76, 77 | 1.96 | 1.17–3.28 | 6 | 0.01 | I2=0.0%, P=0.95 | 206 |
| High PANDAR | 2 | 86, 87 | 3.11 | 2.72–3.55 | 6 | <0.01 | I2=0.0%, P=0.79 | 246 |
| High PVT1 | 2 | 89, 90 | 2.17 | 1.31–3.60 | 6 | <0.01 | I2=0.0%, P=0.87 | 191 |
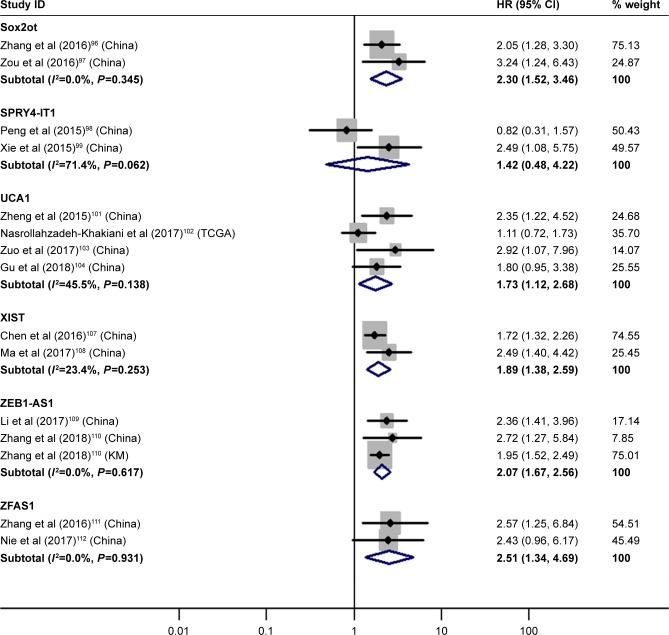
| High Sox2ot | 2 | 96, 97 | 2.30 | 1.52–3.46 | 7 | <0.01 | I2=0.0%, P=0.35 | 287 |
| Low SPRY4-IT1 | 2 | 98, 99 | 1.42 | 0.48–4.22 | 7 | 0.53 | I2=71.4%, P=0.06 | 236 |
| High UCA1 | 4 | 101–104 | 1.73 | 1.12–2.68 | 7 | 0.01 | I2=45.5%, P=0.14 | 399 |
| High XIST | 2 | 107, 108 | 1.89 | 1.38–2.59 | 7 | <0.01 | I2=23.4%, P=0.25 | 204 |
| High ZEB1-AS1 | 2 | 109, 110 | 2.07 | 1.67–2.56 | 7 | <0.01 | I2=0.0%, P=0.62 | 831 |
| High ZFAS1 | 2 | 111, 112 | 2.51 | 1.34–4.69 | 7 | <0.01 | I2=0.0%, P=0.93 | 158 |
Abbreviations: AFAP1-AS1, AFAP1 antisense RNA 1; ANRIL, CDKN2B antisense RNA 1; CASC15, cancer susceptibility 15; CCAT2, colon cancer associated transcript 2; GAPLINC, gastric adenocarcinoma associated, positive CD44 regulator, long intergenic noncoding RNA; GC, gastric cancer; H19, H19, imprinted maternally expressed transcript; HOTAIR, HOX transcript antisense RNA; HOTTIP, HOXA distal transcript antisense RNA; LINC00673, long intergenic non-protein coding RNA 673; lncRNA, long noncoding RNA; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; MEG3, maternally expressed 3; PANDAR, promoter of CDKN1A antisense DNA damage activated RNA; PVT1, Pvt1 oncogene; Sox2ot, SOX2 overlapping transcript; SPRY4-IT1, SPRY4 intronic transcript 1; UCA1, urothelial cancer associated 1; XIST, X inactive specific transcript; ZEB1-AS1, ZEB1 antisense RNA 1; ZFAS1, ZNFX1 antisense RNA 1.
AFAP1 antisense RNA 1 (AFAP1-AS1), CDKN2B antisense RNA 1 (ANRIL), cancer susceptibility 15 (CASC15), colon cancer-associated transcript 2 (CCAT2), gastric adenocarcinoma associated, positive CD44 regulator, long intergenic noncoding RNA (GAPLINC) and H19, imprinted maternally expressed transcript (H19) demonstrated significantly prognostic value
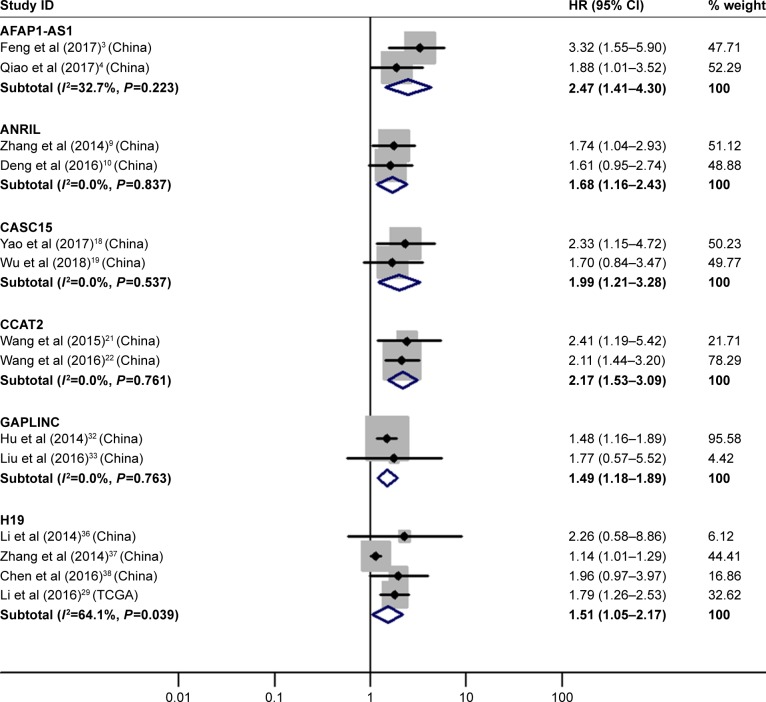
Two articles3,4 reported the relationship between high AFAP1-AS1 expression and OS, indicating that GC patients with its high expression had significantly worse OS than those with its low expression (HR=2.47, 95% CI=1.41–4.30, P<0.01).
Two researches9,10 covered the connections between high ANRIL expression and OS, suggesting that GC patients with its high expression had significantly poorer OS than those with its low expression (HR=1.68, 95% CI=1.16–2.43, P<0.01).
Two investigations18,19 analyzed the associations between high CASC15 expression and OS, showing that GC patients with its high expression had significantly shorter OS than those with its low expression (HR=1.99, 95% CI=1.21–3.28, P<0.01).
