Abstract
Background
Following the emergence of Schmallenberg virus (SBV) in Ireland in 2012, a sentinel herd surveillance program was established in the south of Ireland with the primary aim of investigating the species composition and abundance of Culicoides on livestock farms in the region.
Methods
Ultraviolet-light trapping for Culicoides was carried out on 10 sentinel farms. Each site was sampled fortnightly over 16 weeks (21st July to 5th November 2014). One Onderstepoort Veterinary Institute UV light trap was run overnight at each site and catches were transferred immediately into 70% ethanol. Culicoides were morphologically identified to species level. Collection site habitats were characterised using the Phase 1 habitat survey technique (Joint Nature Conservation Committee).
Results
A total of 23,929 individual Culicoides from 20 species was identified, including one species identified in Ireland for the first time, Culicoides cameroni. The most abundant species identified were Culicoides obsoletus/Culicoides scoticus (38%), Culicoides dewulfi (36%), Culicoides pulicaris (9%), Culicoides chiopterus (5%) and Culicoides punctatus (5%), comprising 93% of all Culicoides specimens identified. Collection site habitats were dominated by improved grassland and a combination of broadleaf woodland and native woodland species.
Conclusions
The most abundant species of Culicoides identified were the putative vectors of bluetongue virus (BTV) and SBV in northern Europe. Their presence and abundance demonstrates the potential for future transmission of arboviruses among livestock in this region.
Electronic supplementary material
The online version of this article (10.1186/s13071-018-3010-6) contains supplementary material, which is available to authorized users.
Keywords: Culicoides, Ecological habitats, Schmallenberg virus, Bluetongue virus, Arbovirus, Vector, Sentinel herd surveillance, Ireland
Background
Arthropod-borne viruses (arboviruses) are transmitted by several insect vectors including mosquitoes and Culicoides biting midges [1]. Culicoides biting midges have been implicated in the transmission of over 50 arboviruses worldwide [1] including bluetongue virus (BTV; Orbivirus, Reoviridae), Schmallenberg virus (SBV; Orthobunyavirus, Peribunyaviridae) and African horse sickness virus (AHS; Orbivirus, Reoviridae). Currently, the only arbovirus known to be primarily transmitted by Culicoides to and between humans is Oropouche virus (OROV; Orthobunyavirus, Peribunyaviridae) [2]. The recent unprecedented emergence of arboviruses transmitted by Culicoides species in northern Europe, such as SBV and multiple serotypes of BTV, has highlighted Europe’s susceptibility to exotic arboviruses transmitted by biting midges from distant geographical regions.
Since 1998, there have been regular outbreaks of bluetongue disease in many parts of southern Europe with the Afro-Asiatic species, Culicoides imicola, implicated as the principal vector in the transmission of the virus [3]. However, BTV serotype 8 (BTV-8) emerged in northern Europe (the Netherlands) for the first time in 2006 [4]. The virus was successfully transmitted by northern Palaearctic species of Culicoides, specifically four members of the subgenus Avaritia; Culicoides obsoletus Meigen, 1818, Culicoides scoticus Downes & Kettle, 1952, Culicoides dewulfi Goetghebuer, 1936 and Culicoides chiopterus Meigen, 1830, and two members of the subgenus Culicoides; Culicoides pulicaris Linnaeus, 1758 and Culicoides punctatus Meigen, 1804 [5]. Subsequently, BTV was responsible for significant losses in livestock industries in a number of European countries between 2006 and 2008 [6]. Infection with BTV in ruminants can cause severe illness characterised by fever, inflammation of blood vessels (vasculitis), oedema and death in severe cases. More recently, BTV-8 re-emerged in France in 2015 [7] and outbreaks of bluetongue disease continue to occur in domestic livestock in France [8]. In October 2017, a consignment of cattle from France was imported into the UK, with some individuals testing PCR-positive for BTV [9]. Culicoides vector surveillance was immediately expanded to determine the risk of onwards transmission and monitoring of the situation is on-going (M. E. England, personal communication).
Schmallenberg virus is a novel Simbu serogroup Orthobunyavirus which emerged for the first time in northern Europe (Germany and the Netherlands) in 2011 [10]. The presence and abundance of suitable Culicoides vector species in northern Europe facilitated the rapid spread of SBV across the continent in 2012 resulting in a pan-European epizootic of Schmallenberg disease after a single vector-season [11]. The detection of SBV in field-caught Culicoides in a number of countries implicated a similar range of Culicoides species in the transmission of SBV as BTV [12–16]. Infection with SBV in ruminants can cause a drop in milk yield in dairy cattle, and abortions, stillbirths and congenital malformations in cattle, sheep and goats [10]. Following the initial European Schmallenberg epizootic in 2011/2012, the virus continued to circulate at a low level in a number of countries between 2013 and 2015. In contrast, there was little evidence of SBV circulation in Ireland in the three years (2013–2015) following the initial emergence of SBV in Ireland in 2012 [17, 18]. However, in 2016, SBV re-emerged and recirculated at a significant level in Ireland and the UK resulting in a second outbreak of congenital Schmallenberg disease in ruminants in late 2016 and early 2017 [19].
The emergence and re-emergence of BTV and SBV in Europe has highlighted the need for active surveillance systems for emerging and re-emerging infectious diseases. Arbovirus surveillance programs which combine serological, virological and vector studies are considered a particularly effective model for arbovirus surveillance [17]. A sentinel herd surveillance program (bovine serological and Culicoides entomological and virological studies) was, therefore, established on livestock farms located in the south of the Republic of Ireland (ROI) in order to monitor post-epizootic SBV circulation between 2013 and 2017 [17, 19].
A Department of Agriculture, Food and the Marine (DAFM) Culicoides survey conducted in Ireland between 2007 and 2009 as part of the National BTV Vector Surveillance Programme indicated the presence of several suspected Culicoides arbovirus vector species [20]. The most abundant species identified in this study were four members of the subgenus Avaritia (C. obsoletus/C. scoticus C. dewulfi and C. chiopterus) and two members of the subgenus Culicoides (C. pulicaris and C. punctatus) accounting for approximately 80–90% of all Culicoides identified. These species were found ubiquitously and in abundance throughout the ROI. The results of the DAFM study are consistent with similar studies in Northern Ireland and Scotland [21, 22]. Currently, a total of thirty Culicoides species are listed on the Irish Culicoides checklist [5]. However, limited data are available regarding the species and abundance of Culicoides biting midges in the south of Ireland. In the DAFM study, only two collection sites (one in Co. Kerry and one in Co. Waterford) out of a total of ten randomly selected sites distributed throughout the ROI were used to investigate the species composition of Culicoides in the same region as in the present study. Moreover, the DAFM study was conducted three years prior to the emergence of SBV in Ireland in 2012.
The south of Ireland is of particular interest compared to the rest of the country as it is where SBV first emerged in Ireland in 2012 [23] and re-emerged four years later in 2016 [19]. As a result, the south of Ireland is considered one of the most likely regions for BTV and other exotic arboviruses to enter Ireland. Therefore, detailed knowledge of the Culicoides composition in this region is essential to rapidly assess the risk of introduction and transmission of Culicoides-borne arboviruses such as BTV in Ireland. An in-depth Culicoides survey was established in ten sentinel farms in the south of Ireland in 2014 with the aim of investigating the species composition, abundance and broad ecological preferences of Culicoides biting midges in this region.
Methods
Collection sites
As part of a Schmallenberg virus sentinel herd surveillance study, 26 livestock farms located in the south of Ireland were used to monitor post-epizootic SBV circulation in Ireland [17, 19]. Ten of these farms were selected, based on their geographical location to cover as great an area of the south of Ireland as possible, for collection and monitoring of Culicoides (Table 1 and Fig. 1).
Table 1.