Two studies21,22 focused on the correlation between high CCAT2 expression and OS, manifesting that GC patients with its high expression had significantly worse OS than those with its low expression (HR=2.17, 95% CI=1.53–3.09, P<0.01).
Two papers32,33 paid attention to the pertinence between high GAPLINC expression and OS, demonstrating that GC patients with its high expression had significantly poorer OS than those with its low expression (HR=1.49, 95% CI=1.18–1.89, P<0.01).
Four literature29,36–38 described the relativity between high H19 expression and OS, proving that GC patients with its high expression had significantly shorter OS than those with its low expression (HR=1.51, 95% CI=1.05–2.17, P=0.03; Figure 2).
Figure 2.
Forest plot of pooled analyses of OS in association with high AFAP1-AS1, ANRIL, CASC15, CCAT2, GAPLINC and H19 expression levels.
Note: Weights are from random-effects analysis.
Abbreviations: AFAP1-AS1, AFAP1 antisense RNA 1; ANRIL, CDKN2B antisense RNA 1; CASC15, cancer susceptibility 15; CCAT2, colon cancer associated transcript 2; GAPLINC, gastric adenocarcinoma associated, positive CD44 regulator, long intergenic noncoding RNA; H19, H19, imprinted maternally expressed transcript. OS, overall survival.
HOX transcript antisense RNA (HOTAIR) demonstrated significantly prognostic value
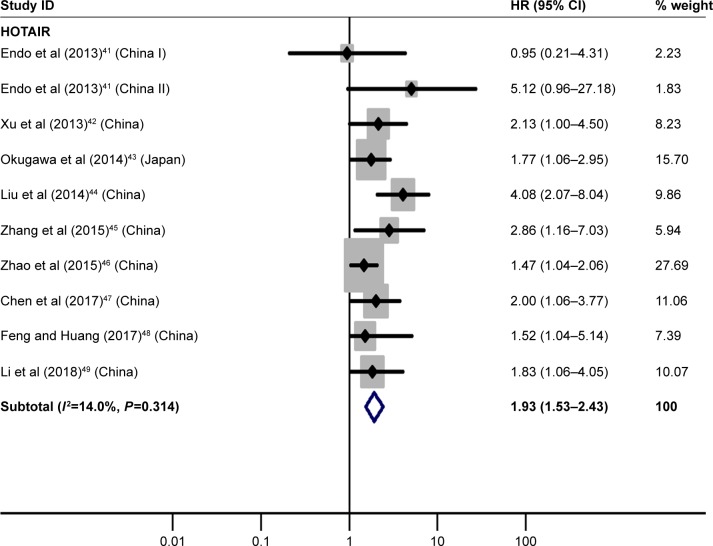
Nine essays41–49 discussed the relation between high HOTAIR expression and OS, illuminating that GC patients with its high expression had significantly worse OS than those with its low expression (HR=1.93, 95% CI=1.53–2.43, P<0.01; Figure 3).
Figure 3.
Forest plot of pooled analysis of OS in association with high HOTAIR expression levels.
Note: Weights are from random-effects analysis.
Abbreviations: HOTAIR, HOX transcript antisense RNA; OS, overall survival.
Publication bias
The Begg’s funnel plot was used to estimate publication bias, and its P-value was 0.20, so there was no significant publication bias in the pooled analysis of OS about high HOTAIR expression (Figure 4).
Figure 4.
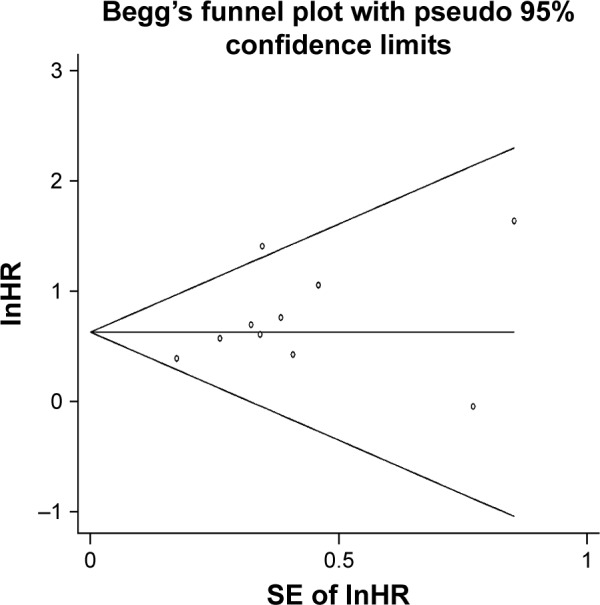
Beggs’s funnel plot of publication bias for pooled analysis of OS in association with high HOTAIR expression levels.
Abbreviations: HOTAIR, HOX transcript antisense RNA; OS, overall survival; SE, standard error.
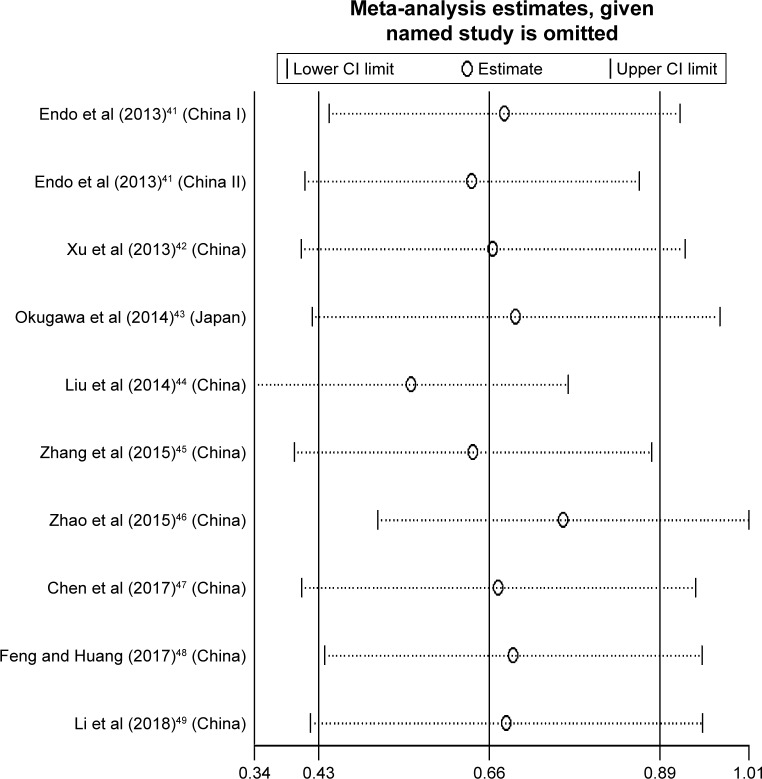
Sensitivity analysis
The sensitivity analysis was performed to examine how sensitive the merged HR was if the single study was removed. After this process, no individual study significantly affected the combined HR with 95% CI (Figure 5).
Figure 5.
Sensitivity analysis of pooled analysis of OS in association with high HOTAIR expression levels.
Abbreviations: HOTAIR, HOX transcript antisense RNA; OS, overall survival.
HOXA distal transcript antisense RNA (HOTTIP), long intergenic non-protein coding RNA 673 (LINC00673), metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), maternally expressed 3 (MEG3), promoter of CDKN1A antisense DNA damage activated RNA (PANDAR) and Pvt1 oncogene (PVT1) demonstrated significantly prognostic value
The details are shown in Table 3 and Figure 6.
Figure 6.
Forest plot of pooled analyses of OS in association with high HOTTIP, LINC00673, MALAT1, PANDAR, PVT1 expression levels, or low MEG3 expression levels.
Note: Weights are from random-effects analysis.
Abbreviations: GEO, Gene Expression Omnibus; HOTTIP, HOXA distal transcript antisense RNA; LINC00673, long intergenic non-protein coding RNA 673; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; MEG3, maternally expressed 3; OS, overall survival; PANDAR, promoter of CDKN1A antisense DNA damage activated RNA; PVT1, Pvt1 oncogene; TCGA, The Cancer Genome Atlas.
SOX2 overlapping transcript (Sox2ot), urothelial cancer-associated 1 (UCA1), X inactive specific transcript (XIST), ZEB1 antisense RNA 1 (ZEB1-AS1) and ZNFX1 antisense RNA 1 (ZFAS1) demonstrated significantly prognostic value
The details are shown in Table 3 and Figure 7.
Figure 7.
Forest plot of pooled analyses of OS in association with high Sox2ot, UCA1, XIST, ZEB1-AS1, ZFAS1 expression levels, or low SPRY4-IT1 expression levels.
Note: Weights are from random-effects analysis.
Abbreviations: OS, overall survival; Sox2ot, SOX2 overlapping transcript; SPRY4-IT1, SPRY4 intronic transcript 1; UCA1, urothelial cancer associated 1; XIST, X inactive specific transcript; ZEB1-AS1, ZEB1 antisense RNA 1; ZFAS1, ZNFX1 antisense RNA 1.
Discussion
Current situation
So far, the clinical treatment of GC remains limited. In the past score years, there has been little progress in both traditional and new treatment methods. Therefore, novel biomarkers that can improve the prognosis of GC patients are in need. Recently, there is an increasing evidence that lncRNAs can hinder the growth and metastasis of cancer. For example, Xu et al123 reported that upregulating long stress-induced noncoding 5 (LSINCT5) significantly promoted the growth of the GC cell, while downregulating LSINCT5 suppressed its growth. Dan et al124 conducted the cancer model experiments using mice, proving that MEG3 overexpression could suppress GC growth and metastasis in vivo by suppressing miR-21 expression. More importantly, several abnormally expressed lncRNAs have been discovered to touch upon the development of GC and perhaps possess prognostic potency in this illness. In view of the above consequences, we conducted this meta-analysis about the prognostic value of lncRNAs in GC.
Research finding
In the present research, a total of 51 articles reporting 19 lncRNAs, which were latent prognostic biomarkers and 6,095 GC patients were included, among which 18 lncRNAs (except SPRY4 intronic transcript 1 [SPRY4-IT1]) manifested a significantly prognostic value. Meanwhile, strong heterogeneity was only shown in two (H19 and SPRY4-IT1) analyses about lncRNAs, during which there was no significant associations between SPRY4-IT1 expression and OS. Further analysis suggested that AFAP1-AS1, CCAT2, LINC00673, PANDAR, PVT1, Sox2ot, ZEB1-AS1 and ZFAS1 were strong candidates in predicting prognosis of GC patients.
Molecular mechanisms
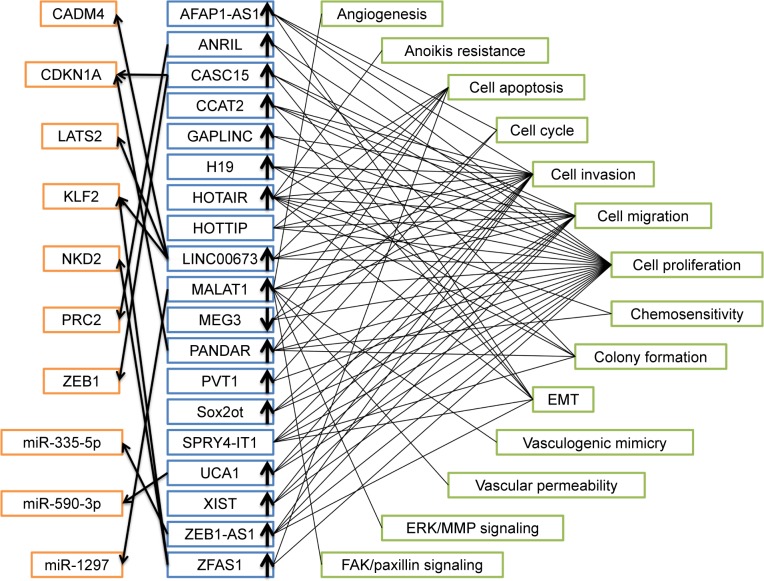
Figure 8 shows the summary of lncRNAs with aberrant expression, potential targets and pathways included in this study. It is noteworthy that there existed inconsistent outcomes about expression of HOTTIP and SPRY4-IT1 compared with normal controls, so these two lncRNAs were not shown to be up or down expressed. Unexpected results were findings that CDKN1A was target of both CASC15 and PANDAR and KLF2 was target of both LINC00673 and ZFAS1. In addition, cell proliferation was the most related cell function of these lncRNAs.
Figure 8.
Summary of lncRNAs with altered expression, potential targets and pathways entered in this study.
Abbreviations: AFAP1-AS1, AFAP1 antisense RNA 1; ANRIL, CDKN2B antisense RNA 1; CASC15, cancer susceptibility 15; CCAT2, colon cancer associated transcript 2; EMT, epithelial–mesenchymal transition; GAPLINC, gastric adenocarcinoma associated, positive CD44 regulator, long intergenic noncoding RNA; H19, H19, imprinted maternally expressed transcript; HOTAIR, HOX transcript antisense RNA; HOTTIP, HOXA distal transcript antisense RNA; LINC00673, long intergenic non-protein coding RNA 673; lncRNA, long noncoding RNA; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; MEG3, maternally expressed 3; PANDAR, promoter of CDKN1A antisense DNA damage activated RNA; PVT1, Pvt1 oncogene; Sox2ot, SOX2 overlapping transcript; SPRY4-IT1, SPRY4 intronic transcript 1; UCA1, urothelial cancer associated 1; XIST, X inactive specific transcript; ZEB1-AS1, ZEB1 antisense RNA 1; ZFAS1, ZNFX1 antisense RNA 1.