Characteristics of the ten sentinel farms located in the south of the Republic of Ireland included in the surveillance study for Culicoides in 2014 (July-November)
| Farm ID | Location | Grid reference | Altitude (m) | Farm animals | No. of collections with Culicoides/total no. collections | Mean no. of Culicoides per collection | Maximum no. of Culicoides per collection | |
|---|---|---|---|---|---|---|---|---|
| Latitude | Longitude | |||||||
| 1 | Clonakilty, Co. Cork | 51.65° | -8.85° | 76 | 230 Bovines; 100 Ovinesa |
5/8 | 52.2 | 121 |
| 2 | Charleville, Co. Cork | 52.33° | -8.79° | 128 | 180 Bovines | 7/8 | 593.3 | 2858 |
| 3 | Carrignavar, Co. Cork | 52.02° | -8.44° | 177 | 212 Bovines | 7/8 | 240.6 | 589 |
| 4 | Cahir, Co. Tipperary | 52.44° | -7.96° | 45 | 390 Bovines | 7/8 | 618.4 | 2216 |
| 5 | Dunmanway, Co. Cork | 51.71° | -9.19° | 131 | 169 Bovines | 6/8 | 269.2 | 874 |
| 6 | Hospital, Co. Limerick | 52.46° | -8.49° | 79 | 241 Bovines | 8/8 | 197.5 | 1265 |
| 7 | Fermoy, Co. Cork | 52.18° | -8.24° | 37 | 324 Bovines; 26 Ovinesa |
7/8 | 327.1 | 1322 |
| 8 | Tallow, Co Waterford | 52.09° | -7.94° | 70 | 677 Bovines | 7/8 | 435.1 | 1282 |
| 9 | Macroom, Co. Cork | 51.88° | -9.01° | 81 | 106 Bovines | 6/8 | 353.0 | 975 |
| 10 | Mallow, Co. Cork | 52.13° | -8.76° | 84 | 142 Bovines | 8/8 | 375.9 | 1155 |
| All | Cork (7); Waterford (1); Tipperary (1); Limerick (1) | 51.65° to 52.44° | -9.19° to -7.94° | 37–177 | 106–677 Bovines; 26–100 Ovines |
68/80 | 354.2 | 2858 |
aOvines grazed separately from bovines
Fig. 1.
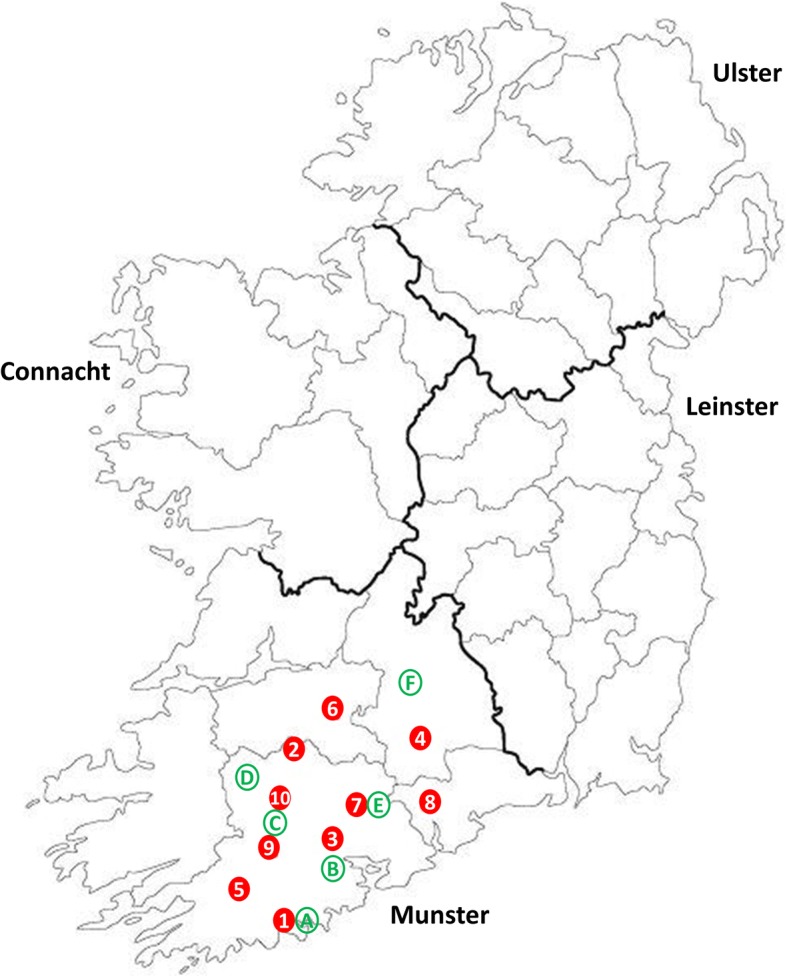
Map showing the location of the ten sentinel farms and six weather station locations in the south of the Republic of Ireland. Each numbered red dot (1–10) corresponds with each of the ten sentinel farms studied and each of the six alphabetically labelled green circles (A-F) corresponds with the location of the local weather stations. Seven farms were located in county Cork, the county where Schmallenberg virus was first identified in Ireland in 2012 [16], and one farm each was located in the adjoining counties Limerick, Tipperary and Waterford, respectively
Specimen collection
Onderstepoort Veterinary Institute-type ultraviolet (UV) light suction traps were used to collect Culicoides; one trap was operated overnight (dusk until dawn) on each farm, approximately 250m away from livestock. Each farm was sampled fortnightly over a period of 16 weeks (21st July - 5th November 2014) during the 2014 vector season, corresponding to eight trap collections per farm and a total of 80 collections during the study period. Insects attracted to the UV light suction traps during operation were collected into beakers. Insects were transferred into 70% ethanol the following morning for storage pending specimen identification.
Identification of specimens
Trap collections were identified morphologically to species-level under a dissecting microscope using the keys of Campbell & Pelham-Clinton [24] and Mathieu et al. [25]. Female Culicoides were further classified as unpigmented (nulliparous), pigmented (parous), gravid and blood-fed individuals. It is not possible to distinguish between C. obsoletus and C. scoticus females based on morphological characteristics, so they were grouped as C. obsoletus/C. scoticus. In contrast, it is possible to distinguish male C. obsoletus and C. scoticus specimens; hence, males of these species were morphologically identified and counted individually.
Culicoides specimens with damaged/missing abdomens (n = 478) were identified to species level but the parity status of those females was recorded as not-specified (N/S). Specimens that were too damaged to identify to species level (n = 75) were counted as unidentified Culicoides.
A number of specimens of Culicoides clastrieri Callot, Kremer & Deduit, 1962 were morphologically identified in trap catches suggesting a possible new Culicoides record for Ireland. However, considering that it can be difficult to separate C. clastrieri and C. festivipennis by wing morphology, molecular analyses were employed to explore the taxonomy of these two species. To do this, a region of the cytochrome c oxidase subunit 1 (cox1) gene of two female specimens morphologically identified as C. clastrieri from two different farms, were sequenced (adapted from Folmer et al. [26] and Hebert et al. [27]). These sequences were compared with available sequences in GenBank using BLAST [28].
Habitat surveys
The habitat surrounding the trap at each of the ten locations was characterised using the Phase 1 habitat survey technique [29]. Each farm site was visited and the land use surrounding the trap (approximately 500 m) was categorised according to Phase 1 habitat survey classifications [29]. The relative density (low; +, medium; ++, high; ++) of habitat classes within the surveyed area was recorded. Target notes recorded the location of important on-farm features such as manure storage points (slurry pits, lagoons, dung heaps) and the location of the OVI trap. Altitude and the number of livestock on each farm were also noted.