Merits
The current study had several merits: 1) nearly all articles appraising the associations between OS of GC patients and lncRNA expression were searched and are clearly shown in Table 1; 2) most of our meta-analyses revealed no or low heterogeneity (I2≤50.0%), indicating relatively consistent results of the meta-analyses and 3) all the included studies had a relatively large sample size (≥30), decreasing the error of low sample size to some degree.
Limitations
However, the limitations of this work could not be ignored: 1) only English papers were included in the present research, which may exclude potentially relevant articles; 2) most of the patients were from China, which cannot adequately represent the prognosis of global patients; 3) only the meta-analysis of HOTAIR was composed of nine articles,41–49 and other merged analyses about lncRNAs were from relatively small article number (two to four) and 4) the papers omitted due to no mention of OS may provide a lot of information on which lncRNAs hold promise for a prognostic value.
Inspirations
This study left several inspirations for us: 1) lncRNAs were arranged in an alphabetical order as shown in Table 1, via which the recently research frequency could be distinctly seen by clinical workers and scientific researchers; 2) the detailed outcomes of OS from the pooled analyses are shown in Table 3, through which combined detection of lncRNAs might better predict the survival time of GC patients and 3) for the molecular mechanisms of the included lncRNAs, their connections are shown in Figure 8, which might play enlightening roles in future basic experiments on lncRNAs in GC.
Conclusion
This meta-analysis suggested that the abnormally expressed lncRNAs (AFAP1-AS1, ANRIL, CASC15, CCAT2, GAPLINC, H19, HOTAIR, HOTTIP, LINC00673, MALAT1, MEG3, PANDAR, PVT1, Sox2ot, UCA1, XIST, ZEB1-AS1 and ZFAS1) were significantly associated with the survival of GC patients, among which AFAP1-AS1, CCAT2, LINC00673, PANDAR, PVT1, Sox2ot, ZEB1-AS1 and ZFAS1 were strong candidates in predicting prognosis of GC patients.
Footnotes
Author contributions
Yue Zhang contributed toward study concept and design. Song Gao and Zhi-Ying Zhao were involved in acquisition of data. Song Gao, Zhi-Ying Zhao and Rong Wu carried out analysis and interpretation of data. Yue Zhang performed drafting of the manuscript. Song Gao, Zhi-Ying Zhao, Rong Wu, Yue Zhang and Zhen-Yong Zhang assisted with revision of manuscript. Yue Zhang and Zhen-Yong Zhang helped in supervision of work. All authors read and approved the final manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Li F, Huang C, Li Q, Wu X. Construction and comprehensive analysis for dysregulated long non-coding RNA (lncRNA)-associated competing endogenous RNA (ceRNA) network in gastric cancer. Med Sci Monit. 2018;24:37–49. doi: 10.12659/MSM.905410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Sun J, Song Y, et al. The novel long noncoding RNA AC138128.1 may be a predictive biomarker in gastric cancer. Med Oncol. 2014;31(11):262. doi: 10.1007/s12032-014-0262-7. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y, Zhang Q, Wang J, Liu P. Increased lncRNA AFAP1-AS1 expression predicts poor prognosis and promotes malignant phenotypes in gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21(17):3842–3849. [PubMed] [Google Scholar]
- 4.Qiao CF, Zhang Y, Jin L, Du XG, Qiao ZJ. High expression of lncRNA AFAP1-AS1 promotes cell proliferation and invasion by inducing epithelial-to-mesenchymal transition in gastric cancer. Int J Clin Exp Pathol. 2017;10(1):393–400. [Google Scholar]
- 5.Qi F, Liu X, Wu H, et al. Long noncoding AGAP2-AS1 is activated by SP1 and promotes cell proliferation and invasion in gastric cancer. J Hematol Oncol. 2017;10(1):48. doi: 10.1186/s13045-017-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Zhang J, Hou L, et al. LncRNA AK023391 promotes tumorigenesis and invasion of gastric cancer through activation of the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2017;36(1):194. doi: 10.1186/s13046-017-0666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan ZY, Liu W, Yan C, et al. Identification of a five-lncRNA signature for the diagnosis and prognosis of gastric cancer. Tumour Biol. 2016;37(10):13265–13277. doi: 10.1007/s13277-016-5185-9. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Wang R, Zhang T, Dong X. Hypoxia/lncRNA-AK123072/EGFR pathway induced metastasis and invasion in gastric cancer. Int J Clin Exp Med. 2015;8(11):19954–19968. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang EB, Kong R, Yin DD, et al. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget. 2014;5(8):2276–2292. doi: 10.18632/oncotarget.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng W, Wang J, Zhang J, Cai J, Bai Z, Zhang Z. TET2 regulates LncRNA-ANRIL expression and inhibits the growth of human gastric cancer cells. IUBMB Life. 2016;68(5):355–364. doi: 10.1002/iub.1490. [DOI] [PubMed] [Google Scholar]
- 11.Saito T, Kurashige J, Nambara S, et al. A long non-coding RNA activated by transforming growth factor-β is an independent prognostic marker of gastric cancer. Ann Surg Oncol. 2015;22(suppl 3):S915–S922. doi: 10.1245/s10434-015-4554-8. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Zhang L, Zhang Y, Zhou F. Increased expression of LncRNA BANCR is associated with clinical progression and poor prognosis in gastric cancer. Biomed Pharmacother. 2015;72:109–112. doi: 10.1016/j.biopha.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Wang Y, Sun L, et al. Long noncoding RNA BC005927 upregulates EPHB4 and promotes gastric cancer metastasis under hypoxia. Cancer Sci. 2018;109(4):988–1000. doi: 10.1111/cas.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lü MH, Tang B, Zeng S, et al. Long noncoding RNA BC032469, a novel competing endogenous RNA, upregulates hTERT expression by sponging miR-1207-5p and promotes proliferation in gastric cancer. Oncogene. 2016;35(27):3524–3534. doi: 10.1038/onc.2015.413. [DOI] [PubMed] [Google Scholar]
- 15.Sun TT, He J, Liang Q, et al. LncRNA GClnc1 promotes gastric carcinogenesis and may act as a modular scaffold of WDR5 and KAT2A complexes to specify the histone modification pattern. Cancer Discov. 2016;6(7):784–801. doi: 10.1158/2159-8290.CD-15-0921. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Chunyan Q, Zhou Y, et al. BCAR4 increase cisplatin resistance and predicted poor survival in gastric cancer patients. Eur Rev Med Pharmacol Sci. 2017;21(18):4064–4070. [PubMed] [Google Scholar]
- 17.Zhou J, Huang H, Tong S, Huo R. Overexpression of long non-coding RNA cancer susceptibility 2 inhibits cell invasion and angiogenesis in gastric cancer. Mol Med Rep. 2017;16(4):5235–5240. doi: 10.3892/mmr.2017.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao XM, Tang JH, Zhu H, Jing Y. High expression of LncRNA CASC15 is a risk factor for gastric cancer prognosis and promote the proliferation of gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21(24):5661–5667. doi: 10.26355/eurrev_201712_14010. [DOI] [PubMed] [Google Scholar]
- 19.Wu Q, Xiang S, Ma J, et al. Long non-coding RNA CASC15 regulates gastric cancer cell proliferation, migration and epithelial mesenchymal transition by targeting CDKN1A and ZEB1. Mol Oncol. 2018;12(6):799–813. doi: 10.1002/1878-0261.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu JN, Shangguan YM. Long non-coding RNA CARLo-5 upregulation associates with poor prognosis in patients suffering gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21(3):530–534. [PubMed] [Google Scholar]
- 21.Wang CY, Hua L, Yao KH, Chen JT, Zhang JJ, Hu JH. Long non-coding RNA CCAT2 is up-regulated in gastric cancer and associated with poor prognosis. Int J Clin Exp Pathol. 2015;8(1):779–785. [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YJ, Liu JZ, Lv P, Dang Y, Gao JY, Wang Y. Long non-coding RNA CCAT2 promotes gastric cancer proliferation and invasion by regulating the E-cadherin and LATS2. Am J Cancer Res. 2016;6(11):2651–2660. [PMC free article] [PubMed] [Google Scholar]
- 23.Pei YF, Zhang YJ, Lei Y, Wu DW, Ma TH, Liu XQ. Hypermethylation of the CHRDL1 promoter induces proliferation and metastasis by activating Akt and Erk in gastric cancer. Oncotarget. 2017;8(14):23155–23166. doi: 10.18632/oncotarget.15513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao YP, Qiu JH, Zhang DB, Yu CG. Long non-coding RNA DANCR, a prognostic indicator, promotes cell growth and tumorigenicity in gastric cancer. Tumour Biol. 2017;39(6) doi: 10.1177/1010428317699798. 1010428317699798. [DOI] [PubMed] [Google Scholar]
- 25.Xu TP, Wang YF, Xiong WL, et al. E2F1 induces TINCR transcriptional activity and accelerates gastric cancer progression via activation of TINCR/STAU1/CDKN2B signaling axis. Cell Death Dis. 2017;8(6):e2837. doi: 10.1038/cddis.2017.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng W, Wu J, Fan H, Lu J, Feng J. LncRNA EGOT promotes tumorigenesis Via Hedgehog Pathway in gastric cancer. Pathol Oncol Res. 2017 doi: 10.1007/s12253-017-0367-3. [DOI] [PubMed] [Google Scholar]
- 27.Xu TP, Huang MD, Xia R, et al. Decreased expression of the long non-coding RNA FENDRR is associated with poor prognosis in gastric cancer and FENDRR regulates gastric cancer cell metastasis by affecting fibronectin1 expression. J Hematol Oncol. 2014;7:63. doi: 10.1186/s13045-014-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Zhang P, Zhu H, Li S, Chen X, Shi L. Long noncoding RNA FEZF1-AS1 indicates a poor prognosis of gastric cancer and promotes tumorigenesis via activation of Wnt signaling pathway. Biomed Pharmacother. 2017;96:1103–1108. doi: 10.1016/j.biopha.2017.11.113. [DOI] [PubMed] [Google Scholar]
- 29.Li CY, Liang GY, Yao WZ, et al. Integrated analysis of long non-coding RNA competing interactions reveals the potential role in progression of human gastric cancer. Int J Oncol. 2016;48(5):1965–1976. doi: 10.3892/ijo.2016.3407. [DOI] [PubMed] [Google Scholar]
- 30.Chong DQ, Shan JL, Yang CS, Wang R, Du ZM. Clinical prognostic value of A FOXM1 related long non-coding RNA expression in gastric cancer. Eur Rev Med Pharmacol Sci. 2018;22(2):417–421. doi: 10.26355/eurrev_201801_14190. [DOI] [PubMed] [Google Scholar]
- 31.Feng L, Zhu Y, Zhang Y, Rao M. LncRNA GACAT3 promotes gastric cancer progression by negatively regulating miR-497 expression. Biomed Pharmacother. 2018;97:136–142. doi: 10.1016/j.biopha.2017.10.074. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, Wang J, Qian J, et al. Long noncoding RNA GAPLINC regulates CD44-dependent cell invasiveness and associates with poor prognosis of gastric cancer. Cancer Res. 2014;74(23):6890–6902. doi: 10.1158/0008-5472.CAN-14-0686. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Zhao X, Zou H, Bai R, Yang K, Tian Z. Hypoxia promotes gastric cancer malignancy partly through the HIF-1α dependent transcriptional activation of the long non-coding RNA GAPLINC. Front Physiol. 2016;7:420. doi: 10.3389/fphys.2016.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun M, Jin FY, Xia R, et al. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319. doi: 10.1186/1471-2407-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang F, Xue X, Zheng L, et al. Long non-coding RNA GHET1 promotes gastric carcinoma cell proliferation by increasing c-Myc mRNA stability. FEBS J. 2014;281(3):802–813. doi: 10.1111/febs.12625. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Yu B, Li J, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5(8):2318–2329. doi: 10.18632/oncotarget.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang EB, Han L, Yin DD, Kong R, De W, Chen J. c-Myc-induced, long, noncoding H19 affects cell proliferation and predicts a poor prognosis in patients with gastric cancer. Med Oncol. 2014;31(5):914. doi: 10.1007/s12032-014-0914-7. [DOI] [PubMed] [Google Scholar]
- 38.Chen JS, Wang YF, Zhang XQ, et al. H19 serves as a diagnostic biomarker and up-regulation of H19 expression contributes to poor prognosis in patients with gastric cancer. Neoplasma. 2016;63(2):223–230. doi: 10.4149/207_150821N454. [DOI] [PubMed] [Google Scholar]
- 39.Chen JF, Wu P, Xia R, et al. STAT3-induced lncRNA HAGLROS over-expression contributes to the malignant progression of gastric cancer cells via mTOR signal-mediated inhibition of autophagy. Mol Cancer. 2018;17(1):6. doi: 10.1186/s12943-017-0756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen WM, Huang MD, Kong R, et al. Antisense long noncoding RNA HIF1A-AS2 is upregulated in gastric cancer and associated with poor prognosis. Dig Dis Sci. 2015;60(6):1655–1662. doi: 10.1007/s10620-015-3524-0. [DOI] [PubMed] [Google Scholar]