Meteorological data
Meteorological data (maximum, minimum and mean daily temperature in °C) were retrieved from the Irish Meteorological Service Online [30] and from Dr Patrick Touhy, Teagasc (personal communication) for six weather stations (A-F) located within the region (Fig. 1). One weather station each was located on Farm 1 (weather station A) and Farm 7 (weather station E), and four other weather stations (B, C, D and F) were located within a 25 km radius of one of the other study farms (Fig. 1). The correlation (Pearson’s correlation r) between mean fortnightly temperature at the six weather stations (mean of data from the 6 weather stations) and Culicoides abundance during the 16 week study period was calculated using GraphPad Prism 7 software (GraphPad Software Inc., CA, USA).
Results
A total of 23,929 Culicoides, representing twenty species, was collected from 10 farms in 68 successful trap collections; twelve trap collections from 8 farms (range 1–3 catch collections per farm) yielded no Culicoides specimens (Tables 2, 3). Culicoides were found ubiquitously across all sites; however, there was large variation in the total number of Culicoides collected on each farm during the 16-week study period ranging from 257 to 4285 Culicoides per farm (Additional file 1: Figure S1). Female Culicoides (84%; n = 19,936) were more abundant than males (n = 3918; 16%) in trap catches, equating to a male to female sex ratio of 1:5.
Table 2.
Species of Culicoides, sorted according to their abundance (number and percentage) and gender, on ten Irish farms collected in the south of the Republic of Ireland during part of the 2014 vector-active season (July-November)
| Culicoides (C.) species | Female | Male | Total | No. of farms with species confirmed | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| C. obsoletus/C. scoticus | 8103 | 89 | 1004a | 11 | 9107 | 38 | 10 | |
| C. dewulfi | 6755 | 78 | 1928 | 22 | 8683 | 36 | 10 | |
| C. pulicaris | 1879 | 85 | 325 | 15 | 2204 | 9 | 10 | |
| C. punctatus | 1056 | 94 | 67 | 6 | 1123 | 5 | 10 | |
| C. chiopterus | 781 | 72 | 305 | 28 | 1086 | 5 | 10 | |
| Sub-total (vector species) | 18,574 | 84 | 3629 | 16 | 22,203 | 93 | ||
| C. achrayi | 1003 | 82 | 220 | 18 | 1223 | 5 | 10 | |
| C. festivipennis | 175 | 83 | 35 | 17 | 210 | < 1 | 9 | |
| C. impunctatus | 88 | 88 | 12 | 12 | 100 | < 1 | 8 | |
| C. nubeculosus | 31 | 70 | 13 | 30 | 44 | < 1 | 5 | |
| C. circumscriptus | 35 | 90 | 4 | 10 | 39 | < 1 | 7 | |
| C. salinarius | 13 | 81 | 3 | 19 | 16 | < 1 | 5 | |
| C. fascipennis | 5 | 83 | 1 | 17 | 6 | < 1 | 3 | |
| C. delta | 3 | 75 | 1 | 25 | 4 | < 1 | 3 | |
| C. cameroni | 3 | 100 | 0 | 0 | 3 | < 1 | 1 | |
| C. brunnicans | 2 | 100 | 0 | 0 | 2 | < 1 | 2 | |
| C. newsteadi | 1 | 100 | 0 | 0 | 1 | < 1 | 1 | |
| C. riethi | 1 | 100 | 0 | 0 | 1 | < 1 | 1 | |
| C. stigma | 1 | 100 | 0 | 0 | 1 | < 1 | 1 | |
| C. reconditus | 1 | 100 | 0 | 0 | 1 | < 1 | 1 | |
| Sub-total (other Culicoides spp.) | 1362 | 82 | 289 | 18 | 1651 | 7 | ||
| Total | 19,936 | 84 | 3918 | 16 | 23,854b | 100 | ||
a690 Culicoides obsoletus and 314 Culicoides scoticus males
b75 unidentifiable Culicoides (74 females and 1 male) not included in the table
Table 3.
Current Irish Culicoides species list (n = 31) and species confirmed in the present study (n = 20), including one species recorded in Ireland for the first time
| Genus | Species | Authority | Confirmed |
|---|---|---|---|
| Culicoides (Avaritia) | chiopterus | Meigen, 1830 | ✓ |
| dewulfi | Goetghebuer, 1936 | ✓ | |
| obsoletus | Meigen, 1818 | ✓ | |
| scoticus | Downes & Kettle, 1952 | ✓ | |
| Culicoides (Beltranmyia) | circumscriptus | Kieffer, 1918 | ✓ |
| salinarius | Kieffer, 1914 | ✓ | |
| Culicoides (Culicoides) | delta | Edwards, 1939 | ✓ |
| grisescens | Edwards, 1939 | ||
| impunctatus | Goetghebuer, 1920 | ✓ | |
| newsteadi | Austen, 1921 | ✓ | |
| pulicaris | Linnaeus, 1758 | ✓ | |
| punctatus | Meigen, 1804 | ✓ | |
| Culicoides (Monoculicoides) | nubeculosus | Meigen, 1830 | ✓ |
| parroti | Kieffer, 1922 | ||
| riethi | Kieffer, 1914 | ✓ | |
| stigma | Meigen, 1818 | ✓ | |
| Culicoides (Oecacta) | |||
| sensu Szadziewski et al., 2016 | brunnicans | Edwards, 1939 | ✓ |
| vexans | Campbell & Pelham-Clinton, 1960 | ||
| duddingstoni | Kettle & Lawson, 1955 | ||
| festivipennis | Kieffer, 1914 | ✓ | |
| heliophilus | Edwards, 1921 | ||
| kibunensis | Tokunaga, 1937 | ||
| pictipennis | Staeger, 1839 | ||
| poperinghensis | Callot, Kremer & Paradis, 1962 | ||
| Unspecified | cameroni | Staeger, 1839 | ✓a |
| furcillatus | Goetghebuer, 1953 | ||
| reconditus | Campbell & Pelham-Clinton, 1960 | ✓ | |
| segnis | Campbell & Pelham-Clinton, 1960 | ||
| Culicoides (Silvaticulicoides) | achrayi | Kettle & Lawson, 1955 | ✓ |
| fascipennis | Staeger, 1839 | ✓ | |
| pallidicornis | Kieffer, 1919 | ||
aSpecies recorded for the first time in Ireland
Culicoides cameroni Campbell & Pelham-Clinton, 1960, was identified and recorded in the ROI for the first time (Fig. 2). The most abundant species identified were members of the subgenus Avaritia (C. obsoletus/C. scoticus: 38%; C. dewulfi: 36%; and C. chiopterus: 5%), C. pulicaris: 9% and C. punctatus: 5%, comprising 93% of all Culicoides collected (Table 2 and Additional file 1: Figure S1). While C. obsoletus and C. scoticus females are indistinguishable morphologically, males were identified and counted separately; C. obsoletus males (n = 690) were more abundant than C. scoticus males (n = 314) (Table 2). The remaining Culicoides were principally Culicoides achrayi Kettle & Lawson, 1955 (5.1%) and Culicoides festivipennis Kieffer, 1914 (0.9%) (Table 2 and Additional file 1: Figure S1). The results of the molecular analyses on two specimens morphologically identified as Culicoides clastrieri Callot, Kremer & Deduit, 1962, revealed that both individuals had 99% match with both C. festivipennis and C. clastrieri and, therefore, we were unable to confirm C. clastrieri as a new record for ROI. Due to the apparent uncertainty surrounding the morphological and molecular distinction of these two species, C. festivipennis refers to individuals which were morphologically identified either as C. festivipennis or C. clastrieri. Seventy-five damaged Culicoides could not be identified to species level (74 females and 1 male). The overall species composition and relative abundance of the most frequently identified Culicoides species on the ten sentinel farms are illustrated in Additional file 1: Figure S1.
Fig. 2.