- 41.Endo H, Shiroki T, Nakagawa T, et al. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One. 2013;8(10):e77070. doi: 10.1371/journal.pone.0077070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu ZY, Yu QM, Du YA, et al. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9(6):587–597. doi: 10.7150/ijbs.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okugawa Y, Toiyama Y, Hur K, et al. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35(12):2731–2739. doi: 10.1093/carcin/bgu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu XH, Sun M, Nie FQ, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang ZZ, Shen ZY, Shen YY, et al. HOTAIR long noncoding RNA promotes gastric cancer metastasis through suppression of poly r(C)-binding protein (PCBP) 1. Mol Cancer Ther. 2015;14(5):1162–1170. doi: 10.1158/1535-7163.MCT-14-0695. [DOI] [PubMed] [Google Scholar]
- 46.Zhao W, Dong S, Duan B, et al. HOTAIR is a predictive and prognostic biomarker for patients with advanced gastric adenocarcinoma receiving fluorouracil and platinum combination chemotherapy. Am J Transl Res. 2015;7(7):1295–1302. [PMC free article] [PubMed] [Google Scholar]
- 47.Chen WM, Chen WD, Jiang XM, et al. HOX transcript antisense intergenic RNA represses E-cadherin expression by binding to EZH2 in gastric cancer. World J Gastroenterol. 2017;23(33):6100–6110. doi: 10.3748/wjg.v23.i33.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng X, Huang S. Effect and mechanism of lncRNA HOTAIR on occurrence and development of gastric cancer. J Cell Biochem. 2017 doi: 10.1002/jcb.26594. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Li J, Zhang B, Zeng H. Long-chain non-coding RNA HOTAIR expression in tissue samples correlates with gastric cancer survival. Int J Clin Exp Med. 2018;11(2):856–862. [Google Scholar]
- 50.Ye H, Liu K, Qian K. Overexpression of long noncoding RNA HOTTIP promotes tumor invasion and predicts poor prognosis in gastric cancer. Onco Targets Ther. 2016;9:2081–2088. doi: 10.2147/OTT.S95414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y, Ma B, Yan Y, et al. Long non-coding RNA HOXA transcript at the distal tip as a biomarker for gastric cancer. Oncol Lett. 2017;14(1):1068–1072. doi: 10.3892/ol.2017.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao R, Zhang Y, Zhang X, et al. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol Cancer. 2018;17(1):68. doi: 10.1186/s12943-018-0817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie M, Sun M, Zhu YN, et al. Long noncoding RNA HOXA-AS2 promotes gastric cancer proliferation by epigenetically silencing P21/PLK3/DDIT3 expression. Oncotarget. 2015;6(32):33587–33601. doi: 10.18632/oncotarget.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun M, Nie F, Wang Y, et al. LncRNA HOXA11-AS promotes proliferation and invasion of gastric cancer by scaffolding the chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res. 2016;76(21):6299–6310. doi: 10.1158/0008-5472.CAN-16-0356. [DOI] [PubMed] [Google Scholar]
- 55.Ma B, Wang J, Song Y, et al. Upregulated long intergenic noncoding RNA KRT18P55 acts as a novel biomarker for the progression of intestinal-type gastric cancer. Onco Targets Ther. 2016;9:445–453. doi: 10.2147/OTT.S98613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou B, Jing XY, Wu JQ, Xi HF, Lu GJ. Down-regulation of long non-coding RNA LET is associated with poor prognosis in gastric cancer. Int J Clin Exp Pathol. 2014;7(12):8893–8898. [PMC free article] [PubMed] [Google Scholar]
- 57.Shan Y, Ying R, Jia Z, et al. LINC00052 promotes gastric cancer cell proliferation and metastasis via activating the Wnt/β-catenin signaling pathway. Oncol Res. 2017;25(9):1589–1599. doi: 10.3727/096504017X14897896412027. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Chen WM, Huang MD, Sun DP, et al. Long intergenic non-coding RNA 00152 promotes tumor cell cycle progression by binding to EZH2 and repressing p15 and p21 in gastric cancer. Oncotarget. 2016;7(9):9773–9787. doi: 10.18632/oncotarget.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang ZZ, Zhao G, Zhuang C, et al. Long non-coding RNA LINC00628 functions as a gastric cancer suppressor via long-range modulating the expression of cell cycle related genes. Sci Rep. 2016;6:27435. doi: 10.1038/srep27435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang E, Yin D, Han L, et al. E2F1-induced upregulation of long non-coding RNA LINC00668 predicts a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically silencing of CKIs. Oncotarget. 2016;7(17):23212–23226. doi: 10.18632/oncotarget.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ba MC, Long H, Cui SZ, et al. Long noncoding RNA LINC00673 epigenetically suppresses KLF4 by interacting with EZH2 and DNMT1 in gastric cancer. Oncotarget. 2017;8(56):95542–95553. doi: 10.18632/oncotarget.20980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang M, Hou J, Wang Y, et al. Long noncoding RNA LINC00673 is activated by SP1 and exerts oncogenic properties by interacting with LSD1 and EZH2 in gastric cancer. Mol Ther. 2017;25(4):1014–1026. doi: 10.1016/j.ymthe.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Zeng S, Xie X, Xiao YF, et al. Long noncoding RNA LINC00675 enhances phosphorylation of vimentin on Ser83 to suppress gastric cancer progression. Cancer Lett. 2018;412:179–187. doi: 10.1016/j.canlet.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 64.Fei ZH, Yu XJ, Zhou M, Su HF, Zheng Z, Xie CY. Upregulated expression of long non-coding RNA LINC00982 regulates cell proliferation and its clinical relevance in patients with gastric cancer. Tumour Biol. 2016;37(2):1983–1993. doi: 10.1007/s13277-015-3979-9. [DOI] [PubMed] [Google Scholar]
- 65.Miao Y, Sui J, Xu SY, Liang GY, Pu YP, Yin LH. Comprehensive analysis of a novel four-lncRNA signature as a prognostic biomarker for human gastric cancer. Oncotarget. 2017;8(43):75007–75024. doi: 10.18632/oncotarget.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen X, Chen Z, Yu S, et al. Long noncoding RNA LINC01234 functions as a competing endogenous RNA to regulate CBFB expression by sponging miR-204-5p in gastric cancer. Clin Cancer Res. 2018;24(8):2002–2014. doi: 10.1158/1078-0432.CCR-17-2376. [DOI] [PubMed] [Google Scholar]
- 67.Qin QH, Yin ZQ, Li Y, Wang BG, Zhang MF. Long intergenic non-coding RNA 01296 aggravates gastric cancer cells progress through miR-122/MMP-9. Biomed Pharmacother. 2018;97:450–457. doi: 10.1016/j.biopha.2017.10.066. [DOI] [PubMed] [Google Scholar]