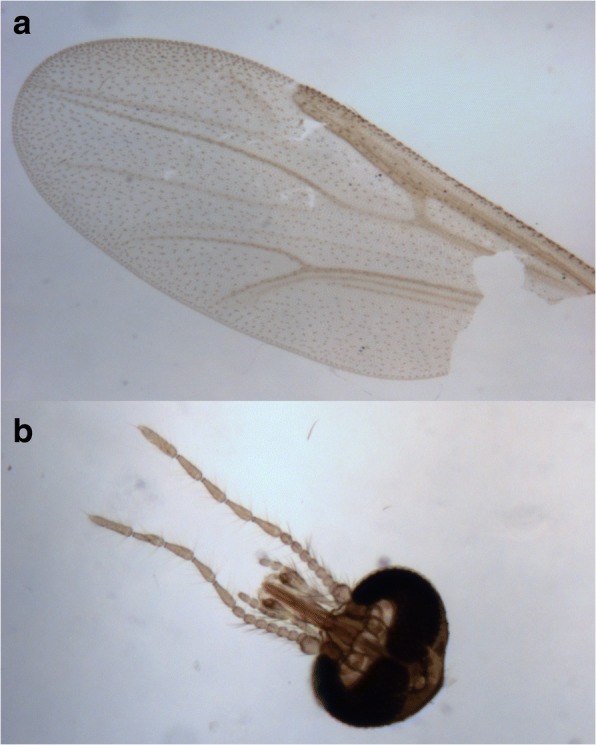
Photograph of wing (a) and head/antennae (b) of Culicoides cameroni identified in Ireland for the first time (photomicrograph taken at 10× magnification)
Mean fortnightly temperatures were highly correlated between the six weather stations (r range 0.90–0.99, P < 0.01). The abundance of Culicoides collected on the 10 farms during each fortnightly collection period was highly correlated with mean fortnightly temperature in the region (r = 0.87, P < 0.01) (Fig. 3). The abundance of C. obsoletus/C. scoticus, C. dewulfi, C. pulicaris, C. punctatus and other Culicoides species at each fortnightly collection were each significantly correlated with mean fortnightly temperatures in the region (r = 0.79, 0.77, 0.80, 0.77 and 0.76, respectively; all P < 0.05). In contrast, there was no significant correlation between mean fortnightly temperature and the relative abundance of C. chiopterus at each fortnightly collection (r = 0.46, P > 0.05). The majority (88%) of Culicoides were collected within the first 10 weeks (between 21st July and 28th September) of the study period (Fig. 3) which correlated with warmer temperatures.
Fig. 3.
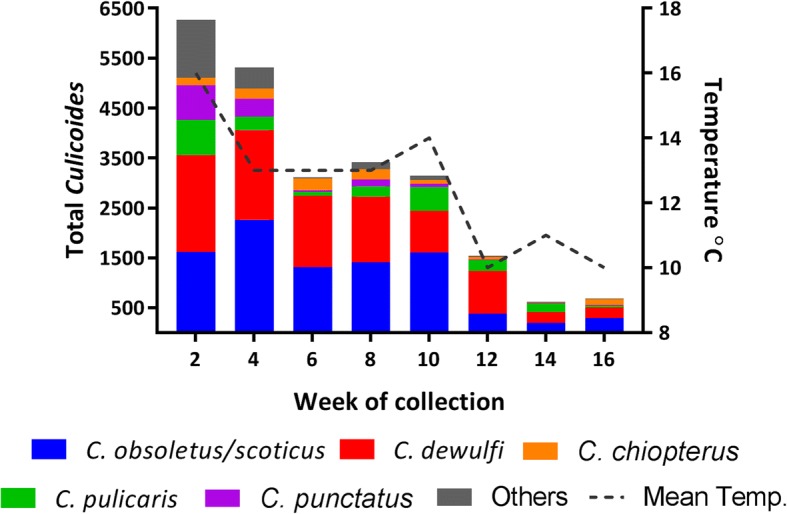
Total Culicoides abundance on 10 sentinel farms in the south of Ireland (between 21st July and 5th November 2014) in relation to week of collection and mean temperature at six weather stations located in the region
The total number of Culicoides species identified ranged from 10 to 15 species per farm (mean = 13; Table 2) and this was weakly correlated (non-significantly) with total Culicoides abundance on each farm (r = 0.34, P > 0.05). There was no correlation between the number of host species per farm and the total number of Culicoides per farm (r = 0.17, P > 0.05). The six major Culicoides arbovirus vector species were identified on all 10 farms.
The parity status was determined for 98% (n = 19,458) of all female Culicoides collected (Additional file 2: Table S1). The majority of the female arbovirus vector species collected was unpigmented (46%) and pigmented (33%), followed by gravid (12%) and blood-fed (5%). For other Culicoides species, gravid Culicoides (33%) were the most abundant, followed by unpigmented (28%), pigmented (28%) and blood-fed (10%). The change in parity rate across the collection period is shown in Fig. 4. The overall abundance of each parity group (unpigmented, pigmented, gravid and blood-fed) of Culicoides were each significantly highly correlated with mean fortnightly temperatures (r range 0.74–0.83, P < 0.05).
Fig. 4.
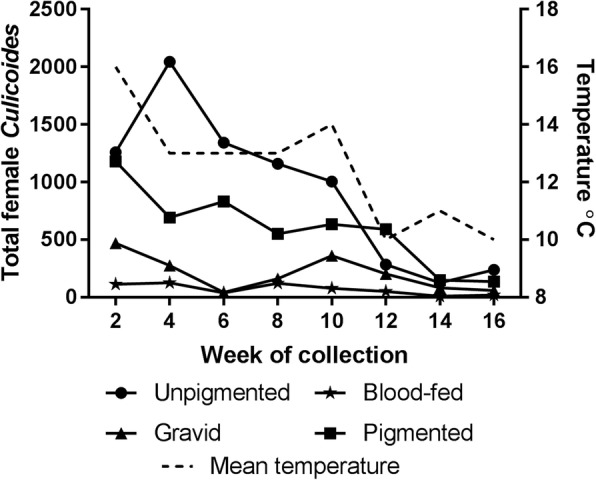
Change in Culicoides parity rate of C. obsoletus/C. scoticus and C. dewulfi and mean temperature across the collection period (between 21st July and 5th November 2014) on 10 sentinel farms located in the south of the Republic of Ireland
Collection site habitat classes are summarised in Table 4. All 10 farms were dominated by improved grassland. Broadleaf woodland was present on most farms; coniferous woodland was also present at a lower density. Boundaries on farms were predominantly native woodland species. Non-native shrub/tree species boundaries were also present on farms to a lesser extent, typically around domestic buildings. The majority of farms had a water course running through the site and almost half of farms had a dense network of cow roadway systems. There were varying densities of broadleaf and coniferous scattered trees across the ten farms. On average, OVI-traps were stationed approximately 250 m (range 0–500 m) from livestock, approximately 120 m (range 20–155 m) from the main farmyard and approximately 130 m (range 20–155 m) from a manure storage point. Cattle were the most abundant host species present on study farms. Sheep were also present in fewer numbers.
Table 4.
Farm and OVI-trap collection site habitat characteristics with total Culicoides abundance and catch break-down (%) per farm and relative density (low; +, medium; ++, high; +++) of habitat classes
| Farm ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Total no. Culicoides | 257 | 4001 | 1687 | 4285 | 1651 | 1549 | 2257 | 3040 | 2105 | 3022 |
| C. obsoletus/C. scoticus (%) | 25 | 27 | 33 | 36 | 43 | 27 | 43 | 62 | 43 | 32 |
| C. dewulfi (%) | 32 | 35 | 51 | 42 | 30 | 55 | 24 | 17 | 44 | 39 |
| C. pulicaris (%) | 19 | 12 | 4 | 10 | 16 | 4 | 7 | 6 | 4 | 14 |
| C. punctatus (%) | 6 | 13 | 2 | 2 | 1 | 2 | 12 | 1 | 2 | 2 |
| C. chiopterus (%) | 12 | 2 | 5 | 8 | 1 | 10 | 6 | 1 | 6 | 3 |
| Other Culicoides species | 6 | 11 | 4 | 2 | 9 | 2 | 7 | 13 | 2 | 10 |
| Animals (No.) | ||||||||||
| Bovines | 230 | 180 | 212 | 390 | 169 | 241 | 324 | 677 | 106 | 142 |
| Ovines | 100 | 26 | ||||||||
| Altitude (m) | 76 | 128 | 177 | 45 | 131 | 79 | 37 | 70 | 81 | 84 |
| Grassland | ||||||||||
| Improved | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Semi-improved | ++ | + | + | + | ||||||
| Marshy | + | |||||||||
| Woodland/Scrub | ||||||||||
| Broadleaf | ++ | ++ | ++ | ++ | +++ | + | +++ | + | ||
| Coniferous | + | + | ++ | + | ++ | |||||
| Scrub | + | + | ++ | + | ||||||
| Boundaries/Margins | ||||||||||
| Native woodland species | + | +++ | ++ | +++ | +++ | + | + | ++ | +++ | +++ |
| Non-native shrub/tree species | + | + | + | + | + | + | + | + | ||
| Stone walls | +++ | ++ | ||||||||
| Scattered trees | ||||||||||
| Broadleaf | + | + | + | ++ | + | ++ | +++ | + | + | |
| Coniferous | + | + | + | + | ||||||
| Cow roadways | +++ | ++ | + | + | + | +++ | +++ | +++ | + | ++ |
| Waterways/ditches | ||||||||||
| Stream | P | P | P | |||||||
| River | P | P | P | P | P | |||||
| Dry ditch | P | P | P | P | P | P | P | |||
| OVI-trap distance (m) from | ||||||||||
| Farmyard | 100 | 150 | 100 | 100 | 10 | 150 | 500 | 20 | 25 | 20 |
| Manure storage point | 150 | 150 | 75 | 150 | 50 | 150 | 500 | 20 | 50 | 20 |
Abbreviation: P, present
Discussion
The most abundant Culicoides species identified in the present study (C. obsoletus/C. scoticus, C. chiopterus, C. dewulfi, C. pulicaris and C. punctatus) are the putative vectors of BTV and SBV in northern Europe. Moreover, the majority (80%) of females identified within these species were host-seeking females (unpigmented and pigmented). The presence and dominance of putative arbovirus vectors demonstrates the potential for future transmission of BTV and SBV and other Culicoides-borne viruses among livestock in this region.
Nearly twice as many C. obsoletus male specimens were identified compared to C. scoticus male specimens suggesting that C. obsoletus was relatively more abundant. However, separation of C. obsoletus females from C. scoticus females would be required to fully assess the relative abundance of these two species. As females of these two species are indistinguishable morphologically, molecular assays would need to be employed to separate the species. Current methods for this are labour intensive as each Culicoides female has to be processed individually and, as such, males of these species are more commonly used as an indication of relative abundance.
Culicoides obsoletus/C. scoticus were the most abundant species identified in the present study. These species are known to breed in a wide variety of habitats including acid grassland, leaf litter, dung heaps and cow pats [31, 32]. These findings are consistent with the relatively high density of such habitats on farms in the present study. A similarly high abundance of C. dewulfi was also recorded on study farms. Culicoides dewulfi and C. chiopterus are particularly associated with high soil moisture and cattle manure [32, 33] and in this study, cattle were the most abundant livestock species present on these farms. Culicoides pulicaris and C. punctatus were present in lower numbers compared to C. obsoletus/C. scoticus and C. dewulfi. A previous study in Ireland indicated that C. pulicaris and C. punctatus tend to be more active earlier in the vector-active season (May) in Ireland [34] compared to the time when the present study was conducted (July-November).
In the present study, Culicoides abundance on the 10 farms was highly correlated with ambient temperatures in the region. This finding supports the previous results of McCarthy et al. [34] (Republic of Ireland), Jess et al. [22] (Northern Ireland) and Sanders et al. [21] (Scotland). Mean fortnightly temperatures were positively correlated with the relative abundance of C. obsoletus/C. scoticus, C. dewulfi, C. pulicaris, C. punctatus and other Culicoides species in the region but were not significantly correlated with relative abundance of C. chiopterus. This is most likely due to the fact that C. chiopterus was the least abundant species in the present study accounting for only 4.5% of all Culicoides trapped and identified. The small numbers of C. chiopterus may have influenced the outcome of the statistical analyses due to the small sample size. Some species such as C. chiopterus are frequently underrepresented in light traps, but further trapping could provide larger samples for analyses of these species.
In the present study, there was wide variation in total Culicoides abundance between farms. The greatest number of Culicoides was collected on Farm 2 and Farm 4, accounting for 17 and 18% of all Culicoides collected, respectively. In contrast, Farm 1 yielded the least number of Culicoides accounting for only 1% of all Culicoides collected. Ecological habitats, and possibly local meteorological conditions not captured in the present study such as wind speed and precipitation, may have influenced the Culicoides abundances on these farms. For example, Farm 1 was located close to the coast; this, coupled with the fact that this farm had a low density of woodland and trees, is likely to have resulted in the OVI-trap being more exposed to windy conditions, thus, resulting in smaller catch collections. Farm 2 and Farm 4 had noticeably higher densities of woodland and native woodland species in farm boundaries in comparison to Farm 1, which are likely to have provided shelter from the wind and suitable substrate (e.g. leaf litter) for larval development and emergence. Furthermore, the difference in distance between traps and animals (which can be quite variable and often not standardised in these types of studies) may have also been an important factor to explain differences in Culicoides abundances between farms in the present study.
The total Culicoides abundance between farms did not appear to be influenced by differences in host availability on individual farms. Total Culicoides abundance was not correlated with total number of cows and sheep per farm. The distance from trap to animal varied on each farm and may have contributed to differences in Culicoides abundance between farms. Additionally, it is difficult to be specific about host availability as all farms were commercial dairy herds with rotational grazing systems; the proximity of animals to the trap on each collection day may have varied during the study period as animals grazed different farm paddocks during each rotation. Therefore, the number of animals present on the farm can give a broad indication of host availability, but the animals may not always be in close proximity to the trap.
The total number of Culicoides species identified ranged between 10–15 species per farm. This was weakly correlated with total Culicoides abundance per farm, for example Culicoides species diversity was wide on Farm 10 (n = 15 Culicoides species; n = 4293 individuals) and narrow on Farm 1 (n = 10 Culicoides species; n = 264 individuals). The identification of C. cameroni in the present study constitutes a new Irish record. This new record in Ireland will update the current checklist of Irish Culicoides [5] to a total of 31 species. Culicoides cameroni is not considered an arbovirus vector species and has been recorded in the UK previously. There was some evidence to suggest that C. clastrieri was also present on farms in the present study (based on morphological identification); however, molecular analyses were unable to distinguish this species from C. festivipennis based on sequencing of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene. This has highlighted the need for further taxonomic investigation of these two species with specimens from a range of locations, as they appear to be almost indistinguishable based on the cox1 gene.
The parity dynamics of female Culicoides provides an indication of the changes in population age across the collection period. In the present study, the overall abundance of each parity group of Culicoides was significantly correlated with mean fortnightly temperatures demonstrating the influence of local meteorological conditions on the Culicoides life-cycle. There was a notable increase in the abundance of nulliparous Culicoides in the fourth week of the collection period (11th - 17th August). During the sixth week of collection (25th - 31st August), there was an increase in the abundance of pigmented Culicoides demonstrating the change in population age during the season.