- 68.Zou Z, Ding Q, Li P, et al. Overexpression of lincRNA-ROR predicts poor prognosis in patients with gastric cancer. Int J Clin Exp Pathol. 2016;9(9):9467–9472. [Google Scholar]
- 69.Hu Y, Pan J, Wang Y, Li L, Huang Y. Long noncoding RNA linc-UBC1 is negative prognostic factor and exhibits tumor pro-oncogenic activity in gastric cancer. Int J Clin Exp Pathol. 2015;8(1):594–600. [PMC free article] [PubMed] [Google Scholar]
- 70.Guo W, Dong Z, Shi Y, et al. Methylation-mediated downregulation of long noncoding RNA LOC100130476 in gastric cardia adenocarcinoma. Clin Exp Metastasis. 2016;33(5):497–508. doi: 10.1007/s10585-016-9794-x. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Y, Liu Y, Lin L, et al. The lncRNA MACC1-AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1. Mol Cancer. 2018;17(1):69. doi: 10.1186/s12943-018-0820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qi Y, Ooi HS, Wu J, et al. MALAT1 long ncRNA promotes gastric cancer metastasis by suppressing PCDH10. Oncotarget. 2016;7(11):12693–12703. doi: 10.18632/oncotarget.7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J, Gao J, Tian W, Li Y, Zhang J. Long non-coding RNA MALAT1 drives gastric cancer progression by regulating HMGB2 modulating the miR-1297. Cancer Cell Int. 2017;17:44. doi: 10.1186/s12935-017-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, Wu Z, Yuan J, et al. Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. 2017;395:31–44. doi: 10.1016/j.canlet.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 75.Chen F, Tian Y, Pang EJ, Wang Y, Li L. MALAT2-activated long non-coding RNA indicates a biomarker of poor prognosis in gastric cancer. Cancer Gene Ther. 2015 doi: 10.1038/cgt.2015.6. [DOI] [PubMed] [Google Scholar]
- 76.Sun M, Xia R, Jin F, et al. Downregulated long noncoding RNA MEG3 is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumour Biol. 2014;35(2):1065–1073. doi: 10.1007/s13277-013-1142-z. [DOI] [PubMed] [Google Scholar]
- 77.Guo W, Dong Z, Liu S, et al. Promoter hypermethylation-mediated downregulation of miR-770 and its host gene MEG3, a long non-coding RNA, in the development of gastric cardia adenocarcinoma. Mol Carcinog. 2017;56(8):1924–1934. doi: 10.1002/mc.22650. [DOI] [PubMed] [Google Scholar]
- 78.Nie FQ, Ma S, Xie M, Liu YW, De W, Liu XH. Decreased long non-coding RNA MIR31HG is correlated with poor prognosis and contributes to cell proliferation in gastric cancer. Tumour Biol. 2016;37(6):7693–7701. doi: 10.1007/s13277-015-4644-z. [DOI] [PubMed] [Google Scholar]
- 79.Quan Y, Zhang Y, Lin W, et al. Knockdown of long non-coding RNA MAP3K20 antisense RNA 1 inhibits gastric cancer growth through epigenetically regulating miR-375. Biochem Biophys Res Commun. 2018;497(2):527–534. doi: 10.1016/j.bbrc.2018.02.072. [DOI] [PubMed] [Google Scholar]
- 80.Lai Y, Xu P, Liu J, et al. Decreased expression of the long non-coding RNA MLLT4 antisense RNA 1 is a potential biomarker and an indicator of a poor prognosis for gastric cancer. Oncol Lett. 2017;14(3):2629–2634. doi: 10.3892/ol.2017.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y, Zhang D, Wu K, Zhao Q, Nie Y, Fan D. Long noncoding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Mol Cell Biol. 2014;34(17):3182–3193. doi: 10.1128/MCB.01580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin Z, Lai S, He X, et al. Decreased long non-coding RNA MTM contributes to gastric cancer cell migration and invasion via modulating MT1F. Oncotarget. 2017;8(57):97371–97383. doi: 10.18632/oncotarget.22126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fu JW, Kong Y, Sun X. Long noncoding RNA NEAT1 is an unfavorable prognostic factor and regulates migration and invasion in gastric cancer. J Cancer Res Clin Oncol. 2016;142(7):1571–1579. doi: 10.1007/s00432-016-2152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo X, Yang Z, Zhi Q, et al. Long noncoding RNA OR3A4 promotes metastasis and tumorigenicity in gastric cancer. Oncotarget. 2016;7(21):30276–30294. doi: 10.18632/oncotarget.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang YQ, Zhang QY, Weng WW, et al. Upregulation of the non-coding RNA OTUB1-isoform 2 contributes to gastric cancer cell proliferation and invasion and predicts poor gastric cancer prognosis. Int J Biol Sci. 2016;12(5):545–557. doi: 10.7150/ijbs.13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma P, Xu T, Huang M, Shu Y. Increased expression of LncRNA PANDAR predicts a poor prognosis in gastric cancer. Biomed Pharmacother. 2016;78:172–176. doi: 10.1016/j.biopha.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 87.Liu J, Ben Q, Lu E, et al. Long noncoding RNA PANDAR blocks CDKN1A gene transcription by competitive interaction with p53 protein in gastric cancer. Cell Death Dis. 2018;9(2):168. doi: 10.1038/s41419-017-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bi M, Yu H, Huang B, Tang C. Long non-coding RNA PCAT-1 over-expression promotes proliferation and metastasis in gastric cancer cells through regulating CDKN1A. Gene. 2017;626:337–343. doi: 10.1016/j.gene.2017.05.049. [DOI] [PubMed] [Google Scholar]
- 89.Kong R, Zhang EB, Yin DD, et al. Long noncoding RNA PVT1 indicates a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically regulating p15 and p16. Mol Cancer. 2015;14:82. doi: 10.1186/s12943-015-0355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuan CL, Li H, Zhu L, Liu Z, Zhou J, Shu Y. Aberrant expression of long noncoding RNA PVT1 and its diagnostic and prognostic significance in patients with gastric cancer. Neoplasma. 2016;63(3):442–449. doi: 10.4149/314_150825N45. [DOI] [PubMed] [Google Scholar]
- 91.Sun J, Song Y, Chen X, et al. Novel long non-coding RNA RP11-119F7.4 as a potential biomarker for the development and progression of gastric cancer. Oncol Lett. 2015;10(1):115–120. doi: 10.3892/ol.2015.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song W, Liu YY, Peng JJ, et al. Identification of differentially expressed signatures of long non-coding RNAs associated with different metastatic potentials in gastric cancer. J Gastroenterol. 2016;51(2):119–129. doi: 10.1007/s00535-015-1091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu Y, Ma Z, He Y, Liu W, Su Y, Tang Z. LncRNA-SNHG1 contributes to gastric cancer cell proliferation by regulating DNMT1. Biochem Biophys Res Commun. 2017;491(4):926–931. doi: 10.1016/j.bbrc.2017.07.137. [DOI] [PubMed] [Google Scholar]