Limited data are available regarding the species and abundance of Culicoides biting midges in the south of Ireland. In 2007, DAFM initiated the National BTV Vector Surveillance Program which collected Culicoides on a weekly basis at ten randomly selected sites located throughout the ROI between April 2007 and December 2009. Two sites were located in the south of Ireland; one in Co. Kerry and one in Co. Waterford [20]. Similar to the results of the present study, in the DAFM study the Avaritia and Culicoides punctatus and Culicoides pulicaris were the most abundant species identified (during the same 16-week study period as the present study) in 2007 (91%), 2008 (95%) and 2009 (81%).
However, it is interesting to note that the percentage composition of these species in the DAFM study differed considerably from the results of the present study, particularly in relation to the species C. obsoletus/C. scoticus, C. chiopterus, C. pulicaris and C. punctatus. In the present study C. obsoletus/C. scoticus accounted for 38% of all Culicoides identified. However, in the DAFM study the percentage composition of C. obsoletus/C. scoticus was lower in 2007 (18%), 2008 (14%) and 2009 (5%) during the same 16-week study period. The abundance of C. chiopterus was notably higher in the present study (5%) compared to the DAFM study in 2007 (0.9%), 2008 (0.3%) and 2009 (0.4%). The combined percentage of the C. pulicaris and C. punctatus in the present study (14%) was lower than the combined per cent for the same two species in the DAFM study in 2007 (37%), 2008 (20%) and 2009 (54%). While relative abundance of C. dewulfi in the present study (36%) was similar to the results of the DAFM study in 2007 (35%) and 2009 (22%), it was lower than that recorded in 2008 (60%). Given that the trapping methodology and insect traps used were the same in both studies, and the same 16 weeks of each year are compared directly here, it is interesting to note that during the five years between when the DAFM study was completed (2007–2009) and when the present study was completed (2014) the relative abundance of C. obsoletus/C. scoticus and C. chiopterus appears to have increased while the combined per cent of C. pulicaris and C. punctatus appears to have decreased. The population of each species may be affected differently by external factors such as predation, adverse weather conditions and changes in farming practices. As populations may take several years to recover following a decline, continued monitoring of Culicoides populations in the south of Ireland over a longer period of time coupled with data on changes in external factors affecting populations would be required to determine if this trend was continuing and the cause.
A number of studies have demonstrated that light-trapping surveillance does not always provide an accurate reflection of the biting population of Culicoides present. Frequently, certain individuals/species of Culicoides can be underrepresented in light-trap collection samples [35, 36]. In the present study, five-times more female Culicoides were identified compared to males despite the fact that male-to-female sex ratios in biting midge populations are assumed to be close to 1:1 [37]. While trapping techniques such as emergence traps typically show realistic sex ratios, skewed sex ratios in light traps have been reported in biting midges previously [32, 38]. Light traps are often female-biased with males regularly representing less than 5% of Culicoides collected [35, 37, 39]. A number of hypotheses have been suggested to explain this phenomenon such as; light sources predominantly attract females more than males [40], males disperse over shorter distances from breeding sites compared to females [41] and females have a longer life span than males [42]. However, when considering disease risk, it is the female Culicoides that are most important and light traps provide an efficient and practical tool to investigate the faunal composition and abundance of Culicoides in an area. Indeed, ultraviolet light suction traps have been the most commonly used collection method in Culicoides surveys and national arbovirus surveillance programs since 2000 in southern Europe and since 2008 in northern and central Europe [35].
The possible underrepresentation of C. chiopterus should also be considered in the present study. The percentage composition of C. chiopterus in light-trap catches has been shown to be low in a number of studies previously, for example, in the ROI [34], in the UK [43], and in the Netherlands [44]. As a result, it has been assumed that C. chiopterus is likely to play a minor role in arbovirus transmission. However, Carpenter et al. [36] proposed that the role of C. chiopterus as an arbovirus vector may be markedly underestimated. A number of studies which collected Culicoides directly (e.g. drop trap, sticky tape trap, direct aspiration) from host species (bovine, ovine and equine) revealed high abundances of C. chiopterus in direct catch collections [36, 45, 46]. Hence, the low abundance of C. chiopterus in ultraviolet-light trap collections in the present study may not provide an accurate representation of this species in the surrounding area. Therefore, further studies that employ direct collection techniques may provide additional information about the species and abundance of Culicoides feeding on livestock in Ireland.
The effects of climate change and changes in meteorological conditions on the distribution and abundance of Culicoides and their ability to transport arboviruses into new regions [47] continues to pose a threat to livestock in many countries. Wind movement is considered an important factor in the transmission of exotic arboviruses from endemic regions into new regions. A model demonstrated that the majority of SBV infections in Ireland [48] and the UK [49] in 2012 occurred as a result of infected midges being transported through downwind movement facilitated by prevailing winds from continental Europe. A similar study implicated both downwind and upwind movements in the spatial and temporal pattern of BTV-8 movement across northwest Europe in 2006 [50]. It has also been proposed that the re-emergence of SBV in Ireland in 2016 was a result of favourable easterly wind conditions which may have facilitated the transport of virus-infected Culicoides into Ireland from neighbouring countries [19]. The on-going outbreak of BTV in France (2015-present) poses a continuing threat to livestock farms in Ireland and the UK, as has recently been the case [8, 9]. It is likely that, should BTV emerge in Ireland it will most likely occur as a result of wind dispersal of virus-infected Culicoides from neighbouring countries. A model has been developed by DAFM in conjunction with The Irish Meteorological Office (Met Éireann) to monitor weather conditions which may favour a possible incursion of Culicoides from the UK and continental Europe (D. J. Barrett, personal communication). The emergence of BTV or other exotic arboviruses in Ireland could result in dramatic disease epizootics due to the immunologically naïve status of animals in the region.
While virus-infected insects are the most likely route of entry of exotic arboviruses into Ireland, the possibility of importing virus-infected animals should also be considered. Bluetongue virus serotype 8 emerged on a cattle farm in Northern Ireland during the 2008 vector-free period following the importation of pregnant cattle from the Netherlands. All 20 cows tested negative for viral RNA (reverse transcription PCR) at importation, but three calves from two cows tested positive for BTV at birth [51]. Fortunately, this incident was rapidly isolated and there was no further transmission beyond the original herd. Between 2011 and 2016, on average, 6470 live animals (range: 2497–12,996) were imported into the ROI annually (excluding livestock imported for immediate slaughter), the majority of which (mean = 78%, range: 67–91% across the six years) were imported from the UK [52]. The remainder of livestock imports originated primarily in Denmark (4.7%), Germany (4.6%), France (3.8%) and the Netherlands (3.7%) [52]. It would be prudent to continue monitoring livestock imports as a possible route of introduction of exotic arboviruses into Ireland. In the context of BTV, SBV and other exotic arboviruses, continued monitoring of the dynamics of Culicoides biting midges on farms in Ireland is recommended, particularly considering the apparent change in the Culicoides species composition and abundance in the south of Ireland since 2009 and the on-going threat of a possible incursion of BTV-infected Culicoides from Europe. Evaluating Culicoides abundances throughout the year would also provide valuable information on adult Culicoides activity; knowing when the adult midges are active (and inactive) would indicate when disease transmission is most likely to occur. This information can be used by policy makers to inform decisions regarding animal movement restrictions and international trade.
Conclusions
The most abundant Culicoides species identified in this study are the putative vectors of a number of arboviruses in northern Europe. The presence and abundance of these species highlight that disease transmission could occur and be maintained following a new incursion of BTV, SBV or other exotic Culicoides-transmitted arboviruses into these areas.
Additional files
Figure S1. Between-site variations in total Culicoides abundance on 10 sentinel farms (Farms 1–10) in the south of the Republic of Ireland. (TIF 250 kb)
Table S1. Physiological/parity status of female Culicoides with the potential to transmit arboviruses collected on 10 Irish farms during part of the 2014 vector-active season (July-November). (DOCX 19 kb)
Acknowledgements
We acknowledge the contribution of Dr Kieran McCarthy and Anne Bateman (National University Ireland, Galway) for their knowledge and support. Ultraviolet light traps were provided by the Department of Agriculture, Food and the Marine and National University Ireland, Galway. The assistance of John Heffernan and Mike Dineen (Teagasc) with sample collection is greatly appreciated. We also thank Dr Emma Howson (The Pirbright Institute) for her assistance with DNA extraction and sequencing. We thank the farmers for access to their farms and their data.
Funding
This research was funded by the Teagasc project MKAB-6520 and the Teagasc Walsh Fellowship Scheme. The Culicoides morphological identification which was conducted at the Pirbright Institute, UK, was funded by the UK Culicoides Reference Laboratory (Defra).
Availability of data and materials
All data supporting the conclusions of this article are included within the article and its additional files. The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AHS
African horse sickness virus
- BTV
bluetongue virus
- Co.
County
- cox1
cytochrome c oxidase subunit 1
- OROV
Oropouche virus
- OVI
Onderstepoort Veterinary Institute
- ROI
Republic of Ireland
- SBV
Schmallenberg virus
- UV
ultraviolet
Authors’ contributions
All authors participated in the design of the study. ÁC collected insect samples and completed habitat surveys. ME oversaw the Culicoides morphological identification with assistance from ÁC. ÁC and ME wrote the first draft of the manuscript, with all authors involved in reviewing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Áine B. Collins, Email: aine.collins@agriculture.gov.ie
John F. Mee, Email: john.mee@teagasc.ie
Michael L. Doherty, Email: michael.doherty@ucd.ie
Damien J. Barrett, Email: damien.barrett@agriculture.gov.ie
Marion E. England, Email: marion.england@pirbright.ac.uk
References
- 1.Mellor PS, Boorman J, Baylis M. Culicoides biting midges: their role as arbovirus vectors. Annu Rev Entomol. 2000;45:307–340. doi: 10.1146/annurev.ento.45.1.307. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter S, Groschup MH, Garros C, Felippe-Bauer ML, Purse BV. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102–113. doi: 10.1016/j.antiviral.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PPC, Baylis M. Climate change and the recent emergence of bluetongue in Europe. Nat Rev Micro. 2005;3:171–181. doi: 10.1038/nrmicro1090. [DOI] [PubMed] [Google Scholar]
- 4.ISID (International Society for Infectious Diseases). Bluetongue, Ovine - Netherlands: confirmed; 2006. ProMED-mail, 20060818.2311 http://www.promedmail.org/post/20060818.2311. Accessed 9 Jan 2018.
- 5.Chandler. An update of the 1998 Checklist of Diptera of the British Isles [Updated 16 January 2017]; 2017 http://www.dipteristsforum.org.uk/documents/BRITISH_ISLES_CHECKLIST.pdf. Accessed 22 Apr 2018.
- 6.Carpenter S, Wilson A, Mellor PS. Culicoides and the emergence of bluetongue virus in northern Europe. Trends Microbiol. 2009;17:172–178. doi: 10.1016/j.tim.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Sailleau C, Bréard E, Viarouge C, Vitour D, Romey A, Garnier A, et al. Re-emergence of bluetongue virus serotype 8 in France, 2015. Transbound Emerg Dis. 2017;64:998–1000. doi: 10.1111/tbed.12453. [DOI] [PubMed] [Google Scholar]
- 8.ISID (International Society for Infectious Diseases). Bluetongue - Europe: (France) Bovine, Ovine, Serotype 8, Update; 2017. ProMED-mail, 20170223.4859510. http://www.promedmail.org/post/20170223.4859510. Accessed 22 Apr 2018.
- 9.ISID (International Society for Infectious Diseases). Bluetongue - Europe (03): UK (England, Scotland) ex France, bovine, BTV-8. ProMED-mail, 20170223.4859510 https://www.promedmail.org/post/5400555. Accessed 22 Apr 2018.
- 10.Hoffmann B, Scheuch M, Hoper D, Jungblut R, Holsteg M, Schirrmeier H, et al. Novel orthobunyavirus in cattle, Europe, 2011. Emerg Infect Dis. 2012;18:469–472. doi: 10.3201/eid1803.111905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.EFSA (European Food Safety Authority) (2013). “Schmallenberg” virus: analysis of the epidemiological data (May 2013), Supporting Publications; 2013 EN-429 [22 pp.] http://www.efsa.europa.eu/en/publications. Accessed 22 Apr 2018.
- 12.Elbers A, Meiswinkel R, van Weezep E, van Kooi E. Schmallenberg virus detected by RT-PCR in Culicoides biting midges captured during the 2011 epidemic in the Netherlands. Emerg Infect Dis. 2013;19:106–109. doi: 10.3201/eid1901.121054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goffredo M, Monaco F, Capelli G, Quaglia M, Federici V, Catalani M, et al. Schmallenberg virus in Italy: a retrospective survey in Culicoides stored during the bluetongue Italian surveillance program. Prev Vet Med. 2013;111:230–236. doi: 10.1016/j.prevetmed.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen LD, Kirkeby C, Bødker R, Kristensen B, Rasmussen TB, Belsham G, Bøtner A. Rapid spread of Schmallenberg virus-infected biting midges (Culicoides spp.) across Denmark in 2012. Transbound Emerg Dis. 2014;61:12–16. doi: 10.1111/tbed.12189. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen LD, Kristensen B, Kirkeby C, Rasmussen TB, Belsham GJ, Bødker R, Bøtner A. Culicoids as vectors of Schmallenberg virus. Emerg Infect Dis. 2012;18:1204. doi: 10.3201/eid1807.120385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nd R. Deblauwe I, Deken Rd, Vantieghem P, Madder M, Geysen D, et al. Detection of Schmallenberg virus in different Culicoides spp. by real-time RT-PCR. Transbound Emerg Dis. 2012;59:471–475. doi: 10.1111/tbed.12000. [DOI] [PubMed] [Google Scholar]
- 17.Collins ÁB, Barrett D, Doherty ML, Larska M, Mee JF. Post-epidemic Schmallenberg virus circulation: parallel bovine serological and Culicoides virological surveillance studies in Ireland. BMC Vet Res. 2016;12:234. doi: 10.1186/s12917-016-0865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins ÁB, Grant J, Barrett D, Doherty M, Mee JF. Schmallenberg virus: predicting within-herd seroprevalence using bulk-tank milk antibody titres and exploring individual animal antibody titres using empirical distribution functions (EDF) Prev Vet Med. 2017;143:68–78. doi: 10.1016/j.prevetmed.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Collins ÁB, Barrett DJ, Doherty ML, McDonnell M, Mee JF. Significant re-emergence and recirculation of Schmallenberg virus in previously exposed dairy herds in Ireland in 2016. Transbound Emerg Dis. 2017;12:234. doi: 10.1111/tbed.12685. [DOI] [PubMed] [Google Scholar]
- 20.DAFM (Department of Agriculture Food and the Marine). National BTV Vector Surveillance Program 2007-2009; 2008-2010. https://www.agriculture.gov.ie/bluetongue/. Accessed 22 Apr 2018.