- 94.Yan K, Tian J, Shi W, Xia H, Zhu Y. LncRNA SNHG6 is associated with poor prognosis of gastric cancer and promotes cell proliferation and EMT through epigenetically silencing p27 and sponging miR-101-3p. Cell Physiol Biochem. 2017;42(3):999–1012. doi: 10.1159/000478682. [DOI] [PubMed] [Google Scholar]
- 95.Zhang H, Lu W. LncRNA SNHG12 regulates gastric cancer progression by acting as a molecular sponge of miR-320. Mol Med Rep. 2018;17(2):2743–2749. doi: 10.3892/mmr.2017.8143. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y, Yang R, Lian J, Xu H. LncRNA Sox2ot overexpression serves as a poor prognostic biomarker in gastric cancer. Am J Transl Res. 2016;8(11):5035–5043. [PMC free article] [PubMed] [Google Scholar]
- 97.Zou JH, Li CY, Bao J, Zheng GQ. High expression of long noncoding RNA Sox2ot is associated with the aggressive progression and poor outcome of gastric cancer. Eur Rev Med Pharmacol Sci. 2016;20(21):4482–4486. [PubMed] [Google Scholar]
- 98.Peng W, Wu G, Fan H, Wu J, Feng J. Long noncoding RNA SPRY4-IT1 predicts poor patient prognosis and promotes tumorigenesis in gastric cancer. Tumour Biol. 2015;36(9):6751–6758. doi: 10.1007/s13277-015-3376-4. [DOI] [PubMed] [Google Scholar]
- 99.Xie M, Nie FQ, Sun M, et al. Decreased long noncoding RNA SPRY4-IT1 contributing to gastric cancer cell metastasis partly via affecting epithelial-mesenchymal transition. J Transl Med. 2015;13:250. doi: 10.1186/s12967-015-0595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang E, He X, Yin D, et al. Increased expression of long noncoding RNA TUG1 predicts a poor prognosis of gastric cancer and regulates cell proliferation by epigenetically silencing of p57. Cell Death Dis. 2016;7:e2109. doi: 10.1038/cddis.2015.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng Q, Wu F, Dai WY, et al. Aberrant expression of UCA1 in gastric cancer and its clinical significance. Clin Transl Oncol. 2015;17(8):640–646. doi: 10.1007/s12094-015-1290-2. [DOI] [PubMed] [Google Scholar]
- 102.Nasrollahzadeh-Khakiani M, Emadi-Baygi M, Nikpour P. Augmented expression levels of lncRNAs ecCEBPA and UCA1 in gastric cancer tissues and their clinical significance. Iran J Basic Med Sci. 2017;20(10):1149–1158. doi: 10.22038/IJBMS.2017.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zuo ZK, Gong Y, Chen XH, et al. TGFβ1-induced LncRNA UCA1 upregulation promotes gastric cancer invasion and migration. DNA Cell Biol. 2017;36(2):159–167. doi: 10.1089/dna.2016.3553. [DOI] [PubMed] [Google Scholar]
- 104.Gu L, Lu LS, Zhou DL, Liu ZC. UCA1 promotes cell proliferation and invasion of gastric cancer by targeting CREB1 sponging to miR-590-3p. Cancer Med. 2018;7(4):1253–1263. doi: 10.1002/cam4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen M, Wu X, Ma W, et al. Decreased expression of lncRNA VPS9D1-AS1 in gastric cancer and its clinical significance. Cancer Biomark. 2017;21(1):23–28. doi: 10.3233/CBM-170172. [DOI] [PubMed] [Google Scholar]
- 106.Cai J, Wang D, Bai ZG, Yin J, Zhang J, Zhang ZT. The long noncoding RNA XIAP-AS1 promotes XIAP transcription by XIAP-AS1 interacting with Sp1 in gastric cancer cells. PLoS One. 2017;12(8):e0182433. doi: 10.1371/journal.pone.0182433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen DL, Ju HQ, Lu YX, et al. Long non-coding RNA XIST regulates gastric cancer progression by acting as a molecular sponge of miR-101 to modulate EZH2 expression. J Exp Clin Cancer Res. 2016;35(1):142. doi: 10.1186/s13046-016-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma L, Zhou Y, Luo X, Gao H, Deng X, Jiang Y. Long non-coding RNA XIST promotes cell growth and invasion through regulating miR-497/MACC1 axis in gastric cancer. Oncotarget. 2017;8(3):4125–4135. doi: 10.18632/oncotarget.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li Y, Wen X, Wang L, et al. LncRNA ZEB1-AS1 predicts unfavorable prognosis in gastric cancer. Surg Oncol. 2017;26(4):527–534. doi: 10.1016/j.suronc.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 110.Zhang LL, Zhang LF, Guo XH, Zhang DZ, Yang F, Fan YY. Downregulation of miR-335-5p by long noncoding RNA ZEB1-AS1 in gastric cancer promotes tumor proliferation and invasion. DNA Cell Biol. 2018;37(1):46–52. doi: 10.1089/dna.2017.3926. [DOI] [PubMed] [Google Scholar]
- 111.Zhang JJ, Chen JT, Yao KH, Hua L, Wang CY, Hu JH. Up-regulated expression of long non-coding RNA ZFAS1 associates with aggressive tumor progression and poor prognosis in gastric cancer patients. Int J Clin Exp Pathol. 2016;9(2):2059–2063. [Google Scholar]
- 112.Nie F, Yu X, Huang M, et al. Long noncoding RNA ZFAS1 promotes gastric cancer cells proliferation by epigenetically repressing KLF2 and NKD2 expression. Oncotarget. 2017;8(24):38227–38238. doi: 10.18632/oncotarget.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lai Y, Xu P, Li Q, et al. Downregulation of long noncoding RNA ZMAT1 transcript variant 2 predicts a poor prognosis in patients with gastric cancer. Int J Clin Exp Pathol. 2015;8(5):5556–5562. [PMC free article] [PubMed] [Google Scholar]
- 114.McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014;11(11):664–674. doi: 10.1038/nrgastro.2014.143. [DOI] [PubMed] [Google Scholar]
- 115.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 116.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 117.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 119.Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108(12):2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31(43):4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li T, Mo X, Fu L, Xiao B, Guo J. Molecular mechanisms of long non-coding RNAs on gastric cancer. Oncotarget. 2016;7(8):8601–8612. doi: 10.18632/oncotarget.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 123.Xu MD, Qi P, Weng WW, et al. Long non-coding RNA LSINCT5 predicts negative prognosis and exhibits oncogenic activity in gastric cancer. Medicine (Baltimore) 2014;93(28):e303. doi: 10.1097/MD.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dan J, Wang J, Wang Y, et al. LncRNA-MEG3 inhibits proliferation and metastasis by regulating miRNA-21 in gastric cancer. Biomed Pharmacother. 2018;99:931–938. doi: 10.1016/j.biopha.2018.01.164. [DOI] [PubMed] [Google Scholar]