- 21.Sanders CJ, Shortall CR, Gubbins S, Burgin L, Gloster J, Harrington R, et al. Influence of season and meteorological parameters on flight activity of Culicoides biting midges. J Appl Ecol. 2011;48:1355–1364. doi: 10.1111/j.1365-2664.2011.02051.x. [DOI] [Google Scholar]
- 22.Jess S, Thompson G, Clawson S, Forsythe I, Rea I, Gordon A, et al. Surveillance of biting midges (Culicoides spp.) in Northern Ireland: influence of seasonality, surrounding habitat and livestock housing. Med Vet Entomol. 2018;32:48–60. [DOI] [PubMed]
- 23.Bradshaw B, Mooney J, Ross P, Murphy C, O’Donovan T, Sanchez C, et al. Schmallenberg virus cases identified in Ireland. Vet Rec. 2012;171:540–1. [DOI] [PubMed]
- 24.Campbell JA, Pelham-Clinton EC. A taxonomic review of the British species of Culicoides Latrielle (Diptera: Ceratopognidae) Proc R Soc Edinb. 1960;67:181–203. [Google Scholar]
- 25.Mathieu B, Cêtre-Sossah C, Garros C, Chavernac D, Balenghien T, Carpenter S, et al. Development and validation of IIKC: an interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the Western Palaearctic region. Parasit Vectors. 2012;5:137. doi: 10.1186/1756-3305-5-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 27.Hebert PD, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the Neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci USA. 2004;101:14812–14817. doi: 10.1073/pnas.0406166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madden T. The BLAST sequence analysis tool. In: McEntyre J, Ostell J, editors. The NCBI Handbook. Bethesda: National Center for Biotechnology Information; 2003.
- 29.JNCC (Joint Nature Conservation Committee). Handbook for Phase 1 habitat survey - a technique for environmental audit; 2010. Petersborough, UK: Joint Nature Conservation Committee,. http://jncc.defra.gov.uk/page-2468. Accessed 22 Apr 2018.
- 30.Anon. Met Eireann, summary weather reports; 2014. https://www.met.ie/climate/available-data/historical-data. Accessed 22 Apr 2018.
- 31.Harrup L, Purse B, Golding N, Mellor P, Carpenter S. Larval development and emergence sites of farm-associated Culicoides in the United Kingdom. Med Vet Entomol. 2013;27:441–449. doi: 10.1111/mve.12006. [DOI] [PubMed] [Google Scholar]
- 32.Kettle D, Lawson J. The early stages of British biting midges Culicoides Latreille (Diptera: Ceratopogonidae) and allied genera. Bull Entomol Res. 1952;43:421–467. doi: 10.1017/S000748530002945X. [DOI] [Google Scholar]
- 33.Steinke S, Lühken R, Kiel E. Assessment of the abundance of Culicoides chiopterus and Culicoides dewulfi in bovine dung: a comparison of larvae extraction techniques and emergence traps. Vet Parasitol. 2014;205:255–62. [DOI] [PubMed]
- 34.McCarthy TK, Bateman A, Nowak D, Higgins T, Geraghty F, Sheehy E, et al. BTV Vector Surveillance 2007–2009, Annual Technical Report 2009/2010. https://www.agriculture.gov.ie/media/migration/animalhealthwelfare/diseasecontrols/bluetonguedisease/BTVVectorSurveillance0910FinalReport.pdf. Accessed 22 Apr 2018.
- 35.Viennet E, Garros C, Lancelot R, Allène X, Gardès L, Rakotoarivony I, et al. Assessment of vector/host contact: comparison of animal-baited traps and UV-light/suction trap for collecting Culicoides biting midges (Diptera: Ceratopogonidae), vectors of orbiviruses. Parasit Vectors. 2011;4:119. doi: 10.1186/1756-3305-4-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter S, Szmaragd C, Barber J, Labuschagne K, Gubbins S, Mellor P. An assessment of Culicoides surveillance techniques in northern Europe: have we underestimated a potential bluetongue virus vector? J Appl Ecol. 2008;45:1237–1245. [Google Scholar]
- 37.González MA, Alarcón-Elbal PM, Venter GJ, López S. Flight and swarming behaviour of Culicoides species (Diptera: Ceratopogonidae) on a livestock farm in northern Spain. Vet Ital. 2017;53:157–166. doi: 10.12834/VetIt.371.1663.4. [DOI] [PubMed] [Google Scholar]
- 38.Root DS, Gerhardt RR. Seasonal emergence patterns of Culicoides (Diptera: Ceratopogonidae) in eastern Tennessee. J Ag Entomol. 1991;8:127–35.
- 39.Venter GJ, Labuschagne K, Hermanides KG, Boikanyo SNB, Majatladi DM, Morey L. Comparison of the efficiency of five suction light traps under field conditions in South Africa for the collection of Culicoides species. Vet Parasitol. 2009;166:299–307. doi: 10.1016/j.vetpar.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 40.Venter G, Hermanides K. Comparison of black and white light for collecting Culicoides imicola and other livestock-associated Culicoides species in South Africa. Vet Parasitol. 2006;142:383–385. doi: 10.1016/j.vetpar.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Mullen GR, Durden LA. Medical and Veterinary Entomology. London: Academic Press; 2009. Biting Midges (Ceratopogonidae) pp. 169–188. [Google Scholar]
- 42.Hunt G, Schmidtmann E. Care, maintenance, and experimental infection of biting midges. In: Marquardt WC, editor. The Biology of Disease Vectors. 2nd ed. London: Academic Press; 2005.
- 43.Holmes P, Boorman J. Light and suction trap catches of Culicoides midges in southern England. Med Vet Entomol. 1987;1:349–359. doi: 10.1111/j.1365-2915.1987.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 44.Meiswinkel R, van Rijn P, Leijs P, Goffredo M. Potential new Culicoides vector of bluetongue virus in northern Europe. Vet Rec. 2007;161:564–565. doi: 10.1136/vr.161.16.564. [DOI] [PubMed] [Google Scholar]
- 45.Townley P, Baker K, Quinn P. Preferential landing and engorging sites of Culicoides species landing on a horse in Ireland. Equine Vet J. 1984;16:117–120. doi: 10.1111/j.2042-3306.1984.tb01876.x. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen BO. Some observations on biting midges (Diptera: Ceratopogonidae) attacking grazing cattle in Denmark. Insect Syst Evol. 1971;2:94–98. doi: 10.1163/187631271X00103. [DOI] [Google Scholar]
- 47.Wittmann EJ, Baylis M. Climate change: effects on Culicoides-transmitted viruses and implications for the UK. Vet J. 2000;160:107–17. [DOI] [PubMed]
- 48.McGrath G, More SJ, O’Neill R. Hypothetical route of the introduction of Schmallenberg virus into Ireland using two complementary analyses. Vet Rec. 2018;182:226. [DOI] [PMC free article] [PubMed]
- 49.Sedda L, Rogers DJ. The influence of the wind in the Schmallenberg virus outbreak in Europe. Sci Rep. 2013;3:3361. doi: 10.1038/srep03361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sedda L, Brown HE, Purse BV, Burgin L, Gloster J, Rogers DJ. A new algorithm quantifies the roles of wind and midge flight activity in the bluetongue epizootic in northwest Europe. Proc R Soc Lond B Biol Sci. 2012;1737:2354. doi: 10.1098/rspb.2011.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menzies F, McCullough S, McKeown I, Forster J, Jess S, Batten C, et al. Evidence for transplacental and contact transmission of bluetongue virus in cattle. Vet Rec. 2008;163:203–209. doi: 10.1136/vr.163.7.203. [DOI] [PubMed] [Google Scholar]
- 52.DAFM (Department of Agriculture, Food and the Marine). AIM bovine statistics annual reports; 2012–2015. https://www.agriculture.gov.ie/animalhealthwelfare/animalidentificationmovement/cattle/. Accessed 22 Apr 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Between-site variations in total Culicoides abundance on 10 sentinel farms (Farms 1–10) in the south of the Republic of Ireland. (TIF 250 kb)
Table S1. Physiological/parity status of female Culicoides with the potential to transmit arboviruses collected on 10 Irish farms during part of the 2014 vector-active season (July-November). (DOCX 19 kb)
Data Availability Statement
All data supporting the conclusions of this article are included within the article and its additional files. The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.


